Abstract
We evaluated adverse events, biodistribution and shedding of oncolytic vaccinia virus encoding CD40 ligand in two Beagles, in preparation for a phase 1 trial in canine cancer patients. Dog 1 received one dose of vaccinia virus and was euthanized 24 hours afterwards, while dog 2 received virus four times once weekly and was euthanized 7 days after that. Dogs were monitored for adverse events and underwent a detailed postmortem examination. Blood, saliva, urine, feces, and organs were collected for virus detection. Dog 1 had mild fever and lethargy while dog 2 experienced a possible seizure 5.5 hours after first virus administration. Viral DNA declined quickly in the blood after virus administration in both dogs but was still detectable 1 week later by quantitative polymerase chain reaction. Only samples taken directly after virus infusion contained infectious virus. Small amounts of viral DNA, but no infectious virus, were detected in a few saliva and urine samples. Necropsies did not reveal any relevant pathological changes and virus DNA was detected mainly in the spleen. The dogs in the study did not have cancer, and thus adverse events could be more common and viral load higher in dogs with tumors which allow viral amplification.
Introduction
As in humans, cancer is one of the most common reasons for death in dogs. Surgery, chemotherapy, and radiation therapy are the most commonly used treatment options in veterinary oncology but in parallel with the human situation new approaches are needed especially for advanced metastatic solid tumors which are often incurable with traditional therapies.
Dogs with spontaneous cancer serve as a good model for human cancers.1–4 Dogs share the same environment with their owners, their immune system is intact, their size is close to humans and cancer progression is spontaneous. These are key advantages over laboratory rodents. Critically, like cancer in human patients but in contrast to rodent models, cancer arising in dogs develops over several years, resulting in similar complexity, clonality, and immune suppression as seen in man. The biological behavior also has many similarities, including metastatic patterns, relapse, and treatment resistance. In addition, the same cancer-associated genes and histological features have been found in both species.2,5
Oncolytic virotherapy, where replication competent viruses are armed with immunostimulatory transgenes, is a promising new treatment approach.6–9 Before directly killing cancer cells, immunostimulatory genes are expressed by infected cells to awaken the host immune system, which is suppressed by the tumor microenvironment in progressing clinically evident tumors. Then, infected tumor cells are killed by oncolysis, releasing a broad variety of tumor antigens into the environment for the adaptive immune system to sample.
The oncolytic Western Reserve vaccinia virus used in the present study, vvdd-hCD40L-tdTomato,10 has thymidine kinase (TK) and vaccinia growth factor (VGF) deletions to render the virus tumor specific. Removal of TK makes the virus dependent on cellular nucleotides, typically found in abundance in tumors.8 As an important biosafety improvement over previous designs, our virus features a 150 bp deletion of TK instead of mere disruption of the open reading frame of the gene by the transgene cassette, rendering reversion to wild type by recombination impossible.10 Deletion of VGF, the viral analogue of cellular epidermal growth factor (EGF), renders the virus dependent on an activated EGFR-ras pathway, which is a common feature of human solid tumors,7,8 and also found in canine tumors.11 In addition to TK and VGF disruptions which increase virus safety and biosafety, the virus expresses tdTomato, a red fluorescent protein facilitating in vivo tracking of virus-infected cells,12 as well as the immune-stimulatory human CD40 ligand. CD40L is a member of the tumor necrosis factor (TNF) family and enhances antigen-specific T-cell response by activating antigen presenting cells.13 It also has direct antiproliferative and proapoptotic effects on human bladder, cervical and ovarian carcinoma cells.14,15 In addition, gene therapy with adenovirus expressing human DC40L has been successfully used for the treatment of canine malignant melanoma demonstrating that human CD40L is active in dogs.16
We have previously described the activity of vvdd-hCD40L-tdTomato in canine and feline cancer cells lines in vitro and in mouse xenografts.17 The objective of the present study was to examine safety and biodistribution of intravenously administered vvdd-hCD40L-tdTomato in two healthy beagle dogs in preparation for a phase 1 dose escalation study with pet dogs that suffer from incurable cancers.
Results
With the exception of possible seizure, virus administration was well tolerated
To evaluate possible adverse events associated with virus administration, we monitored dogs closely according to VCOG-CTCAE v1.0 guidelines.18 Dog 1 developed transient grade 1 fever (rectal temperature 39.5 °C) 8 hours after virus infusion. The fever resolved in 3 hours but the dog was quieter than usual until he was euthanized the next morning. Dog 2 had a mild increase in rectal temperature as well, although below the threshold of a grade 1 elevation (Figure 1). In addition, dog 2 had a possible grade 3 seizure 5.5 hours after the first virus administration. The actual event was not seen by the personnel. Instead, barking was heard from the kennel and when the researcher arrived less than 1 minute later, dog 2 was lying on his side on the floor and dog 1 was attacking him. When dog 1 was removed, dog 2 did not stand up immediately. It was lifted onto the examination table, at which point the physical and neurological examination did not detect any abnormalities. In the absence of further evidence, we scored the event as a grade 3 seizure, reduced the subsequent virus doses to 0.8 × 108 tissue culture infective dose (TCID50)/kg, and monitored the dogs by video cameras for 24 hours after each virus administration. No further seizures were observed.
Figure 1.
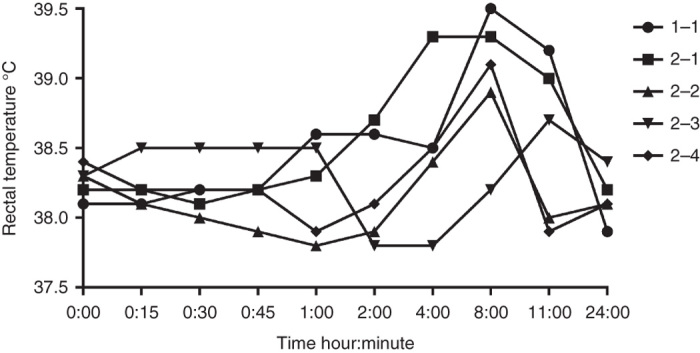
Rectal temperature of the study dogs over time after each virus administration. The number after the dog number indicates dose number.
In addition to physical examination, we assessed adverse events in hematological and biochemical parameters and in urinalysis. Laboratory tests indicated a grade 1 increase in alkaline phosphatase in dog 1 (Supplementary Table S1). Dog 2 exhibited a low albumin level (grade 1) already prior to virus injection, and although there was some further decrease, the values did not exceed grade 1. In addition, both dogs had low baseline bilirubin and cholesterol levels. Hematology and urinalysis did not reveal any obvious changes. Total white blood cell and differential counts are shown in Figure 2.
Figure 2.
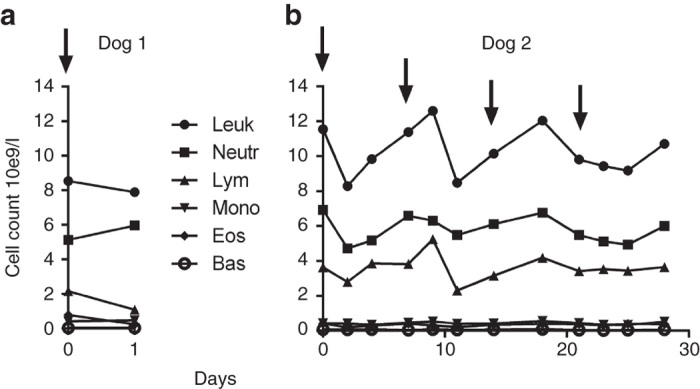
Total white blood cell and differential count of study dogs after receiving oncolytic vaccinia virus. Arrows indicate virus administrations. (a) Dog 1 and (b) Dog 2. Bas, basophils; Eos, eosinophils; Leuk, leukocytes; Lymph, lymphocytes; Mono, monocytes; Neu, neutrophils.
Viral DNA was identified in all blood samples
We measured both viral genome and infectious virus in multiple blood samples collected after virus infusion. Viral DNA was detected by quantitative polymerase chain reaction (qPCR) in the blood of both dogs at all time points (Figure 3), with highest amounts of viral DNA in the blood sample taken immediately after virus infusion. The viral load declined quickly during the first 4 hours after infusion, but viral DNA was still detectable in the blood 1 week later. Using a transduction test and plaque assay, infectious virus in blood was only detectable immediately after virus infusion (Figure 4). However, although the transduction test and plaque assay were clearly positive for infectious virus, the amount of virus was too low to be reliably quantifiable with tissue culture infective dose TCID50 analysis, suggesting low titers.
Figure 3.
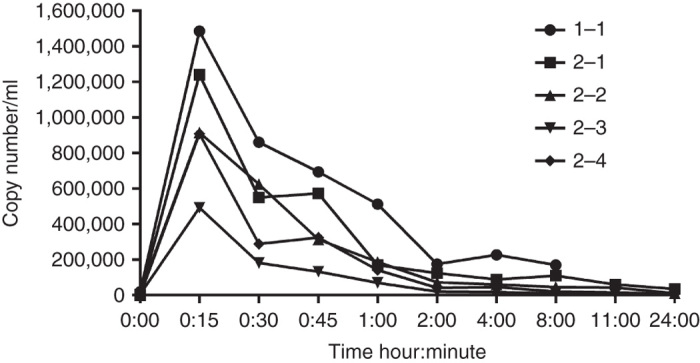
Vaccinia DNA measured by qPCR in the blood of study dogs after vaccinia virus infusions. The number after the dog number indicates dose number.
Figure 4.
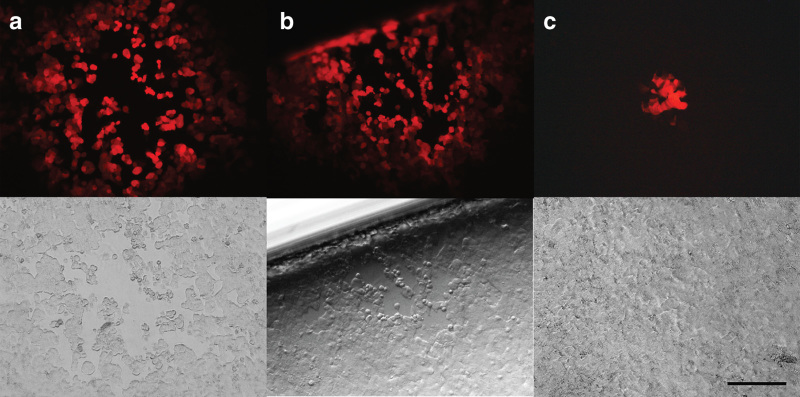
Plaques detected on A549 cell monolayers overlaid with blood samples taken immediately after vvdd-tdTom-CD40L administration. Presence of infectious virus was demonstrated by fluorescence microscope (the red color in the upper row indicates infection by vvdd-hCD40L-tdTomato coding tdTomato) and with light microscopy (lysis of the monolayer present in the lower row indicates productive replication of virus rescued from dog blood). (a) Dog 1 after first dose and (b,c) dog 2 after third dose. Scale bar = 200 µm.
Low levels of viral DNA were detected in saliva and urine
Furthermore, we monitored viral shedding into saliva, urine, and feces to find out if virus could be spread via them into the environment. In qPCR, detectable but not quantifiable amounts (i.e., above the limit of reliable detection but below the limit of reliable quantitation) of viral DNA were found in three of the five saliva samples taken 24 hours after virus administration, and in two urinary samples (7 days after second virus dose and 24 hours after fourth virus dose in dog 2) (Table 1). Transduction test and plaque assay did not reveal infectious virus in any of the samples. Viral DNA was not detected in feces, or in the other saliva or urine samples collected at baseline, and 1, 2, or 4 days after virus administration.
Table 1. Positive secretion samples for vaccinia DNA by qPCR in the study dogs.
| Secretion | Dog | Timepoint |
|---|---|---|
| Saliva | 2 | 1 day after second virus dose |
| 1 day after third virus dose | ||
| 1 day after fourth virus dose | ||
| Urine | 1 | 1 day after first virus dose |
| 2 | 7 days after second virus dose |
Number of DNA copies was <100/10 µl of purified DNA in all samples.
qPCR, quantitative polymerase chain reaction.
Vaccinia DNA was mainly identified in the spleen
We also assessed biodistribution of the virus in canine organs postmortem. In the tissue samples, virus DNA was detected mainly in the spleen (Table 2). However, detectable, but not quantifiable amounts were also found in most organs of dog 1 and in the lungs of dog 2, suggesting effective systemic dissemination of the virus following intravenous delivery.
Table 2. Tissue samples positive for vaccinia DNA by qPCR in the study dogs.
| Dog | Tissue | Copies/mg of genomic DNA |
|---|---|---|
| 1 | Spleen | 9.9 × 105 |
| Adrenal glands, aorta, bone and joint, epididymis, esophagus, gallbladder, gingiva, heart, kidneys, pancreas, sciatic nerve, pituitary gland, prostate, salivary gland, muscle, testes, thyroid, tongue, trachea | <100 | |
| 2 | Spleen | 3.9 × 106 |
| Lungs | <100 |
qPCR, quantitative polymerase chain reaction.
Blood cytokines showed no obvious alterations
As viral infections usually generate a proinflammatory response and cytokine production in the body, we measured selected cytokines in serial blood samples. However, cytokine levels were generally below detection level in blood and did not change after virus injection. In fact, only interleukin (IL)-2, -6, and -12p40 could be detected in dog 2. In dog 1, IL-2 was detectable in the baseline sample but declined below the limit of detection immediately after the infusion. Interferon γ (IFN-γ), TNF-α, monocyte chemotactic protein-1 (MCP-1), IL-8, and -10 were below detection limits in both dogs throughout the experiment.
Administration of vaccinia virus stimulated neutralizing antibodies
Neutralizing antibodies (NAbs) may influence on the treatment efficacy of oncolytic viruses and thus, we measured them. NAbs increased over time (Figure 5). At 2 weeks, the serum dilution that was able to block >50% of gene expression was 1:16; while at 4 weeks, a 1:4,096 dilution was sufficient.
Figure 5.
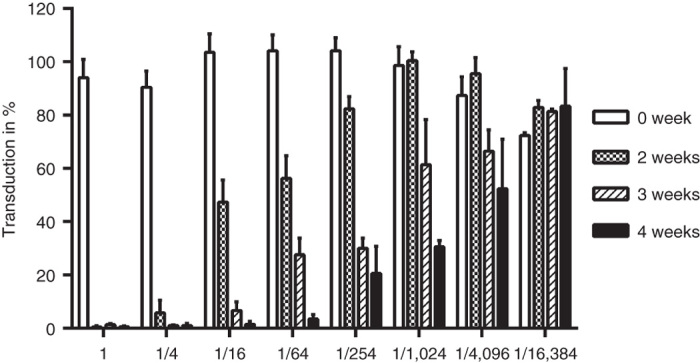
Transduction inhibition by serum neutralizing antibodies in dog 2. Blood samples were collected at baseline (0 week), 2, 3, and 4 weeks after first virus dose. A549 cells were challenged in triplicate with a mixture of vvdd-luc preincubated with heat inactivated serum. Luciferase expression was measured 20 hours after infection. Values are means and error bars show standard deviation.
Virus administration did not induce any pathological changes
Toxic effects of the virus were evaluated in necropsy in addition to physical examination and laboratory measurements. The postmortem examination did not reveal any specific findings. Dog 1, however, exhibited aspermatogenesis as an incidental finding probably not related to treatment. Immunohistochemistry for vaccinia virions and β-galactosidase (an inactive but immunogenic form of which is present in the virus) was performed on the spleen of both dogs, since these organs had shown the highest viral DNA loads. With the antivaccinia antibody, viral antigen was detected in the cytoplasm of a few macrophages in the red pulp, suggesting phagocytosis of vaccinia virus particles (Figure 6). Staining with the anti-β-galactosidase antibody yielded a negative result as expected, since β-galactosidase is expressed only after viral replication, a feature that should not occur in nontumor bearing animals since the virus has been constructed to be tumor specific. Lack of β-galactosidase expression, compatible with lack of virus replication, is in accord with the molecular tests (e.g., qPCR) reported above.
Figure 6.
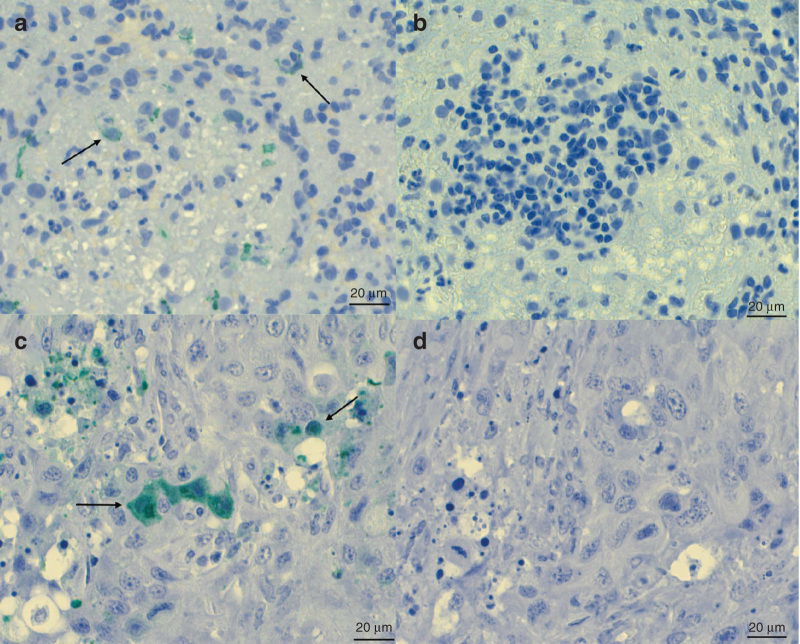
Detection of vaccinia virus antigen by immunocytochemistry. (a,b) Dog 1, spleen. (a) A few macrophages are found to express viral antigen (arrows). (b) In the negative control section (omission of primary antibody), no reaction is seen. (c,d) Mouse tumor xenografts treated with intratumoral injection of vaccinia virus are shown as a positive control. (c) Tumor cells express viral antigen (arrows). (d) In the negative control section (omission of primary antibody), no reaction is seen. HRP-Green, hematoxylin counterstain. Scale bar = 20 µm.
Discussion
Here, we report intravenous administration of oncolytic vaccinia virus, vvdd-hDC40L-tdTomato, to two laboratory Beagles. The infusion was well tolerated and—with the possible exception of the unconfirmed grade 3 seizure in dog 2—only mild adverse events were observed. The postmortem examination did not reveal any significant pathological changes, and none associated with vaccinia virus administration.
The onset of the suspected seizure in dog 2 was not witnessed by the personnel, and no signs associated with a typical grand mal seizure, such as unconsciousness, tonic-clonic motor activity, drooling, urinating, or defecating, was observed during the brief encounter with the still-prone animal, or upon physical inspection after the animal had recovered. However, because dog 2 did not stand up immediately after dog 1 was removed and because his limbs were extended, we classified the event as a grade 3 (involving altered consciousness, as dogs with generalized seizures always exhibit decreased consciousness) seizure per VCOG-CTCAE v1.0 guidelines,18 as opposed to a dog fight, which the two dogs had been found engaged in on some previous occasions.
Seizures have not been reported in human patients receiving oncolytic vaccinia virus.19–25 However, if it did occur in dog 2, the most likely explanation could relate to a hypersensitivity reaction and cytokine production associated with virus administration or other components in the virus preparation, since proinflammatory cytokines modulate brain exitability and predispose to seizures.26 Idiopathic epilepsy is a well-known disease in dogs, and in Harlan colonies the incidence is ~0.1 % (S. Hillen, personal communication). However, dog 2 or his close relatives did not have any reported history of epilepsy. An additional theory relates to the association of high fever with convulsions in humans, especially in children.27 Dog 2 only had minor increase in rectal temperature (39.3 °C), not even reaching grade 1, and thus this explanation seems unlikely.
The virus preparation used in our study contained detectable levels of host cell protein and bovine serum albumin (25.7 and 3.4 µg/ml, corresponding to a total of 52.38 µg in the first injection of dog 2). All foreign proteins can induce an immune response in the host and the risk is increased the less homology there is between foreign and host protein, however, the magnitude of reaction is difficult to evaluate.28 For example, although human and dog albumin show only 91.7% homology,29 human albumin is nevertheless safely and effectively used in the treatment of critically ill dogs with hypoalbuminemia. In a retrospective study, none of 418 dogs receiving human albumin at a dose of 1 g/kg/day (corresponding to a total amount of 145 mg of human albumin in dog 2 in 15 minutes, i.e., 2,700-fold more than there was noncanine protein present in the virus injection) developed severe hypersensitivity reactions or seizures that would require disruption of infusion or treatment.30 We therefore believe that it is unlikely that a small amount of human or bovine protein residues in the virus preparation would induce a seizure. Rather, if a seizure occurred, the relatively high dose of vaccinia virus present in the preparation may be a more likely cause.
Fever and lethargy are well-known symptoms in association with viral infection. The body temperature of dog 1 was at the lower limit of grade 1 fever (39.5 °C; VCOG-CTCAE v1.0 grade 1 fever 39.5–40.0 °C) and he had grade 1 lethargy. Dog 2 exhibited a mild grade 0 increase in rectal temperature after the first virus administration (39.3 °C) and no lethargy. Although his temperature was not high enough to qualify as an adverse event, in theory this may have been associated with the possible seizure, as it is known that epileptic seizures can increase body temperature.31 Fever and lethargy are amongst the most common adverse events reported in cancer patients receiving oncolytic virotherapy19–25 and result from the innate immune response to virus.9,32
Hematology and differential blood count did not show any obvious changes in either dog in the study. Histologic evaluation of the bone marrow collected in the necropsies did not reveal any signs of toxicity either. This is in contrast to the results of Park et al.24 who reported transient decrease in lymphocytes, platelets and red blood cells during the first 3 days after oncolytic vaccinia virus (JX-594) treatment of patients with primary or metastatic liver cancer. In addition, they reported neutrophilia in 9 of 14 patients. Heo et al.20 also reported lymphopenia in 4 of 30 patients and a decrease in hemoglobin in 3 of 30 liver cancer patients treated with JX-594. Lymphopenia was also found in healthy beagle dogs receiving recombinant oncolytic vesicular stomatitis virus VSV-IFNβ-NIS,33 but the lymphocyte drop was only seen in one of six healthy dogs receiving oncolytic adenovirus.34 Viral infections are often associated with lymphopenia, and it has been suggested that this phenomenon is associated with type 1 IFNs, the principal cytokines involved in viral infection, leading to attachment of lymphocytes to the endothelium, without migration into tissues.35 An obvious reason for the lack of hematological effects in our dogs is the lack of tumors in them, resulting in no amplification of input virus.
As expected, the vaccinia virus genome copy number was highest in the blood samples taken immediately after virus infusion and declined quickly thereafter. In contrast to studies in human cancer patients,20,21,24 there was no secondary viremia peak, which is compatible with tumor-restricted replication of the virus and thus lack of replication in these dogs. We found infectious virus only in blood collected immediately after virus infusion. One aspect which may impact on the infectiousness of vaccinia in circulation relates to the complement system and the innate immune response. Sampath et al.36 showed that complement severely inhibited vaccinia virus delivery, and we also found decreased infectivity of vaccinia virus when incubated with canine blood. It may be of importance the infectious viruses could be detected in intact dog blood; systemic exposure to infectious tumor selective virus could be useful for treatment of metastatic disease.
We observed aspermatogenesis in dog 1, but consider it highly unlikely that this was a consequence of virus administration, since the dog was euthanized 24 hours after virus infusion and in dogs each spermatogenic cycle lasts ~2 weeks, and spermatogenesis 2 months.37 Considering the fact that despite its role as a breeder, dog 1 did not have any offspring (S. Hillen, personal communication), aspermatogenesis was likely present prior to virus injection.
A few human cancer patients receiving oncolytic vaccinia virus have been reported to develop pox-like skin lesions.19,20 Skin lesions were also reported in laboratory beagles receiving intradermal inoculation of TK-deleted vaccinia virus (VTK-79).38 The dogs receiving the virus were in close contact with dogs that did not receive the virus. None of those developed antibodies to the virus, suggesting that virus did not spread from inoculated to noninoculated dogs.
Although our virus is tumor specific and thus not capable of causing systemic vaccinia infections, we assessed both dogs for potential virus shedding into the environment, by testing saliva, urine and feces for the virus. Vaccinia virus is quite stable in organic material in the environment. For example, it was possible to detect infective vaccinia virus from a scab collected from a small pox vaccination site and saved for 13 years in an envelope in the laboratory cupboard.39 In addition, infectious small pox virus was found in the household of a vaccinated person 10 days after he had left the house.40 The dogs in our study exhibited small amounts of vaccinia genomes in a few saliva and urine samples. However, the dogs used in the study did not carry tumors, and it is possible that the viral load may be higher in dogs with tumors that allow the virus to amplify. Consequently, our trial protocol will request the owners to prevent the dogs from licking humans, in case a pet dog will be treated with oncolytic vaccinia virus.
Urine may also pose a risk for spreading of the virus in environment, since pet dogs urinate outside and it is difficult to collect all urine. In one study, it was reported that vaccinia virus remained infectious for 4 months at +4 °C in water.41 In our dogs, only two urine samples were positive for vaccinia DNA and neither contained infectious virus.
In feces, which have been reported in mice to retain infectious vaccinia virus for up to 20 days at room temperature,42 we did not identify vaccinia virus genomes. Also, feces pose less of a biosafety issue as stools are easier to collect and discard.
Shedding of JX-594, an oncolytic vaccinia virus of the Wyeth strain featuring a TK-deletion and expression of hGM-CSF and LacZ genes, was not found in urine or throat swabs from 14 patients in a phase 1 trial,24 but virus was detected in throat swabs in a subsequent trial (EudraCt 2011-000051-16). Shedding of another oncolytic vaccinia virus, GL-ONC-1, was detected in urine, feces and sputum in 1 out of 27 patients.22 A third vaccinia virus, vvDD-CDSR, was not detected in any of the excretion samples analyzed.25 Thus, the available evidence suggests that vaccinia virus genomes can occasionally be detected in excretions of both dogs and humans, representing probably DNA fragments from virus digested by white blood cells, but identifying infectious virus is rare.
As reported for humans,19–24 vaccinia virus induced NAbs also in our study. The effects of antiviral antibodies in cancer gene therapy remain unclear. Clinical evidence from humans treated with oncolytic adenoviruses seems to contradict the classical viewpoint that antibodies would compromise gene delivery and efficacy, since baseline antibody titers seem to correlate with efficacy instead of the opposite.43–45 Nevertheless, it may be of relevance that vaccinia virus presents also as an extracellular enveloped form which can avoid antibody formation,36 facilitating spread of the virus to metastatic lesions.
Since we only had two dogs included into this preliminary experiment, the power of the study in minimal and the results cannot be extrapolated to other dogs. However, our results show that it is possible to give high dose of oncolytic vaccinia virus intravenously for a healthy dog without lethal or otherwise serious adverse events even multiple times. Further safety evaluation could be done with healthy laboratory dogs; however, in our opinion dogs with naturally occurring incurable cancers offer more reliable and ethical model, since the virus replicates in tumor tissues. Thus, we have opened a phase 1 clinical evaluate safety, adverse events, and shedding of the virus in tumor-bearing client-owned dogs. Virus is given intratumorally and the starting dose will be lower than in this study. The dogs will be carefully monitored for seizures, to shed light on the possible linkage of treatment with the unexpected event described here. Also, a phase 1 clinical study of another oncolytic vaccinia virus, V-VET1 (also called LIVP6.1.1), has been started in pet dogs in the United States in 2012, evaluating intravenous administration of the virus. V-VET1 is derived from Lister strain vaccinia virus and has a TK disruption.46
In conclusion, administration of a high dose of oncolytic Western Reserve vaccinia virus vvdd-hCD40L-tdTomato seems feasible in dogs. The present virus design allows adverse events, efficacy, and biosafety to be monitored meticulously in trials.
Materials and Methods
Dogs
Two healthy adult male HsdRcc:DOBE Beagle dogs (Harlan Laboratories, Gannat, France) were used (Supplementary Table S2). Both were retired breeding males. The experiment was approved by the National Animal Experiment Board of the Regional State Administrative Agency of Southern Finland (DNO 4953), and by the Finnish Board for Gene Technology (17/M/2011).
Virus and cell lines
A vaccinia virus Western Reserve strain deleted for TK and VGF and expressing human CD40L and tdTomato (vvdd-hCD40L-tdTomato) was designed as previously described.10
The virus was produced by standard sucrose gradient centrifugation in good manufacturing practice quality A549 cells (human lung adenocarcinoma epithelial cell line received from National Cancer Institute) in growth media (GM) consisting of Dulbecco’s modified Eagle medium GM (DMEM, Lonza, Verviers, Belgium), 1% L-glutamine, 1% penicillin-streptomycin, and 2% fetal calf serum (FCS) in 5% CO2 at 37 °C.47 The infectious titer was measured by a standard median TCID50 test in A549 cells.48
African green monkey kidney cells (Vero cells) from the American Type Culture Collection grown in DMEM with 4.5 g/l of glucose (Lonza) containing 1% L-glutamine, 1% penicillin-streptomycin, and 10% FCS in 5% CO2 at 37 °C were used for the detection of infectious virus.
Study design
Dog 1 was randomized for the evaluation of acute toxicity and received a single infusion of vvdd-hCD40L-tdTomato. Dog 2 was randomized to evaluate toxicity following repeated virus administration and received four infusions of vvdd-hCD40L-tdTomato once per week. Dog 1 was euthanized 24 hours after virus infusion and dog 2 1 week after the fourth virus infusion. A full postmortem examination was undertaken on both dogs immediately after death.
The dose we chose to use in our study was 1.2 × 108 TCID50/kg, which is approximately three times the maximum dose used (assuming a 75 kg patient) in a phase 1 dose escalation study with another oncolytic vaccinia virus, JX-594, in patients with primary or metastatic liver cancer.24
The virus was diluted in 50 ml of saline (Baxter, Norfolk, UK) and infused via intravenous catheter into a peripheral vein over 15 minutes (200 ml/hour). Vein access was confirmed before virus administration with 10 ml of saline. After virus administration, the saline bag and infusion line were flushed with 25 ml of saline at the same infusion rate to ensure that the dogs received the total planned dose of the virus.
The dogs were monitored for adverse events according to Veterinary cooperative oncology group—Common terminology criteria for adverse events v1.0 (VCOG-CTCAE v1.0).18 Attitude, respiratory and heart rate, color of mucous membranes, capillary refill time, rectal temperature, and blood pressure were monitored prior to virus administration and then every 15 minutes and 1, 2, 4, 8, 11, and 24 hours after virus administration. The dogs were monitored for swelling, itching and vomiting, the most common clinical signs of hypersensitivity in this species. After the first 24 hours, dog 2 had his attitude, appetite, weight, respiratory, and heart rate, color of mucous membranes, capillary refill time, and rectal temperature monitored daily.
Sample collection
Blood (3 ml) was collected into tubes containing potassium (K2) ethylenediaminetetraacetic acid (EDTA) for virus detection and cytokine measurements from the jugular, cephalic or saphenous vein before and immediately after virus administration, and 1, 2, 4, 11, and 24 hours as 2, 4, and 7 days after each virus infusion. Saliva was collected with two cotton tips held in each side of the mouth for 30 seconds. Urine (5 ml) was collected by cystocentesis for each sample except for two times when the urine was collected from the kennel floor immediately after voiding due to unsuccessful cystocentesis on an empty bladder. Feces (5–10 g) were collected rectally or from the kennel latrine after defecation when rectal collection failed. Saliva, urine, and feces were collected on days 1, 2, 4, and 7 after virus infusion.
Blood samples were centrifuged for 10 minutes at 2,400g immediately after collection, plasma and cell pellet was separated, initially frozen at −18 °C and transferred on the same day to −80 °C until analysis. Urine and fecal samples were frozen and stored following the same protocol.
In addition, blood was collected for (K2) EDTA tubes (1 ml) for hematology before virus administration and 2, 4, and 7 days after and for serum tube (6 ml) for clinical chemistry once weekly. In dog 1, samples were collected right before virus administration and euthanasia. Serum was separated after a minimum of 30 minutes incubation in room temperature by centrifuging 10 minutes 2,400g and both hematology and clinical chemistry samples were analyzed the day they were collected.
qPCR
DNA was extracted from whole blood using a commercial kit (Gentra Puregene Blood Kit, QIAGEN, Germantown, MD). Saliva was diluted in 2,500 µl of phosphate-buffered saline (BioWhittaker, Lonza) by squeezing the cotton swabs against the tube walls. One milliliter of the solution was used for DNA extraction (Gentra Puregene Blood Kit for Body Fluids, QIAGEN) according to the manufacturer’s instructions. DNA from the urine was extracted by the same method, using 1 ml of urine per sample. For feces, 180–220 mg was used for DNA extraction. To lyse bacteria and other pathogens and to absorb qPCR reaction inhibitors, samples were first subjected to the QIAamp DNA Stool kit (QIAGEN GmbH, Hilden, Germany) using Buffer ASL and InhibitEX tablets. Subsequently, the Gentra Puregene Tissue Kit (QIAGEN) was used, following the manufacturer’s instructions. The same tissue kit was used for tissue samples (see postmortem examination), using 50–100 mg of each tissue. The concentration of the extracted DNA was measured by spectrophotometry (Nanodrop 8000, Thermo Scientific, Wilmington, DE). Positive controls spiked with vaccinia virus were utilized to monitor the virus quantitation methods. Tissue and excretion samples from healthy dogs were extracted according to the same protocols and served as negative controls.
qPCR amplification was based on primers and probe targeting the HA J7R gene (OPHA-probe AGT GCT TGG TAT AAG GAG, OPHA-F89 GAT GAT GCA ACT CTA TCA TGT A and OPHA-R219 GTA TAA TTA TCA AAA TAC AAG ACG TC; Applied Biosystems UK, Cheshire, UK). Canine β-actin served as housekeeping gene (Canine actin Probe TCC TGG CCT CAC TGT CCA CCT TCC AGC, Canine actin FW GCG CAA GTA CTC TGT GTG GAT, and Canine actin RV GTC GTA CTC CTG CTT GCT GAT; Oligomer, Helsinki, Finland). All samples were run in triplicate. A standard curve was generated for absolute quantification by using HA-vaccinia plasmid kindly donated by Prof Vapalahti, Haartman Institute, University of Helsinki. Water was used as a negative control. The limit of detection for analysis was 1 copy/30 µl whole blood, 1 copy/100 µl urine, and 1 copy/5 mg feces. The limit of quantification was 1 copy/3 ml whole blood, 1 copy/10 ml urine, and 1 copy/500 mg feces. It was not possible to define detection or quantification limits for saliva, since the amount of collected saliva was very low.
Detection of infectious virus
All positive saliva and urine samples and selected blood samples were evaluated for infective virus by plaque assay. A549 and Vero cells were plated on a 6 well plate at 400,000 cells per well in GM with 10% FCS. Samples (200 µl) were each diluted in 6 ml GM total after three freeze-thaw cycles and 1 ml per well was used for infection. To maximize infection, infected cells were centrifuged at 600g for 20 minutes at room temperature and then incubated for 30 minutes in 5% CO2 at 37 °C. After infection, GM with 10% FCS was added and plates were incubated for 3 days, upon which plaques were visualized by Coomassie blue staining. To confirm that canine saliva, urine, and blood did not prevent viral and cell growth, we performed plaque assays with secretions alone and with secretions spiked with virus. We also incubated plaque assay cells with 300 µl blood in each well for 1 hour prior to infection. TCID50 was evaluated in selected blood samples in Vero cells as described above, using dilutions of 10−2–10−9, and the results were read on day 10.
Cytokine measurement and NAb
IFN-γ, TNF-α, IL-2, -6, -8, -10, and -12, and MCP-1 was measured in the plasma collected in EDTA tubes, with the Procarta Immunoassay Magnetic Bead kits (Affymetrix Panomix, Santa Clara, CA). To inactivate virus prior to measurement, 50 µl QuantiGene Plex Assay Kit Lysis Mixture (Affymetrix Panomix) was added to 100 µl plasma. Fluorescence was measured by the Bio-Plex 200 System (Bio Rad, Hercules, CA) device following the manufacturer’s instructions.
NAbs were measured in serum samples from dog 2 collected 2, 3, and 4 week after first virus infusion. A549 cells were plated on a 96-well plate (50,000 cells per well in DMEM with 10% FCS). The following day, serum was heated for 90 minutes at 56 °C to inactivate complement, and a serial fourfold dilution was prepared in plain DMEM. Diluted serum and 100 plaque-forming unit (PFU) of luciferase expressing double deleted vaccinia virus (vvdd-luc) at 4 × 103 PFU/ml were incubated for 60 minutes at room temperature on an orbital shaker and added in triplicates to each well after removing old media and washing of the cells. After incubation for 60 minutes in 5% CO2 at 37 °C, 100 µl of DMEM with 10% FCS was added to the wells, and the plates were incubated for 20 hours in 5% CO2 at 37 °C. After that, GM was removed, cells were lysed and luciferase activity was measured with the Luciferase Assay System (Promega, Madison, WI) using TopCount luminometer (PerkinElmer, Waltham, MA) following the manufacturer’s instructions. The neutralizing antibody titer was defined as the highest dilution of the virus preventing ≥50% of the luciferase expression.
Postmortem examination, histology and immunohistology Necropsy was performed on both beagle dogs immediately after death, and several samples from skeletal muscle, bones, nervous, endocrine, cardiovascular, respiratory, hemolymphatic, gastrointestinal, and urogenital systems and the skin collected for histological examination. Tissue specimens were fixed in 4% paraformaldehyde for 48 hours and routine paraffin wax embedded. Sections (4 µm) were prepared and stained with hematoxylin and eosin for histological examination. Samples from the same organs were collected in liquid nitrogen for DNA extraction and detection of the virus by qPCR.
A rabbit antivaccinia polyclonal antibody (1:1,000; Quartett, Berlin, Germany) and a rabbit anti-β-galactosidase polyclonal antibody (1:3,000; EMD Millipore, Temecula, CA) were used as primary antibodies for the immunohistochemical detection of vaccinia virus. β-galactosidase is a protein expressed by lacZ transgene located in VGF region of vvdd-hCD40L-tdTomato under the control of the p7.5 promoter.49 The horseradish peroxidise method was applied, using the Lab Vision Biotinylated Goat anti-Rabbit HRP kit (Thermo Fisher Scientific, Runcorn, UK) as a secondary antibody and detection system. Binding of the antibodies was visualized with the Polydetector HRP Green Substrate-Chromogen kit (Bio SB, Santa Barbara, CA) and Harris hematoxylin counterstaining. PFA fixed and paraffin wax embedded tumors from mice treated with vaccinia virus served as positive controls for the antibodies. Only the spleen from both beagle dogs was stained, since qPCR had shown that it contained the highest amount of vaccinia genome. Sections incubated without the primary antibody and sections of spleen from an untreated dog served as negative controls.
Acknowledgments
We thank Saila Pesonen, Udo Hetzel, Jere Lindén, Kati Holmsten, and Santeri Suokas for expert assistance. This study was supported by Aniwel Graduate School, University of Helsinki, Oncos Therapeutics Ltd., the European Research Council, ASCO Foundation, HUCH Research Funds (EVO), Sigrid Juselius Foundation, Academy of Finland, Biocentrum Helsinki, Biocenter Finland, Finnish Cancer Organizations, University of Helsinki.
Footnotes
A.H. is a shareholder in Oncos Therapeutics, Ltd. and an employee and a shareholder in TILT Biotherapeutics, Ltd. S.P. is an employee in Oncos Therapeutics, Ltd. A Kipar is currently working at the Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich, Switzerland; S.P. in Oncos Therapeutics, Ltd, Finland; K.G. in GeneQuine Biotherapeutics, Hamburg, Germany; and M.V.-K. in Institute of Biotechnology, University of Helsinki, Finland.
References
- Ranieri, G, Gadaleta, CD, Patruno, R, Zizzo, N, Daidone, MG, Hansson, MG et al. (2013). A model of study for human cancer: Spontaneous occurring tumors in dogs. Biological features and translation for new anticancer therapies. Crit Rev Oncol Hematol 88: 187–197. [DOI] [PubMed] [Google Scholar]
- Vail, DM and MacEwen, EG (2000). Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest 18: 781–792. [DOI] [PubMed] [Google Scholar]
- Hemminki, A, Kanerva, A, Kremer, EJ, Bauerschmitz, GJ, Smith, BF, Liu, B et al. (2003). A canine conditionally replicating adenovirus for evaluating oncolytic virotherapy in a syngeneic animal model. Mol Ther 7: 163–173. [DOI] [PubMed] [Google Scholar]
- Laborda, E, Puig-Saus, C, Rodriguez-García, A, Moreno, R, Cascalló, M, Pastor, J et al. (2014). A pRb-responsive, RGD-modified, and hyaluronidase-armed canine oncolytic adenovirus for application in veterinary oncology. Mol Ther 22: 986–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, GD, Zhai, W, Yang, HC, Fan, RX, Cao, X, Zhong, L et al. (2013). The genomics of selection in dogs and the parallel evolution between dogs and humans. Nat Commun 4: 1860. [DOI] [PubMed] [Google Scholar]
- Elsedawy, NB and Russell, SJ (2013). Oncolytic vaccines. Expert Rev Vaccines 12: 1155–1172. [DOI] [PubMed] [Google Scholar]
- Buller, RM, Chakrabarti, S, Cooper, JA, Twardzik, DR and Moss, B (1988). Deletion of the vaccinia virus growth factor gene reduces virus virulence. J Virol 62: 866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCart, JA, Ward, JM, Lee, J, Hu, Y, Alexander, HR, Libutti, SK et al. (2001). Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res 61: 8751–8757. [PubMed] [Google Scholar]
- Cerullo, V, Koski, A, Vähä-Koskela, M and Hemminki, A (2012). Chapter eight–Oncolytic adenoviruses for cancer immunotherapy: data from mice, hamsters, and humans. Adv Cancer Res 115: 265–318. [DOI] [PubMed] [Google Scholar]
- Parviainen, S, Ahonen, M, Diaconu, I, Hirvinen, M, Karttunen, Å, Vähä-Koskela, M et al. (2014). CD40 ligand and tdTomato-armed vaccinia virus for induction of antitumor immune response and tumor imaging. Gene Ther 21: 195–204. [DOI] [PubMed] [Google Scholar]
- Bergkvist, GT and Yool, DA (2011). Epidermal growth factor receptor as a therapeutic target in veterinary oncology. Vet Comp Oncol 9: 81–94. [DOI] [PubMed] [Google Scholar]
- Singer, JT, Phennicie, RT, Sullivan, MJ, Porter, LA, Shaffer, VJ and Kim, CH (2010). Broad-host-range plasmids for red fluorescent protein labeling of gram-negative bacteria for use in the zebrafish model system. Appl Environ Microbiol 76: 3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereta, M, Bereta, J, Park, J, Medina, F, Kwak, H and Kaufman, HL (2004). Immune properties of recombinant vaccinia virus encoding CD154 (CD40L) are determined by expression of virally encoded CD40L and the presence of CD40L protein in viral particles. Cancer Gene Ther 11: 808–818. [DOI] [PubMed] [Google Scholar]
- Vardouli, L, Lindqvist, C, Vlahou, K, Loskog, AS and Eliopoulos, AG (2009). Adenovirus delivery of human CD40 ligand gene confers direct therapeutic effects on carcinomas. Cancer Gene Ther 16: 848–860. [DOI] [PubMed] [Google Scholar]
- Diaconu, I, Cerullo, V, Hirvinen, ML, Escutenaire, S, Ugolini, M, Pesonen, SK et al. (2012). Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res 72: 2327–2338. [DOI] [PubMed] [Google Scholar]
- Westberg, S, Sadeghi, A, Svensson, E, Segall, T, Dimopoulou, M, Korsgren, O et al. (2013). Treatment efficacy and immune stimulation by AdCD40L gene therapy of spontaneous canine malignant melanoma. J Immunother 36: 350–358. [DOI] [PubMed] [Google Scholar]
- Autio, K, Ahonen, M, Hemminki, A, Knuuttila, A and Kipar, A (2013). Abstract: Effect of oncolytic vaccinia virus on malignant canine and feline cell lines in vitro and in vivo. Vet Comp Onc 11: e48. [Google Scholar]
- Veterinary Cooperative Oncology Group. (2004). Veterinary cooperative oncology group - common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.0. Vet Comp Onc 2: 195–213. [DOI] [PubMed] [Google Scholar]
- Breitbach, CJ, Burke, J, Jonker, D, Stephenson, J, Haas, AR, Chow, LQ et al. (2011). Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477: 99–102. [DOI] [PubMed] [Google Scholar]
- Heo, J, Reid, T, Ruo, L, Breitbach, CJ, Rose, S, Bloomston, M et al. (2013). Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 19: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, TH, Moon, A, Burke, J, Ribas, A, Stephenson, J, Breitbach, CJ et al. (2011). A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther 19: 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaime, JCJ, Young, AM, Mateo, J, Yap, TA, Denholm, KA, Shah, KJ et al. (2012). Phase 1 clinical trial of a genetically modified and oncolytic vaccinia virus GL-ONC1 with green fluorescent protein imaging. J Clin Oncol 30 (Suppl abstract 2530). [Google Scholar]
- Mastrangelo, MJ, Maguire, HC Jr, Eisenlohr, LC, Laughlin, CE, Monken, CE, McCue, PA et al. (1999). Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther 6: 409–422. [DOI] [PubMed] [Google Scholar]
- Park, BH, Hwang, T, Liu, TC, Sze, DY, Kim, JS, Kwon, HC et al. (2008). Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol 9: 533–542. [DOI] [PubMed] [Google Scholar]
- Zeh, HJ, O’Malley, M, Jones, H, Kirn, DH, Moon, A, Tae, HH et al. (2011). Abstract: clinical trial results: intralesional injection of a tumor selective oncolytic vaccinia virus. Mol Ther 19:229.21289636 [Google Scholar]
- Galic, MA, Riazi, K and Pittman, QJ (2012). Cytokines and brain excitability. Front Neuroendocrinol 33: 116–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo, M, Midulla, F and Moretti, C (2014). Actual insights into the clinical management of febrile seizures. Eur J Pediatr 173: 977–982. [DOI] [PubMed] [Google Scholar]
- Wang, X, Hunter, AK and Mozier, NM (2009). Host cell proteins in biologics development: Identification, quantitation and risk assessment. Biotechnol Bioeng 103: 446–458. [DOI] [PubMed] [Google Scholar]
- Kaneko, K, Fukuda, H, Chuang, VT, Yamasaki, K, Kawahara, K, Nakayama, H et al. (2008). Subdomain IIIA of dog albumin contains a binding site similar to site II of human albumin. Drug Metab Dispos 36: 81–86. [DOI] [PubMed] [Google Scholar]
- Viganó, F, Perissinotto, L and Bosco, VR (2010). Administration of 5% human serum albumin in critically ill small animal patients with hypoalbuminemia: 418 dogs and 170 cats (1994-2008). J Vet Emerg Crit Care (San Antonio) 20: 237–243. [DOI] [PubMed] [Google Scholar]
- Platt, S and Olby, NJ (2004). Neurological emergencies. In: Platt, S, Olby, NJ (eds.). BSAVA Manual of Canine and Feline Neurology, 3rd edn. BSAVA: Quedgeley. pp. 320–336. [Google Scholar]
- Cerullo, V, Diaconu, I, Romano, V, Hirvinen, M, Ugolini, M, Escutenaire, S et al. (2012). An oncolytic adenovirus enhanced for toll-like receptor 9 stimulation increases antitumor immune responses and tumor clearance. Mol Ther 20: 2076–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBlanc, AK, Naik, S, Galyon, GD, Jenks, N, Steele, M, Peng, KW et al. (2013). Safety studies on intravenous administration of oncolytic recombinant vesicular stomatitis virus in purpose-bred beagle dogs. Hum Gene Ther Clin Dev 24: 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, BF, Curiel, DT, Ternovoi, VV, Borovjagin, AV, Baker, HJ, Cox, N et al. (2006). Administration of a conditionally replicative oncolytic canine adenovirus in normal dogs. Cancer Biother Radiopharm 21: 601–606. [DOI] [PubMed] [Google Scholar]
- Kamphuis, E, Junt, T, Waibler, Z, Forster, R and Kalinke, U (2006). Type I interferons directly regulate lymphocyte recirculation and cause transient blood lymphopenia. Blood 108: 3253–3261. [DOI] [PubMed] [Google Scholar]
- Sampath, P, Li, J, Hou, W, Chen, H, Bartlett, DL and Thorne, SH (2013). Crosstalk between immune cell and oncolytic vaccinia therapy enhances tumor trafficking and antitumor effects. Mol Ther 21: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, JM, Avelar, GF and França, LR (2009). The seminiferous epithelium cycle and its duration in different breeds of dog (Canis familiaris). J Anat 215: 462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel, MJ and Paoletti, E (1988). Immune response to vaccinia virus and recombinant virus products in dogs. Am J Vet Res 49: 1932–1934. [PubMed] [Google Scholar]
- Wolff, HL and Croon, JJ (1968). The survival of smallpox virus (variola minor) in natural circumstances. Bull World Health Organ 38: 492–493. [PMC free article] [PubMed] [Google Scholar]
- Lederman, E, Miramontes, R, Openshaw, J, Olson, VA, Karem, KL, Marcinak, J et al. (2009). Eczema vaccinatum resulting from the transmission of vaccinia virus from a smallpox vaccinee: an investigation of potential fomites in the home environment. Vaccine 27: 375–377. [DOI] [PubMed] [Google Scholar]
- Essbauer, S, Meyer, H, Porsch-Ozcürümez, M and Pfeffer, M (2007). Long-lasting stability of vaccinia virus (orthopoxvirus) in food and environmental samples. Zoonoses Public Health 54: 118–124. [DOI] [PubMed] [Google Scholar]
- Abrahão, JS, Trindade, Gde S, Ferreira, JM, Campos, RK, Bonjardim, CA, Ferreira, PC et al. (2009). Long-lasting stability of Vaccinia virus strains in murine feces: implications for virus circulation and environmental maintenance. Arch Virol 154: 1551–1553. [DOI] [PubMed] [Google Scholar]
- Westphal, M, Ylä-Herttuala, S, Martin, J, Warnke, P, Menei, P, Eckland, D et al. ASPECT Study Group. (2013). Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol 14: 823–833. [DOI] [PubMed] [Google Scholar]
- Särkioja, M, Pesonen, S, Raki, M, Hakkarainen, T, Salo, J, Ahonen, MT et al. (2008). Changing the adenovirus fiber for retaining gene delivery efficacy in the presence of neutralizing antibodies. Gene Ther 15: 921–929. [DOI] [PubMed] [Google Scholar]
- Pesonen, S, Nokisalmi, P, Escutenaire, S, Särkioja, M, Raki, M, Cerullo, V et al. (2010). Prolonged systemic circulation of chimeric oncolytic adenovirus Ad5/3-Cox2L-D24 in patients with metastatic and refractory solid tumors. Gene Ther 17: 892–904. [DOI] [PubMed] [Google Scholar]
- Gentschev, I, Patil, SS, Adelfinger, M, Weibel, S, Geissinger, U, Frentzen, A et al. (2013). Characterization and evaluation of a new oncolytic vaccinia virus strain LIVP6.1.1 for canine cancer therapy. Bioengineered 4: 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal, GJ and Abrahams, M (2004). Growing poxviruses and determining virus titer. In: Isaacs, SN (ed.). Vaccinia Virus and Poxvirology, 1st edn. Human Press: New Jersey. pp. 101–112. [DOI] [PubMed] [Google Scholar]
- Hemminki, O, Bauerschmitz, G, Hemmi, S, Lavilla-Alonso, S, Diaconu, I, Guse, K et al. (2011). Oncolytic adenovirus based on serotype 3. Cancer Gene Ther 18: 288–296. [DOI] [PubMed] [Google Scholar]
- Guse, K, Sloniecka, M, Diaconu, I, Ottolino-Perry, K, Tang, N, Ng, C et al. (2010). Antiangiogenic arming of an oncolytic vaccinia virus enhances antitumor efficacy in renal cell cancer models. J Virol 84: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.