Abstract
Invasion of a suitable host hepatocyte by mosquito-transmitted Plasmodium sporozoites is an essential early step in successful malaria parasite infection. Yet, precisely how sporozoites target their host cell and facilitate productive infection remains largely unknown. Here, we found that the hepatocyte EphA2 receptor was critical for establishing a permissive intracellular replication compartment, the parasitophorous vacuole. Sporozoites productively infected hepatocytes with high EphA2 expression and deletion of EphA2 protected mice from liver infection. Lack of host EphA2 phenocopied the lack of the sporozoite proteins P52 and P36. Our data suggests that P36 engages EphA2, which is likely to be a key step to establish the permissive replication compartment.
Malaria infections inflict a tremendous global health burden (1). Their causative agents, Plasmodium parasites, are transmitted to mammals as sporozoites by the bite of Anopheles mosquitoes. After entry into a capillary, sporozoites are carried to the liver where they pass through multiple cells before recognizing and invading hepatocytes. During invasion, the sporozoite forms a protective parasitophorous vacuole (PV) derived of hepatocyte plasma membrane, which ensconces the parasite, establishes the intrahepatocytic replication niche, and supports successful infection. It has been demonstrated that highly sulfated proteoglycans home sporozoites into the liver parenchyma (2, 3) and hepatocyte CD81 and scavenger receptor B1 are important for hepatocyte infection (4-6). Beyond this, the molecular mechanisms underlying infection remain poorly understood.
Hepatocytes exhibit differential susceptibility to infection. Sporozoites preferentially enter polyploid hepatocytes (7). Also, BALB/cByJ mice are more susceptible to Plasmodium yoelii sporozoite infection than BALB/cJ mice (8). To assess potential host receptors that might contribute to differential susceptibility, we used an antibody array to assess the levels of 28 activated receptors in the livers of BALB/cJ and BALB/cByJ mice. Nine receptors, including EphA2, were present in significantly (P<0.01) and substantially elevated levels in highly susceptible BALB/cByJ mice (Table S1). Polyploid hepatocytes expressed higher levels of EphA2 (Fig. S1).
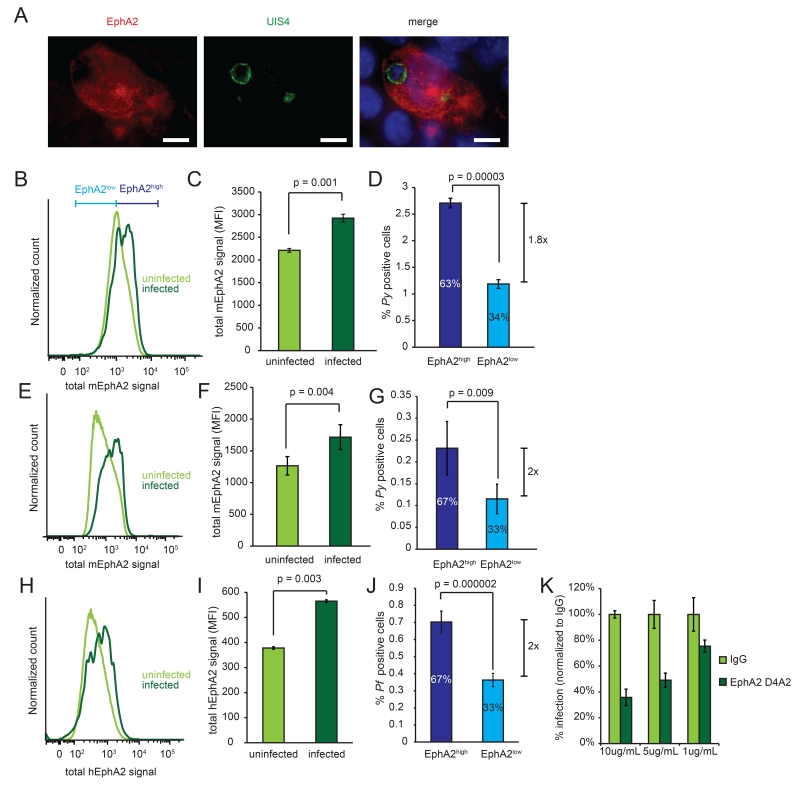
In metazoans, Eph receptors and their cognate Ephrin ligands mediate cell-cell contact (9), making EphA2 a candidate to mediate hepatocyte-sporozoite interaction. Furthermore, an Ephrin-like fold is present in the parasite 6-Cys protein family (10). Although Hepa1-6 cells held consistent EphA2 expression across passages, variation within a culture was substantial (Fig. S2) and we thus postulated that if EphA2 mediates sporozoite invasion, there might be variable susceptibilities within a culture of Hepa1-6 cells. When we infected Hepa1-6 cells with P. yoelii sporozoites, we observed parasites in hepatocytes that expressed high levels of EphA2 after 24h (Fig. 1A). This was also observed 1.5h after infection by flow cytometry (Fig. 1B, Fig. S3A), as parasite-infected cells exhibited significantly increased levels of both total (Fig. 1C) and surface (Fig. S3B-D) EphA2. Similarly, the frequency of infection in the top 50% of EphA2-expressing cells (EphA2high) was elevated compared to infection in cells with the lowest 50% of EphA2 levels (EphA2low) (Fig. 1D). When we included only the top 40%, 30% or 20% or 10% of EphA2 expressing cells in the EphA2high gate, the preference was even more dramatic (Fig. S3E). We next challenged BALB/c mice with 106 P. yoelii sporozoites and isolated hepatocytes after 3 h. We again observed a strong parasite preference for EphA2high hepatocytes (Fig. 1E, F, G). Finally, we asked if the preference for infection of EphA2high hepatocytes was conserved in the human parasites by infecting HC-04 hepatocytes with Plasmodium falciparum. We observed elevated levels of EphA2 in infected cells and a higher proportion of sporozoite-containing cells in the EphA2high population (Fig. 1H-J).
Fig. 1.
Plasmodium sporozoites invade hepatocytes with high EphA2 expression. (A) Hepa1-6 cells were infected with P. yoelii sporozoites and visualized by immunofluorescence 24 h post infection. Scale bar is 5μM. (B, C, D) Hepa1-6 cells were infected with 105 P. yoelii sporozoites. (B) Distribution of EphA2 1.5h after infection. (C) EphA2 levels were compared between parasite-infected and uninfected cells. (D) Parasite-infection rates within the highest and lowest 50% fraction of EphA2-expressing cells (designated EphA2high and EphA2low). The percentages represent the proportion of infected cells within each subset. (E, F, G) BALB/c mice were infected with 106 P. yoelii sporozoites by i.v. injection. Analysis of hepatocytes performed as in B-D. (H, I, J) HC04 cells were infected with 105 P. falciparum sporozoites. Analysis performed as in B-D. (K) Hepa1-6 cells were incubated with αEphA2 or IgG control 30 min before infection with 105 P. yoelii sporozoites. Infection rate was normalized to the rate with IgG. All data represent three independent experiments.
EphA2 has an extracellular ligand-binding region and an intracellular kinase domain, which mediates downstream signaling. To assess if interaction with the extracellular portion of EphA2 is critical for Plasmodium infection, we infected hepatocytes in the presence of an antibody that binds extracellular EphA2. This reduced sporozoite infection in a dose-dependent manner (Fig. 1K). In contrast, inhibiting the kinase domain of EphA2 did not inhibit infection (Fig. S4). Thus, the extracellular portion of EphA2 facilitates Plasmodium invasion of hepatocytes.
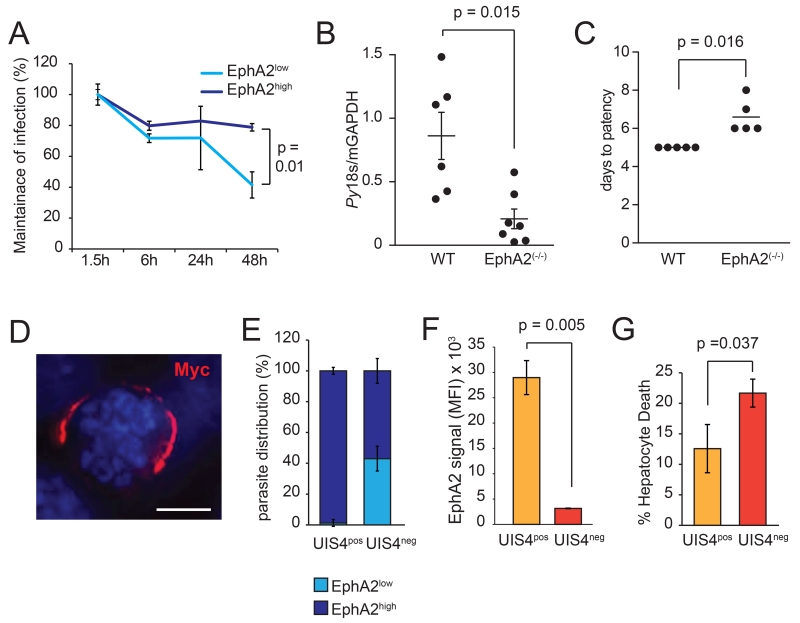
To ask if EphA2 levels were important for liver stage parasite survival and development, we measured infection rates in EphA2high and EphA2low cells over the course of 48 h, normalizing each infection rate to the rate at 1.5h post-infection. While EphA2high –infected cells were maintained throughout the course of infection, the number of EphA2low–infected cells decreased over time (Fig. 2A). This could not be accounted for by division rates as we observed lower levels of host cell division among EphA2low cells, suggesting actual underestimation of the impact of EphA2 on infected cell survival (Fig. S5). When we infected EphA2(−/−) mice or wild-type mice with 105 P. yoelii sporozoites, we observed a dramatic decrease in liver stage burden after 42 h in EphA2(−/−) mice (Fig. 2B), which caused a delay in the onset of blood stage infection by 1-3 days (Fig. 2C). Thus, without EphA2, the host is far less susceptible to productive parasite liver infection.
Fig. 2.
EphA2 impacts PVM formation. (A) Time course showing maintenance of P.yoelii infection in Hepa1-6 cells. Maintenance of infection was defined as the infection rate at a given point divided by the infection rate at 1.5 h post-infection. (B) EphA2(−/−) or age-matched wild-type mice were infected with 105 P. yoelii sporozoites. Infection was assessed 42 h post-infection by qPCR. Error bars show SEM. (C) EphA2(−/−) or strain-matched wild-type mice were infected with 102 P. yoelii sporozoites. Patency was monitored daily by thin smear. (D) PyUIS-Myc parasite in Hepa1-6 cells 24 h post-infection. Scale bar is 5μM. (E, F) PyUIS-Myc parasites were used to infect Hepa1-6 cells and analyzed for the presence of PyUIS-Myc after 24 h. (G) PyUIS4-Myc-infected Hepa1-6 cells were assessed for permeability after 48 h. All data represent three independent experiments.
The PV membrane (PVM) is critical for liver stage development. One liver stage PVM resident protein, UIS4, is highly expressed after invasion when it is exported to the PVM (11), making it a useful marker. We constructed a P. yoelii parasite line, which expressed a UIS4-Myc fusion protein driven by the endogenous UIS4 promoter (Fig. 2D). This allowed us to monitor PVM prevalence in infected cells by flow cytometry (UIS4pos). Most of the UIS4pos - infected host cells were EphA2high (Fig. 2E). Similarly, the level of EphA2 expression was higher in UIS4pos-infected cells than in UIS4neg-infected cells (Fig. 2F). Thus, sporozoites not only preferentially entered EphA2high cells, but invasion with PVM formation was far more effective in these cells. UIS4neg-infected hepatocytes suffered a higher frequency of cell death (Fig. 2G).
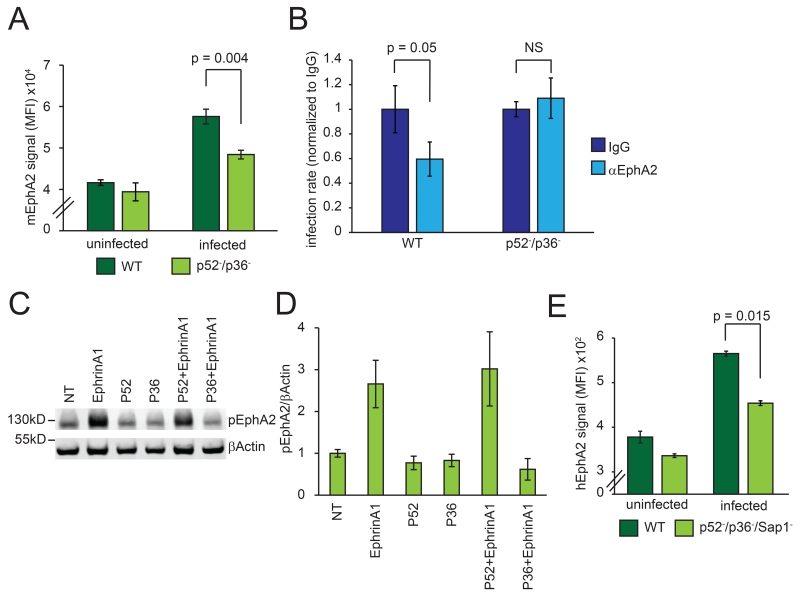
Two members of the parasite protein 6-Cys family, P52 and P36, are expressed in sporozoites and are important for invasion of hepatocytes (15-17) with PVM formation (15). In mouse livers, parasites without P52 or P36 are almost entirely eliminated within 3 h (Fig. S6). We asked if lack of P52 and P36 phenocopies the lack of host EphA2 and found that p52−/p36− P. yoelii sporozoites exhibited a reduced preference for EphA2high cells (Fig. 3A).
Fig. 3.
P36 interacts with EphA2. (A) 2 × 105 WT or p52−/p36− P. yoelii parasites were used to infect Hepa 1-6 cells. Levels of EphA2 were monitored in infected and uninfected cells. (B) P. yoelii sporozoites were used to infect Hepa 1-6 cells after 30 min treatment with IgG or α-EphA2 antibody. Infection levels were normalized to infection rate in the presence of IgG. (C, D) Hepa1-6 cells were incubated with recombinant P52 or P36 alone or in combination with 10 minutes of EphrinA1 treatment. Western blot shows levels of pEphA2 (pY772). (E) 2 × 105 WT or p52−/p36−/sap1− P. falciparum sporozoites were used to infect 6 × 105 HC04 cells. EphA2 levels were measured in infected and uninfected cells. All data represent three independent experiments.
The 6-Cys protein P12 shows structural similarity to the mammalian ligand for EphA2, EphrinA1, while sharing no amino acid sequence similarity (10). We showed that an interaction in the extracellular region of EphA2 was required for sporozoite entry using an EphA2 blocking antibody (Fig. 1K). Therefore, we asked if the presence of P36 and P52 was required for the antibody to block sporozoite entry. While the EphA2 antibody blocked infection with wild-type P. yoelii sporozoites, p52−/p36− sporozoite entry was not impacted (Fig. 3B). These data suggest that P36 or P52 directly engage EphA2 at the point of host cell invasion. We next tested if P52 or P36 could directly impede the interaction between EphrinA1 and EphA2 on the hepatocyte surface, which results in EphA2 activation. When we added EphrinA1 in the presence of P36 to Hepa1-6 cells, P36 blocked activation of EphA2 (Fig. 3C, D). P52 however, did not block EphrinA1-mediated activation of EphA2 (Fig. 3C, D). To assess if the interaction between EphA2 and P36 is conserved in human parasites, we assessed levels of EphA2 in P. falciparum wild-type or P52/P36 deficient parasite-infected HC04 cells. The Pf P52/P36-deficient sporozoites partially lost selectivity for EphA2high HC04 cells when compared to Pf wild-type sporozoites. Thus, P36 engages EphA2, but does not trigger its activation in rodent and human parasites.
We have shown that both host EphA2 and parasite 6-Cys proteins have a role in invasion and establishment of the growth-permissive intracellular niche during sporozoite infection of hepatocytes. Without either component, the parasite can still enter hepatocytes, but does so without a PVM, which can lead to death of the infected hepatocyte. The convergence of infection-permissive phenotypes is best explained by an interaction between parasite P36 and hepatocyte EphA2 at the point of sporozoite invasion when the PVM is formed. This role for EphA2 in hepatocyte infection does not preclude additional critical hepatocyte receptors for infection. Interventional strategies aimed at either EphA2 or sporozoite 6-Cys proteins might block parasite infection before the onset of clinical malaria.
Supplementary Material
Acknowledgments
We are grateful to W. Betz for mosquito and sporozoite production. We thank the Center for Infectious Disease Research vivarium staff for their work with mice. This work was funded by an R01 to SHIK and AK (1R01GM101183-01A1). AK is also a recipient of a Transition to Independence Award (1K99AI111785-01A1) which partially funded this work. AK, NA, AND, VV, ND, HK and LSA performed experiments. AK, DNS and SHIK supervised the research. AK and SHIK wrote the paper.
References and Notes
- 1.Kappe SH, Vaughan AM, Boddey JA, Cowman AF. Science. 2010;328:862–866. doi: 10.1126/science.1184785. [DOI] [PubMed] [Google Scholar]
- 2.Coppi A, et al. Cell host & microbe. 2007;2:316–327. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frevert U, et al. The Journal of experimental medicine. 1993;177:1287–1298. doi: 10.1084/jem.177.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silvie O, et al. Nature medicine. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 5.Yalaoui S, et al. Cell host & microbe. 2008;4:283–292. doi: 10.1016/j.chom.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues CD, et al. Cell host & microbe. 2008;4:271–282. doi: 10.1016/j.chom.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Austin LS, Kaushansky A, Kappe SH. Cellular microbiology. 2014;16:784–795. doi: 10.1111/cmi.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaushansky A, et al. Infection and immunity. 2014 [Google Scholar]
- 9.Lisabeth EM, Falivelli G, Pasquale EB. Cold Spring Harbor perspectives in biology. 2013;5 doi: 10.1101/cshperspect.a009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arredondo SA, et al. Proc Natl Acad Sci U S A. 2012;109:6692–6697. doi: 10.1073/pnas.1204363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller AK, et al. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser K, Camargo N, Kappe SH. The Journal of experimental medicine. 2003;197:1045–1050. doi: 10.1084/jem.20022100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Annoura T, et al. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:2158–2170. doi: 10.1096/fj.13-241570. [DOI] [PubMed] [Google Scholar]
- 14.van Dijk MR, et al. PLoS pathogens. 2010;6:e1000853. doi: 10.1371/journal.ppat.1000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labaied M, et al. Infection and immunity. 2007;75:3758–3768. doi: 10.1128/IAI.00225-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dijk MR, et al. Proc Natl Acad Sci U S A. 2005;102:12194–12199. doi: 10.1073/pnas.0500925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.VanBuskirk KM, et al. Proc Natl Acad Sci U S A. 2009;106:13004–13009. doi: 10.1073/pnas.0906387106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.