Abstract
Objective
The mechanisms leading to worse outcomes in African-American (AA) women with preeclampsia/eclampsia remain unclear. Our objective was to identify racial differences in maternal comorbidities, peripartum characteristics, and maternal and fetal outcomes.
Methods/Results
When compared to white women with preeclampsia/eclampsia, AA women had an increased unadjusted risk of inpatient maternal mortality (OR 3.70, 95% CI: 2.19-6.24). After adjustment for covariates, in-hospital mortality for AA women remained higher than that for white women (OR 2.85, 95% CI: 1.38-5.53), while, the adjusted risk of death among Hispanic women did not differ from that for white women. We also found an increased risk of intrauterine fetal death (IUFD) among AA women. When compared to white women with preeclampsia, AA women had an increased unadjusted odds of IUFD (OR 2.78, 95% CI: 2.49-3.11), which remained significant after adjustment for covariates (adjusted OR 2.45, 95% CI: 2.14-2.82). In contrast, IUFD among Hispanic women did not differ from that for white women after adjusting for covariates.
Conclusions and Relevance
Our data suggest that African American women are more likely to have risk factors for preeclampsia and more likely to suffer an adverse outcome during peripartum care. Future research should examine whether controlling co-morbidities and other risk factors will help to alleviate racial disparities in outcomes in this cohort of women.
Keywords: hypertension, mortality, epidemiology
Introduction
African Americans (AA) experience a threefold higher risk of mortality from pregnancy related complications when compared to white women1-4. The mortality rate for AA and white mothers is 38.9 and 12.0 per 100,000 live births4 and has remained unchanged for the last 50 years with approximately 44% of these deaths deemed preventable5
Hypertensive disorders of pregnancy, including preeclampsia/eclampsia, are among the causes of maternal death deemed most preventable by the National Partnership for Maternal Safety.6 In AA women with hypertensive disorders of pregnancy, previous studies have demonstrated higher complication rates and mortality even after controlling for socioeconomic differences2, 7, 8. Higher case fatality rates from preeclampsia/eclampsia in African-American women contribute to these observed mortality differences2. However, no large, population based studies have examined why these differences in maternal and fetal outcomes exist. We hypothesized that AA women with preeclampsia/eclampsia were more likely to suffer higher rates of severe maternal comorbidities and complications including congestive heart failure (CHF), cardiac arrest, stroke, etc. which may then contribute to worsened peripartum maternal and fetal outcomes. Identifying severe maternal comorbidities may help clinicians devise strategies for improving both maternal and fetal outcomes in this patient population.
In this study, we used data from the National Inpatient Sample to examine racial differences in the incidence of preeclampsia/eclampsia in the United States. We then compared differences between white, African American (AA), and Hispanic women with respect to maternal comorbidities, characteristics of pregnancy and delivery, complications and maternal and fetal mortality, in parturients with preeclampsia and eclampsia.
Methods
Data Source
We performed a retrospective cohort analysis using data from the National Inpatient Sample (NIS) from 2004 to 2012. The NIS database provides discharge data on approximately eight million hospital stays annually, is maintained by the Healthcare Utilization Project (HCUP) of the Agency for Healthcare Quality and Research, and is the largest inpatient care database in the United States9. The NIS randomly samples 20% of all discharges from all HCUP hospitals (N= 4,378 in 2012). National estimates are obtained by weighting NIS data to provide data estimates for 95% of all inpatient hospitalization in the United States. The NIS dataset includes demographic information, comorbidities, hospital characteristics, procedures performed, inpatient mortality, and disposition.9 Hospitals can also be stratified based on region, ownership/control (profit/nonprofit), teaching status, bed size and location (urban/rural). Because the NIS has no data elements that identify individual patients, the Committee on Clinical Investigations at both Beth Israel Deaconess Medical Center and the University of Chicago declared this study exempt.
Data in the NIS is validated using both internal and external quality assessments. External validation was excellent when compared against the American Hospital Association Annual Survey Database, the National Hospital Discharge Survey from the National Center for Health Statistics and the MedPAR Inpatient Data from the Centers of Medicare and Medicaid Services10.
Study Population
The study population included all patients in the NIS with a diagnosis of preeclampsia/eclampsia from 2004 through 2012, as identified by the presence of International Classification of Diseases, Ninth Revision (ICD-9-CM) codes on discharge.
Exposure, Outcome and Covariates
The exposure of interest was the patient’s race (white, AA, or Hispanic). NIS defines race as white, black, Hispanic, Asian or Pacific Islander, Native American and other. The primary outcome was inpatient mortality during hospitalization. IUFD was defined as fetal demise during hospitalization. Potential confounders were identified and adjusted for in our final analysis.
Covariates were identified based upon known risk factors for preeclampsia that include parity, multiple gestation, diabetes (including gestational diabetes), obesity and preexisting hypertension
To account for potential confounding due to known risk factors for preeclampsia we adjusted for age, characteristics of pregnancy (parity, preterm labor), delivery type (Caesarean section versus vaginal), and maternal characteristics (diabetes, gestational diabetes, obesity). To adjust for socioeconomic status we utilized median household income of residents in the patient’s zip code, payer type (Medicare, Medicaid, private insurance, self-pay, no charge and other), region and hospital type.
Missing data
In our study cohort, race was missing in 18.36% of patient admissions. We used two approaches to account for these missing data. We first performed the analysis only on patients in our cohort who had complete case information (complete case analysis). We then performed multiple imputation to generate missing values for race using a weighted sequential hot deck method which does not place any restrictions on missing data patterns to create five complete datasets11. Pooled values were then utilized to calculate race-specific outcomes. This multiple imputation approach is recommended by HCUP to overcome any inherent biases in the complete case analysis approach12.
Statistical Analysis
All analyses were performed using SAS 9.3 (SAS Institute, Cary, NC) and SUDAAN 10.0 (Research Triangle Institute, Research Triangle Park, NC). For all analyses, weighted estimates were used to adjust for design effects of the sampling. Categorical variables were presented as frequencies or proportions and compared using the chi-square test. An unadjusted logistic regression model was used to estimate the risk of inpatient mortality among patients with preeclampsia/eclampsia relative to race. We then used a multivariate adjusted model, with terms for patient age, median household income, region, hospital type and region, mode of delivery, parity, diabetes, year and payer type. To account for in-hospital clustering we used generalized estimating equations with robust variance estimates. All tests were two sided and p-values <0.05 were considered statistically significant.
Results
Demographics and Patient Characteristics
Demographics, clinical characteristics, and comorbidities of our study cohort are presented in Table 1. We identified 1,175,046 weighted patient discharges with preeclampsia/eclampsia. The incidence of preeclampsia was 6.04% in African American women, compared to 2.58% in Hispanic women and 3.75% among white women (p <0.0001). AA women with preeclampsia were younger than white women and more likely to reside in areas in the lowest quartile for median income.
Table 1.
Patient Characteristics at Presentation
| Weighted N (%) | P-Value | ||||
|---|---|---|---|---|---|
| White | Black | Hispanic | |||
| No. of Patients | 568124 (3.75) | 235007 (6.04) | 268014 (2.58) | <0.0001 | |
|
| |||||
| Age (years) | |||||
| 10 – 19 | 28135 (5.48) | 25115 (11.86) | 26767 (11.07) | <0.0001 | |
| 20 – 29 | 256729 (49.99) | 111925 (52.88) | 123190 (50.97) | ||
| 30 – 39 | 203769 (39.68) | 65958 (31.16) | 81266 (33.62) | ||
| 40 – 49 | 24913 (4.85) | 8675 (4.10) | 10472 (4.33) | ||
| Median Household Income for Patient’s Zip code (percentile) | |||||
| 0 - 25th | 127504 (22.42) | 116237 (49.94) | 106755 (40.22) | <0.0001 | |
| 26th - 50th | 148532 (26.12) | 51382 (22.08) | 65418 (24.65) | ||
| 51st - 75th | 151746 (26.68) | 39509 (16.97) | 55926 (21.07) | ||
| 76th - 100th | 140973 (24.79) | 25627 (11.01) | 37304 (14.06) | ||
| Primary Expected Payer | |||||
| Medicare | 4583 (0.80) | 3503 (1.46) | 1395 (0.51) | <0.0001 | |
| Medicaid | 175778 (30.50) | 147173 (61.51) | 174834 (64.03) | ||
| Private Insurance | 367865 (63.83) | 76687 (32.05) | 73572 (26.94) | ||
| Self-pay | 8885 (1.54) | 6561 (2.74) | 17016 (6.23) | ||
| No Charge | 713 (0.12) | 680 (0.28) | 1796 (0.66) | ||
| Other | 18463 (3.20) | 4667 (1.95) | 4451 (1.63) | ||
|
| |||||
|
Hospital Characteristics
| |||||
| Hospital Region | |||||
| Northeast | 108309 (18.77) | 49173 (20.49) | 36063 (13.20) | <0.0001 | |
| Midwest | 117231 (20.31) | 33122 (13.80) | 11394 (4.17) | ||
| South | 236670 (41.01) | 139447 (58.12) | 119230 (43.63) | ||
| West | 114921 (19.91) | 18200 (7.59) | 106616 (39.01) | ||
|
| |||||
|
Patient Comorbidities
| |||||
| Hypertension | 53826 (9.33) | 40040 (16.69) | 23319 (8.53) | <0.0001 | |
| Obesity | 47199 (8.18) | 28619 (11.93) | 20965 (7.67) | <0.0001 | |
| Diabetes Without Chronic Complications | 15385 (2.67) | 8374 (3.49) | 9518 (3.48) | <0.0001 | |
| Diabetes With Chronic Complications | 2828 (0.49) | 1498 (0.62) | 1257 (0.46) | <0.0001 | |
| Renal Failure | 1252 (0.22) | 1054 (0.44) | 994 (0.36) | <0.0001 | |
| Liver Disease | 1039 (0.18) | 415 (0.17) | 673 (0.25) | <0.0001 | |
|
| |||||
|
Pregnancy and Delivery Characteristics
| |||||
| Multiple Gestation | 39724 (6.88) | 10433 (4.35) | 9635 (3.53) | <0.0001 | |
| Multiparity | 2783 (0.48) | 3426 (1.43) | 3460 (1.27) | <0.0001 | |
| Cesarean Delivery | 324787 (26.28) | 137240 (57.20) | 144268 (52.79) | 0.0001 | |
Patient Comorbidities
When compared to white women, AA women with preeclampsia had a higher rate of hypertension, diabetes, obesity, and acute renal failure (Table 1).
Pregnancy, delivery characteristics and obstetrical complications
AA women with preeclampsia/eclampsia were more likely to be multiparous, to have a placental abruption, and to have undergone a delivery by cesarean section than white women (Table 1). Although AA women with preeclampsia/eclampsia had similar rates of postpartum hemorrhage than white women, Hispanic women had higher rates of postpartum hemorrhage than both African American and white women (Table 2).
Table 2.
Complications
| Weighted N (%) | P-Value | |||
|---|---|---|---|---|
| White | Black | Hispanic | ||
|
Patient Complications
| ||||
| Acute Respiratory Distress Syndrome (ARDS) | 200 (0.03) | 166 (0.07) | 145 (0.05) | 0.08 |
| Pulmonary Edema | 162 (0.03) | 166 (0.07) | 44 (0.02) | 0.0005 |
| Pulmonary Embolism | 331 (0.06) | 235 (0.10) | 174 (0.06) | 0.10 |
| Congestive Heart Failure | 1752 (0.30) | 1275 (0.53) | 728 (0.27) | <0.0001 |
| Peripartum Cardiomyopathy | 1020 (0.18) | 701 (0.29) | 389 (0.14) | 0.0003 |
| Mechanical Ventilation | 2070 (0.36) | 1972 (0.82) | 1380 (0.50) | <0.0001 |
| Coagulopathy | 13675 (2.37) | 5325 (2.22) | 5614 (2.05) | 0.01 |
| Stroke | 573 (0.10) | 397 (0.17) | 343 (0.13) | 0.03 |
| Cardiac Arrest | 85 (0.01) | 85 (0.04) | 78 (0.03) | 0.0001 |
| Died | 123 (0.02) | 189 (0.08) | 105 (0.04) | 0.001 |
|
| ||||
|
Obstetrical Complications
| ||||
| Intrauterine Fetal Death | 3137 (0.54) | 3591 (1.50) | 1811 (0.66) | <0.0001 |
| Placental Abruption | 12814 (2.22) | 7983 (3.33) | 6605 (2.42) | <0.0001 |
| Postpartum Hemorrhage | 32662 (5.66) | 13765 (5.74) | 20696 (7.57) | <0.0001 |
Peripartum Complications
The incidence of peripartum complications are listed in Table 2. When compared to white women, AA women with preeclampsia/eclampsia had a higher likelihood of severe maternal complications including cardiac arrest, acute respiratory distress syndrome (ARDS), pulmonary edema, pulmonary embolism, congestive heart failure and peripartum cardiomyopathy, and mechanical ventilation. (Figure 1).
Figure 1.
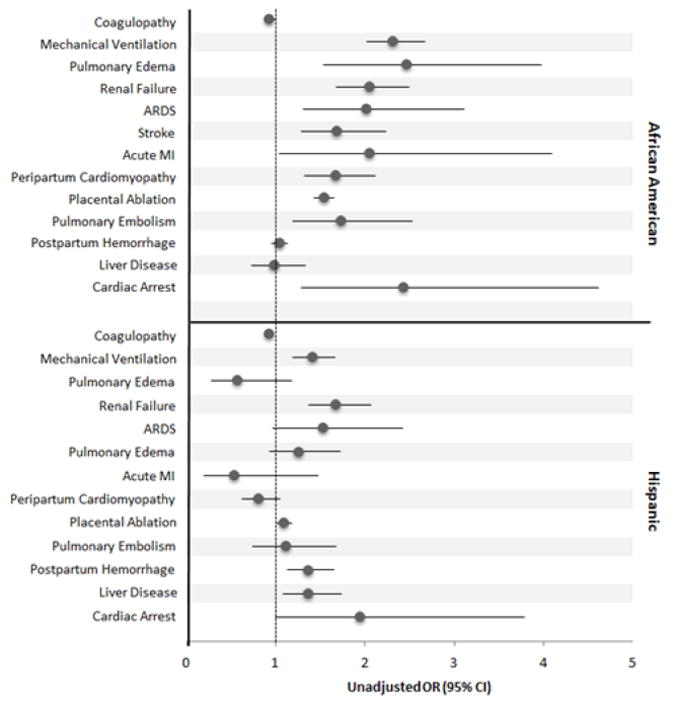
Odds ratios for severe maternal complications in African American and Hispanic women with preeclampsia. African American women were significantly more likely than white women to need mechanical ventilation, develop pulmonary edema, renal failure, ARDS, stroke, acute MI, peripartum cardiomyopathy, placental ablation, pulmonary embolism, or cardiac arrest.
Inpatient Mortality
When compared to white women with preeclampsia/eclampsia, AA women had an increased unadjusted risk of inpatient maternal mortality (OR 3.70, 95% CI: 2.19-6.24). After adjustment for covariates, in-hospital mortality for AA women remained higher than that for white women (OR 2.85, 95% CI: 1.38-5.53). In contrast, the unadjusted and adjusted risk of death among Hispanic women did not differ from that for white women (unadjusted OR 1.81, 95% CI: 0.98–3.36; adjusted OR 1.44, 95% CI: 0.74-2.79). (Table 3). Similar mortality differences between AA and white women were observed after multiple imputation for both unadjusted (OR 3.93, 95% CI: 2.41-6.54) and adjusted analyses (OR 2.88, 95% CI: 1.63-5.10).
Table 3.
Adjusted and Unadjusted Associations between Race and Complications for Patients with Preeclampsia/Eclampsia
| Race | |||
|---|---|---|---|
| White | Black | Hispanic | |
|
Unadjusted OR (95% CI)
| |||
| Maternal Mortality | 1.0 [Reference] | 3.70 [2.19, 6.24] | 1.81 [0.98, 3.36] |
| Intrauterine Fetal Death | 1.0 [Reference] | 2.78 [2.49, 3.11] | 1.22 [1.08, 1.39] |
|
| |||
|
Adjusted OR* (95% CI)
| |||
| Maternal Mortality** | 1.0 [Reference] | 2.85 [1.38, 5.53] | 1.44 [0.74, 2.79] |
| Intrauterine Fetal Death‡ | 1.0 [Reference] | 2.45 [2.14, 2.82] | 0.96 [0.82, 1.13] |
Adjusted for age group, median household income, hospital region, teaching status, mode of delivery, multiparity, diabetes (with and without complications), year, preexisting hypertension, obesity and payer type
Adjusted for age group, median household income, hospital region, teaching status, mode of delivery, multiparity, diabetes (with and without complications), year, obesity and payer type
Intrauterine Fetal Death (IUFD)
We also found an increased risk of intrauterine fetal death (IUFD) among AA women. When compared to white women with preeclampsia, AA women had an increased unadjusted odds of IUFD (OR 2.78, 95% CI: 2.49-3.11), which remained significant after adjustment for covariates (adjusted OR 2.45, 95% CI: 2.14-2.82). In contrast, IUFD among Hispanic women did not differ from that for white women (adjusted OR 0.96, 95% CI: 0.82- 1.13). (Table 3)
Discussion
Using the National Inpatient Sample, we found racial disparities in severe maternal morbidities and peripartum characteristics among preeclamptic women. Our data are consistent with previously published observations that AA women develop preeclampsia at higher rates than their white counterparts2, 7, 8. In addition, we found that AA women with preeclampsia had higher rates of maternal and obstetric complications, and experienced higher unadjusted and adjusted odds of mortality when compared to white women with preeclampsia/eclampsia. Finally, we observed that AA women had higher unadjusted and adjusted odds of IUFD when compared to white women.
Several explanations are possible for our data. AA women had higher odds of severe maternal complications, which may have resulted in the observed differences in maternal and fetal outcomes. Previous work has demonstrated an increased prevalence of cardiovascular disease13-18 among AA women when compared to white women, and also higher mortality rates for congestive heart failure14, 19, coronary artery disease13, 15, 18, 20, hypertension20, 21 and stroke15, 18, 20. The explanation for these previously noted disparities in cardiovascular disease and death rates are incompletely understood but may include genetic differences, and disparities in socioeconomic status, limited access to health care, and limited awareness among practitioners of outcome disparities16, 18, 20, 22-25.
In our analysis, we demonstrate a previously unreported higher incidence of comorbidities among AA women that may be risk factors for preeclampsia/eclampsia, including hypertension, diabetes, obesity, acute renal failure and chronic renal failure. It is possible that more aggressive treatment of these underlying comorbidities may improve outcomes for AA women with preeclampsia. Similar racial disparities in adverse outcomes have been observed for many of these individual complications in non-pregnant patients.20, 26-29 Recent evidence suggests it may be possible to eliminate some racial outcome disparities by medical treatment of certain risk factors (e.g., blood pressure, cholesterol, glycosylated hemoglobin).18, 30-32
We found that the increased likelihood of both maternal and fetal mortality among African American parturients with preeclampsia persisted despite adjustment for socioeconomic and insurance status (SES). This finding suggests that although disparities in socioeconomic status may contribute to racial disparities in outcome, as has been noted in other diseases, other factors may also play a role13, 15, 19, 22, 25, 27, 29.
Hypertension is a major contributor to the etiology of many of maternal complications that we observed, including CHF, pulmonary edema, and stroke33-36. Previous work has demonstrated that AA women not only develop more severe hypertension earlier than white women, but also have higher rates of mortality from hypertension-related end organ damage such as CHF and stroke when compared to other ethnic racial groups37-39. Whether treating maternal hypertension more aggressively in AA women will improve outcomes is unclear. In a recent trial comparing tight (diastolic blood pressure <85mmhg) versus less tight control (DBP<100 mmHg) of blood pressure in mothers with non-proteinuric, preexisting or gestational hypertension, Magee et al demonstrated that although tight control of blood pressure significantly decreased the incidence of severe hypertension (>160/110 mmHg), maternal outcomes or neonatal outcomes did not differ between groups, and progression to preeclampsia occurred at equal rates between groups40. While that trial did enroll AA women, it was not powered to answer the question of whether AA women with hypertension will have improved outcomes with tighter control of blood pressure in pregnancy.
Our study contributes to the existing body of literature on outcomes in AA women with preeclampsia. Work in the last decade has demonstrated increased mortality among AA women with preeclampsia.2, 7, 8 Our data, which draws from a much larger sample, reinforces the presence of racial disparities in outcome and suggests that such disparities may be larger than previously thought. We also provide evidence of an increased incidence of associated comorbidities and severe complications that may account for the increased mortality.
A primary strength of our study was our use of a large, nationally representative sample of patients from the NIS from 2004 to 2012. However, our study also has several limitations. Despite a prevalence of preeclampsia similar to that in previous studies, we were unable to independently verify coding accuracy for race, socioeconomic variables, comorbidities, and outcomes. Also, because the NIS is an administrative database and because of our retrospective study design, we could not verify causal links between race, comorbidities and outcomes. For example, NIS data are unable to determine the time course between disease onset and mortality. Finally, the NIS is only a 20% sample of all hospital discharges, and may not be fully representative of current trends in preeclampsia/eclampsia care.
In conclusion, we have identified racial disparities in maternal and fetal outcomes among women with preeclampsia. Our data suggest that African American women are more likely to have comorbidities associated with preeclampsia, and more likely to suffer an adverse outcome during peripartum care. Future research should examine whether better medical management of comorbidities and treatment of peripartum complications will reduce racial disparities in preeclampsia/eclampsia outcomes.
Acknowledgments
Sources of financial support:
Foundation for Anesthesia Research (FAER), Beth Israel Anesthesia Foundation and the Center for Anesthesia Research Excellence (CARE). S. R. is supported by the NIH KO8 award.
Footnotes
Conflict of interest disclosures:
None.
References
- 1.Chang J, Elam-Evans LD, Berg CJ, Herndon J, Flowers L, Seed KA, Syverson CJ. Pregnancy-related mortality surveillance--United States, 1991--1999. Morbidity and mortality weekly report Surveillance summaries. 2003;52:1–8. [PubMed] [Google Scholar]
- 2.Tucker MJ, Berg CJ, Callaghan WM, Hsia J. The Black-White disparity in pregnancy-related mortality from 5 conditions: differences in prevalence and case-fatality rates. American journal of public health. 2007;97:247–51. doi: 10.2105/AJPH.2005.072975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstetrics and gynecology. 2010;116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 4.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstetrics and gynecology. 2015;125:5–12. doi: 10.1097/AOG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 5.Berg CJ, Harper MA, Atkinson SM, Bell EA, Brown HL, Hage ML, Mitra AG, Moise KJ, Jr, Callaghan WM. Preventability of pregnancy-related deaths: results of a state-wide review. Obstetrics and gynecology. 2005;106:1228–34. doi: 10.1097/01.AOG.0000187894.71913.e8. [DOI] [PubMed] [Google Scholar]
- 6.D’Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstetrics and gynecology. 2014;123:973–7. doi: 10.1097/AOG.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 7.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstetrics and gynecology. 2001;97:533–8. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertension in pregnancy. 2003;22:203–12. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]
- 9.HCUP Nationwide Inpatient Sample (NIS). Healthcare cost and utilization project (HCUP) Rockville (MD): Agency for Healthcare Research and Quality; 1994-2012. [Google Scholar]
- 10.Barrett M, Wilson E, Whalen D. 2007 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. HCUP Methods Series Report # 2010-03. US Agency for Healthcare Research and Quality; Online September 9, 2010. Available: http://www.hcup-us.ahrq.gov/reports/methods.jsp. [Google Scholar]
- 11.Wang CN, Little R, Nan B, Harlow SD. A hot-deck multiple imputation procedure for gaps in longitudinal recurrent event histories. Biometrics. 2011;67:1573–82. doi: 10.1111/j.1541-0420.2011.01558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Houchens R. Missing Data Methods for the NIS and the SID. HCUP Methods Series Report # 2015-01 January 22 USAfHRa 2015 [Google Scholar]
- 13.Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, Havlik R, Hogelin G, Marler J, McGovern P, Morosco G, Mosca L, Pearson T, Stamler J, Stryer D, Thom T. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the national conference on cardiovascular disease prevention. Circulation. 2000;102:3137–47. doi: 10.1161/01.cir.102.25.3137. [DOI] [PubMed] [Google Scholar]
- 14.Davis AM, Vinci LM, Okwuosa TM, Chase AR, Huang ES. Cardiovascular Health Disparities: A Systematic Review of Health Care Interventions. Medical care research and review : MCRR. 2007;64:29S–100S. doi: 10.1177/1077558707305416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie CD, Wigington C, Hong Y. Coronary heart disease and stroke deaths - United States, 2009. Morbidity and mortality weekly report Surveillance summaries. 2013;62(Suppl 3):157–60. [PubMed] [Google Scholar]
- 16.Hayes DK, Greenlund KJ, Denny CH, Neyer JR, Croft JB, Keenan NL. Racial/ethnic and socioeconomic disparities in health-related quality of life among people with coronary heart disease, 2007. Preventing chronic disease. 2011;8:A78. [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 18.Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB. State of disparities in cardiovascular health in the United States. Circulation. 2005;111:1233–41. doi: 10.1161/01.CIR.0000158136.76824.04. [DOI] [PubMed] [Google Scholar]
- 19.Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. The New England journal of medicine. 1999;340:609–16. doi: 10.1056/NEJM199902253400804. [DOI] [PubMed] [Google Scholar]
- 20.Wong MD, Shapiro MF, Boscardin WJ, Ettner SL. Contribution of major diseases to disparities in mortality. The New England journal of medicine. 2002;347:1585–92. doi: 10.1056/NEJMsa012979. [DOI] [PubMed] [Google Scholar]
- 21.Hicks LS, Shaykevich S, Bates DW, Ayanian JZ. Determinants of racial/ethnic differences in blood pressure management among hypertensive patients. BMC cardiovascular disorders. 2005;5:16. doi: 10.1186/1471-2261-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Booske BC, Robert SA, Rohan AM. Awareness of racial and socioeconomic health disparities in the United States: the national opinion survey on health and health disparities, 2008-2009. Preventing chronic disease. 2011;8:A73. [PMC free article] [PubMed] [Google Scholar]
- 23.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the multi-ethnic study of atherosclerosis. Circulation. 2015;131:141–8. doi: 10.1161/CIRCULATIONAHA.114.011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberg BM, Pearson T, Zwanziger J, Mukamel D. Explaining disparities in access to high-quality cardiac surgeons. The Annals of thoracic surgery. 2004;78:18–24. doi: 10.1016/j.athoracsur.2004.01.021. discussion 24-5. [DOI] [PubMed] [Google Scholar]
- 25.Epstein AM, Ayanian JZ. Racial Disparities in Medical Care. New England Journal of Medicine. 2001;344:1471–1473. doi: 10.1056/NEJM200105103441911. [DOI] [PubMed] [Google Scholar]
- 26.Moss M, Mannino DM. Race and gender differences in acute respiratory distress syndrome deaths in the United States: an analysis of multiple-cause mortality data (1979- 1996) Critical care medicine. 2002;30:1679–85. doi: 10.1097/00003246-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Razi RR, Churpek MM, Yuen TC, Peek ME, Fisher T, Edelson DP. Racial disparities in outcomes following PEA and asystole in-hospital cardiac arrests. Resuscitation. 2015;87:69–74. doi: 10.1016/j.resuscitation.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider D, Lilienfeld DE, Im W. The epidemiology of pulmonary embolism: racial contrasts in incidence and in-hospital case fatality. Journal of the National Medical Association. 2006;98:1967–72. [PMC free article] [PubMed] [Google Scholar]
- 29.Soto GJ, Martin GS, Gong MN. Healthcare disparities in critical illness. Critical care medicine. 2013;41:2784–93. doi: 10.1097/CCM.0b013e3182a84a43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke JF, Vijan S, Chekan LA, Makowiec TM, Thomas L, Morgenstern LB. Targeting high-risk employees may reduce cardiovascular racial disparities. The American journal of managed care. 2014;20:725–33. [PubMed] [Google Scholar]
- 31.Chin MH, Walters AE, Cook SC, Huang ES. Interventions to Reduce Racial and Ethnic Disparities in Health Care. Medical care research and review : MCRR. 2007;64:7S–28S. doi: 10.1177/1077558707305413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero CX, Romero TE, Shlay JC, Ogden LG, Dabelea D. Changing trends in the prevalence and disparities of obesity and other cardiovascular disease risk factors in three racial/ethnic groups of USA adults. Advances in preventive medicine. 2012;2012:172423. doi: 10.1155/2012/172423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, Fauad-Tarazi F, Horan MJ, Marcus M, Massie B, et al. The heart in hypertension. The New England journal of medicine. 1992;327:998–1008. doi: 10.1056/NEJM199210013271406. [DOI] [PubMed] [Google Scholar]
- 34.Weintraub NL, Collins SP, Pang PS, Levy PD, Anderson AS, Arslanian-Engoren C, Gibler WB, McCord JK, Parshall MB, Francis GS, Gheorghiade M. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–96. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 35.Gandhi SK, Powers JC, Nomeir A-M, Fowle K, Kitzman DW, Rankin KM, Little WC. The Pathogenesis of Acute Pulmonary Edema Associated with Hypertension. New England Journal of Medicine. 2001;344:17–22. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]
- 36.Johansson BB. Hypertension mechanisms causing stroke. Clinical and experimental pharmacology & physiology. 1999;26:563–5. doi: 10.1046/j.1440-1681.1999.03081.x. [DOI] [PubMed] [Google Scholar]
- 37.Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Archives of internal medicine. 2005;165:2098–104. doi: 10.1001/archinte.165.18.2098. [DOI] [PubMed] [Google Scholar]
- 38.Gutierrez J, Williams OA. A decade of racial and ethnic stroke disparities in the United States. Neurology. 2014;82:1080–2. doi: 10.1212/WNL.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiscella K, Holt K. Racial Disparity in Hypertension Control: Tallying the Death Toll. Annals of Family Medicine. 2008;6:497–502. doi: 10.1370/afm.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Magee LA, von Dadelszen P, Rey E, Ross S, Asztalos E, Murphy KE, Menzies J, Sanchez J, Singer J, Gafni A, Gruslin A, Helewa M, Hutton E, Lee SK, Lee T, Logan AG, Ganzevoort W, Welch R, Thornton JG, Moutquin J-M. Less-Tight versus Tight Control of Hypertension in Pregnancy. New England Journal of Medicine. 2015;372:407–417. doi: 10.1056/NEJMoa1404595. [DOI] [PubMed] [Google Scholar]


