Abstract
Craniosynostosis, a condition in which the cranial sutures prematurely fuse, can lead to elevated intracranial pressure and craniofacial abnormalities in young children. Currently surgical intervention is the only therapeutic option for patients with this condition. Craniosynostosis has been associated with a variety of different gene mutations and chromosome anomalies. Here we describe three cases of partial deletion of chromosome 19p. Two of the cases present with syndromic craniosynostosis while one has metopic ridging. A review of the genes involved in the rearrangements between the three cases suggests several gene candidates for craniosynostosis. CALR and DAND5, BMP regulators involved in osteoblast differentiation, and MORG1, a mediator of osteoclast dysregulation may play a role in abnormal cranial vault development. Additionally, CACNA1A, a gene that when mutated is associated with epilepsy and CC2D1A, a gene associated with non-syndromic mental retardation may contribute to additional phenotypic features seen in the patients we describe. In addition, these findings further support the need for genetic testing in cases of syndromic craniosynostosis.
Keywords: Chromosome 19, Craniofacial syndrome, Craniosynostosis, Microarray, Microdeletion
Introduction
The premature fusion of cranial sutures affects approximately 1 in 2500 newborns in a condition known as craniosynostosis.1, 2 Craniosynostosis can lead to elevated intracranial pressure, developmental delay and ocular/visual compromise.3
Craniosynostosis can be characterized as either syndromic or non-syndromic. Approximately 80% of cases are non-syndromic and there is no common genetic basis.4 In isolated (non-syndromic) craniosynostosis the sagittal suture is most commonly affected, followed by the metopic and coronal sutures. Closure of the metopic suture can result in metopic ridging and metopic craniosynostosis which can be difficult to differentiate but which have importance for treatment.5 Syndromic cases can be inherited but are often sporadic and are the result of de novo autosomal dominant mutations commonly involving fibroblast growth factor receptors (FGFRs) and TWIST genes.6, 7, 8 Gain-of-function mutations in FGFR1 to 3 have been associated with Crouzon, Pfeiffer, Apert and Muenke syndromes, among others. More than 180 syndromes have been reported to manifest craniosynostosis.4 Here we describe three cases of patients with deletions in the region of chromosome 19p13.12–p13.2. Two of the cases with overlapping deletion have developmental delay and craniosynostosis, whereas the other patient with a unique deletion in the region has physiologic closure of the metopic suture with ridging without craniosynostosis. These cases along with one additional case from the literature help define genes that may be associated with syndromic craniosynostosis. Approval of this case series was obtained from the University of Chicago Institutional Review Board (IRB#14-0489). Parental consent forms, which included permission for the use of the facial images of the affected individuals, were obtained for the reported work.
Case 1
The first patient was born to healthy parents after a pregnancy complicated by antenatal ultrasound findings of ventriculomegaly. The patient was born via elective cesarean section at 38 weeks with a birth weight of 2365 g (<5th percentile) and occipitofrontal circumference (OFC) of 36.5 cm (95th percentile). In the newborn period, she developed oxygen desaturations and feeding difficulties. Clinical examination at that time revealed micrognathia, frontal bossing, low set ears, a high palate and scaphocephaly (Fig. 1). A CT scan of the cranial bones revealed partial posterior sagittal craniosynostosis and micrognathia (Fig. 1). Magnetic resonance (MR) imaging of the brain revealed an immature brain with thickened cortex and decreased gyral pattern suggestive of a cortical brain malformation. An ophthalmology examination revealed bilateral optic nerve hypoplasia.
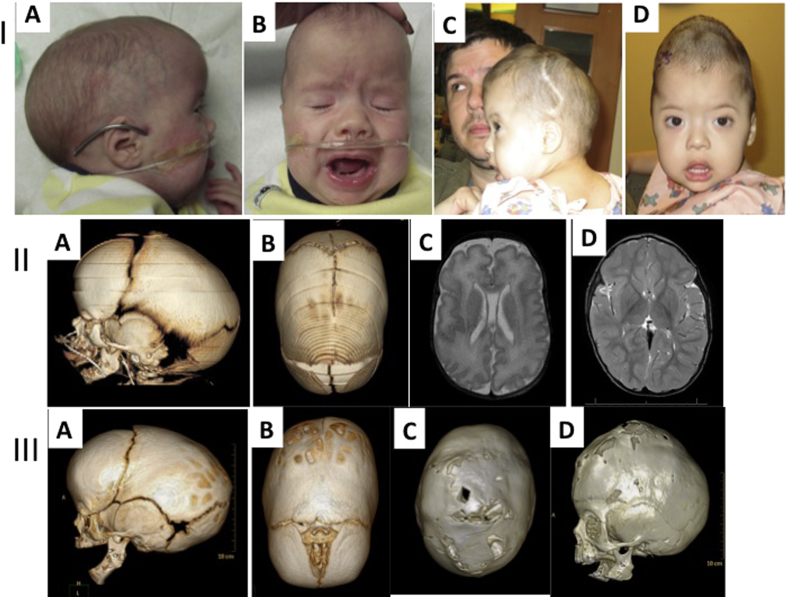
Figure 1.
Case 1. I: A, B; Photos of patient 1 before calvarial vault reconstruction. A. Photo shows typical compensatory scaphocephaly and micrognathia, as well as dysmorphic features including low set ears, frontal bossing of the cranial vault secondary to sagittal suture fusion. C,D; 24 months of age demonstrating turribrachycephaly. II: A, B; CT scan at birth showing the micrognathia (A) and the partial sagittal craniosynostosis (B). C, D; Axial T2 FLAIR MRI at birth shows adequate brain growth. Scan is notable for mild lissencephaly. III: A, B; CT scan at 5 months post mandibular distraction showing persistent micrognathia and progression of the sagittal craniosynostosis. C, D; CT scan at 24 months of age demonstrating craniocerebral mismatch with effacement of the sagittal suture and the coronal sutures.
Due to her severe obstructive sleep apnea (OSA)—with a presurgical apnea–hypopnea index (AHI) of 60—the patient underwent multiple interventions (tongue lip adhesion and mandibular distraction osteogenesis) to improve her airway obstruction. This markedly reduced the patient's supplemental oxygen requirement. With improvement in her airway and nutrition, the patient underwent a subsequent subtotal calvarial vault reconstruction for sagittal craniosynostosis (Fig. 1).
Her growth and development continued to be delayed. At 8 months of age, her weight was 6.61 kg (2nd percentile), and height was 68.2 cm (40th percentile) and OFC was 44.5 cm (75th percentile). At 11 months of age she had developmental delays of gross and fine motor as well as speech. At 20 months of age she developed seizures, which were treated with anti-epileptic medication.
At 26 months of age, 20 months post initial cranial vault expansion, the patient's head circumference failed to progress over a 9-month period. The patient presented with turribrachycephaly and computed tomography (CT) scan evaluation revealed clear signs of craniocerebral mismatch with no identifiable cranial suture patent (Fig. 1). These radiographic signs corroborated the clinical presentation, as the patient was found to have signs and symptoms of increased intracranial pressure. These findings necessitated a revision cranial vault expansion via multidirectional posterior vault distraction.
Microarray results. Cytogenomic microarray revealed a 2.2 Mb microdeletion of chromosome 19p: arr 19p13.12–p13.2 (12,552,241–14,714,485) (National Center for Biotechnology Information (NCBI) build 36.1) containing approximately 73 known genes.
Case 2
The second patient is an international case of a 10-month-old Scandinavian child who is the product of a first pregnancy to healthy parents. She was born following induction of labor for low amniotic fluid at gestational age 41 weeks 3 days. Birth weight was 3 465 g and length was 51 cm. There were no complications at birth. An abnormal head shape, with occipital flattening, was noted by the parents and the pediatrician, but this was thought to be related to positional molding.
She developed persistent vomiting/reflux, necessitating hospitalization on three separate occasions (starting at 2 months of age). Secondary to the reflux and developmental delays a cytogenomic microarray revealed a 700 kb deletion of chromosome 19p13.2 (chr. 19: 13,041,835–13,740,519 NCBI Build 36.1). Parental studies were not obtained.
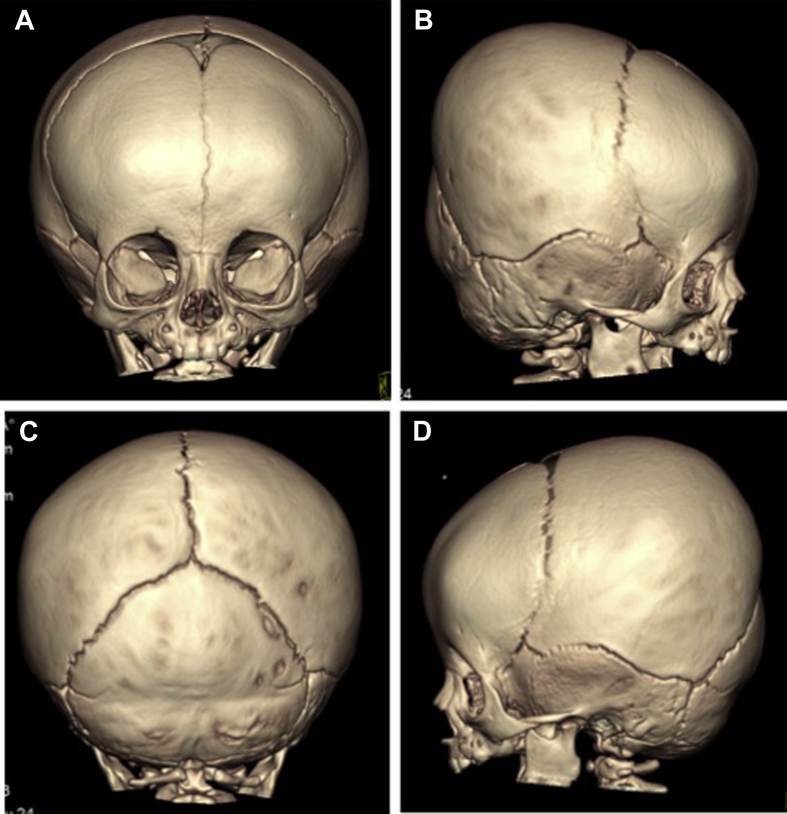
Her head circumference measured 38.1 cm at 4 weeks of age, 42 cm at 4 months of age, and 44.5 cm at 9 months of age (all at the 75th percentile for age). Digital photography (not shown) reveals bitemporal retrusion, mild brachycephaly, and right posterior plagiocephaly. Interestingly, craniofacial CT scan with 3D reformations demonstrates partial synostosis of the bilateral coronal suture systems (Fig. 2). No other findings were remarkable on these imaging studies.
Figure 2.
Case 2. Three-dimensional reformatted CT scan imaging demonstrates partial synostosis of bilateral coronal sutures. A. Frontal view; B. Right lateral view; C. Posterior view; D. Left lateral view. Arrows denote areas of abnormal bridging ossification (synostosis) of coronal sutures.
Case 3
The third patient is a female 7-month-old fraternal twin born via cesarean section at 36 weeks gestation following a pregnancy in which serial ultrasonography identified ventriculomegaly and hydronephrosis. The patient had respiratory distress and cyanosis and feeding difficulties. Birth weight was 2060 g (<5th percentile), length was 41 cm (<5th percentile), and head circumference (HC) was 31.5 cm (<5th percentile). A brain MRI confirmed the enlarged ventricles and, additionally, showed a hypoplastic corpus callosum.
At 6 months of age she was noted to have significant proximal and peripheral hypotonia as well as symmetrically decreased reflexes in the upper and lower extremities. Her ears were slightly low set and posteriorly rotated with mildly pointed superior helices, and her head was triangular shaped with a flat occiput, severe plagiocephaly, biparietal peaking, and bitemporal narrowing. Her skin showed a 1 cm café-au-lait spot in the right groin, a tiny skin tag at the coccyx without dimple, and a 2.5 cm brown flat macule with clear borders on the center of the abdomen. Her weight was 6.5 kg (14th percentile), length was 62.0 cm (8th percentile), and HC was 42.3 cm (41st percentile). Developmentally she had delays in gross motor skills.
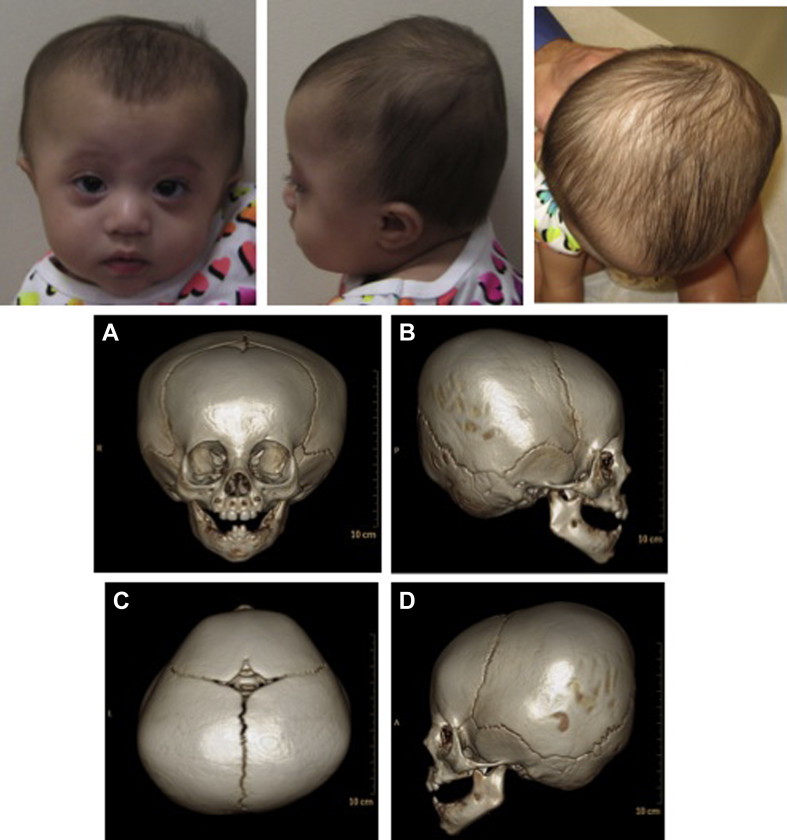
Based on her head shape described above a CT scan was done and showed features consistent with physiologic closure of the metopic suture with ridging, and no craniosynostosis (Fig. 3).
Figure 3.
Case 3. Three-dimensional reformatted CT scan imaging demonstrates head shape and lack of findings consistent with craniosynostosis and consistent with physiologic closure of the metopic suture. A. Frontal view; B. Right lateral view; C. Posterior view; D. Left lateral view. Note occipital flattening and biparietal peaking, physiologic closure of metopic suture and patency of other cranial sutures.
Chromosomal single nucleotide polymorphisms (SNP) array analysis was ordered with showed a 19p13.12 deletion (14,144,824–14,787,018, hg19). This 642 kb deletion was maternally inherited.
Methods
Cytogenomic array analysis was performed using either a custom Agilent oligonucleotide microarray with 180,000 probes and analyzed on human genome build hg18 or the Affymetrix CytoScan HD array including 750,000 SNP probes and 1.9 million non-polymorphic probes analyzed on hg19. Base pair coordinates were estimated from the microarray and the coordinates from hg18 were converted to hg19 for comparison.
Discussion
Microdeletions in the small arm of chromosome 19 are rare and described deletions have significant phenotypes that are often terminal in utero.9 The clinical phenotypes of patients with chromosome 19p deletions are difficult to compare and variable as the deletions are of different sizes and involve different regions of chromosome 19. Many reported cases were described based on karyotype abnormalities with poor resolution of breakpoints. The recent advance of cytogenomic microarray technology has allowed for higher resolution studies and characterization of the exact genomic composition including specific genes within genomic rearrangements.
Three patients with overlapping 19p13.12 microdeletions have been described with mental retardation, ear malformations, brachycephaly and anteverted nares.10 Additionally, an 834 kb deletion in 19p13.2 was described in a five-year-old boy with intellectual disability, febrile seizures and minor dysmorphic features including a slightly protruding forehead, short fingers and ears with overfolded helices.11 An additional case bearing common deletions within the same region to our case series is a case of complex syndromic craniosynostosis (fused left coronal, lambdoid and parieto-temporal sutures) described by Lysy et al.12
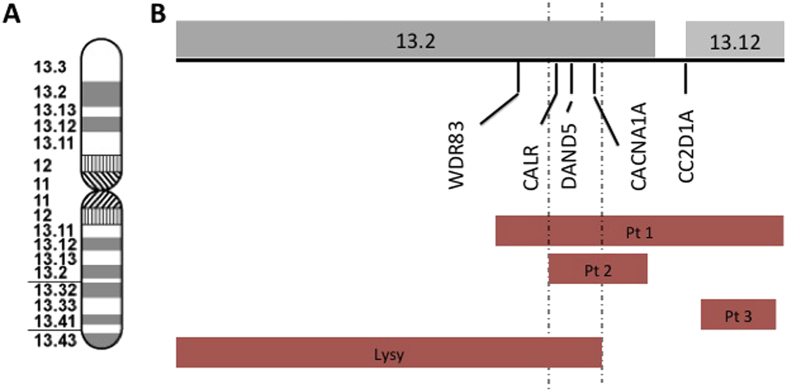
The patient reported by Lysy et al and two of the three patients we describe (Cases 1 and 2, Cases 1 and 2 herein) all share deletions of CALR and DAND5 (Fig. 4)—regulators of bone morphogenetic proteins (BMPs) essential for bone metabolism, organ and skeletal formation and differentiation of osteoblastic cells.13, 14 These common deletions suggest that the absence of these BMP regulatory genes could cause abnormalities in calvarial development leading to craniosynostosis.12 Additionally, MORG1, a gene whose product associates with components of the extracellular signal-regulated kinase (ERK) pathway integral to osteoclast development and activity, is deleted in the patient described by Lysy et al and in our first case.15, 16 Deletion of this helper protein may disrupt the ERK signaling pathway that leads to osteoclast dysregulation.17 This gene was not deleted in the second case reported herein who similarly presents with a 700 kb deletion in chromosome 19p13.2 (within that of Case 1), partial synostosis and developmental delay. Deletion of coding regions on the 5′ end of the gene, however, could affect MORG1 expression. The microdeletion described in this second case is on the 3′ end of the gene, which suggests that MORG1 is not implicated in the craniosynostotic phenotype (Fig. 4). Interestingly, both cases described herein had partial involvement of a particular suture system, whether sagittal (Case 1) or bilateral coronal (Case 2). In the case described by Lysy et al, there is pansynostosis (left unicoronal, lambdoid, and parieto-temporal) which, to our review of the figures in their manuscript, represents a partial, “skip-lesion” phenotype. The authors also comment in their case that the craniosynostosis, in certain areas, is partial. The fact that our first case had craniocerebral disproportion with elevated intracranial pressure, and required revision cranioplasty to expand the cranial vault, corroborates the syndromic nature of this presentation and points to potential lesions in the molecular pathways described above.
Figure 4.
Genomic overlap A. Ideogram of chromosome 19 at 850 band stage. B. Zoomed in view of deletions showing the regions of overlap between the cases and the genes of interest. Dotted lines represent minimum region of overlap between the patients with craniosynostosis.
Further implicating this region's significance in suture homeostasis is the third case presented in this study. Microarray analysis of this patient revealed a deletion distal to the other patients, and therefore not involving the putative genes described above. Despite clinical findings of severe plagiocephaly, biparietal peaking, and bitemporal narrowing, suggestive of premature metopic closure, the patient failed to demonstrate the formal radiographic signs characteristic of metopic craniosynostosis: her findings are more indicative of physiologic closure of the metopic suture with ridging.5
Lastly, the deletion described in our first and second cases also included the CACNA1A and the first and third case included the CC2D1A gene. CACNA1A is a gene encoding a voltage dependent calcium channel subunit expressed in neuronal tissue and mutations in this gene are associated with epilepsy.16, 18 CC2D1A is a gene that regulates the expression of serotonin receptors in neuronal tissue and mutations in this gene have been associated with non-syndromic mental retardation.16 Deletion of these genes likely contributes to the patient's phenotype of developmental disability and seizures.
Conclusion
We now add to the growing body of evidence in the literature supporting the association of specific chromosomal 19p microdeletions (19p13.12–13.2) and cranial suture dysmorphology. Specifically, two craniosynostotic patients are described: one with sagittal craniosynostosis, micrognathia, seizure disorder, hypotonia and developmental delay, and another patient with bilateral coronal synostosis and mild developmental delays. Our third case in this series, with a deletion distal to the first two cases and only hypotonia, plagiocephaly and developmental delay, further targets a putative gene locus, which affects suture homeostasis. Of particular interest, the first two patients described in this report, and that described by Lysy et al, demonstrate initial partial involvement of some sutures, suggesting a progressive process and therefore point to genetic and epigenetic causal factors. The craniosynostosis in the first case required multiple surgeries secondary to craniocerebral mismatch, which is unusual outside the syndromes associated with FGFR. The second case demonstrates bilateral coronal synostosis, which is partial in nature. The genes in the deleted region are associated with seizures, developmental disability and a disruption in the balance between osteoblastic and osteoclastic activity essential for proper bone growth and remodeling. This report not only helps expand the phenotypic characterization of 19p deletions, but also reinforces the importance of cytogenomic microarray in the setting of suspected syndromic craniosynostosis.
Acknowledgments
The authors would like to thank James Cao, Administrative Specialist in the Section of Plastic Surgery, University of Chicago, for his assistance with obtaining test results and figures from Case 2 and with consent form procurement.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Sarah M. Lyon, Email: slyon@uchicago.edu.
Darrel Waggoner, Email: dwaggone@genetics.uchicago.edu.
Sara Halbach, Email: shalbach@bsd.uchicago.edu.
Erik C. Thorland, Email: thorland.erik@mayo.edu.
Russell R. Reid, Email: rreid@surgery.bsd.uchicago.edu.
Conflict of interest
None of the authors have any financial conflicts of interest to disclose.
References
- 1.French L.R., Jackson I.T., Melton L.J., 3rd A population-based study of craniosynostosis. J Clin Epidemiol. 1990;43:69–73. doi: 10.1016/0895-4356(90)90058-w. [DOI] [PubMed] [Google Scholar]
- 2.Singer S., Bower C., Southall P., Goldblatt J. Craniosynostosis in Western Australia, 1980–1994: a population-based study. Am J Med Genet. 1999;83:382–387. doi: 10.1002/(sici)1096-8628(19990423)83:5<382::aid-ajmg8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 3.Lekovic G.P., Bristol R.E., Rekate H.L. Cognitive impact of craniosynostosis. Semin Pediatr Neurol. 2004;11:305–310. doi: 10.1016/j.spen.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Kimonis V., Gold J.-A., Hoffman T.L., Panchal J., Boyadjiev S.A. Genetics of craniosynostosis. Semin Pediatr Neurol. 2007;14:150–161. doi: 10.1016/j.spen.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Birgfeld C.B., Saltzman B.S., Hing A.V. Making the diagnosis: metopic ridge versus metopic craniosynostosis. J Craniofac Surg. 2013;24:178–185. doi: 10.1097/SCS.0b013e31826683d1. [DOI] [PubMed] [Google Scholar]
- 6.el Ghouzzi V., Le Merrer M., Perrin-Schmitt F. Mutations of the TWIST gene in the Saethre–Chotzen syndrome. Nat Genet. 1997;15:42–46. doi: 10.1038/ng0197-42. [DOI] [PubMed] [Google Scholar]
- 7.Britto J.A., Evans R.D., Hayward R.D., Jones B.M. From genotype to phenotype: the differential expression of FGF, FGFR, and TGFbeta genes characterizes human cranioskeletal development and reflects clinical presentation in FGFR syndromes. Plast Reconstr Surg. 2001;108:2026–2039. doi: 10.1097/00006534-200112000-00030. [DOI] [PubMed] [Google Scholar]
- 8.Cohen M.M., Jr. Perspectives on craniosynostosis: sutural biology, some well-known syndromes, and some unusual syndromes. J Craniofac Surg. 2009;20(Suppl. 1):646–651. doi: 10.1097/SCS.0b013e318193d48d. [DOI] [PubMed] [Google Scholar]
- 9.Brewer C., Holloway S., Zawalnyski P., Schinzel A., FitzPatrick D. A chromosomal duplication map of malformations: regions of suspected haplo- and triplolethality–and tolerance of segmental aneuploidy–in humans. Am J Hum Genet. 1999;64:1702–1708. doi: 10.1086/302410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonaglia M.C., Marelli S., Novara F. Genotype–phenotype relationship in three cases with overlapping 19p13.12 microdeletions. Eur J Hum Genet. 2010;18:1302–1309. doi: 10.1038/ejhg.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haberlandt E., Spreiz A., Sigl S.B. Microdeletion 19p13.2 in an almost 5-year-old boy. Am J Med Genet A. 2012;158A:1190–1194. doi: 10.1002/ajmg.a.35291. [DOI] [PubMed] [Google Scholar]
- 12.Lysy P.A., Ravoet M., Wustefeld S. A new case of syndromic craniosynostosis with cryptic 19p13.2–p13.13 deletion. Am J Med Genet A. 2009;149A:2564–2568. doi: 10.1002/ajmg.a.33056. [DOI] [PubMed] [Google Scholar]
- 13.Chen D., Zhao M., Mundy G.R. Bone morphogenetic proteins. Growth Factors Chur Switz. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 14.Kattamuri C., Luedeke D.M., Nolan K. Members of the DAN family are BMP antagonists that form highly stable noncovalent dimers. J Mol Biol. 2012;424:313–327. doi: 10.1016/j.jmb.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He Y., Staser K., Rhodes S.D. Erk1 positively regulates osteoclast differentiation and bone resorptive activity. PLoS ONE. 2011;6:e24780. doi: 10.1371/journal.pone.0024780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer L.R., Zweig A.S., Hinrichs A.S. The UCSC genome Browser database: extensions and updates 2013. Nucleic Acids Res. 2012;41:D64–D69. doi: 10.1093/nar/gks1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Twigg S.R., Vorgia E., McGowan S.J. Reduced dosage of ERF causes complex craniosynostosis in humans and mice and links ERK1/2 signaling to regulation of osteogenesis. Nat Genet. 2013;45:308–313. doi: 10.1038/ng.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sander T., Toliat M.R., Heils A., Becker C., Nürnberg P. Failure to replicate an allelic association between an exon 8 polymorphism of the human alpha(1A) calcium channel gene and common syndromes of idiopathic generalized epilepsy. Epilepsy Res. 2002;49:173–177. doi: 10.1016/s0920-1211(02)00025-6. [DOI] [PubMed] [Google Scholar]