Abstract
Vascular reactive oxygen species (ROS) are known to be involved in atherosclerosis development and progression. NADPH oxidase 4 (Nox4) is a constitutively active ROS-producing enzyme that is highly expressed in the vascular endothelium. Nox4 is unique in its biology and has been implicated in vascular repair, however, the role of Nox4 in atherosclerosis is unknown. Therefore, to determine the effect of endothelial Nox4 on development of atherosclerosis, Apoe E−/− mice +/− endothelial Nox4 (ApoE−/−+EC Nox4) were fed a high cholesterol/high fat (Western) diet for 24 weeks. Significantly fewer atherosclerotic lesions were observed in the ApoE−/− + EC Nox4 mice as compared to the ApoE−/− littermates, which was most striking in the abdominal region of the aorta. In addition, markers of T cell populations were markedly different between the groups; T regulatory cell marker (FoxP3) was increased whereas T effector cell marker (T-bet) was decreased in aorta from ApoE−/− + EC Nox4 mice compared to ApoE−/− alone. We also observed decreased monokine induced by gamma interferon (MIG; CXCL9), a cytokine known to recruit and activate T cells, in plasma and tissue from ApoE−/− + EC Nox4 mice. To further investigate the link between endothelial Nox4 and MIG expression, we utilized cultured endothelial cells from our EC Nox4 transgenic mice and human cells with adenoviral overexpression of Nox4. In these cultured cells, upregulation of Nox4 attenuated endothelial cell MIG expression in response to interferon-gamma. Together these data suggest that endothelial Nox4 expression reduces MIG production and promotes a T cell distribution that favors repair over inflammation, leading to protection from atherosclerosis.
Keywords: NADPH Oxidase 4, reactive oxygen species, atherosclerosis, T regulatory cells, CXCL9
Introduction
Atherosclerosis is a multi-stage disease that initially involves endothelial dysfunction ultimately leading to intimal lipid accumulation, immune cell infiltration, and vascular inflammation. Increased reactive oxygen species (ROS) are considered a key component of atherogenesis. Although there are multiple potential vascular ROS producers[1], the abundance and increased expression of the NADPH oxidase (Nox) family of proteins in diseased vasculature suggest that these proteins are a major source of ROS [2].
There are 7 Nox family members (Nox1–5, Duox1/2) that variably require other accessory proteins (i.e. p22phox, p47phox, p67phox, etc.) found in vascular tissues [3]. However, one family member, NADPH oxidase 4 (Nox4) is unique in that it is constitutively active, and requires only p22phox for activity [4, 5]. In addition, unlike the other Nox members, Nox4 primarily generates hydrogen peroxide rather than superoxide as its ROS product [6, 7] Hydrogen peroxide has a longer half-life than superoxide, more easily crosses membranes, and is important for cell signaling via its largely reversible reaction with protein thiol moieties. Thus, Nox4 catalytic activity has been implicated in a number of cellular signaling processes. [4, 8–10].
In particular, Nox4 expression has been linked to such cell functions as proliferation, differentiation, and oxygen sensing. It is well established that Nox4 expression increases with TGFβ [11], and in models of vascular injury. The timing of increased Nox4 expression seems to coincide with the onset of tissue repair [12]. Consistent with this notion, overexpression of endothelial Nox4 leads to improved blood flow recovery and angiogenesis in response to hindlimb ischemia, whereas Nox4 loss-of-function delayed this process [13, 14]. In humans, vascular Nox4 expression may be decreased in severe disease states[15, 16]. Together, these data led us to hypothesize that endothelial Nox4 plays an important role in reparative responses required for the resolution of vascular disease. To test this, we have utilized endothelial Nox4 expression[13] in a mouse model of atherosclerosis.
Methods
Materials and Methods
Animals
Studies were performed in control (C57/Bl6J) mice and hypercholesterolemic ApoE deficient (ApoE−/− mice) either fed normal chow or a high fat/high cholesterol (Western) diet (Research Diets: D12108) composed of 40 kcal% fat and 1.25% cholesterol. ApoE−/− mice were crossed with endothelial Nox4 expressing transgenic mice[13]. All experiments were done using ApoE−/− +EC Nox4 and littermate (ApoE−/−) controls. All experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Massachusetts Medical School.
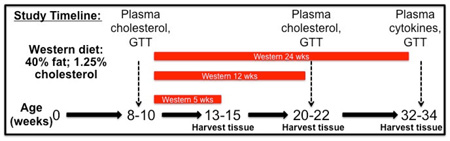
Study Design
Mouse were started on the Western diet at 8–10 weeks of age. Tissues were harvested at 5, 12, and 24 weeks after start of the diet.
Total Cholesterol
Plasma was drawn prior to high fat feeding (baseline) and 12 weeks post-high fat feeding. Total cholesterol was measured using Cayman Cholesterol Assay Kit catalog # 10007640.
Glucose tolerance test
Glucose (1 g/kg) was administrated by intraperitoneal injection. Blood samples were harvested from the tail vein at the indicated time, and glycemia was determined using a glucometer (Bayer).
Oil Red O Aortic Staining
Aorta were harvested immediately following euthanasia and cleaned (fat and connective tissue removed) from the aortic arch to the femoral arteries. Aorta were then cut lengthwise, pinned with insect pins on a black dish and gently washed 1× with PBS for 1 minute at RT. Aorta were then soaked in 60% isopropanol for 1 minute at RT. Oil Red O stain was then applied and let sit for 30min at 37°C. Aorta were then washed with 60% isopropanol for 2 minutes followed by PBS for 2 minutes. Aorta were drained and pictures were immediately taken. Lipid deposits were quantified using image J software in a blinded fashion.
PCR
Total RNA was extracted with the use of Qiagen RNeasy Mini Kits for cells and with the use of TRIzol (Invitrogen) for tissue samples according to the manufacturer’s protocol. Total RNA was reverse- transcribed to cDNA with the use of Qiagen Omniscript RT Kit at 37°C for 60 minutes. The real- time polymerase chain reaction reactions were run on a Bio-RAD iQ5 iCycler using SYBR green or the Invitrogen Taqman System. All gene expression data was normalized to HPRT unless otherwise specified. See Table 1 for SYBR green primer sequences used. Relative gene expression was calculated using 2ΔΔCt.
Table 1.
SYBR green primer sequences.
| gPCR primers | |
|---|---|
| mE-selectin F | ACACATCTGGTGGCAATTCA |
| mE-selectin R | TGTTGTTTGGTTCACCTGGA |
| mP-selectin F | TGTTCTCATGAACTGCAGCC |
| mP-selectin R | ATCCCAGAAGCCAAACATTG |
| mICAM-1 F | GTGATGCTCAGGTATCCATCCA |
| mICAM-1 R | CACAGTTCTCAAAGCACAGCG |
| mVCAM-1 F | AGTTGGGGATTCGGTTGTTCT |
| mVCAM-1 R | CCCCTCATTCCTTACCACCC |
| mF4/80 F | CCCCAGTGTCCTTACAGAGTG |
| mF4/80 R | GTGCCCAGAGTGGATGTCT |
| mMac-1 F | ATGGACGCTGATGGCAATACC |
| mMac-1 R | TCCCCATTCACGTCTCCCA |
| mCD68 F | CCATCCTTCACGATGACACCT |
| mCD68 R | GGCAGGGTTATGAGTGACAGTT |
| mCD3 F | AGTGCAGTTCGGGAACAGAAG |
| mCD3 R | GATTGGCTACTCTGCTGGGT |
| mCD4 F | TCACCTGGAAGTTCTCTGACC |
| mCD4 R | GGAATCAAAACGATCAAACTGCG |
| mFoxP3 F | TTGGTTTACTCGCATGTTCG |
| mFoxP3 R | AGGGATTGGAGCAGCACTTGTTG |
Histology
Aortic roots were excised after 0.9% saline perfusion and fixed overnight in 4%PFA at 4°C. PFA was then rinsed 3× and the tissue was submerged in 30% sucrose with gentle shaking at 4°C. Tissue was then immediately frozen in Tissue Tek OCT, and sections (10uM) were subsequently stained with Masson’s Trichrome and MOMA-2 (1:100). Images were obtained with a 40× objective (Nikon) on an inverted microscope (TE-2000; Nikon) Images were captured with the use of SpotSoftware (Diagnostic Instruments). All images were quantified using 3–5 sections/mouse using image j software.
Plasma Cytokines
Whole blood was collected by cardiac puncture at time of sacrifice in EDTA tubes and immediately spun to separate plasma. Plasma was then snap-frozen and stored at −80°C until further analyzed. Plasma cytokines were measured by ELISA (Luminex 200; Millipore) using the Mouse Magnetic 20-Plex Panel from Life Technologies (Catalog #: LMC0006M).
Cell culture
Cultured human aortic ECs were obtained from Lonza (Basel, Switzerland) and used between passages 3 and 8. Mouse lung endothelial cells (MLECs) were harvested via 2 consecutive selections using ICAM-2 and cultured on 0.2% gelatin-coated dishes. The MLECs were used for experiments between passages 2–6. All endothelial cells were cultured in EGM-2 containing 2% FBS with the included bullet kit supplements (Lonza). Adenoviral constructs were added to the cells for 24h as previously described [13] prior to a 24h treatment with human or mouse interferon gamma (IFNγ R&D systems; 12.5ng/mL. The range of hNox4 overexpression in HAEC with the adenoviral constructs was 4–10 fold (data not shown).
Statistics
Statistics were performed using Prism software (GraphPad, version 6.0). 2-tailed Student’s t tests were used to analyze all normally distributed data. Mann-Whitney rank sum test was used when data were not normally distributed or had unequal group variances. P values less than 0.05 were considered significant. The Grubb’s test was used to detect outliers.
Results
Nox4 expression is altered by a Western diet
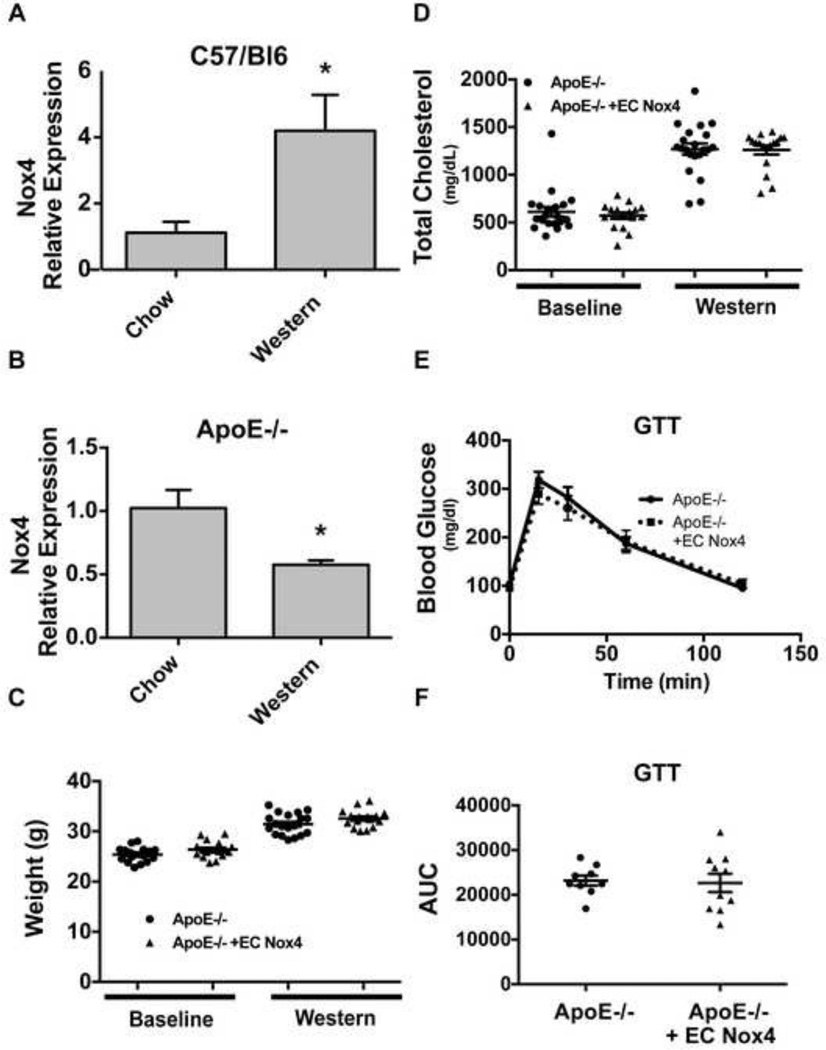
To gain insight into how Nox4 could impact atherosclerosis, we examined its expression in atherosclerosis-resistant versus atherosclerosis-prone models. In response to 24 weeks of a high-fat diet, atherosclerosis-resistant C57/Bl6 (WT) mice demonstrated a significant increase in aortic Nox4 expression (Figure 1A), as previously reported [17]. In contrast, the atherosclerosis-prone ApoE−/− model exhibited reduced Nox4 expression with high-fat feeding (Figure 1B). These data and published reports [15, 16] therefore suggest decreased Nox4 tissue expression may be associated with the development of atherosclerosis.
Figure 1. Nox4 expression is modulated by atherosclerosis, but overexpression does not alter cholesterol or metabolism.
Wild type (A) and ApoE−/− (B) mice were fed with a Western diet for 24 weeks and aortic Nox4 gene expression (normalized to HPRT) was assessed (mean ± SE; n = 4 – 5; *p < 0.05). ApoE−/− mice were bred with the Ve-Cad endothelial specific Nox4 expressing mice [13] and both the control and Nox4 expressing groups of mice were examined for differences in weight (C), cholesterol (D), or glucose tolerance (E,F) after a Western diet (12 weeks; mean ± SE; *p < 0.05).
Endothelial Nox4 expression attenuates atherosclerosis
There is little information about how Nox4 in specific cells modulates the atherosclerotic process. Since Nox4 is the main NADPH oxidase isoform in the endothelium, and endothelial dysfunction has been linked to atherosclerosis, we sought to determine if preventing Nox4 downregulation in the endothelium would impact atherosclerosis. Therefore, we bred endothelial cell Nox4 overexpressing mice (EC Nox4) onto the ApoE−/− background. These mice demonstrated similar endothelial Nox4 expression levels as previously published [13]. We observed no differences in cholesterol levels, weight gain, or glucose tolerance between the two groups after a Western diet (Figure 1C – F).
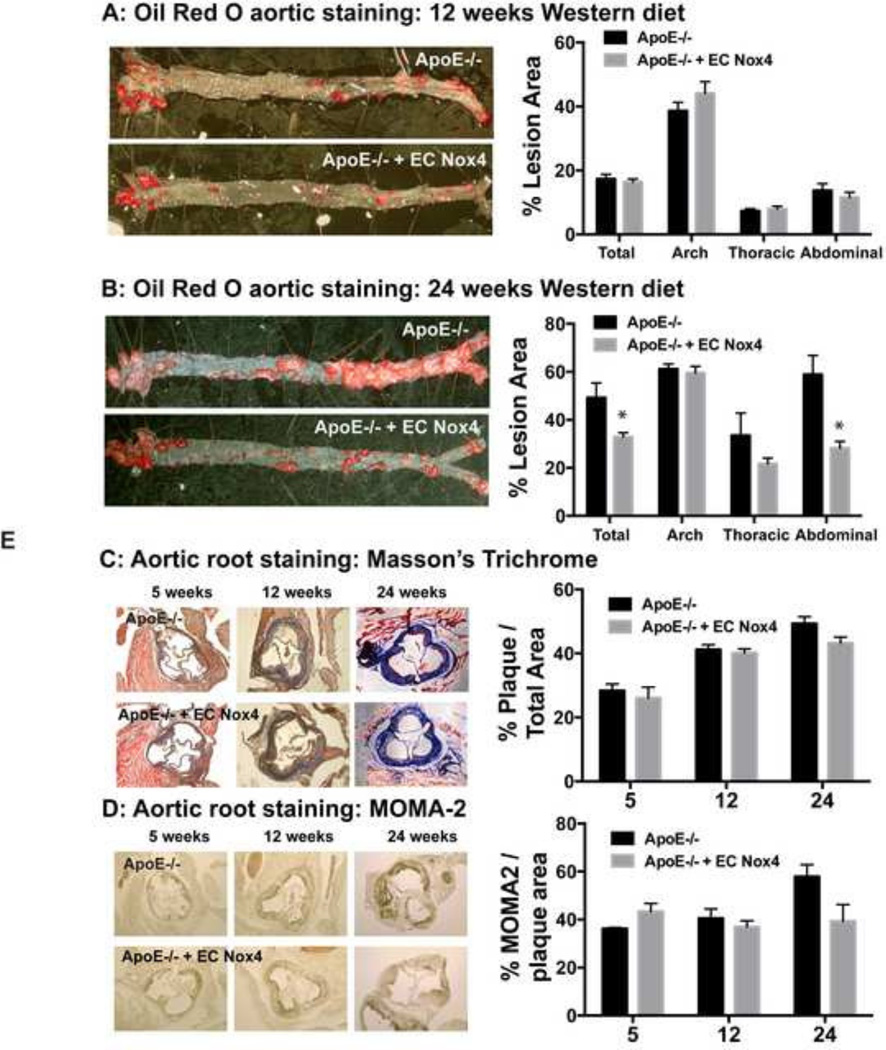
Aortas were harvested at 5, 12 and 24 weeks to assess atherosclerosis at early and late stages. Using Oil Red O staining to visualize atherosclerotic lesions, we observed little lesion development at 5 weeks (data not shown), but there was significant staining primarily in the aortic arch at 12 weeks, regardless of EC Nox4 status (Figure 2A). However, after 24 weeks on a Western diet, there was significantly less lesion area in the ApoE−/− + EC Nox4 group (Figures 2B). Histologic examination of the aortic arch showed no differences in lesion composition (Masson’s trichrome stain) or macrophage infiltration (MOMA-2 staining; Figures 2 C,D). Thus, preservation of endothelial cell Nox4 expression appears to protect ApoE−/− from late-stage atherosclerosis, particularly in the abdominal region.
Figure 2. Endothelial Nox4 protects from atherosclerosis.
Aorta were harvested from ApoE−/− and ApoE−/− + EC Nox4 mice and Oil Red O staining was performed after 12 weeks (A) or 24 weeks (B) of a Western diet. The total aortic lesions and each section of the aorta were quantified for % lesion area (mean ± SE; n = 7–12 / group *p < 0.05). Aortic root sections were harvested at 5,12, and 24 weeks on a Western diet and stained with Masson’s trichrome (C) and MOMA-2 (D) (mean ± SE; n = 3–5 / group; 3–5 sections/mouse).
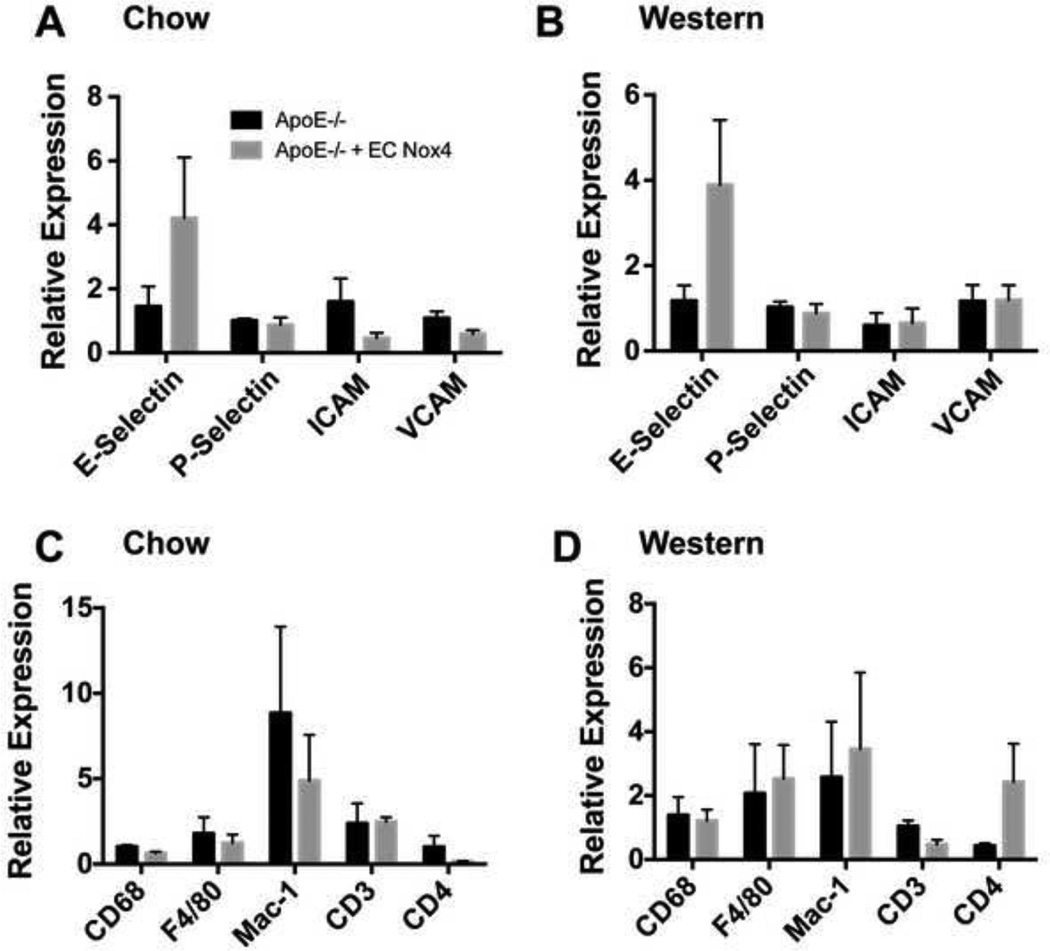
Endothelial Nox4 expression does not alter selectin or adhesion molecule expression, but changes T cell population markers
We harvested the aorta from ApoE−/− + EC Nox4 mice on either a normal chow or Western diet for 24 weeks and examined the expression of selectins and adhesion molecules, known to be involved in atherosclerosis. As shown in Figures 3A and B, there were no significant differences observed between the groups with regards to the expression of E-selectin, P-selectin, VCAM-1, or ICAM-1.
Figure 3. Endothelial Nox4 does not change adhesion molecule expression or macrophage recruitment.
Aorta were harvested from ApoE−/− and ApoE−/− + EC Nox4 mice and expression of different adhesion molecules (A,B) and immune cell markers (C,D) were assessed in mice fed with normal chow diet or a Western diet (normalized to hPRT; mean ± SE; n = 4 – 6).
We also examined gene expression of specific markers for immune cell types known to contribute to atherosclerotic lesion development. We found that EC Nox4 did not impact the expression of markers for monocytes and macrophages (CD68, F4/80, Mac-1) in the aorta regardless of diet (Figures 3C and D).
Endothelial Nox4 alters T cell populations and decreases monokine of interferon gamma (MIG) expression
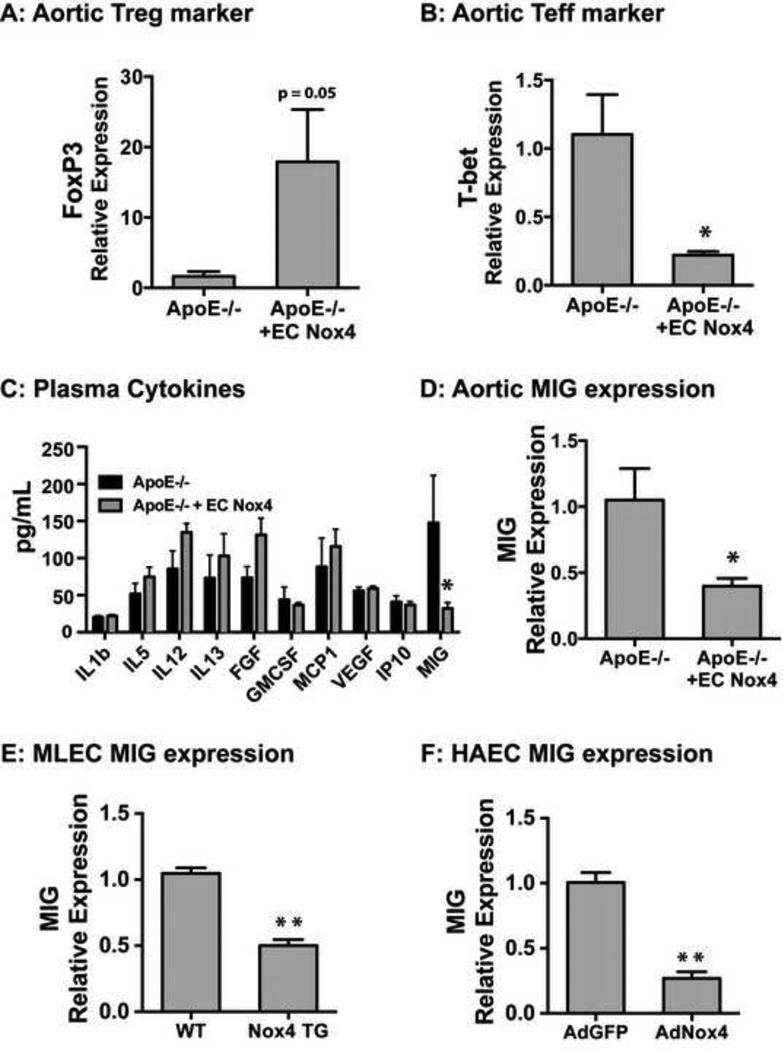
As recent data has indicated T cell populations as mediators of atherosclerosis, we examined aortic gene expression for markers of these T cell subsets. T regulatory cells (Treg; documented by FoxP3 expression), which are known to decrease the severity and pathogenesis of atherosclerosis [18], trended to increase (p = 0.05) in aorta of the ApoE−/− + EC Nox4 mice (Figure 4A), whereas T effector cells (Teff; documented by T-bet expression) were significantly decreased in aorta of the ApoE−/− + EC Nox4 mice (Figure 4B).
Figure 4. Endothelial Nox4 prevents MIG expression.
Aorta were harvested and mRNA of the T regulatory marker FoxP3 (A) and the T effector marker T-bet (B) were measured (mean ± SE; n = 4 – 6; *p < 0.05). Plasma was collected from mice fed with a Western diet for 24 weeks and cytokine levels were measured by ELISA in ApoE−/− and ApoE−/− + EC Nox4 mice (C) (mean ± SE; n = 8 –10; *p < 0.05). Aorta were harvested and MIG mRNA was measured (D). Mouse lung endothelial cells (MLECs) were harvested from both EC Nox4 transgenic mice (Nox4 TG) and littermate controls (WT). MLECs were treated for 24h with mouse IFNγ (12.5 ng/mL) and MIG mRNA expression was measured (normalized to HPRT; mean ± SE; n = 3; *p < 0.05) (E). Human aortic endothelial cells (HAECs) were either transduced by control virus or Nox4 expressing virus. After transduction cells were treated for 24h with human IFNγ (12.5 ng/mL) and harvested for gene expression of MIG (normalized to HPRT; mean ± SE; n = 3; **p < 0.01) (F).
We examined plasma cytokines that could mediate the observed differences in the T cell populations. Plasma monokine of interferon gamma (MIG; CXCL9) levels were significantly reduced (~75%) (Figure 4C) in the transgenic mice. Since endothelial cells are known to produce MIG which typically recruits and activates Teffs, we measured aortic MIG mRNA expression and found that MIG was significantly decreased in aorta from our ApoE−/− + EC Nox4 mice compared to the ApoE−/− controls (Figure 4D).
As MIG was reduced in animals expressing endothelial Nox4, we sought to investigate the relation between Nox4 and MIG expression. We treated mouse lung endothelial cells (MLECs) from EC Nox4 transgenic mice and littermate controls with IFNγ and found that Nox4 significantly decreased IFNγ-induced MIG expression (Figure 4E). Similarly, expression of Nox4 in human aortic endothelial cells (HAECs) prevented IFNγ-mediated MIG expression (Figure 4F). Thus, endothelial Nox4 status modulates endothelial MIG responses to IFNγ.
Discussion
In this study, we found that atherosclerosis-prone ApoE−/− mice exhibited reduced Nox4 expression on an atherogenic (Western) diet, whereas atherosclerosis-resistant C57/Bl6J mice demonstrated preserved Nox4 expression on the same diet. In our model of atherosclerosis, endothelial Nox4 overexpression significantly attenuated disease primarily in the abdominal aorta. Protection from atherosclerosis was associated with a reduction in plasma and tissue levels of the cytokine MIG in addition to increased Treg and decreased Teff markers in the aorta. Thus, our findings suggest that endothelial Nox4 plays a key role in mediating endothelial inflammatory responses in atherosclerosis.
A significant finding of our study was that plasma and tissue MIG expression was decreased in our atherosclerotic transgenic mice. MIG is a cytokine that functions to recruit activated T cells, promoting inflammation. We found that Nox4 inhibited IFNγ induced MIG expression in cultured endothelial cells further suggesting that the decrease in plasma and tissue MIG is a direct effect of endothelial Nox4. Indeed, mice harboring a genetic deletion of the MIG receptor (CXCR3) [19], or a functionally related chemokine (CXCL10) [20], resulted in a similar pattern of protection against atherosclerosis. In these studies, protection was also associated with an increased population of Treg to Teff cells in the aorta.
The fact that we observed reduced MIG in our model suggest that endothelial Nox4 could modulate aortic T cell populations, in part through its impact on MIG. Recent literature has documented that the balance between effector T cells and regulatory T cells can determine the outcome of disease. In this context, Tregs act to decrease inflammation and promote repair, while Teffs serve to promote inflammation [21]. Consistent with this, depleting Tregs enhanced atherosclerosis in both LDLR−/− and ApoE−/− mice [22, 23]. In our study, the expression of T cell markers (FoxP3 and T-bet) indicated a change in the Treg/Teffector balance as a function of Nox4 expression. Taken together, these observations suggest that endothelial Nox4 promotes a T cell distribution that favors repair over inflammation.
Tregs are influenced by the localized environment and multiple factors have been documented to dictate Treg commitment. In this study we demonstrated EC Nox4 mice had decreased plasma and tissue levels of MIG and Nox4 in endothelial cells decreased MIG expression, however this may not be the only factor dictating the localized Tcell response. As we and others have previously shown, endothelial Nox4 produces H2O2 released into the extracellular environment [13]. Recent data has documented a link between ROS and the Treg/Teffector balance, wherein both intracellular and extracellular ROS production can determine T cell fate [24–27]. Therefore, in addition to decreasing MIG production and subsequent T effector recruitment, EC Nox4 expression may also directly modulate the local environment to increase Treg commitment in the Tcell population through ROS signaling.
Our results demonstrate that Nox4 reduces MIG expression, increases the population of Tregs, and decreases Teffs which likely leads to the observed protection against the formation of atherosclerotic lesions. Although the precise function of endothelial Nox4 in human atherosclerosis is yet to be determined, our findings support an adaptive role for Nox4 in response to disease.
Highlights.
NADPH oxidase 4 in the endothelium protects ApoE−/− mice from atherosclerosis.
Plasma monokine of interferon gamma was decreased with endothelial Nox4 expression.
Aorta from Nox4 mice had increased T regulatory and decreased T effector cell markers.
Endothelial Nox4 promotes repair by altering the localized T cell populations.
Acknowledgments
Sources of Funding:
This work was supported by F32HL099282 (SMC), R01HL092122 (JFK), and R01HL098407 (JFK) from the NHLBI.
Abbreviations
- ROS
reactive oxygen species
- Nox4
NADPH oxidase 4
- MIG
monokine induced by interferon gamma
- H2O2
hydrogen peroxide
- WT
wild type
- EC
endothelial cell
- MLEC
mouse lung endothelial cells
- Treg
T regulatory cell
- Teff
T effector cell
- IFNγ
interferon gamma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
NONE
References
- 1.Stocker R, Keaney JF. New insights on oxidative stress in the artery wall. J. Thromb. Haemost. 2005;3:1825–1834. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 2.Sirker A, Zhang M, Shah AM. NADPH oxidases in cardiovascular disease: insights from in vivo models and clinical studies. Basic Res Cardiol. 2011;106:735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lassegue B, San Martin A, Griendling KK. Biochemistry, Physiology, and Pathophysiology of NADPH Oxidases in the Cardiovascular System. Circ. Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martyn KD, Frederick LM, Loehneysen von, K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell. Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Nisimoto Y, Jackson HM, Ogawa H, Kawahara T, Lambeth JD. Constitutive NADPH-dependent electron transferase activity of the Nox4 dehydrogenase domain. Biochemistry. 2010;49:2433–2442. doi: 10.1021/bi9022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takac I, Schröder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. Journal of Biological Chemistry. 2011;286:13304–13313. doi: 10.1074/jbc.M110.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nisimoto Y, Diebold BA, Constentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53:5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helmcke I, Heumüller S, Tikkanen R, Schröder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid. Redox Signal. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 9.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Fórró L, Schlegel W, Krause K-H. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem. J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADPH oxidase 4 localization. J. Cell Biol. 2008;181:1129–1139. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat. Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szöcs K, Lassègue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:21–27. doi: 10.1161/hq0102.102189. [DOI] [PubMed] [Google Scholar]
- 13.Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, Keaney JF. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 15.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 16.Guzik B, Sagan A, Ludew D, Mrowiecki W, Chwala M, Bujak-Gizycka B, Filip G, Grudzien G, Kapelak B, Zmudka K, Mrowiecki T, Sadowski J, Korbut R, Guzik TJ. International Journal of Cardiology. Int. J. Cardiol. Elsevier B.V. 2013;168:2389–2396. doi: 10.1016/j.ijcard.2013.01.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maganto-Garcia E, Tarrio ML, Grabie N, Bu DX, Lichtman AH. Dynamic Changes in Regulatory T Cells Are Linked to Levels of Diet-Induced Hypercholesterolemia. Circulation. 2011;124:185–195. doi: 10.1161/CIRCULATIONAHA.110.006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veillard NR. Differential Influence of Chemokine Receptors CCR2 and CXCR3 in Development of Atherosclerosis In Vivo. Circulation. 2005;112:870–878. doi: 10.1161/CIRCULATIONAHA.104.520718. [DOI] [PubMed] [Google Scholar]
- 20.Heller EA. Chemokine CXCL10 Promotes Atherogenesis by Modulating the Local Balance of Effector and Regulatory T Cells. Circulation. 2006;113:2301–2312. doi: 10.1161/CIRCULATIONAHA.105.605121. [DOI] [PubMed] [Google Scholar]
- 21.Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends in Immunology Elsevier Ltd. 2012;33:49–57. doi: 10.1016/j.it.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klingenberg R, Gerdes N, Badeau RM, Gisterå A, Strodthoff D, Ketelhuth DFJ, Lundberg AM, Rudling M, Nilsson SK, Olivecrona G, Zoller S, Lohmann C, Lüscher TF, Jauhiainen M, Sparwasser T, Hansson GK. Depletion of FOXP3+ regulatory T cells promotes hypercholesterolemia and atherosclerosis. J. Clin. Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ait-Oufella H, Salomon BL, Potteaux S, Robertson A-KL, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, Flavell RA, Hansson GK, Klatzmann D, Tedgui A, Mallat Z. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 24.Amarnath S, Dong L, Li J, Wu Y, Chen W. Endogenous TGF-beta activation by reactive oxygen species is key to Foxp3 induction in TCR-stimulated and HIV-1-infected human CD4+CD25− T cells. Retrovirology. 2007;4:57. doi: 10.1186/1742-4690-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraaij MD, Savage NDL, van der Kooij SW, Koekkoek K, Wang J, van den Berg JM, Ottenhoff THM, Kuijpers TW, Holmdahl R, van Kooten C, Gelderman KA. Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc. Natl. Acad. Sci. U.S.A. 2010;107:17686–17691. doi: 10.1073/pnas.1012016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee K, Won HY, Bae MA, Hong J-H, Hwang ES. Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:9548–9553. doi: 10.1073/pnas.1012645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim H-R, Lee A, Choi E-J, Kie J-H, Lim W, Lee HK, Moon B-I, Seoh J-Y. Attenuation of Experimental Colitis in Glutathione Peroxidase 1 and Catalase Double Knockout Mice through Enhancing Regulatory T Cell Function. Chatenoud, L., editor. PLoS ONE. 2014;9:e95332. doi: 10.1371/journal.pone.0095332. [DOI] [PMC free article] [PubMed] [Google Scholar]