Abstract
A number of gene therapy approaches have been developed for protecting neurons from necrotic neurological insults. Such therapies are limited by the need for transcription and translation of the protective protein, delaying therapeutic impact. As an alternative, we explore the neuroprotective potential of protein therapy, using a fusion protein comprised of the death-suppressing BH4 domain of the Bcl-xL protein and the protein transduction domain of the human immunodeficiency virus Tat protein. This fusion protein decreased neurotoxicity caused by the excitotoxins glutamate and kainic acid in primary hippocampal cultures, and decreased hippocampal damage in vivo in an excitotoxic seizure model.
INTRODUCTION
There has been increasing use of gene therapy approaches, typically utilizing viral vectors (Verma et al., 2005) to protect neurons from in vitro and in vivo models of necrotic neurological insults (such as stroke and ischemia) (Sapolsky, 2003). Unfortunately, drawbacks to this approach include a) an invasive delivery method; b) the fact that viral vectors do not disperse well in target tissues; c) a time lag in the production of adequate amounts of functional protein, particularly given broad inhibition of protein synthesis in challenged neurons (Dwyer et al., 1986; Eck, 1999; Anderson, 1998; Bonadio, 2000; Weise et al., 2000).
An alternative would be protein therapy, using cell-penetrating peptides (CPPs), also known as protein transduction domains (PTDs), to deliver the biologically active therapeutic protein. The most commonly studied CPPs are Antennapedia (Antp) (Prochiantz, 2000; Joliot and Prochiantz, 2004), the herpes simplex virus (HSV) type 1 protein VP22 (Elliot and O’Hare, 1997), and the human immunodeficiency virus (HIV-1) transactivator Tat protein (Schwarze et al., 1999; Embury et al., 2001). CPPs generally have a net positive charge, are less than 30 amino acids long, and can transport cargoes, independent of their size, into cells.
The present study utilizes a CPP-protein therapy approach, using a fusion protein combining the Tat-PTD protein with a fragment of Bcl-xL, a well-characterized member of the anti-apoptotic Bcl-2 family (Gonzalez-Garcia et al., 1995; Motoyama et al., 1995; Parsadanian et al., 1998). Specifically, we have used the conserved N-terminal homology domain (BH4) of Bcl-xL, which appears essential to the neuroprotective effects of the entire protein (Shimizu et al., 2000). We examine its protective potential against models of excitotoxicity in primary hippocampal cultures, and the hippocampus in vivo.
MATERIALS AND METHODS
Preparation of Tat-MYC and Tat-Bcl-xL Fusion Proteins
A fusion protein containing the BH4 domain of Bcl-xL (amino acids 4–23) linked to the 10 amino acid Tat PTD (HIV-Tat48-57) through a β-alanine spacer was obtained (Calbiochem, #197217); previous work has shown that this fusion protein enters target cells (Shimuzu et al., 2000). A control construct, Tat-MYC (2.6 kDa), was graciously provided by Suorjit Paul and Paul Lombroso. For in vitro experiments, 1 mg of protein was dissolved in 653.5 μL dimethyl sulfoxide (DMSO) yielding a concentration of 400 μM Tat-Bcl-xL. For in vivo experiments, 1 mg was dissolved in 65 μL DMSO and 588.5 μL 1x PBS.
Primary Hippocampal Cultures
Mixed neuronal/glial hippocampal cultures were prepared from day 18 fetal rats by standard culturing techniques (Brooke et al., 1997). Cells were plated at a density of 1.2 × 105 cells/cm2 on 96-well plates coated with poly-D-lysine (Sigma, St. Louis, MO), and were used on day 11. Under these conditions, cultures are approximately 20–30% neuronal.
In Vitro excitotoxicity
Fusion proteins (Tat-MYC or Tat-Bcl-xL) were first diluted to a concentration of 40 μM using warm (37°C) minimal essential medium (MEM; Gibco). 10 μL of the appropriate protein or MEM was added to each well (containing 200 μL of media), yielding a concentration of the protein of 2 μM. Two hours later, media were replaced with media containing indicated concentrations of glutamate or kainic acid (Sigma), or media alone. In other experiments, fusion protein was added either at the time of addition of the exctitoxins, or at indicated times thereafter. Other studies using Tat fusion proteins, including Tat-Bcl-xL, have shown that the fusion protein is present at least 24 hours after transduction, both in vitro and in vivo (Lai et al., 2005; Yin et al., 2006). Neuron death was quantified 24 hours after addition of excitotoxin.
Quantification of neuron loss
Neuron loss was quantified immunohistochemically with an ABTS assay (Brooke et al., 1999), an ELISA-based method, making use of MAP2 monoclonal antibodies (Sigma), secondary antibodies (Vectastain ABC kit), and the ABTS kit (Vector, Burlingame, CA, USA). As part of the assay, some wells were treated with a high concentration of excitotoxin (5 mM) known to kill all the neurons (Brooke et al., 1999), but spare some glia. These wells were used as blanks in the assay.
For visualization of MAP2 stain, ABTS solution was removed from each plate, followed by three washes with 1x PBS. Peroxidase labeling was resolved using FAST-DAB solution as a substrate for 6–8 min (Vector Labs Burlingame, CA) according to the manufacturer’s guidelines. Bright field digital images were captured with an Olympus IX70 microscope and Hamamatsu camera (model C4742-95).
In vivo excitotoxicity
Fusion protein (0.03 μg), either Tat-MYC or Tat-Bcl-xL, and kainic acid (0.06 μg) in PBS was injected stereotaxically into the dentate gyrus of the left hemisphere and kainic acid alone was injected into the dentate gyrus of the right hemisphere of adult male Sprague-Dawley rats (275–350g; Charles Rivers) (coordinates from bregma: AP −4.8 mm, ML +/−3.0 mm, and DV −3.0 mm from the surface of the dura). Protein was delivered in 1 μL delivered at a rate of 0.2 μL/min. After delivery, syringes were left in tissue for 5 minutes to allow for diffusion. A second, age-matched group of animals received vehicle alone as positive control.
Twenty-four hours later, rats were perfused, brains removed and post-fixed in 4% paraformaldehyde/20% sucrose solution for approximately 24 hours, sliced into 30 μm sections, and cresyl violet stained. METAMORPH software (Universal Imaging) was used to quantify pixels of intact versus damaged/missing CA3 neurons in 40 coronal sections through the entire hippocampus at ~0.1 mm increments, and performed blind to treatment.
Data analysis
Data analysis was done with SigmaStat, (Jandel Scientific). In vitro data were analysed by one-way ANOVA followed by Tukey post hoc tests (SigmaStat, Jandel Scientific). Cell death is expressed as a percentage of the intrinsic cell death from changing media in control wells incubated without the respective excitotoxin. In vivo data were analyzed by paired t-tests performed comparing hemispheres receiving and not receiving Tat-Bcl-xL or Tat-MYC. A p < 0.05 is considered statistically significant. Data are presented as mean +/− SEM.
RESULTS
Pilot studies indicated that Tat-fusion proteins were not toxic in this system; neither 2 μM Tat-Bcl-xL nor the control protein Tat-MYC were toxic (data not shown). Representative images from in vitro experiments with glutamate illustrate neuronal morphology (Figure 1; neuronal morphology was similar in the subsequent studies utilizing KA; data not shown). Incubation with 50μM glutamate for 24 hours, after a prior two hour incubation with either vehicle or 2 μM Tat-MYC, caused approximately 50% loss of neurons in both treatments and did not differ significantly. However two hour pre-incubation with 2 μM Tat-Bcl-xL prior to glutamate exposure significantly decreased neuron loss (Figure 2).
Figure 1. Representative bright field images of hippocampal neurons exposed to glutamate in vitro.
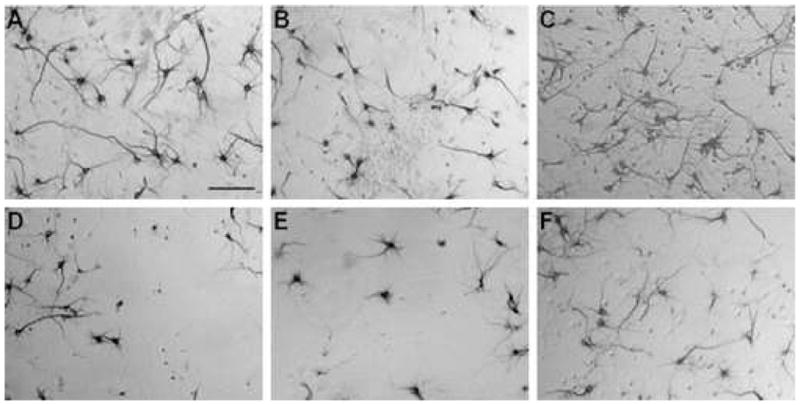
A – F represent neurons incubated under various conditions: A) untreated neurons; B) 2 μM Tat-MYC; C) 2 μM Tat-Bcl-xL; D) 50 μM glutamate; E) 2 μM Tat-MYC followed by 50 μM glutamate; F) 2 μM Tat-Bcl-xL followed by 50 μM glutamate. Scale bar = 100 μm.
Figure 2. Tat-Bcl-xL protects cultured hippocampal neurons against glutamate insult.
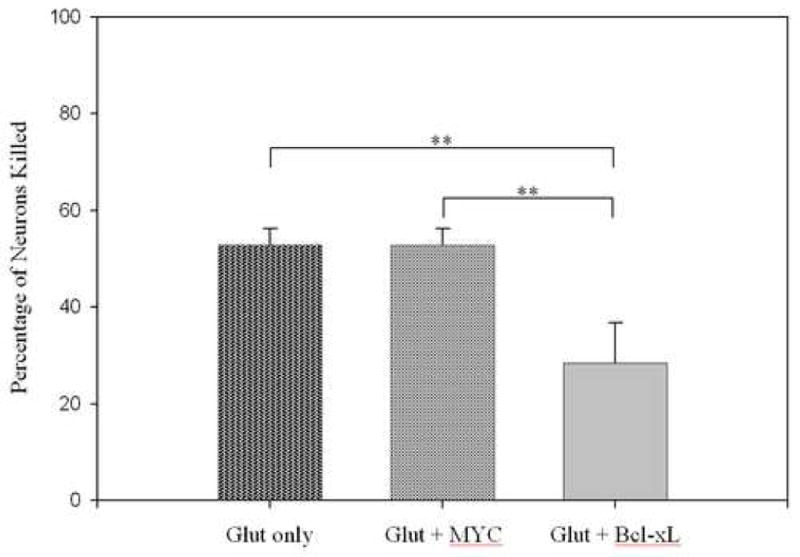
Cultures were incubated with indicated combinations of 50 μM glutamate (Glut), control fusion protein – Tat-MYC (MYC), or experimental Tat-Bcl-xL fusion protein (Bcl-xL). Neuron death is expressed as a percentage of cells killed relative to glutamate-free cultures receiving the same vector treatment. **p < 0.01, n = 12/group.
Incubation with 50 μM KA for 24 hours, after a prior two hour incubation with either vehicle or 2 μM Tat-MYC, caused approximately 50–55% loss of neurons in both treatments and did not differ significantly (Figure 3A), while 100 μM KA caused an approximate 80% loss in both treatments (Figure 3B). However pre-incubation with Tat-Bcl-xL was markedly protective against both KA concentrations.
Figure 3. Tat-Bcl-xL protects cultured hippocampal neurons against kainic acid insult.
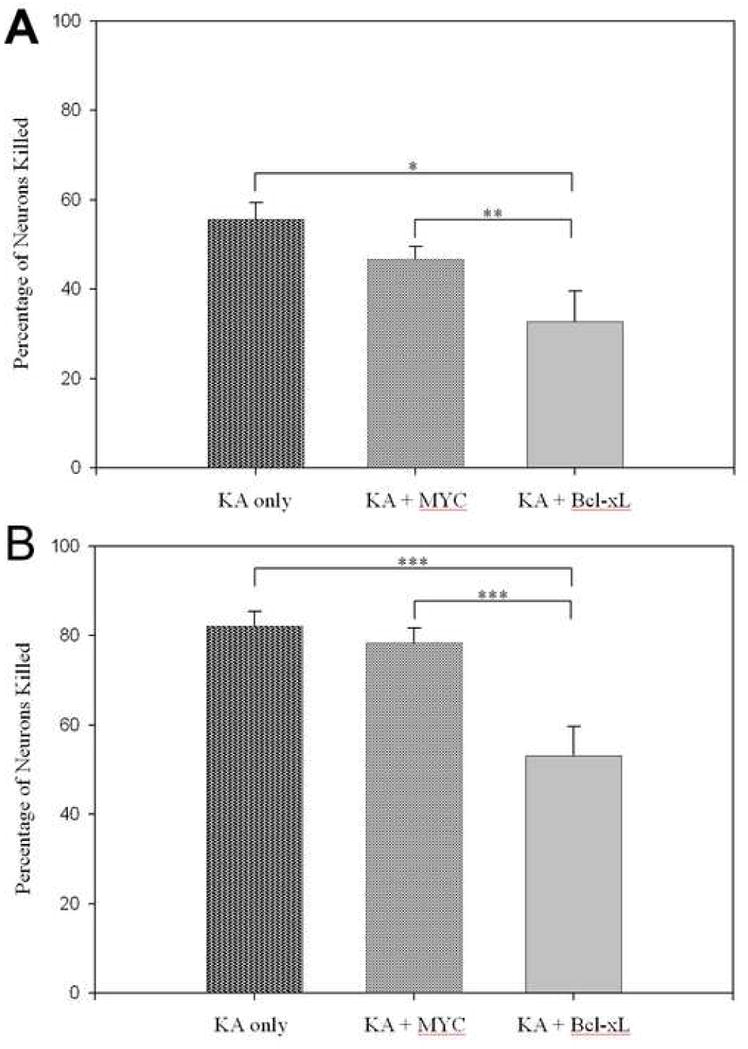
A) Cultures were incubated with indicated combinations of 50 μM kainic acid (KA), 2 μM Tat-MYC (MYC), and 2 μM Tat-Bcl-xl (Bcl-xL). B) Cultures were incubated with indicated combinations of 100 μM kainic acid (KA), 2 μM Tat-MYC (MYC), and 2 μM Tat-Bcl-xl (Bcl-xL). Neuron death is expressed as a percentage of cells killed relative to KA-free cultures receiving the same vector treatment. *p < 0.05, **p < 0.01, ***p < 0.001, n = 24/group.
In the setting of clinical neurology, treatments are typically administered post-injury. Thus, we explored the time window for Tat-Bcl-xL’s protective effects, relative to excitotoxin exposure. Tat-Bcl-xL was protective, whether administered two hours before KA exposure (neurotoxicity in Tat-Bcl-xL/KA wells: 52 +/− 5% that of KA alone; p < 0.01), at the time of KA exposure (Tat-Bcl-xL/KA: 57 +/− 7% of KA alone; p < 0.05), or two hours after KA exposure (43 +/− 8% of KA alone; p <0.05).
We next tested whether Tat-Bcl-xL would protect against KA-induced status epilepticus neurotoxicity in vivo. Saline, Tat-MYC or Tat-Bcl-xL was stereotaxically infused into the dentate gyrus and, 30 minutes later, KA was infused at the same site. The Tat-MYC fusion protein did not show any protection, as the area of intact neurons in the CA3 area is almost equal to that of the contralateral hemisphere receiving only KA. Tat-Bcl-xL, on the other hand, was protective, resulting in approximately 50% more intact neurons in the CA3 area on the hemisphere receiving Tat and KA compared to the contralateral hemisphere that received KA alone (Figure 4).
Figure 4. Neuroprotective effects of Tat-Bcl-xL in a rat model of status epilepticus.
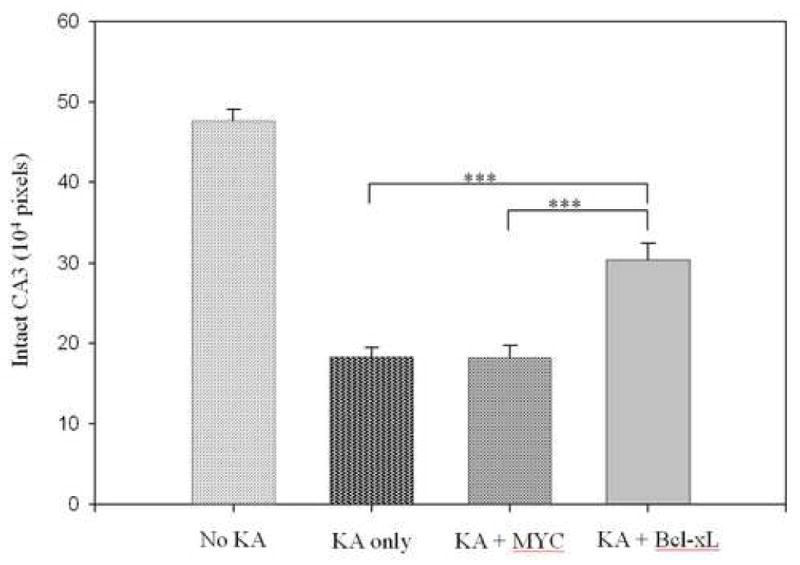
Either 0.03 μg Tat-MYC or Tat-Bcl-xL was infused unilaterally in the dentate gyrus of rats (six each), 30-minutes before infusion of 0.06 μg KA. The control, contralateral hemisphere was infused with KA alone. Vehicle was injected into a second group of animals as a measure of intact CA3 (No KA). ***p < 0.001, n = 6/group.
DISCUSSION
Various gene therapy studies expressing an array of transgenes have demonstrated protection against the neuron loss caused by necrotic neurological insults (Sapolsky, 2003); despite such positive findings, the general approach suffers from the limitation of the lag time between introduction of the vector and the production of functional protein. For example, initial gene expression with HSV vectors takes approximately 2 hours, with latencies even longer for other viral vectors (Oehmig et al., 2004). In exploring the alternative approach of protein therapy, we observe that Tat-Bcl-xL protects against excitotoxins both in vitro and in vivo, even when administered two hours post-insult.
The Bcl-xL gene was chosen for this study because of its anti-apoptotic properties. The protein is a member of the Bcl-2 family, is expressed in adult neurons of the CNS, and can play an essential role in preventing neuron cell death (Gonzalez-Garcia et al., 1995; Motoyama et al., 1995; Kharbanda et al., 1997; Parsadanian et al., 1998; Sastry and Rao, 2000). Bcl-xL, specifically the conserved N-terminal homology domain (BH4), is localized primarily in the mitochondrial membrane and the nuclear envelope (Tsujimoto and Shimizu, 2000), and prevents cytochrome c release and the decline of mitochondrial potential by directly modulating the activity of the voltage-dependent anion channels (Kluck et al., 1997; Yang et al., 1997; Susin et al., 1999; Shimizu et al., 2000); in addition, there are reports of Bcl-xL interacting with caspases (Hu et al., 1998; Pan et al., 1998).
The mechanisms underlying the efficacy of cell penetrating peptides remain unclear, with uncertainty as to the temperature or energy dependency of cell penetration, and whether it involves endocytosis (Derossi et al., 1996; Vives et al., 1997; Futaki et al., 2001; Tung et al., 2002). Amid this uncertainty, the HIV-1-derived Tat peptide (specifically its N-terminal domain) is well characterized for its cell penetrating capacity. It is capable of delivering functional fusion proteins to a variety of cell types (Zhao et al., 2004), including neurons (Schwarze et al., 1999). The fusion of anti-apoptotic Bcl-xL to cell-penetrating Tat holds great promise for being neuroprotective, a property that has been supported in neurodegeneration studies (Dietz et al., 2002; Kilic et al., 2002; Dietz & Bähr, 2005; Diem et al., 2005; Ebert et al., 2005; Dietz et al., 2006) as well as in several other models (Cantara et al., 2004; Klein et al., 2004; Ono et al., 2005; Pizzi et al., 2005; Hotchkiss et al., 2006).
We observed Tat-Bcl-xL to protect against glutamate and KA in vitro, and against KA in vivo. Previous studies with a Tat fusion protein containing the identical BH4 sequence fused to Tat at the same amino acid as in the present case showed that the fusion protein does indeed enter cells and localize to the mitochondria (Shimizu et al., 2000). Our results are in agreement with a report that systemic delivery of Tat-Bcl-xL protects neurons in a stroke model (Cao et al., 2002). Many gene therapy studies utilize viral vectors that are neurotrophic (e.g., HSV-1), with a pronounced preference for infecting neurons. In contrast, peptides such as Tat are not neurotrophic, and presumably penetrate both neurons and glia. However, the insults used were classically neurotoxic, without being gliotoxic; thus, the protection afforded by Tat-Bcl-xL would overwhelmingly be due to decreasing neuron, rather than glial, death.
Tat-Bcl-xL halved the extent of in vitro neurotoxicity induced by approximate LD50 concentrations of glutamate and KA; this is in the range of the extent of protection afforded by many of the anti-apoptotic viral vectors (Sapolsky, 2003). There was also a marked decrease in lesion size in the in vivo KA study, although this capacity for protection may not generalize to other degrees of insult-induced toxicity. Whether a decrease in the extent of neuron death (with either gene or protein therapy) is of sufficient magnitude to translate into sparing of function is often unclear. However, the extent of protection produced by Tat-Bcl-xL in the hippocampus in vivo is of a magnitude previously shown (in gene therapy studies) to be associated with sparing of hippocampal-dependent cognition (McLaughlin et al., 2000; Phillips et al., 2001).
As a final point, there are circumstances where there is a high probability of a necrotic neurological insult occurring (for example, in a person amid a period of repeated transient ischemic attacks), raising the possibility of prophylactic protein or gene therapy. Nonetheless, most insults preclude such intervention, and therapies would have to be carried out following an insult. Thus, the protective effects of Tat-Bcl-xL, even when delivered two hours post KA, is notable; in agreement, systemic administration of Tat-Bcl-xL protects against ischemic damage even when delivered 45 minutes after reperfusion (Cao et al., 2002). Whether there will be differences in the post-insult time window for protection when Bcl-xL is delivered as a fusion protein versus as a gene in a viral vector remains to be studied.
In conclusion, while there are many domains of neurological damage where a gene therapy approach is particularly auspicious, protein therapy may be efficacious in other circumstances. The present report strengthens the feasibility of the latter approach.
Acknowledgments
Technical support was provided by Sheila Brooke. Support was provided by NS37520 and the Adler Foundation (RMS) and by a URO grant (KLJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson W. Human gene therapy. Nature. 1998;392:25–30. doi: 10.1038/32058. [DOI] [PubMed] [Google Scholar]
- Antonawich F, Federoff H, Davis J. BCL-2 transduction, using a herpes simplex virus amplicon, protects hippocampal neurons from transient global ischemia. Exp Neurol. 1999;156:130–137. doi: 10.1006/exnr.1998.7004. [DOI] [PubMed] [Google Scholar]
- Bonadio J. Tissue engineering via local gene delivery. J Mol Med. 2000;78:303–311. doi: 10.1007/s001090000118. [DOI] [PubMed] [Google Scholar]
- Brooke S, Bliss T, Franklin L, Sapolsky R. Quantification of neuron survival in monolayer cultures using an enzyme-linked immunosorbent assay approach, rather than by cell counting. Neurosci Lett. 1999;267:21–24. doi: 10.1016/s0304-3940(99)00315-8. [DOI] [PubMed] [Google Scholar]
- Brooke S, Chan R, Howard S, Sapolsky R. Endocrine modulation of the neurotoxicity of gp120: implications for AIDS-related dementia complex. Proc Natl Acad Sci U S A. 1997;94:9457–9462. doi: 10.1073/pnas.94.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantara S, Donnini S, Giachetti A, Thorpe PE, Ziche M. Exogenous BH4/Bcl-2 peptide reverts coronary endothelial cell apoptosis induced by oxidative stress. J Vasc Res. 2004;41:202–207. doi: 10.1159/000077408. [DOI] [PubMed] [Google Scholar]
- Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp F, Lu A, Ran R, Graham S, Chen J. In Vivo Delivery of a Bcl-xL Fusion Protein Containing the TAT Protein Transduction Domain Protects against Ischemic Brain Injury and Neuronal Apoptosis. J Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornetta K, Morgan R, Anderson W. Safety issues related to retroviral-mediated gene transfer in humans. Hum Gene Ther. 1991;2:5–14. doi: 10.1089/hum.1991.2.1-5. [DOI] [PubMed] [Google Scholar]
- Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem. 1996;271:18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- Diem R, Taheri N, Dietz GP, Kuhnert A, Maier K, Sattler MB, Gadjanski I, Merkler D, Bähr M. HIV-Tat-mediated Bcl-XL delivery protects retinal ganglion cells during experimental autoimmune optic neuritis. Neurobiol Dis. 2005;20:218–226. doi: 10.1016/j.nbd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Bähr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Dietz B, Bähr M. Bcl-x(L) increases axonal numbers but not axonal elongation from rat retinal explants. Brain Res Bull. 2006;70:117–123. doi: 10.1016/j.brainresbull.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Dietz GP, Kilic E, Bähr M. Inhibition of neuronal apoptosis in vitro and in vivo using TAT-mediated protein transduction. Mol Cell Neurosci. 2002;21:29–37. doi: 10.1006/mcne.2002.1165. [DOI] [PubMed] [Google Scholar]
- Dwyer B, Wasterlain C, Fujikawa D, Yamada L. Brain protein metabolism in epilepsy. Adv Neurol. 1986;44:903–918. [PubMed] [Google Scholar]
- Ebert S, Dietz GP, Mitchell TJ, Michel U, Bähr M, Nau R. Limited protection of TAT-Bcl-X(L) against pneumolysin-induced neuronal cell death. Neurosci Lett. 2005;384:349–353. doi: 10.1016/j.neulet.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Eck S. The prospects for gene therapy. Hosp Pract. 1999;34:67–75. doi: 10.3810/hp.1999.10.170. [DOI] [PubMed] [Google Scholar]
- Elliot G, O’Hare P. Intercellular trafficking and protein delivery bya herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- Embury J, Klein D, Pileggi A, Ribeiro M, Jayaraman S, Molano R, Fraker C, Kenyon N, Ricordi C, Inverardi L, Pastori R. Proteins linked to a protein transduction domain efficiently transduce pancreatic islets. Diabetes. 2001;50:1706–1713. doi: 10.2337/diabetes.50.8.1706. [DOI] [PubMed] [Google Scholar]
- Espinoza M, Parer J. Mechanisms of asphyxial brain damage, and possible pharmacologic interventions, in the fetus. Am J Obstet Gynecol. 1991;164:1582–1591. doi: 10.1016/0002-9378(91)91440-8. [DOI] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp Neurol. 2003;179:60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- Futaki S, Suzuki T, Ohashi W, Yagami T, Tanaka S, Ueda K, Sugiura Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem. 2001;276:5836–5840. doi: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia M, Garcia I, Ding L, O’Shea S, Boise L, Thompson C, Nunez G. Bcl-x is expressed in embryonic and postnatal neural tissues and functions to prevent neuronal cell death. Proc Natl Acad Sci U S A. 1995;92:4304–4308. doi: 10.1073/pnas.92.10.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss RS, McConnell KW, Bullok K, Davis CG, Chang KC, Schwulst SJ, Dunne JC, Dietz GP, Bähr M, McDunn JE, Karl IE, Wagner TH, Cobb JP, Coopersmith CM, Piwnica-Worms D. TAT-BH4 and TAT-Bcl-xL peptides protect against sepsis-induced lymphocyte apoptosis in vivo. J Immunol. 2006;176:5471–5477. doi: 10.4049/jimmunol.176.9.5471. [DOI] [PubMed] [Google Scholar]
- Hu Y, Benedict M, Wu D, Inohara N, Nunez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci U S A. 1998;95:4386–4391. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izmailova E, Bertley FM, Huang Q, Makori N, Miller C, Young R, Aldovini A. HIV-1 Tat reprograms immature dendritic cells to express chemoattractants for activated T cells and macrophages. Nat Med. 2003;9:191–197. doi: 10.1038/nm822. [DOI] [PubMed] [Google Scholar]
- Jia W, Wang Y, Qiang D, Tufaro F, Remington R, Cynader M. A bcl-2 expressing viral vector protects cortical neurons from excitotoxicity even when administered several hours after the toxic insult. Mol Brain Res. 1996;42:350–358. doi: 10.1016/s0169-328x(96)00223-9. [DOI] [PubMed] [Google Scholar]
- Joliot A, Prochiantz A. Transduction peptides: from technology to physiology. Nat Cell Biol. 2004;6:189–196. doi: 10.1038/ncb0304-189. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan Z, Saxena S, Weichselbaum R, Nalin C, Kufe D. Role for Bcl-xL as an inhibitor of cytosolic cytochrome C accumulation in DNA damage-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:6939–6942. doi: 10.1073/pnas.94.13.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic E, Dietz GP, Hermann DM, Bähr M. Intravenous TAT-Bcl-Xl is protective after middle cerebral artery occlusion in mice. Ann Neurol. 2002;52:617–622. doi: 10.1002/ana.10356. [DOI] [PubMed] [Google Scholar]
- Kim R. Unknotting the roles of Bcl-2 and Bcl-xL in cell death. Biochem Biophys Res Commun. 2005;333:336–343. doi: 10.1016/j.bbrc.2005.04.161. [DOI] [PubMed] [Google Scholar]
- Klein D, Ribeiro MM, Mendoza V, Jayaraman S, Kenyon NS, Pileggi A, Molano RD, Inverardi L, Ricordi C, Pastori RL. Delivery of Bcl-XL or its BH4 domain by protein transduction inhibits apoptosis in human islets. Biochem Biophys Res Commun. 2004;323:473–478. doi: 10.1016/j.bbrc.2004.08.116. [DOI] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Lai Y, Du L, Dunsmore KE, Jenkins LW, Wong HR, Clark RS. Selectively increasing inducible heat shock protein 70 via TAT-protein transduction protects neurons from nitrosative stress and excitotoxicity. J Neurochem. 2005;94:360–366. doi: 10.1111/j.1471-4159.2005.03212.x. [DOI] [PubMed] [Google Scholar]
- Lawrence M, Ho D, Sun G, Steinberg G, Sapolsky R. Overexpression of Bcl-2 with herpes simplex virus vectors protects CNS neurons against neurological insults in vitro and in vivo. J Neurosci. 1996;16:486–496. doi: 10.1523/JNEUROSCI.16-02-00486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence M, Sun G, Ho D, McIntosh L, Kunis D, McLaughlin J, Sapolsky R, Steinberg G. Herpes simplex viral vectors expressing Bcl-2 are neuroprotective when delivered following a stroke. J Cereb Blood Flow Metab. 1997;17:740–746. doi: 10.1097/00004647-199707000-00003. [DOI] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117:43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Roozendaal B, Dumas T, Gupta A, Ajilore O, Hsieh J, Ho D, Lawrence M, McGaugh JL, Sapolsky R. Sparing of neuronal function postseizure with gene therapy. PNAS. 2000;97:12804–12809. doi: 10.1073/pnas.210350097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- Noguchi H, Matsumoto S. Protein transduction technology: a novel therapeutic perspective. Acta Med Okayama. 2006;60:1–11. doi: 10.18926/AMO/30757. [DOI] [PubMed] [Google Scholar]
- Oehmig A, Fraefel C, Breakefield XO, Ackermann M. Herpes simplex virus type 1 amplicons and their hybrid virus partners, EBV, AAV, and retrovirus. Curr Gene Ther. 2004;4:385–408. doi: 10.2174/1566523043346129. [DOI] [PubMed] [Google Scholar]
- Ono M, Sawa Y, Ryugo M, Alechine AN, Shimizu S, Sugioka R, Tsujimoto Y, Matsuda H. BH4 peptide derivative from Bcl-xL attenuates ischemia/reperfusion injury thorough anti-apoptotic mechanism in rat hearts. Eur J Cardiothorac Surg. 2005;27:117–121. doi: 10.1016/j.ejcts.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- Pan G, O’Rourke K, Dixit VM. Caspase-9, Bcl-XL, and Apaf-1 form a ternary complex. J Biol Chem. 1998;273:5841–5845. doi: 10.1074/jbc.273.10.5841. [DOI] [PubMed] [Google Scholar]
- Parsadanian AS, Cheng Y, Keller-Peck CR, Holtzman DM, Snider WD. Bcl-xL is an antiapoptotic regulator for postnatal CNS neurons. J Neurosci. 1998;18:1009–1019. doi: 10.1523/JNEUROSCI.18-03-01009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, Monje ML, Giuli LC, Meier TJ, Yenari MA, Kunis D, Sapolsky RM. Gene therapy effectiveness differs for neuronal survival and behavioral performance. Gene Therapy. 2001;8:579–585. doi: 10.1038/sj.gt.3301397. [DOI] [PubMed] [Google Scholar]
- Pizzi M, Sarnico I, Boroni F, Benarese M, Steimberg N, Mazzoleni G, Dietz GP, Bähr M, Liou HC, Spano PF. NF-kappaB factor c-Rel mediates neuroprotection elicited by mGlu5 receptor agonists against amyloid beta-peptide toxicity. Cell Death Differ. 2005;12:761–772. doi: 10.1038/sj.cdd.4401598. [DOI] [PubMed] [Google Scholar]
- Prochiantz A. Messenger proteins: homeoproteins, TAT and others. Curr Opin Cell Biol. 2000;12:400–406. doi: 10.1016/s0955-0674(00)00108-3. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Neuroprotective gene therapy against acute neurological insults. Nat Rev Neurosci. 2003;4:61–69. doi: 10.1038/nrn1006. [DOI] [PubMed] [Google Scholar]
- Sastry PS, Rao KS. Apoptosis and the nervous system. J Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Konishi A, Kodama T, Tsujimoto Y. BH4 domain of antiapoptotic Bcl-2 family members closes voltage-dependent anion channel and inhibits apoptotic mitochondrial changes and cell death. Proc Natl Acad Sci U S A. 2000;97:3100–3105. doi: 10.1073/pnas.97.7.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Tenneti L, D’Emilia D, Troy C, Lipton S. Role of caspases in N-methyl-D-aspartate-induced apoptosis in cerebrocortical neurons. J Neurochem. 1998;71:946–959. doi: 10.1046/j.1471-4159.1998.71030946.x. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. Bcl-2 family: life-or-death switch. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- Tung CH, Mueller S, Weissleder R. Novel branching membrane translocational peptide as gene delivery vector. Bioorg Med Chem. 2002;10:3609–3614. doi: 10.1016/s0968-0896(02)00248-1. [DOI] [PubMed] [Google Scholar]
- Verma IM, Weitzman MD. Gene therapy: twenty-first century medicine. Annu Rev Biochem. 2005;74:711–738. doi: 10.1146/annurev.biochem.74.050304.091637. [DOI] [PubMed] [Google Scholar]
- Vile RG, Russell SJ. Retroviruses as vectors. Bdr Med Bull. 1995;51:12–30. doi: 10.1093/oxfordjournals.bmb.a072941. [DOI] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Weise J, Isenmann S, Klocker N, Kugler S, Hirsch S, Gravel C, Bähr M. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–223. doi: 10.1006/nbdi.2000.0285. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Yin W, Cao G, Johnnides MJ, Signore AP, Luo Y, Hickey RW, Chen J. TAT-mediated delivery of Bcl-xL protein is neuroprotective against neonatal hypoxic-ischemic brain injury via inhibition of caspases and AIF. Neurobiol Dis. 2006;21:358–371. doi: 10.1016/j.nbd.2005.07.015. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yenari MA, Cheng D, Sapolsky RM, Steinberg GK. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–1036. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- Zhao M, Weissleder R. Intracellular cargo delivery using tat peptide and derivatives. Med Res Rev. 2004;24:1–12. doi: 10.1002/med.10056. [DOI] [PubMed] [Google Scholar]


