Red Queen dynamics are observed in selected genotypes, whereas the rest of the genotypes remain subordinate in synchronized dynamics.
Keywords: Red Queen, parasitism, coevolution, rare species, cyclic dominance, environmental variability
Abstract
Interactions between hosts and parasites have been hypothesized to cause winnerless coevolution, called Red Queen dynamics. The canonical Red Queen dynamics assume that all interacting genotypes of hosts and parasites undergo cyclic changes in abundance through negative frequency-dependent selection, which means that any genotype could become frequent at some stage. However, this prediction cannot explain why many rare genotypes stay rare in natural host-parasite systems. To investigate this, we build a mathematical model involving multihost and multiparasite genotypes. In a deterministic and controlled environment, Red Queen dynamics occur between two genotypes undergoing cyclic dominance changes, whereas the rest of the genotypes remain subordinate for long periods of time in phase-locked synchronized dynamics with low amplitude. However, introduction of stochastic noise in the model might allow the subordinate cyclic host and parasite types to replace dominant cyclic types as new players in the Red Queen dynamics. The factors that influence such evolutionary switching are interhost competition, specificity of parasitism, and degree of stochastic noise. Our model can explain, for the first time, the persistence of rare, hardly cycling genotypes in populations (for example, marine microbial communities) undergoing host-parasite coevolution.
INTRODUCTION
Antagonistic interactions are major factors that drive evolution on different time scales (1–4). The Red Queen hypothesis has been proposed as a model for antagonistic interactions where species (for example, host-parasite, prey-predator, and victim-exploiter) perpetually coevolve in winnerless dynamics (1, 2, 5, 6). In host-parasite interactions, the Red Queen hypothesis suggests that coevolution occurs as a result of time-lagged negative frequency-dependent selection. The fitness of a common host type decreases because of overinfection by a common parasite type, prompting the increase in the fitness of a rare host type, which after its increase becomes the target of other parasite types that will then increase in consequence (1, 3, 5, 7, 8). Winnerless coevolution is widespread in host-parasite interactions because of nearly symmetrical selection, which means that the evolution of hosts is countered by the evolution of parasites (1, 9). Species that fail to catch up with such coevolutionary races may become extinct (4, 10). The Red Queen hypothesis has gained an important position in evolutionary biology, being suggested to explain the evolution of sex (1, 11), the antagonist-mediated diversity of species (12–14), and the emergence of antibiotic resistance (7).
One of the manifestations of the Red Queen hypothesis is oscillatory dynamics, mathematically defined as out-of-phase population/frequency cycles with similar amplitude (1, 5, 15, 16). In canonical Red Queen dynamics (5), all of the host and parasite genotypes undergo negative frequency-dependent selection (represented by the out-of-phase cycles), but their collective fitness remains the same (fig. S1). However, there are cases where only some genotypes are engaged in Red Queen dynamics, whereas others are engaged in phase-locked (synchronized) cycles with minor amplitude (Fig. 1). Here, we investigate the conditions for such cases using mathematical simulations involving multihost and multiparasite types, and we test for the effects of stochastic noise on coevolutionary dynamics. Stochastic noise represents random physical-environmental perturbations introduced in simulation parameters (17–21). Cycles of alternating dominance in the Red Queen dynamics and the effects of noise on coevolution can be associated with the dynamics observed in marine microbial communities (for example, bacteriophage predation), which have been explained by the “killing-the-winner” hypothesis (15, 22, 23). The Red Queen dynamics also explain coevolution in invertebrate-parasite systems (for example, bacterial infection of Daphnia) (8, 24, 25).
Fig. 1. Population density time series illustrating Red Queen dynamics with phase-locked rare types (K = 40, σ = 0).
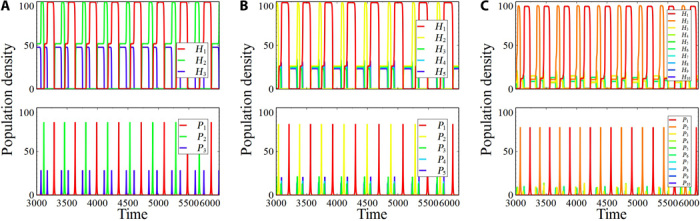
Host 1 (parasite 1) and host 2 (parasite 2) have out-of-phase cycles with dominant amplitude, whereas the rest are inferior and phase-locked. We call the cyclic dominance between the two dominant species types as “Red Queen binary oscillations.” Some inferior phase-locked population plots are not visible because they have almost similar periods and amplitudes as the time series plotted in front of them. The qualitative behaviors of both host and parasite population time series follow Red Queen binary oscillations. (A) n = 3. (B) n = 5. (C) n = 10.
We hypothesize that functional response in host-parasite interactions influences the occurrence of Red Queen dynamics. Functional response represents the rates of infection and host utilization by parasites (26, 27). Type I functional response denotes linear parasitic utilization of hosts. Type II functional response denotes parasitic utilization of hosts following a hyperbolic curve, whereas type III functional response follows a sigmoidal (S-shaped) curve. Type III functional response incorporates host switching and parasite learning, which are characterized by a low parasitism infectivity rate at low host density and by an accelerated parasitism infectivity rate at intermediate host density (27–29). Here, our simulations using type III functional response reveal a novel pattern of coevolution exhibiting the Red Queen dynamics of two host and parasite types, whereas several other genotypes remain subordinate for long periods of time. Discovery of novel coevolutionary dynamics occurring as a result of complex biotic interactions provides an insight into the phenomena seen in epidemiology and evolution, especially the evolution of resistance and virulence (30–32).
RESULTS
To simulate the interaction of n host types and n parasite types (genotypes, strains, varieties, or species), we use an ordinary differential equation representation of an antagonistic system with a type III sigmoidal functional response (see Materials and Methods). We have observed that a limited number of combinations of parameter values lead to Red Queen cycles. A high host basal growth rate (ri), an intermediate death rate in parasites (dj), and high parasite specificity (for example, matching-allele interactions) favor the occurrence of Red Queen oscillatory behavior (Fig. 2 and fig. S2). An increased host basal growth rate provides the host the ability to recover from the effects of parasitism, which is a requirement for inducing Red Queen cycles (Fig. 2A). On the other hand, an intermediate degree of parasite mortality is essential for generating Red Queen cycles because a low parasite death rate results in high levels of parasitism that adversely affect the growth of host populations (Fig. 2B). A high parasite death rate limits the antagonistic effect of the parasites, which is not enough to steer negative frequency-dependent selection in hosts, resulting in equilibrium (Fig. 2B). Furthermore, host-parasite interactions with high parasite specificity are favorable for driving cyclic and never-ending negative frequency-dependent selection, compared to interactions with more uniform parasite specificity (fig. S2).
Fig. 2. Effects of host basal growth rate (ri = r) and parasite mortality rate (dj = d) on the generation of Red Queen binary oscillations (K = 40, σ = 0).
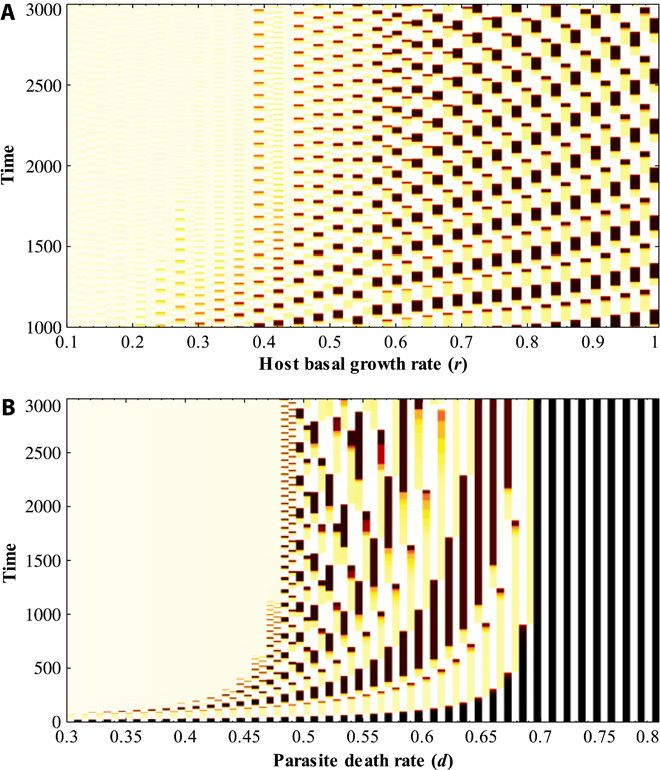
Vertical alternating colors characterize oscillations. (A) A high host basal growth rate r favors the occurrence of Red Queen binary oscillations (for example, r > 0.5; d = 0.5). A lower basal growth rate may result in host extinction or in population dynamics where only one type is dominant. (B) An intermediate parasite mortality rate d favors the occurrence of Red Queen binary oscillations (for example, d = 0.5; r = 1). Relatively low and high death rates may result in host extinction or in population dynamics where only one host type is dominant.
Red Queen binary oscillations
In contrast to the canonical Red Queen dynamics arising from simulations with type II functional response (fig. S1), our simulations involving type III functional response reveal Red Queen dynamics occurring mainly between two host/parasite types that alternate in dominating the population (here called dominant types), with other types playing a subordinate role (that is, remaining rare) (Fig. 1). These dynamics happen not only in low-dimensional systems (for example, one subordinate type, n = 3; Fig. 1A) but also in high-dimensional systems with up to 10 host types and 10 parasite types (Fig. 1, B and C). Specifically, this type of Red Queen dynamics exhibits a population pattern with exactly two out-of-phase host/parasite population densities having high amplitude dynamics, whereas the rest of the hosts/parasites have phase-locked lower population densities that are inferior to the dominant types (Fig. 1). We call these cycles “Red Queen binary oscillations” because of the existence of two dominant types with n-less-two subordinate cyclic types. The two dominant cyclic host/parasite types do not necessarily have a similar length of periodic dominance. The subordinate types are qualitatively identical, with minute differences in their population time series (for example, different cycle peaks).
Modifying the initial conditions of Eqs. 1 and 2 affects the order of dominance in the Red Queen binary oscillations. Using the assumption that only one host type is initially dominant and that the parasite population is initially small [H1(0) = K, Hi(0) = 0.0001 + 0.00001i for i = 2,3,…,n, and Pj(0) = 0.0001 + 0.00001j for j = 1,2,…,n], we have observed that the host types with the highest and lowest initial conditions tend to enter the Red Queen binary oscillations, whereas the rest of the host types become subordinates. However, host-parasite interactions are complex, making it difficult to generalize the order of dominance by simply looking at the rank of initial host and parasite frequencies.
The size of the host’s carrying capacity (K) plays an important role in generating Red Queen binary oscillations (fig. S3). A small K (that is, K < n) promotes intense competition among host types; hence, their population densities converge to equilibrium (region I in fig. S3). As the carrying capacity K increases, the maximum growth potential of each host increases as well, creating an oscillatory competitive outcome (region II in fig. S3). At K = n, population densities start to oscillate (left edge of region IIA). The increase in the maximum host growth potential is represented by the heightened population amplitude (fig. S4). If K is greater than about thrice the number of host types (K > 3n), the Red Queen binary oscillations become apparent at least in a finite time frame (region IIB).
Red Queen dynamics with noise
To test the sensitivity of the deterministic host-parasite interaction model against physical-environmental perturbations, we introduce random noise to the model parameters following the Ornstein-Uhlenbeck process (see Materials and Methods). Under stochastic conditions, it is possible that one of the dominant host/parasite types will be replaced by a subordinate type as a new major player in the Red Queen dynamics (Figs. 3 and 4). Such a replacement is followed again by Red Queen binary oscillations but with different dominant cyclic types. The occurrence of such transition events becomes more frequent as the degree of physical-environmental perturbations increases (figs. S5 and S6). Moderate degrees of stochastic noise preserve the Red Queen binary oscillations; however, large perturbations can cause extinction of species (fig. S5). The replacement of a dominant type by a subordinate type in the Red Queen dynamics is due to changes in the characteristics of each host type (for example, growth rate) and each parasite type (for example, death rate and specificity).
Fig. 3. Illustration of Red Queen dynamics with noise (n = 3, K = 25, σ = 15%).
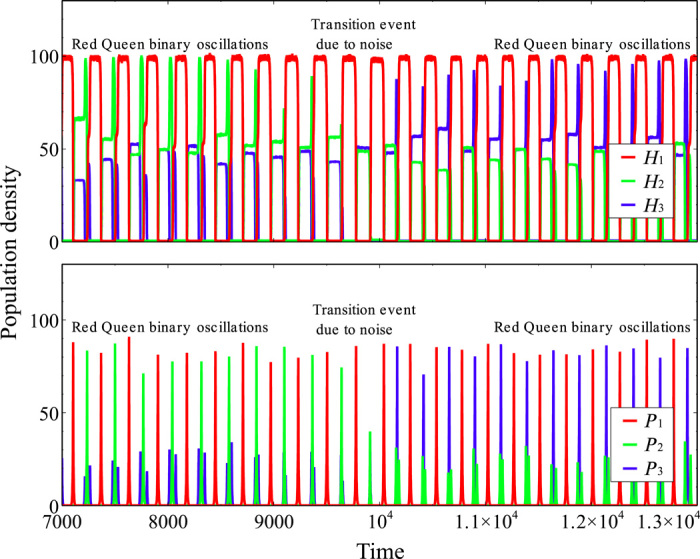
Here, Red Queen dynamics are represented by winnerless coevolution between two dominant cyclic host/parasite types (binary oscillations). The evolution due to random noise is characterized by a stochastic transition event where a rare cyclic host/parasite type (H3, P3) replaces one of the dominant cyclic types (H2, P2). The transition event is a result of the accumulated effect of random noise representing uncertain physical-environmental perturbations.
Fig. 4. Evolutionary landscape separated into two regions by an entrapment barrier.
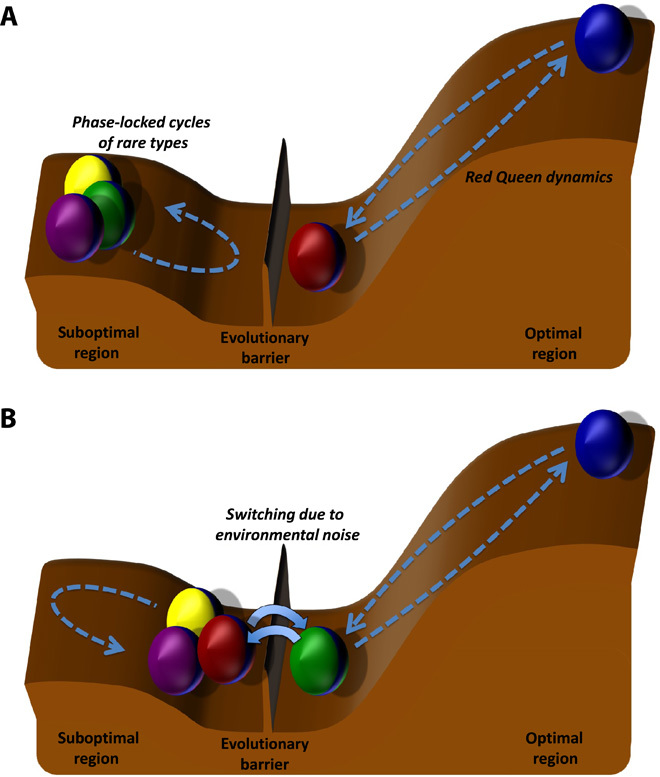
The height of the landscape characterizes the population density of species types (balls). The barrier limits the number of dominant cyclic types and constrains subordinate/rare cyclic types to enter the optimal region where Red Queen dynamics occur. In the optimal region, a dominant cyclic type can attain maximum growth potential near its carrying capacity. The population dynamics of the species oscillate (phase-locked subordinate cycles and Red Queen dynamics) as a result of parasitism (the paths of broken arrow lines illustrate oscillatory behavior). (A) Rare cyclic types remain rare because they are trapped at a suboptimal region. Only two species types undergo cyclic dominance of Red Queen dynamics. The population dynamics of rare cyclic types do not exhibit Red Queen dynamics but rather are phase-locked (synchronized). (B) Physical-environmental variability can allow a rare cyclic type to escape the suboptimal region and enter the optimal region by hurdling the evolutionary entrapment barrier. However, the optimal region only allows two dominant cyclic types; hence, one of the types needs to leave the optimal region to accommodate the succeeding rare type (red and green balls).
DISCUSSION
On the basis of our simulations, replacement of a dominant cyclic host type can happen in two ways: through negative frequency-dependent selection following the Red Queen dynamics and through noise-driven replacement of a dominant cyclic host type by a subordinate cyclic type. We can look at these two ways of evolutionary switching in an evolutionary landscape (Fig. 4). In a controlled and deterministic environment, several subordinate/rare cyclic types can be trapped at a suboptimal region in the evolutionary landscape (Fig. 4A). The optimal region in the landscape denotes the situation where a dominant cyclic type can attain maximum growth potential near its carrying capacity. Physical-environmental variability can aid the trapped subordinate cyclic types to escape and move to a new state of dynamics by hurdling the evolutionary entrapment barrier (Fig. 4B). The entrapment barrier is a constraint that only allows two dominant cyclic genotypes. The formation of the barrier is influenced by the characteristics of hosts and parasites and their interactions, such as the growth rate of hosts, the death rate of parasites, parasite specificity, and the competitive ability of host types. The evolutionary switching events can elucidate the mechanisms that drive different ecological and evolutionary phenomena, such as the fluctuating population in marine microbial communities (15, 22, 23, 33), the evolution of water fleas (8, 24, 25), and the abundance of rare species in nature (34, 35).
The structure of parasitism functional response is essential for generating Red Queen dynamics. In type II and type III functional responses, the rates of infection and host utilization by parasites tend to satiate at high host density. However, type III functional response incorporates population size–based parasite learning and host switching (27–29, 36). This is indicated by the case where a host type with intermediate density is more exposed and prone to parasite infection than a host type with lower density. As a result, certain parameter ranges of the model with type III functional response accommodate only a limited number of dominant cyclic host/parasite types, and the rest are subordinate cyclic types (Fig. 1). This finding is in contrast to the dynamics of models with type II functional response, where the Red Queen dynamics arise in every host type and every parasite type given specific parameter values (we call these canonical Red Queen cycles as “Red Queen n-ary oscillations”) (5). Red Queen n-ary oscillations allow all hosts/parasites to undergo never-ending dominance switching, such that each type can be numerically dominant for certain periods of time (fig. S1). In addition, an interaction system involving multihosts and multiparasites with linear functional response (type I) may result in a different scenario where out-of-phase population cycles with identical amplitude do not exist (37).
With a type III functional response, the rate of infectivity is low at low host population densities, but increases at intermediate host population densities and then slows down at very high host population densities as a result of satiation. Various host-parasite interactions in nature conform to this sigmoidal (S-shaped) functional response curve (38–41). The sigmoidal functional response curve could be a logical consequence of mass-action transmission as it is commonly assumed to be valid in host-parasite systems (38–40). Mass-action transmission implies that the number of susceptible hosts that become infected by parasites depends on the host and parasite population densities.
Consequently, both the Red Queen binary oscillations (resulting from type III functional response) and the Red Queen n-ary oscillations (resulting from type II functional response) bring about parasite-regulated temporal biodiversity (12–14). The Red Queen dynamics, thought to be a form of balancing selection (42), reduce the likelihood of extinction. However, binary and n-ary oscillations differ in the number and dynamics of dominant cyclic host/parasite types. Red Queen binary oscillations entail biodiversity where only a limited number of species types can dominate the system and the rest are phase-locked rare types. The formation of binary oscillations, a special mode of Red Queen dynamics, is one of the possible explanations for the “excess of rare species” phenomenon (34, 35) and synchrony in population abundance (43, 44). Moreover, we hypothesize that restructuring the model of interhost competition and parasitism might give rise to m (where 2 < m < n) sequential dominant host/parasite types.
The relationship between interhost competition and parasitism is necessary for the occurrence of Red Queen binary oscillations. Parasitism is not enough to generate out-of-phase oscillations, but rather strong rivalry among host types should also occur (5). In host-parasite coevolution, interhost competition indicates evolutionary selection among host genotypes. In addition to having increased host basal growth rate (Fig. 2), the resources (K) of competing hosts should be large enough to provide each host the ability to attain its full growth potential (figs. S3 and S4). Hosts with high potential for growth are suitable for stimulating effective parasitism that eventually results in negative frequency-dependent selection. Moreover, increasing the host’s carrying capacity generates oscillations with large amplitude, an effect earlier described as the paradox of enrichment (45).
Random noise could represent stochastic physical-environmental perturbations (17, 20). A moderate level of stochastic noise causes one host/parasite type to leave the Red Queen dynamics and another type to enter the winnerless coevolution process (Fig. 3). Red Queen binary oscillations warrant the existence of subordinate host and parasite types, as well as a limited number of dominant cyclic types, whereas random noise provides an opportunity for the subordinate types to replace the dominant ones (Figs. 3 and 4). A moderate level of stochastic noise affects the model parameters (for example, growth rate and carrying capacity of hosts, mortality rate, specificity, and infectivity of parasites), driving the previously dominant cyclic type to decline and allowing a subordinate cyclic host type to become a new dominant type. The noise-driven changes in parameter values affect the strength of interactions among the hosts and the parasites, resulting in the alteration of players in the Red Queen dynamics. Increasing the degree of physical-environmental perturbations is then expected to increase the occurrence of transition events where a rare cyclic host/parasite type replaces one of the dominant cyclic types (fig. S5).
Many cases of evolutionary switching of dominant populations occur because natural ecosystems are inherently noisy (17–21). Stochastic noise is believed to play a role in regulating the dominant regime in ecological interacting systems (17, 21, 46). Hence, the consequence of the Red Queen binary oscillations with physical-environmental perturbations might be the temporal biodiversity seen in natural ecosystems (for example, see fig. S6) (18, 19, 21). The interplay among biotic and abiotic factors under suitable conditions (genotype-by-genotype-by-environment) shapes the structures and interactions in nature (47–52). However, note that very large noise does not necessarily result in fluctuating densities (fig. S5). Large abiotic perturbations could be outside the survival tolerance range of some species, causing extinction of species (53–55).
In summary, Red Queen binary oscillations illustrate the parasitism-induced evolution of hosts/parasites from one dominant cyclic type to another type (Fig. 1). The population cycles of the two dominant cyclic types have the same amplitude, characterizing a consistent fitness pattern in spite of the continuous evolutionary switching between the two types. The cycle of evolution persists between the two dominant types only, and the remaining types are rare. However, a phase-locked rare type can evolve to become dominant as a result of a considerable degree of physical-environmental noise (Fig. 3). Our mathematical simulation explains the underlying mechanisms of one of the manifestations of Red Queen dynamics acting on host-parasite coevolution. This type of coevolutionary dynamics presents an explanation for the abundance of rare species. Our numerical prediction can guide future empirical studies in finding more evidence for the Red Queen hypothesis and in investigating how winnerless coevolution affects complex ecological interactions in nature (6, 56–58).
MATERIALS AND METHODS
We considered a resource-consumer model of the host-parasite interaction system (5, 29, 37) with up to 10 host types and 10 parasite types (n = 10). The mathematical model with type III functional response is
| (1) |
| (2) |
where i,j = 1,2,…,n (n ≥ 3).
In Eqs. 1 and 2, the population size of a host type (Hi) increases according to basal growth rate (ri) and decreases as a result of parasitic infections (functional response). The sum of all hosts shares a carrying capacity of size K. On the other hand, the population of a parasite type (Pj) increases with parasitic utilization of hosts and decreases with constant death (dj) (numerical response). Let the coefficients φik, cij, and βjik (k = 1,2,…,n) represent the competitive ability between hosts i and k, host i to parasite j conversion due to host utilization, and the effect of host type k (k = 1,2,…,n) on the alteration of the functional response associated with the infection of host i by parasite j, respectively (table S1). This model assumes that hosts are immediately eliminated once infected by a parasite. Infected hosts cannot recover from disease, as is often the case in biological systems involving bacteriophages and castrating parasites and in invertebrate-parasite systems (for example, infection of Daphnia magna by Pasteuria ramosa) (8, 37, 59–62).
The complexity of the dynamics of species interaction increases with the number of variables and parameters. We accordingly focused on the conditions under which Red Queen dynamics are likely to occur in restricted parameter space (see the Supplementary Materials for the discussion of simulation parameter values). Various qualitative dynamics and other modes of Red Queen cycles may arise in an interaction system with type III functional response (for example, fig. S7), but we focused on the parameter conditions under which the Red Queen binary oscillations are likely to occur.
The parameter αij in the functional response represents the parasitism efficiency of parasite j against host i. Because we were concerned with patterns of oscillations (for example, Red Queen dynamics), we restricted the parasitism efficiency matrix A = [αij] to be diagonally dominant such that
| (3) |
for all i.
A diagonally dominant matrix implies that host i is the main host of parasite j = i, whereas all hosts k ≠ i are secondary or nonhosts. This assumption assures that there is neither a unique superior parasite nor an inferior parasite in infectivity, and that there is neither a unique superior host nor an inferior host in parasite resistance. Trade-offs restrict the presence of all-resistant hosts and of superparasites that can infect all hosts (22, 63, 64). The parasitism efficiency matrix can be considered as a representation of the continuum between gene-for-gene and matching-allele models (5, 65–68).
The use of parameter perturbations following an Ornstein-Uhlenbeck process represents physical-environmental stochastic noise. The assumed stochastic differential equation for each variable parameter, say x, is formulated as (20)
| (4) |
The term characterizes drift toward the mean value . The parameter σx is the amplitude of the Gaussian white noise dWx. The variable parameters in our simulations are ri ≥ 0, dj ≥ 0, K > 0, and αij ≥ 0 for all i and j. Each parameter is perturbed by independent white noise.
Supplementary Material
Acknowledgments
We thank the members of the Yoshimura Laboratory, Shizuoka University, the University of the Philippines Los Baños Biomathematics Initiative, and the Numerical Mathematics Training and Research Team (NuMath) for comments and discussions. Funding: This work was partly supported by the Japan Society for the Promotion of Science (grants 22255004, 22370010, 26257405, and 15H04420 to J.Y.; grant 26400388 to S.M.; grants 26840126 and 13J03600 to S.K.; and grant 14J02983 to H.I.), Japanese Government (Monbukagakusho: MEXT Scholarship to J.F.R.), Mitsubishi Corporation (international student scholarship to J.M.T.), Asahi Glass Foundation (S.K.), Swiss National Science Foundation (D.E.), Special Coordination Fund for Promoting Science and Technology “The strategy for construction of low carbon society by CO2 reduction and energy utilization of unused biomass” (to Shizuoka University), and Shizuoka University Environmental Leaders Program (ELSU). Author contributions: J.F.R., S.K., D.E., and J.Y. conceived the study. J.F.R., T.U., S.M., and J.Y. built and analyzed the model. J.F.R., J.M.T., and H.I. ran the simulations and created the figures. J.F.R., T.U., D.E., and J.Y. wrote the manuscript. J.F.R. was the lead author. All authors reviewed the manuscript and gave their final approval for publication. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/3/e1501548/DC1
Supporting text
Table S1. Summary of state variables and model parameters.
Fig. S1. Example of canonical Red Queen dynamics (Red Queen n-ary oscillations) where all host and parasite types undergo dominance switching.
Fig. S2. Oscillatory behavior expected to occur when α has a lower value (that is, there is a higher level of parasite specificity).
Fig. S3. Parameter diagram showing the effects of increasing the host’s carrying capacity K on the oscillation patterns of host and parasite population densities (σ = 0, t = 0 to 50,000).
Fig. S4. Increasing the carrying capacity K increases the amplitude of population density (for example, compare the graphs associated with K = 3 and K = 10; σ = 0).
Fig. S5. Host population density time series showing the effects of stochastic noise (n = 4, K = 16).
Fig. S6. Host population pattern illustrating the effects of stochastic noise on a system with 10 host types and 10 parasite types (K = 40, σ = 10%).
Fig. S7. Additional illustrations of some population dynamics that can arise from our host-parasite interaction system (σ = 0).
REFERENCES AND NOTES
- 1.Brockhurst M. A., Champan T., King K. C., Mank J. E., Paterson S., Hurst G. D. D., Running with the Red Queen: The role of biotic conflicts in evolution. Proc. R. Soc. B 281, 20141382 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mougi A., Iwasa Y., Unique coevolutionary dynamics in a predator–prey system. J. Theor. Biol. 277, 83–89 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Capaul M., Ebert D., Parasite-mediated selection in experimental Daphnia magna populations. Evolution 57, 249–260 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Van Vallen L., A new evolutionary law. Evol. Theory 1, 1–30 (1973). [Google Scholar]
- 5.Rabajante J. F., Tubay J. M., Uehara T., Morita S., Ebert D., Yoshimura J., Red Queen dynamics in multi-host and multi-parasite interaction system. Sci. Rep. 5, 10004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liow L. H., Van Valen L., Stenseth N. C., Red Queen: From populations to taxa and communities. Trends Ecol. Evol. 26, 349–358 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Jefferies J. M. C., Clarke S. C., Webb J. S., Kraaijeveld A. R., Risk of Red Queen dynamics in pneumococcal vaccine strategy. Trends Microbiol. 19, 377–381 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Ebert D., Host-parasite coevolution: Insights from the Daphnia-parasite model system. Curr. Opin. Microbiol. 11, 290–301 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Salathé M., Kouyos R. D., Bonhoeffer S., The state of affairs in the kingdom of the Red Queen. Trends Ecol. Evol. 23, 439–445 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Quental T. B., Marshall C. R., How the Red Queen drives terrestrial mammals to extinction. Science 341, 290–292 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Morran L. T., Schmidt O. G., Gelarden I. A., Parrish R. C. II, Lively C. M., Running with the Red Queen: Host-parasite coevolution selects for biparental sex. Science 333, 216–218 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn A. M., Torchin M. E., Hatcher M. J., Kotanen P. M., Blumenthal D. M., Byers J. E., Coon C. A. C., Frankel V. M., Holt R. D., Hufbauer R. A., Kanarek A. R., Schierenbeck K. A., Wolfe L. M., Perkins S. E., Indirect effects of parasites in invasions. Funct. Ecol. 26, 1262–1274 (2012). [Google Scholar]
- 13.Mouritsen K. N., Poulin R., Parasites boosts biodiversity and changes animal community structure by trait-mediated indirect effects. Oikos 108, 344–350 (2005). [Google Scholar]
- 14.Thomas F., Renaud F., Rousset F., Cezilly F., De Meeus T., Differential mortality of two closely related host species induced by one parasite. Proc. R. Soc. London Ser. B 260, 349–352 (1995). [Google Scholar]
- 15.Weitz J. S., Wilhelm S. W., Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol. Rep. 4, 17 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khibnik A. I., Kondrashov A. S., Three mechanisms of Red Queen dynamics. Proc. R. Soc. London Ser. B 264, 1049–1056 (1997). [Google Scholar]
- 17.Duncan A. B., Gonzalez A., Kaltz O., Stochastic environmental fluctuations drive epidemiology in experimental host–parasite metapopulations. Proc. R. Soc. B 280, 20131747 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benton M. J., Evolutionary biology: New take on the Red Queen. Nature 463, 306–307 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Benton M. J., The Red Queen and the Court Jester: Species diversity and the role of biotic and abiotic factors through time. Science 323, 728–732 (2009). [DOI] [PubMed] [Google Scholar]
- 20.E. Allen, Modeling with Ito Stochastic Differential Equations (Springer, Netherlands, 2007). [Google Scholar]
- 21.Barnosky A. D., Distinguishing the effects of the Red Queen and Court Jester on Miocene mammal evolution in the northern Rocky Mountains. J. Vertebr. Paleontol. 21, 172–185 (2001). [Google Scholar]
- 22.Winter C., Bouvier T., Weinbauer M. G., Thingstad T. F., Trade-offs between competition and defense specialists among unicellular planktonic organisms: The “Killing the Winner” hypothesis revisited. Microbiol. Mol. Biol. Rev. 74, 42–57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Avrani S., Schwartz D. A., Lindell D., Virus-host swinging party in the oceans: Incorporating biological complexity into paradigms of antagonistic coexistence. Mob. Genet. Elements 2, 88–95 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gandon S., Buckling A., Decaestecker E., Day T., Host–parasite coevolution and patterns of adaptation across time and space. J. Evol. Biol. 21, 1861–1866 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Decaestecker E., Gaba S., Raeymaekers J. A. M., Stoks R., Van Kerckhoven L., Ebert D., De Meester L., Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature 450, 870–873 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Smout S., Asseburg C., Matthiopoulos J., Fernández C., Redpath S., Thirgood S., Harwood J., The functional response of a generalist predator. PLOS One 5, e10761 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.H. McCallum, Population Parameters: Estimation for Ecological Models (Blackwell Science, UK, 2000). [Google Scholar]
- 28.Feeney W. E., Langmore N. E., Social learning of a brood parasite by its host. Biol. Lett. 9, 20130443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morozov A. Y., Emergence of Holling type III zooplankton functional response: Bringing together field evidence and mathematical modelling. J. Theor. Biol. 265, 45–54 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Lively C. M., de Roode J. C., Duffy M. A., Graham A. L., Koskella B., Interesting open questions in disease ecology and evolution. Am. Nat. 184, S1–S8 (2014). [DOI] [PubMed] [Google Scholar]
- 31.M. J. Hatcher, A. M. Dunn, Parasites in Ecological Communities: From Interactions to Ecosystems (Cambridge Univ. Press, UK, 2011). [Google Scholar]
- 32.P. Schmid-Hempel, Evolutionary Parasitology: The Integrated Study of Infections, Immunology, Ecology, and Genetics (Oxford Univ. Press, New York, 2011). [Google Scholar]
- 33.Diaz-Muñoz S. L., Koskella B., Bacteria–phage interactions in natural environments. Adv. Appl. Microbiol. 89, 135–183 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Tsai C.-H., Miki T., Chang C.-W., Ishikawa K., Ichise S., Kumagai M., Hsieh C.-H., Phytoplankton functional group dynamics explain species abundance distribution in a directionally changing environment. Ecology 95, 3335–3343 (2014). [Google Scholar]
- 35.Magurran A. E., Henderson P. A., Explaining the excess of rare species in natural species abundance distributions. Nature 422, 714–716 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Wajnberg E., Analysis of variations of handling-time in Trichogramma maidis. Entomophaga 34, 397–407 (1989). [Google Scholar]
- 37.Jover L. F., Cortez M. H., Weitz J. S., Mechanisms of multi-strain coexistence in host–phage systems with nested infection networks. J. Theor. Biol. 332, 65–77 (2013). [DOI] [PubMed] [Google Scholar]
- 38.Ben-Ami F., Ebert D., Regoes R. R., Pathogen dose infectivity curves as a method to analyze the distribution of host susceptibility: A quantitative assessment of maternal effects after food stress and pathogen exposure. Am. Nat. 175, 106–115 (2010). [DOI] [PubMed] [Google Scholar]
- 39.Ben-Ami F., Regoes R. R., Ebert D., A quantitative test of the relationship between parasite dose and infection probability across different host–parasite combinations. Proc. R. Soc. B 275, 853–859 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regoes R. R., Hottinger J. W., Sygnarski L., Ebert D., The infection rate of Daphnia magna by Pasteuria ramosa conforms with the mass-action principle. Epidemiol. Infect. 131, 957–966 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regoes R. R., Ebert D., Bonhoeffer S., Dose-dependent infection rates of parasites produce the Allee effect in epidemiology. Proc. R. Soc. London Ser. B 269, 271–279 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ejsmond M. J., Radwan J., Red Queen processes drive positive selection on major histocompatibility complex (MHC) genes. PLOS Comput. Biol. 11, e1004627 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massie T. M., Blasius B., Weithoff G., Gaedke U., Fussmann G. F., Cycles, phase synchronization, and entrainment in single-species phytoplankton populations. Proc. Natl. Acad. Sci. U.S.A. 107, 4236–4241 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bjørnstad O. N., Cycles and synchrony: Two historical ‘experiments’ and one experience. J. Anim. Ecol. 69, 869–873 (2000). [DOI] [PubMed] [Google Scholar]
- 45.Rosenzweig M. L., Paradox of enrichment: Destabilization of exploitation ecosystem in ecological time. Science 171, 385–387 (1971). [DOI] [PubMed] [Google Scholar]
- 46.Rabajante J. F., Talaue C. O., Equilibrium switching and mathematical properties of nonlinear interaction networks with concurrent antagonism and self-stimulation. Chaos Solitons Fractals 73, 166–182 (2015). [Google Scholar]
- 47.Budria A., Candolin U., How does human-induced environmental change influence host-parasite interactions? Parasitology 141, 462–474 (2014). [DOI] [PubMed] [Google Scholar]
- 48.Lopez Pascua L., Gandon S., Buckling A., Abiotic heterogeneity drives parasite local adaptation in coevolving bacteria and phages. J. Evol. Biol. 25, 187–195 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Mostowy R., Engelstädter J., The impact of environmental change on host–parasite coevolutionary dynamics. Proc. R. Soc. B 278, 2283–2292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ingleby F. C., Hunt J., Hosken D. J., The role of genotype-by-environment interactions in sexual selection. J. Evol. Biol. 23, 2031–2045 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Wolinska J., King K. C., Environment can alter selection in host–parasite interactions. Trends Parasitol. 25, 236–244 (2009). [DOI] [PubMed] [Google Scholar]
- 52.Vrba E. S., Turnover-pulses, the Red Queen, and related topics. Am. J. Sci. 293, 418–452 (1993). [Google Scholar]
- 53.Jackson S. T., Sax D. F., Balancing biodiversity in a changing environment: Extinction debt, immigration credit and species turnover. Trends Ecol. Evol. 25, 153–160 (2010). [DOI] [PubMed] [Google Scholar]
- 54.Barash M. S., Evolution of the Mesozoic oceanic biota: Response to abiotic factors. Oceanology 48, 538–553 (2008). [Google Scholar]
- 55.Bell G., Collins S., Adaptation, extinction and global change. Evol. Appl. 1, 3–16 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poulin R., Forbes M. R., Meta-analysis and research on host–parasite interactions: Past and future. Evol. Ecol. 26, 1169–1185 (2012). [Google Scholar]
- 57.Rigaud T., Perrot-Minnot M.-J., Brown M. J. F., Parasite and host assemblages: Embracing the reality will improve our knowledge of parasite transmission and virulence. Proc. R. Soc. B 277, 3693–3702 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaitala V., Ylikarjula J., Heino M., Dynamic complexities in host–parasitoid interaction. J. Theor. Biol. 197, 331–341 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Ashby B., Gupta S., Parasitic castration promotes coevolutionary cycling but also imposes a cost on sex. Evolution 68, 2234–2244 (2014). [DOI] [PubMed] [Google Scholar]
- 60.T. M. Goater, C. P. Goater, G. W. Esch, J. C. Holmes, Parasitism: The Diversity and Ecology of Animal Parasites (Cambridge Univ. Press, New York, 2013), 510 pp. [Google Scholar]
- 61.Polak M., Luong L. T., Starmer W. T., Parasites physically block host copulation: A potent mechanism of parasite-mediated sexual selection. Behav. Ecol. 18, 952–957 (2007). [Google Scholar]
- 62.Ebert D., Carius H. J., Little T., Decaestecker E., The evolution of virulence when parasites cause host castration and gigantism. Am. Nat. 164 (suppl. 5), S19–S32 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Laine A.-L., Barrès B., Epidemiological and evolutionary consequences of life-history trade-offs in pathogens. Plant Pathol. 62, 96–105 (2013). [Google Scholar]
- 64.Little T. J., Watt K., Ebert D., Parasite-host specificity: Experimental studies on the basis of parasite adaptation. Evolution 60, 31–38 (2006). [PubMed] [Google Scholar]
- 65.Råberg L., Alacid E., Garces E., Figueroa R., The potential for arms race and Red Queen coevolution in a protist host–parasite system. Ecol. Evol. 4, 4775–4785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luijckx P., Fienberg H., Duneau D., Ebert D., A matching-allele model explains host resistance to parasites. Curr. Biol. 23, 1085–1088 (2013). [DOI] [PubMed] [Google Scholar]
- 67.Kwiatkowski M., Engelstädter J., Vorburger C., On genetic specificity in symbiont-mediated host-parasite coevolution. PLOS Comput. Biol. 8, e1002633 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agrawal A., Lively C. M., Infection genetics: Gene-for-gene versus matching-alleles models and all points in between. Evol. Ecol. Res. 4, 79–90 (2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/3/e1501548/DC1
Supporting text
Table S1. Summary of state variables and model parameters.
Fig. S1. Example of canonical Red Queen dynamics (Red Queen n-ary oscillations) where all host and parasite types undergo dominance switching.
Fig. S2. Oscillatory behavior expected to occur when α has a lower value (that is, there is a higher level of parasite specificity).
Fig. S3. Parameter diagram showing the effects of increasing the host’s carrying capacity K on the oscillation patterns of host and parasite population densities (σ = 0, t = 0 to 50,000).
Fig. S4. Increasing the carrying capacity K increases the amplitude of population density (for example, compare the graphs associated with K = 3 and K = 10; σ = 0).
Fig. S5. Host population density time series showing the effects of stochastic noise (n = 4, K = 16).
Fig. S6. Host population pattern illustrating the effects of stochastic noise on a system with 10 host types and 10 parasite types (K = 40, σ = 10%).
Fig. S7. Additional illustrations of some population dynamics that can arise from our host-parasite interaction system (σ = 0).


