Abstract
The virulence of Staphylococcus aureus, in both human and animal hosts, is largely influenced by the acquisition of mobile genetic elements (MGEs). Most S. aureus strains carry a variety of MGEs, including three genomic islands (νSaα, νSaβ, νSaγ) that are diverse in virulence gene content but conserved within strain lineages. Although the mobilization of pathogenicity islands, phages and plasmids has been well studied, the mobilization of genomic islands is poorly understood. We previously demonstrated the mobilization of νSaβ by the adjacent temperate bacteriophage ϕSaBov from strain RF122. In this study, we demonstrate that ϕSaBov mediates the mobilization of νSaα and νSaγ, which are located remotely from ϕSaBov, mostly to recipient strains belonging to ST151. Phage DNA sequence analysis revealed that chromosomal DNA excision events from RF122 were highly specific to MGEs, suggesting sequence-specific DNA excision and packaging events rather than generalized transduction by a temperate phage. Disruption of the int gene in ϕSaBov did not affect phage DNA excision, packaging, and integration events. However, disruption of the terL gene completely abolished phage DNA packing events, suggesting that the primary function of temperate phage in the transfer of genomic islands is to allow for phage DNA packaging by TerL and that transducing phage particles are the actual vehicle for transfer. These results extend our understanding of the important role of bacteriophage in the horizontal transfer and evolution of genomic islands in S. aureus.
Introduction
Genetic variation of bacteria can be achieved through mutations, rearrangements and horizontal gene transfers and recombinations. Increasing genome sequence data have demonstrated that, besides the core genes encoding house-keeping functions such as essential metabolic activities, information processing, and bacterial structural and regulatory components, a vast number of accessory genes encoding antimicrobial resistance, toxins, and enzymes that contribute to adaptation and survival under certain environmental conditions are acquired by horizontal gene transfer of mobile genetic elements (MGEs) [1, 2]. Mobile genetic elements are a heterogeneous group of molecules that include plasmids, bacteriophages, genomic islands, chromosomal cassettes, pathogenicity islands, and integrative and conjugative elements [2–4]. Genomic islands are relatively large segments of DNA ranging from 10 to 200 kb often integrated into tRNA gene clusters flanked by 16–20 bp direct repeats [3]. They are recognized as discrete DNA segments acquired by horizontal gene transfer since they can differ from the rest of the chromosome in terms of GC content (%G+C) and codon usage [3, 5].
Staphylococcus aureus is a major pathogen that colonizes the skin and mucous membranes of humans and animals, causing diseases ranging from mild skin infections to severe invasive diseases such as necrotizing pneumonia, infective endocarditis, and osteomyelitis [6–8]. The pathogenicity of this bacterium is largely influenced by the virulence genes carried on MGEs [9]. Three types of genomic islands (νSaα, νSaβ, νSaγ) are known in S. aureus [2]. Each type of genomic island is polymorphic in gene content but conserved within strain lineages [10–12]. Genomic islands are not as competently mobile as other MGEs, due to the lack of typical genetic elements required for or indicative of mobilization such as integrases, excisionases, terminases, and associated repeat sequences [13, 14]. While efficient mobilization of SaPIs by temperate helper phages has been well documented [15, 16], direct evidence indicating the mechanism of genomic island mobilization has not been well established. Previously, we reported the transfer of νSaβ by temperate phage ϕSaBov, which integrated immediately adjacent to the νSaβ [17]. The induction of ϕSaBov by mitomycin C generated transducing particles harboring overlapping segments of νSaβ in circular and linear forms of phage DNA, which appeared to be followed by sequential integration and homologous recombination events, resulting in transfer of entire ϕSaBov and νSaβ [17]. Here we demonstrate, for the first time, phage-mediated transfer of genomic islands νSaα and νSaγ, which are remotely located from ϕSaBov. Our results also showed that the genetic background of the host and recipient strains impact the ability and efficiency of transfer of MGEs mediated by bacteriophages, suggesting the presence of MGE-specific mechanisms of excision and integration from the donor and the recipient strains, respectively, in concert with functions from bacteriophages.
Materials and Methods
Bacterial strains and growth conditions
All strains and plasmids used in this study are listed in Table 1. Staphylococcus aureus strains were cultured in tryptic soy broth (TSB) or agar (TSA) plates (Difco) supplemented with tetracycline (5 μg/mL, Sigma-Aldrich) as necessary. Escherichia coli were grown in Luria-Bertani (LB) broth and agar plates supplemented with ampicillin (100 μg/mL, Sigma-Aldrich) as necessary. Twenty nine bovine mastitis isolates and 22 human isolates were kindly provided by QIA (Quarantine inspection agency, South Korea) and Patrick Schlievert (University of Iowa), respectively.
Table 1. A list of strains and plasmids used in this study.
| Strain | Description | Reference or source |
|---|---|---|
| Staphylococcus aureus | ||
| RF122 | Bovine isolate, CC151 | 26 |
| RF122 set::tetM | Indicative strain for transfer of νSaα | This study |
| RF122 hla::tetM | Indicative strain for transfer of νSaγ | This study |
| RF122 tst::tetM | Indicative strain for transfer of SaPIbov1 | This study |
| RF122 mdr::tetM | Indicative strain for transfer of SaPI122 | This study |
| RF122 int::tetM | RF122 Δint | [17] |
| RF122 set::tetM, int::cat | Indicative strain for transfer of νSaα in Δint background, RF122 Δint set::tetM | This study |
| RF122 hla::tetM, int::cat | Indicative strain for transfer of νSaγ in Δint background, RF122 Δint hla::tetM | This study |
| RF122 terL::tetM | RF122 ΔterL | [17] |
| RF122 ΔterL pMin164 terL | Complementation of ΔterL | This study |
| Escherichia coli | ||
| DH5α | Cloning host of pMAD and pMin164 | Life Technologies |
| Top10 | Cloning host of pCR4 | Life Technologies |
| Plasmid | ||
| pMAD-CM | Generating deletion mutants | [17] |
| pMAD-tetM | tetM insertion for screening transduction | [17] |
| pCR4-TOPO | TA cloning vector | Life Technologies |
| pMin164 | High copy number vector for complementation | [18] |
Phage induction and transduction
Cultures were incubated at 37°C with 200 rpm until reaching mid-exponential phase, and then mitomycin C (1 μg/mL, Sigma-Aldrich) was added. Cultures were incubated at 30°C with 80 rpm until clear lysis was observed. The lysates were sterilized with syringe filers (0.22 μm, Nalgene). A phage spot test and the plaque-forming unit (PFU) were determined by soft agar (0.5%) overlay method.
For transduction experiments, the recipient strains were cultured to mid-log phase and adjusted to approximately 2×107 CFU/mL. A phage solution containing approximately 108 PFU/mL was added to the culture, and incubated for 30 min at 30°C for the phage absorption, followed by addition of sodium citrate solution (100 mM, pH 4.5). After centrifuging at 4,000 rpm at 4°C for 15min, the pellet was suspended in sodium citrate solution and plated on TSA supplemented with tetracycline (5 μg/mL).
Phage DNA extraction
Phage DNA purification was performed as previously described [17]. Briefly, heterogeneous chromosomal DNA of E. coli K-12 was added to the culture lysates induced by mitomycin C as a control for verification of DNase treatment previously described [17]. Excessive amounts of DNase I (Sigma-Aldrich, 100 unit each) were added to remove chromosomal DNA. The phage particles were precipitated with NaCl (0.5 M final concentration) and polyethylene glycol 8000 (10%, wt/vol), followed by ultracentrifugation at 100,000 × g for 1 h. Phage DNA was extracted from the pellets using DNeasy kit (Qiagen) according to the manufacturers’ instructions.
PCR, outward PCR, and quantitative real time PCR
All primer pairs used in PCR, outward PCR, and quantitative real time PCR are listed in Table 2. PCR was performed to determine the presence of transducing phage particles harboring MGEs using primers specific to MGEs associated with strain RF122 including: νSaα (the set gene), νSaβ (the lukE gene), and νSaγ (the hla gene), SaPIbov1 (the tst gene), SaPI122 (the mdr gene), and ϕSaBov (the int gene). To estimate the frequency of transducing phage particles harboring MGEs, the absolute copy number of each MGE per nanogram of phage DNA was determined using quantitative real time PCR. Briefly, PCR products specific to MGEs (above) were cloned into pCR™4-TOPO® TA Vector (Life Technologies). Plasmids were quantified using a Nanodrop (Thermo Scientific) and the number of DNA copies/ng was calculated as described previously [19]. Quantitative real time PCR reaction was performed using SYBR green I master mix (Applied Biosystems) and a serial dilution of plasmid templates. Standard curves were generated by linear regression analysis calculating the slope, intercept, and correlation coefficient (R2) using Microcal OriginPro (Microcal origin, Version 7.5). Quantification of MGEs was calculated by interpolation the CT from the standard curve.
Table 2. A list of primers used in this study.
| Name | Sequences (5' to 3') |
|---|---|
| Detection of Specific virulence gene in MGEs | |
| intf | CATCACTGGTGGACGCTTTG |
| intr | AATGCATCGAGCGCTTTTTC |
| set1f | GACAGTAGGCAAGCTGCGAAT |
| set1r | TTTCTCTGCCGTCGATTGACT |
| lukEf | TTTTTTTCCATCAGGCGTAACA |
| lukEr | ACGAATGATTTGGCCATTCC |
| hlaf | GCACTTACTGACAATAGTGCC |
| hlar | TCGCCACCTATATATACCGTTTC |
| tstf | TGAATTTTTTTATCGTAAGCCCTTTG |
| tstr | GGAAATGGATATAAGTTCCTTCGCT |
| mdrf | CTTTTCCTAGAAGATACCGCAATGT |
| mdrr | CCCATCCTTCGTGCGTTAGT |
| qRT-PCR | |
| qintf | CATCACTGGTGGACGCTTTG |
| qintr | AATGCATCGAGCGCTTTTTC |
| qsetf | AGACAAGAACGCACGCCTAAA |
| qsetr | TTATGGTTGGAGATTGTGGTGTGT |
| qlukEf | AGGTGGCAATGGCTCATTTA |
| qlukEr | TTGCTGAACCTGACGGACC |
| qhlaf | GGCCAGGCTAAACCACTTTTG |
| qhlar | GCTAATGCCGCAGATTCTGA |
| qtstf | TGAATTTTTTTATCGTAAGCCCTTTG |
| qtstr | GGAAATGGATATAAGTTCCTTCGCT |
| qmdrf | CTTTTCCTAGAAGATACCGCAATGT |
| qmdrr | CCCATCCTTCGTGCGTTAGT |
| rnaIIIf | TGAGTTATTAAGCCATCCCAACTTAA |
| rnaIIIr | AAAATACATAGCACTGAGTCCAAGGA |
| hlgbf | AGGTAAAATAACACCAGTCAGCGTAA |
| hlgbr | TGGTGCATAATCAACGACGTTT |
| Selective marker | |
| tetMf | GCGCGTCGACGATCAAGAAACAAAGGCAACCCA |
| tetMr | GCGCGAATTCTAGGACACAATATCCACTTGTAG |
| Allelic replacement of set, RF122 set::tetM | |
| setupf | GCGCGGATCCACGCCGAAAACTAAAGTGACA |
| setupr | GCGCGTCGACTGCTAA ACTTGCTTTCGCAAT |
| setdnf | GCGCGAATTCTTGAGTCTCTAAGAACGCCGA |
| setdnr | GCGCAGATCTAAAGACATCAAGGCCATGTGT |
| dsetr | TGCGTATAAACACCTGCGTCT |
| Allelic replacement of hla, RF122 hla::tetM | |
| hlaupf | GCGCGAATCCTTACCTCATATAGTGTCATG |
| hlaupr | GCGCGGTCGACGAAAGGTACCATTGCTGGTC |
| hladnf | GCGCGGAATTCGTCAAATTAGAATATTGCAG |
| hladnr | GCGCGAGATCTAATGCCTATAACTAA AAACC |
| dhlar | AATGAATCCTGTCGCTAATGCC |
| Allelic replacement of tst, RF122 tst::tetM | |
| tstupf | GCGCGTCGACACCAATGCGGCAGTCGGTGAT |
| tstupr | GCGCACGCGTATTGGAAAATAACAATGAATGACGGA |
| tstdnf | GCGCGAATTCCACTACTATACCAGTCTAGCAAAT |
| tstdnr | GCGCCCCGGGGTGTACCAACATCTTTAATTTCTTCA |
| dtstr | AGTTCTATTGGAGTAGGTAATTTTTCAG |
| Allelic replacement of mdr, RF122 mdr::tetM | |
| mdrupf | GCGCGTCGACTAAACCTTAAACCCTCTAATTCAGT |
| mdrupr | GCGCACGCGTAGGAGTACTCATAACAGGTGTCGTTA |
| mdrdnf | GCGCGAATTCTCTTAGATACTCCTCTTTGGTT |
| mdrdnr | GCGCCCCGGGAATATTCGGAATAGGCTCGCAG |
| dmdrr | TGGCCATAATCGCGCCAACGA |
| Generating integrase knock-out strain, RF122 Δint | |
| Intupf | GCGCGGATCCGCTCCTTTACGGAGCTTTAA |
| Intupr | GCGCGTCGACAATAAGGGTAGGCGAGCTAC |
| Intdnf | GCGCGAATTCGCATATCTTGGGAACGTTTC |
| Intdnr | GCGCAGATCTAACAGAGAACATGTTGCTAC |
| Allelic replacement of hlgB, RF122 hlgB::tetM | |
| hlgBupf | GCGCGGATCCCATTCGTGCAATCGGTTACC |
| hlgBupr | GCGCGTCGACAGCTAATCGATTTAGAATAG |
| hlgBdnf | GCGCGAATTCGGCTTTGTGAAACCTAATCC |
| hlgBdnr | GCGCAGATCTGGTCGTCACAATTACTGTG |
| dhlgBr | AATGGCAGTATTACTAAG |
| Generating terminaseL knock-out strain, RF122 ΔterL | |
| Terupf | GCGCGGATCCTGTCAACATGGCTTTTTCTG |
| Terupr | GCGCGTCGACTTGCTGAGGGTCTTGTGTTC |
| Terdnf | GCGCGAATTCCTTTCCGACCACGGGTTAA |
| Terdnr | GCGCAGATCTACGAAAGTTTGCCGGAAATA |
| Outwarding PCR | |
| pself | AGCGGTGTGATTCTGGTGAAT |
| p0342r | TGGCGCACTCATCAAAGAGT |
| pSAB1912f | TGG AAG AGA TTT TAT AAC TAA TTT TG |
| pSaPI122r | CAG TGG GGA CAC CTG TGT AA |
| pIntf | CGAGATTTAACGAGGGATAGG |
| p1702r | TTGACACTAGCTTTCCGTTG |
| Verification of chromosomal DNA contamination in phage DNA preparation | |
| waaQf | TAAAGGTGCGGGAACTTTCG |
| waaQr | AAGCGAGATCATCTGCCGAG |
| Complementation of terL | |
| tercompf | GCTAGGATCCATCGGACTCCGTCCCGTCAT |
| tercompr | GCTAGAATTCAGACTACAAAGAGAATCCCG |
Allelic exchange constructs
All PCR primer pairs used in allelic exchange constructs were listed in Table 2. The gene deletion mutants and the insertion of tetracycline resistance gene marker for screening transduction events were generated by allelic exchange using a modified pMAD-CM and pMAD-tetM temperature-sensitive shuttle vector system [17], respectively. Briefly, the gene fragment of upstream and downstream of target gene were cloned in pMAD-CM and pMAD-tetM. Resulting plasmids were electroporated to E. coli DH5α, and then to strain RF122. RF122 harboring the constructed plasmid were cultured at 43°C (non-permissive temperature for the replication of pMAD) to promote the first homologous recombination, followed by culturing 37°C to promote the second recombination, resulting in allelic exchange.
Phage and bacterial genomic DNA sequencing and analysis
Phage capsid DNA was isolated as described above, and bacterial genomic DNA was isolated with a DNeasy kit according to the manufacturer’s instructions (Qiagen). The dsDNA was quantified with a Qubit HS Assay Kit (Invitrogen). Indexed, paired-end libraries were prepared with a Nextera XT DNA Sample Preparation Kit (Illumina). Libraries were cleaned with 1.2× AMPure XP beads (Agencourt) and sequenced using a 300 cycle MiSeq Reagent Kit v2 on an Illumina MiSeq instrument (Illumina). CLC Genomics Workbench v6 software was used to trim and filter reads for quality and to assemble reads de novo. Recombined regions among the RF122 donor (GenBank NC_007622), CTH96 recipient, and transductants were identified through local alignments.
Results
Transducing phage particles induced from RF122 harbor mobile genetic elements (MGEs)
To investigate the possibility that transducing phage particles induced from strain RF122 harbor genes uniquely associated with MGEs, PCR was performed using phage DNA extracted from RF122 following mitomycin C treatment. To ensure the removal of RF122 chromosomal DNA, exogenous E. coli chromosomal DNA was added to the phage-induced lysates and treated with an excessive amount of DNase I. A PCR targeting the waaQ gene of E. coliwas performed to confirm the removal of chromosomal DNA in the phage DNA preparation (Fig 1A). Results of PCR revealed that transducing phage particles induced from RF122 harbor genes associated with MGEs including genomic islands νSaα (the set gene), νSaβ (the lukE gene), and νSaγ (the hla gene), SaPIbov1 (the tst gene), SaPI122 (the mdr gene), and ϕSaBov (the int gene) (Fig 1B). The genes not belonging to MGE (hlgB and rnaIII) were not detected (Fig 1C). The SaPIbov1and ϕSaBov bordered with direct repeat sequences generated amplification products in outward PCR indicating a circular form of phage DNA. Sequencing of amplification products further confirmed these findings (data not shown). The SaPI122, not bordered with direct repeat sequence did not generate an amplification product suggesting a linear form of phage DNA, as expected for cos-type of sticky-end linear molecules (Fig 1D).
Fig 1. The presence of MGEs in transducing particles induced from the RF122 strain.
(A) Verification of chromosomal DNA removal in preparation of phage DNA by adding exogenous chromosomal DNA of E. coli, followed by the treatment with DNase. The presence of MGEs in the phage DNA from transducing particles induced from the RF122 strain was analyzed by PCR amplification using primers specific to (B) MGEs; νSa (set), νSaβ (lukE), νSaγ (hla), SaPIbov1 (tst), SaPI122 (mdr), ϕSaBov (int) and (C) non-MGE (rnaIII, hlgB). (D) Outward PCR analysis of circularization of MGEs flanked with the direct repeat sequence; SaPIbov1, SaPI122, and ϕSaBov.
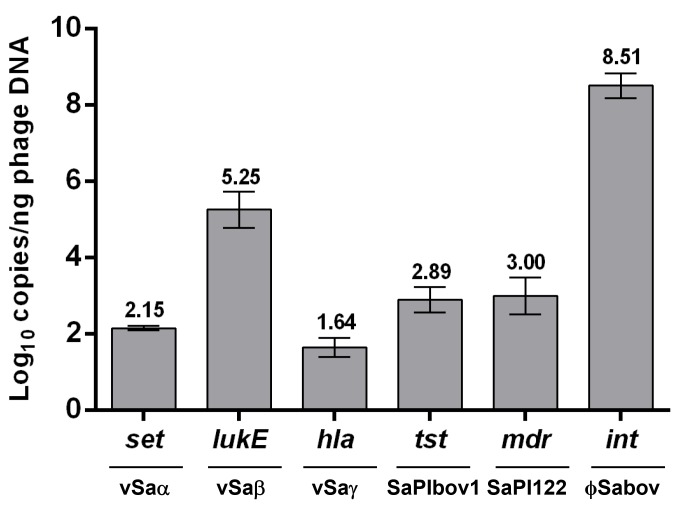
To estimate the frequency of transducing phage particles harboring MGEs, the absolute copy number of each MGE per nanogram of the phage DNA was interpolated from a standard curve generated using quantitative real time PCR (data not shown). The copy number of the ϕSaBov was the highest (8.51 Log10 copies/ng phage DNA), followed by νSaβ (5.25 Log10 copies/ng phage DNA). The copy numbers of νSaα, νSaγ, SaPIbov1, and SaPI122 were lower than the ϕSaBov and νSaβ, and ranged between 1.64–3.00 Log10 copies/ng phage DNA (Fig 2).
Fig 2. Estimation of the absolute copy number of MGEs in phage DNA using quantitative real time PCR.
Sequence analysis of phage DNA
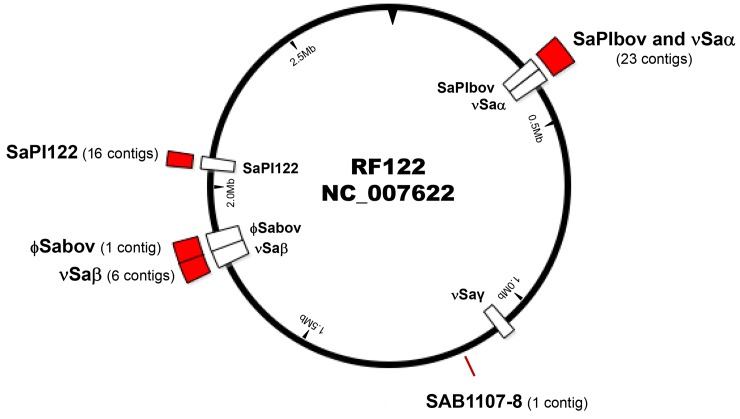
The DNA sequences from phage capsids were determined in order to identify and map the location of the RF122 chromosomal DNA packed into transducing phage particles. A total of 47 contigs (>500 bp) were assembled which clustered in MGEs including νSaα, νSaβ, SaPIbov1, SaPI122, and ϕSaBov, except for a single short contig at SAB1107-8 not known to be located on MGEs (Fig 3). The ϕSaBov was assembled in a single contig, while other MGEs contained multiple contigs ranging 6–23 contigs. A contig corresponding to νSaγ was not found in phage DNA sequencing, which might be due to the low copy number of the νSaγ regions as shown in quantitative real time PCR. These results suggest that the mechanism of excision and packaging of phage DNA is specific to MGEs, rather than a generalized transduction mechanism, which would result in random excision and packaging of host chromosomal DNA.
Fig 3. A schematic map of contigs determined from Illumina MiSeq analysis of phage DNA.
A red box indicates the location and number of contigs detected from sequencing analysis of phage DNA. A white box indicates the location of MGEs (Sa,Sa, Saγ, SaPIbov1, SaPI122, ϕSaBov) present in the RF122 chromosome.
Transfer of MGEs by transducing phage particles induced from RF122
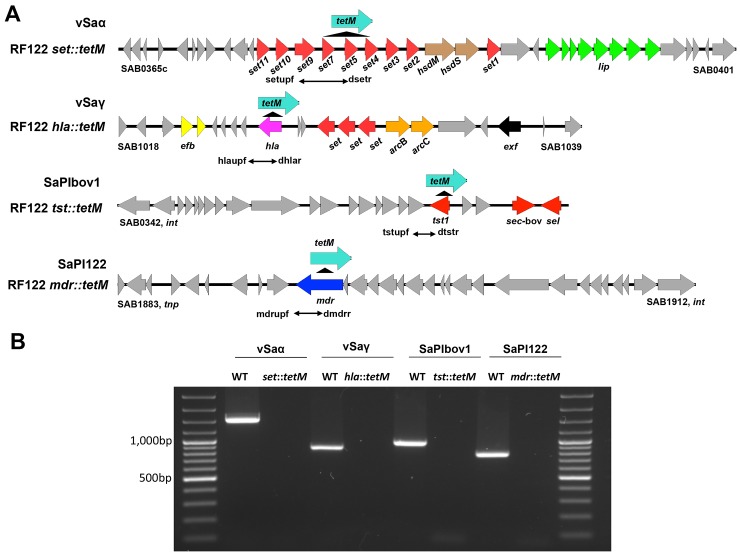
Results of PCR and phage DNA sequencing demonstrated the presence of phage particles harboring MGEs in the RF122 strain. To test the transfer of MGEs by these transducing phage particles to other S. aureus, the tetM gene, conferring tetracycline resistance, was introduced into the MGEs as depicted in Fig 4A by homologous recombination using a modified pMAD system. The insertion of the tetM gene was confirmed by PCR (Fig 4B). The transfer of MGEs was successful mostly in the ST151 lineage (Table 3). Other than ST151 strains, νSaα and SaPIbov1 were transferred only to ST1-SCCmecIV and ST398, respectively. Interestingly, among the ST151 strains, SaPIbov1 was only transferred to recipients (RF114, 38963, CI2135) which do not have SaPIbov1 in the genome. SaPIbov1 was not transferred to the recipients (CTH96, RF113, DS102) which have pre-existing SaPIbov1 in the genome. We also inserted the tetM gene into the non-MGE gene (hlgB) to test random excision and packaging of phage DNA as in true generalized transduction, but transduction was not observed even with the recipients belonging to the ST151 lineage (data not shown).
Fig 4. Schematic maps of the tetM gene insertion in the MGEs present in the RF122 strain.
(A) The tetM gene was inserted into the MGEs present in the RF122 strain, including νSaα, νSaγ, SaPIbov1 and SaPI122, resulting RF122 set::tetM, RF122 hla::tetM, RF122 tst::tetM, and RF122 mdr::tetM, respectively. (B) The insertion of tetM was confirmed by PCR using primer sets designed to present a negative result in the insertional mutants, compared to the RF122 wild type strain (WT).
Table 3. Transduction frequencies of mobile genetic elements.
| Recipient Origin | Recipient Genotypes | Strain | MGE | |||
|---|---|---|---|---|---|---|
| νSaα | νSaγ | SaPIbov1 | SaPI122 | |||
| aBovine (29) | ST151 b(6/6) | CTH96 | c1.30×10−6 | 8.00×10−7 | None | None |
| RF113 | 1.65×10−5 | 7.00×10−7 | None | 2.43×10−6 | ||
| RF114 | 1.10×10−6 | 1.40×10−6 | 3.60×10−7 | None | ||
| 38963 | 2.70×10−6 | 1.00×10−6 | 3.10×10−7 | None | ||
| CI2135 | 2.70×10−6 | 1.50×10−6 | 4.00×10−8 | None | ||
| DS102 | 3.50×10−6 | None | None | None | ||
| ST1 (0/3) | None | None | None | None | ||
| ST188 (0/8) | None | None | None | None | ||
| ST20 (0/5) | None | None | None | None | ||
| ST72 (0/4) | None | None | None | None | ||
| ST398 (1/3) | K31 | None | None | 4.5×10−7 | None | |
| Human (22) | ST1-SCCmecIV (3/7) | MW2 | 2.00×10−8 | None | None | None |
| MN KN | 3.10×10−7 | None | None | None | ||
| C99-529 | 1.00×10−8 | None | None | None | ||
| C99-193 | None | None | None | None | ||
| MN Gary | None | None | None | None | ||
| MN Ask | None | None | None | None | ||
| MN MA | None | None | None | None | ||
| ST8-SCCmecIV (0/7) | None | None | None | None | ||
| ST36-SCCmecII (0/8) | None | None | None | None |
aThe number of tested strains is in parenthesis.
bThe number of strains transduced with any MGE/the number of strains tested.
cTransduction frequencies (CFU/pfu)
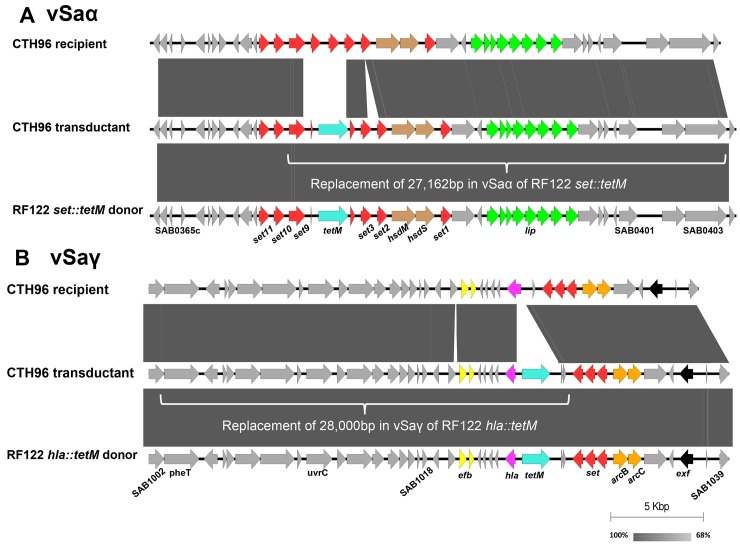
To further confirm the transfer of MGEs, a draft genome sequence of a recipient (CTH96) and transductants (CTH96 νSaα transductant and CTH96 νSaγ transductant) was determined. Based on single nucleotide polymorphisms (SNPs) and the tetM gene found in the CTH96 νSaα transductant, approximately 27,162 bp of νSaα was transferred from donor to recipient, ranging from SAB0378 to SAB0403 and including a part of the set gene locus, hsdM, hsdS, and the lip gene locus (Fig 5A). The sequence comparison of the CTH96 νSaγ transductant suggested that approximately 28,000 bp of νSaγ was transferred from donor to recipient, ranging from SAB1002 to SAB1029 and including phenylalanyl-tRNA synthetase (pheT), excinuclease ABC subunit C (uvrC), recombination and DNA strand exchange inhibitor protein (mutS2), ribonuclease III (rnc), succinate dehydrogenase (sdh), extracellular fibrinogen binding protein (efb), and the hla gene (Fig 5B).
Fig 5.
A schematic map of sequence alignments among RF122 (donor), CTH96 (recipient), and CTH96 transductants of phage induced from RF122 set::tetM (A) and RF122 hla::tetM (B). White brackets indicated the identical sequence between CTH96 transductant and RF122 donor, suggesting gene transfer from RF122 to CTH96. The shading between the entries represents the percent identity (BLASTn) from 68% (light gray) to 100% (dark gray) using Easyfig.
The role of integrase and terminase on ϕSaBov in the transfer of MGEs
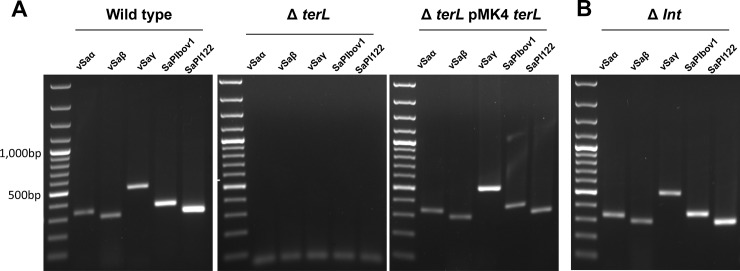
The integrase and terminase encoded in MGEs, including bacteriophages and SaPIs, are important for phage DNA excision, packaging, and integration, in concert with other factors derived from the donor or recipient [15]. However, the integrase and terminase were not present in the genomic islands νSaα and νSaγ. To test the involvement of the integrase (int) and terminase large subunit (terL) encoded by ϕSaBov in the transfer of these genomic islands, the int or terL gene was deleted in the RF122 strain. PCR analysis of phage DNA extracted from these strains showed that disruption of the terL gene completely abrogated phage DNA packaging, which could be restored by complementation of terL (Fig 6A). In contrast, disruption of the int gene did not affect DNA packaging (Fig 6B) nor the transfer of νSaα and νSaγ (Table 4). These results indicate that packaging of genomic islands in ϕSaBov is dependent on the terminase, but does not require integrase.
Fig 6. The roles of terminase large subunit (TerL) and intergrase (Int) on phage DNA excision and packaging.
The terminase large subunit knock-out and integrase knock-out strains were generated from the RF122 strain by allelic replacement. The presence of MGEs in the phage DNA was analyzed by PCR with primers specific to each MGEs; νSaα (set), νSaβ (lukD), νSaγ (hla), SaPIbov1 (tst), and SaPI122 (mdr). A disruption of the TerL completely abolished the phage DNA packaging events. A complementation of TerL by pMin164 terL restored the phage DNA packaging events. (B) A disruption of the Int did not affect the phage DNA packaging events.
Table 4. The role of the integrase encoded in the ϕSaBov on transduction frequencies of MGEs.
| RF122 | RF122Δint | |||
|---|---|---|---|---|
| Recipient strains | νSaα | νSaγ | νSaα | νSaγ |
| CTH96 | a1.45×10−6 | 7.60×10−7 | 1.50×10−6 | 2.00×10−7 |
| RF113 | 1.85×10−5 | 7.20×10−7 | 1.70×10−6 | 1.30×10−7 |
| RF114 | 1.30×10−6 | 1.50×10−6 | 1.00×10−6 | 2.10×10−7 |
| 38963 | 2.50×10−6 | 1.20×10−6 | 2.00×10−6 | 1.50×10−7 |
| CI2135 | 2.90×10−6 | 1.40×10−6 | 2.50×10−6 | 2.00×10−7 |
| DS102 | 3.90×10−6 | None | 4.00×10−6 | None |
aTransduction frequencies (CFU/pfu)
In pac-type phages, the terminase small subunit (TerS) recognizes and binds to the phage-specific packaging (pac) site typically located in or near to the terL gene that initiates a hetero-oligomer complex with TerL resulting in headful phage DNA packaging [15, 20, 21]. Analysis of phage DNA sequence showed an 11 bp consensus sequence present in ϕSaBov, νSaα, and νSaγ (Fig 7), suggesting that MGE-specific phage DNA packaging by ϕSaBov might result from the recognition of pac sites in the MGE by TerS that induce the headful packaging mechanism in concert with TerL and other factors in the ϕSaBov.
Fig 7. An 11 bp consensus sequence identified near to the terL gene using MEME (http://meme-suite.org/index.html) was conserved in MGEs packaged in the transducing phage particles.
Putative pac site in MGEs. Sequence logos separated with colors indicated the frequencies scaled relative to the conservation at each position.
Discussion
A transfer of MGEs by temperate phages requires a series of successful sequential events including excision from the host genome, packaging into transducing phage particles, transfer to a recipient and integration into the recipient genome. To investigate phage DNA excision events, phage DNA analysis using PCR and sequencing was performed. These results revealed that phage DNA excision events by ϕSaBov are highly specific to chromosomal locations of MGEs, suggesting a mechanism of sequence-specific excision events. It has been shown elsewhere that a typical pac-type phage DNA excision event is highly specific to the direct repeat sequence recognized by the integrase, excisionase, and terminase small unit [15, 21]. For MGEs, such as ϕSaBov and SaPIbov1, that are flanked by direct repeat sequences, phage DNA excision events occurred at the direct repeat sequence and formed a circular form of phage DNA. This process was likely controlled by their own integrase, excisionase, and terminases. For MGEs not flanked by direct repeat sequences, including νSaα and νSaγ, phage DNA excision events occurred at various locations within these genomic islands. νSaα and νSaγ do not possess annotated genes for excision events such as integrase, excisionase, and terminase, so phage DNA excision events in these MGEs might be controlled by other elements associated with SaPIs, transposases, integrative and conjugative elements in the host background. To support this possibility, the RN4220 harboring ϕSaBov, which has a different background of integrase, transposases, and integrative and conjugative elements than RF122 and lacks MGEs such as SaPIbov1, SaPI122, did not generate transducing phage particles harboring MGEs (data not shown).
As determined by PCR and DNA sequence analysis of phage DNA, transduction events are specific to MGEs rather than the random events that are expected from generalized transduction. A recent study demonstrated that the TerS encoded by SaPIs induced sequence-specific excision and packaging of phage DNA at unlinked chromosomal segments [15]. In our current study, sequence analysis of MGEs present in transducing particles revealed potential consensus sequences in these MGEs which might mediate DNA excision/packaging events by TerS encoded in the ϕSaBov, SaPIbov1 or SaPI122. Currently, the role of TerS and these potential consensus sequences on horizontal gene transfer is under investigation.
A higher frequency of MGE transfer by ϕSaBov was observed with recipient strains belonging to ST151 than with other strain lineages. These results suggest that the host specificity of phage might be a primary barrier in the transfer of MGE by phage. The host specificity of phage is expected to be determined by interactions between the tail proteins of phage and the target molecules in recipient cells such as proteins and wall teichoic acid (WTA) which were generally conserved in the core genome of the same or related lineages of strain [22–25]. As such, strains belonging to ST151 lineage commonly express receptors to ϕSaBov and thereby become more susceptible to transduction. Interestingly, SaPIbov1 was not transferred to ST151 recipients that already possess SaPIbov1 in the genome. This observation is not likely superinfection immunity due to lysogenic phages since phage was not induced by mitomycin C treatment in these recipient strains [26]. It is possible that the conserved transcriptional regulators in the SaPIs, such as stl and str [27, 28], might negatively regulate transcription of elements required for the integration of the SaPIbov1.
To confirm the transfer of MGEs by ϕSaBov, a draft genome sequence of the transductants for νSaα and νSaγ was determined. The extent of the transfer of MGEs was estimated by comparing single nucleotide polymorphisms and the presence of the tetM gene among donors, recipients, and transductants. Based on these comparisons, it was hypothesized that linear phage DNA segments harboring parts of νSaα and νSaγ were transferred, rather than a transfer of a single contig harboring entire νSaα or νSaγ. This result supports findings that the overall structure of genomic islands can vary as a result of “plug and play” type of recombinational replacements [29]. To our knowledge, this is the first report to demonstrate a mobilization of νSaα and νSaγ. Although the hla gene was largely thought to have evolved with the core chromosome of S. aureus, recombination events are evident in some strains [30, 31]. Our results suggest one mechanism by which the hla gene could be horizontally transferred among S. aureus strains.
Our previous study demonstrated that the int gene encoded by the ϕSaBov is required for integration of phage DNA harboring ϕSaBov and νSaβ into recipient genome, but not for phage DNA excision events of ϕSaBov and νSaβ [17]. Further, the terL encoded in the ϕSaBov is required for packaging phage DNA of ϕSaBov and νSaβ [17]. Similarly, in this study, the int gene encoded by ϕSaBov is not required for phage DNA excision events in νSaα and νSaγ nor for the integration into recipient genome. However, the terL is essential for packaging into transducing phage particles. These results suggest the major role of temperate phage in transfer of νSaα and νSaγ is to package DNA and shuttle it via transducing phage particles.
Although it has long been acknowledged that bacteriophages contribute the horizontal transfer of MGEs by generalized and specialized transduction, the causal link to the transfer of genomic islands by bacteriophages or other means has not been established. In this study, for the first time, we present experimental evidence demonstrating transfer of genomic islands by a temperate phage utilizing MGE-specific DNA excision events from the host genome and presumably recipient-derived factors for integration. These results represent the complexity of underlying mechanism of phage-bacterial interaction in horizontal gene transfer and extend our understanding of the important role of bacteriophage in the horizontal transfer and evolution of genomic islands in S. aureus.
Acknowledgments
This work was partially supported by grants from National Institute of Food and Agriculture, United States Department of Agriculture (2008-35204-04582) to K.S.S.; Center for Biomedical Research Excellence in Pathogen-Host interactions, National Institute of General Medical Sciences, NIH (1P20GM103646-01A1) to K.S.S.; Animal and Plant Quarantine Agency, South Korea Department of Agriculture to K.S.S; National Institute of General Medical Sciences, NIH (GM080602) to D.A.R.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was partially supported by grants from National Institute of Food and Agriculture, United States Department of Agriculture (2008-35204-04582) to K.S.S.; Center for Biomedical Research Excellence in Pathogen-Host interactions, National Institute of General Medical Sciences, NIH (1P20GM103646-01A1) to K.S.S.; Animal and Plant Quarantine Agency, South Korea Department of Agriculture to K.S.S; National Institute of General Medical Sciences, NIH (GM080602) to D.A.R. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schmidt H, Hensel M. Pathogenicity islands in bacterial pathogenesis. Clinical microbiology reviews. 2004;17(1):14–56. Epub 2004/01/17. PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malachowa N, DeLeo FR. Mobile genetic elements of Staphylococcus aureus. Cellular and molecular life sciences: CMLS. 2010;67(18):3057–71. Epub 2010/07/30. 10.1007/s00018-010-0389-4 ; Central PMCID: PMC2929429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juhas M, van der Meer JR, Gaillard M, Harding RM, Hood DW, Crook DW. Genomic islands: tools of bacterial horizontal gene transfer and evolution. FEMS microbiology reviews. 2009;33(2):376–93. Epub 2009/01/31. 10.1111/j.1574-6976.2008.00136.x ; Central PMCID: PMC2704930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth DS, Robinson DA. Integrative and sequence characteristics of a novel genetic element, ICE6013, in Staphylococcus aureus. Journal of bacteriology. 2009;191(19):5964–75. Epub 2009/08/04. 10.1128/jb.00352-09 ; Central PMCID: PMC2747909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Everitt RG, Didelot X, Batty EM, Miller RR, Knox K, Young BC, et al. Mobile elements drive recombination hotspots in the core genome of Staphylococcus aureus. Nature communications. 2014;5:3956 Epub 2014/05/24. 10.1038/ncomms4956 ; Central PMCID: PMC4036114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spaulding AR, Salgado-Pabon W, Kohler PL, Horswill AR, Leung DY, Schlievert PM. Staphylococcal and streptococcal superantigen exotoxins. Clinical microbiology reviews. 2013;26(3):422–47. Epub 2013/07/05. 10.1128/cmr.00104-12 ; Central PMCID: PMC3719495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hidron AI, Low CE, Honig EG, Blumberg HM. Emergence of community-acquired meticillin-resistant Staphylococcus aureus strain USA300 as a cause of necrotising community-onset pneumonia. The Lancet Infectious diseases. 2009;9(6):384–92. Epub 2009/05/27. 10.1016/s1473-3099(09)70133-1 . [DOI] [PubMed] [Google Scholar]
- 8.Sheikh HQ, Aqil A, Kirby A, Hossain FS. Panton-Valentine leukocidin osteomyelitis in children: a growing threat. British journal of hospital medicine (London, England: 2005). 2015;76(1):18–24. Epub 2015/01/15. 10.12968/hmed.2015.76.1.18 . [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald JR. Evolution of Staphylococcus aureus during human colonization and infection. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2014;21:542–7. Epub 2013/04/30. 10.1016/j.meegid.2013.04.020 . [DOI] [PubMed] [Google Scholar]
- 10.Holmes A, McAllister G, McAdam PR, Hsien Choi S, Girvan K, Robb A, et al. Genome-wide single nucleotide polymorphism-based assay for high-resolution epidemiological analysis of the methicillin-resistant Staphylococcus aureus hospital clone EMRSA-15. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(2):O124–31. Epub 2013/08/10. 10.1111/1469-0691.12328 . [DOI] [PubMed] [Google Scholar]
- 11.Hallin M, De Mendonca R, Denis O, Lefort A, El Garch F, Butaye P, et al. Diversity of accessory genome of human and livestock-associated ST398 methicillin resistant Staphylococcus aureus strains. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011;11(2):290–9. Epub 2010/12/15. 10.1016/j.meegid.2010.10.021 . [DOI] [PubMed] [Google Scholar]
- 12.Baba T, Bae T, Schneewind O, Takeuchi F, Hiramatsu K. Genome sequence of Staphylococcus aureus strain Newman and comparative analysis of staphylococcal genomes: polymorphism and evolution of two major pathogenicity islands. Journal of bacteriology. 2008;190(1):300–10. Epub 2007/10/24. 10.1128/jb.01000-07 ; Central PMCID: PMC2223734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobrindt U, Hochhut B, Hentschel U, Hacker J. Genomic islands in pathogenic and environmental microorganisms. Nature reviews Microbiology. 2004;2(5):414–24. Epub 2004/04/22. 10.1038/nrmicro884 . [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Chen CJ, Su LH, Hu S, Yu J, Chiu CH. Evolution and pathogenesis of Staphylococcus aureus: lessons learned from genotyping and comparative genomics. FEMS microbiology reviews. 2008;32(1):23–37. Epub 2007/11/07. 10.1111/j.1574-6976.2007.00086.x . [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Ram G, Penades JR, Brown S, Novick RP. Pathogenicity island-directed transfer of unlinked chromosomal virulence genes. Molecular cell. 2015;57(1):138–49. Epub 2014/12/17. 10.1016/j.molcel.2014.11.011 ; Central PMCID: PMC4289434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Molecular microbiology. 1998;29(2):527–43. Epub 1998/08/28. . [DOI] [PubMed] [Google Scholar]
- 17.Moon BY, Park JY, Hwang SY, Robinson DA, Thomas JC, Fitzgerald JR, et al. Phage-mediated horizontal transfer of a Staphylococcus aureus virulence-associated genomic island. Scientific reports. 2015;5:9784 Epub 2015/04/22. 10.1038/srep09784 ; Central PMCID: PMC4402969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray DL, Prasad GS, Earhart CA, Leonard BA, Kreiswirth BN, Novick RP, et al. Immunobiologic and biochemical properties of mutants of toxic shock syndrome toxin-1. Journal of immunology (Baltimore, Md: 1950). 1994;152(1):87–95. Epub 1994/01/01. . [PubMed] [Google Scholar]
- 19.Yin JL, Shackel NA, Zekry A, McGuinness PH, Richards C, Putten KV, et al. Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) for measurement of cytokine and growth factor mRNA expression with fluorogenic probes or SYBR Green I. Immunology and cell biology. 2001;79(3):213–21. Epub 2001/05/31. 10.1046/j.1440-1711.2001.01002.x . [DOI] [PubMed] [Google Scholar]
- 20.Black LW. DNA packaging in dsDNA bacteriophages. Annual review of microbiology. 1989;43:267–92. Epub 1989/01/01. 10.1146/annurev.mi.43.100189.001411 . [DOI] [PubMed] [Google Scholar]
- 21.Casjens SR. The DNA-packaging nanomotor of tailed bacteriophages. Nature reviews Microbiology. 2011;9(9):647–57. Epub 2011/08/13. 10.1038/nrmicro2632 . [DOI] [PubMed] [Google Scholar]
- 22.Xia G, Wolz C. Phages of Staphylococcus aureus and their impact on host evolution. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2014;21:593–601. Epub 2013/05/11. 10.1016/j.meegid.2013.04.022 . [DOI] [PubMed] [Google Scholar]
- 23.Bartual SG, Otero JM, Garcia-Doval C, Llamas-Saiz AL, Kahn R, Fox GC, et al. Structure of the bacteriophage T4 long tail fiber receptor-binding tip. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(47):20287–92. Epub 2010/11/03. 10.1073/pnas.1011218107 ; Central PMCID: PMC2996694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia G, Corrigan RM, Winstel V, Goerke C, Grundling A, Peschel A. Wall teichoic Acid-dependent adsorption of staphylococcal siphovirus and myovirus. Journal of bacteriology. 2011;193(15):4006–9. Epub 2011/06/07. 10.1128/jb.01412-10 ; Central PMCID: PMC3147540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winstel V, Liang C, Sanchez-Carballo P, Steglich M, Munar M, Broker BM, et al. Wall teichoic acid structure governs horizontal gene transfer between major bacterial pathogens. Nature communications. 2013;4:2345 Epub 2013/08/24. 10.1038/ncomms3345 ; Central PMCID: PMC3903184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nature reviews Microbiology. 2010;8(5):317–27. Epub 2010/03/30. 10.1038/nrmicro2315 . [DOI] [PubMed] [Google Scholar]
- 27.Mir-Sanchis I, Martinez-Rubio R, Marti M, Chen J, Lasa I, Novick RP, et al. Control of Staphylococcus aureus pathogenicity island excision. Molecular microbiology. 2012;85(5):833–45. Epub 2012/06/30. 10.1111/j.1365-2958.2012.08145.x . [DOI] [PubMed] [Google Scholar]
- 28.Ubeda C, Maiques E, Barry P, Matthews A, Tormo MA, Lasa I, et al. SaPI mutations affecting replication and transfer and enabling autonomous replication in the absence of helper phage. Molecular microbiology. 2008;67(3):493–503. Epub 2007/12/19. 10.1111/j.1365-2958.2007.06027.x . [DOI] [PubMed] [Google Scholar]
- 29.Herron-Olson L, Fitzgerald JR, Musser JM, Kapur V. Molecular correlates of host specialization in Staphylococcus aureus. PloS one. 2007;2(10):e1120 Epub 2007/11/01. 10.1371/journal.pone.0001120 ; Central PMCID: PMC2040198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berube BJ, Bubeck Wardenburg J. Staphylococcus aureus alpha-toxin: nearly a century of intrigue. Toxins. 2013;5(6):1140–66. Epub 2013/07/31. ; Central PMCID: PMC3717774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill SR, Fouts DE, Archer GL, Mongodin EF, Deboy RT, Ravel J, et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. Journal of bacteriology. 2005;187(7):2426–38. Epub 2005/03/19. 10.1128/jb.187.7.2426-2438.2005 ; Central PMCID: PMC1065214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.