Abstract
We present a silica nanoparticle (SNP) functionalized with polyphosphate (polyP) that accelerate the body’s natural clotting process. SNPs initiate the blood clotting system’s contact pathway; short-chain polyP accelerates the common pathway via rapid formation of thrombin. This enhances the overall blood-clotting system, both by accelerating fibrin generation and by facilitating the regulatory anticoagulation mechanisms essential for hemostasis. Analyzing the clotting properties of bare SNPs, bare polyP, and polyP-functionalized SNPs in plasma, demonstrates that attaching polyP to SNPs to form polyP-SNPs creates a substantially enhanced synergistic effect that lowers clotting time and increases thrombin production at low concentrations when compared to bare SNPs or polyP alone. PolyP-SNP even retains its clotting function at ambient temperature. The polyP-SNP system has the potential to significantly improve trauma treatment protocols and outcomes in hospital and prehospital settings.
Keywords: hemorrhage, polyphosphate, silicates, nanoparticles, trauma
Controlling hemorrhage is a major focus in the treatment and stabilization of many trauma patients. Uncontrolled blood loss is the leading cause of battlefield deaths, despite the fact that less than 5 % of soldiers who subsequently reach a hospital die of their wounds.[1] In civilian hospitals, hemorrhage results in 15–25 % of trauma deaths.[2] These data suggest that treatment should focus on stopping bleeding prior to hospital arrival. Bleeding management is currently aimed at volume resuscitation and surgical intervention to limit blood loss.[3] However, these measures often do not address the source or mechanism of the bleeding and ultimately can limit the possible options to control it, especially in the prehospital setting.
Currently, there are three major approaches for controlling prehospital hemorrhage. The oldest method employs mechanical devices that compress the wound to minimize the area through which blood can escape the damaged vessel.[4] Agents such as kaolin or chitosan (Scheme 1) are useful as field therapeutics for management of external hemorrhage and are widely utilized by military forces as a first-response treatment.[5],[6] However, these compounds cannot be administered systemically and therefore lack utility for internal injuries with an intrinsic non-compressible hemorrhage.
Scheme 1.
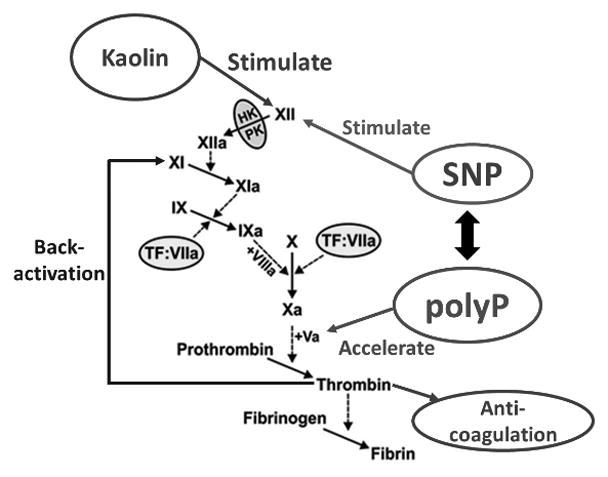
Simplified coagulation cascadsende. SNP surface induces the activation of FXII, while short-chain polyP enhances the rates of activation of FV and FXI leading to an earlier thrombin burst. Rapid thrombin generation leads to back-activation of FXIa, accelerating the coagulation cascade, as well as facilitating increased activity in the anticoagulation pathway, which is designed to limit the spread of coagulation to uninjured vessels. Kaolin acts only to activate FXII via the intrinsic pathway.
Recombinant human Factor VIIa (rFVIIa) is currently licensed for the management of bleeding episodes in patients with hemophilia and certain cases of warfarin overanticoagulation. Off-label use of rFVIIa is also commonly seen in certain other hemorrhagic conditions.[7] Although anecdotal reports of clinical response abound, a significant number of concerns regarding safety remain, due to reported thrombotic complications.[7] Drug storage requirements and the extreme cost of recombinant protein medication further limit field use.[8]
The ideal agent to control non-compressible hemorrhage in the prehospital setting would have the following properties: (1) long shelf-life and stability in weather extremes, (2) reasonable cost, (3) promote key functions of the blood clotting system at the injury site through the common pathway, including coagulation, regulatory anti-coagulation and fibrin formation, and (4) lack of off-target adverse effects. Such an agent could initiate treatment during the transport phase when the patient is at the greatest risk for exsanguination. We propose that targeted short-chain polyphosphate-laden silica nanoparticles (polyP-SNPs) have the potential to fulfill these requirements.
Because it accelerates through the common pathway the key functions of the blood clotting system rather than initiates endogenous coagulation enzyme production, short-chain polyP is a reasonable candidate for the management of hemorrhage.[9] Delivery of a polyP payload on a nanoparticle carrier could potentially be optimized to target the site of injury while minimizing the impact on the systemic circulation. Effective targeting and control would in theory minimize the risk of thrombotic complications that limit procoagulant therapy. In addition to the potential for better biosystem safety, the production cost for polyP is low compared to that of recombinant proteins. Upon attachment to inorganic oxides, polyP also has the potential for long-term stability under a variety of storage conditions.
In response to an injury, human platelets secrete short-chain polyP of approximately 60–100 monomers. Platelet-secreted polyP has a variety of wound-healing therapeutic effects, including enhanced activation of factors XI and V, which ultimately leads to enhanced factor X activity, and limitation of the activity of tissue factor pathway inhibitor, which results in accelerated thrombin generation.[10] The incorporation of polyP also impacts clot structure and leads to resistance to fibrinolysis.[11] Due to a ~90 minute half-life in plasma, the procoagulant effects are temporally limited.[10–12] This endogenous metabolism of polyP offers much better biocompatibility than currently available agents such as kaolin, which are not metabolized.
While long-chain polyP is a potent activator of the contact pathway, short-chain polyP released from platelets has relatively poor capacity to activate factor XII.[9] We employed relatively short-chain polyP for these studies in order to maximize the enhancement of downstream coagulation enzymatic steps (common pathway), while minimizing contact pathway activation.
As noted above, platelets serve as a polyP delivery agent, secrete procoagulants and clotting factors that promote blood coagulation, and initiate the formation of a clot-dissolving enzyme that degrades blood clots during the healing process. In our studies following up on the extensive research originally carried out by the Morrissey group,[9–12] we use free polyP as a benchmark. In the research we report here, delivery agent bifunctionality is introduced by using SNPs as a procoagulant carrier for the polyP.
Silica is generally considered to be a non-toxic material, and it is often used in drug delivery studies.[13] However, due to its negative surface charge, silica is also a contact activator.[5a,14] Consequently, the ideal construction of a polyP-bearing silica nanoparticle would shield the silica from exposure to the systemic circulation, with targeting and exposure of the silica carrier surface and polyP at the site of internal hemorrhage. Current materials used for treating external hemorrhage generally contain particles in the micrometer range, which are too large to easily traverse capillaries and unsuitable for use as intravenous therapeutics.[15] We consequently developed an approach for the synthesis of 50 – 100 nm diameter particles (Figure 1L, Table 1).[5a,15]
Figure 1.
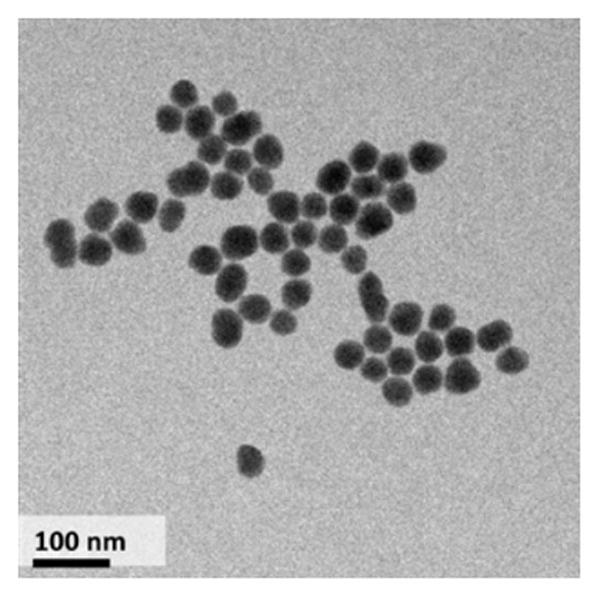
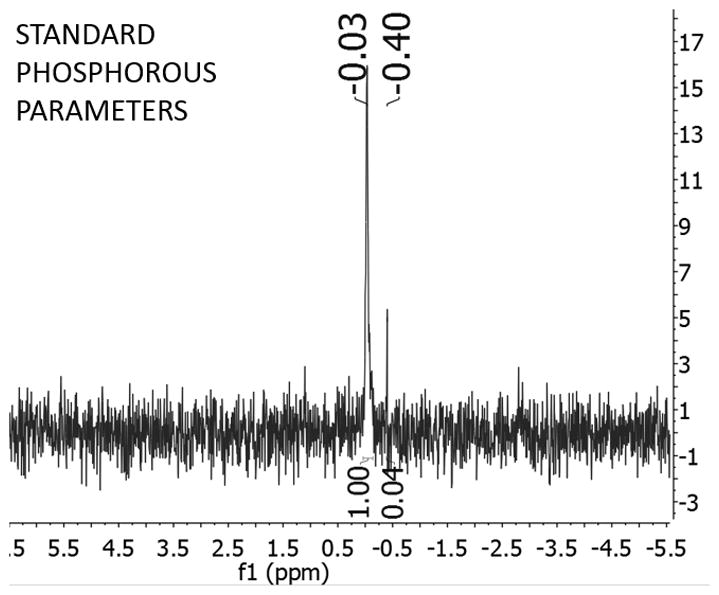
(Left) TEM images of polyP-SNPs; (Right) 31P NMR spectrum of digested polyP-SNPs shows evidence of phosphorous
Table 1.
The zeta potential and surface charge of the particles change when dispersed in water or phosphate buffered saline solution, pH 7.4.
| Compound, Medium[a] | Zeta Potential (μV) | Size (nm) |
|---|---|---|
| SNP, H2O | −24.4 ± 0.3 | 55.97 ± 2.19 |
| SNP, PBS | −52.5 ± 2.1 | 74.00 ± 6.24 |
| PolyP-SNP, H2O | −44.3 ± 0.3 | 53.79 ± 1.75 |
| PolyP-SNP, PBS | −52.0 ± 3.2 | 78.18 ± 2.86 |
In order to attach the highly anionic polyP to an oxide, we followed the model of Lorenz et al. who used zirconia, which like silica has a negative surface potential, as the scaffold for applications in protein separation and purification.[16] This attachment strategy exploits the Lewis acid properties of an oxide surface to bind polyP, overcoming the electrostatic repulsion.[17]
31P NMR and surface charge change qualitatively demonstrated polyP attachment. PolyP-SNPs were digested in acid to break down polyP into phosphate monomers. 31P NMR tests on the digested sample detected the presence of phosphorous. The change in surface charge also suggested the presence of polyP in undigested polyP-SNP (Table 1, SI Figure 2). At physiological pH in phosphate buffered saline solution (PBS), both SNPs and polyP display a negative surface charge. In deionized water, the SNP surface charge ranged from −15 to −25 mV (Table 1, SI Figure 2R). In simulated body fluid (SBF) at physiological pH, SNPs had a surface charge of −50 to −60 mV. PolyP is negatively charged at physiologic pH due to a pKa1 between pH 1–2 (for all internal phosphates) and pKa2 between pH 7.2 and 8.2 (for the two terminal phosphates).[18] Upon functionalization of polyP to the SNP surface, the zeta potential of the polyP-SNP decreased from −20 to −30 mV to roughly −40 to −50 mV in water, confirming the attachment of the polyP. In SBF, both particles exhibited a strongly negative charge below −45 mV.
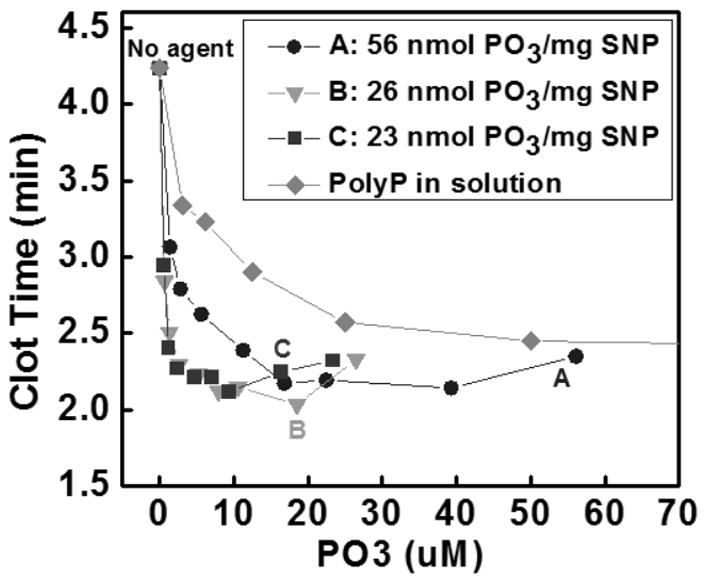
In order to quantify the polyP loaded on the SNP surface, the digested phosphate solutions were measured using both a malachite green assay and inductively coupled plasma atomic emission spectroscopy (ICP-AES). Malachite green identified concentrations of 56, 26, and 23 nmol PO3/mg SNP. ICP-AES determined the 26-nmol PO3 sample had 29.6 nmol PO3/mg SNP. Assuming each polyP chain has 70 PO3 monomers, these data suggest 100 – 200 polyP molecules are attached to each SNP.
By not using tissue factor to initiate clotting, we focused our clotting assays on the intrinsic (common) and the contact activation pathways. Negatively charged particles, such as the aluminosilicate kaolin (QuikClot® Combat Gauze™) used for external injuries, activate the contact pathway,.[4,5a] Because it is a poor clot initiator, polyP attachment shields SNPs in the systemic circulation, which is beneficial for use to control haemorrhage intravenously. We measured the impact of both bare SNPs and polyP-SNPs on clot time using thromboelastography (TEG, Figure 2T, SI Figures 4, 5) on pooled normal plasma (PNP). Both particles decreased the time to initial clot formation (R) in a concentration-dependent manner, with polyP-SNP being more potent at concentrations below 0.5 mg/mL. The clot time reported in Figures 2 and 4 refers to the time to initial clot formation, which is key in illustrating how polyP-SNP successfully accelerates clotting. The agent used did not affect the overall clot size formed, only the time required to reach peak clot size. However, polyP has previously been shown to improve overall clot formation, in part by limiting the effect of fibrinolysis in plasma containing tissue plasminogen activator.[11–12]
Figure 2.
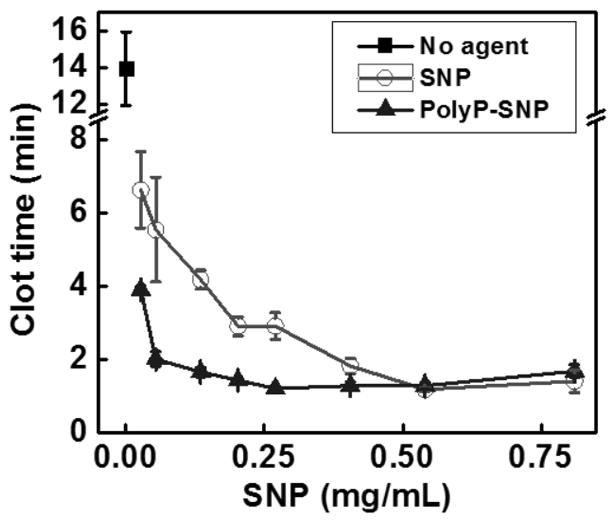
(Top) PolyP-SNP cuts clot time (R value using TEG) roughly in half versus SNP below 0.3 mg/ml. (Bottom) PolyP loaded onto silica lowers clot time compared to bare polyP when measured by fibrometry.
Figure 4.
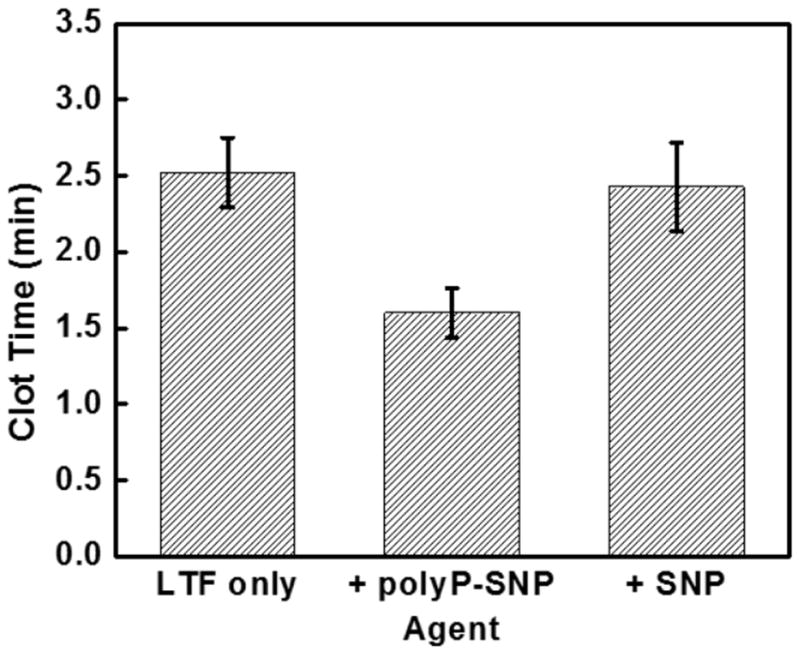
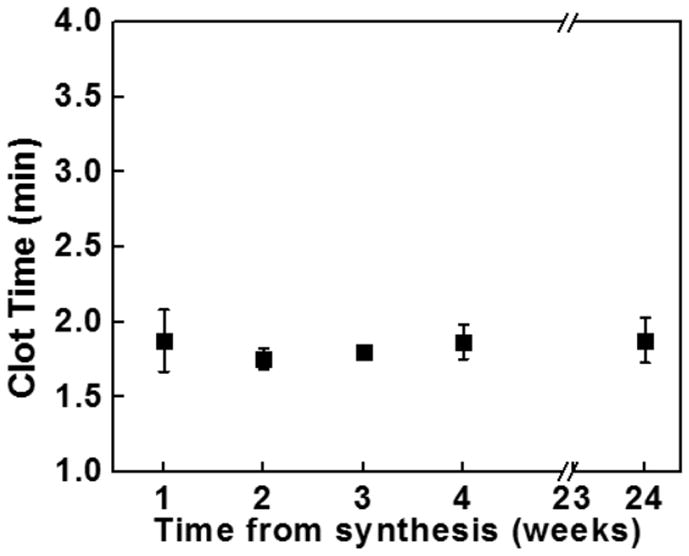
(Top) Adding PolyP-SNP to LTF shortens clot time in FXII-deficient plasma compared to LTF only or LTF + SNP. LTF required to initiate clotting. (Bottom) PolyP-SNP suspended in aqueous solution retains its procoagulant function after weeks of storage at ambient conditions.
Next we evaluated the procoagulant activity of polyP-SNPs formed with differing loads of polyP. The ability to promote coagulation was measured by adding polyP-SNP or polyP in solution to PNP. Coagulation was evaluated by measuring time to clot formation on a fibrometer (Figure 2B). In comparing polyP payload, polyP-SNPs were more potent at activating the contact pathway than polyP in solution.
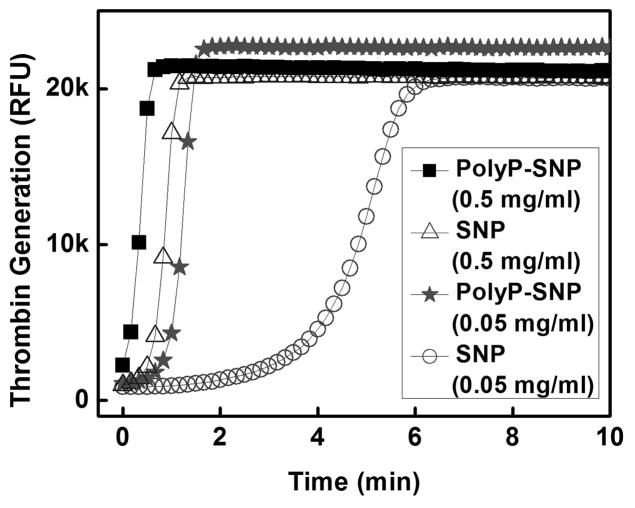
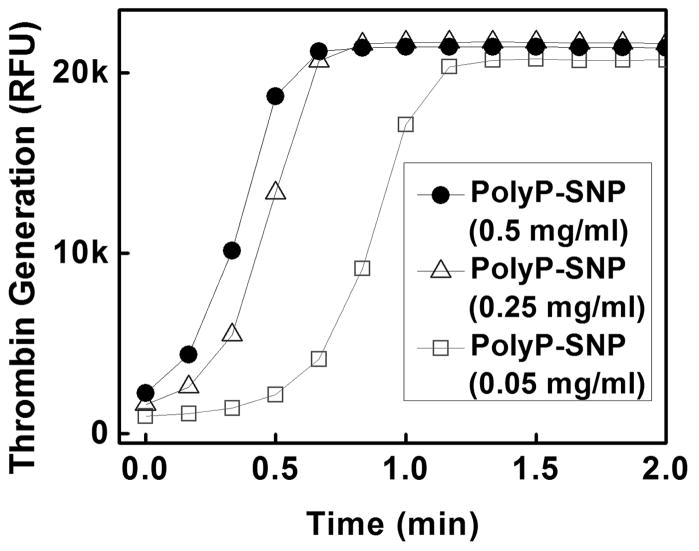
In order to further explore the relative activities of our materials, we evaluated the ability of the materials to generate thrombin, the terminal enzyme of the coagulation cascade and the primary determinant of the rate of fibrin formation.[9a] PolyP-SNP again substantially outperformed its bare counterpart (Figure 3T).
Figure 3.
(Top) Thrombin generation is more rapid with polyP-SNP than bare SNP. (Bottom) polyP-SNPs are able to generate a rapid thrombin burst even at low concentrations. Thrombin generation is measured using a thrombin-sensitive fluorescent dye.
We next evaluated whether polyP-SNPs were able to enhance the generation of downstream coagulation enzymes (common pathway). We eliminated any potential impact on contact activation by utilizing factor XII-deficient plasma and initiating coagulation using a small amount of relipidated tissue factor (LTF, 63 pM). As expected, bare SNPs did not affect TEG clot time in this system (Figure 4T). In contrast, polyP-SNP did shorten the time to physical clot formation. This indicates that the polyP is accessible for binding to the relevant downstream coagulation proteins. Additionally, this response could also be evaluated in the clinical setting by comparing tests such as prothrombin time (PT) and partial thromboplastin time (PTT).
One of the problems facing emergency medical personnel is that current intravenous treatments have a significantly short half-life at ambient temperature. Even pure polyP nanoparticles remain stable for hours.[9b] In comparison, attaching polyP to silica greatly enhanced the stability and procoagulant function of polyP from hours to weeks. After bench-top storage at room temperature in both powder and aqueous suspensions, polyP-SNP clotting times remained constant for weeks (Figure 4B). The strong negative surface charge of polyP-SNPs also minimized aggregation in aqueous suspensions over the same time period. Injectable drugs with long-term shelf-life can be used by emergency medical personnel prior to hospital arrival without concern that the particles will degrade without refrigeration. This suggests that the polyP-SNP system may be the first prehospital intravenous injection designed to treat internal injuries by accelerating the clotting system at bleeding sites.
In this study, we successfully attached polyP to the surface of small-diameter SNPs and demonstrated that these polyP-SNPs are more potent than bare SNPs at promoting coagulation, likely due to polyP’s ability to accelerate the common pathway for active clotting processes. PolyP-SNPs, like polyP in solution, are able to enhance downstream coagulation reactions resulting in a shorter time to clot formation. Even after long-term storage at regular room temperature, the polyP-SNP system retained its procoagulant ability. The polyP-SNP construct is consequently promising as a prohemostatic agent. Further exploration of methods to limit contact activation in vivo will be necessary for use as a systemic agent.
Supplementary Material
Footnotes
This work was funded by the U.S. Army Medical Research and Material Command under Contract Number WQ81XWH-11-2-0021, by the U. S. Army Medical Research & Materiel Command and the Telemedicine & Advanced Technology Research Center under Contract Number W911NF-10-2-0114, and by NIH grant R01 HL047014. The MRL Shared Experimental Facilities are supported by the MRSEC Program of the NSF under Award No. DMR 1121053; a member of the NSF-funded Materials Research Facilities Network. A.M.-M. thanks the Spanish MINECO for financial support within the framework of the project number CTQ2011-29336-C03-02.
Supporting information (SI) for this article: experimental details describing the synthesis and characterization of all new compounds as well as clotting assays conducted.
Contributor Information
Damien Kudela, Email: dkudela@chem.ucsb.edu, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106, USA.
Stephanie A. Smith, Department of Biochemistry, College of Medicine, University of Illinois at Urbana-Champaign, Urbana, IL, 61801 USA
Anna May-Masnou, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106, USA. Departament d’Enginyeria Química, Universitat de Barcelona, c/Martí i Franquès, 1-11, 08028, Barcelona, Catalunya, Spain.
Gary B. Braun, Sandford-Burnham Medical Research Institute, 10901 North Torrey Pines Road, La Jolla, CA 92037, USA
Alessia Pallaoro, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106, USA.
Chi K. Nguyen, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106, USA
Tracy T. Chuong, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106, USA
Sara Nownes, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106, USA.
Riley Allen, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106, USA.
Nicholas R. Parker, Department of Mechanical Engineering, University of California, Santa Barbara, Santa Barbara, CA 93106, USA
Prof. Hooman H. Rashidi, Department of Pathology & Laboratory Medicine, University of California, Davis, Sacramento, CA 95817, USA
Prof. James H. Morrissey, Email: jhmorris@illinois.edu, Department of Biochemistry, College of Medicine, University of Illinois at Urbana-Champaign, Urbana, IL, 61801 USA
Prof. Galen D. Stucky, Email: stucky@chem.ucsb.edu, Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, CA 93106, USA. Materials Department, University of California, Santa Barbara, Santa Barbara, CA 93106, USA
References
- 1.a) Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, Butler FK. Ann Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Bellamy RF. Mil Med. 1984;149:55–62. [PubMed] [Google Scholar]; c) Champion HR, Bellamy RF, Roberts CP, Leppaniemi AJ. Trauma. 2003;54:S13–S19. doi: 10.1097/01.TA.0000057151.02906.27. [DOI] [PubMed] [Google Scholar]
- 2.Pfeifer R, Tarkin IS, Rocos B, Pape H-C. Injury. 2009;40:907–911. doi: 10.1016/j.injury.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Johansson PI, Stensballe J. Transfusion. 2010;50:701–710. doi: 10.1111/j.1537-2995.2009.02458.x. [DOI] [PubMed] [Google Scholar]
- 4.Kheirabadi BS, Terrazas IB, Hanson MA, Kragh JF, Jr, Dubick MA, Blackbourne LH. J Trauma Acute Care Surg. 2013;74:1260–1265. doi: 10.1097/TA.0b013e31828cc983. [DOI] [PubMed] [Google Scholar]
- 5.a) Baker SE, Sawvel AM, Zheng N, Stucky GD. Chem Mater. 2007;19:4390–4392. [Google Scholar]; b) Johnson D, Gegel B, Burgert J, Gasko J, Cromwell C, Jaskowska M, Steward R, Taylor A. ISRN Emergency Medicine. 2012;2012:927678. [Google Scholar]
- 6.a) Kozen BG, Kircher SJ, Henao J, Godinez FS, Johnson AS. Acad Emerg Med. 2008;15:74–81. doi: 10.1111/j.1553-2712.2007.00009.x. [DOI] [PubMed] [Google Scholar]; b) Li Y, Sawvel AM, Jun Y-S, Nownes S, Ni M, Kudela D, Stucky GD, Zink D. Toxicol Res. 2013;2:136–144. [Google Scholar]; c) Bowman PD, Wang X, Meledeo MA, Dubick MA, Kheirabadi BS. J Trauma. 2011;71:727–732. doi: 10.1097/TA.0b013e3182033579. [DOI] [PubMed] [Google Scholar]
- 7.Barletta JF, Ahrens CL, Tyburski JG, Wilson RF. J Trauma. 2005;58:646–651. doi: 10.1097/01.ta.0000154561.97961.ad. [DOI] [PubMed] [Google Scholar]
- 8.Mannucci PM, Mancuso ME, Santagostino E. Blood. 2012;119:4108–4114. doi: 10.1182/blood-2012-01-394411. [DOI] [PubMed] [Google Scholar]
- 9.a) Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Rienstra CM, Morrissey JH. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Donovan AJ, Kalkowski J, Smith SA, Morrissey JH, Liu Y. Biomacromolecules. 2014;15:3976–3984. doi: 10.1021/bm501046t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SA, Morrissey JH. J Thromb Haemost. 2008;6:1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SA, Morrissey JH. Blood. 2008;112:2810–2016. doi: 10.1182/blood-2008-03-145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Proc Natl Acad Sci USA. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Cauda V, Engelke H, Sauer A, Arcizet D, Brauchle C, Radler J, Bein T. Nano Lett. 2010;10:2484–2492. doi: 10.1021/nl100991w. [DOI] [PubMed] [Google Scholar]; b) Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ. Acc Chem Res. 2013;46:792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis J. J Exp Biol. 1961;39:249–258. doi: 10.1038/icb.1961.25. [DOI] [PubMed] [Google Scholar]
- 15.Singh N, Karambelkar A, Gu L, Lin K, Miller JS, Chen CS, Sailor MJ, Bhatia SN. J Am Chem Soc. 2011;133:19582–19585. doi: 10.1021/ja206998x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz B, Marmé S, Müller WEG, Unger K, Schröder HC. Anal Biochem. 1994;216:118–126. doi: 10.1006/abio.1994.1015. [DOI] [PubMed] [Google Scholar]
- 17.Wei W-CJ, Wang S-C, Ho F-Y. J Am Ceram Soc. 1999;82:3385–3392. [Google Scholar]
- 18.Lee A, Whitesides GM. Anal Chem. 2010;82:6838–6846. doi: 10.1021/ac1008018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.a) Myles T, Yun TH, Hall SW, Leung LLK. J Biol Chem. 2001;276:25143–25149. doi: 10.1074/jbc.M011324200. [DOI] [PubMed] [Google Scholar]; b) Xiao Y, Lubin AA, Heeger AJ, Plaxco KW. Angew Chem Int Ed. 2005;44:5456–5459. doi: 10.1002/anie.200500989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.