Summary
Objective
Total testosterone concentrations are influenced by sex hormone-binding globulin (SHBG) concentrations, which are decreased by obesity and increased with ageing. Therefore, we sought to understand and compare the associations of ageing and obesity with SHBG.
Design
We performed a retrospective, cross-sectional analysis of the associations of obesity and age on SHBG and testosterone measurements in men being evaluated for hypogonadism.
Patients, Measurements and Analysis
A total of 3671 men who underwent laboratory testing for testosterone deficiency from the Veterans Administration Puget Sound Health Care System from 1997 through 2007 was included. Univariate and multivariate linear regression modelling of the associations between age and body mass index (BMI) and SHBG was performed.
Results
Obesity was associated with a significantly lower SHBG [β = −1·26 (95% CI −1·14, −1·38) nmol/l] per unit increase in BMI. In contrast, ageing was associated with a significantly increased SHBG [β = 0·46 (95% CI 0·39, 0·53) nmol/l per year] (P < 0·001 for both effects). The association of obesity with lower SHBG was two to three times larger than the association of ageing with increased SHBG in both univariate and multivariate modelling. On average, obese men (BMI >30 kg/m2) had significantly lower SHBG and total testosterone concentrations than nonobese men [(mean ± SD) SHBG: 36 ± 22 vs 50 ± 27 nmol/l and total testosterone: 10·5 ± 5·4 nmol/l vs 14·1 ± 7·4 nmol/l; (P < 0·001 for both comparisons)], but calculated free testosterone concentrations did not differ between obese and nonobese men.
Conclusions
We found that the association between obesity and lowered SHBG is greater than the association of ageing with increased SHBG. These competing effects may impact total testosterone measurements for the diagnosis of low testosterone, particularly in obese men.
Introduction
The estimated prevalence of symptomatic male hypogonadism is 2–6% in men older than 30 years and the prevalence increases with age.1–4 A recent analysis of testosterone prescribing practices in the United States and United Kingdom demonstrated that testosterone use has nearly quadrupled in the United States since 2000.5 Some of this increase may be due to overdiagnosis of testosterone deficiency in older or obese men in whom sex hormone-binding globulin (SHBG) concentrations are altered.
To diagnose hypogonadism in men, the 2010 Endocrine Society guidelines recommend documenting symptoms or signs compatible with androgen deficiency in combination with a low serum total testosterone concentration measured in the morning.6 Because most circulating testosterone is bound to SHBG or albumin and only 1–3% of circulating testosterone is unbound or ‘free’, changes in SHBG can greatly affect the interpretation of total testosterone concentrations.7 Therefore, the Endocrine Society guidelines suggest measuring free testosterone using an accurate method if there is concern that the patient may have altered SHBG concentrations. As testosterone concentrations decline with age, SHBG concentrations increase, whereas SHBG concentrations are reduced with obesity and the metabolic syndrome.8–13 However, the relative impact of obesity and ageing on SHBG and total testosterone concentrations has not been clearly quantified in samples that include large numbers of older and obese men. From a clinical standpoint, knowledge regarding the magnitude of the associations between SHBG, ageing and obesity in these populations may be helpful in understanding the value of total testosterone concentration as an initial screening test and determining in which settings the measurement of free testosterone would be useful for the diagnosis of low testosterone.
We hypothesized that ageing would be associated with an increased SHBG, and that association would be greater than the association of obesity (BMI >30) with a reduced SHBG. To determine these associations with precision, we examined them in the setting of a very large sample of men undergoing an evaluation for low testosterone at a large veterans’ hospital over a 10-year span. We focused on the influences of obesity and age as these are increasingly common conditions in the veterans’ population and the US population and are known to influence SHBG concentrations.
Materials and methods
Patients
We searched the electronic medical record at the Veterans Administration Puget Sound Health Care System (VAPSHCS) from 1 January 1997 through 31 December 2007 for men evaluated for low testosterone by laboratory testing. The VAPSHCS includes several satellite outpatient clinics and a 504-bed teaching hospital serving veterans from Washington, Idaho, and Alaska. We identified 3671 men who were underwent laboratory testing of testosterone using a serum testosterone panel that included simultaneous measurements of total testosterone, SHBG, albumin and a calculated free testosterone. If multiple panels were performed on an individual patient, we used only the initial panel for our analysis. We excluded men who were already on androgen replacement therapy at the time of initial laboratory assessment. Blood samples were collected between 8AM (0800) and 5PM (1700) in men with suspected testosterone deficiency. The timing of measurement is not in accordance with more recent clinical guidelines recommending measurement of testosterone in the morning, but is consistent with clinical practice.6 All available data were extracted and aggregated from the electronic medical record by a single research co-ordinator and were de-identified to remove any previous linkage of laboratory data to individual patients. The VAPSHCS Institutional Review Board and Research and Development Committee approved the study and authorized a waiver of informed consent for this research.
Hormone assays
All samples were measured in the clinical laboratory at the VAPSHCS. Total testosterone concentrations were measured using an Elecsys® platform-based immunoassay (Roche Diagnostics, Indianapolis, IN, USA). The total testosterone assay range was 0·07–52·5 nmol/l (normal range 9·8–28 nmol/l). SHBG was measured using the Elecsys® electrochemiluminescence platform-based immunoassay (Roche Diagnostics, Indianapolis, IN, USA). The assay range for SHBG was 0·35–200 nmol/l (normal range 10–80 nmol/l). Albumin was measured with the VITROS® 5.1 analytic platform assay (Ortho-Clinical Diagnostics, Rochester, NY, USA) the assay range was 1–6 gm/dl (normal range 2–5·2 gm/dl). The Vermeulen formula14 was used to calculate free testosterone measures, and the normal range for free testosterone was 118 to 673 pmol/l using mid-range SHBG and albumin values. In this assay, calculated free testosterone concentrations correlate well (R2 = 0·93) with free testosterone concentrations measured by equilibrium dialysis/tandem mass spectrometry.
Statistical analysis
Multiple linear, polynomial, log-transformed and multivariate regression analyses with and without interaction terms were performed to determine the optimal model to understand the associations between obesity, age, free testosterone, albumin and SHBG. Total testosterone was not included in the multivariate model due to colinearity with SHBG. Ultimately, simple linear regression without an interaction was found to be the most informative regression model. Therefore, the linear univariate models are presented for obesity and age in graphic form, and the multivariate model is presented in tabular form. To better understand the effects of age and obesity on SHBG and testosterone, men were divided into 4 groups: (i) nonobese younger, (BMI <30 and age <65; n = 1350); (ii) obese younger (BMI ≥30 and age <65; n = 1207); (iii) nonobese older (BMI<30 and age ≥65; n = 674); and (iv) obese older men (BMI ≥30 and age ≥65; n = 440). SHBG, total testosterone and calculated free testosterone concentrations in the groups were compared using an anova with a Scheffe post hoc test with a Bonferroni correction for multiple comparisons. Comparison of the proportion of men defined as having a low testosterone within a given group was performed with an extended chi-squared test. All analyses were performed with stata (College Park, TX, USA) version 10. For all analyses, a P-value of <0·05 was considered significant. Results are reported as means ± SD unless otherwise noted.
Results
Subject characteristics
Of the 3671 men included for evaluation, the mean BMI was 30 ± 7 kg/m2 and the mean age was 60 ± 12 years (range 20–98). Nearly one-half of the men (45%) were obese. The population was 59·4% white, 17·1% black, 10·1% Asian, 3·2% Native American and 10·2% unknown.
Effect of BMI and ageing on SHBG
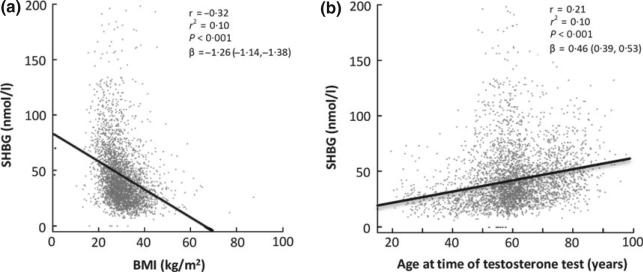
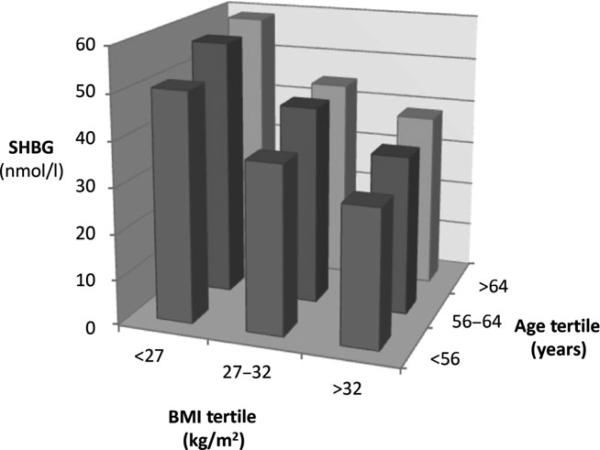
Linear regression analysis demonstrated that BMI was negatively associated with SHBG concentrations, while ageing was positively associated with SHBG concentrations (Fig. 1a,b). For each unit increase in BMI, SHBG decreased by 1·26 (95% CI: −1·14, −1·38) nmol/l, while for each year increase in age, SHBG increased 0·46 (95% CI: 0·39, 0·53) nmol/l (P < 0·001 for both effects). Examination of this cohort by tertiles of BMI and age demonstrated that the progressive decline in SHBG with increasing BMI was greater than the progressive increase in SHBG with age across all tertiles (Fig. 2). Overall, BMI explained 2·5 times more of the variability in SHBG than did ageing (r2 = 0·1 for BMI and r2 = 0·04 for age). In the multivariate model (Table 1), age and BMI were the most significant factors influencing SHBG concentration, with free testosterone and albumin concentration only slightly affecting SHBG.
Fig. 1.
BMI (a) has a greater influence on SHBG than ageing (b).
Fig. 2.
SHBG concentrations progressively decrease with increasing BMI, independent of ageing when viewed as tertiles.
Table 1.
Multivariate linear regression of factors associated with SHBG
| Variable (unit) | β (95% CI) | Cumulative R2 | Δ R 2 | P-value |
|---|---|---|---|---|
| BMI (kg/m2) | –1·23 (–1·35, –1·11) | 0·10 | – | <0·001 |
| Age (year) | 0·31 (0·24, 0·37) | 0·14 | 0·04 | <0·001 |
| Albumin (mg/dl) | –8·3(–10·1, –6·4) | 0·16 | 0·02 | <0·001 |
| Calculated free testosterone (pmol/l) | –0·07 (–0·09, –0·05) | 0·17 | 0·01 | <0·001 |
For the analysis above, the total R2 value for all variables in the model is reported as ‘cumulative’ R2. The individual R2 value for any specific variable can be found under the column ‘ΔR2’.
When the men were considered in groups (Table 2), SHBG concentrations were significantly lower in obese men than in nonobese men (36 ± 22 nmol/l [mean ± SD] vs 50 ± 27 nmol/l, P < 0·001) and higher in older men than in younger men (49 ± 24 nmol/l vs 42 ± 27 nmol/l, P < 0·001). The lowest SHBG concentrations were observed in obese, younger men (mean 34 ± 23 nmol/l), while the highest concentrations were seen in nonobese, older men (mean 54 ± 25 nmol/l) (Table 3).
Table 2.
Age, BMI, SHBG, total testosterone and calculated free testosterone stratified by BMI or age. All values are means ± SD
| Age |
BMI |
||||||
|---|---|---|---|---|---|---|---|
| All men | <65 | ≥65 | P-value | <30 | ≥30 | P-value | |
| Number of men | 3671 | 2557 | 1114 | 2024 | 1647 | ||
| Average age (years) | 60 ± 12 | 54 ± 9 | 74 ± 6 | <0·001 | 61 ± 13 | 59 ± 11 | <0·001 |
| Average BMI (kg/m2) | 30 ± 7 | 30·4 ± 6·8 | 29·1 ± 6·1 | <0·001 | 25·5 ± 3·3 | 35·6 ± 5·3 | <0·001 |
| SHBG (nmol/l) | 44 ± 26 | 42 ± 27 | 49 ± 24 | <0·001 | 50 ± 27 | 36 ± 22 | <0·001 |
| Total testosterone (nmol/l) | 12·5 ± 6·8 | 13·0 ± 7·1 | 11·5 ± 6·0 | <0·001 | 14·1 ± 7·4 | 10·5 ± 5·4 | <0·001 |
| Calculated free testosterone (pmol/l) | 215 ± 111 | 229 ± 94 | 184 ± 94 | <0·001 | 229 ± 121 | 204 ± 97 | <0·001 |
| % with total testosterone <9·8 nmol/l | 36·2 | 34·4 | 40·4 | <0·001 | 26·3 | 48·4 | <0·001 |
| % with free testosterone <118 pmol/l | 15·3 | 13·1 | 20·2 | <0·001 | 14·6 | 16·2 | 0·173 |
Table 3.
Age, BMI SHBG, total testosterone and calculated free testosterone by group. All values are means ± SD
| Nonobese, younge (Group 1) | Obese, younger (Group 2) | Nonobese, older (Group 3) | Obese, older (Group 4) | P-value | |
|---|---|---|---|---|---|
| n | 1350 | 1207 | 674 | 440 | |
| Average age (years) | 53·8 ± 9 | 54·6 ± 8 | 75·1 ± 7† | 71·9 ± 5† | <0·001 |
| Average BMI (kg/m2) | 25·6 ± 3·4‡ | 35·8 ± 5·5 | 25·4 ± 3·2‡ | 35·1 ± 4·5† | <0·001 |
| SHBG (nmol/l) | 49 ± 2† | 34 ± 23† | 54 ± 25† | 41 ± 20† | <0·001 |
| Total testosterone (nmol/l) | 15 ± 7·7† | 10·7 ± 5·5 | 12·5 ± 6·3‡ | 10·0 ± 5·5ϕ | <0·001 |
| Calculated free testosterone (pmol/l) | 246 ± 128† | 208 ± 97† | 187 ± 100ε | 174 ± 83ε | <0·001 |
| % with total testosterone <9·8 nmol/l | 23·2† | 47 | 32·6† | 52·2ϕ | <0·001 |
| % with calculated free testosterone <118 pmol/l | 12·4 | 14 | 18·8ε | 22·2ε | <0·001 |
Different compared to all other groups.
Different compared to Group 2.
Different compared to Groups 1 and 3.
Different compared to Groups 1 and 2.
Effect of BMI and ageing on total testosterone and calculated free testosterone
Increasing BMI and ageing were both associated with declines in total testosterone concentrations. Among men of all ages, higher total testosterone concentrations were observed in the nonobese groups compared to obese groups (14·1 ± 7·4 vs 10·5 ± 5·4 nmol/l P < 0·001) (Table 2). Similarly, higher total testosterone concentrations were observed in younger men than older men (mean 13·0 ± 7·1 vs 11·5 ± 6·0 nmol/l, P < 0·001). The highest total testosterone concentrations were observed in nonobese, younger men (15·0 ± 7·7 nmol/l), while the lowest were in older, obese men (10·0 ± 5·5 nmol/l) (Table 3).
Among nonobese men, calculated free testosterone concentrations were higher in younger men than older men (229 ± 94 vs 184 ± 94 pmol/l, P < 0·001) (Table 2). This difference was also observed when comparing obese, younger men to obese, older men (208 ± 97 vs 174 ± 83 pmol/l, P < 0·001) (Table 3). However, the proportion of men with a low calculated free testosterone was not significantly different when stratified solely by BMI, with 14·6% of nonobese and 16·2% of obese men having a calculated free testosterone of <118 pmol/l (P = 0·173) (Table 2).
Discussion
Total testosterone measurements are known to be affected by SHBG concentrations with obesity decreasing and ageing increasing SHBG. We sought to quantify the relative associations of obesity and ageing on SHBG and total testosterone in a large sample of men undergoing clinical evaluation for hypogonadism. In this study of 3671 men, we found that the negative effect of BMI on SHBG was significantly greater (approximately 2·5 fold) than the positive effect of age on SHBG, both by linear regression and examination of tertiles of BMI and age. Our findings are congruent with two other recent cross-sectional analyses in Chinese men that demonstrated a negative correlation between lower SHBG and increasing BMI,13,15 but our study is much larger and includes more older, obese men and is therefore more reflective of many men being evaluated for hypogonadism.
The mechanism by which obesity is associated with lowered SHBG has not been fully elucidated, but may involve suppression of hepatic SHBG synthesis by elevated concentrations of insulin.16,17 In our multivariate regression model, age and BMI were the most significant factors associated with serum SHBG concentration, accounting for 14% of the variation in SHBG, with albumin and calculated free testosterone only accounting for small additional percentages. This finding suggests that other factors, possibly genetic or environmental factors such as serum insulin concentrations, are the main determinants of serum SHBG. Future analyses examining the impact of insulin and other metabolic hormones on SHBG would be of interest. Indeed, several prior studies have demonstrated associations between low SHBG and the metabolic syndrome.15,18–20 For example, a recent cross-sectional analysis of community-dwelling men by Grossman et al.21 examined the influences of obesity and diabetes on calculated free testosterone, total testosterone and SHBG. Among 240 men in the study, 80 had a diagnosis of type 2 diabetes mellitus, yet the association between low total testosterone and diabetes was not significant after adjusting for BMI and age. Similarly, the association of increasing age with increasing SHBG has been clearly demonstrated in prior studies,22–24 but the reason for this increase is unknown.
The prevalence of low free testosterone in our study was 15·3%, which is higher than that observed in other cross-sectional studies.1,2 However, this is to be expected as our study focused on men in whom testosterone deficiency was suspected by the clinician ordering the testosterone level. As expected, the prevalence of low free testosterone increased with both BMI and age. Interestingly, when all men were stratified on the basis of BMI, there was no significant difference in the proportion of nonobese vs obese men with low free testosterone, despite a significant difference in the proportions of those with total testosterone concentrations <9·8 nmol/l between the two groups. This observation highlights the potential importance of assessment of free testosterone concentrations in obese men before making the diagnosis of male hypogonadism to avoid potential misclassification, as suggested by others.22–24 Of course, as the diagnosis of hypogonadism requires the presence of signs and/or symptoms of hypogonadism in addition to low measured levels of testosterone, the utility of the calculated free testosterone in this sample of men is unclear as we do not have information on symptoms in this population of men. However, it does seem likely that the use of calculated free testosterone might prevent the overdiagnosis of testosterone deficiency in some men, particularly in obese men, who may have low serum SHBG as the main cause of their low total testosterone. It is worth noting that older men likely have a lower threshold of normal for total testosterone. For example, one recent observational study conducted in Australia found that in a reference group of 394 older men in excellent health, the lower limit (bottom 2·5 percentile) for total testosterone was 6·4 nmol/l and for calculated free testosterone was 103 pmol/l.25. It is also worth noting that the percentage of men in this cohort that were categorized as hypogonadal using a low total testosterone (<9·8 nmol/l) was 36%, but only 15% of men were categorized as hypogonadal when a low free testosterone of (<118 pmol/l) threshold was used (Table 2).
This study has strengths and weaknesses. Strengths of this study include the very large sample size, the broad range of BMI and age, which allows for robust statistical inferences regarding the factors influencing SHBG concentrations, and the measurement of all samples with an accurate assay methodology performed in a single central laboratory in a ‘real-world clinical setting’. Limitations of this study include its retrospective and observational design. In addition, our assessment of free testosterone was calculated based on the total testosterone and SHBG concentrations measured with an immunoassay, rather than the gold standards of equilibrium dialysis for the measurement of free testosterone and liquid chromatography/tandem mass spectrometry for total testosterone, which may have introduced some bias. On the other hand, the calculated free testosterone used in our analyses has been shown to correlate highly with free testosterone measured by equilibrium dialysis and represents a practical measure that is used clinically at our and other institutions. Multiple equations have been developed to best calculate free testosterone concentrations, and there is a debate regarding which is the most accurate.26–31 In a recent report that compared a number of published formulas for calculated free testosterone, all formulas (including the Vermeulen formula used in the present report) appeared to have acceptable bias relative to free testosterone by equilibrium dialysis.32 In addition, the testosterone measurements in this study were obtained throughout the day and were not uniformly collected early in the morning, per recent guidelines, potentially leading to an increased incidence of low testosterone measurements.
Another limitation of our study was that we did not have information pertaining to clinical symptoms related to hypogonadism (e.g. loss of libido, sexual interest, presence of erections, muscle mass and strength). Therefore, our conclusions apply only to the measurement of low testosterone and not to the diagnosis of hypogonadism. However, the absence of this information does not affect our conclusion that obesity has a much greater effect on lowering SHBG than ageing has on increasing SHBG. Lastly, a longitudinal design with multiple measurements would be more informative than our current cross-sectional analysis, but such data were not available.
Conclusions
Obesity has a greater association with lowered SHBG than ageing has on increasing SHBG. On average, obese men of all ages have lower SHBG and total testosterone concentrations than nonobese men. These effects on SHBG may impact the utility of total testosterone measurements in the diagnosis of low testosterone, particularly in obese men.
Acknowledgements
L.A.C was supported by a training grant from the National Institutes of Health (NIH), T32HL0007028 and a VA Fellowship in Advanced Geriatrics; S.T.P. was supported by the National Institute of Aging (NIH), Grant AG037603A; A.M.M. was supported by the Department of Veterans Affairs Puget Sound Health Care System, Geriatric Research Education and Clinical Center.
Footnotes
Disclosures
Nothing to declare.
References
- 1.Araujo AB, Esche GR, Kupelian V, et al. Prevalence of symptomatic androgen deficiency in men. The Journal of Clinical Endocrinology and Metabolism. 2007;92:4241–4247. doi: 10.1210/jc.2007-1245. [DOI] [PubMed] [Google Scholar]
- 2.Araujo AB, O'Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. The Journal of Clinical Endocrinology and Metabolism. 2004;89:5920–5926. doi: 10.1210/jc.2003-031719. [DOI] [PubMed] [Google Scholar]
- 3.Wu FC, Tajar A, Beynon JM, et al. Identification of late-onset hypogonadism in middle-aged and elderly men. The New England Journal of Medicine. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 4.Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of Clinical Endocrinology and Metabolism. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 5.Layton JB, Li D, Meier CR, et al. Testosterone lab testing and initiation in the United Kingdom and the United States, 2000-2011. The Journal of Clinical Endocrinology and Metabolism. 2014;20:48–54. doi: 10.1210/jc.2013-3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 7.DeVan ML, Bankson DD, Abadie JM. To what extent are free testosterone (FT) values reproducible between the two Washingtons, and can calculated FT be used in lieu of expensive direct measurements? American Journal of Clinical Pathology. 2008;129:459–463. doi: 10.1309/6PYTC60ALVQQ59RQ. [DOI] [PubMed] [Google Scholar]
- 8.Saboor Aftab SA, Kumar S, Barber TM. The role of obesity and type 2 diabetes mellitus in the development of male obesity-associated secondary hypogonadism. Clinical Endocrinology. 2013;78:330–337. doi: 10.1111/cen.12092. [DOI] [PubMed] [Google Scholar]
- 9.Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. The Journal of Clinical Endocrinology and Metabolism. 1996;81:1821–1826. doi: 10.1210/jcem.81.5.8626841. [DOI] [PubMed] [Google Scholar]
- 10.Mohr BA, Bhasin S, Link CL, et al. The effect of changes in adiposity on testosterone levels in older men: longitudinal results from the Massachusetts Male Aging Study. European Journal of Endocrinology/European Federation of Endocrine Societies. 2006;155:443–452. doi: 10.1530/eje.1.02241. [DOI] [PubMed] [Google Scholar]
- 11.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. The Journal of Clinical Endocrinology and Metabolism. 1994;79:997–1000. doi: 10.1210/jcem.79.4.7962311. [DOI] [PubMed] [Google Scholar]
- 12.Vermeulen A. Decreased androgen levels and obesity in men. Annals of Medicine. 1996;28:13–15. doi: 10.3109/07853899608999068. [DOI] [PubMed] [Google Scholar]
- 13.Cao J, Chen TM, Hao WJ, et al. Correlation between sex hormone levels and obesity in the elderly male. The Aging Male: The Official Journal of the International Society for the Study of the Aging Male. 2012;15:85–89. doi: 10.3109/13685538.2012.666585. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. The Journal of Clinical Endocrinology and Metabolism. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Huang X, Liao M, et al. Both total testosterone and sex hormone-binding globulin are independent risk factors for metabolic syndrome: results from Fangchenggang Area Male Health and Examination Survey in China. Diabetes/Metabolism Research and Reviews. 2013;29:391–397. doi: 10.1002/dmrr.2405. [DOI] [PubMed] [Google Scholar]
- 16.Peter A, Kantartzis K, Machann J, et al. Relationships of circulating sex hormone-binding globulin with metabolic traits in humans. Diabetes. 2010;59:3167–3173. doi: 10.2337/db10-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plymate SR, Jones RE, Matej LA, et al. Regulation of sex hormone binding globulin (SHBG) production in Hep G2 cells by insulin. Steroids. 1988;52:339–340. doi: 10.1016/0039-128x(88)90136-5. [DOI] [PubMed] [Google Scholar]
- 18.Gong Y, Xiao H, Bai J, et al. Association between sex hormone levels and abnormal metabolism in a population of elderly Chinese men. The Aging Male: The Official Journal of the International Society for the Study of the Aging Male. 2013;16:8–16. doi: 10.3109/13685538.2013.765402. [DOI] [PubMed] [Google Scholar]
- 19.Elbadawy HM, Gailledrat M, Desseaux C, et al. Gene transfer of integration defective anti-HSV-1 meganuclease to human corneas ex vivo. Gene Therapy. 2014;21:272–281. doi: 10.1038/gt.2013.82. [DOI] [PubMed] [Google Scholar]
- 20.Chubb SA, Hyde Z, Almeida OP, et al. Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study. European Journal of Endocrinology/European Federation of Endocrine Societies. 2008;158:785–792. doi: 10.1530/EJE-07-0893. [DOI] [PubMed] [Google Scholar]
- 21.Ng Tang Fui M, Hoermann R, Cheung AS, et al. Obesity and age as dominant correlates of low testosterone in men irrespective of diabetes status. Andrology. 2013;1:906–912. doi: 10.1111/j.2047-2927.2013.00124.x. [DOI] [PubMed] [Google Scholar]
- 22.Halmenschlager G, Rhoden EL, Riedner CE. The influence of age on bioavailable and free testosterone is independent of body mass index and glucose levels. World Journal of Urology. 2011;29:541–546. doi: 10.1007/s00345-011-0724-x. [DOI] [PubMed] [Google Scholar]
- 23.Leifke E, Gorenoi V, Wichers C, et al. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clinical Endocrinology. 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 24.Muller M, den Tonkelaar I, Thijssen JH, et al. Endogenous sex hormones in men aged 40-80 years. European Journal of Endocrinology/European Federation of Endocrine Societies. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 25.Yeap BB, Alfonso H, Chubb SA, et al. Reference ranges and determinants of testosterone, dihydrotestosterone, and estradiol levels measured using liquid chromatography-tandem mass spectrometry in a population-based cohort of older men. Journal of Clinical Endocrinology and Metabolism. 2012;97:4030–4039. doi: 10.1210/jc.2012-2265. [DOI] [PubMed] [Google Scholar]
- 26.Anawalt BD, Hotaling JM, Walsh TJ, et al. Performance of total testosterone measurement to predict free testosterone for the biochemical evaluation of male hypogonadism. The Journal of Urology. 2012;187:1369–1373. doi: 10.1016/j.juro.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wartofsky L, Handelsman DJ. Standardization of hormonal assays for the 21st century. The Journal of Clinical Endocrinology and Metabolism. 2010;95:5141–5143. doi: 10.1210/jc.2010-2369. [DOI] [PubMed] [Google Scholar]
- 28.Sartorius G, Ly LP, Sikaris K, et al. Predictive accuracy and sources of variability in calculated free testosterone estimates. Annals of Clinical Biochemistry. 2009;46:137–143. doi: 10.1258/acb.2008.008171. [DOI] [PubMed] [Google Scholar]
- 29.de Ronde W, van der Schouw YT, Pols HA, et al. Calculation of bioavailable and free testosterone in men: a comparison of 5 published algorithms. Clinical Chemistry. 2006;52:1777–1784. doi: 10.1373/clinchem.2005.063354. [DOI] [PubMed] [Google Scholar]
- 30.Ly LP, Handelsman DJ. Empirical estimation of free testosterone from testosterone and sex hormone-binding globulin immunoassays. European Journal of Endocrinology/European Federation of Endocrine Societies. 2005;152:471–478. doi: 10.1530/eje.1.01844. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S, Pencina M, Jasuja GK, et al. Reference ranges for testosterone in men generated using liquid chromatography tandem mass spectrometry in a community-based sample of healthy nonobese young men in the Framingham Heart Study and applied to three geographically distinct cohorts. The Journal of Clinical Endocrinology and Metabolism. 2011;96:2430–2439. doi: 10.1210/jc.2010-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clinical Endocrinology. 2010;73:382–388. doi: 10.1111/j.1365-2265.2010.03804.x. [DOI] [PubMed] [Google Scholar]