Abstract
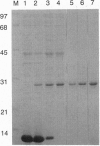
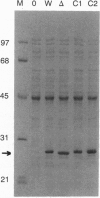
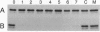
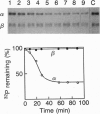
The predicted amino acid sequence encoded by the open reading frame 221 (orf221) of bacteriophage lambda exhibited a high degree of similarity to the catalytic subunits of a variety of protein serine/threonine phosphatases belonging to PP1, PP2A, and PP2B groups. Cloning and expression of the orf221 gene in Escherichia coli provided direct evidence that the gene codes for a protein serine/threonine phosphatase. The single-subunit recombinant enzyme was purified in soluble form and shown to possess a unique repertoire of biochemical properties--e.g., an absolute requirement for Mn2+, resistance to okadaic acid, inhibitors 1 and 2, and ability to dephosphorylate casein, adenovirus E1A proteins, and the alpha subunit of phosphorylase kinase. No phosphotyrosine phosphatase activity was observed. Mutational and biochemical analyses identified the conserved residues 73-77 and Cys138 to be important for activity. The name PP-lambda is proposed for this unusual prokaryotic enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barik S., Banerjee A. K. Phosphorylation by cellular casein kinase II is essential for transcriptional activity of vesicular stomatitis virus phosphoprotein P. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6570–6574. doi: 10.1073/pnas.89.14.6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S., Banerjee A. K. Sequential phosphorylation of the phosphoprotein of vesicular stomatitis virus by cellular and viral protein kinases is essential for transcription activation. J Virol. 1992 Feb;66(2):1109–1118. doi: 10.1128/jvi.66.2.1109-1118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barik S., Das A. An analysis of the role of host factors in transcription antitermination in vitro by the Q protein of coliphage lambda. Mol Gen Genet. 1990 Jun;222(1):152–156. doi: 10.1007/BF00283037. [DOI] [PubMed] [Google Scholar]
- Barik S., Ghosh B., Whalen W., Lazinski D., Das A. An antitermination protein engages the elongating transcription apparatus at a promoter-proximal recognition site. Cell. 1987 Sep 11;50(6):885–899. doi: 10.1016/0092-8674(87)90515-0. [DOI] [PubMed] [Google Scholar]
- Berndt N., Cohen P. T. Renaturation of protein phosphatase 1 expressed at high levels in insect cells using a baculovirus vector. Eur J Biochem. 1990 Jun 20;190(2):291–297. doi: 10.1111/j.1432-1033.1990.tb15575.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Brewis N. D., Hughes V., Mann D. J. Protein serine/threonine phosphatases; an expanding family. FEBS Lett. 1990 Aug 1;268(2):355–359. doi: 10.1016/0014-5793(90)81285-v. [DOI] [PubMed] [Google Scholar]
- Cohen P. T., Cohen P. Discovery of a protein phosphatase activity encoded in the genome of bacteriophage lambda. Probable identity with open reading frame 221. Biochem J. 1989 Jun 15;260(3):931–934. doi: 10.1042/bj2600931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. T., Collins J. F., Coulson A. F., Berndt N., da Cruz e Silva O. B. Segments of bacteriophage lambda (orf 221) and phi 80 are homologous to genes coding for mammalian protein phosphatases. Gene. 1988 Sep 15;69(1):131–134. doi: 10.1016/0378-1119(88)90385-x. [DOI] [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Costantino N., Zuber M., Court D. Analysis of mutations in the ninR region of bacteriophage lambda that bypass a requirement for lambda N antitermination. J Bacteriol. 1990 Aug;172(8):4610–4615. doi: 10.1128/jb.172.8.4610-4615.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A. How the phage lambda N gene product suppresses transcription termination: communication of RNA polymerase with regulatory proteins mediated by signals in nascent RNA. J Bacteriol. 1992 Nov;174(21):6711–6716. doi: 10.1128/jb.174.21.6711-6716.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont D. J., Branton P. E. Phosphorylation of adenovirus E1A proteins by the p34cdc2 protein kinase. Virology. 1992 Jul;189(1):111–120. doi: 10.1016/0042-6822(92)90686-j. [DOI] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990 Mar;9(3):675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M., Alberts A., Brindle P., Meinkoth J., Feramisco J., Deng T., Karin M., Shenolikar S., Montminy M. Transcriptional attenuation following cAMP induction requires PP-1-mediated dephosphorylation of CREB. Cell. 1992 Jul 10;70(1):105–113. doi: 10.1016/0092-8674(92)90537-m. [DOI] [PubMed] [Google Scholar]
- Herrmann C. H., Su L. K., Harlow E. Adenovirus E1A is associated with a serine/threonine protein kinase. J Virol. 1991 Nov;65(11):5848–5859. doi: 10.1128/jvi.65.11.5848-5859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe P. H., Draetta G., Leof E. B. Transforming growth factor beta 1 inhibition of p34cdc2 phosphorylation and histone H1 kinase activity is associated with G1/S-phase growth arrest. Mol Cell Biol. 1991 Mar;11(3):1185–1194. doi: 10.1128/mcb.11.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Cohen P. Protein phosphatases: properties and role in cellular regulation. Science. 1983 Jul 22;221(4608):331–338. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ludlow J. W., Glendening C. L., Livingston D. M., DeCarprio J. A. Specific enzymatic dephosphorylation of the retinoblastoma protein. Mol Cell Biol. 1993 Jan;13(1):367–372. doi: 10.1128/mcb.13.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. The "megaprimer" method of site-directed mutagenesis. Biotechniques. 1990 Apr;8(4):404–407. [PubMed] [Google Scholar]
- Smith C. L., Debouck C., Rosenberg M., Culp J. S. Phosphorylation of serine residue 89 of human adenovirus E1A proteins is responsible for their characteristic electrophoretic mobility shifts, and its mutation affects biological function. J Virol. 1989 Apr;63(4):1569–1577. doi: 10.1128/jvi.63.4.1569-1577.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele F. R., Washburn T., Rieger R., O'Tousa J. E. Drosophila retinal degeneration C (rdgC) encodes a novel serine/threonine protein phosphatase. Cell. 1992 May 15;69(4):669–676. doi: 10.1016/0092-8674(92)90230-a. [DOI] [PubMed] [Google Scholar]
- Sternberg N., Enquist L. Analysis of coliphage lambda mutations that affect Q gene activity: puq, byp, and nin5. J Virol. 1979 Apr;30(1):1–13. doi: 10.1128/jvi.30.1.1-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura S., Yasui A., Tsuiki S. Expression of rat protein phosphatase 2C (IA) in Escherichia coli. Biochem Biophys Res Commun. 1989 Aug 30;163(1):131–136. doi: 10.1016/0006-291x(89)92109-8. [DOI] [PubMed] [Google Scholar]
- Tonks N. K., Cohen P. The protein phosphatases involved in cellular regulation. Identification of the inhibitor-2 phosphatases in rabbit skeletal muscle. Eur J Biochem. 1984 Nov 15;145(1):65–70. doi: 10.1111/j.1432-1033.1984.tb08522.x. [DOI] [PubMed] [Google Scholar]
- Uemura T., Shiomi K., Togashi S., Takeichi M. Mutation of twins encoding a regulator of protein phosphatase 2A leads to pattern duplication in Drosophila imaginal discs. Genes Dev. 1993 Mar;7(3):429–440. doi: 10.1101/gad.7.3.429. [DOI] [PubMed] [Google Scholar]
- Wang D. M., Dalie B., Harter M. L. The adenovirus E1A 243R protein purified from Escherichia coli under nondenaturing conditions is found in association with dnaK. Protein Expr Purif. 1992 Feb;3(1):8–17. doi: 10.1016/1046-5928(92)90050-7. [DOI] [PubMed] [Google Scholar]
- Zhang A. J., Bai G., Deans-Zirattu S., Browner M. F., Lee E. Y. Expression of the catalytic subunit of phosphorylase phosphatase (protein phosphatase-1) in Escherichia coli. J Biol Chem. 1992 Jan 25;267(3):1484–1490. [PubMed] [Google Scholar]