Abstract
Purpose
We evaluated acute toxicity profiles and dosimetric data for children with salivary gland tumors treated with adjuvant photon/electron-based radiation therapy (X/E RT) or proton therapy (PRT).
Methods and Materials
We identified 24 patients who had received adjuvant radiotherapy for salivary gland tumors. Data was extracted from the medical records and the treatment planning systems. Toxicity was scored according to the Common Terminology Criteria for Adverse Effects 4.0.
Results
Eleven patients received X/E RT and 13 PRT, with a median prescribed dose of 60 Gy in each group. In the X/E RT group, 54% of patients developed acute grade II/III dermatitis, 27% grade II/III dysphagia, and 91% grade II/III mucositis, and the median weight loss was 5.3% with one patient requiring feeding tube placement. In the PRT group, 53% had acute grade II/III dermatitis, 0% grade II/III dysphagia, and 46% grade II/III mucositis, with a median weight gain of 1.2%. Additionally, PRT was associated with lower mean doses to several normal surrounding midline and contralateral structures.
Conclusion
In this retrospective study of pediatric salivary tumors, PRT was associated with a favorable acute toxicity and dosimetric profile. Continued follow-up is needed to identify long-term toxicity and survival data.
Keywords: Salivary gland tumors, parotid tumors, proton therapy, pediatrics
INTRODUCTION
Salivary gland tumors are rare in children, with an estimated annual incidence of 0.8 per million [1, 2]. About half of such tumors are malignant [2–4], and most are well to moderately differentiated without nodal involvement or distant metastasis [5, 6]. The 5–year survival rates for pediatric patients with malignant salivary gland tumors range from 85% to 98% [1, 6, 7].
For patients with high-risk disease, treatment consists of upfront surgical resection followed by radiotherapy (RT) to improve locoregional control [1, 2]. This approach, however, is associated with risks of long-term toxicity such as xerostomia, trismus, development of secondary cancers, and craniofacial growth abnormalities [7, 8]. These risks are particularly significant for children, not only because of their long life expectancies but also because irradiation of growing tissues is associated with higher rates of late toxicity.
Proton radiotherapy (PRT), an alternative to conventional photon/electron-based RT (X/E RT), has a characteristic energy deposition profile (the Bragg curve) that eliminates exit dose and reduces exposure to normal surrounding tissue. The use of PRT in treating pediatric tumors is therefore a topic of great interest. Early reports of clinical outcomes after PRT for rhabdomyosarcoma and brain tumors in pediatric cohorts have been encouraging [9–16]. However, use of PRT for pediatric salivary gland tumors has not been reported. Here we evaluate acute toxicity profiles and dosimetric data for pediatric patients with parotid or submandibular tumors treated with adjuvant X/E RT or PRT.
MATERIALS AND METHODS
After receiving institutional review board approval, we retrospectively reviewed all patients up to 18 years of age who had received adjuvant RT for primary salivary gland tumors between 1996 and 2014 at a single institution. We identified 20 cases with parotid tumors and four with submandibular tumors. Eleven patients received X/E RT (from 1996 through 2013), and 13 received PRT (from 2009 through 2014). Of the X/E RT patients, eight patients received primarily electron beam therapy (EBT) and three received intensity-modulated radiotherapy (IMRT). Of the PRT patients, eight received passive scatter proton therapy (PSPT) and five received intensity-modulated proton therapy (IMPT). All 24 patients were included in the toxicity analysis, but cross-sectional plan images were not available for three of the patients who had received EBT. These three patients were excluded from the dosimetric comparisons.
Follow-up
Acute toxicity was monitored weekly during RT. All subsequent institutional and outside-facility clinical notes were reviewed to identify disease recurrence and survival data. Toxicity scores were retrospectively assigned according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events 4.0 [17]. The index date for outcome intervals was the RT start date.
Dosimetric analysis
For the 21 patients included in the dosimetric analysis, the approved RT plan was obtained and the following structures were delineated on each axial slice: pituitary gland, spinal cord, optic nerves, eyes, uninvolved salivary glands (parotid and submandibular), oral cavity, hemi-mandibles, thyroid gland, larynx, and ipsilateral structures including temporal lobe, cochlea, temporomandibular joint, and medial and lateral pterygoid muscles. Mean and maximum doses, in Gy, were recorded for each structure. The integral dose, defined as dose to a particular structure multiplied by the volume of that structure (Gy × L), was also calculated. The inhomogeneity coefficient was calculated by dividing the minimum dose in 5% of target volume by the minimum dose in 95% of target volume [18]. The percentage of each structure receiving at least 5 Gy, 15 Gy, and 30 Gy was recorded (V5, V15, and V30). For a post hoc comparison, we also chose one case (originally planned for EBT) and designed plans for IMRT, PSPT, and IMPT. For photon/electron cases, Pinnacle version 8.0m planning system (Philips Medical Systems, Fitchburg, WI) was utilized. For proton cases, Eclipse treatment planning system V.8.9 (Varian Medical Systems, Palo Alto, CA) was employed. For all proton treatment planning a relative biological effectiveness (RBE) value of 1.1 was used.
Statistical analysis
Statistical analyses were performed using Stata/MP 14 statistical software [19]. Fisher’s exact test was used to assess measures of association in frequency tables. The equality of group medians was assessed using the Mann-Whitney test. A P-value of 0.05 or less was considered to be statistically significant. Statistical tests were based on a two-sided significance level.
RESULTS
Demographic profiles, tumor and treatment characteristics are shown in Table 1. Surgical procedures used were submandibular gland resection (n=4), superficial parotidectomy (n=7), and total parotidectomy (n=13). Sixteen patients underwent neck dissection, and seven of those patients were found to have nodal metastases; four of those patients subsequently received X/E RT and three PRT. Complications of surgery included transient facial nerve deficits in five patients and persistent facial nerve deficits in eight.
Table 1.
Patient, tumor, and treatment characteristics
| All patients n=24 (%) | Photon/electron therapy n=11 (%) | Proton therapy n=13 (%) | P value | |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 14 | 15 | 13 | 0.41 |
| Range | 6–18 | 7–18 | 6–18 | |
| Follow up (months) | ||||
| Median | 35 | 92 | 8 | <0.05 |
| Range | 2–218 | 2–218 | 2–48 | |
| Gender | ||||
| Male | 11 (46) | 5 (45) | 6 (46) | 1.00 |
| Female | 13 (54) | 6 (55) | 7 (54) | |
| Ethnicity | ||||
| Caucasian | 13 (54) | 4 (36) | 9 (69) | |
| Hispanic | 5 (21) | 2 (18) | 3 (23) | 0.35 |
| African American | 4 (17) | 3 (27) | 1 (8) | |
| Other | 2 (8) | 2 (18) | 0 | |
| Tumor site | ||||
| Parotid | 20 (83) | 9 (82) | 11 (85) | 1.00 |
| Submandibular | 4 (17) | 2 (18) | 2 (15) | |
| Histology | ||||
| Mucoepidermoid carcinoma | 12 (50) | 5 (45) | 7 (54) | |
| Adenoid cystic carcinoma | 5 (21) | 2 (18) | 3 (23) | |
| Adenocarcinoma | 2 (8) | 0 (0) | 2 (15) | 0.35 |
| Acinic cell carcinoma | 2 (8) | 2 (18) | 0 | |
| Pleomorphic adenoma | 1 (4) | 0 | 1 (8) | |
| Myoepithelioma | 1 (4) | 1 (9) | 0 | |
| Undifferentiated carcinoma | 1 (4) | 1 (9) | 0 | |
| Tumor grade | ||||
| Low/intermediate | 11 (46) | 5 (45) | 7 (54) | 0.87 |
| High | 5 (21) | 3 (27) | 2 (15) | |
| Unknown | 8 (33) | 3 (27) | 4 (31) | |
| Tumor stage | ||||
| T1–2 | 17 (71) | 9 (82) | 10 (77) | 0.24 |
| T3–4 | 3 (13) | 0 | 3 (23) | |
| Tx | 2 (8) | 2 (18) | 0 | |
| Nodal stage | ||||
| N0 | 17 (71) | 7 (64) | 10 (77) | 0.66 |
| N1 | 7 (29) | 4 (36) | 3 (23) | |
| RT dose | ||||
| Median | 60 | 60 | 60 | 0.88 |
| Range | 54–66 | 56.4–66 | ||
| Treatment of ipsilateral neck | ||||
| Yes | 12 50) | 8 (73) | 4 (33) | 0.10 |
| No | 12 (50) | 3 (27) | 9 (69) | |
| Weight change (%) | ||||
| Median | −1.6 | −5.3 | 1.2 | 0.13 |
| [Range] | [−16.5] – [+8.2] | [−16.5] – [+3.1] | [−9.4] – [+8.2] | |
The primary indications for RT were close (<1 mm) or positive surgical margins (n=21), extra-glandular extension (n=2), or tumor spillage (n=1). The clinical target volume (CTV) comprised the tumor bed in 14 patients, tumor bed plus at-risk areas (e.g., neck, skull base, facial or trigeminal nerves) in eight patients, and tumor bed plus a 1-cm expansion in two patients. To ensure appropriate coverage for patient set-up uncertainties and penumbra, a 3-mm expansion was used for X/E RT while a beam-specific expansion was used for PRT [20].
The median prescribed dose to the CTV was 60 Gy in 30 fractions in each group (range 54–66 Gy). Eighteen patients received 60 Gy in 30 fractions; higher doses were prescribed for three patients with positive surgical margins, and lower doses were prescribed for three patients with benign or favorable tumor histology. Field reductions were used for six patients in the X/E RT group (at 60 Gy in one, at 56 Gy in two, and at 50 Gy in three) and for eight patients in the PRT group (at 60 Gy in one, at 57 Gy in three, at 54 Gy in one, and at 50 Gy in three). A second field reduction was used for three patients receiving X/E RT (at 54 Gy, 50 Gy, and 40 Gy) and for two patients receiving PRT (both at 54 Gy). The ipsilateral neck was treated in eight of the 11 X/E RT patients (73%) and in four of the 13 PRT patients (31%). Of the eight X/E RT patients who were included in the dosimetric analysis, six (75%) were treated to the ipsilateral neck. The three IMRT plans utilized primarily 6 MV photon beams. The EBT plans predominantly utilized a mix of electron beams (6, 9, 12, 16, or 20 MeV). Seven EBT plans included 6 MV photons for skin sparing (electron-photon ratio of 4:1), while one EBT plan used exclusively electrons. No patients required anesthesia to complete radiation treatment. One patient in each group received concurrent cisplatin-based chemotherapy.
Toxicity profiles are summarized in Table 2. All patients completed RT without interruption. No difference in rates of stage II/III dermatitis (P=1.00) or otitis externa (P=0.58) was seen between the two groups. The X/E RT group, however, experienced significantly more grade II/III mucositis compared to the PRT group (91% vs. 46%, P<0.05). Additionally, an increased incidence of dysphagia in the X/E RT group (27% vs. 0% in PRT group, P=0.08) as well as an increased weight lost during treatment in the X/E RT group (−5.3% from baseline vs. +1.2% from baseline in PRT group, P=0.13, Table 1) trended toward significance. Two patients in the X/E RT group experienced chronic toxicity, one with trismus and hypothyroidism and one with craniofacial growth abnormalities, stenosis of the ear canal, and hypoacusis. Long-term toxicity data in the PRT group is not yet available. At a median follow-up time of 35 months (range 2 months to 18 years; medians 8 years for the X/E RT group vs. 8 months for the PRT group), no disease recurrence or deaths were observed in either group.
Table 2.
Acute toxicities by CTCAE 4.0 (Grade II/III)
| All patients | Photon/electron therapy n=11 (%) | Proton therapy n=13 (%) | P value | |
|---|---|---|---|---|
| Dermatitis* | 13 (54) | 6 (55) | 7 (54) | 1.00 |
| Dysphagia‡ | 3 (13) | 3 (27) | 0 | 0.08 |
| Otitis externa€ | 3 (13) | 2 (18) | 1 (8) | 0.58 |
| Mucositis§ | 16 (67) | 10 (91) | 6 (46) | <0.05 |
grade II/III dermatitis=brisk erythema, moderate edema, or moist desquamation
grade II/III dysphagia= pain requiring change in diet and/or nutritional support
grade II/III otitis= discharge from ear canal
grade II/III mucositis= patchy or confluent ulcerations
Dosimetric data is summarized in Table 3. Both mean and maximum doses were lower with PRT than with X/E RT for the optic nerves, pituitary, spinal cord, thyroid, and larynx; and for the contralateral eye, hemimandible, parotid and submandibular glands; and ipsilateral pterygoid and uninvolved salivary glands. Total body integral dose, in units of Gy x L, was 20.9 for PRT versus 50.7 for X/E RT (P<0.05); similarly, integral doses to several surrounding structures was also significantly decreased for PRT compared to X/E RT (Figure 1). PRT was also associated with lower V5, V15, and V30 values for many normal surrounding structures (Supplemental Figure 1).
Table 3.
Mean and maximum doses to surrounding structures
| Mean Dose, Gy | Maximum Dose, Gy | |||||
|---|---|---|---|---|---|---|
| X/E RT n=8 | PRT n=13 | P Value* | X/E RT n=8 | PRT n=13 | P Value* | |
| Eyei | 1.3 | 0.6 | 0.10 | 5.4 | 1.7 | 0.41 |
| Eyec | 1.5 | 0.0 | <0.05 | 3.7 | 0.0 | <0.05 |
| Optic nervec | 2.0 | 0.0 | <0.05 | 3.1 | 0.0 | <0.05 |
| Optic nervei | 2.1 | 0.0 | <0.05 | 3.8 | 0.4 | <0.05 |
| Pituitary gland | 3.8 | 0.0 | <0.05 | 5.1 | 0.0 | <0.05 |
| Temporal lobei | 4.6 | 3.2 | 0.69 | 43.0 | 47.3 | 0.96 |
| Parotid glandc | 4.6 | 0.0 | <0.05 | 10.2 | 0.0 | <0.05 |
| Spinal cord | 9.3 | 0.2 | <0.05 | 39.4 | 8.1 | <0.05 |
| Hemimandiblec | 11.9 | 0.0 | <0.05 | 32.4 | 0.6 | <0.05 |
| Submandibular glandc | 13.5 | 0.0 | <0.05 | 22.8 | 0.1 | <0.05 |
| Oral cavity | 20.7 | 4.6 | <0.05 | 55.9 | 61.0 | 0.54 |
| Thyroid | 22.5 | 1.5 | <0.05 | 54.4 | 18.5 | 0.26 |
| Cochleai | 31.0 | 15.8 | 0.27 | 37.5 | 26.0 | 0.91 |
| Hemimandiblei | 42.8 | 32.9 | <0.05 | 68.6 | 64.7 | 0.07 |
| Larynx | 44.3 | 11.3 | <0.05 | 62.7 | 31.2 | <0.05 |
| Temporomandibular jointi | 45.8 | 50.6 | 0.96 | 57.0 | 59.9 | 0.78 |
| Pterygoid musclesi | 50.8 | 38.9 | <0.05 | 64.9 | 62.4 | 0.07 |
| Uninvolved salivary glandi | 61.3 | 50.2 | <0.05 | 67.5 | 63.0 | 0.07 |
Abbreviations: X/E RT, photon/electron radiotherapy; PRT, proton radiotherapy
contralateral to tumor
ipsilateral to tumor
P values from Mann-Whitney tests.
Figure 1.
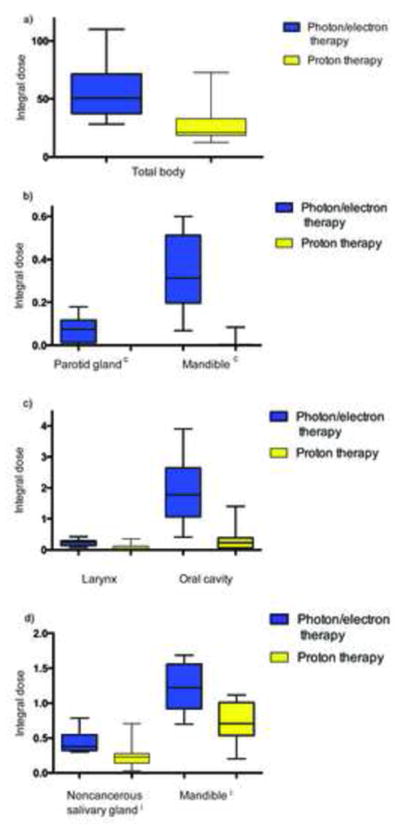
Box plot showing median, quartile, and minimum/maximum integral doses (in Gy×L) to the total body (panel a) and surrounding normal structures (panels b, c, and d). All P values <0.05. Abbreviations: c, contralateral; i, ipsilateral.
Post hoc treatment plans were generated for one patient, who had been treated with EBT for a right parotid gland tumor, to visualize differences in dose distributions among EBT, IMRT, PSPT, and IMPT plans (Figure 2). Compared with the EBT and IMRT plans, PSPT and IMPT were associated with reduced mean and maximum doses to several normal surrounding structures (Supplemental Figure 2). The inhomogeneity coefficients for the respective plans were 1.21 (EBT), 1.02 (IMRT), 1.06 (PSPT), and 1.06 (IMPT).
Figure 2.
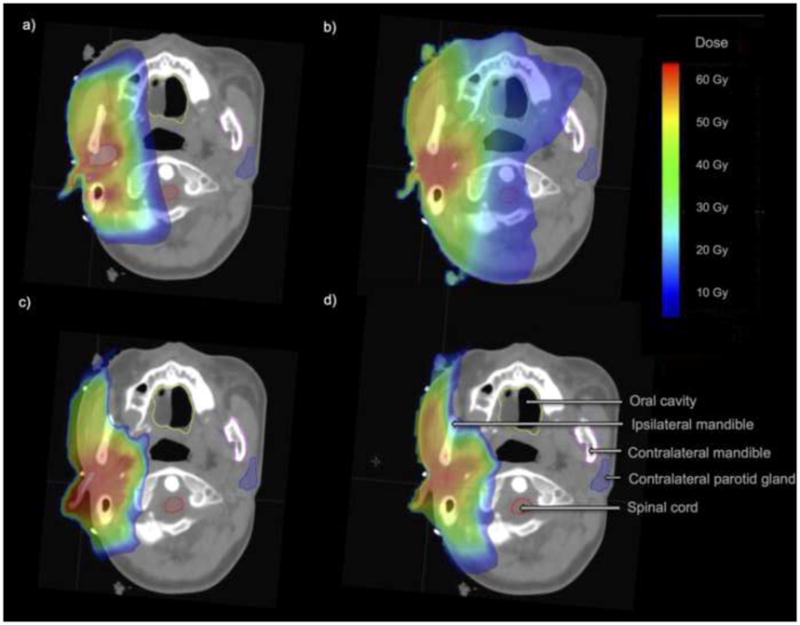
Isodose line comparison of treatment plans for a patient with a right parotid gland tumor. The patient was treated with electron beam therapy (panel a), and post hoc plans were created for intensity-modulated radiotherapy (b), passive scatter proton radiotherapy (c), and intensity-modulated proton radiotherapy (d). The inhomogeneity coefficients for the respective plans were 1.21 (EBT), 1.02 (IMRT), 1.06 (PSPT), and 1.06 (IMPT).
DISCUSSION
Several key findings are able to be made from this retrospective analysis of adjuvant RT for pediatric salivary gland tumors. First, compared to X/E RT, patients who received PRT experienced significantly less grade II/III acute mucositis and a trend toward significantly less grade II/III dysphagia and weight loss. Second, rates of grade II/III dermatitis and otitis externa were similar between cohorts. Third, PRT was associated with reduced dose to several surrounding normal structures relative to X/E RT. And fourth, total body integral dose was significantly reduced in patients receiving PRT.
This is one of only several studies to report on outcomes following PRT in the pediatric population, and the only one to do so for salivary gland tumors. The dosimetric advantage of PRT over X/E RT has been well documented in previous studies [21, 22]. The clinical benefit of PRT, however, is less clear. Reports on PRT for pediatric ependymomas [16], medullablastomas [23], and gliomas [10] show encouraging rates of acute toxicity. But as a newly emerging technique, data on long-term toxicity in the pediatric population is sparse. Results from the ongoing phase II trial of pediatric rhabdomyosarcomas treated with PRT may be of most value in assessing clinical outcomes [15]. In this trial, 57 patients with a median of age of 3.5 years were treated with PRT to a median dose of 50.4 Gy. Preliminary data demonstrates favorable rates of acute and late toxicity with 17% of patients developing acute grade III toxicity (dermatitis in 9% of all patients, odynophagia in 10% of head and neck patients, and mucositis in 6% of head and neck patients) and 7% of patients developing late grade III toxicity (cataract, chronic otitis, and retinopathy in one patient each). With a median follow up time of 47 months, no secondary malignancies were reported. Five-year rates of event-free survival, overall survival, and local control were 69%, 78%, and 81% respectively, which is similar to reported data in comparable photon studies.
Although PRT is a specialized technique not available to a large number of patients, its reach is expanding. The number of operating proton centers in the United States, for example, has increased from three to 14 in the past decade, with 11 more currently under construction [24]. As PRT becomes more accessible, studies reporting clinical outcomes are increasingly important. Indeed, PRT will continue to be a topic of great interest going forward, particularly in the pediatric population. Clinical protocols have recently been generated at the Heidelberg Ion Therapy Center, for example, with the intent of further clarifying the benefits of PRT (as well as carbon ion therapy) in the treatment of pediatric tumors [13].
In this study of pediatric salivary tumors, we show that PRT is associated with favorable rates of mucositis while rates of dermatitis and otitis externa were comparable X/E RT. These findings highlight the advantage of PRT, which is in reduced exit but not entrance dose as characterized by the Bragg curve. In fact, the lack of proximal conformality with PSPT may occasionally actually increase entrance dose relative to X/E RT [25], but this is expected that this will be overcome with advanced PRT techniques such as IMPT.
Chronic toxicities were seen in the X/E RT cohort, but long-term follow up data is not yet available for the PRT cohort. While only continued follow up will determine chronic toxicity, dose-restriction guidelines exist for several relevant structures and may predict for long-term complications. Studies have shown that a mean dose of 40 Gy or more to the pterygoid muscles is predictive of late trismus [26, 27]. This dose was exceeded by the mean dose in the X/E RT group (50.8 Gy vs. 38.9 Gy in PRT, P<0.05). Similarly, the Children’s Oncology Group found a mean cochlear dose of 30 Gy to be predictive of long-term hearing loss [28]. Although not significantly higher than in PRT, this dose was exceeded by the X/E RT cohort (31.0 Gy vs. 15.8 Gy in PRT, P=0.27). While no clear dose threshold to bone has been established, studies have demonstrated a dose-effect relationship between irradiation of cartilaginous growth plates in the mandible and maxilla and subsequent asymmetric facial development during puberty [29–31]. In this study, mean dose to the ipsilateral and contralateral mandible was significantly higher in the X/E RT cohort (42.8 Gy vs. 32.9 Gy in PRT, P<0.05 and 11.9 Gy vs. 0 Gy in PRT, P<0.05, respectively).
One of the feared complications of childhood RT is the development of secondary malignancies. As cancer therapies have improved, increasing numbers of childhood cancer survivors are living into adulthood, with an increased life-time risk of developing second neoplasms [32]. Clear dose thresholds have been difficult to elucidate because of the heterogeneity in types and locations of secondary malignancies. Still, a dose-effect relationship likely exists. For example, Dorr et al., in a study of 85 patients with a variety of tumors, found that most secondary malignancies occurred in areas exposed to >6 Gy [33]. In our study, patients in the X/E RT group had higher V5 values for several surrounding normal structures, and as such may be at higher risk of developing secondary malignancies.
For several normal surrounding structures, mean dose was higher in the X/E RT cohort compared to PRT, but remained lower than estimated thresholds. Quantitative Analysis of Normal Tissue Effects in Clinic (QUANTEC) guidelines, for example, suggest that xerostomia can typically be avoided when at least one parotid gland receives a mean dose of ≤20 Gy [34]. This was not exceeded in either cohort (4.6 Gy to the contralateral parotid gland in X/E RT vs. 0 Gy in PRT, P<0.05). However, these dose guidelines are for adults; whether children have similar organ-dose tolerance is not clear and is currently being investigated by the Pediatric Normal Tissue Effects in the Clinic (PENTEC) group. Bhandare et al. [35] found the risk of hypothyroidism was minimal with thyroid doses <45 Gy, higher than the mean thyroid dose in either cohort (22.5 Gy in X/E RT vs. 1.5 Gy in PRT, P<0.05).
There are several limitations of this study evaluating outcomes for pediatric salivary gland tumors treated with PRT. First, a direct comparison between X/E RT and PRT is limited by the retrospective nature of the study, the small number of patients in each cohort, and the short follow up times for patients receiving PRT. Salivary gland tumors in the pediatric population, however, are quite rare and only recently have been considered for treatment with PRT. Indeed, this report on pediatric salivary gland tumors is one of the largest to date and, to our knowledge, the only one to document outcomes following PRT. Second, discrepant rates of treatment to the ipsilateral neck (73% in X/ERT vs. 33% in PRT, P=0.10) may confound toxicity and dosimetric data, particularly in rates of acute dysphagia and dose to the thyroid gland and larynx. Given the limited number of patients in each cohort, however, statistical analysis of patients grouped by radiation field (i.e. with or without neck treatment) could not be performed. Furthermore, differences in ipsiliateral neck treatment would not be expected to affect dose to other surrounding structures including the oral cavity, mandible, parotid glands, muscles of mastication, or auditory structures. Third, given the rarity of the tumor in the pediatric population, the X/E RT cohort included both EBT and IMRT plans. Although IMRT has largely become the mainstay of head and neck RT, its application to salivary tumors is still under investigation and has thus not entirely replaced EBT [36–38]. EBT may actually be particularly appropriate given the superficial location of the tumor. Still, it is not clear what dosimetric and toxicity differences would be seen if PRT was compared to IMRT or EBT separately. Again, however, given the limited number of patients, subgroup analysis could not be performed.
CONCLUSION
Previous studies have shown encouraging outcomes following PRT in several pediatric tumors. This is the only study to our knowledge, however, to evaluate PRT for pediatric salivary tumors. We assessed acute toxicity, early tumor control, and potential for reduction in late morbidity, finding PRT to be associated with a favorable acute toxicity and dosimetric profile. Continued follow-up is required to evaluate long-term toxicity and survival data.
Supplementary Material
Mean V5 (a), V15 (b), and V30 (c) values to surrounding structures. P values for V5 are as follows: eyei, 0.12; eyec, 0.17; optic nervec, 0.49; optic nervei, 0.17; temporal lobei, 0.69; pituitary gland, 0.09; parotid glandc, <0.05; thyroid, 0.08; spinal cord, <0.05; hemimandiblec, <0.05; submandibular glandc, <0.05 ; cochleai, 0.61 ; TMJi, 0.15; oral cavity, <0.05; larynx, 0.08; hemimandiblei, 0.14; pterygoid musclesi, 0.91; uninvolved salivary glandi, 0.90. P values for V15 are as follows: pituitary gland, 0.17; hemimandiblec, 0.07; submandibular glandc, 0.23; thyroid, 0.06; oral cavity, <0.05; spinal cord, <0.05; cochleai, 0.64; TMJi, 0.22; larynx, 0.11; pterygoid musclesi, 0.91; hemimandiblei, 0.09; uninvolved salivary glandi, 0.47. P values for V30 are as follows; hemimandiblec, 0.59; submandibular glandc, 0.67; temporal lobei, 1.00; oral cavity, <0.05; spinal cord, <0.05; thyroid, 0.12; cochleai, 0.77; larynx, <0.05; TMJi, 0.09; hemimandiblei, 0.14; pterygoid musclesi, 0.86; uninvolved salivary glandi, 0.77. Abbreviations: c, contralateral; i, ipsilateral; TMJ, temporomandibular joint.
Mean (a) and maximum (b) doses, in Gy, are compared among various types of radiotherapy plans for a patient with a right parotid gland malignancy. Abbreviations: c, contralateral; i, ipsilateral; TMJ, temporomandibular joint.
SUMMARY.
We retrospectively analyzed 24 cases of pediatric salivary gland tumors and found that proton therapy was associated with a favorable acute toxicity and dosimetric profile.
Acknowledgments
The authors would like to thank Christine Wogan for editorial assistance and Pamela Allen for statistical assistance.
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thariat J, et al. The role of radiation therapy in pediatric mucoepidermoid carcinomas of the salivary glands. J Pediatr. 2013;162(4):839–43. doi: 10.1016/j.jpeds.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 2.Fang QG, et al. Epithelial salivary gland tumors in children: a twenty-five-year experience of 122 patients. Int J Pediatr Otorhinolaryngol. 2013;77(8):1252–4. doi: 10.1016/j.ijporl.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 3.Deng R, et al. Salivary gland neoplasms in children. J Craniofac Surg. 2013;24(2):511–3. doi: 10.1097/SCS.0b013e3182801866. [DOI] [PubMed] [Google Scholar]
- 4.Laikui L, et al. Epithelial salivary gland tumors of children and adolescents in west China population: a clinicopathologic study of 79 cases. J Oral Pathol Med. 2008;37(4):201–5. doi: 10.1111/j.1600-0714.2007.00618.x. [DOI] [PubMed] [Google Scholar]
- 5.Sultan I, et al. Salivary gland carcinomas in children and adolescents: a population- based study, with comparison to adult cases. Head Neck. 2011;33(10):1476–81. doi: 10.1002/hed.21629. [DOI] [PubMed] [Google Scholar]
- 6.Ryan JT, et al. Primacy of surgery in the management of mucoepidermoid carcinoma in children. Head Neck. 2011;33(12):1769–73. doi: 10.1002/hed.21675. [DOI] [PubMed] [Google Scholar]
- 7.Kupferman ME, et al. Outcomes of pediatric patients with malignancies of the major salivary glands. Ann Surg Oncol. 2010;17(12):3301–7. doi: 10.1245/s10434-010-1165-2. [DOI] [PubMed] [Google Scholar]
- 8.Tribius S, et al. Xerostomia after radiotherapy. What matters--mean total dose or dose to each parotid gland? Strahlenther Onkol. 2013;189(3):216–22. doi: 10.1007/s00066-012-0257-2. [DOI] [PubMed] [Google Scholar]
- 9.Yock T, et al. Proton radiotherapy for orbital rhabdomyosarcoma: clinical outcome and a dosimetric comparison with photons. Int J Radiat Oncol Biol Phys. 2005;63(4):1161–8. doi: 10.1016/j.ijrobp.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 10.Greenberger BA, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2014;89(5):1060–8. doi: 10.1016/j.ijrobp.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 11.Bishop AJ, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys. 2014;90(2):354–61. doi: 10.1016/j.ijrobp.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald SM, et al. Proton radiotherapy for childhood ependymoma: initial clinical outcomes and dose comparisons. Int J Radiat Oncol Biol Phys. 2008;71(4):979–86. doi: 10.1016/j.ijrobp.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 13.Combs SE, et al. Treatment of pediatric patients and young adults with particle therapy at the Heidelberg Ion Therapy Center (HIT): establishment of workflow and initial clinical data. Radiat Oncol. 2012;7:170. doi: 10.1186/1748-717X-7-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Childs SK, et al. Proton radiotherapy for parameningeal rhabdomyosarcoma: clinical outcomes and late effects. Int J Radiat Oncol Biol Phys. 2012;82(2):635–42. doi: 10.1016/j.ijrobp.2010.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladra MM, et al. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2015;33(2):228. doi: 10.1200/JCO.2014.56.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amsbaugh MJ, et al. Proton therapy for spinal ependymomas: planning, acute toxicities, and preliminary outcomes. Int J Radiat Oncol Biol Phys. 2012;83(5):1419–24. doi: 10.1016/j.ijrobp.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0. May 29, 2009. NIH publication # 09-7473. [Google Scholar]
- 18.Kataria T, et al. Homogeneity Index: An objective tool for assessment of conformal radiation treatments. J Med Phys. 2012;37(4):207–13. doi: 10.4103/0971-6203.103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StataCorp. Statistical Software. College Station, TX: StataCorp LP; 2015. Stata: Release 14. [Google Scholar]
- 20.van der Giessen PH. Peridose, a software program to calculate the dose outside the primary beam in radiation therapy. Radiother Oncol. 2001;58(2):209–13. doi: 10.1016/s0167-8140(00)00326-1. [DOI] [PubMed] [Google Scholar]
- 21.Ladra MM, et al. A dosimetric comparison of proton and intensity modulated radiation therapy in pediatric rhabdomyosarcoma patients enrolled on a prospective phase II proton study. Radiother Oncol. 2014;113(1):77–83. doi: 10.1016/j.radonc.2014.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak KR, et al. A dosimetric comparison of proton and intensity-modulated photon radiotherapy for pediatric parameningeal rhabdomyosarcomas. Int J Radiat Oncol Biol Phys. 2009;74(1):179–86. doi: 10.1016/j.ijrobp.2008.06.1942. [DOI] [PubMed] [Google Scholar]
- 23.Moeller BJ, et al. Low early ototoxicity rates for pediatric medulloblastoma patients treated with proton radiotherapy. Radiation Oncology. 2011;6:58. doi: 10.1186/1748-717X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Proton Therapy Center. [Accessed June 25, 2015];Proton Therapy Around the World. http://www.proton-cancer-treatment.com/proton-therapy/proton-therapy-around-the-world/
- 25.Price P, Sikora K. Treatment of Cancer. 6. Boca Raton, FL: Taylor and Francis Group; 2015. [Google Scholar]
- 26.van der Molen L, et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol. 2013;106(3):364–9. doi: 10.1016/j.radonc.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Teguh DN, et al. Trismus in patients with oropharyngeal cancer: relationship with dose in structures of mastication apparatus. Head Neck. 2008;30(5):622–30. doi: 10.1002/hed.20760. [DOI] [PubMed] [Google Scholar]
- 28.Grewal S, et al. Auditory late effects of childhood cancer therapy: a report from the Children’s Oncology Group. Pediatrics. 2010;125(4):e938–50. doi: 10.1542/peds.2009-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otmani N. Oral and maxillofacial side effects of radiation therapy on children. J Can Dent Assoc. 2007;73(3):257–61. [PubMed] [Google Scholar]
- 30.Dawson WB. Growth impairment following radiotherapy in childhood. Clin Radiol. 1968;19(3):241–56. doi: 10.1016/s0009-9260(68)80001-7. [DOI] [PubMed] [Google Scholar]
- 31.Merchant TE, et al. Differential attenuation of clavicle growth after asymmetric mantle radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(2):556–61. doi: 10.1016/j.ijrobp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Meadows AT, Silber J. Delayed consequences of therapy for childhood cancer. CA Cancer J Clin. 1985;35(5):271–86. doi: 10.3322/canjclin.35.5.271. [DOI] [PubMed] [Google Scholar]
- 33.Dorr W, Herrmann T. Cancer induction by radiotherapy: dose dependence and spatial relationship to irradiated volume. J Radiol Prot. 2002;22(3A):A117–21. doi: 10.1088/0952-4746/22/3a/321. [DOI] [PubMed] [Google Scholar]
- 34.Lee TF, Fang FM. Quantitative analysis of normal tissue effects in the clinic (QUANTEC) guideline validation using quality of life questionnaire datasets for parotid gland constraints to avoid causing xerostomia during head-and-neck radiotherapy. Radiother Oncol. 2013;106(3):352–8. doi: 10.1016/j.radonc.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Bhandare N, et al. Primary and central hypothyroidism after radiotherapy for head-and-neck tumors. Int J Radiat Oncol Biol Phys. 2007;68(4):1131–9. doi: 10.1016/j.ijrobp.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Schoenfeld JD, et al. Salivary gland tumors treated with adjuvant intensity-modulated radiotherapy with or without concurrent chemotherapy. Int J Radiat Oncol Biol Phys. 2012;82(1):308–14. doi: 10.1016/j.ijrobp.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 37.Jereczek-Fossa BA, et al. Prospective study on the dose distribution to the acoustic structures during postoperative 3D conformal radiotherapy for parotid tumors: dosimetric and audiometric aspects. Strahlenther Onkol. 2011;187(6):350–6. doi: 10.1007/s00066-011-2170-5. [DOI] [PubMed] [Google Scholar]
- 38.National Comprehensive Cancer Network. Head and Neck Cancers, Clinical Practice Guideliens in Oncology (Version 2.2014) 2015 Apr 15; Available from: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean V5 (a), V15 (b), and V30 (c) values to surrounding structures. P values for V5 are as follows: eyei, 0.12; eyec, 0.17; optic nervec, 0.49; optic nervei, 0.17; temporal lobei, 0.69; pituitary gland, 0.09; parotid glandc, <0.05; thyroid, 0.08; spinal cord, <0.05; hemimandiblec, <0.05; submandibular glandc, <0.05 ; cochleai, 0.61 ; TMJi, 0.15; oral cavity, <0.05; larynx, 0.08; hemimandiblei, 0.14; pterygoid musclesi, 0.91; uninvolved salivary glandi, 0.90. P values for V15 are as follows: pituitary gland, 0.17; hemimandiblec, 0.07; submandibular glandc, 0.23; thyroid, 0.06; oral cavity, <0.05; spinal cord, <0.05; cochleai, 0.64; TMJi, 0.22; larynx, 0.11; pterygoid musclesi, 0.91; hemimandiblei, 0.09; uninvolved salivary glandi, 0.47. P values for V30 are as follows; hemimandiblec, 0.59; submandibular glandc, 0.67; temporal lobei, 1.00; oral cavity, <0.05; spinal cord, <0.05; thyroid, 0.12; cochleai, 0.77; larynx, <0.05; TMJi, 0.09; hemimandiblei, 0.14; pterygoid musclesi, 0.86; uninvolved salivary glandi, 0.77. Abbreviations: c, contralateral; i, ipsilateral; TMJ, temporomandibular joint.
Mean (a) and maximum (b) doses, in Gy, are compared among various types of radiotherapy plans for a patient with a right parotid gland malignancy. Abbreviations: c, contralateral; i, ipsilateral; TMJ, temporomandibular joint.


