Abstract
Pseudomonas syringae pv. syringae B728a, causal agent of brown spot on bean, is an economically important plant pathogen that utilizes extracellular signaling to initiate a lifestyle change from an epiphyte to a pathogen. LuxR regulatory proteins play an important role in the transcriptional regulation of a variety of biological processes involving two-component signaling, quorum sensing, and secondary metabolism. Analysis of the B728a genome identified 24 LuxR-like proteins, three of which are encoded by salA, syrF, and syrG located adjacent to the syringomycin gene cluster. The LuxR-like proteins encoded by these three genes exhibit a domain architecture that places them in a subfamily of LuxR-like proteins associated with regulation of secondary metabolism in B728a. Deletion mutants of salA, syrF, and syrG failed to produce syringomycin and displayed reduction of virulence on bean. The transcriptional start sites of salA, syrG, and syrF were located 63, 235, and 498 bp upstream of the start codons, respectively, using primer extension analysis. The predicted -10/-35 promoter regions of syrF and syrG were confirmed using site-directed mutagenesis and GFP reporters that showed conserved promoter sequences around the -35 promoter region. Overexpression analysis and GFP reporters identified SyrG as an upstream transcriptional activator of syrF, where both SyrG and SyrF activate promoters of syringomycin biosynthesis genes. This study shows that syrG and syrF encode important transcriptional regulators of syringomycin biosynthesis genes.
Introduction
P. syringae pv. syringae (Pss) B728a [1, 2] is an aggressive plant pathogen of bean that causes brown spot. Pss B728a is highly adapted to its host where it has the ability to function as an epiphyte on leaf surfaces before invading apoplastic tissues as a plant pathogen. The bacterium’s pronounced epiphytic phase produces large bacterial populations residing on the surfaces of bean leaves, where it persists until it utilizes extracellular signaling to initiate a lifestyle change from an epiphyte to a pathogen [3]. Epiphytic populations provide a source of inoculum that is used to colonize the apoplast under appropriate conditions and multiply by using nutrients available in living host cells [4]. During apoplastic colonization, Pss B728a extensively expresses genes associated with pathogenicity and virulence that include type III secretion systems, exopolysaccharides, siderophores, an ice nucleation protein, cell wall-degrading enzymes, and phytotoxins [5]. The molecular basis for the switch from an epiphyte to pathogen is complex requiring the intricate interaction and regulation of multiple virulence factors, which makes Pss B728a an important model in the study of molecular plant pathogenesis [1, 2]. The lipopetide phytotoxins, syringomycin and syringopeptin, are considered important virulence factors that contribute to the disease development of brown spot on bean. The phytotoxins function by inserting into the cell membrane of the host to form small pores that result in electrolyte leakage and cell death [6–8]. Genes responsible for the biosynthesis of syringomycin and syringopeptin are encoded on two adjacent gene clusters referred to as the syr-syp gene cluster [6, 7, 9]. The syr-syp gene cluster found in Pss B728a displays similar size and gene organization as Pss B301D. Adjacent to the syr-syp gene cluster are three transcriptional regulatory genes, salA, syrF, and syrG that were identified as encoding LuxR-like proteins [10]. These LuxR-like proteins have been implicated in virulence and syringomycin regulation [10].
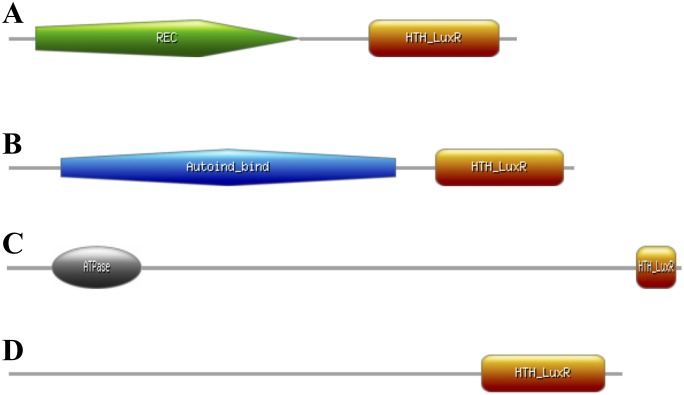
LuxR proteins are a family of prokaryotic transcriptional regulators that are defined by having a helix-turn-helix (HTH) DNA binding motif on the C-terminus region of the protein and an N-terminus response regulatory domain [11–13]. The LuxR superfamily can be grouped into four subfamilies based on domain architecture and the mechanism of regulatory activation, illustrated in Fig 1 [12]. The first subfamily consists of regulators that are part of a two-component sensory transduction system that are activated by the phosphorylation of an aspartate residue on the N-terminal region of the protein, typically by a transmembrane kinase. An example of this subfamily of LuxR is NarL [14, 15], which activates the nitrate reductase operon in E. coli. NarL is comprised of two domains, an N-terminal receiver domain that is controlled by phosphorylation and a C-terminal effector domain that elicits a physiological response. Phosphorylation occurs at the N-terminal domain to form dimers that recognize heptamer sequences in the promoter regions of gene targets [12, 14, 15]. Regulators activated by N-acyl homoserine lactone comprise the second subfamily of LuxR proteins, which includes LuxR [12, 16], TraR [12, 17], CarR [12, 18], ExpR [19], LasR [12, 20], PhzR [21], and RhlR [22]. LuxR is involved in the activation of bioluminescence related genes and is essential for quorum sensing in Vibrio fischeri [23]. These regulators have a C-terminal HTH DNA binding domain and an N-terminal autoinducer-binding domain that interacts with acyl-homoserine lactone, which is a signaling molecule involved in quorum sensing. The third subfamily of regulators is referred to as large ATP-binding regulators of the LuxR family (LAL) [24–26]. Experimentally characterized LALs include GdmRI [25], GdmRII [25], MalT [24], and PikD [26]. The most studied LAL is MalT, which is the transcriptional activator of the maltose regulon in E. coli but requires two co-factors for activation [24]. This subfamily of LuxR proteins is significantly different because they are relatively large in size (800 to 1,200 amino acids), contain an N-terminal ATP-binding motif, and contain a C-terminal HTH DNA binding domain [24–26]. These LuxR proteins require the binding of ATP to the N-terminal region for activation. The fourth subfamily of regulators represents the simplest form of the LuxR superfamily because they harbor the typical C-terminal HTH DNA binding domain but lack an N-terminal regulatory domain. GerE was one of the first transcriptional regulators placed into this group of LuxRs [27]. This regulator was involved in the transcriptional regulation of genes associated with spore formation and maturation in Bacillus subtilis [27].
Fig 1. Domain organization of LuxR proteins that are classified into four sub-families based on domain architecture and mechanism of regulatory activation.
A. GacA is a LuxR-like protein in Pss B728a part of a global signal transduction system characterized as having an N-terminal receiver domain activated by phosphorylated and an C-terminal HTH DNA-binding domain that is characteristic of the first sub-family of LuxR-like proteins. B. AhlR is part of quorum sensing system in Pss B728a with AhlI. It has an N-terminal auto-inducer binding domain where hexanoyl-homoserine lactone binds to activate transcription of ahlI and has a C-terminal HTH DNA-binding domain. This domain organization is typical of the second sub-family of LuxRs associated with quorum sensing. C. Psyr_0993, which has not been characterized in Pss B728a, shares homology to malT in E. coli. These genes encode a subfamily of LuxR-like proteins have an N-terminal AAA ATPase domain that requires ATP for transcriptional activation and has a C-terminal HTH DNA binding domain. D. SyrG, which has been implicated in virulence and syringomycin production in Pss B728a lacks any defined N-terminal regulatory domain and has a C-terminal HTH DNA binding domain. This domain organization is typically seen in the fourth subfamily of LuxR-like proteins, which have not been fully defined functionally. LuxR-like proteins characterized in this family of LuxRs have been associated with secondary metabolism in Pss B728a.
Bioinformatic investigation of the 6.09-Mb genome of Pss B728a revealed 24 genes encoding LuxR-like proteins dispersed throughout the genome, homologs of these LuxR-like proteins were also found in the genome of Pss B301D. The genes gacA (Psyr_2897), Psyr_1294, Psyr_1384, Psyr_1940, Psyr_2114, Psyr_3299, Psyr_3890, Psyr_4376, Psyr_4618, and Psyr_5088 encode proteins that were identified as belonging to a subfamily of LuxRs that are typically part of a two-component sensory transduction system. This subfamily is one of the largest groups of LuxR proteins found in Pss B728a. The LuxR-like proteins encoded on ahlR (Psyr_1622), Psyr_1858, and Psyr_4216 are classified as belonging to the second subfamily of LuxR proteins, associated with quorum sensing. The third and smallest group of LuxRs found in Pss B728a belongs to the third subfamily of LuxR proteins, referred to as LAL, which includes only one LuxR protein encoded on Psyr_0993. Psyr_0993 has not been functionally defined in Pss B728a but does encode a protein that exhibits domain architecture typical of this subfamily of LuxR proteins. The proteins that are encoded by salA (Psyr_2601), sylA (Psyr_1702), syrG (Psyr_2602), syrF (Psyr_2607), syrR (Psyr_2575), Psyr_2045, Psyr_2578, Psyr_3767, Psyr_4266, and Psyr_4278 exhibit domain architecture that is typical of the fourth subfamily of LuxR proteins, which is the second largest group of LuxRs found in the Pss B728a genome. These transcriptional regulators seemingly play a key role in the regulation of genes associated with secondary metabolism, pathogenicity, and virulence of Pss B728a.
The LuxR-like proteins SalA, SyrF, and SyrG are part of the fourth subfamily of LuxR proteins, which are not completely defined [10, 12]. SalA is part of a complex regulatory network that is involved in the biosynthesis, secretion, and regulation of syringomycin, syringopeptin, and syringolin [28]. All genes identified to be part of the SalA regulon are absent from the genome of Pst DC3000. This transcriptional regulator is under the control of the GacS/GacA global signal transduction system, which controls expression of genes essential for plant pathogenesis [29]. Also, it has been demonstrated that SalA is required for the functional activation of both syrG and syrF [10]. Both salA and syrF genes are necessary for the biosynthesis of syringomycin and syringopeptin, which led to the conclusion that the regulatory networks involving syringomycin and syringopeptin overlap, but are not identical [10]. Meanwhile, SalA mediates the regulation of syringomycin and syringopeptin through the regulation of syrF [12]. Protein sequence analysis also revealed that both SyrF and SyrG have somewhat similar protein sequences with 49% identity [10]. The sequence similarity is significant given that SalA only has 27% and 26% identity to the protein sequence of SyrF and SyrG. The similarity of SyrF and SyrG may indicate similar regulatory gene targets. Previous research established that both salA and syrF genes are required for syringomycin production, where syrG gene expression is highly induced in the apoplast and is associated with virulence [5, 10]. It is surmised that SyrG plays a critical role in the regulation of genes associated with pathogenesis given that mutants of syrG displayed a significant reduction in virulence [10]. It appears that the regulatory role of SyrG in virulence is complex and may involve molecular mechanisms that may reside outside the syr-syp gene cluster.
Despite previous evidence that salA, syrF, and syrG genes have an effect on virulence and syringomycin production [10], the regulatory role of SyrG in regards to syringomycin production, and the production of other secondary metabolites, remains unknown. In addition, the previous study performed by Lu et al. [10] utilized site directed insertional mutants of salA, syrF, and syrG in Pss B301D to determine their effect on virulence and syringomycin production. It is hypothesized that these insertional mutants still produce truncated proteins with reduced functional activity displaying low levels of toxin production instead of eliminating it completely. It was important to test this hypothesis by determining the effect salA, syrF, and syrG in Pss B728a has on virulence and syringomycin production with clean deletions mutants. Also, it is hypothesized that the LuxR-like protein SyrG is involved in the regulation of genes essential for the pathogenic lifestyle of Pss B728a while under transcriptional control of SalA. This hypothesis was tested utilizing phenotypic characterization and quantitative real-time PCR analysis in an effort to identify new components of the SyrG regulon. It was demonstrated in this study that the syrG gene has a stronger influence on virulence and phytotoxin production than previously reported [10]. It was illustrated that SyrG is required for virulence but is not required for the replication of Pss B728a in planta. The LuxR-like protein, SyrG, is not involved in the transcriptional regulation of known virulence genes associated with the biosynthesis of achromobactin, alginate, levansucrase, pyoverdine, syringolin, and syringafactin. However, both SyrG and SyrF are required for syringomycin production with SyrG being an important transcriptional regulator of genes associated with the biosynthesis of syringomycin in Pss B728a. It has been established that SalA controls the expression of both syrG and syrF [10], but it is unknown how SyrG affects the expression of syrF and vice versa. It is important to further define these transcriptional regulators in order to fully understand the complex nature of SalA, SyrG, and SyrF regulons in regards to virulence and the plant-pathogen interaction.
Materials and Methods
Bacterial strains, plasmids and media
Bacterial strains and plasmids used in this study are listed in Table 1. One Shot® TOP10 chemically competent E. coli cells were used for cloning reactions following manufacturer’s protocols (Invitrogen, Carlsbad, CA). Pss B728a and mutant strains were cultured from 20% glycerol stocks stored at -80°C onto nutrient broth-yeast extract (NBY) [30], or on King’s B (KB) [31] at 26°C. Bioassays for syringomycin were grown on Hrp minimal medium (HMM) agar [32, 33]. The following antibiotic concentractions (μg/ml) were added to media: rifampicin, 100; kanamycin, 75; tetracycline, 20; ampicillin, 100; gentamycin, 5; spectinomycin, 100.
Table 1. Strains and plasmids.
| Designation | Relevant characteristics | Source |
|---|---|---|
| Bacterial Strains | ||
| Escherichia coli | ||
| One Shot® TOP10 | F- mcrA Δ(mrr-hsdRMS-mcrBC)φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (StrR) endA1 nupG | Invitrogen |
| P. syringae pv. syringae | ||
| B728a | Wild-type, bean pathogen; Rifr | [1] |
| B728aΔsalA | salA mutant derivative of B728a, Rifr | [34, 35] |
| B728aΔsyrF | syrF mutant derivative of B728a, Rifr | This study |
| B728aΔsyrG | syrG mutant derivative of B728a, Rifr | This study |
| B728aΔsyrFΔsyrG | syrF and syrG mutant derivative of B728a, Rifr | This study |
| B728aΔgacS | gacS mutant derivative of B728a, Rifr | [36] |
| Plasmids | ||
| pE2602 | pENTR/D-TOPO 6.80 kb region carrying syrG, Kmr | This study |
| pE2607 | pENTR/D-TOPO 6.62-kb region carrying syrF, Kmr | This study |
| pKD13 | Template plasmid containing FRT-flanked nptII | [37] |
| pLVCD | Gateway destination vector for mating with P. syringae; pBR322 derivative with mob genes from RSF1010; Tcr Apr Cmr | [38] |
| pLV2602 | pLVCD carrying syrG; Tcr Apr | This study |
| pLV2607 | pLVCD carrying syrF; Tcr Apr | This study |
| pLV2602-FP | pLVCD carrying upstream and downstream regions of syrG fused to nptII; Tcr Apr Kmr | This study |
| pLV2607-FP | pLVCD carrying upstream and downstream regions of syrF fused to nptII; Tcr Apr Kmr | This study |
| pPROBE-KT’ | Promoter-probe vector with pVS1/p15a replicon and gfp reporter, Kmr | [39] |
| pPKT::syrG752 | pPROBE-KT’ carrying syrG along with 752-bp upstream; Kmr | This study |
| pPKT::syrG552 | pPROBE-KT’ carrying syrG along with 552-bp upstream; Kmr | This study |
| pPKT::syrG452 | pPROBE-KT’ carrying syrG along with 452-bp upstream; Kmr | This study |
| pPKT::syrG352 | pPROBE-KT’ carrying syrG along with 352-bp upstream; Kmr | This study |
| pPKT::syrG352-10 | pPROBE-KT’ carrying syrG along with 352-bp upstream with the potential -10 region replaced with CTGCAG; Kmr | This study |
| pPKT::syrG352-35 | pPROBE-KT’ carrying syrG along with 352-bpupstream with the potential -35 region replaced with CTGCAG; Kmr | This study |
| pPKT::syrG252 | pPROBE-KT’ carrying syrG along with 252-bp upstream; Kmr | This study |
| pPKT::syrG202 | pPROBE-KT’ carrying syrG along with 202-bp upstream; Kmr | This study |
| pPKT::syrG102 | pPROBE-KT’ carrying syrG along with 102-bp upstream; Kmr | This study |
| pPKT::syrG52 | pPROBE-KT’ carrying syrG along with 52-bp upstream, Kmr | This study |
| pPKT::syrF | pPROBE-KT’ carrying syrF along with 1.3-kb upstream; Kmr | This study |
| pPKT::syrF1000 | pPROBE-KT’ carrying syrF along with 1.0-kb upstream; Kmr | This study |
| pPKT::syrF800 | pPROBE-KT’ carrying syrF along with 800-bp upstream; Kmr | This study |
| pPKT::syrF600 | pPROBE-KT’ carrying syrF along with 600-bp upstream; Kmr | This study |
| pPKT::syrF600-10 | pPROBE-KT’ carrying syrF along with 600-bp upstream with the potential -10 region replaced with CTGCAG; Kmr | This study |
| pPKT::syrF600-35 | pPROBE-KT’ carrying syrF along with 600-bp upstream with the potential -35 region replaced with CTGCAG; Kmr | This study |
| pPKT::syrF500 | pPROBE-KT’ carrying syrF along with 500-bp upstream; Kmr | This study |
| pPKT::syrF252 | pPROBE-KT’ carrying syrF along with 252-bp upstream; Kmr | This study |
| pPKT::syrF202 | pPROBE-KT’ carrying syrF along with 202-bp upstream; Kmr | This study |
| pPKT::syrF152 | pPROBE-KT’ carrying syrF along with 152-bp upstream; Km | This study |
| pPKT::syrF102 | pPROBE-KT’ carrying syrF along with 102-bp upstream; Kmr | This study |
| pPKT::syrF52 | pPROBE-KT’ carrying syrF along with 52-bp upstream; Kmr | This study |
| pMEKm12 | E. coli and P. syringae pv. syringae overexpression vector, Kmr | [40] |
| pMK::syrG | pMEKm12 carrying the syrG gene in-frame fused to malE; Kmr | This study |
| pMK::syrG583 | pMEKm12 carrying 583-bp of the syrG N-terminal region fused to malE; Kmr | This study |
| pMK::syrF | pMEKm12 carrying the syrF gene in-frame fused to malE; Kmr | This study |
| pMK::syrF583 | pMEKm12 carrying 583-bp of the syrF N-terminal region fused to malE; Kmr | This study |
| pRK2073 | Helper plasmid; Spr Trmr | [41] |
General DNA manipulations
For methodologies that involve the use of Gateway cloning technology ([42] and regulatory aspects of lambda site specific recombination), targeted genes were PCR amplified and cloned into the pENTR/D-TOPO vector following the manufacturer’s protocols (Invitrogen). Recombination between pENTR constructs and Gateway destination vectors was performed employing the use of LR clonase in accordance with the manufacturer’s protocol (Invitrogen). Plasmids were introduced into E. coli by chemical transformation or electroporation [43]. Plasmids were incorporated into P. syringae by tri-parental mating utilizing the helper plasmid pRK2073 [41]. Complementation of Pss B728a derivative mutants was achieved by the electroporation of the complement construct. Standard PCR procedures and cycling conditions were used [32, 36].
Restriction enzymes, and T4 DNA ligase were purchased from New England Biolabs (Beverly, MA). Phusion High-Fidelity DNA polymerase was purchased from Thermo Scientific Inc. (Waltham, MA). In-Fusion® HD cloning kit was purchased from Clontech Laboratories (Mountain View, CA). The design and purchase of oligonucleotides was acquired using PrimerQuest and OligoAnalyzer applications of Integrated DNA technologies (Coralville, IA). The oligonucleotide sequences are listed in S1 Table. Plasmids were introduced into E. coli by chemical transformation or electroporation [43]. Plasmids were transferred to P. syringae by triparental mating using the helper plasmid pRK2073 [41]. Standard PCR procedures and cycling conditions were used.
Bioinformatic analysis
Protein sequences were retrieved using the Pseudomonas Genome Database [44]. The Conserved Domain Database at NCBI (http://www.ncbi.nlm.nih.gov/Structure/cdd/) was used to identify conserved domains of protein sequences. Additionally, database searches were performed using a Basic Local Alignment Search Tool (BLAST) to identify homologous sequences SyrG and SyrF in pseudomonad genomes. A clustalW alignment of homologous protein sequences was generated using the CLC Genomics Workbench (V5.5, CLC Bio.) [32].
Construction of markerless deletion mutants in Pss B728a
For targeted deletion mutants in Pss B728a, the gene of interest (GOI) along with 3 to 4-kb of flanking DNA was PCR amplified using Phusion® high fidelity polymerase (ThermoScientific). The purified PCR product was cloned into a Gateway entry vector pENTR/D-TOPO (Invitrogen) and transformed chemically into E. coli One Shot® TOP10 cells. LR clonase II (Invitrogen) was used to carry out recombination between the pENTR construct and the Pseudomonas suicide vector, pLVC-D [38].
Site-directed mutagenesis occurred by linearization of the pLVC-D plasmid (pLVC-D:flank-GOI-flank) using inverse PCR with primers that exclude the GOI, and purified using a Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, WI). A linear kanamycin cassette (nptII) flanked by the FLP recognition target sites, was amplified from pKD13 plasmid using primers with 15 bp extensions that were homologous to regions adjacent to the GOI [37]. The kanamycin cassette was cloned into the purified linearized pLVC-D construct using the In-Fusion® HD cloning kit (Clontech) according to the manufacturer’s protocol and chemically transformed into E. coli One Shot® TOP10 cells for confirmation of the construct. The resulting pLVC-D construct (pLVC-D:flank-nptII-flank) was moved into Pss B728a by triparental mating with the helper plasmid pRK2073 [41]. Colony PCR and qRT-PCR was used to confirm double recombination of the kanamycin cassette into Pss B728a, replacing the GOI. The kanamycin marker was later removed by the introduction of the pBH474 vector carrying the FLP recombinase gene. FLP recombination resulted in the loss of the nptII marker, giving a markerless deletion mutant in Pss B728a. The Sucs pBH474 plasmid was cured from the B728a deletion mutant cells by culturing in NBY + 5% sucrose liquid medium.
Construction of complementing and overexpressing plasmids
For the complementation of B728a derivative mutants a copy of the targeted gene and the predicted promoter region was PCR amplified from Pss B728a with a BamHI and SacI restriction enzyme sited on each end of the PCR product using primers listed in S1 Table. The PCR product was digested with BamHI and SacI. Additionally, the broad-host-range promoter-probe vector, pPROBE-KT’ was digested with BamHI and SacI [39]. Digested PCR products and vector were purified using Wizard® SV Gel and PCR Clean-Up System (Promega). Purified digested products were quantified using micro-spectrophotometry (Nano-Drop Technologies, Inc.). Ligation of the vector and insert was performed using T4 DNA ligase (New England Biolabs) and chemically transformed into E. coli One Shot® TOP10 cells (Invitrogen) for confirmation of construct. Both constructs, pPROBE-KT’:syrF and pPROBE-KT’:syrG, were introduced into B728a derivative mutants by electroporation.
The overexpression of SyrF, SyrG, and their respective truncated proteins missing portions of the C-terminal region were cloned into the expression vector, pMEKm12 [40]. A total of two overexpression constructs was generated for each gene of interest. For syrF, the targeted gene, and 583-bp of the N-terminal sequence was PCR amplified from Pss B728a with BamHI and XbaI restriction enzyme sites flanking the PCR products. For syrG, the targeted gene, and 583-bp of the N-terminal sequence was PCR amplified from Pss B728a with BamHI and XbaI restriction enzyme sites flanking the PCR products. The PCR products and the expression vector, pMEKm12, were digested with BamHI and XbaI. Digested PCR products and vector were purified using Wizard® SV Gel and PCR Clean-Up System (Promega). Purified digested products were quantified using micro-spectrophotometry (Nano-Drop Technologies, Inc.). Ligation of the vector and insert was performed using T4 DNA ligase (New England Biolabs) and chemically transformed into E. coli One Shot® TOP10 cells (Invitrogen) for confirmation of construct. Overexpression constructs were introduced into Pss B728a by electroporation.
Pathogenicity assays
The ability of derivative mutants (B728aΔsalA, B728aΔsyrF, B728aΔsyrG, and B728aΔsyrFΔsyrG) to cause disease and multiply in planta was evaluated by vacuum infiltration on 2-week old Blue Lake 274 (Burpee Seeds, Warminster, PA) bean plants (Phaseolus vulgaris L.) and 4-week old Nicotiana benthamiana. The method for vacuum infiltration was described previously [32, 36]. Pss B728a was used as a positive control and Pss B728aΔgacS served as the negative control. Each strain was evaluated on at least three plants of each species, with triplicate biological replicates. The virulence of derivative mutants was evaluated by measurement of necrotic lesion surface area found on leaves 3 days post inoculation. Lesion surface areas were calculated using ImageJ software (version 1.49e; http://rsbweb.nih.gov/ij/). A total of three leaves were evaluated for each biological replicate.
To evaluate the ability of derivative mutants to replicate in planta, population analysis was performed for B728a, B728aΔsalA, B728aΔsyrF, B728aΔsyrG, B728aΔsyrFΔsyrG, and B728aΔgacS on Day 0 and Day 3 after vacuum infiltration of bean plants. From each infiltrated plant, a trifoliate leaf was detached and infiltrated tissue was removed using the bottom of a sterile 2 mL microcentrifuge tube (Bio Plas Inc., San Franscisco, CA). A total of 10 leaf discs were removed per leaf and rinsed with sterile deionized water. The leaf discs were ground using a sterile mortar and pestle with Silwet Phosphate Magnesium Buffer (SPM) [32]. Serial dilutions were prepared with SPM buffer and plated on KB agar with appropriate antibiotics followed by incubation at 26°C for 48 h. Colonies were counted and calculated as CFU per squared cm.
Syringomycin assays
The production of syringomycin by Pss B728a and derivative mutant strains were evaluated using a bioassay previously described [45] for syringomycin production on HMM agar. Bacterial strains were grown overnight in 2 ml NBY at 26°C with shaking at 180 rpm. Cells were washed and resuspended in sterile deionized water to OD600 = 0.3 (~2 x 108 CFU/ml), and 5 μl aliquots of bacterial suspension were spotted on HMM. After an incubation period of 3 days at 26°C the plates were lightly sprayed with a cell suspension of Geotrichum candidum strain F-260 using a sterile chromatography sprayer. After 24 h, quantification of syringomycin production was determined by measuring the diameter of inhibition zones and compared to the parental strain of Pss B728a. This experiment was repeated in triplicate.
RNA isolation
Pss B728a and derivative mutant strains were cultured overnight with shaking at 26°C in 5 ml of liquid NBY medium. Cells were harvested by centrifugation, washed and resuspended in sterile deionized water to a concentration of approximately 2 x 108 CFU per ml. Cell suspensions (100 μl) were spread onto HMM agar and incubated at 26°C for 48 h. Total RNA was purified using an RNeasy Mini Kit along with the RNAprotect reagent following the manufacturer’s protocol (Qiagen Inc., Valencia, CA). For studies that evaluated the influence of the apoplast on gene expression, bacterial strains were introduced into bean leaves by vacuum infiltration. Approximately, 48 hours post-inoculation, a total of 40–80 leaves were harvested individually and endophytic bacteria were extracted using an acidic ethanol/phenol solution (9 mL buffer-saturated phenol (pH 6.6), 171 mL absolute ethanol, 420 mL sterile distilled water). The leaves were individualy removed from the plants and cut into squares (~3 x 3 mm2) while submerged in the ethanol/phenol, and the plant tissue and liquid were sonicated for 10 min. The solution was filtered through sterile cheese cloth and centrifuge to remove bacterial cells at 5,500 x g for 10 min. The cell and plant debris pellet was resuspended in approximately 5 mL of the supernatant and filtered through a Luer-Lock syringe packed with sterile cheesecloth and fitted with a Millipore Millex 25 mm Durapore® PVDF 5 μm filter unit. Cells were pelleted, flash frozen and stored up to 2 weeks at -20C prior to RNA extraction. For these apoplastic conditions, two RNA samples were obtained each biological replicate, with each RNA sample including samples from 40–80 leaves. RNA samples were treated with TURBO™ DNase (Ambion, Austin, TX) to remove residual DNA. The RNA was tested for DNA contamination using RT-PCR where RNA is used as the template with no reverse transcription reaction. The RNA quality and quantification was evaluated utilizing an Agilent 2100 Bioanlyzer (Agilent Technologies, Inc.), selecting samples with RNA Integrity Number (RIN) above 8.0 [32].
For qRT-PCR analysis, selected RNA samples were converted to cDNA by reverse transcription using Super Script Vilo™ cDNA Synthesis kit (Invitrogen) as described by Greenwald et al. [32], and diluted to 10 ng/μl. Reverse transcription was performed with the following temperature cycle: 10 min at 25°C, 60 min at 42°C, and 5 min at 85°C.
qRT-PCR analysis
An Applied Biosystems 7500 Fast Real-Time PCR System was used in conjunction with SYBR® Select Master Mix (Invitrogen) for qRT-PCR analysis. For each 20 μl reaction the following was used: 10 μl SYBR® Select Master Mix, 8.20 μl nuclease-free water, 0.4 μl of both the forward and reverse primers (200 nM), and 1 μl of template cDNA (10 ng/μl). Primers used for qRT-PCR analysis as listed in S2 Table along with primers specific for recA and 16s-rRNA as internal control genes that were used to normalize gene expression [28]. For each primer pair, the linearity of detection was confirmed to have a correlation coefficient of at least 0.98 (r2>0.98) over the detection area by measuring a 5-fold dilution curve with cDNA generated from bacterial RNA. Conditions for qRT-PCR involved an incubation temperature of 95°C for two minutes, followed by 40 cycles involving 3 seconds at 95°C and 30 seconds at 60°C. A melting curve analysis was used for each qRT-PCR reaction to validate that a single primer product was amplified. qRT-PCR was performed to determine the effects of apoplastic and HMM conditions on the expression of genes that encode LuxR-like proteins in parental strain Pss B728a. The expression of genes associated with syringomycin biosynthesis, epiphytic fitness and secondary metabolism was also determined in parental strain Pss B728a compared to salA, syrF, and syrG deletion mutants in HMM media conditions.
Data was analyzed using the comparative Ct method [46], where an increase or decrease of transcript levels is determined by comparing the Ct values of the samples of interest to the Ct values of a control sample. Fold change in gene expression was calculated using the following equation: 2-ΔΔCt = (Ct gene-of-interest−Ct internal control) Treated sample–(Ct gene-of-interest−Ct internal control) Untreated sample [46]. A 2-fold or more change in Ct for the sample of interest when compared to the control sample was considered to be significant [36]. A decrease in fold change was computed by taking the negative inverse of the fold change value [46].
Operon analysis of salA, syrF, and syrG in Pss B728a using RT-PCR
RT-PCR analysis was performed to define the operons that encompass salA, syrF, and syrG using RNA isolated from Pss B728a. Primers designed for salA, syrF, syrG, and neighboring genes (S1 Table) were used to identify if they were transcribed as monocistronic or polycistronic mRNA. Total RNA was prepared from Pss B728a by growing cells on HMM agar for 48 h at 26°C and harvesting total RNA using an RNeasy Mini Kit along with the RNAprotect reagent following the manufacturer’s protocol (Qiagen Inc., Valencia, CA). RNA samples were treated with TURBO™ DNase (Ambion, Austin, TX) to remove residual DNA. The RNA was tested for DNA contamination using RT-PCR where RNA is used as the template with no reverse transcription reaction. The RNA quality and quantification was evaluated using an Agilent 2100 Bioanlyzer (Agilent Technologies, Inc.), and selecting samples with an RNA Integrity Number (RIN) above 8.0 [32].
Using approximately 100 ng of total RNA from Pss B728a, RT-PCR was performed using an Applied Biosystems 7500 Fast Real-Time PCR System with a OneStep RT-PCR kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions. Reverse transcription was performed by incubating at 50°C for 30 min. After reverse transcription, RT-PCR was carried out using the following temperature cycle: 95°C for 15 minutes, followed by 30 cycles involving 30 seconds at 94°C and 30 seconds at 55°C. After RT-PCR, amplified products were subjected to electrophoresis.
Primer extension analysis
Primer extension was performed using the Primer Extension System (Promega, Madison, WI), and a sequence marker that was created using the Sequenase Version 2.0 DNA sequencing kit following the manufacturer’s instructions (Affymetrix, Santa Clara, CA). Oligonucleotides salAPE, syrFPE, and syrGPE were radiolabeled with [γ-32P]ATP (Perkin Elmer, Inc., Boston, MA) at the 5’ end. Primer extension was performed with 1.0 pmol of the labeled primer and 15 μg of total RNA from Pss B728a. Total RNA from Pss B728a was prepared as described previously [32]. The plasmids pLV2602, and pLV2607 were used as templates to create sequencing ladders of the upstream regions of salA, syrF, and syrG.
Computer analysis
Nucleotide sequences that were 100-bp upstream of identified transcriptional start sites were analyzed using the Softberry Bprom algorithm (http://linux1.softberry.com/berry.phtml) to identify putative σ70-dependent promoters. These putative promoter sequences were aligned with T-Coffee [47].
Construction of GFP translational fusions and mutagenesis
To define and characterize the promoter regions of syrF and syrG, promoter fragments were PCR amplified from Pss B728a genomic DNA using primers listed in S1 Table and cloned into a gfp broad-host-range promoter-probe vector, pPROBE-KT’, resulting in translational fusions to gfp. BamHI and SacI restriction enzyme sites flanked all promoter fragments. Amplified promoter fragments were digested with BamHI and SacI along with the broad-host-range promoter-probe vector, pPROBE-KT’[39]. Digested PCR products and vector were purified using Wizard® SV Gel and PCR Clean-Up System (Promega) and quantified utilizing micro-spectrophotometry (Nano-Drop Technologies, Inc.). Ligation of the vector and insert was performed using T4 DNA ligase (New England Biolabs) and chemically transformed into E. coli One Shot® TOP10 cells (Invitrogen) for confirmation of constructs. Additionally, salA, syrB1, and syrP promoters were cloned in pPROBE-KT’. Cloned pPROBE-KT’ constructs were introduced into B728a derivative mutants by electroporation.
GFP assays
Quantitative GFP assays were performed as described by Miller et al. [39]. E. coli cells were cultured overnight in LB with the appropriate antibiotics at 37°C with shaking. For Pss B728a, cells were cultured overnight in NBY with the appropriate antibiotics at 26°C with shaking. Cells were harvested, washed, and resuspended in 10 mM phosphate buffer to a concentration of 2 x 109 cells per mL. GFP fluorescence was measured on Tecan SpectraFluor (Tecan) at an excitation wavelength of 485 nm, and an emission wavelength of 525 nm. Intensity readings were represented by arbitrary units and normalized to a cell density of 109 cells per mL.
Results
SalA, SyrF, and SyrG are novel LuxR transcriptional regulators with homologs found exclusively in Pseudomonas syringae genomospecies 2
An investigation of the Pss B728a genome identified 24 genes encoding LuxR-like regulatory proteins, which were also found in the genome of Pss B301D, displaying a high degree of nucleotide conservation between these two bacterial strains. Three genes of interest are salA, syrG, and syrF that are located adjacent to the syringomycin gene cluster. A conserved domain search of salA, syrG, and syrF using NCBI Conserved Domains database (http://www.ncbi.nlm.nih.gov/Structure/cdd/) confirmed the presence of a conserved helix-turn-helix DNA binding motif. The HTH DNA binding motif on the C-terminal region of the proteins is typical of LuxR regulatory proteins, but they lacked an N-terminal autoinducer-binding domain and receiver domain. BLAST analysis was also performed on SalA, SyrF, and SyrG protein sequences to determine the degree of conservation in pseudomonad strains. It was observed that all three regulatory proteins are exclusively found in P. syringae genomospecies 2, with the C-terminal region being highly conserved with regulatory protein SyrG (S1 Fig).
Expression of the syrG and syrF genes in the apoplast of bean relative to HMM medium
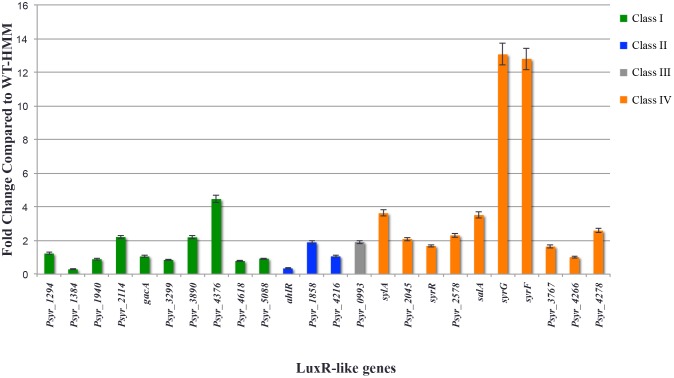
Analysis of the 6.09 Mb Pss B728a genome identified 24 genes dispersed throughout the genome ecoding LuxR-like proteins. Some of these proteins have been implicated in virulence and secondary metabolism in Pss B728a. To identify the LuxR-like proteins that are important to the plant-pathogen interaction, qRT-PCR analysis was used to determine transcript abundance of the genes identified as LuxR-like proteins in the apoplast of bean when compared to parental strain B728a in conditions conducive for hrp gene expression. The results indicated that in Pss B728a, change in gene expression between these conditions was the greatest for both syrG and syrF genes in the apoplast of bean relative to HMM liquid medium (Fig 2). This result is consistent with earlier reports of syrG gene expression levels in the apoplast [5, 10]. Higher expression levels of these genes in the apoplast is attributed to the presence of plant signal molecules known to activate expression of syr genes [6]. The relative expression of syrG and syrF when compared to other luxR genes indicate that the SyrG and SyrF proteins are involved in the transcriptional regulation of genes that potentially are critical to plant pathogenesis.
Fig 2. Expression analysis in the apoplast of bean of genes encoding LuxR-like proteins in Pss B728a.
The genes that encode proteins that are classified in the first subfamily of LuxR (Class I) are shown in the green and are typically associated with two component signal transduction systems. Shown in blue are genes that encode LuxR-like proteins implicated in quorum sensing based on domain architecture. Psyr_0993, which is shown as gray, is the only gene that encodes a protein characterized as a LAL or LuxR-like proteins that require ATP for activation. The final subfamily of LuxR-like proteins are encoded on genes shown in orange bars, which lack an N-terminal regulatory domain and are associated with secondary metabolism. Out of all 24 LuxR-like proteins found in the genome of Pss B728a, the genes encoding SyrG and SyrF are the most highly expressed in the apoplast when compared to HMM liquid medium. The values are represented as the average fold change of three technical replicates of three biological samples. Gene expression was normalized to the 16s-rRNA and recA internal control genes. Vertical bars indicate standard errors of the average values over triplicate runs.
SalA, SyrF, and SyrG influence virulence of Pss B728a on bean plants
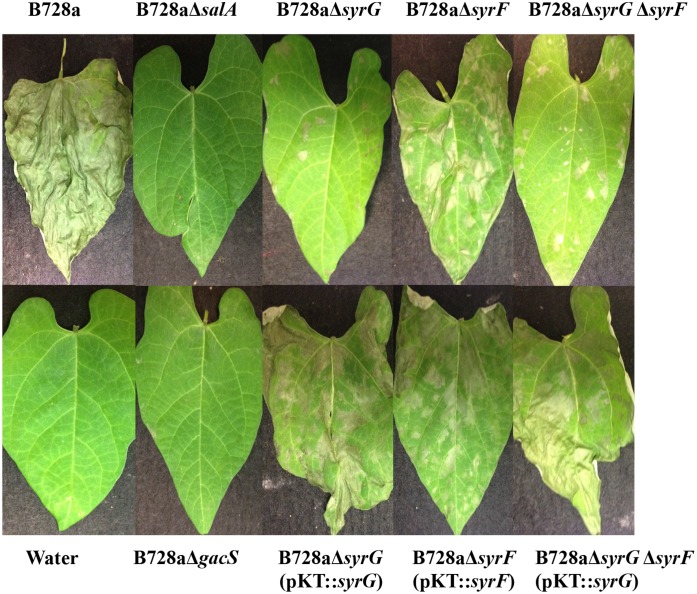
The generation of a clean deletion mutant of syrG and syrF in Pss B728a was achieved by using the mutation strategy described in the Materials and Methods. The bacterial strains B728aΔsyrG, B728aΔsyrF, and B728aΔsyrGΔsyrF displayed colony morphologies and growth curve patterns similar to parental strain B728a (data not shown). The derivative mutants B728aΔsyrG, B728aΔsyrF, and B728aΔsyrGΔsyrF were significantly reduced in virulence relative to the parental strain Pss B728a shown in Fig 3. The salA mutant failed to produce watersoaked necrotic lesions typical of Pss B728a. The salA mutant was comparable to B728aΔgacS in regards to virulence by lacking the ability to produce necrotic lesions and cause disease on bean. B728aΔsyrG was reduced in virulence by approximately 95% when compared to the parental strain Pss B728a. Mutants of syrG displayed small, non-spreading lesions with the average surface area of 45.1 mm2 on bean leaves. In contrast, the syrF mutant was able to produce large necrotic lesions, but displayed approximately 61% reduction in virulence on bean with the average necrotic lesion surface area of 284.5 mm2. The double deletion mutant of syrG and syrF exhibited disease symptoms comparable to the syrG mutant. Virulence of syrG and syrF derivative mutants was partially restored in trans by complementation of syrG and syrF (Fig 3).
Fig 3. Pathogenicity assays to evaluate the contribution of syrG and syrF to virulence on bean.
Bean leaves were inoculated by vacuum infiltration with bacterial suspensions containing 107 CFU/cm2 of either B728a, B728aΔsyrG, B728aΔsyrF, B728aΔsyrGΔsyrF, B728aΔgacS, B728aΔsyrG (pKT::syrG), B728aΔsyrF (pKT::syrF), or B728aΔsyrGΔsyrF (pKT::syrG). Plants were maintained at room temperature in a growth chamber for 72 h. Necrotic lesion surface areas were calculated using ImageJ software. This experiment was performed in triplicate, and representative results are shown.
Bacterial populations of infected bean plants were monitored over a 3-day period. At 3 days post-inoculation, bacterial titers for parental strain Pss B728a was 6.5 x 107 CFU/cm2, while B728aΔsalA, B728aΔsyrG and B728aΔsyrF were 2.0 x 106 CFU/cm2, 3.2 x 107 CFU/cm2 and 1.5 x 107 CFU/cm2, respectively. B728aΔgacS, which fails to produce disease on bean, maintained a population equivalent to day 0 of 2.2 x 104 CFU/cm2 3 days post-inoculation. The bacterial population of B728aΔsyrG and B728aΔsyrF were not significantly different from the parental strain Pss B728a, indicating that the syrG and syrF genes are not required for multiplication in planta. In contrast, B728aΔsalA displayed a 10-fold reduction in bacterial titers compared to parental strain B728a. B728aΔsalA is able to replicate in planta, but at a reduce rate when compared to B728a. In the case of B728aΔgacS, the bacterium remains viable but is limited on its ability to multiply in planta.
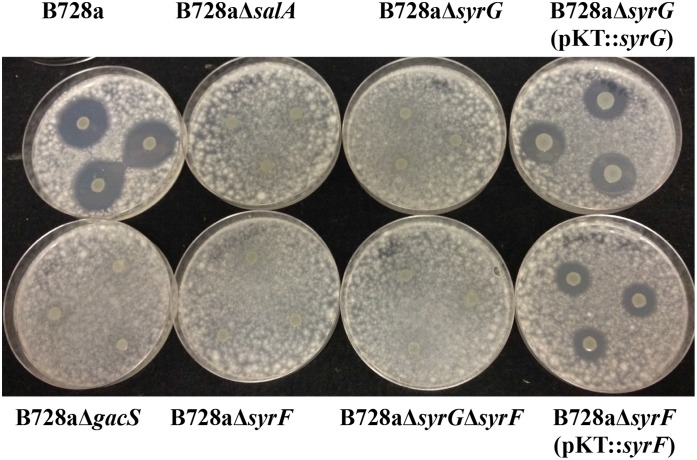
Deletion mutants of the salA, syrF, and syrG genes in Pss B728a affect syringomycin production
The bioassay used to evaluate the influence deletion of salA, syrG, and syrF has on syringomycin production was determined by measuring zones of antifungal activity to G. candidum as compared to parental strain Pss B728a grown on HMM agar (Fig 4). All the deletion mutants, including the double mutant Pss B728aΔsyrFΔsyrG, displayed no measurable antifungal activity toward G. candidum. Antifungal activity toward G. candidum was partially restored when B728a mutant derivatives were complemented in trans with the vector pPROBE-KT’ carrying an intact copy of the syrF or syrG gene. These results differed from a previous study by Lu et al. [10] with site directed insertional mutants of salA, syrF, and syrG in Pss B301D. It is hypothesized that the insertional mutants of syrF and syrG produce truncated proteins with reduced functional activity displaying low levels of toxin production. In contrast, clean deletion mutants of syrF and syrG were generated in Pss B301D. These derivative mutants displayed a loss of syringomycin production similar to the derivative mutants generated in Pss B728a (data not shown).
Fig 4. Bioassy to evaluate syringomycin production in parental strain B728a and derivative mutants.
Bacterial strains were grown on HMM for 4 days. Plates were oversprayed with Geotrichum candidum and incubated 24 h at 26°C to observe zones of inhibition indicative of syringomycin production. The experiment was repeated in triplicate.
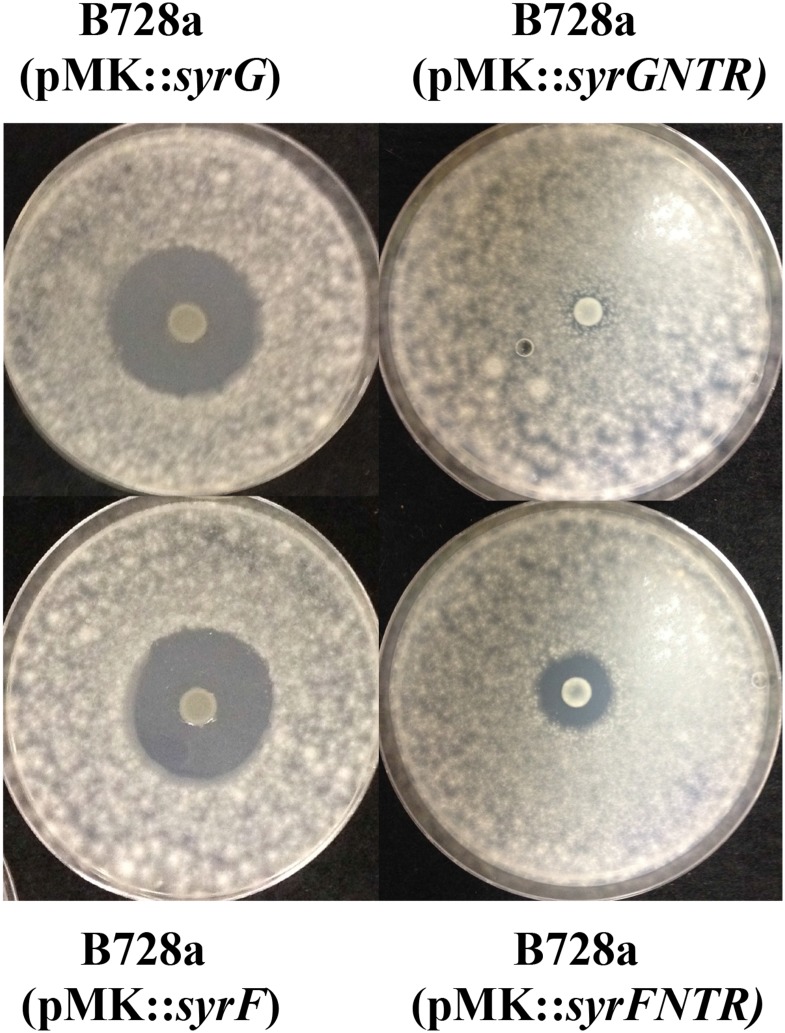
Overexpression of N-terminal truncated proteins of SyrG and SyrF has an effect on syringomycin production
To test the hypothesis that insertional mutants of syrF and syrG are leaky mutations and to demonstrate the HTH DNA binding domain of SyrG and SyrF are essential for binding to syr-syp promoters, the SyrG and SyrF proteins lacking the C-terminal HTH DNA-binding domain were overexpressed in Pss B728a. The overexpression of SyrG and SyrF had no effect on syringomycin production. However, overexpression of the N-terminal regions lacking the C-terminal HTH DNA-binding domains of SyrG and SyrF in Pss B728a resulted in a marked reduction of syringomycin zones of inhibition to G. candidum from 16 mm to 0.5 mm and 3 mm, respectively (Fig 5). The overexpression of the N-terminal regions of SyrG and and SyrF resulted in a 97% and 81% reduction in syringomycin production, which can be attributed to nonfunctional heterodimers formed between wild-type proteins and the truncated proteins. These nonfunctional heterodimers lack the ability to properly bind to syr-syp promoters, which is essential for the transcriptional activation of genes required for phytotoxin production. The truncation of SyrG displayed the greatest reduction in syringomycin production, which is comparable to the overexpression of an N-terminal truncated SalA in Pss B301D [12].
Fig 5. Effect of overexpression of N-terminal region (NTR) of SyrG and SyrF on syringomycin production in Pss B728a.
Bacterial strains were grown on HMM for 4 days. Plates were oversprayed with Geotrichum candidum and incubated 24 h at 26°C to observe zones of inhibition indicative of syringomycin production. The experiment was repeated in triplicate.
The effect of syrG and syrF deletion mutants on genes associated with virulence
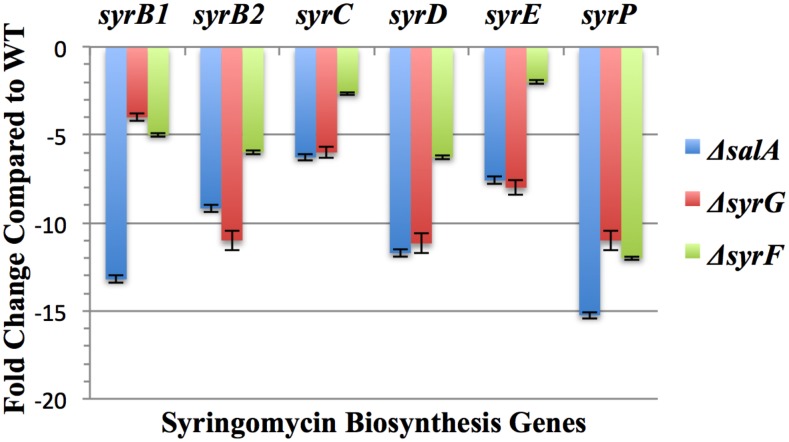
Previous experiments have established both syrG and syrF have an influence on syringomycin production and virulence in Pss B728a. Quantitative real-time PCR [48] was used to identify the effect syrG and syrF deletion mutants have on genes associated with syringomycin production and virulence. A total of 23 genes were evaluated using qRT-PCR that included genes involved in the biosynthesis of syringomycin, syringopeptin, achromobactin, alginate, levansucrase, syringolin, syringafactin, and pyoverdine. Both syrG and syrF had an effect on the expression of syringomycin biosynthesis genes (Fig 6). A deletion mutant of syrG resulted in a 4- to 11-fold decrease in transcript abundance of syrB1, syrB2, syrC, syrD, syrE and syrP. In regards to syrF, the deletion mutant resulted in a 2- to 12-fold decrease of transcript abundance of syringomycin biosynthesis genes. The results also indicated that both SyrG and SyrF are involved in the transcriptional regulation of genes associated with syringomycin production. Mutants of syrG and syrF did not appear to have an effect on the expression of genes associated with achromobactin, alginate, levansucrase, syringolin, syringofactin, or pyoverdine biosynthesis (data not shown). Both syrG and syrF mutants failed to have an effect on known virulence genes outside of the syr-syp gene cluster.
Fig 6. Quantitative real-time PCR analysis of syringomycin biosynthesis genes in ΔsalA, ΔsyrG, and ΔsyrF mutants of Pss B728a.
The values represent the average fold change in gene expression from parental strain Pss B728a; the results are the averages of three technical replicates from three biological samples grown in HMM liquid medium. Gene expression levels were normalized to 16s-rRNA and recA internal control genes, and vertical bars indicate standard errors of the average values over triplicate runs. Negative values indicate a decrease in transcript abundance by taking the negative inverse of a fold change value less than 1.
The effect of syrF and syrG deletion mutants on LuxR-like homologs in Pss B728a
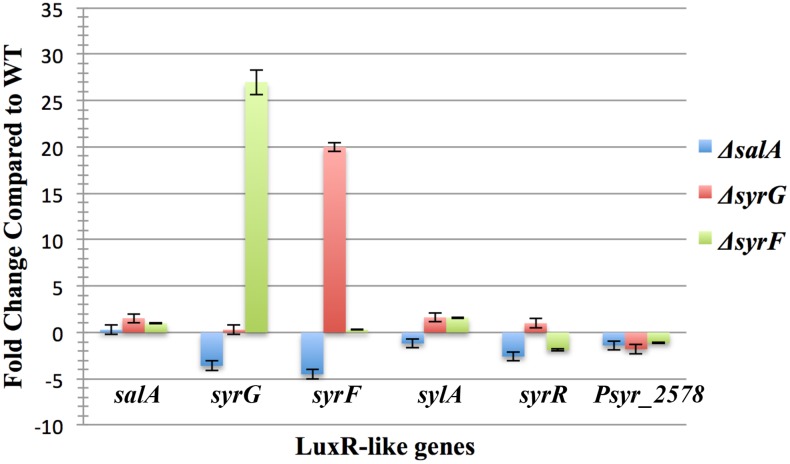
To determine the effect syrF and syrG mutants have on LuxR-like homologs in Pss B728a, qRT-PCR analysis was performed using primers specific for salA, syrG, syrF, sylA, syrR, and Psyr_2578. SylA and SyrR are LuxR-like proteins that have been implicated in the regulation of syringolin and syringofactin [5, 35, 49]. Results from qRT-PCR analysis show that syrG and syrF require a functional salA gene for activation (Fig 7). Mutants of salA displayed a 3.6-, and 4.5-fold decrease in transcript abundance of syrG and syrF, respectively. Mutants of syrG displayed a 27-fold increase in transcript abundance of syrF, and mutants of syrF displayed a 20-fold increase in transcript abundance of syrG. Data obtained from qRT-PCR analysis indicated that both syrG and syrF negatively regulate expression of each other’s gene. SyrG and SyrF did not have an effect on the expression of the LuxR-like genes sylA, syrR, and Psyr_2578 indicating they are not part of the SyrG or SyrF regulatory networks.
Fig 7. Quantitative real-time PCR analysis of LuxR-like genes in ΔsalA, ΔsyrG, and ΔsyrF mutants of Pss B728a.
The values represent the average fold change in gene expression from parental strain Pss B728a; the results are the averages of three technical replicates from three biological samples grown in HMM liquid medium. Gene expression levels were normalized to 16s-rRNA and recA internal control genes, and vertical bars indicate standard errors of the average values over triplicate runs. Negative values indicate a decrease in transcript abundance by taking the negative inverse of a fold change value less than 1.
The syrF gene is in an operon with oprM, whereas salA and syrG are monocistronic mRNA transcripts
The syr-syp gene cluster consists of genes involved in the biosynthesis, regulation and secretion of syringomycin and syrinopeptin. These genes are organized into two operons that were defined by Wang et al. [50] in Pss B301D. Located adjacent to the syr-syp gene cluster are the LuxR-like regulatory genes salA, syrF, and syrG. RT-PCR analysis (data not shown) revealed that both salA and syrG are transcribed as moncistronic mRNA with their own native promoter regions. There were no salA-syrG products observed with RT-PCR using primers specific to the 3’ and 5’ sequences of these genes, respectively. The genes that are organized in a polycistronic operon are syrF and oprM. A syrF-oprM product was obtained with RT-PCR using primers oprMF and syrFR that were specific for the 3’ and 5’ sequences of these genes, respectively.
Characterization of transcriptional start sites of the syrG and syrF genes
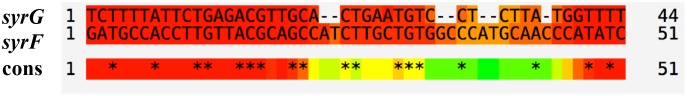
After identifying the monocistronic and polycistronic transcripts of salA, syrF, and syrG, the transcriptional start sites of the respective genes were defined using primer extension analysis. For salA, the salAPE primer was radiolabeled and used to identify the transcriptional start site. Primer extension analysis revealed the transcription start site was at the thymine residue, which was 63 bp upstream to the translational start codon of salA. This result is similar to the transcriptional start site identified for salA in Pss B301D by Wang et al. [50]. However, the prior study in Pss B301D did not identify the transcriptional start sites for syrF and syrG. For syrF, the syrFPE primer was used to identify the transcriptional start site at the cytosine residue, which was revealed to be 498 bp upstream of the translation start codon. The syrGPE primer was used to identify the transcriptional start site at the thymine residue, 235 bp upstream of the translation start codon. The transcriptional start site of syrG suggests a putative promoter region sequence, CTGAGAN17TCTTAT (Fig 8). Similarly, the transcriptional start site of syrF suggests a putative promoter region sequence, TTGTTAN23TGCAAC. In addition, computer analysis of promoter sequences identified conserved sequences observed around the -35 promoter regions of syrG and syrF, illustrated by the nucleotide sequence alignment of the predicted promoter regions (Fig 9). These putative promoter regions were predicted using defined transcriptional start sites and BPROM promoter prediction software [51]. Both promoters share high similarity to the consensus promoter sequence of σ70 found in Gram-negative bacteria [52].
Fig 8. Comparison of putative promoter sequences of salA, syrG and syrF.
Predicted promoter sequences are based on defined transcriptional start sites and using BPROM promoter prediction software. Conserved sequence motifs corresponding to -35 and -10 promoter regions are underlined and compared to a σ70 dependent promoter sequence. The defined promoter region of salA is distinctly different from the predicted promoter regions of syrG and syrF.
Fig 9. Alignment of syrG and syrF promoter sequences in Pss B728a.
The predicted promoter sequences of syrG and syrF were aligned using T-COFFEE and conserved sites are shown as asterisks. The color code is based on CORE index, using consistency among pairwise alignments for estimating reliability. Sequences shown in red indicate high reliability, where green is indicative of low reliability.
Identification of essential syrG and syrF promoter regions
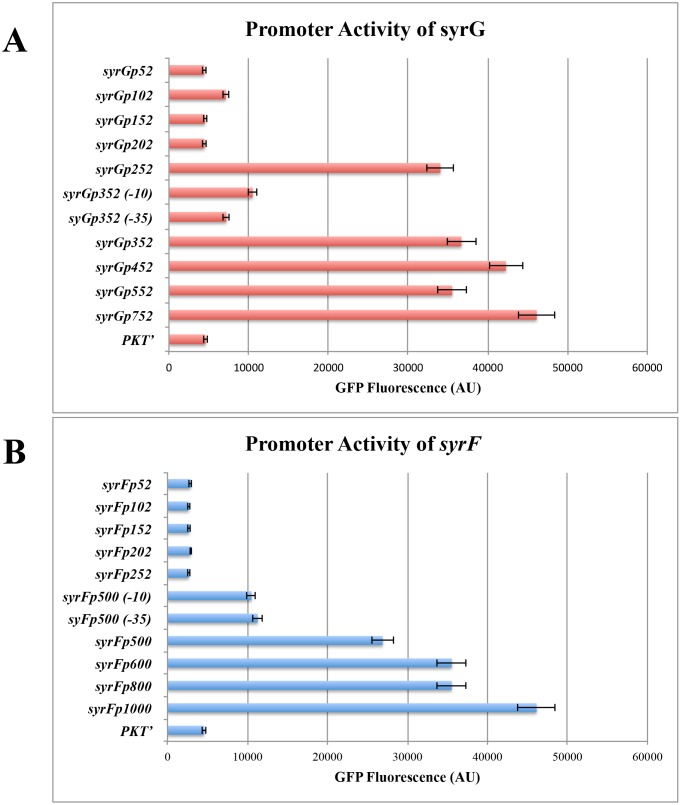
The effects of promoter deletion mutants on the expression of syrG::gfp and syrF::gfp fusions are shown in Fig 10. For syrG::gfp fusions, deletion constructs were generated from 752-bp, 552-bp, 452-bp, 352-bp, 252-bp, 202-bp, 152-bp, 102-bp, and 52-bp upstream of the translational start site of syrG. For syrF::gfp fusions, deletion constructs were generated from 1000-bp, 800-bp, 600-bp, 500-bp, 252-bp, 202-bp, 152-bp, 102-bp, and 52-bp upstream of the translational start site of syrF. Results indicated that the 100-bp region of 252- to 352-bp upstream of the start codon of syrG is critical for the expression of the syrG::gfp fusion. In addition, the 100-bp region of 500- to 600-bp upstream of the start codon of syrF is critical for the expression of the syrF::gfp fusion. When the predicted -10 promoter region TCTTAT of syrG and TGCAAC of syrF was substituted with CTGCAG, expression of the syrG::gfp and syrF::gfp reporters in Pss B728a decreased by 71% and 70%, respectively. When the predicted -35 promoter region CTGAGA of syrG and TTGTTA of syrF was substituted with CTGCAG, expression of the syrG::gfp and syrF::gfp reporters in Pss B728a decreased by 80% and 68%, respectively.
Fig 10. Analysis of the promoter regions of syrG (A) and syrF (B) by testing the effect deletion mutants have on the expression of syrG::gfp and syrF::gfp transcriptional fusions.
All gfp constructs were electroporated into cells of Pss B728a to measure GFP fluorescence (AU). All measurements were averages over three technical replicates of three biological samples. Horizontal bars represent the standard error of the average values.
LuxR-type transcription regulator SalA is a positive regulator of syrF and syrG promoters
The effects of deletion mutants of salA, syrG, and syrF have on salA::gfp, syrG::gfp, and syrF::gfp reporter gene activities are shown in Table 2. In the parental strain Pss B728a, the salA::gfp construct (pPKT::salA) displayed the relative GFP fluorescence of 43044 AU/109 CFU. Fluorescence of a salA::gfp fusion was reduced by 76% in a salA derivative mutant of Pss B728a. Deletion mutants of syrG and syrF did not significantly reduce the GFP fluorescence of a salA::gfp transcriptional fusion. Reporter gene activities of syrG::gfp and syrF::gfp were significantly reduced by 91% and 95% in B728aΔsalA as compared to activities in Pss B728a. This indicates that SalA is a transcriptional activator that functions upstream of syrG and syrF.
Table 2. Effect of salA, syrG, and syrF mutants on gfp reporter gene activity for salA, syrG, syrF, and syrB1.a.
| Strain (reporter fusion) | Gene | GFP fluorescence ± SE |
|---|---|---|
| B728a (salA::gfp) | salA | 43,044 ± 203 |
| B728aΔsalA (salA::gfp) | salA | 10,055 ± 357 |
| B728aΔsyrG (salA::gfp) | salA | 42,991 ± 425 |
| B728aΔsyrF (salA::gfp) | salA | 38,106 ± 454 |
| B728a (syrG::gfp) | syrG | 45,776 ± 773 |
| B728aΔsalA (syrG::gfp) | syrG | 4,175 ± 237 |
| B728aΔsyrG (syrG::gfp) | syrG | 6,121 ± 449 |
| B728aΔsyrF (syrG::gfp) | syrG | 47,875 ± 389 |
| B728a (syrF::gfp) | syrF | 44,857 ± 718 |
| B728aΔsalA (syrF::gfp) | syrF | 2,202 ± 223 |
| B728aΔsyrG (syrF::gfp) | syrF | 6,163 ± 239 |
| B728aΔsyrF (syrF::gfp) | syrF | 7,713 ± 281 |
| B728a (syrB1::gfp) | syrB1 | 47,266 ± 2,158 |
| B728aΔsalA (syrB1::gfp) | syrB1 | 9,653 ± 626 |
| B728aΔsyrG (syrB1::gfp) | syrB1 | 24,347 ± 522 |
| B728aΔsyrF (syrB1::gfp) | syrB1 | 29,458 ± 345 |
a The pPROBE-KT’ vector was used to construct pPKT fusions of specific genes to the gfp reporter. GFP fluorescence were averaged over three technical replicates of three biological samples followed by the standard error of the averaged values.
SyrG is a positive regulator of syrF and both syrG and syrF are involved in the expression of syrB1
The effect deletion mutants of syrG and syrF have on the syrG::gfp and syrF::gfp reporter gene activities is shown in Table 2. The parental strain Pss B728a harboring the syrG::gfp transcriptional fusion displays a relative GFP fluorescence of 45776 AU/109 CFU. GFP fluorescence of syrG::gfp decreased to 6121 AU/109 CFU in syrG derivative mutants of Pss B728a, where the GFP fluorescence syrG::gfp was not significantly reduced in syrF deletion mutants. These results indicated that SyrG is required to activate its own gene expression, but SyrF does not have an affect on the promoter activities of syrG. The parental strain Pss B728a harboring the syrF::gfp transcriptional fusion displayed a relative GFP fluorescence of 44857 AU/109 CFU. The relative GFP fluorescence of syrF::gfp decreased to 6163 and 7713 AU/109 CFU in deletion mutants of syrG and syrF, respectively. Both SyrG and SyrF are required for activation of the syrF gene.
The parental strain Pss B728a harboring the syrB1::gfp transcriptional fusion displays a relative GFP fluorescence of 47266 AU/109 CFU (Table 2). GFP fluorescence of syrB1::gfp decreased to 9653, 24347, 29458 AU/109 CFU in deletion mutants of salA, syrG, and syrF, respectively. Both SyrG and SyrF are involved in the expression of syrB1.
Overexpression of SyrF restores syringomycin production in syrG deletion mutants of Pss B728a
The derivative mutants of syrG and syrF in Pss B728a displayed a significant loss of syringomycin production when compared to the parental strain Pss B728a. The overexpression of SyrF had the ability to partially restore syringomycin production in syrG derivative mutants. Syringomycin inhibition zones increase from 0 mm to 25 mm, resulting in a 25% increase in syringomycin production when compared to a syrG derivative mutant. However, the overexpression of SyrG failed to restore syringomycin production in syrF derivative mutants (data not shown). These results indicated that SyrG is an upstream activator of syrF.
Discussion
The genome of Pss B728a is relatively large in size (6.09 Mb), and encodes 24 LuxR-like proteins. Homologs of these LuxR-like proteins were also found in the genome of Pss B301D displaying a high degree of sequence conservation compared to Pss B728a. These LuxR-like proteins have been associated with a variety of biological processes that includes quorum sensing, virulence, and secondary metabolism in Pss B728a [5, 10, 28, 29, 35, 36, 49, 53]. The superfamily of LuxR-like proteins may be categorizied into four subfamilies based on domain architecture and mechanism of regulatory activation shown in Fig 1. Located adjacent to the syringomycin gene cluster are salA, syrG, and syrF, which encode three LuxR-like proteins classified into a subfamily of LuxR proteins not fully characterized. These proteins lack a defined N-terminal regulatory domain, but possess a highly conserved C-terminal HTH DNA binding domain. The HTH DNA binding motif is known to interact with the promoter elements of targeted regulatory genes to induce or repress transcription [12, 49]. Sequence analysis of SalA, SyrG, and SyrF showed that these LuxR-like proteins are closely related to FixJ and NarL. Both FixJ and NarL are LuxR-like proteins belong to a subfamily of LuxR-like proteins that are part of a two componenet signal transduction system that requires phosphorylation of the N-terminal receiver domain for activation [14, 15, 27]. However, SalA, SyrG, and SyrF share the greatest sequence homology to the LuxR-like protein GerE that is involved in the regulation of spore formation in B. subtilis [27]. The GerE protein also lacks an N-terminal regulatory domain [27]. LuxR-like proteins that lack a N-terminal regulatory domain are part of a LuxR subfamily that is not completely defined that may act as transcriptional activators and repressors. Analysis of the crystal structure of GerE revealed that it is comprised of four alpha helices, of which the central pair forms a HTH DNA-binding motif in the C-terminal region of the protein [27]. LuxR-like proteins exhibiting a similar domain organization have been associated with secondary metabolism in Pss B728a. For example, SlyA activates the transcription of slyB and sylC, which are involved in the biosynthesis of syringolin [49]. SyrR and Psyr_2578 encode two LuxR-like proteins that are located adjacent to syfA and syfB. Both syfA and syfB are required for biosynthesis of syringafactin [5, 35, 44].
The importance of SalA, SyrG, and SyrF in regards to pathogenicity on bean plants was demonstrated by qRT-PCR analysis and pathogenicity assays. Quantitative real-time PCR analysis revealed that both syrG and syrF genes are expressed at high levels in the apoplast of bean relative to HMM liquid medium. This result indicated that both SyrG and SyrF are involved in regulation of genes important to establishing the plant-pathogen interaction or pathogenesis. Consequently, pathogenicity assays showed that mutants of salA, syrG, and syrF displayed a significant reduction in virulence by approximately 100%, 95%, and 61%, respectively. Disease development as observed for a mutant of syrG was comparable to a mutant of salA, whereas a mutant of syrF produced larger necrotic lesions on bean. A double mutant of syrF and syrG in B728a displayed symptomology comparable to the mutant of syrG. This result indicated that syrG is involved in regulating a broader range of genes critical to plant pathogenesis, and that it acts upstream of the LuxR-like protein SyrF in a regulatory cascade. Mutants of syrG and syrF were able to produce bacterial populations similar to the parental strain B728a in planta, indicating that syrG and syrF are not required for replication in the apoplast.
Reduction in virulence observed for syrG and syrF mutants can be attributed largely to the reduction in syringomycin and syringopeptin production. Syringomycin is considered one of the major virulence determinants of Pss B728a, along with syringopeptin [45]. Both syrG and syrF are required for syringomycin production (shown in Fig 4). Syringomycin production was partially restored when a functional copy of syrG and syrF was expressed in trans. Quantitative real-time PCR analysis also showed that mutants of syrG and syrF resulted in a significant decrease in the expression of syringomycin biosynthesis genes. These results were surprising given that a previous study by Lu et al. [10] using site-directed insertional mutants of salA, syrF, and syrG in Pss B301D, which displayed reductions of 100%, 83%, and 40% in syringomycin production, respectively, as compared to the parental strain. In contrast, clean deletion mutants of syrF and syrG were generated in Pss B301D; these derivative mutants corresponded to syrF and syrG mutants of Pss B728a with comparable reductions in syringomycin production (data not shown). It was hypothesized that the insertional mutants of syrF and syrG in Pss B301D produce truncated proteins with reduced functional activity as displayed by low levels of toxin production. This hypothesis was tested by overexpressing of the N-terminal regions of SyrG and SyrF in Pss B728a, which resulted in a significant reduction of syringomycin production (Fig 5). The overexpression of the N-terminal regions of SyrG and and SyrF resulted in 97% and 81% reduction in syringomycin production, which can be attributed to the formation of a nonfunctional heterodimers unable to bind to the promoter regions of genes associated with syringomycin production. Similar results were seen in V. fischeri when the overexpression of the N-terminal domain of LuxR displayed a reduction in luminescence [54]. The amino acids between 116 and 161 in the N-terminal domain were critical for LuxR to form dimers and activate transcription of the luxICDABE operon [54]. The truncation of SyrG displayed the greatest reduction in syringomycin production, which was comparable to the overexpression of the N-terminal region of SalA in B301D [12]. Wang et al. [12] showed that the overexpression of the N-terminal region of SalA and SyrF resulted in a significant decrease in expression of syrB1:uidA and sypA:uidA reporters. These results indicated that SyrG regulates a broader range of genes involved in toxin production as compared to SyrF.
Quantitative real-time PCR analysis was used to identify new components of the SyrG and SyrF regulons that had not been identified previously. The syrG and syrF regulatory genes do not appear to affect expression of genes involved in the production of alginate, achromabactin, levansucrase, syringolin, syringafactin, and pyoverdine. These results are not suprising given that a microarray study performed by Wang et al. [12] showed that genes involved in siderophore production, environmental stress, quorum sensing, global regulation, phytohormone synthesis and alginate production were not part the SyrF regulon. Both syrG and syrF mutants failed to have an effect on known virulence genes outside of the syr-syp gene cluster. But it seems that SyrG and SyrF negatively regulate the expression of each other’s gene to illustrate the complexity of their regulatory roles. Accordingly, it was established that both the SyrG and SyrF regulons overlap, and appear to be in competition for the binding and transcriptional activation of genes associated with syringomycin production.
It was revealed that the promoter sequences of syrG and syrF were highly similar to each other, but were distinctly different from the promoter region of salA. Alignment of these promoter sequences also identified a conserved sequence observed around the -35 region of the promoter. It was hypothesized that these conserved sequences are the binding site for SalA. Previous studies by Lu et al. [10] showed that SalA is required for the functional activation of syrG and syrF. In addition, Wang et al. [50] established that SalA binds to the promoter region of SyrF to activate transcription. It is unknown if similar conserved sequences observed around the -35 promoter region of syrG and syrF are found in the promoter region of sylA, given that sylA is under the transcriptional control of SalA [49]. It has been shown that the promoter of related genes, under the control of a LuxR regulatory protein, display conserved sites for binding of sigma factors and/or transcriptional regulators [50, 55, 56]. Wang et al. [50] identified a conserved 20-bp syr-syp box sequence around the -35 region promoter region in both syringomycin and syringopeptin biosynthesis genes. This syr-syp box was not identified in the promoters of syrG and syrF, which indicates that SalA has a promoter binding site sequence dissimilar from the syr-syp box to coordinate expression of the syrG and syrF regulatory genes. It was established that SyrF forms dimers that recognize the syr-syp box as a binding site [12, 50], but it remains unknown if SyrG recognizes the syr-syp box as a putative binding site. Similar binding sites occur in promoters of genes regulated by LuxRs, such as the las-rhl box identified in the promoters of genes controlled by quorum sensing in P. aeruginosa [49].
GFP reporter assays were used to determine the functional activity of promoters transcriptionally fused to gfp in Pss B728a mutant derivatives. A similar study by Ramel et al. [49] showed that the promoters of syringolin biosynthesis genes required sylA for activation. Our GFP assays showed that SalA is an upstream transcriptional activator of syrG and syrF. In addition, results demonstrated that SyrG is located upstream of SyrF in a regulatory cascade. Consequently, overexpression of syrF was able to restore syringomycin production in syrG derivative mutants, whereas overexpression of syrG was not able to restore syringomycin production in syrF derivative mutants. Thus, the regulatory effect of SyrG on the promoters of syringomycin biosynthesis genes may be indirect, which is consistent with the report of Wang et al. [12] that SyrF binds to the promoter of syrB1.
Characterization of the salA, syrG, and syrF genes is important to define the complex regulatory mechanism used for the expression of genes associated with virulence in Pss B728a. Based on previous studies [10, 12, 50] it was speculated that SyrG was responsible for the transcriptional activation of a broader range of genes associated with virulence than SyrF. However, this study did not identify virulence genes outside of the syr-syp gene cluster under the transcriptional control of SyrG. Nevertheless, this study demonstrated that SyrG is important for the transcriptional regulation of syringomycin and may be involved in an overlapping regulon with SyrF to control phytotoxin production. At the very least, SyrG appears to function as an upstream transcriptional activator of syrF. In conclusion, despite the complexity of the interactions between the SalA, SyrG, and SyrF regulators, they exhibit significant roles in toxigenesis during plant pathogenesis.
Supporting Information
SyrG is conserved only in Pseudomonas syringae genomospecies 2 and not in P. syringae pv. tomato DC3000 genomospecies 1. Strains include: P. syringae pv. syringae (Pss) strains B301D, HS191, B728a, 642, and FF5, P. syringae pv. japonica M301072; P. syringae pv. aptata DSM 50252, P. syringae pv. aceris M302273, and P. syringae strain Cit7. There is also a high level of conservation observed in the C-terminal region of the protein where there is a HTH DNA binding motif known to interact with the promoter regions of targeted genes. The sequence of SyrG in Pss B301D, a closely related strain to Pss B728a, is one amino acid different from the sequence in Pss B728a.
(PDF)
(PDF)
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Texas A&M AgriLife Research project nol TEX0-1-8832. The open access publishing fees for this article have been covered by the Texas A&M University Online Access to Knowledge Fund (OAKFund), supported by the University Libraries and the Office of the Vice President for Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Feil H, Feil WS, Chain P, Larimer F, DiBartolo G, Copeland A, et al. Comparison of the complete genome sequences of Pseudomonas syringae pv. syringae B728a and pv. tomato DC3000. Proc Natl Acad Sci U S A. 2005;102(31):11064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ravindran A, Jalan N, Yuan JS, Wang N, Gross DC. Comparative genomics of Pseudomonas syringae pv. syringae strains B301D and HS191 and insights into intrapathovar traits associated with plant pathogenesis. MicrobiologyOpen. 2015;4(4):553–73. 10.1002/mbo3.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monier JM, Lindow SE. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl Environ Microbiol. 2004;70(1):346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirano SS, Upper CD. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae-a pathogen, ice nucleus, and epiphyte. Microbiol Mol Biol Rev. 2000;64(3):624–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Lund SP, Scott RA, Greenwald JW, Records AH, Nettleton D, et al. Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proc Natl Acad Sci U S A. 2013;110(5):E425–34. 10.1073/pnas.1221892110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender CL, Alarcón-Chaidez F, Gross DC. Pseudomonas syringae phytotoxins: mode of action, regulation, and biosynthesis by peptide and polyketide synthetases. Microbiol Mol Biol Rev. 1999;63(2):266–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross H, Loper JE. Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep. 2009;26(11):1408–46. 10.1039/b817075b [DOI] [PubMed] [Google Scholar]
- 8.Hutchison ML, Gross DC. Lipopeptide phytotoxins produced by Pseudomonas syringae pv. syringae: comparison of the biosurfactant and ion channel-forming activities of syringopeptin and syringomycin. Mol Plant-Microbe Interact. 1997;10(3):347–54. [DOI] [PubMed] [Google Scholar]
- 9.Scholz-Schroeder BK, Soule JD, Gross DC. The sypA, sypB, and sypC synthetase genes encode twenty-two modules involved in the nonribosomal peptide synthesis of syringopeptin by Pseudomonas syringae pv. syringae B301D. Mol Plant-Microbe Interact. 2003;16(4):271–80. [DOI] [PubMed] [Google Scholar]
- 10.Lu SE, Scholz-Schroeder BK, Gross DC. Characterization of the salA, syrF, and syrG regulatory genes located at the right border of the syringomycin gene cluster of Pseudomonas syringae pv. syringae. Mol Plant-Microbe Interact. 2002;15(1):43–53. [DOI] [PubMed] [Google Scholar]
- 11.Subramoni S, Venturi V. LuxR-family 'solos': bachelor sensors/regulators of signalling molecules. Microbiology. 2009;155(Pt 5):1377–85. 10.1099/mic.0.026849-0 [DOI] [PubMed] [Google Scholar]
- 12.Wang N, Lu SE, Records AR, Gross DC. Characterization of the transcriptional activators SalA and SyrF, Which are required for syringomycin and syringopeptin production by Pseudomonas syringae pv. syringae. J Bacteriol. 2006;188(9):3290–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang M, Giel JL, Cai T, Zhong Z, Zhu J. The LuxR family quorum-sensing activator MrtR requires its cognate autoinducer for dimerization and activation but not for protein folding. J Bacteriol. 2009;191(1):434–8. 10.1128/JB.01247-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maris AE, Sawaya MR, Kaczor-Grzeskowiak M, Jarvis MR, Bearson SM, Kopka ML, et al. Dimerization allows DNA target site recognition by the NarL response regulator. Nat Struct Biol. 2002;9(10):771–8. [DOI] [PubMed] [Google Scholar]
- 15.Zhang JH, Xiao G, Gunsalus RP, Hubbell WL. Phosphorylation triggers domain separation in the DNA binding response regulator NarL. Biochemistry. 2003;42(9):2552–9. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan HB, Greenberg EP. Overproduction and purification of the luxR gene product: Transcriptional activator of the Vibrio fischeri luminescence system. Proc Natl Acad Sci U S A. 1987;84(19):6639–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piper KR, Beck von Bodman S, Farrand SK. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993;362(6419):448–50. [DOI] [PubMed] [Google Scholar]
- 18.Welch M, Todd DE, Whitehead NA, McGowan SJ, Bycroft BW, Salmond GP. N-acyl homoserine lactone binding to the CarR receptor determines quorum-sensing specificity in Erwinia. EMBO J. 2000;19(4):631–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussain MB, Zhang HB, Xu JL, Liu Q, Jiang Z, Zhang LH. The acyl-homoserine lactone-type quorum-sensing system modulates cell motility and virulence of Erwinia chrysanthemi pv. zeae. J Bacteriol. 2008;190(3):1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gambello MJ, Iglewski BH. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J Bacteriol. 1991;173(9):3000–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selin C, Fernando WG, de Kievit T. The PhzI/PhzR quorum-sensing system is required for pyrrolnitrin and phenazine production, and exhibits cross-regulation with RpoS in Pseudomonas chlororaphis PA23. Microbiology. 2012;158(Pt 4):896–907. 10.1099/mic.0.054254-0 [DOI] [PubMed] [Google Scholar]
- 22.Brint JM, Ohman DE. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J Bacteriol. 1995;177(24):7155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shadel GS, Baldwin TO. Positive autoregulation of the Vibrio fischeri luxR gene. LuxR and autoinducer activate cAMP-catabolite gene activator protein complex-independent and -dependent luxR transcription. J Biol Chem. 1992;267(11):7696–702. [PubMed] [Google Scholar]
- 24.Danot O. A complex signaling module governs the activity of MalT, the prototype of an emerging transactivator family. Proc Natl Acad Sci U S A. 2001;98(2):435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He W, Lei J, Liu Y, Wang Y. The LuxR family members GdmRI and GdmRII are positive regulators of geldanamycin biosynthesis in Streptomyces hygroscopicus 17997. Arch Microbiol. 2008;189(5):501–10. 10.1007/s00203-007-0346-2 [DOI] [PubMed] [Google Scholar]
- 26.Wilson DJ, Xue Y, Reynolds KA, Sherman DH. Characterization and analysis of the PikD regulatory factor in the pikromycin biosynthetic pathway of Streptomyces venezuelae. J Bacteriol. 2001;183(11):3468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducros VM, Lewis RJ, Verma CS, Dodson EJ, Leonard G, Turkenburg JP, et al. Crystal structure of GerE, the ultimate transcriptional regulator of spore formation in Bacillus subtilis. J Mol Biol. 2001;306(4):759–71. [DOI] [PubMed] [Google Scholar]
- 28.Lu SE, Wang N, Wang J, Chen ZJ, Gross DC. Oligonucleotide microarray analysis of the salA regulon controlling phytotoxin production by Pseudomonas syringae pv. syringae. Mol Plant-Microbe Interact. 2005;18(4):324–33. [DOI] [PubMed] [Google Scholar]
- 29.Kitten T, Kinscherf TG, McEvoy JL, Willis DK. A newly identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol Microbiol. 1998;28(5):917–29. [DOI] [PubMed] [Google Scholar]
- 30.Vidaver AK. Synthetic and complex media for the rapid detection of fluorescence of phytopathogenic pseudomonads: effect of the carbon source. Appl Microbiol. 1967;15(6):1523–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King EO, Ward MK, Raney DE. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954;44(2):301–7. [PubMed] [Google Scholar]
- 32.Greenwald JW, Greenwald CJ, Philmus BJ, Begley TP, Gross DC. RNA-seq analysis reveals that an ECF σ factor, AcsS, regulates achromobactin biosynthesis in Pseudomonas syringae pv. syringae B728a. PLoS One. 2012;7(4):e34804 10.1371/journal.pone.0034804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huynh TV, Dahlbeck D, Staskawicz BJ. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science. 1989;245(4924):1374–7. [DOI] [PubMed] [Google Scholar]
- 34.Yu X, Lund SP, Greenwald JW, Records AH, Scott RA, Nettleton D, et al. Transcriptional analysis of the global regulatory networks active in Pseudomonas syringae during leaf colonization. MBio. 2014;5(5):e01683–14. 10.1128/mBio.01683-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenwald JW. An in-depth analysis of iron and pathogenicity regulatory networks in Pseudomonas syringae pv. syringae B728A [dissertation]. College Station (TX): Texas A&M University; 2011. [Google Scholar]
- 36.Records AR, Gross DC. Sensor kinases RetS and LadS regulate Pseudomonas syringae type VI secretion and virulence factors. J Bacteriol. 2010;192(14):3584–96. 10.1128/JB.00114-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000;97(12):6640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marco ML, Legac J, Lindow SE. Pseudomonas syringae genes induced during colonization of leaf surfaces. Environ Microbiol. 2005;7(9):1379–91. [DOI] [PubMed] [Google Scholar]
- 39.Miller WG, Leveau JH, Lindow SE. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol Plant-Microbe Interact. 2000;13(11):1243–50. [DOI] [PubMed] [Google Scholar]
- 40.Lu SE, Scholz-Schroeder BK, Gross DC. Construction of pMEKm12, an expression vector for protein production in Pseudomonas syringae. FEMS Microbiol Lett. 2002;210(1):115–21. [DOI] [PubMed] [Google Scholar]
- 41.Leong SA, Ditta GS, Helinski DR. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982;257(15):8724–30. [PubMed] [Google Scholar]
- 42.Landy A. Dynamic, structural, and regulatory aspects of lambda site-specific recombination. Annu Rev Biochem. 1989;58:913–49. [DOI] [PubMed] [Google Scholar]
- 43.Sawahel W, Sastry G, Knight C, Cove D. Development of an electro-transformation system for Escherichia coli DH10B. Biotechnol Tech.; 1993. p. 261–6. [Google Scholar]
- 44.Winsor GL, Lam DK, Fleming L, Lo R, Whiteside MD, Yu NY, et al. Pseudomonas Genome Database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res. 2011;39(Database issue):D596–600. 10.1093/nar/gkq869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scholz-Schroeder BK, Hutchison ML, Grgurina I, Gross DC. The contribution of syringopeptin and syringomycin to virulence of Pseudomonas syringae pv. syringae strain B301D on the basis of sypA and syrB1 biosynthesis mutant analysis. Mol Plant-Microbe Interact. 2001;14(3):336–48. [DOI] [PubMed] [Google Scholar]
- 46.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8. [DOI] [PubMed] [Google Scholar]
- 47.Notredame C, Higgins DG, Heringa J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302(1):205–17. [DOI] [PubMed] [Google Scholar]
- 48.Thakur PB, Vaughn-Diaz VL, Greenwald JW, Gross DC. Characterization of five ECF sigma factors in the genome of Pseudomonas syringae pv. syringae B728a. PLoS One. 2013;8(3):e58846 10.1371/journal.pone.0058846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramel C, Baechler N, Hildbrand M, Meyer M, Schadeli D, Dudler R. Regulation of biosynthesis of syringolin A, a Pseudomonas syringae virulence factor targeting the host proteasome. Mol Plant-Microbe Interact. 2012;25(9):1198–208. 10.1094/MPMI-03-12-0070-R [DOI] [PubMed] [Google Scholar]
- 50.Wang N, Lu SE, Yang Q, Sze SH, Gross DC. Identification of the syr-syp box in the promoter regions of genes dedicated to syringomycin and syringopeptin production by Pseudomonas syringae pv. syringae B301D. J Bacteriol. 2006;188(1):160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agaras B, Sobrero P, Valverde C. A CsrA/RsmA translational regulator gene encoded in the replication region of a Sinorhizobium meliloti cryptic plasmid complements Pseudomonas fluorescens rsmA/E mutants. Microbiology. 2013;159(Pt 2):230–42. 10.1099/mic.0.061614-0 [DOI] [PubMed] [Google Scholar]
- 52.Browning DF, Busby SJ. The regulation of bacterial transcription initiation. Nat Rev Microbiol. 2004;2(1):57–65. [DOI] [PubMed] [Google Scholar]
- 53.Quiñones B, Dulla G, Lindow SE. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol Plant-Microbe Interact. 2005;18(7):682–93. [DOI] [PubMed] [Google Scholar]
- 54.Choi SH, Greenberg EP. Genetic dissection of DNA binding and luminescence gene activation by the Vibrio fischeri LuxR protein. J Bacteriol. 1992;174(12):4064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saha S, Lindeberg M. Bound to succeed: Transcription factor binding-site prediction and its contribution to understanding virulence and environmental adaptation in bacterial plant pathogens. Mol Plant-Microbe Interact. 2013;26(10):1123–30. 10.1094/MPMI-04-13-0090-CR [DOI] [PubMed] [Google Scholar]
- 56.Finney AH, Blick RJ, Murakami K, Ishihama A, Stevens AM. Role of the C-terminal domain of the alpha subunit of RNA polymerase in LuxR-dependent transcriptional activation of the lux operon during quorum sensing. J Bacteriol. 2002;184(16):4520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SyrG is conserved only in Pseudomonas syringae genomospecies 2 and not in P. syringae pv. tomato DC3000 genomospecies 1. Strains include: P. syringae pv. syringae (Pss) strains B301D, HS191, B728a, 642, and FF5, P. syringae pv. japonica M301072; P. syringae pv. aptata DSM 50252, P. syringae pv. aceris M302273, and P. syringae strain Cit7. There is also a high level of conservation observed in the C-terminal region of the protein where there is a HTH DNA binding motif known to interact with the promoter regions of targeted genes. The sequence of SyrG in Pss B301D, a closely related strain to Pss B728a, is one amino acid different from the sequence in Pss B728a.
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.