Abstract
Introduction
Glycolysis affects glucose determination in vitro. The placement of sample tubes in ice-water slurry with plasma separation within 30 minutes is recommended, or alternatively the use of a glycolysis inhibitor. The aim of our two-steps study was to evaluate which Terumo tube is best for glucose determination in routine clinical setting.
Materials and methods
In the first study, blood from 100 volunteers was collected into lithium heparin (LH), NaF/Na heparin (FH) and NaF/citrate buffer/Na2EDTA (FC-Mixture) tubes. LH sample was treated as recommended and considered as reference, while FH and FC-Mixture samples were aliquoted, maintained at room temperature (RT) for 1, 2 and 4 hours; centrifuged and plasma analysed in triplicate. In the second study, samples from 375 volunteers were collected in LH, FH and FC-Mixture tubes and held at RT before centrifugation from 10 to 340 minutes, depending on each laboratory practice. Samples were analysed in one analytical run.
Results
In the first study, FH glucose concentrations were 5.15 ± 0.66 mmol/L, 5.05 ± 0.65 mmol/L and 5.00 ± 0.65 mmol/L (P < 0.001) in tubes stored at RT for 1, 2 and 4 hours, respectively. Mean biases in all time points exceeded the analytical goal for desirable bias based on biological variation criteria. FC-Mixture glucose concentrations were 5.48 ± 0.65 mmol/L, 5.46 ± 0.6 mmol/L and 5.46 ± 0.64 mmol/L in tubes stored at RT for 1, 2 and 4 hours, respectively. Mean biases for FC-Mixture glucose in all time points reached optimal analytical goals. In the second study, the biases for LH and FH glucose compared to reference FC-Mixture glucose exceeded the preset analytical goals, regardless of the blood collection to centrifugation time interval.
Conclusions
FC-mixture tubes glucose concentrations were preserved up to 4h storage at RT. We confirmed that NaF alone does not allow immediate glycolysis inhibition in real life pre-centrifugation storage conditions (up to 340 minutes). FC-Mixture should be used exclusively for glucose determination in laboratories unable to implement the recommended blood samples’ treatment.
Key words: glucose, pre-analytical phase, sodium fluoride, citrate acidification, stability
Introduction
Laboratory plasma glucose testing is fundamental for the diagnosis of diabetes mellitus (DM), impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) and in particular for the screening and diagnosis of gestational diabetes mellitus (GDM) where HbA1c can’t be used (1-3). Therefore is evident that the plasma glucose measurement needs to be accurate and precise for correct patient classification according to the well-established international guidelines (4).
The principally used analytical methods for glucose determination are enzymatic assays, based on the hexokinase (recommended) or on the glucose oxidase reaction (5). These methods are highly standardized with an inter-laboratory imprecision (CV) < 2.6% (1, 4, 5).
Thus, in glucose measurement, the analytical variation is highly reduced while it is the pre-analytical variation that is responsible for most of total variation in glucose determination (4, 6). It has been reported an in vitro decrease in plasma glucose samples, not immediately centrifuged, of 5 - 7% per hour due to glycolysis (7).
To minimize in vitro glycolysis, the American Diabetes Association (ADA) and the National Academy of Clinical Biochemistry (NACB) recommend to immediately place the sample tube in ice-water slurry and to separate plasma from cells within 30 minutes (8) and, if this is not possible, as in most Italian laboratories, to use a sample tube containing rapid glycolysis inhibitor (such as citrate buffer) (8).
In order to perform this task, sodium fluoride (NaF) exerting its action on enolase, an enzyme which catalysed a down-stream step of glycolysis, played a fundamental role in the past. However, the inhibitory effect of NaF on glycolysis appears only after 2-3 hours from blood collection, with the effect that the glucose concentration in this lapse of time decreases (5, 7, 9).
In the last years the use of blood-collection tubes with NaF and citrate buffer have been proposed: the acidification of blood below pH 5.9 inhibits the hexokinase and phosphofructokinase, enzymes involved in the up-stream steps of glycolysis, therefore ensuring a quicker inhibition of it (10, 11).
Since the site of blood drawing is far from the central laboratory for most of Italian laboratories (even hundreds of kilometres), which in turn prevents the implementation of ADA-NACB guidelines for sample treatment, the “Italian joint SIBioC-SIPMeL Study Group on Diabetes Mellitus” has performed a two-steps study using VenosafeTM tubes (Terumo, Rome, Italy): 1) an experimental pilot project to evaluate the effect of different glycolysis inhibitors on the stability of glycaemia in samples maintained at room temperature (RT) for 1, 2 and 4 hours; and 2) a multi-centric study to verify how different real life conditions of blood collection (i.e. use of lithium-heparin or sodium fluoride as anticoagulants) and processing (i.e. time between blood collection and centrifugation) could influence plasma glucose determination.
Our hypothesis is that the use of an immediate glycolysis inhibitor has to be regarded as necessary to perform a correct diagnosis or monitoring of DM.
Materials and methods
Experimental study
Study population
The study has been performed in the Clinical Chemistry Laboratory of Spedali Civili of Brescia between April 2013 and May 2014, following the ethical standards of the revised Declaration of Helsinki. One hundred fasting and non-fasting volunteers (29 males, 71 females), median age 44 years (range: 18-75 years), recruited from the staff of the laboratories of Spedali Civili of Brescia and their relatives, have been subjected to venous blood collection from antecubital fossa of the arm between 9.00 a.m. and 2.00 p.m. by a single skilled phlebotomist through tubing 21 G*3/4’’ (Terumo, Rome, Italy, ref. MN*SV21) and holder (Terumo, Rome, Italy, ref. XX-VF010HGS).
Study design
A total of 11 mL of blood was collected from each volunteer in three different VenosafeTM tubes (Terumo, Rome, Italy):
Lithium heparin (LH) 4 mL draw (ref. VF-054SHL)
NaF/Na Heparin (FH) 4 mL draw (ref. VF-054SFH)
NaF/citrate buffer/Na2EDTA (FC-Mixture) 3 mL draw (ref. VF-053SFC32).
The LH sample was subjected to the ADA - NACB recommended gold-standard treatment (8): after blood collection it was immediately placed in ice-water slurry and centrifuged at 2500 x g for 15 minutes at 4 °C using J6-MI centrifuge (Beckman Coulter, Milan, Italy). Plasma was finally separated from blood cells and kept in 1.5 mL tubes at 4 °C until analysis.
The FH and FC-Mixture whole blood samples were immediately separated after blood collection in three aliquots (1.3 mL of FH and 1 mL of FC-Mixture whole blood sample, respectively) and kept in three 1.5 mL tubes placed at room temperature (20 - 25 °C). After a storage period of 1, 2 and 4 hours, they were centrifuged at 2500 x g for 15 minutes at 4 °C to obtain complete separation of plasma from blood cells. Plasma was then stored in 1.5 mL tubes at 4 °C until analysis.
Methods
Glucose testing of all samples of the same subject was performed in triplicate in the same analytical run, in order to reduce analytical variability (CV intra-assay < 0.99%). Glucose concentrations were measured by hexokinase method on a Dimension Vista 1500 system (Siemens Healthcare Diagnostics, Milan, Italy).
Multi-centre study
Study population
The multi-centre study involved 15 Italian laboratories (Metabolic Diseases and Diabetology Unit-INRCA-IRCCS, Ancona; Clinical Chemistry Laboratory, Spedali Civili of Brescia, Brescia; Clinical Laboratory & Molecular Diagnostics, INRCA National Institute, Ancona; Clinical Chemistry and Haematology Laboratory, St. Bortolo Hospital, Vicenza; Laboratory of Clinical Pathology, Hospital SS. Annunziata, Chieti; Department of Clinical and Experimental Medicine, University Hospital of Messina; Laboratory Medicine, Azienda ULSS No. 9, Treviso; Clinical Biochemistry Laboratory, Bracciano Hospital, Rome; Laboratory of Clinical Chemistry and Hematology, University Hospital of Parma, Parma; Clinical Laboratory, AOUI Verona, Verona; ASUR 8, Recanati, Macerata; Servizio di Medicina di laboratorio, San Raffaele Scientific Institute, Milan; Ravenna Spoke Laboratory, AUSL Romagna; Laboratory of Clinical Chemistry - Hospital Agency Pugliese, Ciaccio, Catanzaro; Department of Laboratory Medicine, University Hospital, Padova). Blood samples were collected from the antecubital vein of 375 fasting and non-fasting volunteers (142 males and 233 females), aged 51 years (range: 18-91 years), to cover a wide range of plasma glucose concentrations (2.44 - 25.17 mmol/L). To minimize venipuncture bias in each laboratory, all venipunctures were performed by a single skilled phlebotomist.
Study design
A total of 11 mL of blood was collected from each volunteer in three different VenosafeTM LH, FH and FC-Mixture tubes (Terumo, Rome, Italy). Immediately after venipuncture the blood was thoroughly mixed with the anticoagulant by inverting the tubes several times, according to manufacturer’s recommendations (12). Sample tubes were kept at room temperature (variable from each laboratory from 15-25 °C) and then centrifuged.
The time from blood sample collection to centrifugation was established by each laboratory and was recorded (between 10 and 340 minutes for all the samples). 138 samples had a collection-centrifugation interval shorter than 60 minutes (min), 100 samples an interval between 60 and 120 min, 77 samples between 121 and 180 min and 60 samples had a collection-centrifugation interval longer than 180 min. Since the recommended treatment of blood samples according to ADA-NACB guidelines cannot be performed in most Italian laboratories, in this multi-centre study the FC-Mixture tube, containing citrate buffer, was established to be the reference tube for an accurate glucose determination (8).
Methods
Glucose determinations in all plasma samples (FC-Mixture, LH and FH) of the same volunteer were performed in a single analytical run, according to the instruments’ recommendations used in each laboratory: Cobas 6000, Cobas Integra, C702, C501 and Hitachi 912 (Roche Diagnostics, Monza, Italy); ADVIA 2400 and Dimension Vista 1500 (Siemens Healthcare Diagnostics, Milan, Italy); Unicel DxC 600i and AU5800 (Beckman Coulter, Milan, Italy); Vitros fusion (Ortho Clinical Diagnostics, Milan, Italy). All the above-mentioned instruments use the hexokinase enzymatic method, with the exception of dry chemistry in Vitros system which uses the glucose oxidase method. The mean coefficient of variation (CV) of participating laboratories was 1.5 ± 0.34%.
Statistical analysis
Continuous variables were tested for normality by the Kolmogorov–Smirnov test and are expressed as mean ± standard deviation (SD) if normally distributed or as median and interquartile range (IQR) if not normally distributed, respectively (13). Repeated measures ANOVA and pair-wise comparison using Bonferroni correction or Friedman test were used to test the difference between glucose concentrations in FH, FC-Mixture and LH tubes and the gold standard treatment according to ADA – NACB guidelines in the experimental study and to test the difference between glucose concentration in FH, LH and FC-Mixture tubes in the multi-centre study; as well as to test differences in serial glucose measurements performed after 1, 2 and 4 hours storage at RT in the experimental study. If P of ANOVA was significant, a post-hoc comparison was made using the paired t-Student test.
In the experimental study bias from reference glucose was calculated as:
B = [ (Gluval / Gluref) x 100 ] - 100where Gluval represents glucose concentration (mean of triplicate measurements) in FH and FC-Mixture tubes and Gluref represents glucose concentration (mean of triplicate measurements) in the reference tube (LH with gold standard treatment according ADA – NACB guidelines).
In the multicentric study bias from reference glucose was calculated as:
B = [ (Gluval / GluFCMix) x 100 ] - 100where Gluval represents glucose concentration in LH and FH tubes while GluFCMIX represents glucose concentration in real life reference tube (FC-Mixture, according ADA – NACB guidelines).
The acceptance criteria for bias and for total allowable error (TEa) were defined according biological variation studies (14) (desirable bias ≤ 1.8%, optimal bias ≤ 0.92% and TEa ≤ 4.0%). Total allowable error (TEa) limits were defined using the following equation:
TEa = Biasdes + 1.96 + (CVdes / RADsq3)where Biasdes is the desirable bias according biological variability (1.8%) and CVdes is the desirable imprecision according biological variability (2.3%) and RADsq3 is the correction of imprecision for triplicate measurements, that is TEa of 4.0%.
In the experimental project Bland-Altman analysis was used to assess the agreement between glucose concentrations in FH, FC-Mixture and in reference LH tubes with ADA-NACB gold standard treatment.
P-values < 0.05 were considered statistically significant. Statistical analysis was performed using the MedCalc statistical software version 15.11.0 (MedCalc, Ostend, Belgium).
Results
Experimental project
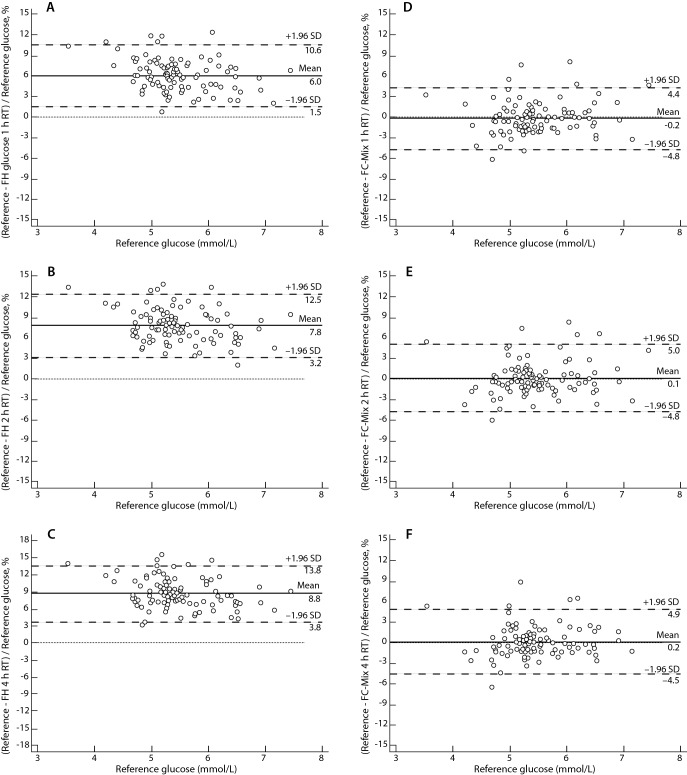
Mean plasma glucose concentration measured in 100 LH sample tubes treated according the ADA - NACB guidelines was 5.47 ± 0.65 mmol/L. Mean plasma glucose concentration in FH sample tubes was 5.14 ± 0.66 mmol/L, 5.05 ± 0.65 mmol/L and 5.00 ± 0.65 mmol/L, with a mean relative bias of -6.04%, -7.84% and -8.76%, exceeding the analytical goal for desirable bias based on biological variation criteria (≤ 1.8%) after 1, 2 and 4 h at RT, respectively. Mean plasma glucose concentration in FC-Mixture tubes was 5.48 ± 0.65 mmol/L, 5.46 ± 0.65 mmol/L and 5.46 ± 0.64 mmol/L with a mean absolute bias 0.20%, -0.10% and -0.22%, reaching the optimal analytical goal for bias based on biological variation criteria (≤ 0.92%) after 1, 2 and 4 h at RT, respectively. The ANOVA has shown statistically significant differences between glucose concentrations in LH, FH and FC-Mixture tubes (P < 0.001). Pair-wise comparisons showed that glucose concentrations in FH tubes after 1, 2 and 4 h at RT were significantly lower than glucose in LH tubes treated according to ADA-NACB (P < 0.001). Contrarily, no statistically significant differences were observed in glucose concentrations comparing FC-Mixture and LH samples after 1 h (P = 0.499), 2 h (P = 0.614) and 4 h storage at RT (P = 0.283). Figure 1 shows the Bland-Altman plot of glucose in FH, FC-Mixture tubes compared to reference glucose in LH tubes treated according to ADA-NACB. Finally, 89.7% of FC-Mixture samples and 9.3% of FH samples were within the desirable TEa limits derived from biological variation data.
Figure 1.
Comparison of glucose concentrations in sample tubes evaluated, stored at room temperature, with reference glucose concentrations using Bland-Altman plots. Reference glucose concentrations were obtained from lithium-heparin tubes placed in ice-water slurry and centrifuged within 30 minutes, according to ADA-NACB guidelines. A – Comparison of reference glucose with sodium fluoride-sodium heparin (FH) glucose stored for 1 hour. B - Comparison of reference glucose with FH glucose stored for 2 hours. C - Comparison of reference glucose with FH glucose stored for 4 hours. D - Comparison of reference glucose with sodium fluoride-citrate buffer-sodium EDTA (FC-Mixture) glucose stored for 1 hour. E - Comparison of reference glucose with FC-Mixture glucose stored for 2 hours. F - Comparison of reference glucose with FC-Mixture glucose stored for 4 hours. Solid line (mean) – mean difference. Dashed lines (SD) – standard deviation. Dotted line – line of equality.
Multi-centre study
Results from the multi-centre study, mimicking real life conditions, were divided in four different groups according to time between blood collection and centrifugation, for data analysis purposes.
There were statistically significant differences (P < 0.001) between median glucose (IQR) in LH tubes and FH tubes compared to paired reference glucose in FC-Mixture tubes, regardless of the interval between blood collection and centrifugation: for intervals of ≤ 60 min: LH 5.49 (5.27 - 5.66) mmol/L, FH 5.55 (5.32 - 5.71) mmol/L vs. FC-Mixture 5.77 (5.55 - 5.93) mmol/L. For the blood collection to centrifugation time interval of 61 - 120 min, median plasma glucose was 5.10 (4.93 - 5.21) mmol/L in LH tubes, 5.21 (5.05 - 5.32) mmol/L in FH tubes and 5.60 (5.47 - 5.73) mmol/L in FC-Mixture tubes. For the time interval of 121-180 min, median plasma glucose was 4.71 (4.63 - 4.88) mmol/L in LH tubes, 5.05 (4.88 - 5.21) mmol/L in FH tubes and 5.49 (5.43-5.77) mmol/L in FC-Mixture tubes. After 180 min median plasma glucose was 5.43 (4.98 - 5.83) mmol/L in LH tubes, 5.68 (5.43 - 6.27) mmol/L in FH tubes and 6.02 (5.87 - 6.82) mmol/L in FC-Mixture tubes. Data of glucose stored at room temperature at different time between blood drawing and centrifugation are summarized in Figure 2.
Figure 2.
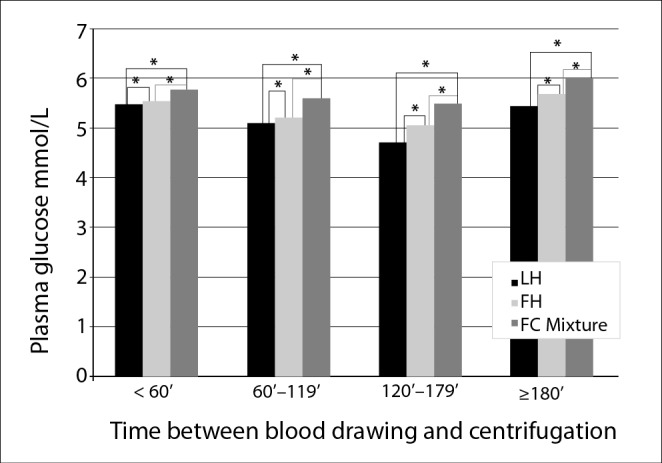
Median glucose concentrations in evaluated tubes stored at room temperature at different time periods between blood drawing and centrifugation. LH - lithium-heparin tube. FH - Sodium-fluoride and sodium-heparin containing tube. FC-Mixture – Sodium-fluoride, citrate buffer and sodium EDTA containing tube.*Wilcoxon pair-wise test: P < 0.001.
Mean bias respect to FC-Mixture tubes in FH tubes was -4.9%, -6.8%, -8.0% and -6.4% at ≤ 60 min, 61-120 min, 121-180 min and > 180 min (P < 0.001), respectively. Plasma glucose in LH tubes showed a lower bias (P < 0.001): -5.7%, -9.2%, -13.8% and -16% when samples were stored at room temperature for ≤ 60 min, 61-120 min, 121-180 min, and 181 min or more, respectively.
Discussion
Plasma glucose concentrations measured in NaF/Na2 heparin tubes stored at RT up to 4 h were lower than glucose in LH sample tubes placed in an ice-water slurry immediately after blood drawing and centrifuged at 2000 x g 15 min at 4 °C with plasma separated within 30 min (according to the ADA-NACB guidelines) (8). On the contrary, in FC-mixture tubes, containing not only NaF but also citrate buffer and Na2EDTA, glucose concentration was preserved up to 4h at RT.
This result can be explained by the fact that NaF exerts a particular inhibiting action on enolase, an enzyme involved in the final phase of the glycolytic process and, therefore, its action of inhibition of the glycolysis occurs quite late, approximately 1-2 hours from blood collection (15). Accordingly, blood glucose measured in the NaF/Na2-heparin containing tubes, stored at RT for 4 hours, was lower compared to glucose measured in the same tubes stored for 2 hours. Furthermore, both were significantly lower than glucose in the same tubes stored at RT and centrifuged after 1 hour. As previously reported (15, 16), our results confirm that NaF blocks the glycolysis in a later stage.
On the other hand, as observed by Gambino et al. (10), FC-Mixture tubes allow maintaining a glucose concentration which matches the one obtained with the reference procedure, even after 4 hours of RT storage. The glycemia reduction in the NaF/Na2 heparin samples, kept at RT for 2 hours, resulted be superior to the that observed by Gambino (7.8% compared to 4.5% after 2 hours at 37 °C) (10) as well as by Fobker (3.6% after 2 hours at RT) (17).
The novelty of our study was to test in real life routine of 15 different Italian laboratories, several conditions of storage and time from blood drawing to centrifugation of the commonly used tubes for glucose determination (i.e. lithium-heparin and sodium-fluoride). Moreover, since the majority of laboratories can’t routinely implement the ADA-NACB recommendations, our multi-centre study evaluated the effect on glucose determination of using an immediate glycolysis inhibitor such as citrate buffer (10, 17-19), i.e. Terumo FC-Mixture, compared to the most commonly used LH and FH tubes.
The underestimation of glucose in the LH and FH tubes resulted to be statistically significant already in the group of tubes centrifuged within 60 minutes from blood collection and the bias increased with the longer time intervals. In the LH tubes the average bias tends to increase almost linearly with time (from -5.7% in the samples centrifuged within 60 min to -16% in those centrifuged after more than 180 min), whereas in the FH tubes, as expected, the glycaemia decrease stops after two hours of storage.
Therefore, in accordance to the pilot-study, the multi-centre experience confirms that the NaF alone does not allow an immediate glycolysis inhibition. We suggest that FC-Mixture should be used exclusively for glucose determination, because all the centres involved in the study noticed the presence of a higher level of haemolysis compared with the LH and FH tubes.
The haemolysis can be attributed to the high acidity of the additive present in the FC mixture tubes.
The evaluation of serum indexes was not intended in our study. However, since some laboratories had the possibility to resume their data on haemolysis index, in 395 of 1125 FC-Mixture samples tubes it was possible to verify the haemolysis index, showing that 99% of the tubes had a concentrations of free haemoglobin < 2.0 g/L. As previously reported at this haemoglobin concentration, no analytical interference has been observed in glucose determination (20).
Over the course of the time the laboratories improved the analytical performances in the glucose determination. However, the reduction of glucose in vitro under the effect of glycolysis is quantitatively relevant and can invalidate the accuracy of the results. An accurate determination of glucose is therefore fundamental for the diagnosis of DM and particularly GDM, when HbA1c cannot substitute the determination of plasma glucose for the diagnosis (1, 2, 21). If the organization of the laboratory does not allow a quick centrifugation of the tube, it is necessary to use a tube containing glycolysis inhibitors (8). However, the use of NaF alone does not allow an immediate glycolysis blockage (7-9). In the epidemiologic studies that defined the cut-offs for the diagnosis of DM, IFG or IGT, tubes containing the NaF as an inhibitor were employed (5, 8). Over the course of those studies, no waiting times have been specified before the separation of the plasma from the blood cells in which the glucose was reduced under the effect of the glycolysis, probably leading to an underestimation in the predominance of the diabetes.
In the light of the present and recently published results (17, 22-24), it might emerge the impellent need of undertaking new standardized and pre-analytically well-performed studies using FC-mixture tubes with the aim of redefining DM cut-offs. The use of acidified tubes could bring to an increase of the average glucose value of the population, as it was highlighted in recent studies (25, 26).
In conclusion, the authors of the present study agree on the fact that, following a good laboratory practice, in order to do a correct diagnosis of DM, and above all of GDM, trying to avoid wrong classifications of patients, the use of an immediate inhibitor of the glycolysis has to be regarded as necessary (27, 28). The FC mixture in Terumo Venosafe Glycemia tubes (10, 17, 22), from the data presented seem to be suitable for this purpose, while NaF alone is not.
Aknowledgments
We are grateful to all the volunteers, who kindly donated their blood for the experimental work. We thank Terumo Italia s.r.l. for providing the tubes and sampling devices free of charge. We acknowledge also the following collegues for having taken part to the multi-centre study: dr M. Boemi, dr F. Busco, dr R. Galeazzi, dr S. Giovagnetti (Ancona), dr I. Cataldo (Chieti), prof A. Di Benedetto (Messina), dr G. Ferrai (Treviso), dr G. Lavalle (Rome), prof G. Lippi (Parma), dr I. Maccaroni (Recanati), dr G. Pizzagalli, prof F. Ceriotti, (Milan), dr S. Valenti (Ravenna), dr A. Vero, (Catanzaro), dr M. Zaninotto (Padova).
Footnotes
None declared.
References
- 1.American Diabetes Association . Classification and diagnosis of diabetes. Diabetes Care. 2015;38:S8–16. 10.2337/dc15-S005 [DOI] [PubMed] [Google Scholar]
- 2.The HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 3.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, et al. Normal fasting plasma glucose level and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–62. 10.1056/NEJMoa050080 [DOI] [PubMed] [Google Scholar]
- 4.Bruns DE, Knowler WC. Stabilization of glucose in blood samples: why it matters. Clin Chem. 2009;55:850–2. 10.1373/clinchem.2009.126037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks DB. Carbohydrates. In: Burtis CA, Ashwood ER, Bruns DE, eds. Tietz textbook of clinical chemistry and molecular diagnostics, 5th ed. St. Louis: Elsevier Saunders, 2012. p.709-30. [Google Scholar]
- 6.Gambino R. Glucose: a simple molecule that is not simple to quantify. Clin Chem. 2007;53:2040–1. 10.1373/clinchem.2007.094466 [DOI] [PubMed] [Google Scholar]
- 7.Chan YA, Swaminathan R, Cockram C. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem. 1989;35:315–7. [PubMed] [Google Scholar]
- 8.Sacks DB, Arnold M, Bakris GI, Bruns DE, Horvath AR, Kirkman MS, et al. Guidelines and recommendation for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2011;34:e61–99. 10.2337/dc11-9998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gambino R. Sodium fluoride: an ineffective inhibitor of glycolysis. Ann Clin Biochem. 2013;50:3–5. 10.1258/acb.2012.012135 [DOI] [PubMed] [Google Scholar]
- 10.Gambino R, Piscitelli J, Ackattupathil TA, Theriault JL, Andrin RD, Sanfilippo ML, et al. Acidification of blood is superior to sodium fluoride as an inhibitor of glycolysis. Clin Chem. 2009;55:1019–21. 10.1373/clinchem.2008.121707 [DOI] [PubMed] [Google Scholar]
- 11.van den Berg SA, Thelen MH, Salden LP, van Thiel SW, Boonen KJ. It takes acid, rather than ice, to freeze glucose. Sci Rep. 2015;5:8875. 10.1038/srep08875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terumo Europe NV. VenosafeTM Glycaemia tube with unique FC-mixture. Terumo Italia srl. VF-05EN-0505MAR-I1(06.05)EAU.
- 13.Simundic AM. Practical recommendations for statistical analysis and data presentation in Biochemia Medica journal. Biochem Med (Zagreb). 2012;22:15–23. 10.11613/BM.2012.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minchinela J, Ricós C, Perich C, Fernández-Calle P, Alvarez V, Domenech M, et al. Biological variation database, and quality specifications for imprecision, bias and total error (desirable and minimum). The 2014 update. Available at: https://www.westagard.com/biodatabase-2014-update.htm. Accessed November 1st, 2015.
- 15.Mikesh LM, Bruns DE. Stabilization of glucose in blood specimens: mechanism of delay in fluoride in fluoride inhibition of glycolysis. Clin Chem. 2008;54:930–2. 10.1373/clinchem.2007.102160 [DOI] [PubMed] [Google Scholar]
- 16.Bruns DE. Are fluoride-containing tubes still needed for glucose testing? Clin Biochem. 2013;46:289-90. 10.1016/j.clinbiochem.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 17.Fobker M. Stability of glucose in plasma with different anticoagulants. Clin Chem Lab Med. 2014;52:1057–60. 10.1515/cclm-2013-1049 [DOI] [PubMed] [Google Scholar]
- 18.del Pino IG, Constanso I, Vazquez Mourin L, Barbuzano Safont C, Rodriguez Vazquez P. Citric/citrate buffer: an effective antiglycolytic agent. Clin Chem Lab Med. 2013;51:1943–9. 10.1515/cclm-2012-0735 [DOI] [PubMed] [Google Scholar]
- 19.Gambino R, Bruns D. Stabilization of glucose in blood: out with the old, in with the new. Clin Chem Lab Med. 2013;51:1883–5. 10.1515/cclm-2013-0341 [DOI] [PubMed] [Google Scholar]
- 20.Lippi G, Salvagno GL, Montagnana M, Brocco G, Guidi GC. Influence of hemolysis on routine clinical chemistry testing. Clin Chem Lab Med. 2006;44:311–6. 10.1515/CCLM.2006.054 [DOI] [PubMed] [Google Scholar]
- 21.International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winter T, Greiser A, Nauck M, Petersmann A. Long-term stability of glucose: 96-h study using Terumo Glycaemia tubes. Clin Chem Lab Med. 2016;•••: E-pub ahead of print 10.1515/cclm-2015-0548 [DOI] [PubMed] [Google Scholar]
- 23.Juricic G, Kopcinovic LM, Saracevic A, Bakliza A, Simundic AM. Liquid citrate acidification introduces significant glucose bias and leads to misclassification of patients with diabetes. Clin Chem Lab Med. 2016;54:363–71. 10.1515/cclm-2015-0358 [DOI] [PubMed] [Google Scholar]
- 24.Dimeski G, Yow KS, Brown NN. What is the most suitable blood collection tube for glucose estimation? Ann Clin Biochem. 2015;52(Pt 2):270–5. 10.1177/0004563214544708 [DOI] [PubMed] [Google Scholar]
- 25.Norman M, Jones J. The shift from fluoride/oxalate to acid citrate/fluoride blood collection tubes for glucose testing – The impact upon patients results. Clin Biochem. 2014;47:683–5. 10.1016/j.clinbiochem.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 26.Riedefelt P, Akerfeldt T, Helmersson-Karlqvist J. Increased plasma glucose levels after change of recommendation from NaF to citrate blood collection tubes. Clin Biochem. 2014;47:625–8. 10.1016/j.clinbiochem.2014.02.022 [DOI] [PubMed] [Google Scholar]
- 27.Peake MJ, Bruns DE, Sacks DB, Horwath AR. It’s time for a better blood collection tube to improve reliability of glucose results. Diabetes Care. 2013;36:e2. 10.2337/dc12-1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szoke D, Valente C, Panteghini M. Better blood collection tubes for plasma glucose: ready for prime time? Clin Chem Lab Med. 2014;52:e87–9. 10.1515/cclm-2013-1006 [DOI] [PubMed] [Google Scholar]