Abstract
Patients with sarcoidosis undergo spontaneous remission or may be effectively controlled with glucocorticoids alone in many cases. Progressive and refractory pulmonary sarcoidoisis constitute more than 10% of patients seen at specialized centers. Pulmonary fibrosis and associated complications, such as infections and pulmonary hypertension are leading causes of mortality. No universal definition of refractoriness exists, we therefore propose classifying patients as having refractory disease when the following criteria are fulfilled: (1) progressive disease despite at least 10 mg of prednisolone or equivalent for at least three months and need for additional disease-modifying anti-sarcoid drugs due to lack of efficacy, drug toxicity or intolerability and (2) treatment started for significant impairment of life due to progressive pulmonary symptoms. Both criteria should be fulfilled. Treatment options in addition to or instead of glucocorticoids for these patients include second- (methotrexate, azathioprine, leflunomide) and third-line agents (infliximab, adalimumab). Other immunmodulating agents can be used, but the evidence is very limited. Newer agents with anti-fibrotic properties, such as pirfenidone or nintedanib, might hold promise also for the pulmonary fibrosis seen in sarcoidosis. Treating physicians have to actively look for potentially treatable complications, such as pulmonary hypertension, cardiac disease or infections before patients should be classified as treatment-refractory. Ultimately, lung transplantation has to be considered as treatment option for patients not responding to medical therapy. In this review, we aim to propose a new definition of refractoriness, describe the associated clinical features and suggest the therapeutic approach.
Keywords: pulmonary sarcoidosis, biological agents, corticosteroids, interstitial lung disease
Introduction
Sarcoidosis is a multisystem disease characterized by the presence of non-caseating granulomas that affects the lungs in over 90% of patients (1). The disease pathogenesis has not been completely elucidated. However, our current understanding is that it likely includes exposure to one or more, possibly airborne-transmitted antigens (bacterial, organic, anorganic), which then trigger an abnormal immune response in a genetically predisposed host (2). About two thirds of patients will undergo remission within several months to a few years, whereas about one third will require short- or long-term treatment (1). The intriguing observations of chest radiographs in sarcoidosis published by Scadding in 1961 (3) are still considered the gold standard because of the limited use of radiation and its association with resolution or progression of the disease. However, inter- observer variability and lack of association with functional clinical parameters (e. g. pulmonary function tests, six minute walk test) are limiting factors (4). Computed chest tomography (CT) has been found useful in distinguishing areas of potentially reversible lung damage from areas of presumed fibrotic lung (4). Even more importantly, chest CT with or without contrast often allows for the identification of mediastinal or hilar adenopathy, allowing for a potential diagnosis of sarcoidosis via endobronchial ultrasound (EBUS) guided transbronchial needle aspiration. It thereby prevents the need for mediastinoscopy in as many as 87% of patients (5). Especially in patients with parenchymal disease and higher risk for complications, such as pneumothorax, EBUS alone may yield a diagnosis and obviate the need for transbronchial biopsies (6).
Treatment recommendations do exist for pulmonary and extra-pulmonary manifestations of sarcoidosis (7–10), however, the available evidence to guide treatment is limited. Therapeutic options include first-line (glucocorticoids), second-line (disease-modifying anti-sarcoid drugs, DMASD) and third-line (biological agents) therapies. Treatment should always be tailored to the individual patient and adapted to the presence or absence of clinical symptoms or worsening of objective functional parameters, such as pulmonary function tests.
Refractory disease has not been universally defined and treatment recommendations are not existent for this, albeit small, group of patients. In addition, it is challenging to define refractory disease with no approved therapies other than glucocorticoids (and Acthar gel, which has been approved based on very limited evidence) by the U. S. Food and Drug Administration and because of the systemic nature of the disease. In specialized centers, refractory or progressive patients can nevertheless constitute a significant proportion of all patients. In a survey from a World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) Task Force, more than 10% of patients required and increase in systemic medication 5 years after the initial diagnosis (11). Data from the ACCESS studies revealed that among all characteristics of the included patients, dyspnea and need for initial systemic therapy were associated with systemic therapy at two years of follow-up (12). In addition, organ involvement is different according to race, sex and age. African Americans, for example, tend to have more skin involvement (other than erythema nodosum), hematological abnormalities as well as lymph node, liver and bone marrow affection (13). However, it is important to note that organ involvement per se does not necessarily translate into chronicity or refractoriness.
Also, it is clear that refractory sarcoidosis often includes significant fibrosis. Fibrotic sarcoidosis is associated with increased mortality (14,15). Patients in this group die not only from progression of their inflammatory disease, but also from complications of the fibrosis, including pulmonary hypertension (PH) and infections (15).
The overlapping, but nevertheless different concept of “advanced pulmonary sarcoidosis” has evolved over the last couple of years (16). Advanced pulmonary sarcoidosis includes fibrocystic changes with either airflow limitation or severe restrictive impairment and can harbour active or inactive lesions (as evidenced by 18F-positrone emission-computed tomography (PET-CT)). The associated complications include chronic respiratory insufficiency, pulmonary hypertension, pulmonary aspergillosis or other infections (16). Acute worsening events are frequent with a median of three episodes per year (17). Advanced pulmonary sarcoidosis can be seen as potential cause of treatment-refractory disease and the aforementioned complications have to be actively looked for.
In this review, we aim to define treatment-refractory disease, propose clinical and functional parameters associated with refractoriness and, ultimately, suggest a step-wise approach for the management of refractory patients.
Methods and search strategy
Pubmed and Google scholar were searched using the terms “refractory sarcoidosis”, “progressive sarcoidosis”, and combinations of “sarcoidosis” and “methotrexate, azathioprine, leflunomide, rituximab, infliximab and adalimumab”. Articles were reviewed by abstract and included when deemed appropriate by at least two authors. Additional references were identified from included articles and personal archives of the authors. Reviews, case series and clinical studies published in peer-reviewed journals were included. Articles published in English, Spanish and German were included for analysis. Non-english language articles were reviewed and translated by one author (PK) and their content discussed with at least one other author (KS, RPB or NJS). These articles were also included when at least two authors agreed upon their appropriateness.
Definition of refractory pulmonary sarcoidosis
In our article we strive to characterize the term refractory (that is: treatment-refractory) pulmonary sarcoidosis. As of yet no universally accepted definition of refractory pulmonary sarcoidosis exists. However, patients with progressive disease despite adequate therapy consisting of corticosteroids are commonly referred to as having refractory disease according to the literature (18). While corticosteroids remain the first line of treatment and are being used as such, no validated protocol exists with regard to dosage or treatment duration, thereby complicating what is to be considered as treatment refractory (1,19,20). A systematic review from 2005 found that dosages vary widely and that no clear evidence of benefit exists beyond two years of treatment (21). Indications for therapy, response and thereby optimal treatment duration are especially difficult to measure when looking at quality of life rather than objective evidence of organ dysfunction. However, validated questionnaires exist (e. g. Sarcoidosis Health Questionnaire and Fatigue Assessment Scale) and guidelines on their use have been published (22). Factors confounding assessment of response to therapy are coexistence of other diseases or complications due to immunosuppressive therapy. It becomes clear that along with the question on how to define refractory sarcoidosis, one must aim to answer questions about what the optimal dose and duration of a treatment ought to be and how to assess its response.
In light of this complex background we propose the following definition of refractory pulmonary sarcoidosis:
Progressive pulmonary disease despite glucocorticosteroid therapy at adequate dosage defined as at least 10 mg of prednisone (23) once a day and duration of therapy of at least 3 months after initial dosages of 20–40 mg per day for 1–3 months and need for additional therapy due to lack of efficacy, glucocorticoid toxicity or severe side effects
Treatment started for impaired quality of life due to progressive pulmonary symptoms with or without additional disease manifestations (e.g. disfiguring disease, neurosarcoidosis, etc.)
To be considered as treatment-refractory, pulmonary disease patients should fulfill both criteria and other causes such as pulmonary arterial hypertension, infection or cardiac sarcoidosis have to be excluded.
This definition is based on similar descriptions of refractory pulmonary sarcoidosis in the literature and aims to simplify the approach to any given patient. In addition, it is the authors’ personal experience and by no means a recommendation which is based on solid evidence, that patients fulfilling these criteria are those that are usually more difficult to treat in clinical practice. While there are no standardized end-points to define refractoriness, it is the authors’ intention to propose a definition which serves clinicians as a guideline on when to intensify treatment. Clearly, this definition has to be validated in well-designed clinical trials.
Features associated with refractory disease
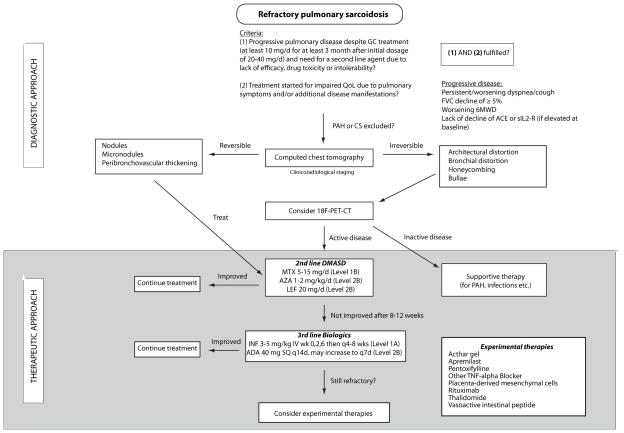
As many as 10% of patients undergoing treatment for sarcoidosis will continue to have active disease (18). Most deaths from sarcoidosis in the western hemisphere occur because of advanced pulmonary fibrosis with pulmonary arterial hypertension (PAH) and less commonly cardiac, central nervous system (CNS) and hepatic involvement (24,25). This contrasts with reports from Japan citing cardiac complications as cause of death in 77% to 85% of sarcoidosis-related deaths as recently reviewed by Lynch et al. (26). Given the high rate of pulmonary involvement and potential of progression to end-stage fibrosis we will focus our review on the diagnosis of pulmonary refractory sarcoidosis and describe clinical findings that could constitute refractoriness (Table 1). Finally, we will propose a diagnostic and therapeutic algorithm for refractory pulmonary sarcoidosis patients (Figure 1).
Table 1.
Clinical, functional, laboratory and imaging features associated with refractory pulmonary sarcoidosis
| Test | Finding | Comments |
|---|---|---|
| Clinical findings | Persisting/worsening dyspnea or cough despite treatment Rarely chest pain, wheezing, hemoptysis |
Exclude fatigue, infection and pulmonary hypertension as causes of worsening symptoms |
| Non-invasive tests | Decline of FVC of ≥ 5% Lack of improvement of 6MWD |
Limited data in sarcoidosis Influenced by multiple factors |
| Biomarkers | Lack of decline of ACE and/or sIL2-R | Only useful when elevated at baseline Serial measurements necessary |
| Computed tomography | Lack of improvement / progression from reversible to irreversible findings Potentially reversible findings: Micronodules, nodules, peribronchovascular thickening Irreversible findings: Architectural distortion, bronchial distortion, honeycombing, bullae |
Trial of intensified treatment warranted If exclusively fibrosis, assess for complications and treat |
| 18F-PET-CT | Search for sites of active inflammation | Consider more intensified immunosuppressive therapy |
Abbreviations used: 18F-PET-CT, 18F-positrone emission computed tomography; 6MWD, 6-minute walking distance; ACE, angiotensin-converting enzyme; FVC, forced vital capacity; sIL2-R, soluble interleukin 2-receptor
Figure 1. Proposed diagnostic and therapeutic approach to refractory pulmonary sarcoidosis.
Abbreviations used: 18F-PET-CT, 18F-positrone emission computed chest tomography; 6MW; 6-minute walking test; ADA, adalimumab; AZA, azathioprine; CS, cardiac sarcoidosis; DMASD, disease-modifying anti-sarcoid drugs; INF, infliximab; IV, intravenously; LEF, leflunomide; MTX, methotrexate; PAH, pulmonary arterial hypertension; QoL, quality of life; SQ, subcutaneously.
Level of evidence: A: at least one double-blind placebo-controlled randomized trial with positive results with one or more case series supporting the results; level B: majority of the case series showing positive results; level C: case series with mixed reports of effectiveness or only a small number of case reports. Recommendations are further differentiated into strong (1A, 1B, 1C) or weak (2A, 2B, 2C)
Clinical symptoms
Symptoms of mediastinal-pulmonary sarcoidosis can encompass a large range of clinical findings. The most common symptom is a persistent cough, followed by dyspnea (which is rare early in the disease course) and rarely chest pain, wheezing or hemoptysis. Crackles on exam are rare and correlate with changes of fibrosis. The variety in clinical symptoms is mirrored in the wide range of histopathological and radiographic presentations. However, these objective and subjective data do not always correlate.
Patients considered to have refractory disease will typically have persistent symptomatology (dyspnea, cough etc.) despite an adequate therapeutic trial with glucocorticoids (see definition above). Sarcoidosis patients who respond well to higher (20–40 mg per day) glucocorticoid doses at first but have recurrent symptoms upon reduction to targeted doses of less than 10 mg per day after three months will also be candidates for the introduction of a second-line agent and thus meet the definition of “treatment-refractory”. The presence of additional progressive extra-pulmonary symptoms requiring immunosuppressive treatment in a situation of stable pulmonary disease would also constitute systemic refractory sarcoidoisis stratified by the index organ involved.
Non-invasive testing (Pulmonary function testing and 6-minute walk distance)
There is no diagnostic pattern in PFTs and the volume of forced vital capacity (FVC) does not correlate clinically with symptoms of dyspnea (27). The most prevalent pattern of pulmonary function tests (PFTs) in patients with sarcoidosis is restrictive (36, 37). Obstruction can nevertheless also be present, either in isolation or concomitantly (29–31). This correlates with the histopathological pattern of sarcoidosis either involving the lung parenchyma, the airways or both. Obstruction in sarcoidosis may be less responsive to immunosuppressive therapy (32) and can be a significant source of refractory disease. In sarcoidosis, there is no definite consensus on how to follow changes in FVC in response to treatment (28). In idiopathic pulmonary fibrosis (IPF), it was shown that a decline of 5% or more was associated with an increase in mortality (33) and a decline of 2–6% has been found to represent the minimal clinically important difference (34). While these thresholds may not necessarily hold true for sarcoidosis, we suggest that, in the absence of better data, a decline of ≥5% (absolute change of predicted) FVC is consistent with refractoriness in sarcoidosis patients. The caveat to this approach is the test variability and the impact of obesity and long-term use of glucocorticosteroids as well as the presence or absence of sleep apnea on the testing. Our unpublished data suggest that a significant reduction in DLCO may indicate refractory pulmonary disease in the absence of PAH as a cause of drop in DLCO.
The 6-minute walk distance (6MWD) is an established measurement in many pulmonary conditions. Although sarcoidosis is not listed as an indication for testing by the American Thorcic Society (35), it has nevertheless been used in this population (36). While results (improvement or lack of improvement) have been mixed in clinical trials which incorporated it as outcome measure, refractory patients should either have declining values as compared to baseline or increase only with supplemental oxygen. One has to keep in mind, however, that the 6MWD is influenced by multiple factors, making it difficult to discriminate the cause of declining values in some cases (36).
Role of imaging in refractory pulmonary Sarcoidosis
Many patients who respond readily to glucocorticoid treatment alone may never need advanced imaging in addition to conventional chest radiographs. Those patients who have persisting or worsening clinical symptoms and functional findings despite adequate treatment will require computed chest tomography or other imaging modalities.
Studies that evaluated the CT features that are associated with sarcoidosis aimed to differentiate reversible features such as nodules or peribronchovascular thickening from findings of potentially irreversible damage such as honeycombing, bronchial distortion or bullae (28, 29). Prior to intensifying therapy in a patient nonresponsive to corticosteroids we recommend evaluating for CT scan findings suggestive of end stage fibrosis, possibly followed by a PET scan to screen for sites of active inflammation. The suspicion of fibrotic changes would be supported by stable PFTs over several years (38). CT is also the most important imaging modality to assess for complications of advanced pulmonary sarcoidosis, such as aspergilloma, pulmonary hypertension or obstruction (4). In a recent study, Walsh and colleagues applied a staging system combining fibrosis grading in high-resolution computed tomography (HRCT) and the composite physiogical index (CPI) to a sarcoidosis population (14). This staging system was found to be predictive of mortality and could easily be used by radiologists and clinicians likewise.
A newer imaging modality correlating with inflammatory activity is 18F-FDG-PET. It may especially predict worsening of pulmonary involvement as well as show areas of active disease involvement, expected to improve with treatment. Not all insurances cover 18F-FDG-PET in sarcoidosis (39–43). 18F-FDG PET has been shown to demonstrate active inflammantion even in stage IV CXR, thereby being a useful marker of active disease and potential response to therapy (42).
All patients suspected of having refractory pulmonary sarcoidosis should receive imaging by CT assessing for reversible/irreversible including clinicoradiological staging as proposed by Walsh et al. If irreversible features are identified, we suggest the consideration of 18F-PET-CT to look for active lesions which could be amenable to treatment. If no active lesions are identified, complications need to be addressed and treated. Nevertheless, even in inactive sarcoidosis by imaging, a therapeutic trial with second-line agents might be justified for individual patients. Prospective validation studies are required to evaluate the role of CT and PET in predicting the need for aggressive therapy in refractory disease.
Biomarkers
Biomarkers as a measurement of disease activity have not yet been incorporated in clinical practice, but are being investigated. Examples include soluble interleukin-2 receptor (sIL2-R), chitotriosidase and exhaled neopterin (44). Angiotensin-converting enzyme (ACE) has not been found to reliably correlate with disease activity (45), which might be due to different genotypes influencing ACE levels (46). Furthermore, treatment with corticosteroids influences ACE levels. Nevertheless, serial measurement of ACE and sIL2-R levels were found to be useful in individual patients to monitor treatment effects (47). Interestingly, measurement of serum amyloid has been found to correlate with disease activity in pulmonary sarcoidosis (48). However, its current use in clinical practice can not yet be recommended. In an interesting study with small sample size, an unbiased 20-gene signature was found to be superior to a more restricitive 31-gene T-cell receptor signaling profile in peripheral blood to distinguish complicated from uncomplicated sarcoidosis (49). Complicated sarcoidosis in this study was defined as cardiac, neurologic or severe pulmonary sarcoidosis. The fact that the T-cell receptor signaling centered gene expression profile was inferior compared with the gene signature derived from genome-wide peripheral blood gene expression analysis underlines the fact that the pathogenesis of sarcoidosis might be T-cell driven, but refractoriness might involve more complex mechanisms (49). In summary, we recommend serial measurements of ACE and/or sIL2-R at baseline and to monitor treatment response when baseline levels were elevated.
Additional biomarkers are under investigation. Peripheral blood lymphocytopenia has been found to correlate with severity of disease manifestations, including pulmonary sarcoidosis (50). In addition, C-reactive protein levels have been found to indicate more a more severely affected subgroup of patients. Interestingly, these patients may have better responses to Infliximab when this treatment in indicated (51). Experimental studies identified additional cytokines that might be important in the disease pathogenesis and different phenotypes. Interleukin(IL)-7 (increased) and IL-5 (decreased) have been found to be altered compared to healthy controls. IL-5 was significantly more decreased in non-fibrotic pulmonary sarcoidosis patients, IL-7 was increased in the fibrotic phenotype, but not statistically significant (52). While still experimental, these results might identify different phenotypic subsets of sarcoidosis patients. All these biomarkers, however, still have to be validated in translational clinical studies and have not been established in clinical practice as of yet. To conlude, patients with refractory disease are characterized by persisting or worsening clinical symptoms, decline of PFT despite treatment, and/or worsening of findings on imaging or changes from potentially reversible to irreversible features despite adequate doses of glucocorticoid treatment, keeping in mind that the adequate dose has been defined based on expert opinion and consensus rather than convincing evidence from clinical studies. No universal biomarkers which are useful in terms of diagnosis or response to treatment have been defined, ACE and sIL2-R are the most frequently used, given that levels are increased at baseline.
Our proposed diagnostic approach is depicted in the upper part of figure 1 and the clinical/investigational findings associated with refractoriness are presented in table 1. In the next section, we will describe our therapeutic approach for patients with refractory disease.
Therapeutic options in refractory sarcoidosis
Glucocorticosteroids in doses ranging from 20 – 40 mg/day are the mainstay of treatment and effective in a majority of sarcoidosis patients with possible tapering to a maintencance dose of < 10 mg/d within a few months. Acthar gel, an ACTH-analogue and FDA-approved medication, is not routinely used by sarcoidologists, since the evidence base is weak, but studies are ongoing to investigate its role in chronic pulmonary sarcoidosis (NCT 02188017). The clinician, often a pulmonologist or rheumatologist, will nevertheless encounter patients who do not readily respond to GC treatment and are therefore considered treatment-refractory.
We will focus the following section on additional, second- and third-line therapeutic agents to be considered in patients with refractory pulmonary sarcoidosis. The therapeutic approach to refractory sarcoidosis is summarized in the lower part of figure 1.
Second line disease modifying anti-sarcoid drugs
The evidence for using these agents including methotrexate (MTX), azathioprine (AZA), leflunomide (LEF), cyclosporine (CYA), antimalarials (hydroxychloroquine, HCQ, and chloroquine, CHQ) and mycophenolate mofetil (MMF) is astonishingly low-grade. Nevertheless, many physicians treating sarcoidosis patients have gained experience using these agents in sarcoidosis patients. The choice of which one to use depends on physician preference and the predominant organ ivolvement rather than objective evidence. We will summarize the existing evidence and our personal experience with the use of non-steroidal agents. Table 2 gives an overview of specific dose and monitoring recommendations.
Table 2.
Treatment recommendations for non-steroidal and biological agents
| Agent | Starting dose (maximum dose) | Comments | Level of evidence |
|---|---|---|---|
| Disease-modifying anti-sarcoid drugs | |||
| Methotrexate | 5–15 mg/week SC/PO (25 mg/week) | Liver and renal insufficieny Use of folic acid (at least 5 mg/week PO) recommended CBC, LFT and Creatinine Risk of hypersensitivity pneumonitis probably <5% |
1B |
| Azathioprine | 1 mg/kg/d (two divided doses) PO 2 mg/kg/d | Mutations of TPMT CBC, LFT Co-medication with allopurinol contraindicated |
2B |
| Leflunomide | 10–20 mg/d PO | CBC, LFT Lung toxicity reported within first 3 months Silent liver fibrosis |
2B |
| Hydroxychloroquine / Chloroquine | 5–7 mg/kg/d PO | Retinopathy (ophthalmologic examination) Cardiomyopathy |
2B |
| Mycophenolate mofetil | 500 mg/d PO (2–3 g/d) | CBC, LFT Diarrhea most common adverse effect |
2C |
| Biological agents | |||
| Infliximab | 3–5 mg/kg IV week 0,2,6 and every 4–8 weeks thereafter | Tuberculosis screening Infections Autoimmunity Hepatitis reactivation CBC, LFT |
1A |
| Adalimumab | 40 mg SC every other week (40 mg SC every week) | 2B | |
| Rituximab | 1 g IV d 0 and 14 | Infections Hepatitis reactivation Test serum immunoglobulins every 3 months Progressive multifocal leukencephalopathy reported |
2B |
Abbreviations used: CBC, complete blood count; IV, intravenously; LFT, liver function test; PO, per os; SC, subcutaneously; TPMT, thiopurine methyltransferase
Level of evidence: A: at least one double-blind placebo-controlled randomized trial with positive results with one or more case series supporting the results; level B: majority of the case series showing positive results; level C: case series with mixed reports of effectiveness or only a small number of case reports. Recommendations are further differentiated into strong (1A, 1B, 1C) or weak (2A, 2B, 2C).
Methotrexate is typically the first steroid-sparing agent used in sarcoidosis and its first reported use in a refractory sarcoidosis patient can be found in the literature as early as 1968 (53). Evidence for MTX use derives from one randomized double-blind placebo-controlled trial (54) and few open-label clinical trials (55–58), additional case series or singular case reports can be found numerously in the literature. In most studies, its use was associated with a possible dose-reduction of glucocorticoids and/or improvement in pulmonary function tests. In 2013, the World Association of Sarcoidosis and Other Granulomatous Diseases (WASOG) has published recommendations for the use of MTX in sarcoidosis (59). The risk of hypersensitivity pneumonitis in sarcoidosis seems to be lower than in patients with rheumatoid arthritis, in which this side effect is estimated to occur in up to 5% of patients (7). In summary, the usual starting dose ranges from 5 – 15 mg / week with supplementation of folic acid of at least 5 mg / week.
Azathioprine has been studied only in open-label clinical trials (57,60,61) and one retrosepective analysis (62) with mixed results. The open-label trials showed small improvements in forced vital capacitiy in a limited number of patients and steroid-sparing capacities and/or disease regression in 54% in one study (57). The retrospective analysis compared MTX versus AZA and found similar clinical benefits (prednisone dose reduction, improvement of FEV1 and VC) but more infectious complications with AZA (62). This important study provides evidence for the efficacy of both MTX and AZA in a larger number of patients in terms of steroid-sparing capacity and clinical improvement.
Leflunomide has been studied in pulmonary (and extra-pulmonary) sarcoidosis in two open-label trials (63,64) and has shown steroid-sapring properties in a standard dose of 20 mg/d. In one study, it improved forced vital capacity. Gastrointestinal side effects (nausea and diarrhea) and elevation of hepatic enzymes were the most common side-effects. In our experience, loading with LEF (100 mg on three consecutive days) is usually not necessary. LEF can, however, cause interstitial lung disease (ILD). In a systematic review by Raj and Nugent (65), ILD caused by LEF was found most often within the first 3 months after initiation, the most common finding were bilateral ground glass opacities onn chest CT and diffuse alveolar damage on histopathologic examination.
Other second-line agents are less commonly used according to the literature. Mycophenolate mofetil (MMF) has not been studied in detail in sarcoidosis. The first use in a sarcoidosis patient with refractory uveitis appeared in a paper published in the Lancet in 1998 (66). MMF has shown efficacy in severe cutaneous sarcoidosis in a case series reported by Kouba and colleagues in 2003 (67). In addition, there are few reports on its use for renal sarcoidosis (68). In a retrospective study of 37 patients with pulmonary sarcoidosis, MMF did not improve pulmonary function tests but was steroid-sparing in some patients (69). Its role for pulmonary sarcoidosis is therefore not clear as of yet.
The antimalarials hydroxchloroquine (HCQ) and chloroquine (CHQ) are thought to modulate immune response through inhibition of toll-like receptors-7 and -9 (70). There are limited data for their use in pulmonary sarcoidosis. In one randomized trial, maintenance therapy with CHQ was associated with slower deterioration of PFTs and fewer relapses (71). In a recent analysis, it was confirmed that the risk of retinopathy with prolonged use is higher depending on the cumulative dose (72). A doasage of HCQ of < 5 mg/kg actual body weight should be used and ophthalmologic evaluation at baseline and annually after five years is considered mandatory with their use (73).
Third-line biological agents
Experimental evidence and limited clinical studies suggest that influencing the abnormal immune response in sarcoidosis by biological agents might be useful in certain patients. However, one has to note that the results from clinical trials with different tumor necrosis factor-α blockers are conflicting despite a clear rationale. Data are available for the Tumor necrosis factor alpha blockers infliximab, adalimumab, etanercept and golimumab, the B-cell-depleting agent rituximab and the interleukin 12/23-antagonist ustekinumab. Additional immunmodulatory drugs, such as thalidomide, pentoxifylline and apremilast, are considered experimental and their role has yet to be defined. Data on biological and experimental agents were recently reviewed (7, 9), we will therefore focus this section on agents investigated in refractory pulmonary sarcoidosis.
Infliximab is the best studied biological agent in sarcoidosis. Over the last ten years, its efficacy has been reported in numerous case reports and retrospective studies of patients with severe pulmonary and extra-pulmonary disease. However, there are only two prospective, double-blind placebo-controlled randomized clinical trials for pulmonary sarcoidosis (74,75). These trials involved 138 and 19 patients, respectively, with chronic pulmonary disease despite glucocorticoid therapy. Infliximab led to improvement in forced vital capacity (FVC) and radiographical progression (74) or FVC alone (75). In addition, we found that patients with elevated C-reactive protein levels seemed to benefit more from the intervention (51). Of note, the beneficial effects of Infliximab for extra-pulmonary sarcoidosis, including neurosarcoidosis, have been described in the literature despite the absence of higher quality clinical trials which are difficult to perform due to the very varied clinical presentations. In patients requiring prednisone doses higher than 15–20 mg, Infliximab has not been shown to improve the difference in FVC after two years of treatment (76).
Adalimumab is an alternative option and experiences derived from larger case series as well as results from open label clinical trials are encouraging. We recently published an open-label clinical trial (77) which involved 11 patients with refractory sarcoidosis. Treatment success was defined as improvements in FVC, 6MWD, Borg dyspnea score or possible reduction of concomitant medication. After 24 and 52 weeks, 9/11 (82%) and 8/11 (80%) had improved in at least one of the variables tested. Of practical importance, the dose use in this trial was 40 mg weekly, which is higher than the standard dose of 40 mg every other week approved by the FDA for rheumatoid arthritis.
The WASOG provides recommendations for the use of the TNF-blockers Infliximab and Adalimumab in sarcoidosis, these are, however, based on the limited data available and expert opinion of leading sarcoidologists (78). Recommendations regarding dosage (see also Table 2), discontinuation as well as general recommendations for the use of anti-TNF alpha blockers were formulated.
The trial for progressive pulmonary sarcooidosis including Etanercept, a soluble TNF receptor, was terminated prematurely due to ineffectiveness (79). However, occasional case reports have also noted efficacy for diverse disease manifestations (80).
A recent trial involving Ustekinumab, a combined anti interleukin (IL) 12/23 antibody, and Golimumab, a TNF alpha blocker, was designed to assess the clinical benefits in pulmonary and cutaneous sarcoidosis. The primary end-point, improvement of change in predicted percentage of FVC was not reached. There was a tendency of Golimumab to improve some of the cutaneous manifestations of sarcoidosis, but this did not reach statistical significance either.
Ultimately, Rituximab, a monoclonal antibody directed against CD20-positive B-cells, which is used in malignancies and many autoimmune diseases (including rheumatoid arthritis, vasculitis, refractory systemic lupus erythematosus, primary Sjögren’s syndrome among many others) was reported as salvage treatment in cases of refractory disease. It was demonstrated that, despite being a predominantly T-cell mediated disease, B-cell depletion might still be useful in sarcoidosis patients. In a recent study including 42 patients with sarcoidosis, almost 30% (n=12) of patients showed positive serum titers of antinuclear antibodies when compared with healthy vokunteers (81). We recently reported a phase I/II clinical trial (82) of 10 patients with refractory pulmonary sarcoidosis showed improvements in FVC of >5% in one patient and >10% in 4 patientsafter 24 weeks. 6MWD improved in 5 of 10 patients after 24 weeks. Thus, there were some patients with improvements while others did not benefit. No clear recommendation can therefore be given for the optimal subgroup of patients most likely to benefit from Rituximab treatment.
Importantly, treating physicians not familiar with the use of anti-TNF therapies have to keep in mind that autoimmune reactions to these agents can, albeit rarely, occur. Induction of antinuclear antibodies is common, but clinically apparent systemic lupus erythematosus, demyelinating disorders and also sarcoidosis have been reported (83). Development of anti-drug antibodies can occur which might be prevented by using a combination therapy with low-dose methotrexate or other DMASDs.
Other treatments
Optimal treatment of refractory sarcoidosis not only consists of medical treatment of the disease with second- and third-line agents (see above), but also needs to address complications of fibrotic lung disease, such as infections (e. g. aspergilloma), bronchiectasis and pulmonary hypertension. Physical therapies including pulmonary rehabilitation and physical training programs might improve quality of life and maintain functional parameters despite disease progression (84,85).
Ultimately, lung transplantation has to be considered as an option for the most severely affected patients and early referral to the transplant team should be considered in severely affected patients. As reviewed by Shlobin and Nathan (86), lung transplantation in sarcoidosis patients might be underutilized. Importantly, despite reported recurrences of sarcoidosis post-transplant, mortality is comparable to other conditions (86).
Newer treatment options, namely the anti-fibrotic agent pirfenidone and nintedanib, a multikinase inhibitor, yielded promising results in idiopathic pulmonary fibrosis (IPF) (reviewed in (87)), but have not been investigated in advanced sarcoidosis.
Summary
Sarcoidosis is a heterogenous disease with variable clinical picture and disease course. Clinical, laboratory and functional evaluation has to be comprehensive in patients not responding to standard therapy and other causes (cardiac dyspnea, pulmonary hypertension, infections) have to be excluded first before patients should be labeled as “treatment-refractory”.
We propose a new definition of refractory disease based on (1) responsiveness to/tolerance of glucocorticoids and (2) progressive pulmonary symptoms despite established therapeutic options.
In refractory cases, disease-modifying anti-sarcoid drugs and biologicals can be used. Methotrexate is preferred by most sarcoidologists, azathioprine and leflunomide are viable alternatives. The different advantages and disadvantages of each agent have to be discussed individually with the patient since none of these medications has been approved for use by the U.S. Food and Drug administration (FDA) or the European Medicines Agency (EMA).
Third-line options include anti-TNF alpha blockers. First choice is Infliximab because of two available double-blind placebo controlled clinical trials, Adalimumab has also been used successfully in many patients with refractory disease. The reason why there are some agents of the same class (Etanercept and Golimumab) which do not seem to be equally effective, is not convincingly explained until now.
In the future, patients with fibrotic sarcoid lung disease might benefit from newer drugs used in idiopathic pulmonary fibrosis, such as pirfenadine and nintedanib. These agents have not been studied in fibrotic sarcoidosis as of yet.
Lung transplantation is a viable option for the most severely affected patients and should be considered relatively early in the disease course of refractory patients. A multidsciplinary approach is required to identify optimal candidates for transplantation and ultimately approve outcomes.
Equally important in the management of patients with refractory sarcoidosis is the recognition and appropriate treatment of complications, such as infections (aspergilloma, bacterial infections), bronchiectasis, bronchial stenosis and pulmonary hypertension.
Footnotes
Conflicts of interests:
Peter Korsten, Katharina Strohmayer and Nadera J Sweiss: None declared
Robert P Baughman: Dr Baughman has research grants for sarcoidosis from Celgene, Pfizer, Forest, Gilead, Bayer, Mallinckrodt and Novartis. He has been a consultant for Celgene, Novartis and Mallinckrodt.
All authors state that they have not received any funding for this work.
References
- 1.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 1999 Sep;16(2):149–73. [PubMed] [Google Scholar]
- 2.Iannuzzi MC, Fontana JR. Sarcoidosis: clinical presentation, immunopathogenesis, and therapeutics. JAMA. 2011 Jan 26;305(4):391–9. doi: 10.1001/jama.2011.10. [DOI] [PubMed] [Google Scholar]
- 3.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years’ observation. Br Med J. 1961 Nov 4;2(5261):1165–72. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nunes H, Uzunhan Y, Gille T, Lamberto C, Valeyre D, Brillet P-Y. Imaging of sarcoidosis of the airways and lung parenchyma and correlation with lung function. Eur Respir J. 2012 Sep;40(3):750–65. doi: 10.1183/09031936.00025212. [DOI] [PubMed] [Google Scholar]
- 5.Navani N, Lawrence DR, Kolvekar S, Hayward M, McAsey D, Kocjan G, et al. Endobronchial ultrasound-guided transbronchial needle aspiration prevents mediastinoscopies in the diagnosis of isolated mediastinal lymphadenopathy: a prospective trial. Am J Respir Crit Care Med. 2012 Aug 1;186(3):255–60. doi: 10.1164/rccm.201203-0393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014 Sep;146(3):547–56. doi: 10.1378/chest.13-2339. [DOI] [PubMed] [Google Scholar]
- 7.Baughman RP, Nunes H, Sweiss NJ, Lower EE. Established and experimental medical therapy of pulmonary sarcoidosis. Eur Respir J. 2013 Jun;41(6):1424–38. doi: 10.1183/09031936.00060612. [DOI] [PubMed] [Google Scholar]
- 8.Korsten P, Mirsaeidi M, Sweiss NJ. Nonsteroidal therapy of sarcoidosis. Curr Opin Pulm Med. 2013 Sep;19(5):516–23. doi: 10.1097/MCP.0b013e3283642ad0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korsten P, Tampe B, Konig MF, Nino J, Sawaqed S, Sawaqed R, et al. To treat or not to treat, this is the question: an evidence-based approach to pulmonary sarcoidosis. Minerva Pneumol. 2015 [Google Scholar]
- 10.Sweiss NJ, Patterson K, Sawaqed R, Jabbar U, Korsten P, Hogarth K, et al. Rheumatologic manifestations of sarcoidosis. Semin Respir Crit Care Med. 2010 Aug;31(4):463–73. doi: 10.1055/s-0030-1262214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baughman RP, Nagai S, Balter M, Costabel U, Drent M, du Bois R, et al. Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2011 Jul;28(1):56–64. [PubMed] [Google Scholar]
- 12.Baughman RP, Judson MA, Teirstein A, Yeager H, Rossman M, Knatterud GL, et al. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM Mon J Assoc Physicians. 2006 May;99(5):307–15. doi: 10.1093/qjmed/hcl038. [DOI] [PubMed] [Google Scholar]
- 13.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Bresnitz EA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001 Nov 15;164(10 Pt 1):1885–9. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 14.Walsh SL, Wells AU, Sverzellati N, Keir GJ, Calandriello L, Antoniou KM, et al. An integrated clinicoradiological staging system for pulmonary sarcoidosis: a case-cohort study. Lancet Respir Med. 2014 Feb;2(2):123–30. doi: 10.1016/S2213-2600(13)70276-5. [DOI] [PubMed] [Google Scholar]
- 15.Nardi A, Brillet P-Y, Letoumelin P, Girard F, Brauner M, Uzunhan Y, et al. Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 2011 Dec;38(6):1368–73. doi: 10.1183/09031936.00187410. [DOI] [PubMed] [Google Scholar]
- 16.Valeyre D, Nunes H, Bernaudin J-F. Advanced pulmonary sarcoidosis. Curr Opin Pulm Med. 2014 Sep;20(5):488–95. doi: 10.1097/MCP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 17.Baughman RP, Lower EE. Frequency of acute worsening events in fibrotic pulmonary sarcoidosis patients. Respir Med. 2013 Dec;107(12):2009–13. doi: 10.1016/j.rmed.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011 Mar 1;183(5):573–81. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Judson MA. An approach to the treatment of pulmonary sarcoidosis with corticosteroids: the six phases of treatment. Chest. 1999 Apr;115(4):1158–65. doi: 10.1378/chest.115.4.1158. [DOI] [PubMed] [Google Scholar]
- 20.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008 Sep;63(Suppl 5):v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 21.Paramothayan NS, Lasserson TJ, Jones PW. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst Rev. 2005;(2):CD001114. doi: 10.1002/14651858.CD001114.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baughman RP, Drent M, Culver DA, Grutters JC, Handa T, Humbert M, et al. Endpoints for clinical trials of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2012 Oct;29(2):90–8. [PubMed] [Google Scholar]
- 23.Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med. 2010 May;104(5):717–23. doi: 10.1016/j.rmed.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Gideon NM, Mannino DM. Sarcoidosis mortality in the United States 1979–1991: an analysis of multiple-cause mortality data. Am J Med. 1996 Apr;100(4):423–7. doi: 10.1016/S0002-9343(97)89518-6. [DOI] [PubMed] [Google Scholar]
- 25.Mirsaeidi M, Machado RF, Schraufnagel D, Sweiss NJ, Baughman RP. Racial difference in sarcoidosis mortality in the United States. Chest. 2015 Feb 1;147(2):438–49. doi: 10.1378/chest.14-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lynch JP, Hwang J, Bradfield J, Fishbein M, Shivkumar K, Tung R. Cardiac involvement in sarcoidosis: evolving concepts in diagnosis and treatment. Semin Respir Crit Care Med. 2014 Jun;35(3):372–90. doi: 10.1055/s-0034-1376889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeager H, Rossman MD, Baughman RP, Teirstein AS, Judson MA, Rabin DL, et al. Pulmonary and psychosocial findings at enrollment in the ACCESS study. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2005 Jun;22(2):147–53. [PubMed] [Google Scholar]
- 28.Keir G, Wells AU. Assessing pulmonary disease and response to therapy: which test? Semin Respir Crit Care Med. 2010 Aug;31(4):409–18. doi: 10.1055/s-0030-1262209. [DOI] [PubMed] [Google Scholar]
- 29.Hansell DM, Milne DG, Wilsher ML, Wells AU. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology. 1998 Dec;209(3):697–704. doi: 10.1148/radiology.209.3.9844661. [DOI] [PubMed] [Google Scholar]
- 30.Handa T, Nagai S, Fushimi Y, Miki S, Ohta K, Niimi A, et al. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest. 2006 Dec;130(6):1851–6. doi: 10.1378/chest.130.6.1851. [DOI] [PubMed] [Google Scholar]
- 31.Harrison BD, Shaylor JM, Stokes TC, Wilkes AR. Airflow limitation in sarcoidosis--a study of pulmonary function in 107 patients with newly diagnosed disease. Respir Med. 1991 Jan;85(1):59–64. doi: 10.1016/s0954-6111(06)80211-8. [DOI] [PubMed] [Google Scholar]
- 32.Chambellan A, Turbie P, Nunes H, Brauner M, Battesti J-P, Valeyre D. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest. 2005 Feb;127(2):472–81. doi: 10.1378/chest.127.2.472. [DOI] [PubMed] [Google Scholar]
- 33.Zappala CJ, Latsi PI, Nicholson AG, Colby TV, Cramer D, Renzoni EA, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J. 2010 Apr;35(4):830–6. doi: 10.1183/09031936.00155108. [DOI] [PubMed] [Google Scholar]
- 34.Du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med. 2011 Dec 15;184(12):1382–9. doi: 10.1164/rccm.201105-0840OC. [DOI] [PubMed] [Google Scholar]
- 35.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002 Jul 1;166(1):111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 36.Baughman RP, Lower EE. Six-minute walk test in managing and monitoring sarcoidosis patients. Curr Opin Pulm Med. 2007 Sep;13(5):439–44. doi: 10.1097/MCP.0b013e328273bc2b. [DOI] [PubMed] [Google Scholar]
- 37.Corte TJ, Wort SJ, Gatzoulis MA, Macdonald P, Hansell DM, Wells AU. Pulmonary vascular resistance predicts early mortality in patients with diffuse fibrotic lung disease and suspected pulmonary hypertension. Thorax. 2009 Oct;64(10):883–8. doi: 10.1136/thx.2008.112847. [DOI] [PubMed] [Google Scholar]
- 38.Baughman RP, Winget DB, Bowen EH, Lower EE. Predicting respiratory failure in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 1997 Sep;14(2):154–8. [PubMed] [Google Scholar]
- 39.Adams H, Keijsers RG, Korenromp IHE, Grutters JC. FDG PET for gauging of sarcoid disease activity. Semin Respir Crit Care Med. 2014 Jun;35(3):352–61. doi: 10.1055/s-0034-1376866. [DOI] [PubMed] [Google Scholar]
- 40.Mostard RLM, Vöö S, van Kroonenburgh MJPG, Verschakelen JA, Wijnen PaHM, Nelemans PJ, et al. Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med. 2011 Dec;105(12):1917–24. doi: 10.1016/j.rmed.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Sobic-Saranovic D, Grozdic I, Videnovic-Ivanov J, Vucinic-Mihailovic V, Artiko V, Saranovic D, et al. The utility of 18F-FDG PET/CT for diagnosis and adjustment of therapy in patients with active chronic sarcoidosis. J Nucl Med Off Publ Soc Nucl Med. 2012 Oct;53(10):1543–9. doi: 10.2967/jnumed.112.104380. [DOI] [PubMed] [Google Scholar]
- 42.Mostard RLM, Van Kuijk SMJ, Verschakelen JA, van Kroonenburgh MJPG, Nelemans PJ, Wijnen PAHM, et al. A predictive tool for an effective use of (18)F-FDG PET in assessing activity of sarcoidosis. BMC Pulm Med. 2012;12:57. doi: 10.1186/1471-2466-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keijsers RG, Verzijlbergen EJ, van den Bosch JM, Zanen P, van de Garde EM, Oyen WJ, et al. 18F-FDG PET as a predictor of pulmonary function in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2011 Oct;28(2):123–9. [PubMed] [Google Scholar]
- 44.Harlander M, Salobir B, Zupančič M, Dolenšek M, Bavčar Vodovnik T, Terčelj M. Serial chitotriosidase measurements in sarcoidosis--two to five year follow-up study. Respir Med. 2014 May;108(5):775–82. doi: 10.1016/j.rmed.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Bargagli E, Mazzi A, Rottoli P. Markers of inflammation in sarcoidosis: blood, urine, BAL, sputum, and exhaled gas. Clin Chest Med. 2008 Sep;29(3):445–458. viii. doi: 10.1016/j.ccm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Fløe A, Hoffmann HJ, Nissen PH, Møller HJ, Hilberg O. Genotyping increases the yield of angiotensin-converting enzyme in sarcoidosis--a systematic review. Dan Med J. 2014 May;61(5):A4815. [PubMed] [Google Scholar]
- 47.Vorselaars ADM, van Moorsel CHM, Zanen P, Ruven HJT, Claessen AME, van Velzen-Blad H, et al. ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respir Med. 2015 Feb;109(2):279–85. doi: 10.1016/j.rmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Rothkrantz-Kos S, van Dieijen-Visser MP, Mulder PGH, Drent M. Potential usefulness of inflammatory markers to monitor respiratory functional impairment in sarcoidosis. Clin Chem. 2003 Sep;49(9):1510–7. doi: 10.1373/49.9.1510. [DOI] [PubMed] [Google Scholar]
- 49.Zhou T, Zhang W, Sweiss NJ, Chen ES, Moller DR, Knox KS, et al. Peripheral blood gene expression as a novel genomic biomarker in complicated sarcoidosis. PloS One. 2012;7(9):e44818. doi: 10.1371/journal.pone.0044818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sweiss NJ, Salloum R, Gandhi S, Ghandi S, Alegre M-L, Sawaqed R, et al. Significant CD4, CD8, and CD19 lymphopenia in peripheral blood of sarcoidosis patients correlates with severe disease manifestations. PloS One. 2010;5(2):e9088. doi: 10.1371/journal.pone.0009088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweiss NJ, Barnathan ES, Lo K, Judson MA, Baughman R T48 Investigators. C-reactive protein predicts response to infliximab in patients with chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2010 Jul;27(1):49–56. [PubMed] [Google Scholar]
- 52.Patterson KC, Franek BS, Müller-Quernheim J, Sperling AI, Sweiss NJ, Niewold TB. Circulating cytokines in sarcoidosis: phenotype-specific alterations for fibrotic and non-fibrotic pulmonary disease. Cytokine. 2013 Mar;61(3):906–11. doi: 10.1016/j.cyto.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lacher MJ. Spontaneous remission or response to methotrexate in sarcoidosis. Ann Intern Med. 1968 Dec;69(6):1247–8. doi: 10.7326/0003-4819-69-6-1247. [DOI] [PubMed] [Google Scholar]
- 54.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2000 Mar;17(1):60–6. [PubMed] [Google Scholar]
- 55.Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med. 1995 Apr 24;155(8):846–51. [PubMed] [Google Scholar]
- 56.Vucinic VM. What is the future of methotrexate in sarcoidosis? A study and review. Curr Opin Pulm Med. 2002 Sep;8(5):470–6. doi: 10.1097/00063198-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 57.Baughman RP, Lower EE. Alternatives to corticosteroids in the treatment of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 1997 Sep;14(2):121–30. [PubMed] [Google Scholar]
- 58.Goljan-Geremek A, Bednarek M, Franczuk M, Puścińska E, Nowiński A, Czystowska M, et al. Methotrexate as a single agent for treating pulmonary sarcoidosis: a single centre real-life prospective study. Pneumonol Alergol Pol. 2014;82(6):518–33. doi: 10.5603/PiAP.2014.0069. [DOI] [PubMed] [Google Scholar]
- 59.Cremers JP, Drent M, Bast A, Shigemitsu H, Baughman RP, Valeyre D, et al. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med. 2013 Sep;19(5):545–61. doi: 10.1097/MCP.0b013e3283642a7a. [DOI] [PubMed] [Google Scholar]
- 60.Lewis SJ, Ainslie GM, Bateman ED. Efficacy of azathioprine as second-line treatment in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 1999 Mar;16(1):87–92. [PubMed] [Google Scholar]
- 61.Müller-Quernheim J, Kienast K, Held M, Pfeifer S, Costabel U. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J. 1999 Nov;14(5):1117–22. doi: 10.1183/09031936.99.14511179. [DOI] [PubMed] [Google Scholar]
- 62.Vorselaars ADM, Wuyts WA, Vorselaars VMM, Zanen P, Deneer VHM, Veltkamp M, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest. 2013 Sep;144(3):805–12. doi: 10.1378/chest.12-1728. [DOI] [PubMed] [Google Scholar]
- 63.Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2004 Mar;21(1):43–8. doi: 10.1007/s11083-004-5178-y. [DOI] [PubMed] [Google Scholar]
- 64.Sahoo DH, Bandyopadhyay D, Xu M, Pearson K, Parambil JG, Lazar CA, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J. 2011 Nov;38(5):1145–50. doi: 10.1183/09031936.00195010. [DOI] [PubMed] [Google Scholar]
- 65.Raj R, Nugent K. Leflunomide-induced interstitial lung disease (a systematic review) Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2013 Oct;30(3):167–76. [PubMed] [Google Scholar]
- 66.Kilmartin DJ, Forrester JV, Dick AD. Rescue therapy with mycophenolate mofetil in refractory uveitis. Lancet. 1998 Jul 4;352(9121):35–6. doi: 10.1016/S0140-6736(05)79515-5. [DOI] [PubMed] [Google Scholar]
- 67.Kouba DJ, Mimouni D, Rencic A, Nousari HC. Mycophenolate mofetil may serve as a steroid-sparing agent for sarcoidosis. Br J Dermatol. 2003 Jan;148(1):147–8. doi: 10.1046/j.1365-2133.2003.05042.x. [DOI] [PubMed] [Google Scholar]
- 68.Hilderson I, Van Laecke S, Wauters A, Donck J. Treatment of renal sarcoidosis: is there a guideline? Overview of the different treatment options. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2014 Oct;29(10):1841–7. doi: 10.1093/ndt/gft442. [DOI] [PubMed] [Google Scholar]
- 69.Hamzeh N, Voelker A, Forssén A, Gottschall EB, Rose C, Mroz P, et al. Efficacy of mycophenolate mofetil in sarcoidosis. Respir Med. 2014 Nov;108(11):1663–9. doi: 10.1016/j.rmed.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costedoat-Chalumeau N, Dunogué B, Morel N, Le Guern V, Guettrot-Imbert G. Hydroxychloroquine: a multifaceted treatment in lupus. Presse Médicale Paris Fr 1983. 2014 Jun;43(6 Pt 2):e167–180. doi: 10.1016/j.lpm.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 71.Baltzan M, Mehta S, Kirkham TH, Cosio MG. Randomized trial of prolonged chloroquine therapy in advanced pulmonary sarcoidosis. Am J Respir Crit Care Med. 1999 Jul;160(1):192–7. doi: 10.1164/ajrccm.160.1.9809024. [DOI] [PubMed] [Google Scholar]
- 72.Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014 Dec;132(12):1453–60. doi: 10.1001/jamaophthalmol.2014.3459. [DOI] [PubMed] [Google Scholar]
- 73.Marmor MF, Melles RB. Hydroxychloroquine and the retina. JAMA. 2015 Feb 24;313(8):847–8. doi: 10.1001/jama.2014.14558. [DOI] [PubMed] [Google Scholar]
- 74.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, du Bois R, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med. 2006 Oct 1;174(7):795–802. doi: 10.1164/rccm.200603-402OC. [DOI] [PubMed] [Google Scholar]
- 75.Rossman MD, Newman LS, Baughman RP, Teirstein A, Weinberger SE, Miller W, et al. A double-blinded, randomized, placebo-controlled trial of infliximab in subjects with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2006 Oct;23(3):201–8. [PubMed] [Google Scholar]
- 76.Judson MA, Baughman RP, Costabel U, Mack M, Barnathan ES. The potential additional benefit of infliximab in patients with chronic pulmonary sarcoidosis already receiving corticosteroids: a retrospective analysis from a randomized clinical trial. Respir Med. 2014 Jan;108(1):189–94. doi: 10.1016/j.rmed.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 77.Sweiss NJ, Noth I, Mirsaeidi M, Zhang W, Naureckas ET, Hogarth DK, et al. Efficacy Results of a 52-week Trial of Adalimumab in the Treatment of Refractory Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2014;31(1):46–54. [PMC free article] [PubMed] [Google Scholar]
- 78.Drent M, Cremers JP, Jansen TL, Baughman RP. Practical eminence and experience-based recommendations for use of TNF-α inhibitors in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2014;31(2):91–107. [PubMed] [Google Scholar]
- 79.Utz JP, Limper AH, Kalra S, Specks U, Scott JP, Vuk-Pavlovic Z, et al. Etanercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest. 2003 Jul;124(1):177–85. doi: 10.1378/chest.124.1.177. [DOI] [PubMed] [Google Scholar]
- 80.Marques I, Giovannonni G, Marta M. Mononeuritis multiplex as the first presentation of refractory sarcoidosis responsive to etanercept. BMC Neurol. 2014 Dec 11;14(1):237. doi: 10.1186/s12883-014-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobak S, Yilmaz H, Sever F, Duran A, Sen N, Karaarslan A. The prevalence of antinuclear antibodies in patients with sarcoidosis. Autoimmune Dis. 2014;2014:351852. doi: 10.1155/2014/351852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sweiss NJ, Lower EE, Mirsaeidi M, Dudek S, Garcia JGN, Perkins D, et al. Rituximab in the treatment of refractory pulmonary sarcoidosis. Eur Respir J. 2014 May;43(5):1525–8. doi: 10.1183/09031936.00224513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atzeni F, Talotta R, Salaffi F, Cassinotti A, Varisco V, Battellino M, et al. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun Rev. 2013 May;12(7):703–8. doi: 10.1016/j.autrev.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 84.Marcellis R, Van der Veeke M, Mesters I, Drent M, De Bie R, De Vries G, et al. Does physical training reduce fatigue in sarcoidosis? Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2015;32(1):53–62. [PubMed] [Google Scholar]
- 85.Strookappe B, Elfferich M, Swigris J, Verschoof A, Veschakelen J, Knevel T, et al. Benefits of physical training in patients with idiopathic or end-stage sarcoidosis-related pulmonary fibrosis: a pilot study. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG World Assoc Sarcoidosis Granulomatous Disord. 2015;32(1):43–52. [PubMed] [Google Scholar]
- 86.Shlobin OA, Nathan SD. Management of end-stage sarcoidosis: pulmonary hypertension and lung transplantation. Eur Respir J. 2012 Jun;39(6):1520–33. doi: 10.1183/09031936.00175511. [DOI] [PubMed] [Google Scholar]
- 87.Tzouvelekis A, Bonella F, Spagnolo P. Update on therapeutic management of idiopathic pulmonary fibrosis. Ther Clin Risk Manag. 2015;11:359–70. doi: 10.2147/TCRM.S69716. [DOI] [PMC free article] [PubMed] [Google Scholar]