Abstract
Relational memory is a canonical form of episodic memory known to rely on the hippocampus. Several lines of evidence suggest that relational memory has a developmental trajectory in which it is fragile, inflexible, and error-prone until around 6 years of age, which seems to mirror maturational changes in the morphology of the hippocampus. However, recent findings from Richmond and Nelson (2009) challenge this idea as they provided evidence of adult-like relational memory in 9-month old infants. In this study, the authors measured the eye-movements of infants and showed that they preferentially gazed at correct, as opposed to incorrect, face-scene pairings at test. The goal of the present study was to evaluate the development of relational memory by assessing 4-year-olds using Richmond and Nelson's task and stimuli, but gathering two dependent measures of relational memory: overt response as well as eye-movements. The results show that overall, preferential looking at correct face-scene pairings was at chance; however, preferential looking was observed when the correct face-scene pair was later explicitly identified. Thus, while eye movements do index explicit memory in 4-year-olds, behavioral data are necessary to obtain a full picture of the development of relational memory in childhood.
Memory provides the foundation of our identity and a means by which we can learn from old experiences and prepare for new ones. In particular, forming relational memories – e.g., what happened where – is the basis of our ability to use knowledge flexibly in novel situations, and it is the hallmark of episodic memory (Eichenbaum, 1999). Hippocampal lesion studies in humans and animals (Cohen & Eichenbaum, 1993; Squire 2004) as well as neuroimaging studies in healthy adults (reviewed in Davachi 2006), have demonstrated the critical role of the hippocampus in the formation and flexible use of relational representations. An important question is how these structures mature over the course of development, thereby affecting the development of relational memory.
The most common way to measure relational memory in adults is through old/new recognition paradigms that require an overt response. In addition, recent work shows that eye movement measurements can be used as an alternative dependent measure of relational memory. A large number of studies have shown that eye movements (e.g., the number of fixations and number of regions sampled) over previously viewed scenes decrease incrementally as the scene is repeated (Smith, Hopkins, & Squire, 2006), reflecting behavioral habituation. However, if a region within the scene is altered – a relational change – then normal adults show an increase in eye fixations to the altered region, suggesting that memory for the original item-location relationships has modulated viewing patterns (Hayhoe, Bensinger, & Ballard, 1998; Henderson & Hollingworth, 2003; Ryan, Althoff, Whitlow, & Cohen, 2000; Smith, Hopkins, & Squire, 2006). Similarly, when previously studied faces are presented amongst novel faces, viewing patterns discriminate the novel from the familiar faces (Ryan, Hannula, and Cohen, 2007).
Like other forms of relational memory, the increased eye-sampling of relational changes in scenes or faces is linked to hippocampal processing. Ryan and colleagues (2000) recorded the eye movements of amnesic patients with bilateral hippocampal damage and matched controls while they looked at scenes containing changes. The results showed that the amnesic patients failed to show the eye-sampling bias to relational changes in previously studied scenes (Ryan, et al., 2000). Moreover, an fMRI study found that hippocampal activations were correlated with the eye-sampling bias to relational changes in a scene (Hannula & Ranganath, 2009).
In addition to reflecting relational changes within scenes or faces, memory for relations between items can also lead to altered viewing patterns when viewing familiar versus recombined face-scene combinations. Hannula and colleagues (2007) presented both healthy and amnesic adults with a series of face displays superimposed on scenes. A familiar scene was then presented along with three familiar faces, only one of which had been previously viewed with that scene. Relational memory for face-scene pairs was demonstrated by disproportionate viewing of the face previously studied with the scene, but these relational memory effects on eye-movement were not present in adults with amnesia. These alterations in eye movements can proceed with or without awareness (Hannula & Ranganath, 2009; Hannula, Ryan, Tranel, & Cohen, 2007; Holm, Eriksson, & Andersson, 2008) and independent of task instruction (Ryan et al., 2007; Hannula et al. 2007), suggesting that eye movements can serve as an indirect measure of hippocampal-dependent relational memory.
Though the critical role of the hippocampus in the formation of relational memories is apparent, less is known about how maturational changes in these structures contribute to changes in relational memory during development. Assessing memory in preverbal infants and children poses a challenge, and different tasks have led to varied conclusions regarding the age at which adult-like relational memory develops. Infants at 3 months demonstrate memory in tasks using mobile conjugate reinforcement (Rovee-Collier, Griesler, & Earley, 1985) and at 6 months in deferred imitation tasks (Meltzoff, 1988; Barr, Dowden, & Hayne 1996). However, changes in context (Hayne, Boniface, & Barr, 2000) or test stimuli (Hartsher & Rovee-Collier, 1997; Hayne et al., 2000) eliminate retention among younger infants compared to older ones. Manipulating the length of retention can further alter the memory performance of young infants, with 24-month olds retaining information longer than 18-month olds (Herbert & Hayne, 2000). Tasks assessing place learning, which is known to rely on the hippocampus, show that children do not successfully learn the relations among landmarks until 22-months (Newcombe, Huttenlocher, Drummey, & Wiley, 1998).
However, such findings should not be taken as evidence that relational memory is mature at age 2. Sluzenski, Newcombe, and Kovacs (2006) had 4- and 6-year-old children view animals paired with patterned backgrounds. When later asked to identify which animal/background pairs they had seen, both age groups performed equally well on recognition tests for individual items but 6-year olds out-performed 4-year-olds at remembering animal/background combinations. Thus, even if rudimentary relational memory systems are present during infancy, the resultant memories appear to relatively inflexible and fragile. It is only later, around age 6, that relational memories appear to be nearly adult-like. This may reflect the fact that the human hippocampus undergoes dramatic maturational changes through the first two years of life with slower continuing change after that, appearing to end around age 12 (Utsunomiya, Takana, Okazaki, & Mitsudome, 1999). Thus there may be a mirroring of structure and function: as the hippocampus matures, relational memory performance gradually improves.
The logic of this argument is clear, and yet a recent study failed to support it. Richmond and Nelson (2009) presented 9-month-olds with face-scene combinations, and then assessed memory for face-scene combinations by measuring eye fixation patterns. Much like adults tested in a similar paradigm (Hannula et al., 2007), infants looked longer at familiar face-scene combinations compared to rearranged combinations. These findings suggest that relational memory is mature at a younger age than was previously thought, and that an immature hippocampus is sufficient to support this function. The puzzle that these data present is why infants show apparently mature relational memory when assessed via eye movements, but older children fail to exhibit adult-like relational memory when assessed via traditional explicit responses.
There are two possible answers to this conundrum. One is that eye-movements provide an accurate measure of past experience that is independent of explicit response. Recent findings in adults by Hannula and colleagues (2012) support this idea. On this account, the “eyes know” but explicit report may lag behind. A second possibility is that eye-movements provide an index of past experience – sometimes accurate and sometimes not-- that is mirrored in overt response. On this account, problems with relational memory in preschool children might arise from overly-difficult tasks that fail to tap relational memory because they also require other abilities, e.g., inhibition. Unfortunately 9-month-old infants are unable to provide an overt response, so data from this age range cannot tell us whether the ability to consciously remember face-scene pairs coincides with patterns of eye-movements in children, or not.
To answer this question, we assessed relational memory in 4-year-olds. We adapted the face-scene paradigm used by Richmond and Nelson (2009) to include an explicit response condition, where memory performance was examined using eye tracking, a 3-alternative forced choice response, and a forced choice yes/no response. We additionally included a no response condition, where only eye movements were recorded, to control for the possibility that the task instructions requiring an explicit response might interfere with automatic eye-movements. Four-year old children studied a series of face-scene pairs, and relational memory for face-scene pairs was tested by displaying three familiar faces in front of a familiar scene. Fixations on each of the 3 faces were recorded. In the explicit response condition, children were also asked to verbally identify which face had been studied with that scene. Because of findings suggesting that age-related differences in memory performance for item-background combinations were due to higher rates of false alarms among 4-year-olds compared to 6-year-olds (Lloyd, Doydum, & Newcombe, 2009), we also included a subsequent yes-no recognition task where a familiar scene was presented with a single familiar face and children were asked to judge whether the face-scene pair had been previously studied together, or not.
Method
Participants
Sixty-two 4-year-olds were recruited from the suburbs of Philadelphia from a database of families who had previously expressed interest in research participation. The ‘explicit response’ condition included 16 boys and 17 girls with an average age of 54 months, 7 days, (SD = 3.10 months) and the ‘no response’ condition included 12 boys and 6 girls with an average age of 54 months, 3 days (SD = 3.09 months). Seven children were discarded from the ‘explicit response condition’ due to computer error, and 1 for calibration error. Two children were excluded from the ‘no response’ condition because they failed to complete the task. One file was corrupt and discarded from the analysis of fixation time during study trials but was included in all other analyses. Children were rewarded with a small toy for their participation.
Equipment
A Tobii X60 eye-tracker was used to record participants’ on-screen fixations. Eye- trackers use infrared light sources and cameras to detect corneal reflections and record the X and Y coordinates of participants’ eye-positions at 60 Hz. The tracker rested on the desk directly below the computer monitor on which stimuli were presented. Calibration was performed using Tobii Studio software. A 5-point calibration designed for infants and children was used in which participants were asked to follow a bouncing kitten with their eyes to each of the 5 locations in which it appeared onscreen. Calibration was repeated until accurate fixations were recorded for each of the five points. Stimulus presentation and data recording were performed with E-prime software. Fixations were defined based on the criteria used by Richmond and Nelson (2009), as periods of looking during which the eyes did not shift more than 200 pixels in 50 milliseconds. Fixation data was coded using MatLab and fixations per face per trial were identified with the previously described fixation criteria. Trials in which no fixations were recorded were excluded from further analysis.
Materials
Stimuli were randomly selected from the same face and scene photographs used by Richmond and Nelson (2009). The present study included 72 photographs of natural or man-made scenes (650 × 500 pixels), and 72 photographs of faces (180 × 230 pixels), half of which were male and half female. Scenes and faces were used without repetition across trials.
Design
Participants were seated in a car seat on top of a chair so that the center of the monitor was approximately at eye-level. The position of the chair was adjusted until the eye-tracker accurately detected the child's corneal reflection.
We adapted the relational memory eye-tracking paradigm previously used with adults (Hannula et al, 2007) and infants (Richmond and Nelson, 2009), but included an explicit recognition response in addition to recording fixation data in order to examine the relationship between automatic eye-movements and explicit recall when assessing relational memory. Participants were tested in one of two experimental conditions. In the explicit response condition, children's memory performance was examined using eye-tracking and both a 3-alternative forced choice response, followed by a forced choice yes/no response. In the no response condition, no overt response was required and only eye-movements were recorded.
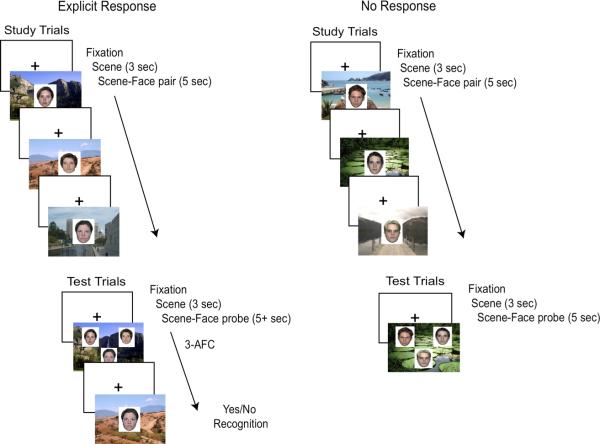
The task was to look at and remember a sequence of three scene-face associations (see Figure 1). For each study trial, a scene was presented for 3000 ms, after which a face was presented in the center of the scene for an additional 5000 ms. This was followed by an intertrial interval consisting of a fixation cross which remained on-screen until the child's attention was directed toward the monitor. This was followed by two more scene-face stimuli and ITI's with the same timing as the first stimulus. After the third and last stimulus a probe screen was presented which contained a familiar scene from the study trials for 3000 ms, followed by the presentation of 3 familiar faces on top of the scene. Although all 3 faces had been viewed during the preceding study trials, only one had been viewed in association with the probe scene. This “matched” face-scene pair was presented in either the first, second, or third study trial an equal number of times, and in a fixed random order. Children were asked to identify which face had been previously matched with the scene by pointing with a wand so that head movement was minimized and calibration was not disrupted, and fixations on each of the three faces were recorded concurrently. The correct face appeared in each of 3 possible locations (left, right, and bottom) an equal number of times, and the location was randomly ordered across the 24 test trials. The probe remained on-screen until the child provided a response.
Figure 1.
A schematic illustration of the trial design.
The 3-alternative forced-choice design did not allow us to gauge false-alarm rate. We were interested in measuring this given prior findings from our laboratory showing that 4-year-olds have high false alarm rates on relational memory tasks, compared to older children (Lloyd et al., 2009). Thus we included a second response requirement during which children were asked to identify with a yes/no recognition response whether a familiar face and a familiar scene had appeared together (or not) during the study portion of the trial. During this trial, one of the 2 scenes not displayed in the probe trial was presented for 3000 ms, and then one of the 2 non-matching faces from the probe trial was presented in the center of the scene until a response was given. Half of the yes/no recognition trials were ‘match’ trials, containing a scene-face pair that had been presented together during the study portion of the trial. The remaining trials were ‘mismatch’ trials in which a familiar face and a familiar scene which had not studied together were presented.
To control for the possibility that the explicit memory retrieval demands might interfere with looking preference, we included a condition in which only eye-movements were recorded (see Figure 1). A separate group of children were tested in this condition. They were instructed to look at and remember each scene and the person in that place. During the probe trial, after presentation of the scene for 3000 ms, the 3 faces were presented in front of the scene for an additional 5000 ms. No explicit memory responses were required. All other aspects of the task, including the visual stimuli and timing during the probe portion of the trial, were identical to that used in the explicit response condition.
To keep the children motivated and interested in the study, each was given a chart with his/her name at the top and with 6 blank squares. For every 4 test trials the child completed, he/she received a sticker for the chart. The child was reminded that the experiment would be completed once the chart was filled.
Results
Behavioral Response Data
Our first analyses examined the accuracy of explicit memory responses. Participants were asked to verbally identify which of the 3 faces presented in the probe matched the scene in the background. A one-sample t-test compared the percentage of correct responses to what would be expected by chance (.33). Overall, children correctly identified the matched face more often than chance (M = .44, SD = .14, t (32) = 4.34, p < .001), indicating that children are able to form relational memories for face-scene combinations at above-chance levels, although they are clearly far from perfectly accurate.
Given the high rate of false alarms found among 4- year olds (Lloyd et al., 2009), an additional set of yes/no recognition trials was included where children were asked to identify whether a single face was previously paired with a given scene. The hit rate was calculated as the proportion of “yes” responses given on trials where “yes” was the correct answer, and the false alarm rate was calculated as the proportion of “yes” responses given on trials where “no” was the correct answer. The average hit rate was high (.90) but the false alarm rate was high as well (.89), suggesting a strong response bias. Children tended to reply “yes” whenever familiar visual information was presented, regardless of whether the faces and scenes were originally viewed together.
Eye-Movement Measures
In addition to the behavioral data, eye-movements were recorded to analyze preferential looking toward the matching face in the face-scene combination, as done for infants by Richmond and Nelson (2009). Trials in which there were no fixations recorded toward any of the faces in the test probe were discarded from analysis. The average number of trials included in analysis for the ‘explicit response’ condition (M =21.91, SD = 2.85) did not differ from the average number of trials included for the ‘no response’ condition (M =21.22, SD = 2.37; t(49) = .87, p = .39). Additionally, in order to ensure that any difference in memory performance between conditions was not due to a difference in viewing time during study trials, we compared the average time fixating study images in each condition. The amount of time (ms) fixating the study images in the ‘explicit response’ condition (M =918.68, SD = 278.89) did not significantly differ from the amount of time fixating the study images in the ‘no response’ condition (M =963.12, SD = 344.61; t(48) = , p = .63). Finally, we compared the average amount of fixation time in seconds toward the test probe in each condition to be certain that the lack of instructions in the ‘no response’ condition did not decrease participants’ motivation to fixate the test images. The amount of looking time during the test probe was actually significantly longer in the ‘no response’ condition (M =66.14, SD = 19.72) than in the ‘explicit response’ condition (M =53.12, SD = 2.43; t(49) = -2.74, p = .01).
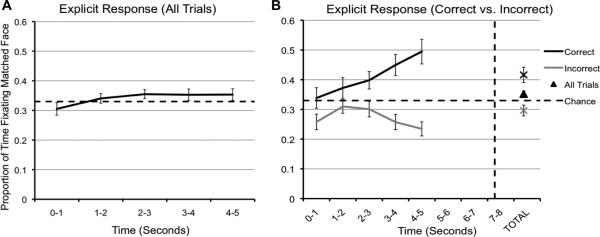
First, data was analyzed for the ‘explicit response’ condition. The proportion of time spent fixating the matching face compared to non-matching faces was calculated for each trial, and fixation data were analyzed using one-sample t-tests to determine whether preferential looking scores were greater than would be expected by chance. Three faces were displayed in the probe image, so fixation scores were compared to a chance performance of .33. Fixation scores were analyzed in 1000 ms time bins for the duration of the 5000 ms probe, and a separate score was calculated for total fixations across the probe (see Figure 2a). The proportion of time spent fixating the matched face was not significantly greater than chance for any of the 1000 ms time bins (all p's >.05), and a one-sample ANOVA revealed no change in fixations across time bins for the first 5000 ms (F(4, 160) = 1.34, p = .26). However, the total proportion of time fixating the matching face across the duration of the probe was significantly greater than chance, t (32) = 2.09, p = .05. Thus, 4-year-olds did not show the same effect shown by infants and adults, but they did fixate the matching face longer overall.
Figure 2.
(a) Data from the explicit response trials. Proportion of time in seconds spent fixating the correct face across all probe trials. (b) The proportion of time spent fixating the correct face as a function of correct vs. incorrect response. The horizontal dashed line represents chance performance (33%), the vertical dotted line represents the average response time.
In the similar eye-tracking study by Hannula and Ranganath (2009), adults were also asked to explicitly identify the matched face. The proportion of time spent fixating the selected face was greater when it was also the correct face compared to when it was the incorrect face beginning between 500 and 1000 ms following stimulus onset, and continuing for the first 2000 ms. In the present study, we separated trials based on whether the verbal response was accurate or inaccurate (see Figure 2b). A repeated-measures ANOVA (response × time) revealed a non-significant effect of time (p < .05), but a significant effect of response accuracy on fixation time (F(1,32) = 21.94, p < .001). The accuracy by time interaction was also significant (F(1, 128) = 4.71, p < .01). Paired t-tests comparing correct to incorrect responses for each time bin revealed that the proportion of time spent fixating the matched face for correctly answered trials was significantly greater than for incorrectly answered trials during the first 1000 ms (t(32) = 2.09, p = .05), the third 1000 ms (t(32) = 2.16, p = .04), the fourth 1000 ms (t(32) = 3.96, p < .001), and the final 1000 ms (t(32) = 5.06, p < .001) following stimulus onset.
We then ran one-sample t-tests to compare fixation times for incorrect and correct responses to chance (.33). The proportion of time fixating the matched face was significantly greater than chance for the third 1000 ms (t(32) = 2.37, p = .02), the fourth 1000 ms (t(32) = 3.41, p < .01), and the final 1000 ms (t(32) = 4.02, p < .001), while the proportion of time spent fixating the matched face on incorrect trials was significantly below chance during the first 1000 ms (t(32) = -2.81, p < .01), the fourth 1000 ms (t(32) = -2.90, p < .01), and the final 1000 ms (t(32) = -4.09, p < .001). The total time fixating the matched face was significantly greater than chance for correctly answered trials (t(32) = 3.41, p < .01), but not for incorrectly answered trials (p >.05). This suggests that children did not automatically fixate the matching face for a greater proportion of time, but instead spent more time fixating the two non-matching faces.
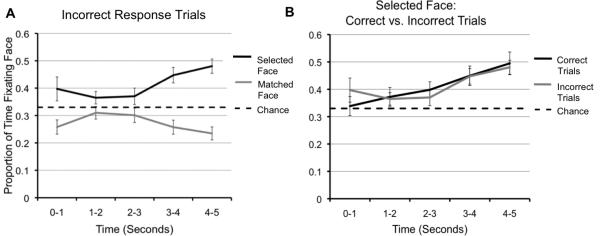
To examine whether children were fixating the face they selected incorrectly rather than the correct face, we compared the proportion of time fixating the selected face to chance (.33) and to the proportion of time fixating the correct face on incorrect response trials for each of the previously designated time bins (see Figure 3a). Children fixated the selected, incorrect face significantly longer than chance during the fourth ((t(32) = 4.14, p < .001) and fifth (t(32) = 5.81, p < .001) time bins, and across the full probe (t(32) = 4.02, p < .001), Further, the time spent fixating the correct face was significantly less than the time spent fixating the selected face during the first (t(32) = -2.20, p = .03), the fourth (t(32) = -3.92, p < .01), and the fifth (t(32) = -5.64, p < .001) time bins, and across the full probe (t(32) = -3.44, p < .01). It appears that children did not fixate the matching face for a greater proportion of time, but instead fixated the face that they believed to be the matching face. Further, this disproportionate viewing of the selected face emerged during the first 1000 ms following stimulus onset, which is when one would expect automatic eye-movements toward the correct face to occur based on prior findings (Hannula et al., 2007; Hannula & Ranganath, 2009; Hannula et al., 2012). An additional analysis comparing the time spent fixating the selected face on incorrect response trials to the time spent fixating the selected face on correct response trials indicated that fixation times did not differ (all p's > .05; see Figure 3b). Thus, children fixate the face they select for a greater proportion of time regardless of whether this is the accurate face or not.
Figure 3.
(a) Data from the explicit response trials answered incorrectly. Proportion of time spent fixating the correct face compared to the proportion of time spent fixating the selected face. Chance performance was 33%. (b) Proportion of time spent fixating the selected face on correct trials compared to the proportion of time spent fixating the selected face on correct trials. Chance performance was 33%.
The results of prior studies suggest that automatic, implicit eye-movements toward the matching face occur independently of task instruction and without conscious awareness (Hannula et al., 2007; Richmond & Nelson, 2009). To assess this we included a second experimental condition where no response was required to examine whether being instructed to verbally identify the matched face affected the fixation scores across trials. In the ‘no response’ condition, the proportion of time spent fixating the matching faces compared to non-matching faces was the only measure included in analysis, and participants were not instructed to explicitly identify the matched face. Using a one-sample t –test, fixation scores were compared to chance (.33) for each 1000 ms time bin across the 5000 ms probe. The proportion of time spent fixating the matched face was not significantly greater than chance for any of the 1000 ms time bins or for the total duration of the trial (all p's >.05), and fixation time was actually lower than chance between 2000 and 3000 ms (t(17) = -2.25, p = .04). A one-sample ANOVA revealed no change in fixations across time bins (F(4, 85) = .69, p = .60). These data suggest that preferential looking toward the matched face only occurs when children are required to explicitly identify the matched face and can do so accurately. We then compared the ‘no response’ data to the ‘explicit response’ data to see if the amount of time fixating the matched face differed by condition (see Figure 4). A multi-factor repeated-measures ANOVA (condition × time) revealed non-significant main effects of time and condition, and a non-significant time by condition interaction (all p's >.05). Thus, fixations toward the matched face were similar whether or not an explicit response was required.
Figure 4.
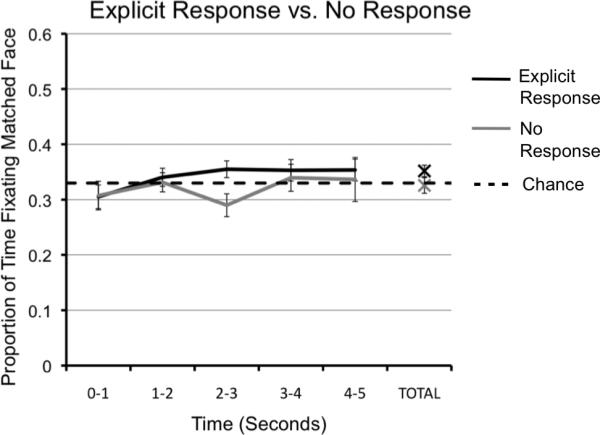
Data from the explicit response trials compared to data from the no response trials. Proportion of time in seconds spent fixating the correct face across all probe trials, separated by condition. Chance performance was 33%.
Finally, we addressed the possibility that the time lapse between the study trial in which the test pair appears and the test probe might affect fixation patterns during the test probe. Richmond and Nelson (2009) included two “lag” conditions: one in which the test pair appeared during the first study trial (Lag 2), and one in which the test pair appeared during the last study trial (Lag 0). Though in both cases the infants looked longer at the matched face on the test probe, the effect emerged slightly later in the Lag 2 condition. We analyzed the eye-data separately depending on whether the test pair appeared in the first, second, or third study trial, and compared the three “lag” groups to see if there was a difference. Analyses revealed that the total proportion of time fixating the matched face did not differ from chance (.33) for any of the 3 lag groups (all p's > .05), and there were no significant main effects for lag or time nor was there an interaction (all p's > .05).
Given prior findings suggesting a preferential looking effect emerged within the first 2000 ms following stimulus onset, we ran post-hoc power analyses with the achieved effect sizes to see whether we failed to find similar results due to an insufficient sample size. The effect sizes for the one-sample t-tests comparing fixation data for the first two time bins of the ‘no response’ condition to chance were very small (d= .21 and d= .01, respectively), and a power analysis suggested sample sizes of N= 180 and N = 78,491, respectively, to achieve a power of .80 using α-level of .05. For the comparison between conditions, the effects sizes are even smaller (d = .01 and d = .03, respectively) with a power analysis suggesting N's of 156,979 and 17,443. We therefore concluded that it would be unlikely to obtain an effect, even if additional participants were included.
Discussion
The goal of this study was to address the question of why infants can show adult levels of relational memory when assessed via eye movements (e.g. Richmond and Nelson, 2009), but older children fail to exhibit adult levels of relational memory when assessed via traditional explicit responses. We reasoned that there were two plausible explanations: (1) the dissociation explanation: that eye-movements provide an accurate measure of past experience from infancy to adulthood but explicit report has a developmental lag; or (2) the association-plus explanation: that eye-movements provide one index of past experience – sometimes accurate and sometimes not-- that is mirrored in overt response. Preschool children's problems with relational memory might arise in tasks that require other abilities such as inhibition. To test these explanations, we examined whether eye-movements indicate relational memory for face-scene combinations in 4-year olds and whether these eye-movements are unassociated with, or associated with the child's ability to explicitly identify previously viewed combinations.
Our findings provide evidence for the association-plus explanation. Preferential looking toward the matched face in the probe trial was only present when an explicit response showed that the matched face was correctly identified. When asked to identify the matched face out of three possible faces, children were more accurate at doing so than chance but still made many errors. However, when asked to determine whether a single familiar face had been previously paired with a familiar scene, children almost exclusively replied “yes,” even when the items were recombined to create a novel face-scene pair. This high rate of false alarms and the moderate, although significant, success on the 3-alternative forced-choice trials suggest that children of this age are only capable of forming weak relational memories and are not yet performing at the level of adults.
Comparison with Prior Findings
Our findings stand in contrast to those of a previous eye-tracking study showing a greater proportion of time spent fixating the matched face-scene pairing (in the absence of an explicit response) in 9-month-old infants (Richmond & Nelson, 2009). The 4-year olds tested in our study showed greater fixations of the matching face-scene pairing only when they had conscious knowledge of the correct pairing. Richmond and Nelson's finding was in fact, the catalyst for the current study as it suggested the existence of adult-like relational memories at a much younger age than was suspected based on a range of prior findings. For instance, research on the development of episodic memory, using a variety of measures, demonstrates a restricted capacity for such memory in infancy, and that these abilities improve with age (Barr et al., 1996; Borovsky & Rovee-Collier, 1990; Meltzoff, 1988; Rovee-Collier et al., 1985). Infant episodic memory also appears to be quite inflexible1 and easily disrupted (Hayne et al., 2000; Hayne, MacDonald, & Barr 1997). The reliability of childhood episodic memory may be linked to the development of inhibitory systems as 6-year-old children experience a lower rate of false alarms than 4-year-olds to recombined pairs and a greater rate of success identifying previously viewed item-background combinations (Lloyd et al., 2009).
Maturational changes in the prefrontal cortices may account for developmental changes in memory, in particular the higher rate of false alarms to recombined familiar items among younger children (Lloyd et al., 2009). And as noted in the introduction, relational memory is closely associated with hippocampal functioning. Imaging research shows dramatic structural changes in the hippocampus during childhood (Utsunomiya et al., 1999), with a developmental trajectory that correlates with improvement in memory performance (Ghetti, DeMaster, Yonelinas, & Bunge, 2010). Evidence of developmental changes in brain regions associated with memory does not mean a capacity for relational memory is not present early in life, but it explains why improvement in memory performance continues during childhood. Further, the ability to successfully encode relational memories may develop at a different rate than the ability to successfully retrieve relational memories, thus leading to high rates of false alarms despite evidence of memory when assessed through different measures.
The current findings fail to support the contentions of a recent study, that eye-movements provide an accurate measure of memory independent of conscious remembering (Hannula, Baym, Warren, and Cohen, 2012). In this study, fixations and explicit memory were recorded while adult participants viewed a previously studied face and novel, morphed versions of the studied face. Fixations toward the studied face were disproportionately greater than toward the similar but incorrect morphed faces very early on - from 1 – 2 s following stimulus onset, and a second before an explicit response was given. Explicit responses were negatively influenced by the visual similarity of foils, while eye movements proved impervious to this manipulation. Our findings show that in 4-year olds, eye movements do not index past experience regardless of what is consciously remembered, but rather, they provide a rapid index of what is later consciously reported.
Research with adults shows a similar divergence between the proportion of time fixating the matched face on correct trials and the selected face on incorrect trials. Adults spent a greater proportion of time fixating the matched face on correct trials than the selected face on incorrect trials, although fixations toward the selected face were greater than chance regardless of accuracy (Hannula & Ranganath, 2009). Similarly, work by Smith and Squire (2008) measuring eye-movements toward previously studied and altered scenes showed that preferential viewing toward the changed region of the scene only occurred when participants were aware of the change. These findings suggest eye-movement measures of memory rely on awareness. However, findings in the adult literature have been interpreted as evidence that eye-movements provide an index of memory that can be measured prior to explicit response, and in some cases, independent of response accuracy (Hannula et al., 2012). The children in the present study spent a greater proportion of time fixating the selected face, whether it was the matched face or not. These findings suggest that children do not automatically fixate the matched face on trials where they believe a different face is the correct response. The eyes appear to provide the same information as the overt memory response, only more quickly.
It is possible that the functional immaturity of the hippocampus in 4-year-olds prevented eye-movements towards familiar items occurring in all cases, as predicted by the findings of (Hannula et al., 2012). However, this fails to explain why eye-movements toward the matched face were present when the matched face was correctly identified. Alternatively, it is possible that in younger children, who are not yet able to form strong relational memories, the request for an explicit memory response interferes with fixations to a greater extent than in adults. Further, the absence of a request for an explicit memory response may allow for the child's attention to be directed elsewhere and not toward the matched face, which would prevent them from fixating the matched face any longer than would occur by chance. Our data show quite the opposite however: in trials where no response was required the children actually fixated the match face less than the non-matched faces. They might have had less motivation to look toward the matched face without specific instructions to identify it verbally. Evidence of developmental changes in novelty preference may contribute to differences in visual attention in 4-year-olds compared to infants. Around 7-8 months, infants demonstrate a preference for stimuli with an intermediate level of complexity, and tend to look away from stimuli that are overly surprising or overly predictable (Kidd, Piantadsoi, & Aslin, 2010). This fact suggests that fixation patterns may depend on the content of the visual stimuli. Developmental changes in visual preference may therefore lead to differences in memory and preferential looking toward the familiar, matched face compared to non-matched faces, regardless of demands for verbal response.
However, the present study provides evidence that eye-movements might be more strongly linked to conscious awareness than was previously believed, and they might not provide a more accurate measure of memory than verbal response. We should therefore be cautious when interpreting eye-movement measures of memory in the absence of explicit response.
Conclusions
Relational memory performance continues to improve during early childhood, but it remains unclear whether this is due to an inability to successfully encode relational memories or to retrieve them, and to what extent the maturation of relevant brain structures plays a role. Research using explicit response measures of memory often offer different results than the behavioral paradigms used with pre-verbal infants, which makes it difficult to accurately compare results. The present data suggest that eye movements do assess relational memory in children, in that it was only when children selected the correct choice that they looked preferentially (and early) at the correct face. Yet, at the same time, the data make clear that eye movements are not the royal road to assessing relational memory, and leave open the question of why Richmond and Nelson's infants showed an overall effect that our 4-year-old children did not, even with the same stimuli. Perhaps adult faces are more compelling for infants, but this idea is purely speculative.
Future research should aim to further examine the specific nature of eye-movements in relation to memory in young children and the impact explicit recall has on such processes, as well as the factors that might contribute to the varying results found in the current memory development literature. It is also important to further examine the relation between eye-movements and explicit memory in older children and adults in order to determine how much the eyes actually know, and whether this is truly different than the memory measured through overt response. Though implicit eye-movements provide a unique, non-verbal measure of memory, they do not appear to successfully measure the same type of memory in young children as is measured by verbal recall.
Acknowledgments
We would like to thank Jennifer Richmond for providing stimuli, Shannon Fitzhugh and Dominique Dumay for their assistance with eye tracking, Elizabeth Klobusicky for her assistance with data analysis, and Melissa Hansen for her assistance with data collection.
Footnotes
It should be noted that flexible retrieval is a key feature of episodic memory and hippocampal function (Norman & O'Reilly, 2003). The absence of this attribute hints that the memory being tested may be semantic or habit-based, rather than episodic. These other forms of memory do not rely on hippocampal function.
References
- Barr R, Dowden A, Hayne H. Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behavior and Development. 1996;19:159–170. [Google Scholar]
- Bauer PJ. Getting explicit memory off the ground: Steps toward construction of a neurodevelopmental account of changes in the first two years of life. Developmental Review. 2004;4:347–373. [Google Scholar]
- Borovsky D, Rovee-Collier C. Contextual constraints on memory retrieval at six months. Child Development. 1990;61:1569–1583. [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behavioural Brain Research. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, Bunge S. Developmental differences in medial temporal lobe function during memory encoding. The Journal of Neuroscience. 2010;30(28):9548–9556. doi: 10.1523/JNEUROSCI.3500-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ryan JD, Tranel D, Cohen NJ. Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. Journal of Cognitive Neuroscience. 2007;19(10):1690–1705. doi: 10.1162/jocn.2007.19.10.1690. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Baym CL, Warren DE, Cohen NJ. The eyes know: Eye movements as a veridical index of memory. Psychological Science. 2012;23(3):278–287. doi: 10.1177/0956797611429799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartsher K, Rovee-Collier C. Infant learning and long-term memory at 6 months: A confirming analysis. Developmental Psychobiology. 1998;30(1):71–85. [PubMed] [Google Scholar]
- Hayhoe MM, Bensinger DG, Ballard DH. Task constraints in visual working memory. Vision Research. 1998;38:125–137. doi: 10.1016/s0042-6989(97)00116-8. [DOI] [PubMed] [Google Scholar]
- Hayne H, MacDonald S, Barr R. Developmental changes in the specificity of memory over the second year of life. Infant Behaviour and Development. 1997;20:233–245. [Google Scholar]
- Hayne H, Boniface J, Barr R. The development of declarative memory in human infants: Age-related changes in deferred imitation. Behavioral Neuroscience. 2000;114(1):77–83. doi: 10.1037//0735-7044.114.1.77. [DOI] [PubMed] [Google Scholar]
- Herbert J, Hayne H. The ontogeny of long-term retention during the second year of life. Developmental Science. 2001;3(1):50–56. [Google Scholar]
- Henderson JM, Hollingsworth A. Eye movements and visual memory: Detecting changes to saccade targets in scenes. Perception & Psychophysics. 2003;65:58–71. doi: 10.3758/bf03194783. [DOI] [PubMed] [Google Scholar]
- Holm L, Eriksson J, Andersson L. Looking as if you know: Systematic object inspection precedes object recognition. Journal of Vision. 2008;8:4, 1–7. doi: 10.1167/8.4.14. [DOI] [PubMed] [Google Scholar]
- Kidd C, Piantadois ST, Aslin RN. The goldilocks effect: Infants’ preference for visual stimuli that are neither too predictable nor too surprising. Proceedings of the 32nd Annual Meeting of the Cognitive Science Society. 2010 [Google Scholar]
- Lloyd ME, Doydum AO, Newcombe NS. Memory binding in early childhood: Evidence for a retrieval deficit. Child Development. 2009;80(5):1321–1328. doi: 10.1111/j.1467-8624.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. Infant imitation and memory: Nine-month-olds in immediate and deferred tests. Child Development. 1988;59:217–225. doi: 10.1111/j.1467-8624.1988.tb03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe N, Huttenlocher J, Drummey AB, Wiley JG. The development of spatial location coding: Place learning and dead reckoning in the second and third years. Cognitive Development. 1998;13:185–200. [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychological Review. 2003;110(4):611–64. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- Richmond J, Nelson CA. Relational memory during infancy: Evidence from eye-tracking. Developmental Science. 2009;12:549–556. doi: 10.1111/j.1467-7687.2009.00795.x. [DOI] [PubMed] [Google Scholar]
- Rovee-Collier C, Griesler P, Earley L. Contextual determinants of retrieval in three-month-old infants. Learning and Motivation. 1985;16:139–157. [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychological Science. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Hannula DE, Cohen N. The obligatory effects of eye-movements. Memory. 2007;15(5):508–525. doi: 10.1080/09658210701391022. [DOI] [PubMed] [Google Scholar]
- Seress L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. MIT Press; Cambridge, MA: 2001. pp. 45–58. [Google Scholar]
- Sluzenski J, Newcombe NS, Kovacs SL. Binding, relational memory, and recall of naturalistic events: A developmental Perspective. Journal of Experimental Psychology. 2006;32(1):89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Smith CN, Hopkins RO, Squire LO. Experience-dependent eye movements, awareness, and hippocampus-dependent memory. The Journal of Neuroscience. 2006;26(44):11304–11312. doi: 10.1523/JNEUROSCI.3071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Squire LR. Experience-dependent eye movements reflect hippocampus-dependent (aware) memory. The Journal of Neuroscience. 2008;28(48):12825–12833. doi: 10.1523/JNEUROSCI.4542-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: A brief history and current perspective. Neurobiology of Learning and Memory. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Takana K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: analysis by MR-based volumetry. American Journal of NeuroRadiology. 1999;20:717–723. [PMC free article] [PubMed] [Google Scholar]