Exquisitely preserved fossil lizards from 99-million-year-old Burmese amber provide new insights into paleotropical diversity.
Keywords: Mesozoic period, fossils, lizards, paleobiology
Abstract
Modern tropical forests harbor an enormous diversity of squamates, but fossilization in such environments is uncommon and little is known about tropical lizard assemblages of the Mesozoic. We report the oldest lizard assemblage preserved in amber, providing insight into the poorly preserved but potentially diverse mid-Cretaceous paleotropics. Twelve specimens from the Albian-Cenomanian boundary of Myanmar (99 Ma) preserve fine details of soft tissue and osteology, and high-resolution x-ray computed tomography permits detailed comparisons to extant and extinct lizards. The extraordinary preservation allows several specimens to be confidently assigned to groups including stem Gekkota and stem Chamaleonidae. Other taxa are assignable to crown clades on the basis of similar traits. The detailed preservation of osteological and soft tissue characters in these specimens may facilitate their precise phylogenetic placement, making them useful calibration points for molecular divergence time estimates and potential keys for resolving conflicts in higher-order squamate relationships.
INTRODUCTION
Fossil lizards are predominantly represented by lithified, disarticulated bones of large-bodied species, although exceptional, fully articulated specimens do exist, preserved as compressions (1) or three-dimensional (3D) mineralized replicas (2, 3). Conversely, amber selectively fossilizes small, delicate specimens, not only conserving the durable cuticular and skeletal remains but often preserving the internal organs and soft tissues with cellular and ultrastructural fidelity (4).
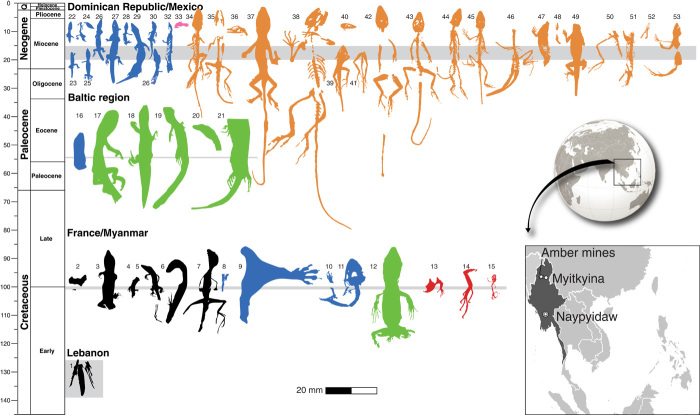
Amber-embedded lizards have been found on three continents from six horizons that span 100 million years (Fig. 1). Amber from Burma, also called burmite, has been radiometrically dated to approximately 99 million years ago (Ma) (5) and contains some of the oldest and most diverse arrays of organismal inclusions, preserving fungi, plants (bryophytes, ferns, conifers, and angiosperms), arthropods, gastropods, worms, feathers, and lizards (6–8). Burmite chemistry and inclusions indicate a coniferous origin; the Burmese amber forest may have been dominated by a Metasequoia-like tree, although there was also a substantial diversity of angiosperms. Many organisms in burmite—bark-growing liverworts, buthid scorpions, roaches (Blattodea), stem group ants, termites, webspinners (Embiodea), scale insects (Coccoidea), Zoraptera, and Onychophora (6)—resemble extant, tropical taxa, indicating that the amber was deposited in a moist tropical paleoenvironment.
Fig. 1. Squamates in amber from the four known localities.
Color key: Black, stem Squamata; blue, stem Gekkota; green, Lacertoidea; red, Acrodonta; orange, Pleurodonta. Early Cretaceous from the Lebanon (Valanginian-Barremian): Squamata, (1) Baabdasaurus xenurus (MNHN Entomology). Albian of France: Squamata, (2) MNHN Arc 237.5. Mid-Cretaceous of Myanmar: Squamata, (3) JZC Bu269, (4) JZC Bu268, (5) JZC Bu267, (6) AND-B-870, (7) JCZ Bu 1848; Gekkota, (8) C. burmae (Poinar collection #B-V-4), (9) JCZ Bu1847, (10) MCZ R-190835, (11) JCZ Bu1802; Lacertoidea, (12) JZC Bu1803; Acrodonta, (13) MCZ R-190836, (14) JCZ Bu266, (15) JCZ Bu154. Eocene (Ypresian) Baltic region: Gekkota, (16) Yantarogekko balticus (GAM 1400); Lacertidae, (17 to 21) Succinilacerta succinea. Miocene (Burdigalian-Langhian) of Dominican Republic: Gekkota, (22 to 27) personal collection of Ettore Morone, (28) Sphaerodactylus dommeli (SMNS Do-3584), (29) S. dommeli (ZFMK 66238), (30) personal collection of Ettore Morone, (31) ZFMK specimen, (32) Sphaerodactylus ciguapa (MCZ R-186380); Serpentes, (33) scales from typhlophid snake; Anolis, (34) AMNH DR-SH-1, (35) A. dominicanus (NMBA, entomology no. P52), (36) SMU 74976, (37 to 39) specimens I, II, and III (Supplementary Materials), (40) SMNS Do-4871-M, (41) M-1062, (42) M-1153, (43) M-3410, (44) M-525, (45) OAAAA, (46) SMNS Do-5566-X, (47) M-2096, (48) RAAA (Supplementary Materials), (49) SMNS Do-5742, (50) SMNS Do-4886-B, (51) M-3016, (52) M-1273. Mexico: Anolis, (53) A. electrum (UCMP 68496 and UCMP 68497). All silhouettes at the same scale.
Previous reports of reptiles in burmite are limited to a fragment of skin (6) and the foot and partial tail of the gecko Cretaceogekko burmae (9). On the basis of recent discoveries of large amounts of burmite containing diverse new inclusions (Fig. 2), we report the most diverse lizard paleofauna preserved in amber, representing multiple species from five major clades of lizards.
Fig. 2. Lizards preserved in mid-Cretaceous Burmese amber.

(A) Squamata (body cavity empty; epidermis translucent) (JZC Bu269); (B) Squamata (epidermis translucent; hind leg with bones) (AND-B-870); (C) Squamata [intact specimen; epidermis with some coloration; skeleton and some organs (for example, tongue) mostly intact] (JZC Bu267); (D) Squamata (integument, some skeletal elements, and internal organs preserved) (JZC Bu1848); (E) Gekkota (hind legs, pelvic area, and base of tail preserved) (JZC Bu1847); (F) Gekkota (hand, including bones and impressions of toe pads) (MCZ R-190835); (G) Gekkota (very well preserved skull and mandibles, vertebral column, and some disarticulated limb and pelvic bones) (JZC Bu1802); (H) Agamidae (large left hind leg, including epidermis) (JZC Bu266 leg); (I) Agamidae (epidermis translucent; pelvic area with left leg and parts of left foot preserved) (MCZ 190836); (J) Lacertoidea (large specimen; integument intact; some skeletal elements preserved as internal casts) (JZC Bu1803); (K) Stem Chamaeleonidae (small, neonatal individual with integument and most of skeleton preserved) (JZC Bu154); (L) Undetermined specimen (very pyritized leg with some epidermis and bones) (JZC Bu268). Scale bar, 1 cm for all photographs except (F) and (I)].
RESULTS
High-resolution x-ray computed tomography (HRCT; movie S1), scanning electron microscopy (SEM), and light microscopy allowed a detailed taxonomic assessment of 12 lizard specimens, 10 of them housed in the amber collection at the American Museum of Natural History (AMNH) and two at the Museum of Comparative Zoology (MCZ), Harvard University. Data from the three most intact specimens were added to two recent data sets representing the major lepidosauromorph clades (10–12) (Supplementary Materials). Phylogenetic analyses using parsimony and Bayesian inference produced near-identical topologies, consistently recovering one fossil as a basal squamate, another as sister to Gekkota, and the third as sister to Chamaeleonidae (Fig. 3 and figs. S1 and S2). Apomorphy-based diagnoses were used to provide tentative taxonomic identifications for the less complete specimens. Clade names follow those used in a recent classification of squamates that included both living and fossil taxa (12).
Fig. 3. Results from analysis of the combined morphological and molecular data sets (10) using parsimony and Bayesian inference.
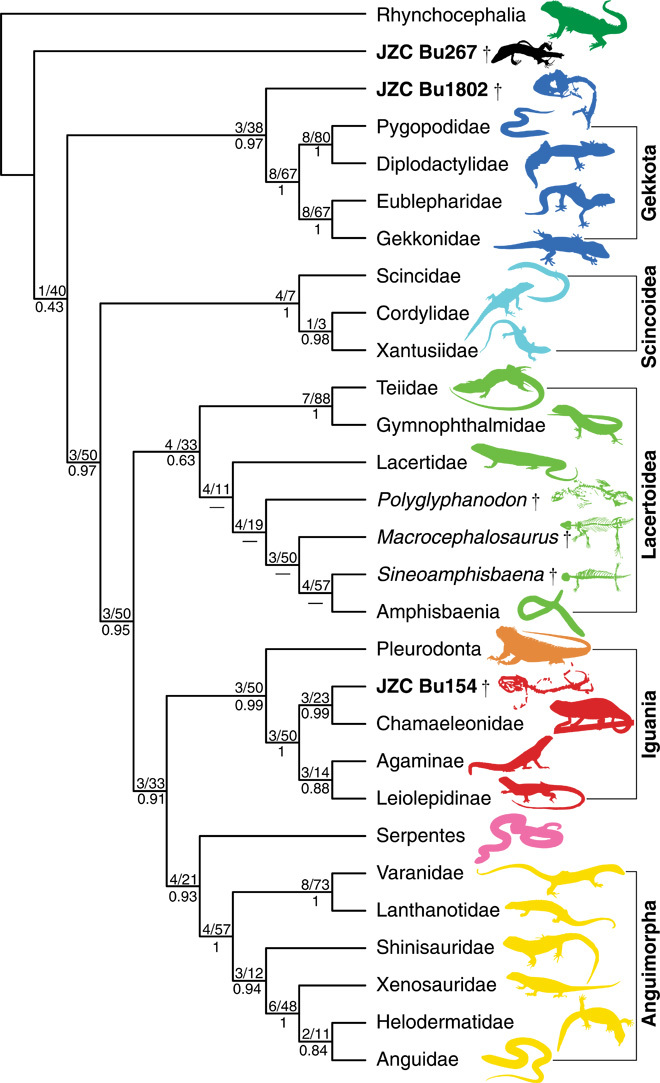
The position of three burmite lizards were consistent in both analyses. JZC Bu267, a basal squamate; JZC Bu1802, a stem gekkotan; JCZ Bu154, a stem chamaeleonid (see also the Supplementary Materials). Values above each node correspond to Bremer and Relative Bremer support, and posterior probabilities are below.
Squamata Incertae sedis
Squamata includes all living lizards and snakes (9900 species) as well as diverse fossil taxa assignable to the crown and stem groups of the major clades to which the living taxa belong. These groups were probably established by the Late Jurassic. Stem squamates were likely present in the Triassic and survived until at least the Early Cretaceous. Amber-embedded specimens are here assigned to stem Squamata on the basis of our preliminary phylogenetic analyses but may well be members of crown Squamata or of more highly nested clades (12).
Specimen JZC Bu269 (Fig. 2A) is preserved as a hollow body cavity surrounded by a clear, multicarinate-scaled epidermis, with retention of some marginal tooth-bearing bones. Specimen AND-B-870 (Fig. 2B) preserves only portions of the trunk, a hind leg and a foreleg, and part of the tail; its scales are transparent and unkeeled. The preservation of JZC Bu267 (Figs. 2C and 4C) is exceptional; it includes the epidermis and soft tissues and even a unique extended tongue tip with a narrow medial projection that does not resemble the form of any described squamate tongues (13). The multicarinate scales of this specimen exhibit a different architecture than those of JZC Bu269, and these also preserve pigmentation. The specimen was infiltrated with sediment in the cervical region, and much of the skeleton is disarticulated and jumbled within the body cavity. About 50% of the cranial elements of JZC Bu267 have been digitally isolated and replicated on a 3D printer for character coding and eventual reconstruction of the skull. Specimen JZC Bu1848 (Figs. 2D and 4D) is mainly pyritized, and its skeleton is not well preserved. The specimen has a rather slender body and remarkably long toes.
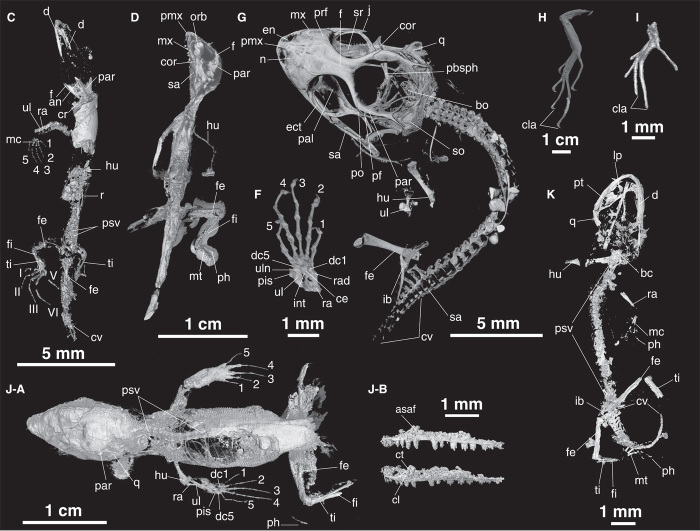
Fig. 4. HRCT images of mid-Cretaceous lizards in Burmese amber.
(C, D, and F to K) Letters correspond to those used for the same specimens in Fig. 2. (C) JZC Bu267; (D) JZC Bu1848; (F) MCZ R-190835; (G) JZC Bu1802; (H) JZC Bu266; (I) MCZ 190836; (J-A) JZC Bu1803; (J-B) digital endocast of the left maxilla of JZC Bu1803 including the tooth row; (K) JZC Bu154. an, angular; bc, basicranium; bo, basioccipital; ce, central; cla, claws; cor, coronoid; cr, cervical rib; cv, caudal vertebra; d, dentary; dc, distal carpal; ect, ectopterygoid; en, external nares; f, frontal; fe, femur; fi, fibula; hu, humerus; ib, innominated bone; int, intermedium; lp, lingual process; mc, metacarpal; mt, metatarsal; mx, maxilla; n, nasal; orb, orbit; pal, palatine; par, parietal; pbsph, parabasisphenoid; pf, postfrontal; ph, phalanx; pis, pisciform; pmx, premaxilla; po, postorbital; prf, prefrontal; psv, presacral vertebra; q, quadrate; r, rib; ra, radius; rad, radiale; sa, surangular; so, supraoccipital; sr, sclerotic ring; ti, tibia; ul, ulna; uln, ulnare; 1 to 5, manual digits; I to V, pedal digits.
JZC Bu267, AND-B-870, and JZC Bu269 display many plesiomorphic squamate characters (for example, notochordal vertebral centrum and paired premaxillae), although these specimens do share some similarities with scincomorphs, including integumentary and osteological features. Two of these specimens (Figs. 2, A and C, and 4C) are similar in overall anatomy, having compressed bodies, stout limbs, and broad feet with narrow, spike-like claws, but differ in size, scale morphology, and osteology. Although the bodies and heads of these fossils lack heavily armored osteoscutes, the scales of two of them are cycloid and multicarinate as in many modern skinks (14) (Fig. 5, A and C). The variation in scale morphology indicates that the three specimens represent separate species instead of ontogenetic variants. All three specimens have multicarinate dorsal cephalic scales, but in JZC Bu269 (Fig. 5A), the scales are pectinate with four to six keels each and well separated from one another by small granular scales. In JZC Bu267 (Fig. 5C), the oval cephalic scales are subimbricate, slightly swollen, and bear one to four keels. JZC Bu269 and JZC Bu267 share a well-developed coronoid process of the dentary (as in Scincidae, Cordylidae, Gerrhosauridae, and Xantusiidae) (15). However, their maxillae and dentaries differ in the approximate number of tooth loci (24 versus 17 in the maxilla and 25 versus fewer than 15 in the dentary, respectively); interdental spaces in JZC Bu269 are narrow, whereas in JZC Bu267, gaps between loci are almost as wide as the tooth crowns. Both specimens have a single posterior process of the maxilla, as in some skinks, but differ in the slope of the anterior margin on the facial process. Specimen JZC Bu269 retains the left portion of the premaxilla, suggesting that this specimen had paired premaxillae as in some skinks and gekkotans (15, 16).
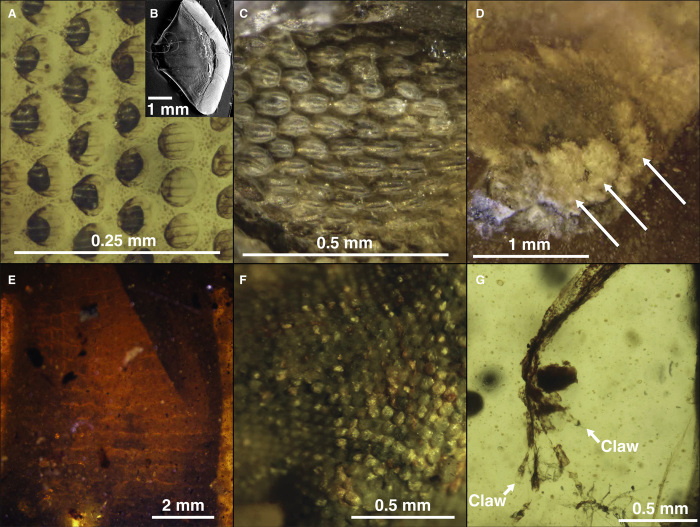
Fig. 5. Photomicrographs of the integument of mid-Cretaceous lizards in Burmese amber.
(A) Postocular head scales of the squamate JZC Bu269. (B) SEM image of a scale from the living skink Lepidothyris hinkeli. (C) Prefrontal scales of the squamate JZC Bu267. (D) Details of the lamellae from toes of the gekkotan JZC Bu1847. (E) Ventral scales of the lacertoidean JZC Bu1803. (F) Detail from the dorsal surface of the talocrural region of JZC Bu266. (G) Small scales and claws from the left forearm in the stem Chamaleonidae (JZC Bu154).
Stem Gekkota
Gekkota is a clade comprising approximately 1650 extant species of geckos and pygopods, largely nocturnal, often scansorial lizards distributed worldwide in warm temperate to tropical areas, as well as numerous Tertiary crown group fossils. Stem Gekkota is represented by a small number of Eurasian fossils from the Late Jurassic to Late Cretaceous (12, 17).
C. burmae is one of two lizards previously known from burmite and was originally assigned with uncertainty to the Gekkonidae sensu lato (9) on the basis of the characteristic toe pads and subequal digits I to IV—known only in gekkotans (17)—and the ungual symmetry that is typical of pad-bearing and climbing geckos (18, 19). Three additional fossils (Figs. 2, E to G, and 4, F and G) unequivocally corroborate the presence of Gekkota in the mid-Cretaceous. Specimen JZC Bu1847 (Fig. 2E) consists of the hindquarters of a large gecko, preserving the right hind leg and entire foot, most of the left hind leg (the ungual portion of digits I to III are lost at the amber surface), basal portion of the tail, and the pelvic region including the cloaca. The interior of the body cavity was replaced with a conglomerate of fine, sandy sediment, almost certainly during encasement.
Specimen MCZ R-190835 (Figs. 2F and 4F) preserves all phalangeal, metacarpal, and carpal elements of the left manus, together with the distal epiphyses of the radius and ulna. Skin impressions were preserved, allowing correlation of the position of toe pads with the phalanges. The phalangeal formula is 2-3-4-5-3, and the pisiform sesamoid contacts the ulnare. Metacarpals 1 and 5 are subequal and are the shortest; metacarpals 2, 3, and 4 are subequal and 1.5× longer than 1 and 5. The longest phalanges are the penultimate one of each digit, and these are markedly arcuate; the unguals are short, which are general features of pad-bearing geckos (19). These burmite geckos both display perfectly preserved toe pads but of significantly different design, confirming not only that mid-Cretaceous geckos were capable of scansorial locomotion but also that alternative pad architectures, comparable to those of living geckos, had already evolved by this time (Fig. 5D).
Specimen JCZ Bu1802 (Figs. 2G and 4G) preserves the entire skull, 26 presacral vertebrae (all cervical and thoracolumbar), the sacrum, about 5 caudal vertebrae (pygal), some ribs, and the left side of the pelvis. The soft tissues either decayed or were scavenged before resin completely covered the skeleton. The snout-vent length (SVL) is 22.5 mm, which, together with several immature characteristics, indicates that it is a hatchling. The skull has features seen only in embryonic and hatchling gekkotans (20), such as a frontal bone lacking a ventral fusion of the subolfactory processes, a large parietal fontanel, and a parabasisphenoid-basioccipital fenestra (basicranial fenestra). The separation between the subolfactory processes is more extensive than in Gobekko cretacicus, the only other Cretaceous gekkotan in which this character can be assessed, in which there is only a narrow gap between these structures, and resembles the embryonic condition of modern species (20). The specimen displays features that are rarely preserved in fossil geckos, which are typically represented by isolated skull bones (dentary, maxilla, or frontals) (17). These include the sclerotic ring, the otostapes (with a stapedial foramen as in many living gekkotans, dibamids, and certain skinks) (21), and two circumorbital bones in the posterodorsal corner of the orbit which correspond to the postfrontal and the postorbital. Two discrete elements in the posterodorsal part of the orbit have bearing on the controversy about the homology of the single element in this position of living geckos, which has been identified as the postfrontal, postorbital, or a fusion of these two bones (22, 23). This fossil exhibits an intermediate condition between more basal stem gekkotans, which have two bones in the posterodorsal corner of the orbit and complete postorbital and supratemporal bars (for example, Norellius and Eichstaettisaurus), and the extant Gekkota, which have only a single bone and reduced temporal arcades.
Crown Lacertoidea
The Lacertoidea includes the approximately 720 living Lacertidae (Old World), Teiidae, and Gymnophthalmidae (New World), mostly fast-moving, active foraging, diurnal lizards. The burrowing amphisbaenians are almost certainly members of this clade (24) but were not included in the classification followed here. Most lacertoid fossils have been assigned to crown Lacertoidea, but our limited data from JZC Bu1803 suggest its placement outside the crown group.
JZC Bu1803 is a large (SVL, 32.2 mm) specimen (Figs. 2J and 4, J-A and J-B) with portions of the skeleton remaining and some integument on the head and back pyritized and exposed. The ventral scales are large, quadrangular, and arranged in regular transverse and longitudinal rows as in most living teiids and lacertids (25, 26) (Fig. 5E). Remarkable features of this specimen are the extremely long digits and claws. The body cavity of this specimen was infiltrated by sediment during encasement, creating a mold of some bones. Observation of these bones was possible by generating digital endocasts of the internal hollow spaces using CT (Fig. 4, J to B). A digital endocast of the left maxilla exposed the entire tooth row section, a dentition including about 14 functional teeth, and an estimated 19 tooth loci. Tooth attachment is pleurodont and morphology is heterodont, with an abrupt transition from conical and recurved teeth (first 12) to tricuspid (with divided crowns, last 7). Tricuspid (or triconodonot) dentition is widespread among squamates (13, 27, 28). Among extant groups, the combination of anterior fang-like and posterior tricuspid teeth with parallel margins, where mesial and distal cusps are shorter than the main apex, most closely resembles that of lacertids and teiids (15, 27). A similar dentition also occurs in the extinct Polyglyphanodontidae (for example, Tripennaculus eatoni and Leptochamops thrinax) (12, 29, 30), although the tooth bases of the amber fossil seem less expanded and cemented than in members of this clade.
Crown Agaminae
Crown Agaminae is a clade of about 450 extant species of diurnal, acrodont, terrestrial to climbing lizards distributed throughout the Old World tropics and warm temperate zones (except Madagascar). Crown group taxa are known from the Eocene onward, and older acrodont fossils have been regarded as stem taxa of this or a more inclusive clade (12).
Specimen JZC Bu266 is an isolated left posterior leg (Figs. 2H and 4H), retaining scale pigmentation (Fig. 5F). Specimen MCZ R-190836 (Figs. 2I and 4I) includes the skin of the left leg and the cloacal and pygal regions. These two specimens appear to be the first agamid remains preserved in amber. Agamids are known from the Paleocene of Myanmar, represented by the large herbivorous lizard Barbaturex morrisoni (31). Whereas most agamid diagnostic characters are cranial (32), the pedal morphology of JZC Bu266 is extremely similar to that of modern Southeast Asia Draconinae (this group includes the familiar Draco or “flying dragon”), a similarity that we interpret as indicative of relationships to this group, although foot structure can be highly convergent in squamates sharing similar habitats. The amber also contains a layer of sand grains upon which a clump of myxomycete (slime mold) sporangia were growing, indicating that the lizard had been active on the ground on a sandy substrate.
MCZ R-190836 preserves some bones and well-defined scales of the foot. Comparison of this specimen with modern lizards reveals similarities in digit proportions and scales to that of modern draconine agamids (for example, Bronchocela) and amphibolurine (for example, Diporiphora and Lophognathus). Similarities with these groups include subequal scale size in the ventral side of the leg and the postcloacals as well as ventral caudal scales that are almost twice the size of the postcloacal scales. The degree of imbrication is similar, but in MCZ R-190836, the scales have more parallel margins (instead of convergent) and a rounded instead of a pointed apex. Another similarity with crown Agaminae is the reduced size of the claws.
Stem Chamaeleonidae
Chamaeleonidae is a clade of more than 200 extant species of diurnal, mostly climbing, acrodont, lingual-feeding lizards with a chiefly Afro-Malagasy distribution. Crown group fossils are known from the Miocene and later; no stem chamaeleonids have previously been reported (33).
Perhaps the most perplexing specimen is a tiny lizard (SVL, 10.6 mm; Figs. 2K and 4K) with short appendages, noncompressed body, barely discernable scales, and well-preserved claws. This specimen’s short and wide skull, large orbits, elongated and robust lingual process, frontal with parallel margins, incipient prefrontal boss, reduced vomers, absent retroarticular process, low presacral vertebral count (between 15 and 17), and extremely short, curled tail suggest an affiliation with Chamaeleonidae. Phylogenetic analyses unambiguously place this specimen as sister to chameleons, although the plesiomorphic iguanian conditions of pleurodont tooth implantation and non-zygodactylous digits indicate that it is outside of the crown group. The fossil record of chameleons is extremely scant and geologically young, with the oldest fossil dating from the Early Miocene (33). Given the widespread fossil record of other acrodont groups during the Cretaceous, the existence of stem group chameleons in this period is likely and is predicted by some (34, 35), but not all (36), recent molecular-based estimates. The ancestor of modern chameleons is predicted to be small-bodied (35), and whereas JZC Bu154 is almost certainly neonatal, it is comparable to the smallest living amniotes, including juveniles of leaf chameleons (for example, Brookesia micra SVL: adults, 15.3 to 19.9 mm; juveniles, 10.2 mm) (37).
DISCUSSION
Amber deposits are especially useful for preserving small, delicate organisms that are seldom represented as lithified remains or, as fragmentary microvertebrate elements, are often overlooked. This is critical because most lizards are small-bodied (38), and small size has been suggested as a feature that has led to the diversification and success of this group (39). The burmite inclusions reveal a previously undocumented diversity of squamates from the tropics of the mid-Cretaceous. Whereas this assemblage contains lizards that do not occur in Myanmar today (for example, chameleons), it does include many of the squamate clades found in the present-day Old World tropics. Thus, the major paleotropical lizard groups—gekkotans, agamids, chameleons, lacertoids, and possibly scincids—were already established in the Old World tropics by the mid-Cretaceous and persisted through the K-Pg (Cretaceous-Paleogene) boundary and the subsequent shift from coniferous to broad-leafed forests. Many squamate clades—for example, Polyglyphanodontidae and early members of Scincidae, Varanidae, Iguanidae, and Acrodonta—experience faunal turnover at the K-Pg boundary (40), so the conservation of the paleotropical lineages provides further support for the view that tropical forests are inherently stable, serving as “museums” of extraordinary biodiversity (41).
The detailed preservation of soft tissues provides unique insights into the anatomy and ecology of Mesozoic lizards. The geckos confirm that not only were adhesive toe pads present by the mid-Cretaceous, but they were already diverse in structure, suggesting a significantly earlier origin of scansorial adaptations in this clade. The stem chamaeleonid reveals the evolutionary sequence of this family’s highly derived features; for example, the presence of a robust elongated lingual process (together with absent retroarticular process) indicates that the ballistic feeding with a projectile tongue evolved before zygodactyly and lateral compression, which are not present in this fossil.
The diversity of characters present in these fossils increases the precision of their phylogenetic placement, having potentially significant implications for dating the evolutionary history of squamates. The lack of Mesozoic representatives for modern lizard groups has limited squamate fossil calibrations to the deepest nodes or near-terminal twigs, resulting in credible intervals that are often extremely wide (10, 34, 35). Although most of the major squamate clades were well established by the mid-Cretaceous, the patterns of taxic and structural diversity within these clades during this time period remain virtually unknown. The burmite lizards occur early enough that one might expect to discover combinations of character states that are not present in Paleogene fossils, which are often fragmentary, or Neogene fossils, many of which are assignable to extant genera (20). The presence of a stem chamaeleonid in burmite, for example, extends the origin of the chameleon lineage back to the mid-Cretaceous and provides a useful calibration for the divergence between chamaeleonids and agamines within Acrodonta. It might also challenge current views about the African origin of the group (35).
Despite concerted efforts to produce a unified squamate phylogeny (42), there remains significant discordance between the morphological and molecular phylogenies (43). The addition of deeply placed, character-rich specimens such as the burmite fossils presented here may help resolve ambiguities in the squamate tree of life.
MATERIALS AND METHODS
Specimen origin
Specimens were derived from mid-Cretaceous outcrops in Kachin Province, northern Myanmar, approximately 100 km west of the town of Myitkyina. Location, history of excavations, and stratigraphy of the Burmese amber deposits were summarized elsewhere (5). These authors estimated an age of approximately early Cenomanian for Burmese amber on the basis of the stratigraphic occurrence of Cretaceous arthropod taxa preserved in Burmese amber and other, better-dated Cretaceous deposits. Soon thereafter, Burmese amber deposits were dated as latest Albian (44) on the basis of the ammonite Monticeras and some spore and pollen samples. A latest Albian age was cited (9), but erroneously, as 97 to 110 Ma (which encompasses more than the entire Albian and into the Cenomanian). Most recently, zircon crystals from the sediments in the Burmese amber outcrops were radiometrically dated at 99 Ma using U-Pb isotopes (5). This places the age of the deposit very close to the Aptian-Cenomanian boundary, which is also the boundary between the Early and Late Cretaceous.
Eight amber pieces containing lizards have catalog numbers of JZC (James Zigras Collection): JZC Bu154, JZC Bu266, JZC Bu267, JZC Bu268, JZC Bu269, JZC Bu1802, JZC Bu1803, and AND-B-870. These were purchased from dealers; somewhere in the chain from excavator to dealer, the rough amber was ground down and polished into cabochons. Notes and measurements were taken of the original cabochons, and then three of the pieces were trimmed and polished to provide better exposure and observation of the lizard inclusions (but avoiding other important syninclusions). For trimming, a 4-inch diameter (1 mm thickness), diamond-edged, water-fed trim saw was used; for grinding and polishing, a Buehler EcoMet water-fed flat lapidary wheel was used with Buehler wet emory disks of decreasing grit (400, 600, 800, 1200, 2400). Photomicrography and measurements were done with a Nikon SMZ1500 stereomicroscope at the AMNH, a Nikon 5-MP digital camera, light-emitting diode fiber optic lights, and NIS-Elements software (including Z-stacking for photos at higher magnifications). Specimens were cataloged in the MCZ collection, the private collection of James Zigras (JZC), and the private collection of Scott Anderson (AND). All private specimens are available for study through the AMNH (D.A.G.). Physical characteristics of the amber pieces (for example, color, hardness, fluorescence, and presence of calcite-filled fractures) as well as features of preservation and the presence of diagnostic arthropod taxa indicate that these pieces derive from the same deposits as collections of Burmese amber having authoritative, definitive provenance (6).
High-resolution x-ray computed tomography
All specimens were inspected using 3D CTs in a GE phoenix v|tome|x s240 system, with a molybdenum target and modification of the current and voltage to maximize the range of densities recorded. 3D rendering and segmentation were performed using VGStudio MAX version 2.2 (Volume Graphics GmbH) and Avizo Lite 9.0.0 (Visualization Sciences Group). A video with 3D volume-rendered movies of selected burmite lizards is included in the supplementary information (movie S1), and CT data will be made available on written request from the authors.
Phylogenetic analyses
Given the ongoing conflict between the morphological and molecular phylogenetic hypotheses in squamates (42), we sought to investigate the phylogenetic position of the amber fossils under different data sets and optimality criteria to provide a measure of the confidence of our results. To this end, three of the most complete specimens were added into two of the most comprehensive morphological squamate data sets (10–12). The first data set contained 34 taxa and had a combined alignment of 233 ordered and unordered morphological characters and 4224 molecular characters, representing three nuclear genes: rag-1, c-mos, and BDNF (brain-derived neurotrophic factor). The second data set contained 231 taxa and 632 ordered and unordered morphological characters.
Parsimony analyses were performed in the computer program TNT version 1.1 64-bit (no taxon limit) (45) following the search strategy: Tree searches with the command “xmult” until 50 independent hits of the most parsimonious trees were found. Each run of xmult consisted of 20 independent Wagner trees with tree bisection and reconnection (TBR), followed by a combination of sectorial searches and 100 rounds of ratchet and tree drifting. At the end, each set of five trees obtained was subjected to tree fusing. Additional collapsible branches were detected using TBR branch swapping in the resulting trees from the 50 hits until the 1000 most parsimonious trees were found. The strict consensus was calculated, and Bremer and Relative Bremer support values were calculated for nodes. Bayesian analysis was performed using MrBayes version 3.2.2 (46); the nuclear genes partitioned by codon and assigned the best-fit model (GTR+I+G for all partitions) identified by MrModeltest (47), whereas the standard discrete model was used for the ordered and unordered morphological characters in both data sets. The analyses were run for 15 million generations; the first 20% was removed as burn-in and a maximum clade credibility tree was produced.
Supplementary Material
Acknowledgments
We are grateful to J. Zigras and S. Anderson for making specimens available for this study and to J. Hanken for purchasing and loaning the two MCZ amber fossils. We also thank M. Hill and H. Towbin (AMNH Microscopy and Imaging Facility) for assistance with CT scanning; K. de Queiroz, D. Blackburn, and M. Bernal for comments on the manuscript; A. Bolet for comments on our interpretation of the fossil material; and N. Longrich for sharing data sets and helping with the analyses. Funding: Funding was provided by the Department of Biological Sciences, the Office of the Dean of the College of Science, and the Office of Research and Sponsored Programs at Sam Houston State University (J.D.D.); grants DEB 0844523 and DEB 1019943 from the U.S. National Science Foundation and the Gerald M. Lemole, M.D. Endowed Chair Fund at Villanova University (A.M.B.). We acknowledge the Willi Hennig Society for subsidizing the computer program TNT. Author contributions: D.A.G. prepared the specimen and performed photomicrography; E.L.S. and D.A.G. performed HRCT scanning and reconstruction; E.L.S., J.D.D., P.W., and A.M.B. performed morphological observations, character scoring, and phylogenetic analyses; J. D. D. and D.A.G. prepared the illustrations. All authors made substantial intellectual contributions to this study. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors. The data are available upon request from D.A.G.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/3/e1501080/DC1
Character scores for amber specimens
Synapomorphies of amber specimens with squamates, gekkotans, and chameleons
Fig. S1. Results from the analysis of the large morphological data set using parsimony.
Fig. S2. Results from the analysis of the large morphological data set using Bayesian inference.
Movie S1. 3D volume-rendered movies of the burmite lizards.
Reference (48)
REFERENCES AND NOTES
- 1.Bolet A., Evans S. E., A new lizard from the Early Cretaceous of Catalonia (Spain), and the Mesozoic lizards of the Iberian Peninsula. Cretaceous Res. 31, 447–457 (2010). [Google Scholar]
- 2.Gao K., Norell M. A., Taxonomic composition and systematics of late Cretaceous lizard assemblages from Ukhaa Tolgod and adjacent localities, Mongolian Gobi Desert. Bull. Am. Mus. Nat. Hist. 249, 4–52 (2000). [Google Scholar]
- 3.Conrad J. L., Norell M. A., A complete late Cretaceous iguanian (Squamata, Reptilia) from the Gobi and identification of a new iguanian clade. Am. Mus. Novit. 3584, 1–47 (2007). [Google Scholar]
- 4.Grimaldi D. A., Bonwich E., Delannoy M., Doberstein S., Electron microscopic studies of mummified tissues in amber fossils. Am. Mus. Novit. 3097, 1–31 (1994). [Google Scholar]
- 5.Shi G., Grimaldi D. A., Harlow G. E., Wang J., Wang J., Yang M., Lei W., Li Q., Li X., Age constraint on Burmese amber based on U–Pb dating of zircons. Cretaceous Res. 37, 155–163 (2012). [Google Scholar]
- 6.Grimaldi D. A., Engel M. S., Nascimbene P. C., Fossiliferous Cretaceous amber from Myanmar (Burma): Its rediscovery, biotic diversity, and paleontological significance. Am. Mus. Novit. 3361, 1–71 (2002). [Google Scholar]
- 7.Poinar G. Jr, Buckley R., Brown A. E., The secrets of Burmite amber. MAPS Dig. 20, 21–29 (2008). [Google Scholar]
- 8.A. Ross, C. Mellish, P. York, B. Crighton, in Biodiversity of Fossils in Amber from the Major World Deposits, D. Penney, Ed. (Siri Scientific Press, Manchester, UK, 2010), pp. 208–235. [Google Scholar]
- 9.Arnold E. N., Poinar G. A., 100 million year old gecko with sophisticated adhesive toe pads, preserved in amber from Myanmar. Zootaxa 1847, 62–68 (2008). [Google Scholar]
- 10.Hutchinson M. N., Skinner A., Lee M. S. Y., Tikiguania and the antiquity of squamate reptiles (lizards and snakes). Biol. Lett. 8, 665–669 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martill D. M., Tischlinger H., Longrich N. R., A four-legged snake from the Early Cretaceous of Gondwana. Science 349, 416–419 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Gauthier J. A., Kearney M., Maisano J. A., Rieppel O., Behlke A. D. B., Assembling the squamate tree of life: Perspectives from the phenotype and the fossil record. Bull. Peabody Mus. Nat. Hist. 53, 3–308 (2012). [Google Scholar]
- 13.Schwenk K., Of tongues and noses: Chemoreception in lizards and snakes. Trends Ecol. Evol. 10, 7–12 (1995). [DOI] [PubMed] [Google Scholar]
- 14.Wagner P., Böhme W., Pauwels O. S. G., Schmitz A., A review of the African red–flanked skinks of the Lygosoma fernandi (Burton, 1836) species group (Squamata: Scincidae) and the role of climate change in their speciation. Zootaxa 2050, 1–30 (2009). [Google Scholar]
- 15.S. E. Evans, in Biology of the Reptilia, vol. 20, Morphology H. The skull of Lepidosauria, C. Gans, A. S. Gaunt, K. Adler, Eds. (Society for the Study of Amphibians and Reptiles, Ithaca, NY, 2008), pp. 1–347. [Google Scholar]
- 16.W. Presch, in Phylogenetic Relationships of the Lizard Families: Essays Commemorating Charles L. Camp, R. Estes, G. Pregill, Eds. (Stanford Univ. Press, Stanford, CA, 1988), pp. 471–492. [Google Scholar]
- 17.Daza J. D., Bauer A. M., Snively E. D., On the fossil record of the Gekkota. Anat. Rec. 297, 433–462 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Russell A. P., Bauer A. M., Ungual asymmetry in the context of pedal symmetry in Ailuronyx (Reptilia Gekkonidae): Modification for an opposable grip. J. Zool. 218, 1–9 (1989). [Google Scholar]
- 19.A. P. Russell, A. M. Bauer, in Biology of the Reptilia, vol. 21, Morphology I. The skull and appendicular locomotor apparatus of Lepidosauria, C. Gans, S. Gaunt, K. Adler, Eds. (Society for the Study of Amphibians and Reptiles, Ithaca, NY, 2008), pp. 1–465. [Google Scholar]
- 20.Daza J. D., Bauer A. M., Snively E., Gobekko cretacicus (Reptilia: Squamata) and its bearing on the interpretation of gekkotan affinities. Zool. J. Linn. Soc. 167, 430–448 (2013). [Google Scholar]
- 21.Greer A. E., On the occurrence of stapedial foramen in living non-gekkonid lepidosaurs. Copeia 1976, 591–592 (1976). [Google Scholar]
- 22.Wise P. A. D., Russell A. P., Development of the dorsal circumorbital bones in the leopard gecko (Eublepharis macularius) and its bearing on the homology of these elements in the Gekkota. Anat. Rec. 293, 2001–2006 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Daza J. D., Bauer A. M., The circumorbital bones of the Gekkota (Reptilia: Squamata). Anat. Rec. 293, 402–413 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Müller J., Hipsley C. A., Head J. J., Kardjilov N., Hilger A., Wuttke M., Reisz R. R., Eocene lizard from Germany reveals amphisbaenian origins. Nature 473, 364–367 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Harvey M. B., Ugueto G. N., Gutberlet R. L. Jr, Review of teiid morphology with a revised taxonomy and phylogeny of the Teiidae (Lepidosauria: Squamata). Zootaxa 3459, 1–156 (2012). [Google Scholar]
- 26.Boulenger G. A., Synopsis of the families of existing Lacertilia. Ann. Mag. Nat. Hist. 14, 117–122 (1884). [Google Scholar]
- 27.R. Kosma, thesis, Leibniz Universität Hannover (2004). [Google Scholar]
- 28.Presch W., A survey of the dentition of the macroteiid lizards (Teiidae: Lacertilia). Herpetologica 30, 344–349 (1974). [Google Scholar]
- 29.Nydam R. L., Voci G. E., Teiid-like scincomorphan lizards from the late Cretaceous (Campanian) of Southern Utah. J. Herpetol. 41, 211–219 (2007). [Google Scholar]
- 30.Gao K., Fox R. C., New teiid lizards from the Upper Cretaceous Oldman Formation (Judithian) of Southeastern Alberta, Canada, with a review of the Cretaceous record of teiids. Ann. Carnegie Mus. 60, 145–162 (1991). [Google Scholar]
- 31.Head J. J., Gunnell G. F., Holroyd P. A., Hutchison J. H., Ciochon R. L., Giant lizards occupied herbivorous mammalian ecospace during the Paleogene greenhouse in Southeast Asia. Proc. Biol. Sci. 280, 20130665 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bell C. J., Mead J. I., Swift S. L., Cranial osteology of Moloch horridus (Reptilia: Squamata: Agamidae). Rec. West. Aust. Mus. 25, 201–237 (2009). [Google Scholar]
- 33.A. Bolet, S. E. Evans, in The Biology of Chameleons, K. A. Tolley, A. Herrel, Eds. (University of California Press, Berkeley, CA, 2013), pp. 175–192. [Google Scholar]
- 34.Jones M. E. H., Anderson C. L., Hipsley C. A., Müller J., Evans S. E., Schoch R. R., Integration of molecules and new fossils supports a Triassic origin for Lepidosauria (lizards, snakes, and tuatara). BMC Evol. Biol. 13, 208 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolley K. A., Townsend T. M., Vences M., Large-scale phylogeny of chameleons suggests African origins and Eocene diversification. Proc. Biol. Sci. 280, 20130184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulcahy D. G., Noonan B. P., Moss T., Townsend T. M., Reeder T. W., Sites J. W. Jr, Wiens J. J., Estimating divergence dates and evaluating dating methods using phylogenomic and mitochondrial data in squamate reptiles. Mol. Phyl. Evol. 65, 974–991 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Glaw F., Köhler J., Townsend T. M., Vences M., Rivaling the world’s smallest reptiles: Discovery of miniaturized and microendemic new species of leaf chameleons (Brookesia) from northern Madagascar. PLOS One 7, e31314 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meiri S., Length–weight allometries in lizards. J. Zool. 281, 218–226 (2010). [Google Scholar]
- 39.A. M. Bauer, in Reptiles and Amphibians, H.G. Cogger, R.G. Zweifel, Eds. (Academic Press, San Diego, ed. 2, 1998), 240 pp. [Google Scholar]
- 40.Longrich N. R., Bhullar B.-A. S., Gauthier J. A., Mass extinction of lizards and snakes at the Cretaceous–Paleogene boundary. Proc. Natl. Acad. Sci. U.S.A. 109, 21396–21401 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.A. R. Wallace, Tropical Nature and Other Essays (Macmillian and Company, London, 1878), 390 pp. [Google Scholar]
- 42.Reeder T. W., Townsend T. M., Mulcahy D. G., Noonan B. P., Wood P. L. Jr, Sites J. W. Jr, Wiens J. J., Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLOS One 10, e0118199 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Losos J. B., Hillis D. M., Greene H. W., Who speaks with a forked tongue? Science 338, 1428–1429 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Cruickshank R. D., Ko K., Geology of an amber locality in the Hukawng Valley, Northern Myanmar. J. Asian Earth Sci. 21, 441–455 (2003). [Google Scholar]
- 45.Goloboff P. A., Farris J. S., Nixon K. C., TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 (2008). [Google Scholar]
- 46.Ronquist F., Huelsenbeck J. P., MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003). [DOI] [PubMed] [Google Scholar]
- 47.J. A. A. Nylander, MrModeltest v2 (Program distributed by the author. Evolutionary Biology Centre, Uppsala University, 2004). [Google Scholar]
- 48.Sherratt E., Castañeda M. d. R., Garwood R. J., Mahler D. L., Sanger T. J., Herrel A., de Queiroz K., Losos J. B., Amber fossils demonstrate deep-time stability of Caribbean lizard communities. Proc. Natl. Acad. Sci. U.S.A. 112, 9961–9966 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/2/3/e1501080/DC1
Character scores for amber specimens
Synapomorphies of amber specimens with squamates, gekkotans, and chameleons
Fig. S1. Results from the analysis of the large morphological data set using parsimony.
Fig. S2. Results from the analysis of the large morphological data set using Bayesian inference.
Movie S1. 3D volume-rendered movies of the burmite lizards.
Reference (48)