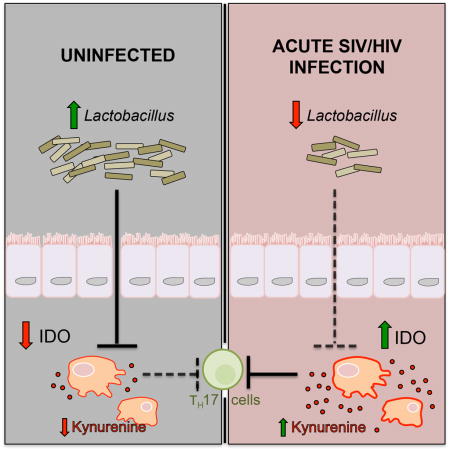
Summary
Gut microbes can profoundly modulate mucosal barrier-promoting Th17 cells in mammals. A salient feature of HIV/SIV immunopathogenesis is the loss of Th17 cells, which has been linked to increased activity of the immunomodulatory enzyme, indoleamine 2,3-dioxygenase 1 (IDO 1). The role of gut microbes in this system remains unknown, and the SIV-infected rhesus macaque provides a well-described model for HIV-associated Th17 loss and mucosal immune disruption. We observed a specific depletion of gut-resident Lactobacillus during acute and chronic SIV infection of rhesus macaques, which was also seen in early HIV-infected humans. This depletion in rhesus macaques correlated with increased IDO1 activity and Th17 loss. Macaques supplemented with a Lactobacillus-containing probiotic exhibited decreased IDO1 activity during chronic SIV infection. We propose that Lactobacillus species inhibit mammalian IDO1 and thus may help to preserve Th17 cells during pathogenic SIV infection, providing support for Lactobacillus species as modulators of mucosal immune homeostasis.
Graphical Abstract
Introduction
Untreated HIV infection is characterized by chronic immune activation, which is postulated to be spurred by decreased mucosal barrier function and subsequent translocation of microbial products into systemic circulation (Klatt et al., 2013). The gut mucosa of HIV-infected individuals exhibits a relative depletion of Th17 cells, a cell type that participates in containment of gut luminal bacteria and that supports barrier integrity via secretion of IL-17 and IL-22, cytokines which act on epithelial cells to promote production of antimicrobial peptides and mucins (Ouyang et al., 2008). Concomitant with Th17 cell depletion in HIV [which also occurs in non-human primate models using the closely related simian immunodeficiency virus (SIV)], has been observed a simultaneous upregulation of the immunomodulatory enzyme, indoleamine 2,3-dioxygenase 1(IDO1) (Favre et al., 2010). IDO1 is a gene induced primarily in macrophages and plasmacytoid dendritic cells by type I and II interferons, cytokines that are highly abundant in circulation during untreated HIV/SIV infection (Hosmalin and Lebon, 2006). IDO1 catalyzes the conversion of tryptophan to kynurenine and other downstream catabolites, which bind the aryl hydrocarbon receptor and that have been shown to inhibit Th17 differentiation (Desvignes and Ernst, 2009; Romani et al., 2008). IDO1 activity, as measured by the ratio of kynurenine to its parent compound tryptophan (Kyn:Trp) in blood plasma, correlates strongly with the depletion of mucosal-barrier promoting Th17 cells in the gut mucosa of HIV-infected subjects (Favre et al., 2010; Vujkovic-Cvijin et al., 2013). It is postulated that this disruption of mucosal immunity accounts for associations between IDO1 activity and microbial translocation (Chen et al., 2014) as well as with peripheral immune activation (Jenabian et al., 2013; Jenabian et al., 2015) and mortality (Byakwaga et al., 2014) in HIV-infected individuals. These observations support a role for IDO1 activity in the pathophysiology of HIV/AIDS, and highlight the need for a greater understanding of the extrinsic and host-mediated factors regulating the IDO1 pathway and the depletion of Th17 cells in HIV/SIV infection.
Human-associated bacteria of the gastrointestinal tract vastly outnumber host cells within each individual, and evidence suggests that complex bidirectional relationships have evolved between gut-resident microbes and the mammalian mucosal immune system. Likely, selective pressures have guided these relationships to facilitate a homeostasis wherein the health of both host and microbe is maintained. Indeed, Th17 cells and regulatory T cells (Treg) are poised at mucosal surfaces where they interact with gut-resident microbes (Lathrop et al., 2011) and regulate their containment in the gut lumen (Raffatellu et al., 2008); conversely, these cells themselves are dynamically modulated by the presence of specific gut microbes (Ivanov et al., 2009; Atarashi et al., 2011). Uncovering pathways by which gut-resident bacteria modulate mucosal T cell differentiation is an active area of research. Putative microbial drivers of mucosal immune homeostasis in HIV/SIV disease, specifically those that may inhibit the immunomodulatory IDO1 axis and/or otherwise preserve the Th17 cell pool, have yet to be identified.
SIV-infected macaques provide a well-described non-human primate model that recapitulates major features of HIV-associated chronic inflammation and mucosal immune disruption, including innate and adaptive immune activation, CD4 T cell depletion in the peripheral blood and gut-associated lymphoid tissue, and a loss of mucosal barrier-promoting Th17 cells (Favre et al., 2009). The etiology of these immune disruptions in the SIV-infected macaque model remain poorly understood, though distinct assemblages of the macaque gut microbiome have been shown to associate with abundance of Th17 cells (Ardeshir et al., 2014). Furthermore, several recent macaque studies have shown that members of the bacterial genus, Lactobacillus, can modulate the Th17 axis, though mechanisms for this process remain poorly understood. Administration of VSL#3, a probiotic cocktail of 7 bacterial species 4 of which are Lactobacillus spp., to chronically SIV-infected pigtail macaques resulted in greater Th17 polyfunctionality as well as a partial resolution of several inflammatory measures (e.g., lower fibrosis, reduced CD4 T cell activation, and increased CD4 T cell recovery) (Klatt et al., 2013; Ortiz et al., 2014). In other studies, ligated ileal loops of acutely SIV-infected rhesus macaques inoculated with Lactobacillus plantarum displayed increased IL-17 expression and epithelial tight junction formation (Hirao et al., 2014). Studies of the macaque gut microbiome have shown few ecological differences in gut bacterial communities between SIV-uninfected and chronically SIV-infected macaques (Klatt et al., 2013; Handley et al., 2012; McKenna et al., 2008), which is in contrast to the pronounced microbiota perturbations observed in human cohorts (Lozupone et al., 2013; Dillon et al., 2014; Mutlu et al., 2014; Dinh et al., 2015; Vujkovic-Cvijin et al., 2013). However, host-microbiome dynamics during the acute phase of lentiviral infection, when IDO1 is maximally induced by interferons, remain unexplored. We found that a transient perturbation of the fecal microbiota of SIV-infected macaques occurs during acute SIV infection, which coincides with maximal IDO1 activity. This perturbation was characterized chiefly by depletion of Lactobacillus members and we provide evidence here that gut-resident Lactobacillus spp. modulate primate IDO1 by inhibiting production of immunomodulatory kynurenine compounds that affect the Th17 cell arm of the mammalian mucosal immune system.
Results
A transient shift in microbiota composition occurs during acute SIV infection of rhesus macaques
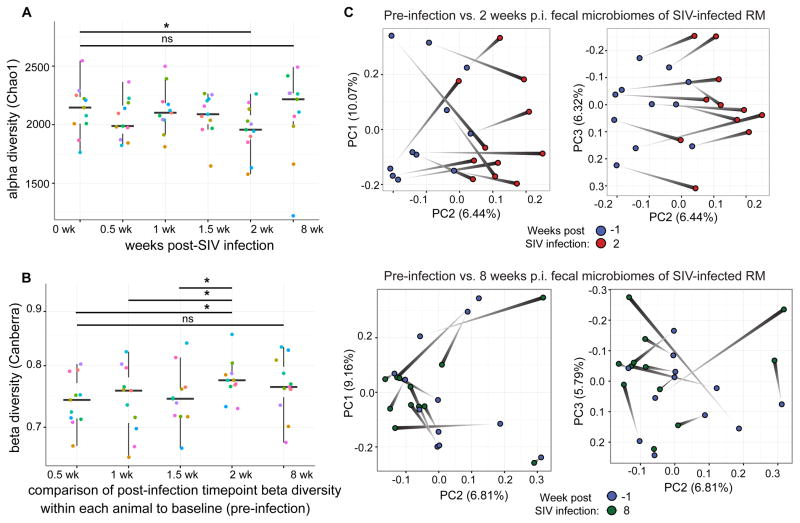
Two temporally independent cohorts of six female rhesus macaques were each experimentally infected with SIVmac251 and followed longitudinally via stool and peripheral blood sampling before infection and at five time points through the acute and chronic infection phases. We profiled fecal bacterial microbiota by sequencing of the V4 region of the 16S rRNA gene and collapsing reads with 97% sequence homology into operational taxonomic units (OTUs). A transient shift in the fecal microbiota at 2 weeks post-SIV infection that subsided by 8 weeks post-infection (Figure 1) was evident. These bacterial community dynamics were characterized by a decrease in alpha diversity (Figure 1A) (a measure for community richness, i.e., the total number of unique taxa, and evenness, i.e., the relative dispersion of abundances of all taxa). This shift was characterized also by an increase in beta diversity (defined as the compositional difference between two ecological communities) within each animal as compared to its own pre-infection time point, using both the Canberra beta diversity metric (which weights pair-wise distances by relative abundance of bacterial OTUs present among both samples; Figure 1B) and the unweighted UniFrac beta diversity metric (which incorporates a phylogenetic relationship weighting of shared OTUs in distance calculations; Figure S1). That a marked magnitude of microbiota compositional change occurred at 2 weeks post infection was supported by principal coordinates ordination (permANOVA P=0.039, Figure 1C). This analysis also demonstrated that a significant change in composition was not evident when comparing pre-infection communities to 8 weeks p.i. (permANOVA P = 0.194), further supporting the observation that the greatest change in microbiota composition occurred during the acute phase of SIV infection in this model. Intriguingly, when comparing rhesus macaque fecal microbiota at each time point to fecal microbiota of chronically HIV-infected humans, there was no increase in similarity of the rhesus macaque fecal microbiota to that of chronically HIV-infected humans after SIV infection (Figure S1B), indicating that SIV infection in this non-human primate model does not cause a microbiota shift that resembles that of the HIV-associated gut microbiota.
Figure 1. Ecological dynamics of microbiota composition across SIV infection in rhesus macaques.
A) Difference between baseline (pre-infection) and post-SIV infection (p.i.) fecal microbiota significantly increases at 2 weeks p.i. and returns by 8 weeks p.i. (Wilcoxon matched-pairs signed rank test *P < 0.05, FDR Q value < 0.10). Each color represents a unique animal.
B) Alpha diversity (richness and evenness) of the rhesus macaque fecal microbiota drops at 2 weeks p.i. (Wilcoxon matched-pairs signed rank test *P < 0.05, FDR Q value < 0.05).
C) Principal Coordinates Analysis (PCoA) plot of fecal microbiota shift from pre-infection to 2 weeks p.i. (P = 0.039 by permANOVA, stratified by individual, Canberra beta diversity metric). No such shift is evident at 8 weeks p.i. (P = 0.194 by permANOVA, Canberra)
The acute SIV-associated microbiota shift is characterized by depletion of Lactobacillus spp. and is paralleled in early HIV infection
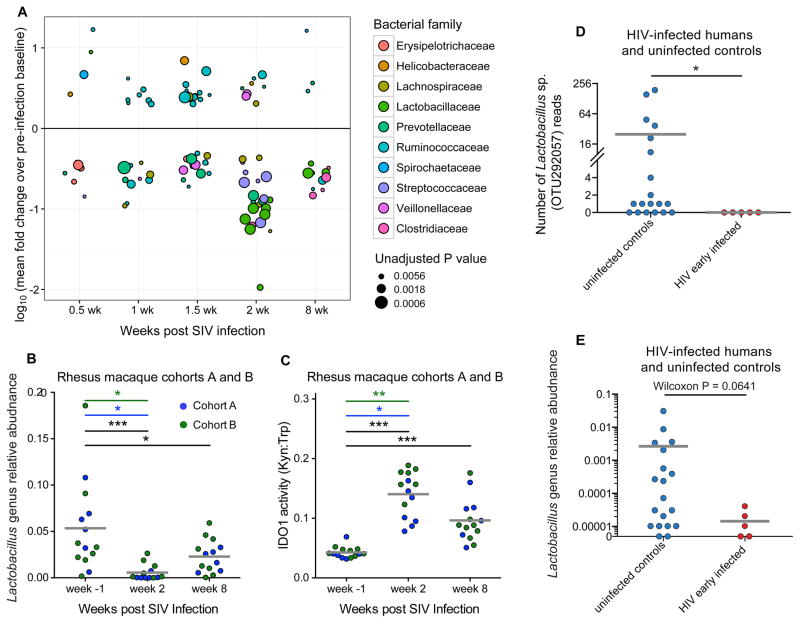
To investigate taxonomic dynamics in microbiota composition as a function of SIV infection (with a particular interest in the 2 week p.i. community), we compared OTU abundances pre-infection to each time point after infection for each animal (Table S1A). Notable differences were observed at 2 weeks p.i., at which time depletion of multiple Lactobacillus and Streptococcus spp. occurred (Figure 2A, Table S1A). Lactobacillus genus depletion was observed at 2 weeks p.i. in each of the temporally independent cohorts of animals as well as when considered together (Figure 2B, Table S1B), and this depletion persisted into the chronic phase of infection 8 weeks p.i. (Figure 2B). Concomitant with these taxonomic depletions at 2 weeks p.i., IDO1 activity was heightened at this time point and did not resolve to baseline (pre-infection) levels during the chronic phase of infection (Figure 2C). This pattern of IDO1 activity was similar in kinetics and relative scale to the acute depletion of Lactobacillus, with a persistent but intermediate depletion extending into the chronic phase of SIV infection. In contrast to the rhesus macaque model used here, the non-pathogenic SIV infection model of the African green monkey (a non-human primate which, when experimentally infected with SIV, neither exhibits SIV-associated chronic inflammation nor develops simian AIDS (Jacquelin et al., 2009)), restores IDO1 activity to pre-infection levels during chronic infection. This coincides with an increased abundance of fecal Lactobacillus at that time (Figure S2), suggesting a role for Lactobacilli in the regulation of mammalian IDO1 activity during SIV infection. Furthermore, interrogation of fecal microbiomes using the same methods described above for rhesus animals was performed on a small cohort of subjects in the early stages of HIV infection (time since infection for all infected subjects < 16 months, median time since last HIV RNA negative test = 175 days, detailed cohort characteristics in Table S1C). Analysis revealed all five OTUs within the Lactobacillaceae family that were detected in our human dataset exhibited a fold decrease in abundance as compared to HIV-uninfected, risk-matched control human subjects (Table S1D). One such OTU was significantly depleted (P = 0.019, FDR Q value < 0.10, Figure 2D), while the Lactobacillus genus trended toward significance when comparing early HIV-infected to uninfected subjects (P = 0.064, Figure 2E), suggesting that depletion of Lactobacilli is a feature of acute lentiviral infection that is conserved among primates including humans.
Figure 2. Gut microbiota taxonomic shifts across time in SIV-infected rhesus macaques and in early HIV infection.
A) Shown are OTUs that differ significantly between pre-infection time point and each p.i. time point within each animal, with Wilcoxon matched-pairs signed rank test P < 0.01 and mean read abundance fold change > 2. Colors indicate the bacterial family to which each OTU belongs; the relative sizes of points are proportional to the inverse log of Wilcoxon P values.
B) Lactobacillus genus is depleted at 2 weeks p.i. and this depletion persists into the chronic phase of SIV infection at 8 weeks p.i. as compared to pre-infection. Wilcoxon matched-pairs signed rank test was used for each comparison.
C) Rhesus macaques exhibit elevated IDO1 activity that persists into the chronic phase of infection. Wilcoxon matched-pairs signed rank test, * P < 0.05, ** P < 0.005, *** P < 0.0005 for RM cohorts A and B considered together (black error bars).
D) Lactobacillus sp. OTU 292057 is significantly depleted in the stool of early HIV-infected human subjects as compared to uninfected risk-matched controls (P = 0.019). Dataset used for this analysis was rarified to 97,000 reads per sample.
E) Fecal Lactobacillus genus abundance trends toward significant depletion in early HIV-infected human subjects as compared to uninfected controls (P = 0.0641). See also Table S1.
Lactobacillus depletion associates with increased IDO1 activity and loss of Th17 cell abundance
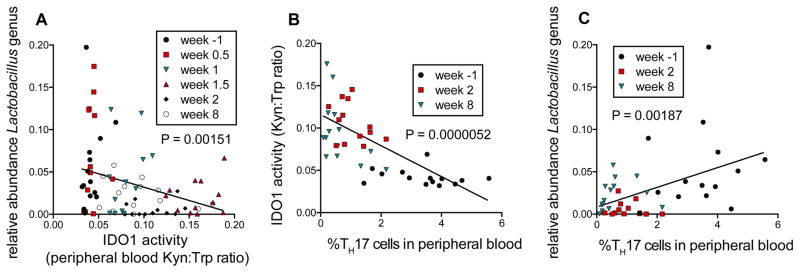
To further narrow the candidate bacterial taxa that may modulate IDO1 activity, we used a linear mixed effects models approach (which accounts for inter-individual variation in longitudinal time-series data) to compare abundances of all bacterial genera detected in all rhesus animals at all time points to respective plasma Kyn:Trp ratios. We found that fecal abundance of the Lactobacillus genus correlated significantly and inversely with IDO1 activity (P=0.00151, Figure 3A), and was the top inverse correlate of all genera detected in our dataset (total=86, Table S2A). Furthermore, IDO1 activity was found to be tightly correlated with peripheral blood Th17 cell loss across acute and chronic SIV infection (P=0.0000052, Figure 3B). Accordingly, the abundance of the Lactobacillus genus exhibited a significant positive correlation with Th17 cell abundances (P=0.00187, Figure 3C) and was amongst the strongest taxonomic correlates of Th17 cell abundances in our dataset (Table S2B). To address the hypothesis that other fecal taxa that correlated positively with IDO1 activity may themselves produce kynurenine compounds, we utilized the UniProt genome database to identify bacterial families that encode enzymes that participate in the kynurenine pathway of tryptophan catabolism. We found that families that encode such enzymes do not preferentially correlate with Kyn:Trp ratios (Figure S3A), suggesting that direct bacterial tryptophan catabolism may not represent the primary driver of Kyn:Trp ratios in the SIV-infected rhesus macaque.
Figure 3. IDO1 activity and Th17 cell dynamics associate with loss of gut-resident Lactobacillus.
A) The abundance of Lactobacillus genus correlates strongly with IDO1 activity as measured by Kyn:Trp ratios in peripheral blood. Linear mixed effects (LME) modeling was used to allow for inclusion of longitudinal data from each animal and to test for correlations between all detected bacterial families and IDO1 activity (peripheral blood Kyn:Trp ratios). Linear regression lines are shown in black.
B) IDO1 activity (Kyn:Trp) correlates strongly and significantly with Th17 cell abundances in peripheral blood of RM, using LME modeling.
C) Lactobacillus abundance correlates with Th17 cell abundance in peripheral blood. As with B), LME modeling was used to test for correlations between all bacterial families detected and peripheral blood Th17 cell abundance, as assessed by flow cytometry gating the percentage of IL-17+ of total CD4+ cells. See also Table S2.
To understand whether the inverse correlation between IDO1 activity and Lactobacillus abundance found in our rhesus macaque cohorts was due to antagonistic effects of kynurenine on Lactobacillus growth, we isolated Lactobacillus spp. from the stool samples obtained from experimental animals. The resulting primary isolates were dominated by L. animalis and L. reuteri (Figure S3B). Physiologic and supraphysiologic concentrations of kynurenine were added to growth cultures of L. animalis and L. reuteri isolates, and growth was measured by light absorption. No effect of kynurenine on the growth of these primary isolates was observed (Figure S3C).
Supplementation of SIV-infected macaques with a Lactobacillus-containing probiotic reduces peripheral blood IDO1 activity
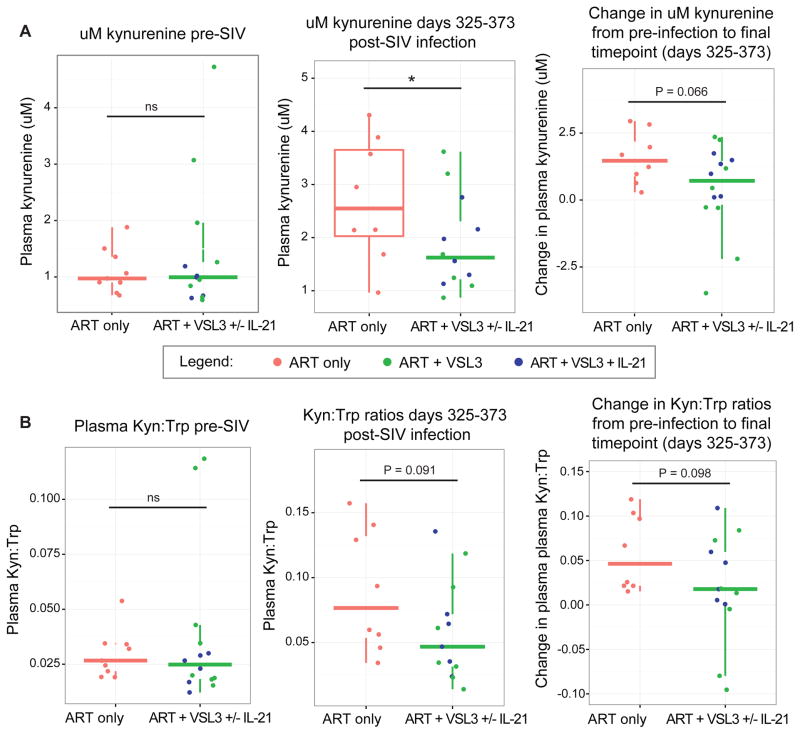
To further investigate cause and effect relationships responsible for the correlation between Lactobacillus genus abundance and activity of the immunomodulatory IDO1 pathway, we asked whether supplementation of SIV-infected macaques with Lactobacillus spp. may modulate IDO1 activity in vivo. Plasma was analyzed from two cohorts of SIV-infected pigtail macaques that had initiated treatment with VSL#3, a probiotic mixture composed of 7 bacterial strains of which 4 are Lactobacillus spp., twelve weeks post SIV infection (Klatt et al., 2013; Ortiz et al., 2014). While only a subset of animals were administered VSL#3, all animals received antiretroviral therapy as detailed in Experimental Procedures. As determined using linear mixed effects models to control for between-cohort differences, VSL#3-treated animals at days 325–373 p.i. exhibited significantly decreased plasma concentrations of kynurenine, an effector molecule produced through IDO1 catabolism of tryptophan, as compared to untreated control animals (Figure 4A). These animals also exhibited trends towards lower ratios of Kyn:Trp (Figure 4B), a marker of flux through the IDO1 catabolic pathway, and differences in both kynurenine concentration and Kyn:Trp ratios at the 325–372 days p.i. time point as compared to the same measurements in pre-infection plasma. While one of the two cohorts treated with VSL#3 received an additional agent (the Th17-promoting IL-21 cytokine, which has no reported relationships to IDO1 activity), there were no differences in Kyn:Trp ratios or kynurenine concentrations between the VSL#3-treated arms of these cohorts (Figure S4).
Figure 4. Effects of supplementation of SIV-infected pigtail macaques with Lactobacillus-containing probiotic on markers of IDO1 activity.
A) Two cohorts of pigtail macaques (PTMs) given daily VSL#3 exhibit a decrease in peripheral blood kynurenine concentrations compared to untreated control PTMs at days 325–372 p.i., P = 0.046, by LME.
B) Trends toward signifiance in Kyn:Trp ratios are seen in PTMs treated with VSL#3 at at days 325–372 p.i. (P = 0.091).
Discussion
We observed a specific depletion of gut-resident Lactobacillius in rhesus macaques, seen most dramatically during acute SIV infection and persisting into the chronic phase. This depletion correlated with increased IDO1 activity and Th17 cell loss across time during SIV infection. The kynurenine product of the IDO1 pathway was found to have no effect on Lactobacillus growth in vitro, while pigtail macaques supplemented with a Lactobacillus-containing probiotic exhibited decreased IDO1 activity during chronic SIV infection. We propose that gut-resident Lactobacillus species inhibit primate IDO1 and thus may help to preserve the Th17 cell compartment during pathogenic SIV infection. If so, Lactobacillus species may be beneficial modulators of mucosal immune homeostasis during the course of such infection.
Furthermore, we profiled the fecal microbiota of several African green monkeys at different stages of SIV infection. In contrast to SIV-infected macaques, African green monkeys exhibit a non-pathogenic course of SIV infection characterized by a resolution of IDO1 activity during the chronic phase and a preservation of the Th17 cell pool (Favre et al., 2009). We found that these animals instead exhibited an increase in fecal Lactobacillus abundance during chronic infection, consistent with a role for Lactobacillus in inhibiting the IDO1 pathway. However, these observations were obtained from a small, cross-sectional sample size of mixed gender (n=4–6 for pre-infection, acute, and chronic SIV-infected animals), whereas our rhesus cohorts were female, and should be interpreted cautiously at this time. Additionally, the power to detect differences between VSL#3-treated and untreated SIV-infected animal groups was enhanced by the combination of two temporally separated VSL#3 supplementation experiments. One of these trials included administration of the cytokine IL-21 in addition to VSL#3. To our knowledge no precedence exists for IL-21 directly affecting IDO1 activity, though this possibility cannot be ruled out. Despite this, plasma kynurenine concentrations and Kyn:Trp ratios did not differ significantly between the cohort that received IL-21 and that which did not, suggesting that the interpretation of the effect being largely due to the VSL#3 probiotic may be appropriate. Furthermore, while Lactobacillus has been previously associated with lower peripheral blood kynurenine in rats (Valladares et al., 2013), mono-administration of a Bifidobacterium sp. contained in VSL#3 did not affect peripheral blood kynurenine levels (Bercik et al., 2010), while products of the other genus composing VSL#3, Streptococcus, have been shown to induce rather than inhibit IDO1 activity (Murr et al., 2001).
While shifts in the gut bacterial microbiota of chronically HIV-infected humans have been well-described and with relative consistency in findings, several studies (including that presented herein) suggest that significant ecological shifts in the gut bacterial microbiota do not occur in macaques in the chronic phase of experimental SIVmac251 (Handley et al., 2012; McKenna et al., 2008) or SIVmac239 (Klatt et al., 2013) infection as compared to uninfected macaques. We observed that a pronounced depletion of Lactobacillus occurs during acute SIV infection and appears to occur also during early HIV infection, though our cohort of HIV-infected humans was small (n=5). This suggests that similarities do exist between the experimental SIV infection model of rhesus macaques and HIV-infected humans in the early phases of infection. However, numerous studies of HIV-associated microbiota shifts have reported no changes in the abundance of gut Lactobacillus during chronic HIV infection in humans (Lozupone et al., 2013; Dillon et al., 2014; Mutlu et al., 2014; Dinh et al., 2015; Vujkovic-Cvijin et al., 2013), while we observed that Lactobacillus depletion persists into chronic SIV infection. As chronic HIV-associated microbiota shifts may be relevant to HIV disease pathogenesis, knowledge of this distinction as a potential caveat to the macaque model of experimental SIV infection may inform interpretation of results from this important model. Regardless, however, of its incapacity to recapitulate chronic HIV-associated microbiota shifts at the taxonomic level, the macaque model offers various notable advantages toward probing host-microbiome immunologic relationships, including genomic proximity to humans and a higher capacity for tissue sampling across time.
Lactobacillus-supplemented yogurt has been investigated as a possible therapeutic agent in African cohorts of treated and untreated HIV-infected adults, with results showing improvements in CD4 count through mechanisms that remain poorly understood (Anukam et al., 2008; Irvine et al., 2010). Extrapolating from the findings of this study, we propose that Lactobacilli may inhibit IDO1 activity of humans as well as of non-human primates, an effect that may have the capacity to mitigate the loss of gut barrier-promoting human Th17 cells. This could allow for maintenance of mucosal integrity, reduction in microbial translocation, and an overall diminution of HIV-associated inflammation. This proposal could be directly tested in clinical trials using existing, over-the-counter probiotics (e.g., VSL#3) containing Lactobacillus spp. and should be carried out with a focus on IDO1 activity as an outcome.
Experimental Procedures
Study cohorts and sample collection
Six female rhesus macaques (Macaca mulatta) were infected with SIVmac251-2010 on April 23, 2013 (Cohort A) and eight more females were infected on July 16, 2013 (Cohort B) with 100 TCID50 administered i.v. Peripheral blood plasma and stool were collected and stored at −80°C at the following days relative to the dates of infection for all rhesus macaque animals: −7, 3, 7, 10, 14, and 56 days, while peripheral blood mononuclear cells (PBMCs) were obtained and cryopreserved at −80°C at days −7, 14, and 56 post-infection. A total of 14 African green monkeys (AGMs) (Chlorocebus sabaeus) were used in this study as part of Cohort C shown in Figure S2. Twelve AGMs were adult males while 2 were female, all aged 6–11 year old. Six AGMs were uninfected, while 8 AGMs were i.r. infected with plasma containing 107 copies of SIVagm.sab92018. Peripheral blood and stool were collected at the following days relative to the dates of infection for all AGMs: −30, 9–13, and 46–56 days. Human subject fecal samples were self-collected and deposited at −80°C without preservative within 6 hours of stool deposition. Peripheral blood plasma was collected at time of deposition and stored at −80°C.
Fecal sample processing, 16S rRNA amplification, and 16S rRNA sequencing
Fecal sample DNA was extracted and DNA was amplified targeting the V4 region of the 16S rRNA gene. Microbiome profiling was performed on eleven animals at all fecal time points collected, while the remaining three animals were profiled at −7, 14, and 56 days p.i. Amplification, pooling, purification, and quantification was performed as described in Supplemental Experimental Procedures. 16S rRNA sequencing data have been deposited at the Qiita database (http://qiita.microbio.me) under Study ID: 10257.
16S rRNA sequence analysis and statistical methods
An open-reference OTU picking scheme was utilized as implemented in the QIIME software package and reads were rarefied to 34,000 per sample. Beta diversity and alpha diversity calculations were performed in QIIME, while the R package ‘adonis’ was used to calculate permANOVA statistics. Custom R scripts were designed to identify discriminating OTUs after SIV infection, and to compare abundances of all bacterial families to peripheral blood IDO1 activity and Th17 cell abundances. Comparisons of RM fecal microbiomes to HIV-infected humans were made using fecal 16S sequencing data published previously (Lozupone et al., 2013) and beta diversity metrics were calculated using QIIME.
Flow cytometry and analysis
PBMCs were isolated from rhesus macaque peripheral blood, cryopreserved, stimulated with phorbol 12-myristate 13-acetate + ionomycin, and analyzed by flow cytometry as described in Supplemental Experimental Procedures.
IDO activity measurements
IDO activity was measured as the ratio of the plasma concentration of its enzymatic product, kynurenine, to concentration of its parent compound, tryptophan. Concentrations of kynurenine and tryptophan were quantitated in the plasma of all animals within the present study by liquid chromatography-tandem mass spectrometry, as previously described (Favre et al., 2010).
VSL#3 trial characteristics and analysis
Two separate studies using pigtail macaques (Macaca nemestrina) infected with 3,000 TCID of SIVmac239 i.v. were combined for analysis of the effects of VSL#3 treatment on IDO1 activity. One cohort, initiated on 6/25/2010 (Klatt et al., 2013), consisted of 4 control animals and 7 VSL#3-treated animals, with VSL#3-treated animals beginning daily administration of probiotic beginning at 100 days post-infection and both groups beginning antiretroviral therapy at day 160 post-infection as described previously (Klatt et al., 2013). The second cohort, initiated on 10/9/12, consisted of 5 control animals and 6 VSL#3/IL-21-treated animals, with antiretroviral therapy commencing on day 98 p.i. for both groups and the VSL#3/IL-21 group receiving daily VSL#3 beginning on day 98 p.i. as well as two courses of rMamu IL-21-IgFc (50 ug/kg at 1 mg/mL in H2O s.c., 5 once-weekly doses) initiated at days 98 and 333 p.i. as described (Ortiz et al., 2014). One animal in the control group for the second cohort was sacrificed before the study end due to severe diarrhea. Linear mixed effects modeling fitted by maximum likelihood (R package ‘lme4’) was used to test the significance of differences between VSL#3-treated and untreated animals, incorporating stratification by study cohort.
Lactobacillus isolation and in vitro experimentation
Lactobacillus primary isolates were isolated by plating frozen stool from animals in Cohort B onto MRS selection agar (Becton, Dickinson and Company). Full-length 16S rRNA sequencing to verify taxonomic identity of resultant isolates was performed using the 27f/1492r 16S rRNA primer pair. Taxonomy was assigned to sequence reads using NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Growth assessment with addition of exogenous kynurenine was performed in MRS liquid media at 37° C using an automated spectrophotometer with OD600 readings taken at regular intervals.
Supplementary Material
Acknowledgments
We thank P. Hunt, A. Sil, and J. Cyster for helpful discussions and K. Raehtz for assistance with AGM studies. The research reported in this publication was supported by CHRP D13-SF-388 (to IVC), NSF 1144247 (to IVC), NIH U19 AI96109 (to JMM), by the Harvey V. Berneking Living Trust (to JMM), and R01HL117715-11 (to IP). Funding for this study was provided in part by the Division of Intramural Research/NIAID/NIH. The content of this publication does not necessarily reflect the views or policies of DHHS, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Collection of human specimens was supported by Bill and Melinda Gates Foundation grant OPP1017716, P01 AI071713 (to FMH), and R01 HD074511 (to CDP). We thank M. Busch, G. Murphy, K. Marson, S. Facente, and A. Welte for aid in developing the human specimen and data repository. IVC, CAS, and SNC performed DNA extraction on rhesus fecal samples.
Footnotes
Author Contributions:
IVC performed 16S rRNA library preparation, sequencing, and analysis of rhesus macaque and AGM cohorts. LAS performed flow cytometry measurements and analysis. DLL and DWF performed DNA extractions and 16S sequencing on human subject samples. IVC, RMD, and LAS designed the rhesus macaque studies. IVC and SNC isolated Lactobacillus primary isolates. AP performed Lactobacillus isolate growth experiments. AAF assisted with microbiome analysis. FMH and CDP oversaw human subject cohort recruitment and sample collection. YH measured kynurenine and tryptophan in macaque plasma. CA and IP designed AGM experiments and provided samples. NRK, JMB, and AMO designed VSL#3 trials in pigtail macaques and provided samples, while IVC performed analyses. IVC, SVL, and JMM wrote the manuscript. SVL and JMM oversaw experiments and analyses. The authors declare they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anukam KC, Osazuwa EO, Osadolor HB, Bruce AW, Reid G. Yogurt containing probiotic Lactobacillus rhamnosus GR-1 and L. reuteri RC-14 helps resolve moderate diarrhea and increases CD4 count in HIV/AIDS patients. Journal of clinical gastroenterology. 2008;42:239–243. doi: 10.1097/MCG.0b013e31802c7465. [DOI] [PubMed] [Google Scholar]
- Ardeshir A, Narayan NR, Méndez-Lagares G, Lu D, Rauch M, Huang Y, Van Rompay KK, Lynch SV, Hartigan-O’Connor DJ. Breast-fed and bottle-fed infant rhesus macaques develop distinct gut microbiotas and immune systems. Sci Transl Med. 2014;6:252ra120. doi: 10.1126/scitranslmed.3008791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. e1. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- Byakwaga H, Boum Y, Huang Y, Muzoora C, Kembabazi A, Weiser SD, Bennett J, Cao H, Haberer JE, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T-cell recovery, and mortality among HIV-infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;210:383–391. doi: 10.1093/infdis/jiu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Shao J, Cai R, Shen Y, Zhang R, Liu L, Qi T, Lu H. Anti-retroviral therapy decreases but does not normalize indoleamine 2,3-dioxygenase activity in HIV-infected patients. PLoS One. 2014;9:e100446. doi: 10.1371/journal.pone.0100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvignes L, Ernst JD. Interferon-gamma-responsive nonhematopoietic cells regulate the immune response to Mycobacterium tuberculosis. Immunity. 2009;31:974–985. doi: 10.1016/j.immuni.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–994. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, Wanke CA, Ward HD. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis. 2015;211:19–27. doi: 10.1093/infdis/jiu409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Lederer S, Kanwar B, Ma ZM, Proll S, Kasakow Z, Mold J, Swainson L, Barbour JD, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, Abbink P, Maxfield LF, Kambal A, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151:253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao LA, Grishina I, Bourry O, Hu WK, Somrit M, Sankaran-Walters S, Gaulke CA, Fenton AN, Li JA, et al. Early mucosal sensing of SIV infection by paneth cells induces IL-1β production and initiates gut epithelial disruption. PLoS Pathog. 2014;10:e1004311. doi: 10.1371/journal.ppat.1004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmalin A, Lebon P. Type I interferon production in HIV-infected patients. J Leukoc Biol. 2006;80:984–993. doi: 10.1189/jlb.0306154. [DOI] [PubMed] [Google Scholar]
- Irvine SL, Hummelen R, Hekmat S, Looman WNC, Habbema JDF, Reid G. Probiotic yogurt consumption is associated with an increase of CD4 count among people living with HIV/AIDS. Journal of clinical gastroenterology. 2010;44:e201. doi: 10.1097/MCG.0b013e3181d8fba8. [DOI] [PubMed] [Google Scholar]
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenabian M-A, El-Far M, Vyboh K, Kema I, Costiniuk CT, Thomas R, Baril J-G, LeBlanc R, Kanagaratham C, Radzioch D. Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. Journal of Infectious Diseases, jiv037. 2015 doi: 10.1093/infdis/jiv037. [DOI] [PubMed] [Google Scholar]
- Jenabian MA, Patel M, Kema I, Kanagaratham C, Radzioch D, Thébault P, Lapointe R, Tremblay C, Gilmore N, et al. Distinct tryptophan catabolism and Th17/Treg balance in HIV progressors and elite controllers. PLoS One. 2013;8:e78146. doi: 10.1371/journal.pone.0078146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, Quiñones M, Deming CB, Perkins M, Hazuda DJ. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest. 2013 doi: 10.1172/JCI66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends in microbiology. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–54. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE. Alterations in the Gut Microbiota Associated with HIV-1 Infection. Cell Host Microbe. 2013;14:329–339. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna P, Hoffmann C, Minkah N, Aye PP, Lackner A, Liu Z, Lozupone CA, Hamady M, Knight R, Bushman FD. The macaque gut microbiome in health, lentiviral infection, and chronic enterocolitis. PLoS Pathog. 2008;4:e20. doi: 10.1371/journal.ppat.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murr C, Gerlach D, Widner B, Dierich MP, Fuchs D. Neopterin production and tryptophan degradation in humans infected by Streptococcus pyogenes. Med Microbiol Immunol. 2001;189:161–63. doi: 10.1007/s430-001-8023-3. [DOI] [PubMed] [Google Scholar]
- Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz M, Klase A, Carmack M, Vinton L, Perkins R, Paiardini Villinger, Brenchley M. Probiotic and IL-21 Treatment Promotes Th17 Cell Recovery in ARV-Treatment of Pigtail Macaques. 21st Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. Mar 3–6, 2014; 2014. Sess. O-6. [Google Scholar]
- Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–28. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–15. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- Valladares R, Bojilova L, Potts AH, Cameron E, Gardner C, Lorca G, Gonzalez CF. Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. 2013;27:1711–720. doi: 10.1096/fj.12-223339. [DOI] [PubMed] [Google Scholar]
- Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, et al. Dysbiosis of the Gut Microbiota Is Associated with HIV Disease Progression and Tryptophan Catabolism. Sci Transl Med. 2013;5:193ra91. doi: 10.1126/scitranslmed.3006438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.