Abstract
Identification of the immune suppressive mechanisms active within the tumor microenvironment led to development of immunotherapeutic strategies aiming to reverse the immunosuppression and to enhance the function of tumor-infiltrating lymphocytes. Of those, cancer therapy with antibodies targeting the immune costimulatory and coinhibitory receptors has demonstrated significant promise in the recent years, with multiple antibodies entering clinical testing. The responses to these agents, however, have not been universal and have not been observed in all cancer types, calling for identification of appropriate predictive biomarkers and development of combinatorial strategies. Pre-existing immune infiltration in tumors has been demonstrated to have a strong association with response to immunotherapies, with the type I interferon (IFN) pathway emerging as a key player in tumor innate immune recognition and activation of adaptive immunity. These findings provide a rationale for evaluation of strategies targeting the type I IFN pathway as a means to enhance tumor immune recognition and infiltration, which could potentially make them susceptible to therapeutics targeting the cosignaling receptors. To this end in particular, oncolytic viruses (OVs) have been demonstrated to enhance tumor recognition by the immune system through multiple mechanisms, which include upregulation of major histocompatibility complex and costimulatory molecules on cancer cells, immunogenic cell death and antigen release, and activation of the type I IFN pathway. Evidence is now emerging that combination therapies using OVs and agents targeting immune cosignaling receptors such as 4-1BB, PD-1, and CTLA-4 may work in concert to enhance antitumor immunity and therapeutic efficacy. Our evolving understanding of the interplay between OVs and the immune system demonstrates that the virus-induced antitumor immune responses can be harnessed to drive the efficacy of the agents targeting cosignaling receptors and provides a strong rationale for integration of such therapies in clinic.
Introduction
The immune system is a key player in elimination and control of early tumor growth and evasion of the immune response by tumors has been recognized to constitute an emerging hallmark of cancer.1 The immune response against tumors constitutes a multistep process, which consists of multiple components involving both the innate and adaptive arms of the immune system. Recognition of cancer cells by the immune system typically begins at the tumor site, where professional antigen-presenting cells (APCs) such as dendritic cells (DCs) take up fragments of dying tumor cells and process them to display the peptides from tumor-associated antigens (TAAs) and other proteins within the context of class I and II major histocompatibility complex (MHC) molecules.2–5 The DCs then migrate to tumor-draining lymph nodes where priming of T cells occurs, though priming of T cells can also take place within tumor-associated tertiary lymphoid structures.6–8 Following chemoattractive signals, activated T cells then migrate to the tumors through the systemic vasculature.9,10 Entry of T cells into the tumors requires the T-cell arrest and extravasation through the tumor vasculature, which is facilitated by the expression of adhesion molecules on the tumor endothelium.11 Finally, lysis of the tumor cells proceeds through recognition of cognate MHC-peptide complexes present on the tumor cell surface.
Successful escape from recognition and/or destruction by the immune system is dependent on a myriad of mechanisms developed by the tumors aiming to prevent and/or suppress the multiple steps in the antitumor immune response.12 Recognition of these mechanisms provides the basis for the development of various immunotherapeutic approaches targeting each step in the immunosuppressive pathways.13 In particular, targeting of the costimulatory and coinhibitory receptors regulating T-cell activation has shown significant promise over the last decade, with durable clinical benefit and even cures seen in patients with metastatic cancers.14–21 The observed clinical efficacy, however, has not been universal, highlighting the marked immunosuppressive nature of these tumors and calling for development of appropriate predictive biomarkers and combinatorial strategies. This presents an opportunity for numerous combinatorial approaches, which most commonly involve combinations of immune checkpoint blocking antibodies with strategies thought to promote presentation of tumor antigens, either through exogenous vaccination or by induction of “in situ” vaccination through therapies thought to induce immunogenic cell death (ICD) and antigen release such as radiation therapy and chemotherapy.22–24
Oncolytic viruses (OVs) represent another class of emerging cancer therapeutics, which for the past 60 years have been evaluated in a variety of cancer types.25 While promising activity of OVs has been demonstrated in a variety of animal models (primarily with intratumoral injection), the clinical results have not been as impressive. The major limitation of OVs is their poor delivery to metastatic cancer sites with systemic administration and the rapid development of neutralizing antibodies by the host, which limits the utility of further systemic administration.26 However, in the few patients that did achieve response to oncolytic virotherapy, the observed clinical benefit was often durable even after completion of therapy, an effect reminiscent of the responses seen with immunotherapeutic approaches.27–29 Indeed, it has become increasingly recognized that modification of tumor cells by OVs may promote their recognition by the immune system, with activation of adaptive immune responses specific not only for viral, but also for tumor antigens.30 Recently, evidence has emerged from both preclinical and clinical studies demonstrating the immune therapeutic potential of OVs, marking a new era in the development of these agents and suggesting a tantalizing possibility that the immunostimulatory properties of OVs can be steered to improve the efficacy of immunomodulatory agents.31 Here, we highlight the mechanisms of tumor immune recognition and resistance, briefly review immunotherapies targeting immune costimulatory and coinhibitory receptors, and discuss the available evidence and rationale behind the use of combinations of OVs with systemic agents targeting the pathways of immune costimulation and coinhibition.
Mechanisms of Immune Suppression by Tumors
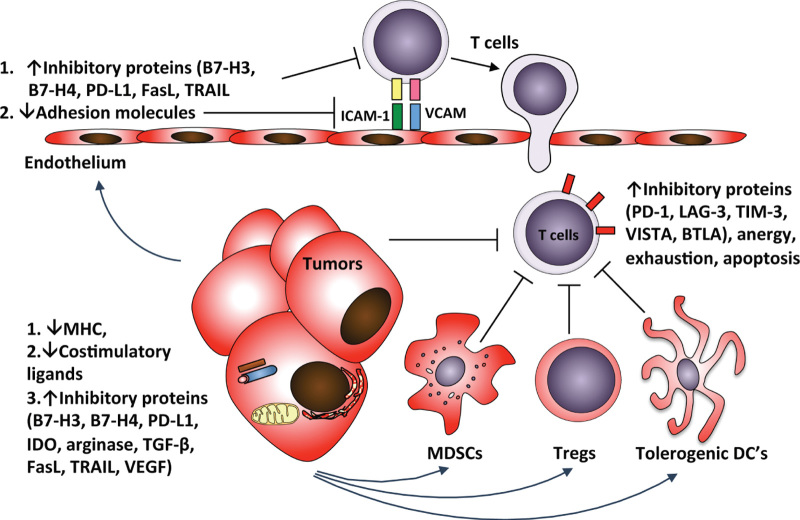
Starting with the process of antigen presentation, tumors have evolved multiple mechanisms to avoid recognition by the immune system (Figure 1).12 A key component in generation of an activating rather than tolerogenic antitumor immune response involves the presence of specific factors that are required for DC maturation.4,5 Tumors can have inhibitory effect on the function of the professional APC’s by preventing effective DC maturation. The resulting immature or incompletely matured DCs fail to activate T cells and may actually induce immune tolerance.32 In addition to blocking antigen presentation, inhibition of DC maturation has a profoundly negative effect on the production of appropriate T-cell–attracting chemokines such as CCL2, CCL3, CCL5, CXCL9, and CXCL10, which play a major rule in recruitment of T cells to the tumor site.9,10
Figure 1.
Barriers to effective adaptive antitumor immune response. Tumors have developed multiple mechanisms to effectively inhibit the antitumor immune response. Starting with tumor immune recognition, lack of appropriate maturation signals leads to generation of tolerogenic DC’s, which prevents effective antigen presentation and generation of tumor-reactive T cells. Inhibition of production of appropriate attractive chemokines fails to recruit T cells to the tumor site. Tumor vasculature establishes a further barrier to tumor-reactive T cells. Tumor effects on vascular endothelial cells lead to downregulation of the adhesion molecules necessary for T-cell arrest and transmigration, as well as to expression of immunosuppressive ligands that act to inhibit T-cell function or even to kill T cells. The T cells that manage to transmigrate into the tumor stroma further encounter additional inhibitory barriers, which include suppression by MDSCs and Tregs through different mechanisms, leading to upregulation of inhibitory receptors, apoptosis, anergy, or exhaustion. Finally, the encounter with tumor cells may not lead to efficient lysis due to downregulation of MHC or specific TAAs on tumor cells, and increased expression of immunosuppressive proteins by the tumor cells that act to inhibit T-cell function. DC, dendritic cell; MDSC, myeloid-derived suppressor cell; MHC, major histocompatibility complex; TAA, tumor-associated antigen.
In addition to the effect on the immune cells within the tumor microenvironment, tumors have profound effect on the vascular endothelium (Figure 1). Through production of factors such as vascular endothelial growth factor, tumors can inhibit the expression of appropriate adhesion molecules required for T-cell adhesion to endothelium,11 and induce the expression of the immune inhibitory or cytotoxic molecules such as PD-L1, B7-H3, and TRAIL on endothelial cells.33–36 Furthermore, for the T cells that do manage to cross the endothelial barrier, the immunosuppressive cell populations within the tumor microenvironment such as Tregs and myeloid-derived suppressor cells act to inhibit T-cell function, induce T-cell exhaustion, or directly kill the T cells.37,38
Finally, tumor cells themselves have developed endogenous mechanisms to avoid recognition and killing by T cells (Figure 1).39 Some tumors have evolved to downregulate or completely lose the expression of MHC, which prevents their recognition by T cells.40 Other tumors may express molecules that can directly kill T cells, such as FasL and TRAIL, or proteins that inhibit T-cell function, such as IDO, PD-L1, B7-H3, and B7-H4.36,41–43 Tumors may also secrete soluble inhibitory mediators such as transforming growth factor β, interleukin-10, and adenosine, and deplete essential metabolites by overexpression of enzymes such as IDO and arginase.42,43
To orchestrate effective escape from the host immune response, most tumors use multiple concurrent inhibitory strategies,12 with some tumors being more immunosuppressive than others. Analysis of different tumor types consistently demonstrates that tumor infiltration with T cells is a good prognostic marker in a variety of tumor types.44–55 Presumably, the T-cell–infiltrated tumors are a marker of an ongoing spontaneous antitumor immune response, which results in better tumor control and leads to more favorable clinical outcomes. Interestingly, the prognostic value of this phenotype has been shown to be more powerful than traditional staging in colorectal cancer,56 and studies are currently underway to validate this marker prospectively.57 Evidence of pre-existing immune infiltration in tumors and presence of tumor-reactive lymphocytes suggest that the immune avoidance mechanisms active in such tumors do not entirely prevent activation of the antitumor immune response. Rather, lack of immune-mediated killing of cancer cells in such tumors attests to the dysfunction of the tumor-infiltrating lymphocytes. The tumor-infiltrating lymphocytes (TILs) are thus kept in check by the immunosuppressive mechanisms active within such tumors, as evidenced by poor functionality of TILs directly isolated from the tumors.58 Expansion of such cells can restore their functionality in vitro, which serves as a basis for success seen with adoptive T-cell therapies.59 Alternatively, direct targeting of the appropriate immunosuppressive mechanisms in the tumor microenvironment with therapeutic antibodies can directly restore the functionality of T cells in vivo, and lead to therapeutic responses in many of these tumors. This is best exemplified by the recent clinical success of antibodies targeting the molecules involved in T-cell costimulation and coinhibition, which will be the major focus of the current review.
Targeting T-Cell Costimulatory and Coinhibitory Receptors for Cancer Therapy
Activation of T cells requires the recognition by the T-cell receptor of cognate antigenic peptides presented by MHC molecules and is regulated by a repertoire of coinhibitory or costimulatory receptors expressed on the T-cell surface.60 These receptors integrate a complex signaling network of ligands present within the tissue microenvironment, and play a crucial role in regulation of T-cell activation, differentiation, survival, and effector function. Most of the receptors belong to the immunoglobulin superfamily and the tumor necrosis factor receptor superfamily. The immunoglobulin superfamily includes costimulatory receptors such as CD28 and ICOS, as well as the coinhibitory receptors such as CTLA-4, PD-1, LAG3, TIM3, BTLA, VISTA, and CD160. The tumor necrosis factor receptor superfamily includes costimulatory members such as GITR, OX40, CD30, CD40, and 4-1BB, as well as several other members, which either have not been identified to have a specific function or may act in either costimulatory or coinhibitory function depending on differential interaction with different ligands through multiple interfaces.60
Identification of the multiple cellular receptors governing the activation status of T cells led to development of agents targeting these receptors in an effort to reverse tumor-induced immunosuppression. This strategy was pioneered by James Allison and colleagues, who were the first to demonstrate that targeting of the inhibitory receptor CTLA-4 with an antibody can result in tumor regressions in animal tumor models.61 Since then, therapeutic antibodies to CTLA-4 (ipilimumab and tremelimumab) have been evaluated in multiple clinical trials, with durable clinical benefit and even unprecedented cures seen in patients with metastatic disease. In a pivotal phase 3 trial in metastatic melanoma, ipilimumab demonstrated improvement in overall survival, which led to its approval by US Food and Drug Administration in 2011 for treatment of metastatic melanoma.18 Both ipilimumab and tremelimumab are currently being evaluated in phase 2 and 3 trials in patients with various other advanced cancers.
The identification of another coinhibitory receptor, PD-1, and its ligands (PD-L1 and PD-L2), prompted their evaluation as therapeutic targets in cancer.62,63 Several therapeutic antibodies to PD-1 and PD-L1 have entered clinical testing over the past few years, with significant activity seen in patients with metastatic melanoma, lung cancer, and renal cell carcinoma, as well as some other cancers, including hematologic malignancies.15,20,64–66 Antibodies targeting additional immune checkpoints such as LAG3 and TIM3 are also entering clinical testing.
In addition to targeting the immune coinhibitory receptors, several clinical trials are ongoing or have been completed with agonistic antibodies targeting the immune activating receptors of the tumor necrosis factor receptor superfamily, such as GITR, 4-1BB, OX40, and CD40, with evidence of immune activation and clinical activity seen with these agents in a variety of malignancies.16,19,67
Tumors Without Pre-Existing Immune Infiltration Respond Poorly to Immunotherapy
Despite the durable therapeutic efficacy seen with antibodies targeting the immune costimulatory and coinhibitory receptors, the responses have not been universal and have not been present in all cancer types. This calls for identification of predictive biomarkers and the development of rational combination strategies, which would make such therapeutic approaches applicable to a larger number of patients and a broader range of tumors. Studies of pretreatment tumor samples in patients with metastatic melanoma have demonstrated that patients with evidence of pre-existing tumor immune infiltration are more likely to respond to immunotherapies with vaccines or immune checkpoint blockade.47–49 Furthermore, gene expression profiling demonstrated that patients with high baseline tumor expression levels of genes related to both innate and adaptive immune response were more likely to favorably respond to immunotherapy.47–49,58
While these studies indicate that tumors with pre-existing TILs are more amenable to immunotherapies, they highlight the significant therapeutic challenge for tumors lacking immune infiltration. It is reasonable to speculate that strategies inducing an increase in TILs should be explored as a means to make tumors more susceptible to immunomodulatory antibodies. At present, it is not fully clear which mechanisms are responsible for increased immune infiltration in some tumors, although transcriptional profiling of lymphocyte-infiltrated tumors demonstrated that tumors infiltrated with CD8+ T cells also upregulate a type I interferon (IFN) transcriptional signature, suggesting that activation of type I IFN pathway may play a role in the innate immune recognition of tumors.68 Indeed, type I IFN receptor-deficient animals showed near complete loss of T-cell priming in transplantable and carcinogen-induced tumor models.68–70 This defect was shown to be largely attributed to the loss of the CD8α+ DCs, a major DC subset responsible for tumor antigen cross-presentation and priming of tumor-specific CD8+ T cells.68,70 These findings thus provide a compelling reason to explore agents targeting the type I IFN pathway as a strategy that can activate innate immune responses in tumors, potentially promoting tumor immune infiltration and overcoming the resistance to systemic agents targeting costimulatory and coinhibitory receptors.
Activation of Innate Immune Response and ICD
The innate immune system has evolved to detect infectious processes through recognition of specific pathogen-associated molecular patterns (PAMPs), which are microbial components inherent to each pathogen.71,72 Major PAMPs identified to date are nucleic acids, proteins, and components of pathogen cell membranes. In addition, innate immune activation involves recognition of endogenous danger signals produced by dying eukaryotic cells, collectively known as damage-associated molecular patterns (DAMPs).73 Some of the characterized DAMPs include intracellular proteins such as high-mobility group box 1 and heat-shock proteins, DNA, adenosine triphosphate, uric acid, heparin sulfate, and mitochondrial components.74 PAMPs and DAMPS in turn are recognized by several classes of pattern-recognition receptors, which include the toll-like receptors (TLRs), retinoic acid-inducible gene-1–like receptors, nucleotide oligodimerization domain–like receptors, absent in melanoma 2, and the receptor for advanced glycation end products.75 Recognition of PAMPs and DAMPs by pattern-recognition receptors activates intracellular signaling cascades, which involve several important adaptors including Interferon β promoter stimulator-1 and stimulator of interferon genes (STING). An important converging point for the multiple pathways is phosphorylation and nuclear translocation of IFN regulatory factor 3, which drives the production of IFN-β.75
Activation of the innate immune response as well as the danger signals released from the dying tumor cells lead to secretion of inflammatory mediators and recruitment of immune cells, a process that collectively has been termed immunogenic cell death (ICD).74,76 To achieve this effect, several strategies targeting the various components of innate immunity have been explored in animal models with promising results, with agents binding TLR being used most commonly. TLR agonists can result in rapid activation of innate immune responses and trigger activation of adaptive immunity. The most commonly used TLR agonists are cytosine-phosphorothioate-guanine oligonucleotides activating TLR9, and polyinosinic:polycytidylic acid (poly I:C), which activates TLR3.77 Intratumoral therapy with TLR agonists has been shown to enhance antitumor immunity of adoptively transferred T cells,78,79 and of immunomodulatory antibodies such as anti-CTLA-4, anti-PD-1, and anti-OX40.80,81 An analogous strategy was employed using 5,6-dimethylxanthenone-4-acetic acid, an agonist of STING, a DNA-sensing protein that has been implicated in tumor sensing by the APC’s. Intratumoral or systemic therapy with 5,6-dimethylxanthenone-4-acetic acid has been demonstrated to activate antitumor immune response, providing a strong rationale for exploration of combination therapies of STING agonists with immunomodulatory antibodies.82
Intralesional administration of type I IFNs has also been explored in several studies, both in animals and patients, with evidence of local and systemic activity. In addition, one study explored a combination of systemic agonistic antibody to 4-1BB and intralesional IFN-α.83 Combination therapy demonstrated evidence of synergy of the combination, with antitumor effects seen not only in the IFN-injected lesions, but also in the lesions implanted at a distant site.83
OVs Cause ICD and Activate Innate Immune Responses in Tumors
OVs represent another class of promising emerging cancer therapeutics, with viruses from several families currently being evaluated in clinical trials.25,26,84 The initial therapeutic appeal of OVs arose from the finding of the inherent ability of such viruses to replicate in cancer cells, while sparing normal tissues. Multiple studies have been performed evaluating the therapeutic potential of OVs over the past 60 years, with defined mechanisms of virus-mediated oncolysis and specificity for cancer cells. In the majority of the cases, the selectivity of OVs for tumor cells exploits the cellular defects that govern oncogenesis, namely, resistance to apoptosis and translational suppression, and avoidance of both innate and adaptive immune responses, which are the same mechanisms that are normally needed to successfully clear virus infection.25 Specifically, defects in activation of the type I IFN pathway have been shown to play a major role in the oncolytic specificity of some OVs, such as vesicular stomatitis virus (VSV) and newcastle disease virus (NDV).26
Approximately 50 years ago, investigators working with influenza virus noted that infection of tumor cells with the virus increased immune responses directed toward tumor cell antigens.85 Since then, multiple studies have demonstrated that in addition to tumor lysis, through release of PAMPs and DAMPs OVs can potently activate innate immune responses in both tumor cells and tumor-infiltrating APCs, characterized by upregulation of MHC and costimulatory molecules as well as secretion of inflammatory mediators, which aid in recruitment of the adaptive immune cells.86 Our evolving understanding of mechanisms of antitumor activity exerted by OVs has led to their increasing exploration as immune therapeutics and as vectors delivering immunotherapeutic transgenes or antigens for vaccination.
The concept of virus rendering tumor cells immunogenic has been explored in multiple clinical trials evaluating virus-modified tumor cells as a vaccination strategy. Perhaps the best studied virus in this setting is NDV.87 The initial studies were performed by Cassel et al. in the 1970’s utilizing vaccination with NDV-infected autologous or allogeneic melanoma cells in the adjuvant setting. These studies saw unprecedented 60% 10-year recurrence-free survival in these patients.88–90 Analogous studies were performed by Schirrmacher and colleagues, utilizing whole-cell autologous irradiated tumor vaccines modified by infection with attenuated NDV. While most of the studies were uncontrolled, evidence of clinical benefit was seen in patients with colorectal, breast, ovarian, and kidney cancer, as well as glioblastoma.87,91
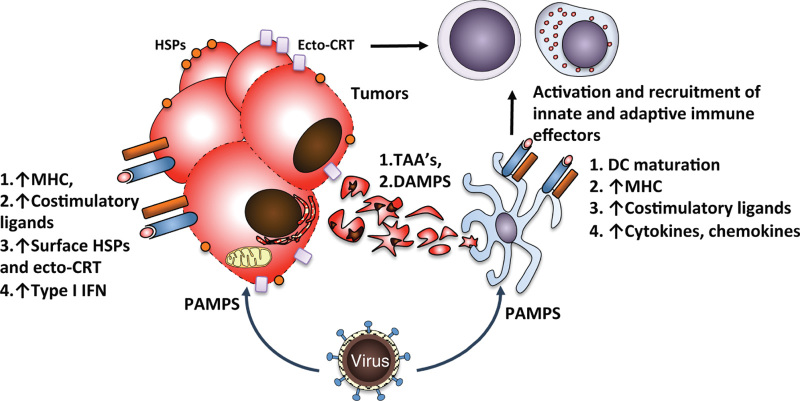
Taken together, activation of the innate immune response by OVs involves several mechanisms, which all likely act in concert to generate antitumor immunity (Figure 2). First, direct infection of tumor cells renders them immunogenic through activation of innate immune response in tumor cells, which includes secretion of inflammatory mediators such as IFN-β, and upregulation of MHC, cell adhesion proteins, and costimulatory molecules, potentially reversing the immune inhibition of inflammation in the tumor microenvironment. Indeed, ex vivo studies of TILs isolated from freshly resected melanomas revealed that while the lymphocytes were nonproliferative when stimulated with autologous melanoma cells, proliferation was restored when they were stimulated with autologous melanoma cells infected with NDV.76 Second, OVs kill tumor cells through several mechanisms, including immunogenic apoptosis, necrosis, and autophagy, all of which have been described to be associated with ICD.86 As a result, tumor cell lysis results in release of TAAs and DAMPs, which promote activation of tumor-infiltrating professional APC’s.86 Finally, the inflammatory mediators secreted by the infected tumor cells as well as direct infection of tumor-infiltrating DC’s further promote DC activation and maturation.92
Figure 2.
Immunogenic cell death and inflammation induced by OVs. Infection of tumor cells by OVs leads to production of PAMPs, which activate cellular stress and innate immune responses resulting in production of type I IFN and upregulation of surface MHC, costimulatory ligands, and danger signals such as calreticulin (ecto-CRT) and HSPs. Lysis of the infected cells leads to release of TAAs and DAMPs, which aid in activation of professional APCs. Direct infection of APCs by some OVs further aids APC maturation and antigen presentation, ultimately leading to activation and recruitment of additional innate and adaptive immune effectors. APC, antigen-presenting cell; DAMP, damage-associated molecular pattern; HSPs, heat-shock proteins; IFN, interferon; MHC, major histocompatibility complex; OV, oncolytic virus; PAMP, pathogen-associated molecular pattern; TAA, tumor-associated antigen.
Exploration of OV Properties for Stimulation of Adaptive Antitumor Immunity
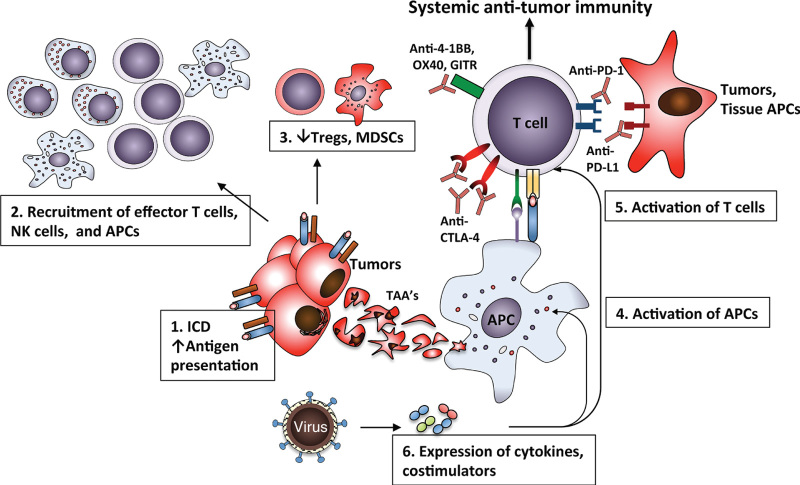
The inflammatory changes in tumors induced by OV infection in essence act as a vaccination event, where in the setting of tumor cell lysis and APC activation, can overcome the immunosuppressive tumor microenvironment and create optimal conditions necessary for effective T-cell priming and activation (Figure 3). Studies also suggest that these changes have a negative effect on suppressive immune populations within the tumors, with reduction of Tregs and myeloid-derived suppressor cells in the infected tumors.93–96 With advent of genetic engineering, multiple studies have explored whether expression of immunostimulatory ligands or cytokines by the OVs can result in stronger adaptive immune response. Most of these vectors have been used for direct intratumoral administration. Expression of OX40 ligand (OX40L) or GITR ligand (GITRL) by adenovirus vectors administered intratumorally led to suppression of growth of the treated tumors in several tumor models.97,98 Similarly, expression of 4-1BBL by oncolytic vaccinia virus has been demonstrated to enhance tumor regression in the B16 melanoma model, an effect that was markedly enhanced when used in combination with nonmyeloablative lymphodepletion.99
Figure 3.
Using OVs to restore systemic antitumor immunity. The immune response activated in the tumor microenvironment by OVs can be harnessed to drive therapeutic efficacy of the agents targeting T-cell activation. Infection of tumors with OVs leads to ICD, enhanced antigen presentation, APC maturation, and recruitment and activation of T cells, with changes in the microenvironment favoring the expansion of the effector rather than suppressive cell populations. Combination of OV with agents targeting costimulatory (e.g., 4-1BB, OX40, GITR) and/or coinhibitory receptors on T cells (e.g., CTLA-4, PD-1) results in more effective systemic antitumor immunity, which is active at the sites not directly accessible to OV infection. APC, antigen-presenting cell; ICD, immunogenic cell death; OV, oncolytic virus.
Several of these vectors have been evaluated in clinic. Recombinant vaccinia virus expressing B7.1 was evaluated for intralesional therapy in patients with advanced melanoma with accessible lesions. Evidence of clinical benefit and development of tumor-specific CD8+ cells was observed in several patients.100 A replication-competent adenovirus encoding CD40L was evaluated for intralesional therapy in patients with various advanced tumors, with evidence of disease control and induction of tumor-specific immune responses.101 A CD40L-expressing vaccinia virus was similarly found to result in enhanced tumor growth inhibition, and recruitment of APC’s and lymphocytes to the tumor site.102
Most of the studies demonstrated that such viruses could induce stronger infiltration of immune cells into the microenvironment of the infected tumors, leading to improved therapeutic efficacy. The question remains as to whether such local changes can induce strong antitumor immune responses, which would be effective not just at the site of viral infection, but also at distant metastatic sites. The latter is favored by several studies, which have demonstrated that tumor lysis by virus is followed by generation of both innate and adaptive immune responses. The best clinical example of this is demonstrated by the studies of talimogene laherparepvec, an oncolytic herpes simplex virus encoding granulocyte-macrophage colony-stimulating factor. Intralesional administration of the virus to patients with advanced melanoma led to tumor immune infiltration and regression not only of the injected lesions, but also at distant sites.28,96 In a similar study, intratumoral injection of another OV, coxsackievirus A21, in patients with melanoma similarly demonstrated responses not only in the injected, but also in distant lesions.103 Analogous to these findings, we have recently demonstrated that in mouse models bearing bilateral tumors, administration of oncolytic NDV to single tumor led to lymphocytic infiltration into the virus-injected and distant tumors.93
OVs as Potentiators of Therapies Targeting Costimulatory and Coinhibitory Receptors
With emerging knowledge of the importance of the type I IFN pathway in the induction of adaptive antitumor immune responses through activation of CD8α+ DC’s as discussed above,68,70 the question re-emerges as to whether OVs could be used to “inflame” tumors and thus drive response to immunotherapies targeting T-cell activation. To date, there have been very few studies evaluating the effects of combining OVs with antibodies targeting immune costimulatory and coinhibitory receptors. Combination of adenovirus encoding interleukin-12 with systemic agonistic antibodies targeting OX40 and 4-1BB demonstrated enhanced development of antitumor cytotoxic T lymphocyte responses and led to tumor rejection in an experimental colorectal cancer model with liver metastases.104 A replication-competent VSV targeted to Her2/neu has been tested in a mouse mammary tumor cell line stably transfected to express human Her2/neu protein.105 Intratumoral treatment with the virus by itself was able to suppress growth of the treated tumors, but resulted in no cures. Combination of this approach with systemic CTLA-4 blockade resulted in cures in the majority of animals, and the effect that was dependent on CD4+ and CD8+ T cells.105 A combination of intratumoral oncolytic vaccinia virus with systemic agonistic antibody to 4-1BB resulted in significant reduction in growth of established subcutaneous MC38 and AT-3 tumors and increased tumor immune infiltration.106 Therapeutic vaccination with replication deficient adenovirus expressing a model antigen in combination with agonistic anti-CD40 antibody and CTLA-4 blockade was demonstrated to delay growth of B16-F10 melanomas expressing the same antigen, resulting in complete tumor regression in some animals.107
While these studies demonstrated evidence of enhanced antitumor activity in the virus-injected tumors when used in combination with costimulatory agonists or coinhibitory antagonists, very few studies have explored whether such strategies would be active against distant tumors, a question of highest therapeutic relevance for patients with metastatic disease. To formally evaluate whether OVs given intratumorally can generate adaptive antitumor immune response that can concurrently be active on the systemic level, we have recently performed a study using NDV, which we selected for it’s known ability to enhance immunogenicity of tumor cells and it’s capacity to induce robust type I IFN response and DC maturation.108 Interestingly, prior clinical studies with NDV demonstrated evidence of durable benefit, reminiscent of responses seen with immunotherapeutic approaches.27 In a challenging bilateral flank B16-F10 melanoma model, intratumoral injection of the virus led to immune infiltration in distant tumors, not directly affected by viral infection, with predominant increase in the numbers of the CD4+ and CD8+ effector but not regulatory T cells.93 Combination of intratumoral NDV with systemic CTLA-4 blockade led to rejection of contralateral tumors, with long-term survival in the majority of animals, an effect that was highly dependent on natural killer cells, CD8 cells, and type I and II IFNs.93 Importantly, the success of therapy was seen in several tumor models, independent of tumor cell line sensitivity to NDV-mediated lysis, highlighting the importance of the virus-induced antitumor immunity rather than direct oncolysis in the observed therapeutic efficacy. Consistent with these findings, intratumoral injection of NDV also induced tumor infiltration with adoptively transferred tumor-specific lymphocytes, highlighting the ability of the virus to reverse the inhibitory effects of tumor microenvironment that allowed for entry and expansion of these cells in tumors.93 In a similar approach, Aida et al., used systemic agonistic anti-GITR antibody together with intratumoral administration of adenovirus expressing IFN-α, which also resulted in synergistic inhibition of the virus-injected and distant tumors.109
Clinical validation of these findings was recently presented at the 2014 ASCO annual meeting, where the initial data from the phase 1 study of combination of intralesional talimogene laherparepvec in combination with systemic ipilimumab in advanced melanoma were discussed.110 In this report, of 18 patients who received the combination therapy, an objective response rate of 41%, including 24% complete responses were observed. Although this is a preliminary report from a noncontrolled study, the response rate is higher than that expected with ipilimumab alone.
Several studies have also targeted the costimulatory and coinhibitory receptors as a means to augment an immune response to virus-encoded vaccines. Additive or synergistic efficacy with CTLA-4 blockade was reported with modified vaccinia Ankara poxvirus encoding mutated p53 protein.111 Similarly, stimulation of 4-1BB and OX40 with agonistic antibodies has been demonstrated to enhance the immune response to another poxvirus-based vaccine,112 while immunization with adenovirus vector expressing Her-2/neu antigen in combination with agonistic antibody to GITR was able to break self-tolerance and induce cytotoxic T lymphocyte responses to the Her-2/neu in a tolerogenic mouse tumor model.113 Combination of lentivirus-encoded tumor antigen vaccine with PD-1 or PD-L1 blocking antibodies has similarly been demonstrated to result in enhanced vaccination efficacy and improved tumor control.114 Expression of 4-1BB ligand (4-1BBL) by vaccinia virus has been shown to enhance the efficacy of vaccination of another vaccinia vector carrying the genes for CEA, B7.1, ICAM-1, and LFA-3 (rV-CEA-TRICOM).115 Similarly, combination of the recombinant vaccinia and fowlpox-based CEA-TRICOM vaccines with systemic CTLA-4 blockade led to enhanced antitumor immunity.116 Interestingly, this effect was dependent on scheduling of vaccine and CTLA-4 blockade, with the highest activity seen when the vaccine and anti-CTLA-4 antibody were administered concurrently.
Considerations
Taken together, these studies thus provide a strong rationale for further evaluation of OVs as potentiators of immunotherapy with agents targeting immune costimulatory or coinhibitory receptors. At present, it is not completely clear whether the immunomodulatory antibody needs to be administered systemically, or whether locoregional administration of the antibody to the tumor site may be sufficient. Data from Levy et al. provides a compelling argument for the latter, where intratumoral administration of significantly lower doses of immunomodulatory antibodies such as anti-CTLA-4 and anti-OX40 was equally effective to systemic treatment, with antitumor efficacy seen at distant sites.117 These findings would argue that expression of immunomodulatory antibody at the tumor site by a genetically engineered OV may prove to be most effective in generation of antitumor immunity. To this end, an adenovirus expressing human anti-CTLA-4 antibody was demonstrated to express significantly higher concentrations of the antibody in the tumor, as compared to plasma.118 While the study did not specifically look at the efficacy of such a virus in animal tumor models, it highlights a possibility that such a strategy may mitigate some of the immunologic adverse effects seen with systemic administration of such antibodies.
While evaluating OVs as immunotherapeutic agents, it is important to balance with words of caution. Despite the hundreds of reports demonstrating the beneficial effects of OVs on antitumor immunity, there is an obvious publication bias toward the agents that appear to be active, and one must wonder how many additional therapies have been ineffective or even detrimental. When designing combinatorial therapies with OVs as well as developing recombinant viruses expressing costimulatory molecules, a strong consideration needs to be given to the potential to worsen the antitumor immunity by skewing the immune response to predominantly target the virus. This is best exemplified by the studies of recombinant VSV as an oncolytic vaccine vector expressing human dopachrome tautomerase (hDCT), a TAA expressed by murine B16 melanoma.119 While VSV was previously demonstrated as a very effective oncolytic agent in a variety of tumor models, in this study the authors demonstrated that therapy with VSV-hDCT induced a predominantly anti-VSV immune response, which prevented the establishment of antitumor immunity and was not therapeutically effective in the model of intracranial B16 melanoma. In the same study, when VSV-hDCT was used as a boosting agent in the animals that were initially primed with adenovirus-hDCT vaccine, there was a dramatic enhancement of immunity to hDCT with significant improvement in survival.119 In another study, in an attempt to enhance VSV-generated antitumor immunity, a VSV vector expressing CD40 ligand (VSV-CD40L) was generated.120 Surprisingly, this vector did not demonstrate any enhancement in antitumor efficacy. The authors went on to demonstrate that VSV-CD40L induced high levels of activated T cells without any specificity for TAAs, suggesting that VSV-associated immune activation distracted the immune system from priming and activation of tumor-specific T cells.120 In contrast, a replication-defective adenovirus expressing CD40L in this system was able to efficiently prime T cells directed against TAAs.120 While these studies utilized oncolytic VSV, it is likely that other OVs may also exhibit similar effects.
A strong consideration must also be given to the route of OV administration. For example, while NDV seems to strongly induce DC maturation and effective antitumor immune response with direct intratumoral administration,93,108 intratumoral VSV has been shown to reduce viability of tumor-associated DC’s and thus interferes with priming of tumor-specific CD8 T-cell responses.121 In contrast, VSV-based vaccine was very effective when given intravenously, an effect that is dependent on the virus vector delivery by the follicular B cells to DCs in spleen and lymph nodes.122 These findings highlight that the immune therapeutic effect of different OVs likely proceeds through different mechanisms, and suggest that one should exercise caution when developing OV-based immunotherapies, as findings with one virus might not be generalizable to others. The success of such therapeutics may highly depend on the choice of the viral vector and costimulatory ligands, route of administration, sequencing and scheduling of agents that are used in combination, and the choice of the tumor models.
Conclusions
Despite the significant promise of OVs and their extensive evaluation as tumor lytic agents over the past 60 years, their therapeutic efficacy in patients has so far been limited. We are now starting to understand the importance of OV-induced antitumor immune response as one of the primary drivers of efficacy, with significant enhancement in clinical benefit in recent trials utilizing OV’s designed to amplify the antitumor immune response. Concurrently, the major breakthroughs in the field of cancer immunotherapy with agents targeting the immune costimulatory and coinhibitory receptors have provided a strong rationale for integration of these approaches. There is emerging evidence that such combination strategies can indeed lead to enhanced antitumor immunity and may translate into therapeutic benefit where either therapy is ineffective alone. Our improved understanding of the interplay between the viral oncolysis and the development of antiviral and antitumor immunity will be crucial for the rational design of engineered OVs and the development of appropriate combinatorial strategies with other immune therapeutics.
Acknowledgments
This work was supported by the National Institutes of Health (CA056821 to J.D.W.), and Swim Across America (J.D.W.). D.Z. is the Bart A Kamen Fellow of the Damon Runyon Cancer Research Foundation and a recipient of the Young Investigator Awards from the ASCO Conquer Cancer Foundation and Bladder Cancer Awareness Network.
Footnotes
J.D.W. received consulting reimbursements from MedImmune, Bristol Myers-Squibb, Merck, and GlaxoSmithKline. J.D.W. and D.Z. are co-inventors of intellectual property related to the use of NDV for cancer immunotherapy.
References
- Hanahan, D and Weinberg, RA (2011). Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Boon, T, Coulie, PG, Van den Eynde, BJ and van der Bruggen, P (2006). Human T cell responses against melanoma. Annu Rev Immunol 24: 175–208. [DOI] [PubMed] [Google Scholar]
- Segal, NH, Parsons, DW, Peggs, KS, Velculescu, V, Kinzler, KW, Vogelstein, B et al. (2008). Epitope landscape in breast and colorectal cancer. Cancer Res 68: 889–892. [DOI] [PubMed] [Google Scholar]
- Mellman, I and Steinman, RM (2001). Dendritic cells: specialized and regulated antigen processing machines. Cell 106: 255–258. [DOI] [PubMed] [Google Scholar]
- Trombetta, ES and Mellman, I (2005). Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol 23: 975–1028. [DOI] [PubMed] [Google Scholar]
- de Chaisemartin, L, Goc, J, Damotte, D, Validire, P, Magdeleinat, P, Alifano, M et al. (2011). Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 71: 6391–6399. [DOI] [PubMed] [Google Scholar]
- Goc, J, Fridman, WH, Sautès-Fridman, C and Dieu-Nosjean, MC (2013). Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology 2: e26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palucka, K, Banchereau, J and Mellman, I (2010). Designing vaccines based on biology of human dendritic cell subsets. Immunity 33: 464–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlin, H, Meng, Y, Peterson, AC, Zha, Y, Tretiakova, M, Slingluff, C et al. (2009). Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res 69: 3077–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciszkiewicz, K, Boissonnas, A, Boutet, M, Combadière, C and Mami-Chouaib, F (2012). Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res 72: 6325–6332. [DOI] [PubMed] [Google Scholar]
- Zitvogel, L, Tesniere, A and Kroemer, G (2006). Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 6: 715–727. [DOI] [PubMed] [Google Scholar]
- Motz, GT and Coukos, G (2013). Deciphering and reversing tumor immune suppression. Immunity 39: 61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, DS and Mellman, I (2013). Oncology meets immunology: the cancer-immunity cycle. Immunity 39: 1–10. [DOI] [PubMed] [Google Scholar]
- Wolchok, JD, Kluger, H, Callahan, MK, Postow, MA, Rizvi, NA, Lesokhin, AM et al. (2013). Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid, O, Robert, C, Daud, A, Hodi, FS, Hwu, WJ, Kefford, R et al. (2013). Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curti, BD, Kovacsovics-Bankowski, M, Morris, N, Walker, E, Chisholm, L, Floyd, K et al. (2013). OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 73: 7189–7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert, C, Thomas, L, Bondarenko, I, O’Day, S, M D, JW, Garbe, C et al. (2011). Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364: 2517–2526. [DOI] [PubMed] [Google Scholar]
- Hodi, FS, O’Day, SJ, McDermott, DF, Weber, RW, Sosman, JA, Haanen, JB et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznol, M, Hodi, FS, Margolin, K, McDermott, DF, Ernstoff, MS, Kirkwood, JM et al. (2008). Phase I study of BMS-663513, a fully human anti-CD137 agonist monoclonal antibody, in patients (pts) with advanced cancer (CA). 2008 ASCO Annual Meeting. Chicago, IL. pp. 3007.
- Topalian, SL, Hodi, FS, Brahmer, JR, Gettinger, SN, Smith, DC, McDermott, DF et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty, GL, Chiorean, EG, Fishman, MP, Saboury, B, Teitelbaum, UR, Sun, W et al. (2011). CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331: 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel, L and Kroemer, G (2009). Anticancer immunochemotherapy using adjuvants with direct cytotoxic effects. J Clin Invest 119: 2127–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Elsas, A, Hurwitz, AA and Allison, JP (1999). Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med 190: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, I, Coukos, G and Dranoff, G (2011). Cancer immunotherapy comes of age. Nature 480: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, SJ, Peng, KW and Bell, JC (2012). Oncolytic virotherapy. Nat Biotechnol 30: 658–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo, R, Miest, T, Shashkova, EV and Barry, MA (2008). Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat Rev Microbiol 6: 529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorence, RM, Roberts, MS, O’Neil, JD, Groene, WS, Miller, JA, Mueller, SN et al. (2007). Phase 1 clinical experience using intravenous administration of PV701, an oncolytic Newcastle disease virus. Curr Cancer Drug Targets 7: 157–167. [DOI] [PubMed] [Google Scholar]
- Andtbacka, RHI, Collichio, FA, Amatruda, T, Senzer, NN, Chesney, J, Delman, KA et al. (2013). OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol 31: suppl; abstr LBA9008. [Google Scholar]
- Heo, J, Reid, T, Ruo, L, Breitbach, CJ, Rose, S, Bloomston, M et al. (2013). Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 19: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, AW, Senzer, N, Cerullo, V, Templeton, NS, Hemminki, A and Nemunaitis, J (2012). Oncolytic viruses for induction of anti-tumor immunity. Curr Pharm Biotechnol 13: 1750–1760. [DOI] [PubMed] [Google Scholar]
- Bauzon, M and Hermiston, T (2014). Armed therapeutic viruses - a disruptive therapy on the horizon of cancer immunotherapy. Front Immunol 5: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman, RM, Turley, S, Mellman, I and Inaba, K (2000). The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med 191: 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frebel, H, Nindl, V, Schuepbach, RA, Braunschweiler, T, Richter, K, Vogel, J et al. (2012). Programmed death 1 protects from fatal circulatory failure during systemic virus infection of mice. J Exp Med 209: 2485–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secchiero, P and Zauli, G (2008). The puzzling role of TRAIL in endothelial cell biology. Arterioscler Thromb Vasc Biol 28: e4; author reply e5–e4; author reply e6. [DOI] [PubMed] [Google Scholar]
- Mazanet, MM and Hughes, CC (2002). B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol 169: 3581–3588. [DOI] [PubMed] [Google Scholar]
- Zang, X, Sullivan, PS, Soslow, RA, Waitz, R, Reuter, VE, Wilton, A et al. (2010). Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Mod Pathol 23: 1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich, DI and Nagaraj, S (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciabene, A, Motz, GT and Coukos, G (2012). T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res 72: 2162–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside, TL (2006). Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol 16: 3–15. [DOI] [PubMed] [Google Scholar]
- Chang, CC, Campoli, M and Ferrone, S (2005). Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res 93: 189–234. [DOI] [PubMed] [Google Scholar]
- Kryczek, I, Zou, L, Rodriguez, P, Zhu, G, Wei, S, Mottram, P et al. (2006). B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med 203: 871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside, TL (2002). Tumor-induced death of immune cells: its mechanisms and consequences. Semin Cancer Biol 12: 43–50. [DOI] [PubMed] [Google Scholar]
- Munn, DH and Mellor, AL (2004). IDO and tolerance to tumors. Trends Mol Med 10: 15–18. [DOI] [PubMed] [Google Scholar]
- Cipponi, A, Wieers, G, van Baren, N and Coulie, PG (2011). Tumor-infiltrating lymphocytes: apparently good for melanoma patients. But why? Cancer Immunol Immunother 60: 1153–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, B, Tinker, AV, Lee, CH, Subramanian, S, van de Rijn, M, Turbin, D et al. (2009). Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol 22: 393–402. [DOI] [PubMed] [Google Scholar]
- Fridman, WH, Pagès, F, Sautès-Fridman, C and Galon, J (2012). The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12: 298–306. [DOI] [PubMed] [Google Scholar]
- Gajewski, TF, Louahed, J and Brichard, VG (2010). Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J 16: 399–403. [DOI] [PubMed] [Google Scholar]
- Hamid, O, Schmidt, H, Nissan, A, Ridolfi, L, Aamdal, S, Hansson, J et al. (2011). A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 9: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, RR, Chasalow, SD, Wang, L, Hamid, O, Schmidt, H, Cogswell, J et al. (2012). An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 61: 1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, HE, Chae, SW, Lee, YJ, Kim, MA, Lee, HS, Lee, BL et al. (2008). Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer 99: 1704–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurquin, C, Lethé, B, De Plaen, E, Corbière, V, Théate, I, van Baren, N et al. (2005). Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med 201: 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oble, DA, Loewe, R, Yu, P and Mihm, MC Jr (2009). Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human melanoma. Cancer Immun 9: 3. [PMC free article] [PubMed] [Google Scholar]
- Pagès, F, Berger, A, Camus, M, Sanchez-Cabo, F, Costes, A, Molidor, R et al. (2005). Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353: 2654–2666. [DOI] [PubMed] [Google Scholar]
- Ruffini, E, Asioli, S, Filosso, PL, Lyberis, P, Bruna, MC, Macrì, L et al. (2009). Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg 87: 365–71; discussion 371. [DOI] [PubMed] [Google Scholar]
- Sheu, BC, Kuo, WH, Chen, RJ, Huang, SC, Chang, KJ and Chow, SN (2008). Clinical significance of tumor-infiltrating lymphocytes in neoplastic progression and lymph node metastasis of human breast cancer. Breast 17: 604–610. [DOI] [PubMed] [Google Scholar]
- Galon, J, Costes, A, Sanchez-Cabo, F, Kirilovsky, A, Mlecnik, B, Lagorce-Pagès, C et al. (2006). Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313: 1960–1964. [DOI] [PubMed] [Google Scholar]
- Galon, J, Pagès, F, Marincola, FM, Angell, HK, Thurin, M, Lugli, A et al. (2012). Cancer classification using the Immunoscore: a worldwide task force. J Transl Med 10: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger, S, Spaapen, RM, Zha, Y, Williams, J, Meng, Y, Ha, TT et al. (2013). Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 5: 200ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, SA and Dudley, ME (2009). Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol 21: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L and Flies, DB (2013). Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13: 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach, DR, Krummel, MF and Allison, JP (1996). Enhancement of antitumor immunity by CTLA-4 blockade. Science 271: 1734–1736. [DOI] [PubMed] [Google Scholar]
- Dong, H, Strome, SE, Salomao, DR, Tamura, H, Hirano, F, Flies, DB et al. (2002). Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8: 793–800. [DOI] [PubMed] [Google Scholar]
- Iwai, Y, Ishida, M, Tanaka, Y, Okazaki, T, Honjo, T and Minato, N (2002). Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA 99: 12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer, JR, Tykodi, SS, Chow, LQ, Hwu, WJ, Topalian, SL, Hwu, P et al. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366: 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon, EB, Balmanoukian, A, Hamid, O, Hui, R, Gandhi, L, Leighl, N et al. (2013). Preliminary clinical safety and activity of MK-3475 monotherapy for the treatment of previously treated patients with non-small cell lung cancer (NSCLC). 15th World Conference on Lung Cancer. Sydney, Australia.
- Berger, R, Rotem-Yehudar, R, Slama, G, Landes, S, Kneller, A, Leiba, M et al. (2008). Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 14: 3044–3051. [DOI] [PubMed] [Google Scholar]
- Schaer, DA, Hirschhorn-Cymerman, D and Wolchok, JD (2014). Targeting tumor-necrosis factor receptor pathways for tumor immunotherapy. J Immunother Cancer 2: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuertes, MB, Kacha, AK, Kline, J, Woo, SR, Kranz, DM, Murphy, KM et al. (2011). Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med 208: 2005–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette, BC, Liang, H, Lee, Y, Chlewicki, L, Khodarev, NN, Weichselbaum, RR et al. (2011). The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res 71: 2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond, MS, Kinder, M, Matsushita, H, Mashayekhi, M, Dunn, GP, Archambault, JM et al. (2011). Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med 208: 1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi, ME (2007). DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81: 1–5. [DOI] [PubMed] [Google Scholar]
- Janeway, CA Jr and Medzhitov, R (2002). Innate immune recognition. Annu Rev Immunol 20: 197–216. [DOI] [PubMed] [Google Scholar]
- Kono, H and Rock, KL (2008). How dying cells alert the immune system to danger. Nat Rev Immunol 8: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko, DV, Agostinis, P, Krysko, O, Garg, AD, Bachert, C, Lambrecht, BN et al. (2011). Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol 32: 157–164. [DOI] [PubMed] [Google Scholar]
- Takeuchi, O and Akira, S (2010). Pattern recognition receptors and inflammation. Cell 140: 805–820. [DOI] [PubMed] [Google Scholar]
- Tesniere, A, Panaretakis, T, Kepp, O, Apetoh, L, Ghiringhelli, F, Zitvogel, L et al. (2008). Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 15: 3–12. [DOI] [PubMed] [Google Scholar]
- Devaud, C, John, LB, Westwood, JA, Darcy, PK and Kershaw, MH (2013). Immune modulation of the tumor microenvironment for enhancing cancer immunotherapy. Oncoimmunology 2: e25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou, Y, Liu, C, Lizée, G, Peng, W, Xu, C, Ye, Y et al. (2011). Antitumor activity mediated by CpG: the route of administration is critical. J Immunother 34: 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos, SM, Pegram, HJ, Westwood, JA, John, LB, Devaud, C, Clarke, CJ et al. (2011). Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol Immunother 60: 671–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangsbo, SM, Sandin, LC, Anger, K, Korman, AJ, Loskog, A and Tötterman, TH (2010). Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother 33: 225–235. [DOI] [PubMed] [Google Scholar]
- Houot, R and Levy, R (2009). T-cell modulation combined with intratumoral CpG cures lymphoma in a mouse model without the need for chemotherapy. Blood 113: 3546–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales, L, Woo, SR and Gajewski, TF (2013). Extremely potent immunotherapeutic activity of a STING agonist in the B16 melanoma model in vivo. J Immunother Cancer 1 (suppl. 1): O15. [Google Scholar]
- Dubrot, J, Palazón, A, Alfaro, C, Azpilikueta, A, Ochoa, MC, Rouzaut, A et al. (2011). Intratumoral injection of interferon-a and systemic delivery of agonist anti-CD137 monoclonal antibodies synergize for immunotherapy. Int J Cancer 128: 105–118. [DOI] [PubMed] [Google Scholar]
- Tedcastle, A, Cawood, R, Di, Y, Fisher, KD and Seymour, LW (2012). Virotherapy–cancer targeted pharmacology. Drug Discov Today 17: 215–220. [DOI] [PubMed] [Google Scholar]
- Lindenmann, J and Klein, PA (1967). Viral oncolysis: increased immunogenicity of host cell antigen associated with influenza virus. J Exp Med 126: 93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, ZS, Liu, Z and Bartlett, DL (2014). Oncolytic Immunotherapy: Dying the Right Way is a Key to Eliciting Potent Antitumor Immunity. Front Oncol 4: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin, D and Palese, P (2012). Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol 7: 347–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batliwalla, FM, Bateman, BA, Serrano, D, Murray, D, Macphail, S, Maino, VC et al. (1998). A 15-year follow-up of AJCC stage III malignant melanoma patients treated postsurgically with Newcastle disease virus (NDV) oncolysate and determination of alterations in the CD8 T cell repertoire. Mol Med 4: 783–794. [PMC free article] [PubMed] [Google Scholar]
- Cassel, WA and Murray, DR (1992). A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med Oncol Tumor Pharmacother 9: 169–171. [DOI] [PubMed] [Google Scholar]
- Cassel, WA and Murray, DR (1988). Treatment of stage II malignant melanoma patients with a Newcastle disease virus oncolysate. Nat Immun Cell Growth Regul 7: 351–352. [PubMed] [Google Scholar]
- Schirrmacher, V and Fournier, P (2009). Newcastle disease virus: a promising vector for viral therapy, immune therapy, and gene therapy of cancer. Methods Mol Biol 542: 565–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melcher, A, Parato, K, Rooney, CM and Bell, JC (2011). Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther 19: 1008–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamarin, D, Holmgaard, RB, Subudhi, SK, Park, JS, Mansour, M, Palese, P et al. (2014). Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med 6: 226ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, J, Galani, IE, Cerwenka, A, Schirrmacher, V and Fournier, P (2011). Antitumor vaccination by Newcastle Disease Virus Hemagglutinin-Neuraminidase plasmid DNA application: changes in tumor microenvironment and activation of innate anti-tumor immunity. Vaccine 29: 1185–1193. [DOI] [PubMed] [Google Scholar]
- Fournier, P, Arnold, A, Wilden, H and Schirrmacher, V (2012). Newcastle disease virus induces pro-inflammatory conditions and type I interferon for counter-acting Treg activity. Int J Oncol 40: 840–850. [DOI] [PubMed] [Google Scholar]
- Kaufman, HL, Kim, DW, DeRaffele, G, Mitcham, J, Coffin, RS and Kim-Schulze, S (2010). Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann Surg Oncol 17: 718–730. [DOI] [PubMed] [Google Scholar]
- Andarini, S, Kikuchi, T, Nukiwa, M, Pradono, P, Suzuki, T, Ohkouchi, S et al. (2004). Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res 64: 3281–3287. [DOI] [PubMed] [Google Scholar]
- Calmels, B, Paul, S, Futin, N, Ledoux, C, Stoeckel, F and Acres, B (2005). Bypassing tumor-associated immune suppression with recombinant adenovirus constructs expressing membrane bound or secreted GITR-L. Cancer Gene Ther 12: 198–205. [DOI] [PubMed] [Google Scholar]
- Kim, HS, Kim-Schulze, S, Kim, DW and Kaufman, HL (2009). Host lymphodepletion enhances the therapeutic activity of an oncolytic vaccinia virus expressing 4-1BB ligand. Cancer Res 69: 8516–8525. [DOI] [PubMed] [Google Scholar]
- Kaufman, HL, Deraffele, G, Mitcham, J, Moroziewicz, D, Cohen, SM, Hurst-Wicker, KS et al. (2005). Targeting the local tumor microenvironment with vaccinia virus expressing B7.1 for the treatment of melanoma. J Clin Invest 115: 1903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen, S, Diaconu, I, Kangasniemi, L, Ranki, T, Kanerva, A, Pesonen, SK et al. (2012). Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res 72: 1621–1631. [DOI] [PubMed] [Google Scholar]
- Parviainen, S, Ahonen, M, Diaconu, I, Hirvinen, M, Karttunen, Å, Vähä-Koskela, M et al. (2014). CD40 ligand and tdTomato-armed vaccinia virus for induction of antitumor immune response and tumor imaging. Gene Ther 21: 195–204. [DOI] [PubMed] [Google Scholar]
- Andtbacka, RHI, Curti, BD, Kaufman, H, Daniels, GA, Nemunaitis, JJ, Spitler, LE et al. (2014). CALM study: A phase II study of an intratumorally delivered oncolytic immunotherapeutic agent, coxsackievirus A21, in patients with stage IIIc and stage IV malignant melanoma. J Clin Oncol 32: 5s (suppl; abstr 3031). [Google Scholar]
- Pan, PY, Zang, Y, Weber, K, Meseck, ML and Chen, SH (2002). OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther 6: 528–536. [DOI] [PubMed] [Google Scholar]
- Gao, Y, Whitaker-Dowling, P, Griffin, JA, Barmada, MA and Bergman, I (2009). Recombinant vesicular stomatitis virus targeted to Her2/neu combined with anti-CTLA4 antibody eliminates implanted mammary tumors. Cancer Gene Ther 16: 44–52. [DOI] [PubMed] [Google Scholar]
- John, LB, Howland, LJ, Flynn, JK, West, AC, Devaud, C, Duong, CP et al. (2012). Oncolytic virus and anti-4-1BB combination therapy elicits strong antitumor immunity against established cancer. Cancer Res 72: 1651–1660. [DOI] [PubMed] [Google Scholar]
- Sorensen, MR, Holst, PJ, Steffensen, MA, Christensen, JP and Thomsen, AR (2010). Adenoviral vaccination combined with CD40 stimulation and CTLA-4 blockage can lead to complete tumor regression in a murine melanoma model. Vaccine 28: 6757–6764. [DOI] [PubMed] [Google Scholar]
- Kato, H, Sato, S, Yoneyama, M, Yamamoto, M, Uematsu, S, Matsui, K et al. (2005). Cell type-specific involvement of RIG-I in antiviral response. Immunity 23: 19–28. [DOI] [PubMed] [Google Scholar]
- Aida, K, Miyakawa, R, Suzuki, K, Narumi, K, Udagawa, T, Yamamoto, Y et al. (2014). Suppression of Tregs by anti-glucocorticoid induced TNF receptor antibody enhances the antitumor immunity of interferon-a gene therapy for pancreatic cancer. Cancer Sci 105: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzanov, I, Milhem, MM, Andtbacka, RHI, Minor, DR, Hamid, O, Li, A, Chastain, M et al. (2014). Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol 32: 5s (suppl; abstr 9029). [Google Scholar]
- Espenschied, J, Lamont, J, Longmate, J, Pendas, S, Wang, Z, Diamond, DJ et al. (2003). CTLA-4 blockade enhances the therapeutic effect of an attenuated poxvirus vaccine targeting p53 in an established murine tumor model. J Immunol 170: 3401–3407. [DOI] [PubMed] [Google Scholar]
- Munks, MW, Mourich, DV, Mittler, RS, Weinberg, AD and Hill, AB (2004). 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology 112: 559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko, HJ, Kim, YJ, Kim, YS, Chang, WS, Ko, SY, Chang, SY et al. (2007). A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res 67: 7477–7486. [DOI] [PubMed] [Google Scholar]
- Sierro, SR, Donda, A, Perret, R, Guillaume, P, Yagita, H, Levy, F et al. (2011). Combination of lentivector immunization and low-dose chemotherapy or PD-1/PD-L1 blocking primes self-reactive T cells and induces anti-tumor immunity. Eur J Immunol 41: 2217–2228. [DOI] [PubMed] [Google Scholar]
- Kudo-Saito, C, Hodge, JW, Kwak, H, Kim-Schulze, S, Schlom, J and Kaufman, HL (2006). 4-1BB ligand enhances tumor-specific immunity of poxvirus vaccines. Vaccine 24: 4975–4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty, M, Schlom, J and Hodge, JW (2007). The combined activation of positive costimulatory signals with modulation of a negative costimulatory signal for the enhancement of vaccine-mediated T-cell responses. Cancer Immunol Immunother 56: 1471–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle, A, Kohrt, H, Sagiv-Barfi, I, Ajami, B, Axtell, RC, Zhou, G et al. (2013). Depleting tumor-specific Tregs at a single site eradicates disseminated tumors. J Clin Invest 123: 2447–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, JD, Hemminki, O, Diaconu, I, Hirvinen, M, Bonetti, A, Guse, K et al. (2012). Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther 19: 988–998. [DOI] [PubMed] [Google Scholar]
- Bridle, BW, Stephenson, KB, Boudreau, JE, Koshy, S, Kazdhan, N, Pullenayegum, E et al. (2010). Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther 18: 1430–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galivo, F, Diaz, RM, Thanarajasingam, U, Jevremovic, D, Wongthida, P, Thompson, J et al. (2010). Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum Gene Ther 21: 439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveille, S, Goulet, ML, Lichty, BD and Hiscott, J (2011). Vesicular stomatitis virus oncolytic treatment interferes with tumor-associated dendritic cell functions and abrogates tumor antigen presentation. J Virol 85: 12160–12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L, Bridle, BW, Chen, L, Pol, J, Spaner, D, Boudreau, JE et al. (2013). Delivery of viral-vectored vaccines by B cells represents a novel strategy to accelerate CD8(+) T-cell recall responses. Blood 121: 2432–2439. [DOI] [PubMed] [Google Scholar]