Research shows that 247Cm was present in meteorites at a level consistent with a single stellar environment of r-process nucleosynthesis.
Keywords: nucleosynthesis, meteorites, uranium isotopes, curium-247, r-process, fine-grained CAI, group II REE pattern
Abstract
High-temperature condensates found in meteorites display uranium isotopic variations (235U/238U), which complicate dating the solar system’s formation and whose origin remains mysterious. It is possible that these variations are due to the decay of the short-lived radionuclide 247Cm (t1/2 = 15.6 My) into 235U, but they could also be due to uranium kinetic isotopic fractionation during condensation. We report uranium isotope measurements of meteoritic refractory inclusions that reveal excesses of 235U reaching ~+6% relative to average solar system composition, which can only be due to the decay of 247Cm. This allows us to constrain the 247Cm/235U ratio at solar system formation to (1.1 ± 0.3) × 10−4. This value provides new clues on the universality of the nucleosynthetic r-process of rapid neutron capture.
INTRODUCTION
All elements beyond the iron peak (above ~70 atomic mass units) are the products of three main processes of stellar nucleosynthesis: the s- (slow neutron capture), r- (rapid neutron capture), and p-process (proton process) (1, 2). Unlike the s- and p-process, which are relatively well understood [neutron capture in asymptotic giant branch (AGB) stars for the s-process and photodisintegration of seed nuclei in supernovae for the p-process] (3, 4), little is known regarding the astrophysical conditions under which r-process nuclides are produced (5–8). The r-process label may comprise some diversity as it was suggested that up to three r-processes were responsible for producing light r-nuclides (A < 140), heavy r-nuclides (A > 140), and actinides (for example, U and Th). The existence of an “actinide” production site is motivated by two main observations: (i) the ages of some old stars inferred from their Th/Eu ratios are negative, meaning that they must have formed with a Th/Eu ratio that was higher than that relevant to the solar system (SS) (9), and (ii) the abundance of the short-lived radionuclide (SLR) 244Pu [t1/2 = 79.3 million years (My)] in meteorites is low compared to another nominal r-process radionuclide, 182Hf (7). However, 244Pu has a long half-life and its stellar yield is uncertain (10), which makes it insensitive to the history of nucleosynthesis before SS formation and whether or not multiple r-process sites contributed to the synthesis of the actinides. In contrast, 247Cm, which decays into 235U, has a much shorter half-life (t1/2 = 15.6 My) and would be very well suited to address this question. Unfortunately, its abundance in the early solar system (ESS) has been the subject of some debate that boils down to knowing whether the uranium isotope variations (that is, 235U/238U ratio) measured in early-formed nebular condensates [calcium- and aluminum-rich inclusions (CAIs)] are due to the decay of 247Cm or isotopic fractionation during condensation. The two cannot be easily distinguished because U has only two long-lived isotopes and both mechanisms would produce similar correlations between light U isotope enrichments and U concentrations.
Recently, excesses in 235U of up to 3.5‰ were documented in four fine-grained CAIs (11). To demonstrate that those variations are due to 247Cm decay, one must show that the δ235U values correlate with the Cm/U parent-to-daughter ratios. Because Cm has no long-lived isotopes, another element must be used as a proxy for Cm in isochron diagrams. On the basis of their near-identical valence states, ionic radii, and volatilities, the light rare earth elements (REEs) are thought to behave similarly to Pu and Cm during nebular processes (12–14). This conclusion is supported by the observed coherent behavior of Pu, Nd, and Sm in pyroxene/melt and phosphate/melt partitioning experiments (15) and during magmatic differentiation in achondrites (12, 14). Although Th has sometimes been used as a proxy for Cm (11, 16), coherent behavior for the Th-Cm and Th-Pu pairs is neither expected (13) nor observed (12), and the light REEs (for example, Nd or Sm) are therefore taken as more reliable proxies of Cm behavior during condensation.
The 235U excesses observed by Brennecka et al. (11) correlate with Nd/U ratios, which the authors interpreted as evidence of live 247Cm in the ESS at a level of (247Cm/235U)ESS = (1.1 to 2.4) × 10−4. However, such 235U enrichments could also reflect mass-dependent fractionation during condensation of solid CAIs from nebular gas (17–19). Indeed, the kinetic theory of gases predicts that the lighter isotope (235U) should condense faster than the heavier isotope (238U), resulting in fractionations that can reach for condensation of atomic U in a low-pressure gas. In both cases of 247Cm decay and fractionation during condensation, one would expect to find correlations between U isotopic variations and the degree of U depletion. It is therefore presently undecided which mechanism is responsible for the U isotope variations documented in CAIs.
Definitive evidence of live 247Cm therefore awaits the discovery of meteoritic material that is highly depleted in uranium and displays 235U excess outside of the ±6‰ window allowed by fractionation at condensation. Because the abundance of 247Cm in the ESS is expected to be low (10), such large excesses of 235U from 247Cm decay will only be resolvable in phases with 144Nd/238U ratios exceeding ~1300. Therefore, numerous slabs of the Allende meteorite were examined, and 15 CAIs (12 fine-grained and 3 coarse-grained) were extracted and digested with acids. Many of these CAIs revealed group II REE patterns and large U depletions indicative of incomplete condensation of refractory lithophile elements (see the Supplementary Materials). A method was developed to measure accurately the U isotopic compositions of samples with low U contents (0.1 to 0.01 ng; see the Supplementary Materials).
RESULTS
As in previous studies, most samples display low 144Nd/238U ratios (that is, <600), and their δ235U values are within 6‰ of the bulk SS value at δ235U = +0.31‰ (relative to CRM-112a) (19, 20). These samples display a trend between δ235U and 144Nd/238U similar to that described previously on similar samples (11) and which had been taken as evidence that 247Cm was alive in the ESS. The samples display significant scatter around the best-fit line that cannot be entirely explained by analytical uncertainty [the mean square weighted deviation (MSWD) of the regression for samples with 144Nd/238U ratios lower than 600 is 46]. Even coarse-grained CAIs that are nondepleted in U (low 144Nd/238U ratios) have δ235U that vary between −0.16 and +1.35‰, well outside of analytical uncertainty.
One sample (named Curious Marie) has an extremely high 144Nd/238U ratio (~13,720) and a 235U excess of +58.9 ± 1.9‰ (equivalent to an absolute 238U/235U ratio of 130.17 ± 0.27; Fig. 1 and Table 1). For comparison, the highest 144Nd/238U ratio and 235U excess measured in CAIs prior to this work were 481 and +3.43‰ (238U/235U = 137.37), respectively (11). Considerable effort was expended to confirm this result (see the Supplementary Materials). The measurement was triplicated using different sample purification schemes and various measurement setups. The tests yielded δ235U values of +52.79 ± 14.91‰ after one purification step with 235U measured on Faraday, +59.12 ± 2.80‰ after two purifications with 235U on an electron multiplier, and +58.97 ± 2.72‰ after three purifications with 235U on an electron multiplier. Extensive testing was also done to ensure that no interferences or matrix effects were affecting the measurement by combining the matrix cut from Curious Marie with CRM-112a, purifying the U by column chemistry, and finding that the measured U isotopic composition is correct (that is, identical to pure CRM-112a) after purification.
Fig. 1. δ235U plotted as a function of the 144Nd/238U ratio in meteoritic samples.
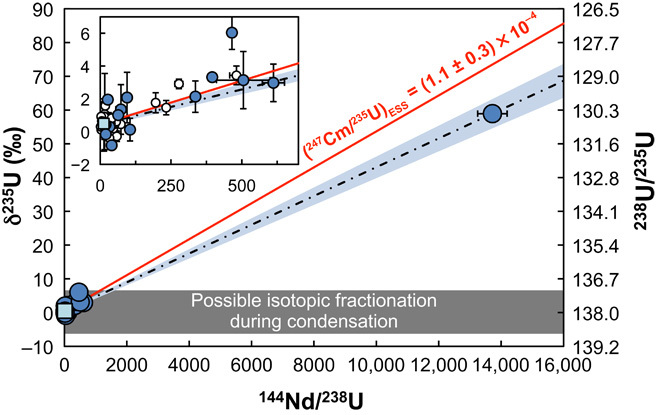
Open circles, previous studies (11, 17–19, 33, 34); blue circles, Allende CAIs from this work; light-blue square, bulk Allende from this work]. The +59‰ δ235U value observed in the Curious Marie CAI is well outside the range of variations expected from fractionation during condensation (gray rectangle) and is thus interpreted as definitive evidence for live 247Cm in the ESS. The scatter in the data (for example, at very low Nd/U ratios) suggests that stable isotopic fractionation during evaporation/condensation also influenced the U isotopic composition of CAIs. The slope of the two-point isochron between Curious Marie and the rest of the samples translates into a 247Cm/235U of (0.9 ± 0.07) × 10−4 at the time of Nd/U fractionation in Curious Marie. Accounting for a possible delay between this fractionation event (possibly related to the extensive alteration of this CAI) and the formation of the SS of 5 ± 5 My, the inferred 247Cm/235U at SS formation is (1.1 ± 0.3) × 10−4 (red line).
Table 1. Type, REE pattern, mass, U content, 144Nd/238U ratio, and U isotopic composition of the samples analyzed in this study.
n is the number of replicate analyses for each sample (starting from digested sample). Nd/U ratios and isotopic compositions are corrected for blank contributions (see table S2). δ235U = [(235U/238U)sample/(235U/238U)CRM-112a − 1] × 103. Error bars are 95% confidence intervals. Nd/U ratio was calculated using the U concentration from the double-spike technique, and Nd concentration from the standard addition technique (see the Supplementary Materials).
| Average 144Nd/238U ratios and U isotopic compositions of CAIs | |||||||||
| Sample | Type | REE pattern | Mass (mg) | U (ng) | n | 144Nd/238U | ± | δ235U (‰) blk corr. | ± |
| Allende | CV3 | 1016 | 21.9 | 2 | 12.2 | 1.5 | 0.49 | 0.16 | |
| FG-1 | Fine-gr. CAI | Group II | 35.2 | 0.77 | 2 | 95.0 | 7.0 | 2.08 | 1.54 |
| Curious Marie | Fine-gr. CAI | Group II | 717.9 | 0.37 | 3 | 13720 | 472 | 58.94 | 1.93 |
| CG-1 | Coarse-gr. CAI | Group I | 48.4 | 0.76 | 2 | 73.2 | 5.3 | 1.35 | 1.52 |
| FG-2 | Fine-gr. CAI | Group II | 50.4 | 1.53 | 2 | 105.4 | 3.9 | 0.12 | 0.69 |
| FG-3 | Fine-gr. CAI | Group II | 98.3 | 4.05 | 2 | 63.5 | 2.7 | 1.01 | 0.50 |
| FG-4 | Fine-gr. CAI | Group II | 349.7 | 15.7 | 2 | 40.5 | 2.9 | −0.83 | 0.25 |
| FG-5 | Fine-gr. CAI | Group II | 48.7 | 2.82 | 2 | 32.9 | 2.4 | 0.30 | 0.59 |
| FG-6 | Fine-gr. CAI | Group II | 54.9 | 0.35 | 2 | 612 | 39 | 2.98 | 1.14 |
| FG-7 | Fine-gr. CAI | Group II | 61.4 | 0.98 | 2 | 336 | 10 | 2.13 | 0.96 |
| FG-8 | Fine-gr. CAI | Group II | 377.3 | 8.50 | 2 | 395 | 6 | 3.32 | 0.21 |
| FG-9 | Fine-gr. CAI | Group II | 330.7 | 23.9 | 2 | 26.8 | 1.9 | 1.95 | 0.14 |
| FG-10 | Fine-gr. CAI | Group II | 15.61 | 0.24 | 2 | 505 | 95 | 3.14 | 1.75 |
| CG-2 | Coarse-gr. CAI | Group V | 200.4 | 23.0 | 2 | 19.1 | 1.4 | 0.43 | 0.14 |
| FG-11 | Fine-gr. CAI | Group II | 68.6 | 0.83 | 2 | 165 | 10. | 6.03 | 1.02 |
| TS32 | Coarse-gr. CAI | Group V | 41.8 | 4.89 | 2 | 197 | 1.4 | −0.16 | 0.30 |
DISCUSSION
Evidence for 247Cm
The finding of such a large excess 235U in a normal CAI [in opposition to fractionated and unknown nuclear (FUN) CAIs, which is a group of refractory inclusions that display FUN effects; see the Supplementary Materials] can only be explained by decay of 247Cm into 235U. Below, we examine other processes that could potentially lead to U isotope variations in ESS materials and show that they all suffer from serious shortcomings:
(i) Isotopic fractionation during secondary processes (for example, aqueous alteration and/or redox processes), either on the meteorite parent body or on Earth, can impart large isotopic fractionation to light elements (such as Li). However, the degree of fractionation generally decreases as the mass of the element increases, and for U on Earth, the variations are limited to ~1.5 to 2‰ (21). Although this process could lead to some scatter in the data around the isochron, it cannot explain a +59‰ anomaly.
(ii) Large isotopic variations of nucleosynthetic origin have been documented for refractory elements in CAIs and are usually readily identified as departures from mass-dependent fractionation. For uranium, which has only two stable isotopes, it is impossible to distinguish between 247Cm decay and nucleosynthetic anomalies. Nevertheless, considering that anomalies on the order of a few permil in heavy elements (for example, Ba, Sm, and Nd) are only found in FUN CAIs (normal CAIs have more subdued anomalies of a few tenths of permil) and given that Curious Marie (δ238U ~ 59‰) is not a FUN CAI (see the Supplementary Materials), the large 235U excess found here cannot be reasonably ascribed to the presence of nucleosynthetic anomalies.
(iii) Finally, U isotopic fractionation could be the result of fractionation during evaporation/condensation processes. During condensation, the light isotope of U condenses faster, leading to large 235U depletion in the condensing gas and the instantaneous solid, but 235U excesses limited to +6‰ (see the Supplementary Materials). Similarly, during evaporation, the highest 235U excess predicted by the kinetic theory of gases is limited to ~+6‰. Consequently, evaporation/condensation processes cannot explain the +59‰ 235U excess observed in Curious Marie, which leaves 247Cm decay as the only possible explanation.
Closure time in Curious Marie and 247Cm/235U ratio in the ESS
The large excess of 235U found in the Curious Marie CAI is definitive evidence that 247Cm was alive in the ESS. An initial (247Cm/235U) ratio of ~0.9 × 10−4 at the time of closure can be calculated using the slope of the isochron in Fig. 1. Even though the samples with low 144Nd/238U ratios define a trend with δ235U, the slope of the isochron in Fig. 1 is mainly leveraged by Curious Marie (144Nd/238U ratio ~ 13,720). The initial 247Cm/235U ratio of (0.9 ± 0.07) × 10−4 thus corresponds to the time when this CAI acquired its high 144Nd/238U ratio. Terrestrial alteration is ruled out because the Allende meteorite is an observed fall that did not experience much terrestrial weathering. The extreme uranium depletion in the Curious Marie CAI is thus most likely due to solar nebula condensation and/or nebular/parent body alteration. All fine-grained CAIs in this study display a typical group II REE pattern, thought to represent a snapshot in time and space of the condensation sequence (22, 23). The most refractory REEs (heavy REEs except Tm and Yb) are depleted in these CAIs because they were sequestered in ultrarefractory dust such as perovskite or hibonite, whereas the more volatile REEs (Eu and Yb) and uranium are also depleted because they stayed in the gas phase when those CAIs formed. In most samples, U and Yb present similar levels of depletion relative to solar composition and the abundance of other refractory lithophile elements (Fig. 2), indicating that those two elements have similar behaviors during evaporation/condensation processes under solar nebula conditions. However, in Curious Marie, the U/Nd ratio is 1000 times lower than solar composition, whereas the Yb/Nd ratio is only depleted by a factor of 50. If U and Yb have similar behaviors during condensation, one would expect the U/Nd ratio to be 50 times lower than solar, not 1000 times as is observed. Compared to other fine-grained CAIs analyzed, Curious Marie is peculiar because it is extremely altered, which is manifested by the extensive replacement of high-T phases by low-T alteration products such as nepheline and sodalite [its Na2O is 15 weight percent (wt %) when other CAIs are all lower than 6.7 wt %; table S1]. Such alteration may have mobilized U, producing a 20-fold U depletion on top of the 50-fold depletion associated with condensation.
Fig. 2. Correlation between U and Yb abundances relative to solar composition and the abundance of Nd (a refractory lithophile element).
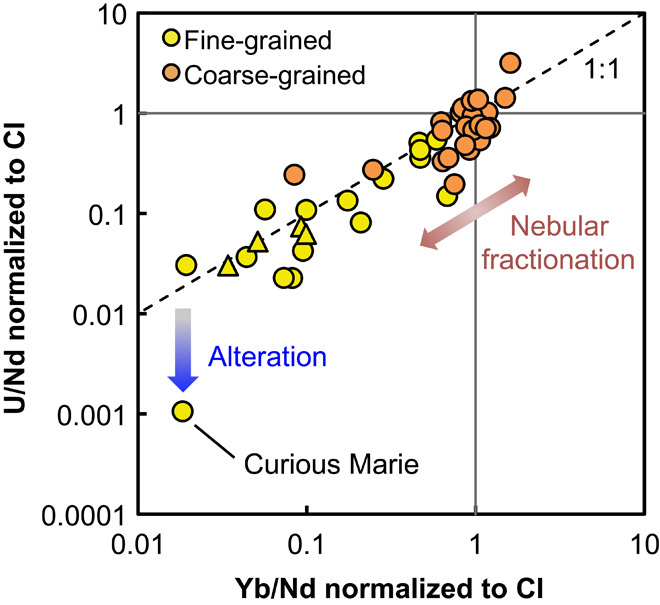
Circles denote data from different studies (35–37) and this study, and triangles denote data from Brennecka et al. (11). This correlation indicates that U and Yb have similar behaviors during evaporation/condensation processes under solar nebula conditions. The Curious Marie CAI plots off the 1:1 line, with a CI-normalized U/Nd ratio 20 times lower than that of Yb/Nd. This CAI is extremely altered (see main text and table S1), and such alteration may have mobilized U, producing a 20-fold U depletion on top of the 50-fold depletion associated with condensation.
Some of these alteration products could have formed (i) in the nebula, (ii) on an earlier generation of water-rich asteroid, or (iii) during aqueous alteration on Allende itself [for example, Ross et al. (24) and Russell and MacPherson (25) and references therein]. Regardless of the location, dating of aqueous alteration products on meteorite parent bodies with extinct radionuclides 36Cl (t1/2 = 0.301 My), 26Al (t1/2 = 0.717 My), 53Mn (t1/2 = 3.74 My), or 129I (t1/2 = 15.7 My) suggests that it took place no later than 10 My after SS formation. This is a conservative upper limit because secondary alteration phases in fine-grained inclusions may have formed very early in the nebula. The half-life of 247Cm is 15.6 My, meaning that a time span of ~5 ± 5 My between SS formation and CAI alteration (and possible U depletion) translates into a correction for the initial 247Cm/235U ratio of 25 ± 25% (the uncertainty on this factor takes into account the uncertainty on the closure age). Our present best estimate of the initial SS 247Cm/235U ratio is thus (1.1 ± 0.3) × 10−4, which is equivalent to (247Cm/238U)ESS = (3.7 ± 0.9) × 10−5 and (247Cm/232Th)ESS = (1.6 ± 0.4) × 10−5. This value is in agreement with the 247Cm/235U ratio of (1.1 to 2.4) × 10−4 obtained by Brennecka et al. (11) based on CAI measurements and an upper limit of ~4 × 10−3 inferred from earlier meteoritic measurements (26). It is also in line with the lower limit derived from modeling of galactic chemical evolution (GCE) (10), which predicts an initial ratio of (5.0 ± 2.5) × 10−5.
Fractionation of U isotopes during evaporation/condensation
Some of the samples with low 144Nd/238U ratios show significant scatter around the isochron (see inset of Fig. 1), outside of analytical uncertainties. In particular, samples with 144Nd/238U ratio as low as ~20 span a range of δ235U values of 3.5‰, a feature that led previous workers (17, 18) to question the conclusion of Brennecka et al. (11) that 247Cm was responsible for U isotope variations. This scatter is most likely due to isotopic fractionation during condensation, suggesting that better isochronous behavior could be obtained if such fractionation could be corrected.
Implications for the r-process
In addition to the dating implications that large 235U/238U variations have on the Pb-Pb ages of the CAIs (11, 21), the existence of 247Cm in the ESS has implications for the nucleosynthesis of r-process elements. The simplest GCE model that successfully reproduces the metallicity distribution of G-dwarfs requires infall of gas onto the galactic disk (10, 27, 28). In such a model, the abundance ratio of an SLR normalized to a stable nuclide is
| (1) |
where N is the abundance, P is the production ratio, τ is the mean life of the SLR (τ = 1/λ), T is the age of the galaxy at the time when the ratio NSLR/NStable is to be calculated, and k is a constant that distinguishes closed-box (k = 0) versus infall models (typically, k = 2 ± 1) (8, 27–29). If T is taken as the presolar age of the galaxy, T* (= TG − TSS = 8.7 Gy), then the ratio NSLR/NStable in the interstellar medium (ISM) at the time of isolation of the protosolar molecular cloud from fresh nucleosynthetic input can be calculated. This value can be compared to the NSLR/NStable ratio in the ESS as obtained from meteoritic measurements. The difference between the two values is often interpreted as a “free-decay interval” (Δ) between the last nucleosynthetic event that produced the SLR and the formation of the SS
| (2) |
At present, the meteoritic abundances of only three short-lived r-nuclides (129I, 182Hf, and 244Pu) have been estimated, yielding three different Δ values (100 ± 7 My, ~35 My, and 158 ± 85 My, respectively) (7). The limit of our understanding of the r-process is illustrated by the fact that there have been as many r-processes proposed as short-lived r-nuclides investigated.
Using the value of (247Cm/232Th)ESS = (1.6 ± 0.4) × 10−5 obtained in this study and the open nonlinear GCE model of Dauphas et al. (28) with k = 1.7, we obtained a free-decay interval of Δ = 87 ± 14 My. This value is in agreement with the Δ value of ~100 My derived from 129I and 244Pu but is much longer than the value of ~35 My obtained from 107Pd and 182Hf (Fig. 3). If 107Pd and 182Hf are indeed pure r-process isotopes, then a multiplicity of r-process environments is needed to explain the inconsistent Δ values (5, 7). This is evident when looking at Fig. 3: 107Pd and 129I have similar (NSLR/NStable)/(PSLR/PStable) ratios but different half-lives, making it impossible for both isotopes to be produced in a single event/process, no matter what model is used for the evolution of the SLR abundances in the giant molecular cloud parental to the SS [for example, free-decay interval versus three-phase mixing ISM (8, 30)]. However, recent nucleosynthetic models have reconsidered the origin of 107Pd and 182Hf and find a significant s-process contribution (70 to 80%) for both isotopes (6, 31). In such a framework, the initial abundance of all r-process SLR in the ESS can be explained by a single r-process making environment, which last injected material into the protosolar molecular cloud ~100 My before SS formation.
Fig. 3. Meteoritic abundance ratios of extinct radionuclides to stable nuclides produced by the same process (for example, 129I/127Ir), normalized to stellar production ratios versus mean lives (τ = t1/2 /ln2).
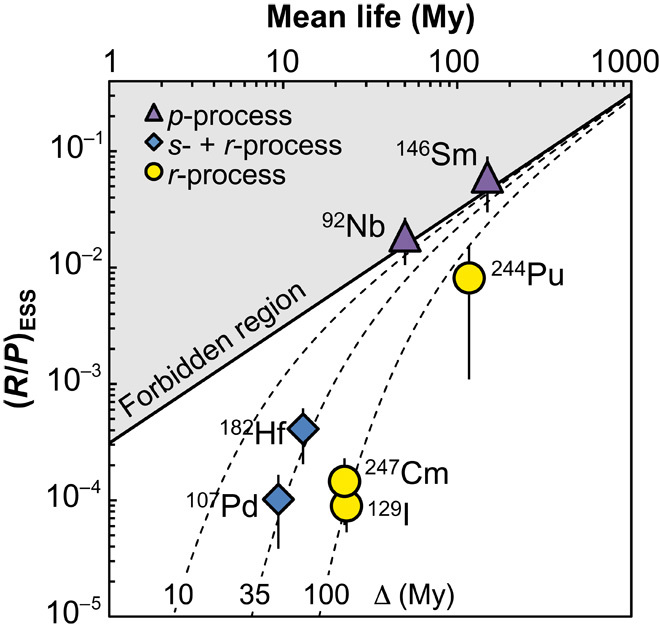
The superscript “r” refers to the r-process component of the cosmic abundance [obtained after subtracting the s-process contribution from solar abundances (3)]. When the normalizing isotope is not stable (for example, X/238U or X/232Th), the R/P ratio [that is, (NSLR/NStable)/(PSLR/PStable)] is corrected for the decay of the long-lived isotope by multiplication by the N/P ratio of the normalizing isotope in the ESS [0.71 for 238U and 0.89 for 232Th, values from Nittler and Dauphas (10)]. Triangles denote p-process isotopes, diamonds denote r-process nuclides with possible large s-process contributions, and circles denote r-process isotopes. The data are compared to model steady-state abundances in the ISM, using the model of Dauphas et al. (28) with k = 1.7 and a presolar age of the galaxy of 8.7 Gy. Dotted curves show model abundances assuming free-decay intervals of 10, 35, and 100 My, respectively. The abundances of all r-process nuclides can be explained by a single r-process environment that was active throughout the history of the galaxy and from which the solar system parent material was isolated about 100 My before SS formation, provided that 182Hf and 107Pd in meteorites originate from the s-process (6, 31). See the Supplementary Materials for source data.
Although some low metallicity halo stars point to the existence of at least three distinct r-processes, these stars formed from a gas that had been enriched in r-process nuclides by synthesis in very low metallicity stars formed early in the history of the galaxy. Meteorite evidence suggests that such r-process multiplicity may only be relevant to exotic conditions that prevailed in the earliest generation of stars in the history of the galaxy and that a single r-process may still be relevant to long-term models of the chemical evolution of the galaxy.
MATERIALS AND METHODS
Twelve fine-grained and three coarse-grained CAIs were selected for this study (fig. S1). One of the coarse-grained CAIs (TS32) was obtained in powder form directly and was described in a previous publication (32). All other CAIs were identified in Allende slabs and extracted with clean stainless steel dental tools before digestion by acids. A small chip of each CAI (all but TS32) was extracted using clean stainless steel dental tools under a stereoscopic zoom microscope, and mounted in epoxy for characterization.
All samples were mapped using a JEOL JSM-5800LV scanning electron microscope (SEM). Images of a selected field of view of each CAI, secondary electron, backscattered electron, and false-color RGB (Mg/Ca/Al) are shown in figs. S2 to S15. The REE patterns of the samples were determined using a Quadrupole LA-ICPMS (laser ablation inductively coupled plasma mass spectrometry) at the Field Museum. Diagnostic group II REE patterns were identified in all 12 fine-grained CAIs, indicating that the Nd/U ratio (a proxy for the Cm/U ratio) of these samples was high and that these samples were therefore well suited to search for 235U excesses coming from 247Cm decay.
After digestion but before column chemistry, a small aliquot of each sample (~2 to 3%) was used to determine U and REE concentrations. The samples were spiked with IRMM-3636 U double spike (233U-236U) and processed through U/TEVA resin (Eichrom) column chromatography, using Optima-grade and sub-boiling double-distilled acids.
Isotopic analyses were performed on a ThermoFinnigan Neptune MC-ICPMS at the Origins Lab (University of Chicago), equipped with an OnToolBooster 150 Jet Pump (Pfeiffer) and using Jet sample cones and X skimmer cones. An Aridus II desolvating nebulizer was used for sample introduction, and the measurements were done using a static cup configuration [see (21)]. When a ~+55‰ 235U excess was discovered in the Curious Marie CAI, a new measurement setup was developed that allowed characterization of the U isotopic composition of 0.1 to 0.01 ng of U with a precision of ±2 to 3‰ (see the Supplementary Materials). Extensive testing was done to ensure that no systematic bias was affecting the data, the details of which can be found in the Supplementary Materials.
Supplementary Material
Acknowledgments
We thank P. Heck, J. Holstein, and the Robert A. Pritzker Center for Meteoritics and Polar Studies at the Field Museum for providing some of the CAI samples; I. Steele for help in operating the SEM at the University of Chicago; and L. Dussubieux for help in operating the LA-ICPMS at the Field Museum. We are grateful to A. M. Davis and B. S. Meyer for discussions. Funding: This work was supported by grants from NASA (Laboratory Analysis of Returned Samples, NNX14AK09G; Cosmochemistry, OJ-30381-0036A and NNX15AJ25G) and NSF (Petrology and Geochemistry, EAR144495; Cooperative Studies of the Earth’s Deep Interior, EAR150259) to N.D. and a NASA grant (Cosmochemistry, NNX13AE73G) to L.G. Author contributions: N.D. and F.L.H.T. designed the research; F.L.H.T. and L.G. selected the samples; F.L.H.T. performed the research; F.L.H.T., N.D., and L.G. wrote the paper. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data are available from the authors upon request. This is Origins Lab contribution number 91.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/3/e1501400/DC1
Materials and Methods
Curious Marie: not a FUN CAI.
The 247Cm-235U chronometer and the initial abundance of 247Cm in the ESS.
GCE model
Fig. S1. Photos of typical fine-grained and coarse-grained CAIs.
Figs. S2 to S15. Secondary electron, backscattered electron, and false-color RGB maps of all samples.
Fig. S16. REE and U-Th abundance patterns of all 12 fine-grained CAIs analyzed in this study.
Fig. S17. Results of the standard addition measurements conducted on the Curious Marie CAI.
Fig. S18. U blank from new U/TEVA resin as a function of the volume of 0.05 M HCl passed through the column.
Fig. S19. Results of precision tests of U isotopic measurements done using various instrumental setups.
Fig. S20. Comparison of the δ235U determined on the CAIs using 80% (x axis) and 20% (y axis) of the sample.
Fig. S21. Flowchart of the tests conducted on the Curious Marie CAI.
Fig. S22. Evolution of the U isotopic composition of the gas, instantaneous solid, and cumulative solid, as a function of the fraction of U condensed.
Table S1. Results of SEM analysis on small chips of CAIs mounted in epoxy (wt %).
Table S2. Summary of U isotopic compositions and concentrations of CAIs and geostandards.
Table S3. Specifics of U isotopic measurements on MC-ICPMS for low U samples.
Table S4. Compilation of chemistry blanks and effect on U “stable” isotope ratio measurements.
Table S5. Summary of the Ti data obtained on geostandards and the Curious Marie CAI.
Table S6. Production ratios of selected SLRs produced by the s-, r-, and p-process and present in the ESS, normalized to a stable isotope produced in the same or similar nucleosynthetic process.
Table S7. Compilation of Cm/U isochron data and free-decay interval data from experimental and theoretical studies.
Table S8. Selected extinct radionuclides produced by the s-, r-, and p-process.
REFERENCES AND NOTES
- 1.Burbidge E. M., Burbidge G. R., Fowler W. A., Hoyle F., Synthesis of the elements in stars. Rev. Mod. Phys. 29, 547–650 (1957). [Google Scholar]
- 2.A. G. W. Cameron, Stellar Evolution, Nuclear Astrophysics, and Nucleogenesis (Dover Publications Inc., New York, ed. 2, 1957). [Google Scholar]
- 3.Bisterzo S., Travaglio C., Gallino R., Wiescher M., Käppeler F., Galactic chemical evolution and solar s-process abundances: Dependence on the 13C-pocket structure. Astrophys. J 787, (2014). [Google Scholar]
- 4.Rauscher T., Dauphas N., Dillmann I., Fröhlich C., Fülöp Z., Gyürky G., Constraining the astrophysical origin of the p-nuclei through nuclear physics and meteoritic data. Rep. Prog. Phys. 76, 066201 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Wasserburg G. J., Busso M., Gallino R., Abundances of actinides and short-lived nonactinides in the interstellar medium: Diverse supernova sources for the r-processes. Astrophys. J 466, L109–L113 (1996). [Google Scholar]
- 6.Meyer B. S., Clayton D. D., Short-lived radioactivities and the birth of the Sun. Space Sci. Rev. 92, 133–152 (2000). [Google Scholar]
- 7.Dauphas N., Multiple sources or late injection of short-lived r-nuclides in the early solar system? Nucl. Phys. A 758, 757–760 (2005). [Google Scholar]
- 8.Huss G. R., Meyer B. S., Srinivasan G., Goswami J. N., Sahijpal S., Stellar sources of the short-lived radionuclides in the early solar system. Geochim. Cosmochim. Acta. 73, 4922–4945 (2009). [Google Scholar]
- 9.Hill V., Plez B., Cayrel R., Beers T. C., Nordström B., Andersen J., Spite M., Spite F., Barbuy B., Bonifacio P., Depagne E., François P., Primas F., First stars. I. The extreme r-element rich, iron-poor halo giant CS 31082-001: Implications for the r-process site(s) and radioactive cosmochronology. Astron. Astrophys. 387, 560–579 (2002). [Google Scholar]
- 10.L. R. Nittler, N. Dauphas, Meteorites and the Chemical Evolution of the Milky Way (University of Arizona Press, Tucson, AZ, 2006), vol. 943, pp. 127–146. [Google Scholar]
- 11.Brennecka G. A., Weyer S., Wadhwa M., Janney P. E., Zipfel J., Anbar A. D., 238U/235U variations in meteorites: Extant 247Cm and implications for Pb-Pb dating. Science 327, 449–451 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Lugmair G. W., Marti K., Sm–Nd–Pu time-pieces in Angra dos Reis meteorite. Earth Planet Sc. Lett. 35, 273–284 (1977). [Google Scholar]
- 13.Boynton W. V., Fractionation in solar nebula, II. Condensation of Th,U, Pu and Cm. Earth Planet Sc. Lett. 40, 63–70 (1978). [Google Scholar]
- 14.Jones J. H., The geochemical coherence of Pu and Nd and the 244Pu/238U ratio of the early solar system. Geochim. Cosmochim. Acta. 46, 1793–1804 (1982). [Google Scholar]
- 15.Jones J. H., Burnett D. S., Experimental geochemistry of Pu and Sm and the thermodynamics of trace element partitioning. Geochim. Cosmochim. Acta. 51, 769–782 (1987). [Google Scholar]
- 16.Tatsumoto M., Shimamura T., Evidence for live 247Cm in the early Solar System. Nature 286, 118–122 (1980). [Google Scholar]
- 17.Amelin Y., Kaltenbach A., Iizuka T., Stirling C. H., Ireland T. R., Petaev M., Jacobsen S. B., U–Pb chronology of the Solar System’s oldest solids with variable 238U/235U. Earth Planet Sc. Lett. 300, 343–350 (2010). [Google Scholar]
- 18.Connelly J. N., Bizzarro M., Krot A. N., Nordlund Å., Wielandt D., Ivanova M. A., The absolute chronology and thermal processing of solids in the solar protoplanetary disk. Science 338, 651–655 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Goldmann A., Brennecka G., Noordmann J., Weyer S., Wadhwa M., The uranium isotopic composition of the Earth and the Solar System. Geochim. Cosmochim. Acta. 148, 145–158 (2015). [Google Scholar]
- 20.Andersen M. B., Elliott T., Freymuth H., Sims K. W. W., Niu Y., Kelley K. A., The terrestrial uranium isotope cycle. Nature 517, 356–359 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Tissot F. L. H., Dauphas N., Uranium isotopic compositions of the crust and ocean: Age corrections, U budget and global extent of modern anoxia. Geochim. Cosmochim. Acta. 167, 113–143 (2015). [Google Scholar]
- 22.Boynton W. V., Fractionation in solar nebula; Condensation of yttrium and rare-earth elements. Geochim. Cosmochim. Acta. 39, 569–584 (1975). [Google Scholar]
- 23.Davis A. M., Grossman L., Condensation and fractionation of rare earths in the solar nebula. Geochim. Cosmochim. Acta. 43, 1611–1632 (1979). [Google Scholar]
- 24.D. K. Ross, J. I. Simon, S. B. Simon, L. Grossman, The 46th Lunar and Planetary Science Conference, The Woodlands, Texas, 16 to 20 March 2015. [Google Scholar]
- 25.S. S. Russell, G. J. MacPherson, in Workshop on parent-body and nebular modification of chondritic materials, Hawaii, 1997, pp. 4054.
- 26.Chen J. H., Wasserburg G. J., The isotopic composition of uranium and lead in Allende inclusions and meteoritic phosphates. Earth Planet Sc. Lett. 52, 1–15 (1981). [Google Scholar]
- 27.Clayton D. D., Galactic chemical evolution and nucleocosmochronology—Standard model with terminated infall. Astrophys. J 285, 411–425 (1984). [Google Scholar]
- 28.Dauphas N., Rauscher T., Marty B., Reisberg L., Short-lived p-nuclides in the early solar system and implications on the nucleosynthetic role of X-ray binaries. Nucl. Phys. A 719, C287–C295 (2003). [Google Scholar]
- 29.Clayton D. D., Nuclear cosmochronology within analytic models of the chemical evolution of the solar neighbourhood. Mon. Not. R. Astron. Soc. 234, 1–36 (1988). [Google Scholar]
- 30.Clayton D. D., Extinct radioactivities; A three-phase mixing model. Astrophys. J 268, 381–384 (1983). [Google Scholar]
- 31.Lugaro M., Heger A., Osrin D., Goriely S., Zuber K., Karakas A. I., Gibson B. K., Doherty C. L., Lattanzio J. C., Ott U., Stellar origin of the 182Hf cosmochronometer and the presolar history of solar system matter. Science 345, 650–653 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Simon S. B., Davis A. M., Grossman L., Origin of compact type A refractory inclusions from CV3 carbonaceous chondrites. Geochim. Cosmochim. Acta. 63, 1233–1248 (1999). [Google Scholar]
- 33.Stirling C. H., Halliday A. N., Porcell D., In search of live 247Cm in the early solar system. Geochim. Cosmochim. Acta. 69, 1059–1071 (2005). [Google Scholar]
- 34.Stirling C. H., Halliday A. N., Potter E.-K., Andersen M. B., Zanda B., A low initial abundance of 247Cm in the early solar system and implications for r-process nucleosynthesis. Earth Planet Sc. Lett. 251, 386–397 (2006). [Google Scholar]
- 35.Grossman L., Ganapathy R., Trace elements in Allende meteorite—I. Coarse-grained, Ca-rich inclusions. Geochim. Cosmochim. Acta. 40, 331–344 (1976). [Google Scholar]
- 36.Grossman L., Ganapathy R., Davis A. M., Trace elements in the Allende meteorite—III. Coarse-grained inclusions revisited. Geochim. Cosmochim. Acta 41, 1647–1664 (1977). [Google Scholar]
- 37.B. Mason, S. R. Taylor, Inclusions in the Allende meteorite, in Smithsonian Contributions to the Earth Sciences (Smithsonian Institution Press, Washington, DC, 1982), vol. 25. [Google Scholar]
- 38.Grossman L., Petrography and mineral chemistry of Ca-rich inclusions in Allende meteorite. Geochim. Cosmochim. Acta. 39, 433–434 (1975). [Google Scholar]
- 39.G. J. MacPherson, S. B. Simon, A. M. Davis, L. Grossman, A. N. Krot, in Chondrites and the Protoplanetary Disk, A. N. Krot, E. R. D. Scott, B. Reipurth, Eds. (University of Michigan, Ann Arbor, MI, 2005), vol. 341, pp. 225. [Google Scholar]
- 40.Tanaka T., Masuda A., Rare-earth elements in matrix, inclusions, and chondrules of Allende meteorite. Icarus 19, 523–530 (1973). [Google Scholar]
- 41.Grossman L., Ganapathy R., Trace elements in Allende meteorite—II. Fine-grained. Ca-rich inclusions. Geochim. Cosmochim. Acta 40, 967–977 (1976). [Google Scholar]
- 42.Jochum K. P., Weis U., Stoll B., Kuzmin D., Yang Q., Raczek I., Jacob D. E., Stracke A., Birbaum K., Frick D. A., Günther D., Enzweiler J., Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanal. Res. 35, 397–429 (2011). [Google Scholar]
- 43.A. Verbruggen et al., “Preparation and certification of IRMM-3636, IRMM-3636a and IRMM-3636b,” JRC Scientific and Technical Reports, 2008. [Google Scholar]
- 44.Weyer S., Anbar A. D., Gerdes A., Gordon G. W., Algeo T. J., Boyle E. A., Natural fractionation of 238U/235U. Geochim. Cosmochim. Acta. 72, 345–359 (2008). [Google Scholar]
- 45.Meisel T., Moser J., Fellner N., Wegscheider W., Schoenberg R., Simplified method for the determination of Ru, Pd, Re, Os, Ir and Pt in chromitites and other geological materials by isotope dilution ICP-MS and acid digestion. Analyst 126, 322–328 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Telus M., Dauphas N., Moynier F., Tissot F. L. H., Teng F.-Z., Nabelek P. I., Craddock P. R., Groat L. A., Iron, zinc, magnesium and uranium isotopic fractionation during continental crust differentiation: The tale from migmatites, granitoids, and pegmatites. Geochim. Cosmochim. Acta. 97, 247–265 (2012). [Google Scholar]
- 47.Dauphas N., Pourmand A., Teng F.-Z., Routine isotopic analysis of iron by HR-MC-ICPMS: How precise and how accurate? Chem. Geol. 267, 175–184 (2009). [Google Scholar]
- 48.Tang H., Dauphas N., Abundance, distribution, and origin of 60Fe in the solar protoplanetary disk. Earth Planet Sc. Lett. 359–360, 248–263 (2012). [Google Scholar]
- 49.Lee T., Mayeda T. K., Clayton R. N., Oxygen isotopic anomalies in Allende Inclusion Hal. Geophys. Res. Lett. 7, 493–496 (1980). [Google Scholar]
- 50.Nagasawa H., Blanchard D. P., Shimizu H., Masuda A., Trace element concentrations in the isotopically unique Allende inclusion, EK 1-4-1. Geochim. Cosmochim. Acta. 46, 1669–1673 (1982). [Google Scholar]
- 51.B. Dominik, E.-K. Jessberger, T. Staudacher, K. Nagel, A. El Goresy, in Lunar and Planetary Institute Science Conference Abstracts (Lunar and Planetary Institute, Houston, 1978), vol. 9, pp. 1249–1266. [Google Scholar]
- 52.Krot A. N., Nagashima K., Wasserburg G. J., Huss G. R., Papanastassiou D., Davis A. M., Hutcheon I. D., Bizzarro M., Calcium-aluminum-rich inclusions with fractionation and unknown nuclear effects (FUN CAIs): I. Mineralogy, petrology, and oxygen isotopic compositions. Geochim. Cosmochim. Acta. 145, 206–247 (2014). [Google Scholar]
- 53.Tang H., Liu M. C., McKeegan K. D., Tissot F. L. H., Dauphas N., Oxygen isotopes and high mg-26 excesses in a u-depleted fine-grained allende cai. Meteorit. Planet. Sci. 50, (2015). [Google Scholar]
- 54.Clayton R. N., Hinton R. W., Davis A. M., Isotopic variations in the rock-forming elements in meteorites. Philos. T. R. Soc. A 325, 483–501 (1988). [Google Scholar]
- 55.Blake J. B., Schramm D. N., 247Cm as a short-lived r-process chronometer. Nature 243, 138–140 (1973). [Google Scholar]
- 56.Bourdon B., Turner S., Henderson G. M., Lundstrom C. C., Introduction to U-series geochemistry. Rev. Mineral. Geochem. 52, 1–21 (2003). [Google Scholar]
- 57.MacPherson G. J., Davis A. M., Refractory inclusions in the prototypical CM chondrite, Mighei. Geochim. Cosmochim. Acta. 58, 5599–5625 (1994). [Google Scholar]
- 58.Dauphas N., Chen J. H., Zhang J., Papanastassiou D. A., Davis A. M., Travaglio C., Calcium-48 isotopic anomalies in bulk chondrites and achondrites: Evidence for a uniform isotopic reservoir in the inner protoplanetary disk. Earth Planet Sc. Lett. 407, 96–108 (2014). [Google Scholar]
- 59.Richter F. M., Davis A. M., Ebel D. S., Hashimoto A., Elemental and isotopic fractionation of Type B calcium-, aluminum-rich inclusions: Experiments, theoretical considerations, and constraints on their thermal evolution. Geochim. Cosmochim. Acta. 66, 521–540 (2002). [Google Scholar]
- 60.Richter F. M., Timescales determining the degree of kinetic isotope fractionation by evaporation and condensation. Geochim. Cosmochim. Acta. 68, 4971–4992 (2004). [Google Scholar]
- 61.Arlandini C., Käppeler F., Wisshak K., Gallino R., Lugaro M., Busso M., Straniero O., Neutron capture in low-mass asymptotic giant branch stars: Cross sections and abundance signatures. Astrophys. J. 525, 886–900 (1999). [Google Scholar]
- 62.Dauphas N., The U/Th production ratio and the age of the Milky Way from meteorites and Galactic halo stars. Nature 435, 1203–1205 (2005). [DOI] [PubMed] [Google Scholar]
- 63.Goriely S., Arnould M., Actinides: How well do we know their stellar production? Astron. Astrophys. 379, 1113–1122 (2001). [Google Scholar]
- 64.S. B. Jacobsen, Chondrites and the Protoplanetary Disk, A. N. Krot, E. R. D. Scott, B. Reipurth, Eds. (ASP, San Francisco, 2005), vol. 341, pp. 548. [Google Scholar]
- 65.Anders E., Grevesse N., Abundances of the elements: Meteoritic and solar. Geochim. Cosmochim. Acta. 53, 197–214 (1989). [Google Scholar]
- 66.Boyet M., Carlson R. W., Horan M., Old Sm–Nd ages for cumulate eucrites and redetermination of the solar system initial 146Sm/144Sm ratio. Earth Planet Sc. Lett. 291, 172–181 (2010). [Google Scholar]
- 67.Brazzle R. H., Pravdivtseva O. V., Meshik A. P., Hohenberg C. M., Verification and interpretation of the I-Xe chronometer. Geochim. Cosmochim. Acta. 63, 739–760 (1999). [Google Scholar]
- 68.Burkhardt C., Kleine T., Bourdon B., Palme H., Zipfel J., Friedrich J. M., Ebel D. S., Hf–W mineral isochron for Ca,Al-rich inclusions: Age of the solar system and the timing of core formation in planetesimals. Geochim. Cosmochim. Acta. 72, 6177–6197 (2008). [Google Scholar]
- 69.J. H. Chen, G. J. Wasserburg, in Earth Processes: Reading the Isotopic Code, A. Basu, S. Hart, Eds. (American Geophysical Union, Washington, D.C., 1996), pp. 1–20. [Google Scholar]
- 70.Harper C. L., Evidence for 92gNb in the early solar system and evaluation of a new p-process cosmochronometer from 92gNb/92Mo. Astrophys. J. 466, 437–456 (1996). [Google Scholar]
- 71.G. B. Hudson, B. M. Kennedy, F. A. Podosek, C. M. Hohenberg, in Lunar and Planetary Institute Science Conference Abstracts (Lunar and Planetary Institute, Houston, TX, 1989), vol. 19, pp. 547–557. [Google Scholar]
- 72.Kleine T., Münker C., Mezger K., Palme H., Rapid accretion and early core formation on asteroids and the terrestrial planets from Hf–W chronometry. Nature 418, 952–955 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Lugmair G. W., Galer S. J. G., Age and isotopic relationships among the angrites Lewis Cliff 86010 and Angra dos Reis. Geochim. Cosmochim. Acta. 56, 1673–1694 (1992). [Google Scholar]
- 74.Prinzhofer A., Papanastassiou D. A., Wasserburg G. J., Samarium-neodymium evolution of meteorites. Geochim. Cosmochim. Acta. 56, 797–815 (1992). [Google Scholar]
- 75.Reynolds J. H., Determination of the age of the elements. Phys. Rev. Lett. 4, 8–10 (1960). [Google Scholar]
- 76.Schönbächler M., Rehkämper M., Halliday A. N., Lee D.-C., Bourot-Denise M., Zanda B., Hattendorf B., Günther D., Niobium-zirconium chronometry and early solar system development. Science 295, 1705–1708 (2002). [DOI] [PubMed] [Google Scholar]
- 77.Schönbächler M., Carlson R. W., Horan M. F., Mock T. D., Hauri E. H., Silver isotope variations in chondrites: Volatile depletion and the initial 107Pd abundance of the solar system. Geochim. Cosmochim. Acta. 72, 5330–5341 (2008). [Google Scholar]
- 78.Yin Q., Jacobsen S. B., Yamashita K., Blichert-Toft J., Télouk P., Albarède F., A short timescale for terrestrial planet formation from Hf–W chronometry of meteorites. Nature 418, 949–952 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/3/e1501400/DC1
Materials and Methods
Curious Marie: not a FUN CAI.
The 247Cm-235U chronometer and the initial abundance of 247Cm in the ESS.
GCE model
Fig. S1. Photos of typical fine-grained and coarse-grained CAIs.
Figs. S2 to S15. Secondary electron, backscattered electron, and false-color RGB maps of all samples.
Fig. S16. REE and U-Th abundance patterns of all 12 fine-grained CAIs analyzed in this study.
Fig. S17. Results of the standard addition measurements conducted on the Curious Marie CAI.
Fig. S18. U blank from new U/TEVA resin as a function of the volume of 0.05 M HCl passed through the column.
Fig. S19. Results of precision tests of U isotopic measurements done using various instrumental setups.
Fig. S20. Comparison of the δ235U determined on the CAIs using 80% (x axis) and 20% (y axis) of the sample.
Fig. S21. Flowchart of the tests conducted on the Curious Marie CAI.
Fig. S22. Evolution of the U isotopic composition of the gas, instantaneous solid, and cumulative solid, as a function of the fraction of U condensed.
Table S1. Results of SEM analysis on small chips of CAIs mounted in epoxy (wt %).
Table S2. Summary of U isotopic compositions and concentrations of CAIs and geostandards.
Table S3. Specifics of U isotopic measurements on MC-ICPMS for low U samples.
Table S4. Compilation of chemistry blanks and effect on U “stable” isotope ratio measurements.
Table S5. Summary of the Ti data obtained on geostandards and the Curious Marie CAI.
Table S6. Production ratios of selected SLRs produced by the s-, r-, and p-process and present in the ESS, normalized to a stable isotope produced in the same or similar nucleosynthetic process.
Table S7. Compilation of Cm/U isochron data and free-decay interval data from experimental and theoretical studies.
Table S8. Selected extinct radionuclides produced by the s-, r-, and p-process.


