Abstract
The immunosuppressive effects of glucocorticoids (GCs) are well-established. However, whether the net effect of GC-elicited alterations in immune function is sufficient to influence a clinically relevant outcome in healthy adults has yet to be shown. The aim of the present study was to investigate whether inter-individual differences in basal salivary cortisol production are associated with increased risk and severity of infection and subsequent illness following experimental exposure to a virus that causes the common cold. The present analyses combine archival data from three viral-challenge studies. Participants were 608 healthy adults, aged 18 to 55 years (49.2% female; 65.8% white), who each completed a three-day saliva collection protocol; was subsequently exposed to a virus that causes the common cold; and monitored for 5 days for objective signs of infection (presence of challenge virus in nasal secretions) and clinical illness (mucus weight, mucociliary clearance time). Basal cortisol production (operationalized as the calculated area-under-the-curve averaged across the 3 days) showed a graded association with infection risk, with those producing higher levels of cortisol being at greater risk. Cortisol also showed a continuous association with duration of viral shedding, an indicator of viral replication and continuing infection, such that higher cortisol concentrations predicted more days of shedding. Cortisol was not, however, related to severity of objective illness. These findings are the first to demonstrate in healthy adults an association between basal cortisol production and an objectively measured and clinically relevant infectious disease outcome.
Keywords: Common Cold Project, HPA, salivary cortisol, viral challenge
1. Introduction
Chronic dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis has been implicated in the pathogenesis of various forms of immune-related disease. Findings from comparative animal research, as well as from human research conducted in vitro, suggest that stress-related elevations in circulating glucocorticoid (GC) hormones may suppress host resistance to infectious disease via downregulatory effects on both innate and adaptive immunity (Bailey et al., 2003; Sheridan et al., 1998). Effects of chronically elevated GCs on natural immunity include inhibition of lymphocyte trafficking during the initial phases of viral infection (e.g., Hermann, Beck, & Sheridan, 1995; Tseng et al., 2005) as well as suppressed synthesis of pro-inflammatory cytokines by macrophages (Brattsand & Linden, 1996). In regard to acquired immunity, GCs have been shown to have modulatory effects on T-helper (Th) activity, simultaneously facilitating a Th2 pattern of cytokine production while inhibiting the production of Th1 cytokines (Elenkov & Chrousos, 1999). One consequence of this shift to a predominantly humoral immune response is attenuation of cell-mediated defense against viral infection (Gern et al., 2000).
The clinical relevance of these in vitro and ex vivo findings from comparative laboratory research has been demonstrated in populations of patients with pathologically elevated levels of circulating GCs. In these populations, hypercortisolemia—whether induced endogenously (i.e., spontaneous Cushing syndrome) or from exogenous administration of therapeutic corticosteroids—is associated with increased risk for infection by opportunistic pathogens (Lionakis & Kontoyiannis, 2003; Sarlis et al., 2000; Stuck et al., 1989). Furthermore, the severity of established infections has been found to increase in a dose-response manner with the degree of hypercortisolemia (Aucott, 1994; Graham & Tucker, 1984). Even relatively short-term exposure to corticosteroids (days rather than weeks, months, or years) has been found to influence the course of upper respiratory virus infection among otherwise healthy adults. Specifically, in a sample of individuals who were experimentally exposed to an upper respiratory virus (rhinovirus), those treated with prophylactic corticosteroids (prednisone) evidenced higher post-challenge viral titers than placebo-treated controls (Gustafson et al., 1996). Paradoxically, however, prophylactic treatment with corticosteroids was unrelated to the overall severity of subsequent upper respiratory illness (Farr et al., 1990; Gustafson et al., 1996).
Importantly, the aforementioned effects of circulating GCs on risk for infectious disease in vivo have been observed among individuals with conditions characterized by supraphysiologic systemic GC concentrations. Moreover, in the case of exogenous corticosteroid therapy, GCs may be present in circulation, and thus in contact with cells of the immune system, for abnormally long periods of time with the extended duration of contact permitting GCs to have a greater immunomodulatory effect (Spencer et al., 2011).
Whether inter-individual variation in endogenous cortisol in non-patient samples likewise affects host resistance to infectious disease has yet to be determined. That changes in circulating cortisol is associated with corresponding alterations in various measures of immunity already has been established. For example, within persons, the number of lymphocytes in the peripheral circulation of healthy adults has been found to vary inversely with normal diurnal fluctuations in plasma cortisol (Eskola et al., 1976; Thomson et al., 1980). In vitro assessments of functional immunity show a diurnal profile, as well, with proliferative response of lymphocytes to mitogen stimulation (Eskola et al., 1976; Hiemke et al., 1995) and stimulated cytokine production (Petrovsky et al., 1998) both reaching a peak later in the day when endogenous levels of cortisol are at their lowest. Between persons, higher concentrations of circulating cortisol have been shown to correlate with lower numbers of circulating lymphocytes and a higher neutrophil-to-lymphocyte ratio (Cole, 2008; Cohen et al., 2012).
The present study combines archived data from three large viral-challenge studies to examine whether inter-individual variation in basal cortisol production—i.e., daily levels not produced in response to a physiological (disease) or psychological stressor—is associated with risk for upper respiratory infection following exposure to an experimentally administered virus that causes a common cold-like illness. Given the findings of previous research, we expect that risk of infection will increase with increasing basal cortisol production. We further expect that the duration of the infection, as indicated by the number of days of viral shedding will increase with increasing cortisol. Due to the inconsistent findings regarding the relation of exogenous corticosteroids with clinical disease severity, we make no prediction with respect to the association of basal cortisol levels with objectively measured signs of upper respiratory illness. Given that hallmark physiological indicators of the common cold (e.g., nasal mucus production, mucosal edema) are thought to result largely from the host’s pro-inflammatory response to the invading virus (Proud, 2008; Turner, 1997), we also will explore the relation of basal cortisol production with local (nasal) pro-inflammatory cytokine response to infection.
2. Methods
2.1. Participants
The present analyses combine archival data from three viral-challenge studies conducted from 1997 to 2001 (Pittsburgh Cold Study 2 [PCS2]), 2000 to 2004 (Pittsburgh Mind-Body Center Study [PMBC]) and from 2007 to 2011 (Pittsburgh Cold Study 3 [PCS3]), respectively. (These and additional data are available at www.CommonColdProject.com). The studies followed a common set of procedures which included a physical exam; blood and urine screenings; questionnaire assessments of demographics; 3 days of saliva collection for assessment of diurnal cortisol; and subsequent participation in a viral-challenge trial. The total sample included 702 healthy adults, aged 18 to 55 years who were recruited from the Pittsburgh, PA metropolitan area via newspaper advertisements and community postings. Four participants were excluded from the present analyses due to missing data on relevant covariates; 47 for missing data on saliva cortisol; and 43 for missing data on viral shedding (see below). The remaining sample was comprised of 608 participants, with a mean age of 30.98 ± 10.84 years. Additional characteristics of the sample are reported in Table 1. Excluded participants did not differ from the present sample in terms of average age (mean difference = 0.63 years, t[700] = 0.53, p = .597), proportions of men and women (44.7% female vs. 49.3% female, X2[1] = 0.71, p = .400), and years of educational attainment (mean difference = −0.06 years, t[700] = −0.30, p = .767). Excluded participants were, however, less likely than those in the present sample to self-identify as white/Caucasian (54.3% vs. 65.8%, X2[1] = 4.72, p = .030). All participants provided informed consent and received financial compensation for study participation. Study procedures were approved by the institutional review boards of the University of Pittsburgh and Carnegie Mellon University.
Table 1.
Sample Characteristics
| Variable | N | % |
|---|---|---|
| Sex | ||
| Male | 309 | 50.8 |
| Female | 299 | 49.2 |
| Race | ||
| White | 400 | 65.8 |
| Black | 181 | 29.8 |
| Other | 27 | 4.4 |
| Study | ||
| PCS2 | 299 | 49.2 |
| PMBC | 119 | 19.6 |
| PCS3 | 190 | 31.3 |
| Virus type | ||
| RV23 | 101 | 16.6 |
| RV39 | 507 | 83.4 |
| Pre-challenge Ab | ||
| < 4 | 378 | 62.2 |
| ≥4 | 230 | 37.8 |
| Season | ||
| Winter | 110 | 18.1 |
| Spring | 267 | 43.9 |
| Summer | 115 | 18.9 |
| Fall | 116 | 19.1 |
| Education | ||
| High school or less | 167 | 27.5 |
| <2 years college, no degree | 185 | 30.4 |
| ≥2 years college + degree | 119 | 19.6 |
| BA/BS or higher degree | 137 | 22.5 |
2.2. Procedures
2.2.1. Screening
Volunteers were included for participation if they were in “good general health”, as determined by medical history and physical examination. They were excluded from study eligibility if they had diabetes, hepatitis, cardiovascular disease, chronic sinusitis, chronic bronchitis, asthma or any other chronic illness; abnormal clinical profiles discovered via urinalysis, complete blood count, or analysis of blood chemistry; previous hospitalization for flu-like illness; used steroids or immunosuppressants within the last 3 months; major nasal or otologic surgery; psychiatric disorder treated in the last year or psychiatric hospitalization in the last 5 years; were pregnant or currently lactating; seropositive for human immunodeficiency virus (HIV); on regular medication (except birth control); had a cold- or flu-like illness within 3 months of viral-challenge; reported any current acute illness; or were living with someone with a compromised immune system or chronic obstructive pulmonary disease (PCS3 only). Baseline immunity to the challenge virus (viral specific antibody titers), demographics, and anthropometrics were also assessed at screening.
2.2.2. Quarantine
Participants were subsequently quarantined in separate rooms for 6 days (baseline [Day 0] and 5 post-challenge days). On Day 0 and prior to viral exposure, participants received an ear, nose, and throat examination, and provided a nasal wash specimen that was cultured for existing viral infection. Baseline objective measures of congestion (nasal mucociliary clearance time) and nasal mucus production were assessed. Volunteers were excluded from participation at this point if they reported having a cold or cold-like symptoms, or retrospectively if a viral pathogen was later isolated from the baseline nasal wash.
After collection of Day 0 baseline data, participants were given nasal drops containing 100–300 50% tissue culture infective dose (TCID50) of either rhinovirus (RV) 39 (n = 507) or RV23 (n = 101; PCS2 only), two viruses that cause a common cold-like illness. Nasal clearance function and mucus production were again assessed on each of the 5 post-challenge days, and daily nasal wash samples were collected for virus culture. Approximately 28 days post-challenge, blood was collected to assay for antibody to the challenge virus. On-site investigators were blinded to all questionnaire and biological measures.
2.2.3. Saliva Collection
Serial saliva samples were collected from subjects on each of three days: two nonconsecutive days occurring 1 to 6 weeks prior to quarantine and on the baseline day in quarantine (Day 0). Both days of pre-quarantine collections took place in participants’ natural environments and while engaging in their usual daily activities. Participants were provided with Salivette ® (Sarstedt, Rommelsdorft, Germany) devices to collect samples of their saliva. The Salivette is comprised of a roll of cotton contained within a specially designed collection tube. When collecting a sample, participants placed the cotton roll in their mouths, chewed on it until it became saturated, replaced it into the inner vial of the Salivette, and then tightly capped the outer tube. Participants were instructed not to eat or brush their teeth for one hour before the scheduled saliva collection time, and to abstain from smoking for 30 minutes before collection time. Participants in PCS2 collected a total of 11 samples during each of the 2 pre-quarantine days and 14 samples on Day 0, whereas participants in PMBC and PCS3 collected 7 samples on each pre-quarantine day and 8 samples on Day 0. (For details on the sampling schedules for each study, see www.commoncoldproject.com/measures-by-study/biological-pathways/salivary-cortisol-pcs2-pmbc-pcs3.html.)
2.3. Measures
2.3.1. Basal cortisol production
PCS2 and PCS3
Cortisol assays were performed by the laboratory of Dr. Clemens Kirschbaum, Dresden, Germany. Cortisol levels in saliva were determined by time-resolved fluorescence immunoassay with a cortisol-biotin conjugate as a tracer1 (Dressendörfer et al., 1992). Intra- and inter-assay variabilities were each less than 12%.
PMBC
Cortisol assays were performed by the Immunologic Monitoring and Cellular Products Laboratory (IMCPL) at the University of Pittsburgh Cancer Institute. Cortisol levels in saliva were determined using a competitive enzyme-linked immunosorbant assay (ELISA) procedure (Salimetrics, State College, PA). Reliability estimates for the salivary cortisol ELISA were established by examining curve-fitting for standard values and retesting previously run or known samples. These checks were conducted periodically, approximately every other month. Standard curves were good, and correlations between re-run samples and original values all exceeded .96. Average deviations between individual pairs of replicates were 4%.
There were no shared samples or standards available to compare results of the fluorescence immunoassay vs. those obtained by ELISA. Previous research, however, has shown the results derived from the two methods to correlate well with one another (r = .95; Dressendörfer et al., 1992).
Cortisol area under the curve (AUC) was computed for each of the 3 collection days using the trapezoid rule (Pruessner et al., 2003). For PMBC and PCS3, pre-challenge cortisol AUC values were computed using all non-missing samples, and Day 0 AUC values were computed using all non-missing samples excluding the wake-up sample. For PCS2, cortisol AUC values were computed using non-missing data from the 7 samples collected on each day that corresponded to the schedule of PMBC and PCS3 saliva sampling (see www.commoncoldproject.com/combining-the-5-studies/variable-modifications.html). For all 3 studies, daily AUC values were computed for participants who were not missing (a) any of the first 3 post-waking samples of the day or (b) more than 2 of the last 4 samples. To be included in the computation, cortisol samples were required to have been collected within 45 minutes of the scheduled collection time. To create a reliable measure of basal cortisol production, AUC values were averaged for all participants with data for at least 2 of the three collection days and then a base 10 logarithm was applied to approximate a normal distribution.
2.3.2. Local (nasal) pro-inflammatory cytokine production
In PCS2 and PCS3, nasal wash fluid was assayed (in duplicate) for interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (PCS3 only) using a commercially available ELISA (Endogen) as per the manufacturer’s instructions. In PMBC, nasal wash samples were assayed for IL-1β, IL-6, and TNF-α the BioSource Ten-plex Bead Immunoassay and methods provided by the manufacturer (BioSource International, Camarillo, CA). There were no shared samples or standards available to compare results of the bead immunoassay vs. those obtained by ELISA. Previous research, however, has shown the results derived from the two methods to correlate well with one another (r = .84–.94; Elshal & McCoy, 2006; Khan, Smith, Reda, Suffredini, & McCoy, 2004).
Daily adjusted nasal cytokine scores were created by subtracting the measurement obtained from the baseline (pre-challenge) day in quarantine from the measurements obtained from each of the five post-challenge days. Adjusted post-challenge nasal cytokine production was operationalized as the AUC (trapezoid method) computed using the 5 adjusted post-challenge scores. Because AUC values for each cytokine were skewed, a base 10 logarithm was applied to approximate a normal distribution.
2.3.3. Infection, total days of viral shedding, and objective markers of clinical illness
Infection was defined as recovery of the challenge virus in nasal lavage samples (cultured using standard procedures; Gwaltney et al., 1989) on any of the 5 post-challenge days (recovered virus criterion) or a 4-fold or greater rise in virus-specific serum neutralizing antibody titer from pre-exposure to 28-days post-exposure (seroconversion criterion) (Cohen et al., 1997; Gwaltney et al., 1989).
Duration of viral shedding was operationalized as the count of post-challenge days on which virus was recovered from participants’ nasal secretions.
Clinical illness severity
Two objective biological measures were used to assess clinical illness severity: nasal mucus production (weight) and nasal mucociliary clearance function. Mucus production was assessed during each day in quarantine by collecting used tissues in sealed plastic bags, weighing the bags, and then subtracting the pre-use weights of the tissues and bags from the measured total (Doyle et al., 1988). Mucociliary clearance function was assessed as the time required for a solution administered into the anterior nose to reach the nasopharynx (Doyle et al., 1988). Baseline-adjusted daily scores for each measure were calculated by subtracting the appropriate baseline score from each of the 5 post-exposure daily scores, with negative values being re-scored to 0. Aggregate scores were created by taking the sum and average, respectively, of adjusted daily mucus weights and nasal clearance times across the 5 post-challenge days. Both measures were examined as continuous variables, with a base 10 logarithm being applied to normalize their respective distributions.
2.3.4. Standard control variables
Five of the nine covariates were collected at screening: age (continuous), sex (female/male), race (dichotomized as white/other; see Table 1), education (represented as 3 dummy-coded variables [bachelor’s degree or higher as reference]: high school or less, some college, and ≥2 years of college with degree), and body mass index (BMI; continuous weight [kg]/height [m]2). The remaining four control variables were season (represented as 3 dummy-coded variables [spring as reference]: winter, summer, and fall) at viral-challenge, Study (represented as 2 dummy-coded variables [PCS3 as reference]: PCS2 and PMBC), virus type (RV23/RV39) and viral-specific immunity (pre-exposure specific antibody to the challenge virus ≥4/<4).
2.4. Data analysis
The data were analyzed using IBM SPSS Statistics software, version 21, and figures were generated using Microsoft Excel 2010. Descriptive statistics are presented as means and standard deviations (SD) or as percentages. Binary multiple logistic regression was used to assess the association of diurnal salivary cortisol with the dichotomous outcomes (infected status and clinical cold), and Poisson regression with a log link function was used to assess the relation of cortisol to the total number of days of post-challenge viral shedding. Multiple linear regression analysis was used to examine the association of salivary cortisol with the two measures of illness severity. Initial analyses were conducted controlling only for virus type and pre-exposure antibody to the challenge virus; main analyses included all nine of the standard control variables described above. All tests were two-tailed, with the criterion for statistical significance being set at p < .05.
3. Results
3.1. Association of salivary cortisol with infected status
Nearly three-quarters of the sample (n = 450, 74.0%) met the seroconversion criterion for infection in that they showed a four-fold increase in antibody to the challenge virus from pre- to 28 days post-exposure. Ninety-two percent (n = 415) of these participants also met the recovered virus criterion for infection—i.e., challenge virus was recovered from at least one of the participants’ five post-challenge nasal wash samples (median = 3, range = 0 to 5). Irrespective of which criterion was used to define infection, the risk for becoming infected with the challenge virus increased with increasing cortisol AUC (see Table 2).
Table 2.
Multivariate associations of daily salivary cortisol AUC with odds of becoming infected with the challenge virus, with infection determined by seroconversion and recovered virus criteria (n = 608).
| Seroconversion Criterion
|
Recovered Virus Criterion
|
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | p | |
| Model 1 | 2.98 | 1.11, 8.03 | .030 | 2.64 | 1.06, 6.57 | .037 |
| Model 2 | 4.14 | 1.17, 14.71 | .028 | 5.03 | 1.58, 16.04 | .006 |
Model 1 covariates: virus type and pre-challenge antibody to the challenge virus
Model 2 covariates: Model 1 + study, age, sex, race, season, body mass index, and educational attainment
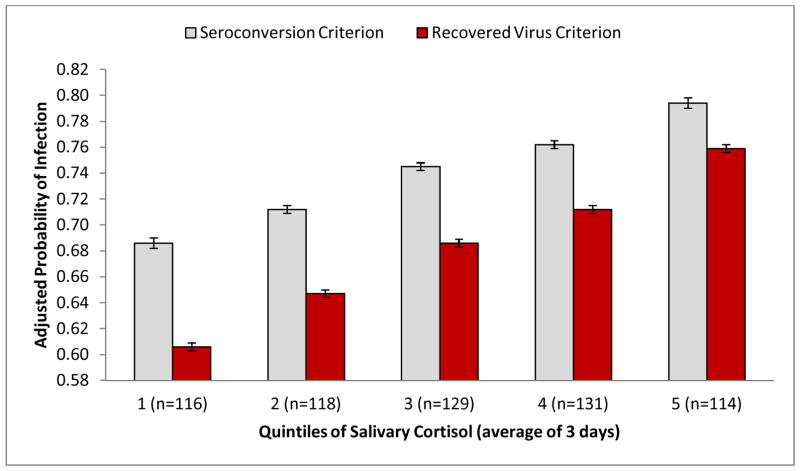
Figure 1 displays the adjusted probability of becoming infected with the challenge virus by quintile of cortisol AUC. As indicated by the Figure, cortisol shows a graded association with risk of infection, with risk increasing with each 20% increase in diurnal cortisol production.
Figure 1.
Adjusted probability of meeting criteria for infection with challenge virus by quintile of salivary cortisol production (3-day average area-under-the-curve; n = 608). Values adjusted for age, sex, race, study, virus type, pre-challenge antibody, body mass index, season, and educational attainment. Bars represent the adjusted probability of meeting the infection criterion derived from multivariate logistic regression; error bars represent the standard error of the probability. Cortisol AUC ranges (nmol/ml) by quintile: 1 = .69–3.94; 2 = 3.98–5.24; 3 = 5.26–6.59; 4 = 6.63–8.11; 5 = 8.15–37.37.
3.2. Association of salivary cortisol with duration of viral shedding
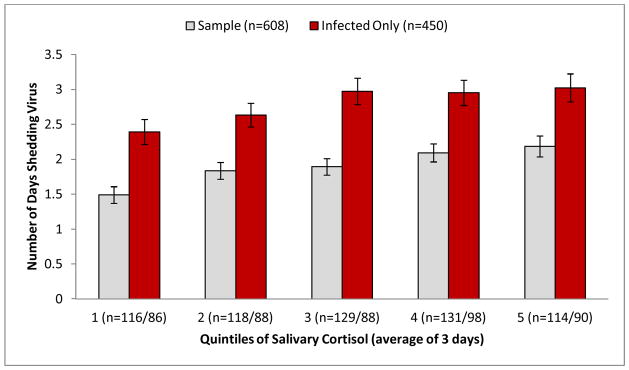
To determine whether the association of salivary cortisol with viral replication is limited to initial susceptibility to infection or can be extended to duration of viral survival, cortisol AUC was examined as a predictor of the number of days of viral shedding. Results of Poisson regression that controlled for virus type and pre-exposure antibody revealed a positive association between daily cortisol production and duration of viral shedding (B = 0.35, 95% CI = 0.09, 0.61, p = .008, n = 608). This association was maintained when the seven additional standard control variables were included in the model (B = 0.54, 95% CI = 0.24, 0.83, p = .001, n = 608). To confirm that the association of cortisol production with duration of viral shedding is independent of the association of cortisol with infected status, the fully-adjusted analysis was conducted a second time, with the sample limited to those who met either of the two criteria for infection. Results of this analysis were similar to those derived from the entire sample (B = 0.34, 95% CI = 0.04, 0.63, p = .024, n = 450). Figure 2 displays the average number of days shedding virus by quintile of salivary cortisol production.
Figure 2.
Average number of days shedding virus by quintile of salivary cortisol production (area-under-the-curve [AUC]) for the entire sample (n = 608) and for the infected subset (n = 450). Bars represent the estimated mean values derived from multivariable Poisson regression; error bars represent the standard errors of the derived means. Values adjusted for age, sex, race, study, virus type, pre-challenge antibody, body mass index, season, and educational attainment. Cortisol AUC ranges (nmol/ml) by quintile: 1 = .69–3.94; 2 = 3.98–5.24; 3 = 5.26–6.59; 4 = 6.63–8.11; 5 = 8.15–37.37
3.3. Association of salivary cortisol with illness severity in infected participants
As expected, duration of viral shedding was correlated with both objective markers of illness. Specifically, more days of shedding was associated with greater mucus production (r = .35, p = .001, n = 450) and slower nasal clearance (r = .12, p = .009, n = 450). However, as indicated by Table 3, the relation of cortisol with duration of viral shedding did not extend to severity of clinical illness.
Table 3.
Multivariate associations of daily salivary cortisol AUC with two objective markers of upper respiratory illness severity. Infected only (n = 450).
| log10 Mucus Weight (g)
|
log10 Nasal Clearance Time (min)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| B | 95% CI | β | p | B | 95% CI | β | p | |
| Model 1 | −0.04 | −0.31, 0.23 | −0.01 | .768 | −0.05 | −0.21, 0.11 | −0.03 | .543 |
| Model 2 | 0.12 | −0.18, 0.43 | 0.04 | .437 | −0.12 | −0.31, 0.07 | −0.07 | .220 |
Model 1 covariates: virus type and pre-challenge antibody to the challenge virus
Model 2 covariates: Model 1 + study, age, sex, race, season, body mass index, and educational attainment
3.4. Exploratory analysis: Association of salivary cortisol with local inflammatory response to infection with the challenge virus
Complete data on nasal pro-inflammatory cytokine production were available for a subset of participants (IL-6 and IL-1β, n = 578; TNF-α, n = 293). Among the infected subsample, basal salivary cortisol was inversely correlated with productions of local IL-6 (r = −.22, p < .001, n = 426) and TNF-α (r = −.14, p = .040, n = 231), but was unrelated to IL-1β (r = −.02, p = .736, n = 425). When examined with multivariate linear regression that included the standard covariates, however, the associations of cortisol with IL-6 and TNF-α were reduced to nonsignificance (ps > .420).
4. Discussion
Glucocorticoids (GCs) are known to have a suppressive effect on immunity via the inhibition of lymphocyte proliferation and trafficking, attenuation of cytotoxic activity, suppression of pro-inflammatory cytokine expression, and facilitation of anti-inflammatory cytokine release (Elenkov & Chrousos, 1999). However, whether the net effect of these GC-elicited alterations in immune function is sufficient to influence a clinically relevant outcome in humans heretofore has not been established.
The present study attempted to fill this gap in the literature by investigating whether inter-individual differences in basal salivary cortisol concentrations are associated with increased risk and severity of infection following experimental exposure to a virus that causes the common cold. Consistent with expectations, daily cortisol levels showed a graded association with risk for infection, with those demonstrating higher levels of cortisol being at greater risk. Cortisol also showed a continuous association with duration of viral shedding, an indicator of viral replication and continuing infection, such that higher salivary cortisol concentrations predicted more days of shedding.
Our findings in regard to rhinovirus infection are consistent with previous research showing an increased risk of infectious disease among individuals in endogenous or exogenously-induced hypercortisolemic states (Lionakis & Kontoyiannis, 2003; Sarlis et al., 2000; Stuck et al., 1989). They are also consistent with the experimental data showing increased post-challenge viral titers among individuals treated with prophylactic prednisone relative to placebo-treated controls (Gustafson et al., 1996). Importantly, the findings of the present study further expand upon this earlier research by being, to our knowledge, the first study of healthy adults to demonstrate an association between inter-individual variability in daily cortisol levels and an objectively measured and clinically relevant infectious disease outcome.
Despite its association with initial risk for infection and duration of viral shedding, basal cortisol concentration was unrelated to the severity of objective illness signs (nasal mucociliary clearance time and nasal mucus weights) among infected participants. This finding replicates those from the previously described research examining the efficacy of prophylactic corticosteroids with respect to rhinovirus-induced upper respiratory illness. Specifically, while corticosteroid treatment was associated with more persistent infection with rhinovirus (Gustafson et al., 1996), exogenous corticosteroids were not reliably related to objective signs of upper respiratory illness (Farr et al., 1990; Gustafson et al., 1996).
Though consistent with each other, the present findings and those of the aforementioned experimental studies contrast with the findings from research conducted among individuals with spontaneous or ectopically-induced Cushing syndrome. Results from these studies suggest that both risk for infection and clinical severity of subsequent disease increase with increasing hypercortisolemia (e.g., Aucott, 1994; Graham & Tucker, 1984). In most cases, however, the opportunistic infections being studied were of mycotic or bacterial origin. By comparison, the pathogen of interest to the present study was an upper respiratory virus (rhinovirus) that causes a common cold-like illness. In contrast to infection with a fungal pathogen such as Aspergillus which causes extensive local cytopathology, rhinoviruses seldom cause direct damage to the upper respiratory endothelium (Jacobs et al., 2013). Rather, the common cold syndrome results from the host’s primarily pro-inflammatory response to the invading rhinovirus (Proud, 2008; Turner, 1997). Thus, the predominantly anti-inflammatory effects of GC hormones that permit continuing damage by Aspergillus or other mycobacterial organisms may attenuate progression of cytokine-driven illness signs such as nasal mucus production and nasal mucosal edema.
We tested the above hypothesis by examining the correlation of salivary cortisol with local pro-inflammatory cytokine production. Though the zero-order correlations of cortisol with IL-6 and TNF-α were consistent with an anti-inflammatory effect, fully-controlled regression analyses showed no associations. This may be due to a subset of the participants in the present sample displaying some degree of glucocorticoid receptor resistance (GCR; Cohen et al., 2012). GCR is thought to explain the lack of association between plasma cortisol and circulating lymphocytes that is evident among individuals undergoing persistent social or life event stress (Cole, 2008; Cohen et al., 2012). As life event stress was not measured consistently across the three studies included in the present analyses, we were unable to determine whether differential stress exposure among participants might explain the lack of association between cortisol and local cytokine production.
Because of the prospective viral challenge design of the study, we were able to rule out reverse causation as an alternative explanation for our findings. Cortisol was measured on 3 occasions prior to participants being exposed to the challenge virus—two days approximately one month before quarantine and on the first day of quarantine, prior to viral challenge—with the resulting AUC values being averaged across the three days to create a relatively stable measure of basal cortisol production. Thus, being infected with the challenge virus could not have stimulated production of excess cortisol. That this potential explanation can be excluded is important as viral challenge has been found to stimulate HPA activity and alter cortisol secretion patterns (Haus & Smolensky, 1999; Spencer et al., 2011). Still, though the present study controlled for a range of relevant factors related both to cortisol production and infectious disease risk, the possibility remains that an unaccounted for third factor may underlie both salivary cortisol production and susceptibility to infection with an upper respiratory viral pathogen. For example, despite our finding only a weak association between salivary cortisol AUC and local (i.e., nasal mucosa) pro-inflammatory cytokine response to viral challenge, the presence of chronic, low-grade systemic inflammation could potentially explain some of the variability in basal cortisol production found in our sample as well as lead to an attenuation of adaptive immune response. However, as participants were screened to be in good general health, this explanation is unlikely.
5. Conclusion
The findings of the present study suggest that inter-individual differences in basal cortisol production may influence the likelihood and duration of infection with an upper respiratory pathogen. Importantly, the study was conducted in a healthy, non-patient population. These results must be interpreted cautiously, however, because they are limited to infection with an upper respiratory virus, specifically 2 types of rhinovirus. Accordingly, we cannot generalize the associations found here to mycobacterial or other types of viral infection. Nevertheless, rhinoviruses are the most frequent cause of the common cold illness and have been linked to exacerbations of chronic respiratory diseases (Jacobs et al 2013). Thus, the importance of understanding factors that influence the likelihood of becoming infected with this upper respiratory viral pathogen should not be minimized.
Highlights.
Risk for upper respiratory viral infection increases with increasing basal cortisol.
Higher basal cortisol is associated with more days of viral shedding.
Basal cortisol is unrelated to severity of clinical illness subsequent to infection.
Acknowledgments
We are grateful to Andrew Baum, Frank Jenkins, Clemens Kirschbaum, Ellen Conser, Janet Schlarb, and James Seroky for their contributions to this research. The data used for this article were collected by the Laboratory for the Study of Stress, Immunity, and Disease at Carnegie Mellon University under the directorship of Sheldon Cohen, PhD; and were accessed via the Common Cold Project (CCP) website (www.commoncoldproject.com). CCP data are made publically available through a grant from the National Center for Complementary and Integrative Health (AT006694). The conduct of the studies was supported by grants from the National Institute of Mental Health (PCS2, MH50429); National Heart, Lung, and Blood Institute (PMBC, HL65111 and HL65112); and the National Institute of Allergy and Infectious Disease (PCS3, AI066367). Secondary support was provided by grants from the National Institutes of Health to the University of Pittsburgh Clinical and Translational Science Institute (PCS2, RR00056; PCS3, RR024153 and TR000005) and from the National Cancer Institute to the University of Pittsburgh Cancer Institute (PMBC, P30CA047904). Supplemental funding for the PMBC study was provided by the John D. and Catherine T. MacArthur Foundation Research Network on Socioeconomic Status & Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aucott JN. Glucocorticoids and infection. Endocrinol Metab Clin North Am. 1994;23:655–670. [PubMed] [Google Scholar]
- Bailey M, Engler H, Hunzeker J, Sheridan JF. The hypothalamic-pituitary-adrenal axis and viral infection. Viral Immun. 2003;16(2):141–157. doi: 10.1089/088282403322017884. [DOI] [PubMed] [Google Scholar]
- Brattsand R, Linden M. Cytokine modulation by glucocorticoids: Mechanisms and actions in cellular studies. Aliment Pharmacol Ther. 1996;10:81–90. doi: 10.1046/j.1365-2036.1996.22164025.x. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. JAMA. 1997;277:1940–1944. doi: 10.1001/jama.277.24.1940. [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle WJ, McBride TP, Swarts JD, Hayden FG, Gwaltney JM. The response of the nasal airway, middle ear, and eustachian tube to experimental rhinovirus infection. Am J Rhinol. 1988;2(4):149–154. doi: 10.2500/105065888781692961. [DOI] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. J Steroid Biochem Mol Biol. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-S. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10(9):359–368. doi: 10.1016/S1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskola J, Frey H, Molnar G, Soppi E. Biological rhythm of cell-mediated immunity in man. Clin Exp Immunol. 1976;26(2):253. [PMC free article] [PubMed] [Google Scholar]
- Farr BM, Gwaltney JM, Hendley JO, Hayden FG, Naclerio RM, McBride T, Doyle WJ, Sorrentino JV, Riker DK, Proud D. A randomized controlled trial of glucocorticoid prophylaxis against experimental rhinovirus infection. J Infect Dis. 1990;162(5):1173–1177. doi: 10.1093/infdis/162.5.1173. [DOI] [PubMed] [Google Scholar]
- Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162(6):2226–2231. doi: 10.1164/ajrccm.162.6.2003019. [DOI] [PubMed] [Google Scholar]
- Graham BS, Tucker WS. Opportunistic infections in endogenous Cushing’s syndrome. Ann Intern Med. 1984;101:334–338. doi: 10.7326/0003-4819-101-3-334. [DOI] [PubMed] [Google Scholar]
- Gustafson LM, Proud D, Hendley JO, Hayden FG, Gwaltney JM. Oral prednisone therapy in experimental rhinovirus infections. J Allergy Clin Immunol. 1996;97(4):1009–1014. doi: 10.1016/S0091-6749(96)80077-7. [DOI] [PubMed] [Google Scholar]
- Gwaltney JM, Colonno RJ, Hamparian VV, Turner RB. Rhinovirus. In: Schmidt NJ, Emmons RW, editors. Diagnostic procedures for viral, rickettsial, and chlamydial infections. American Public Health Association; Washington, DC: 1989. pp. 579–614. [Google Scholar]
- Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 1999;16(5):581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- Hiemke C, Brunner R, Hammes E, Müller H, Zum Büschenfelde KHM, Lohse AW. Circadian variations in antigen-specific proliferation of human T lymphocytes and correlation to cortisol production. Psychoneuroendocrinology. 1995;20(3):335–342. doi: 10.1016/0306-4530(94)00064-H. [DOI] [PubMed] [Google Scholar]
- Hermann G, Beck FM, Sheridan JF. Stress-induced glucocorticoid response modulates mononuclear cell trafficking during an experimental influenza viral infection. J Neuroimmunol. 1995;56(2):179–186. doi: 10.1016/0165-5728(94)00145-E. [DOI] [PubMed] [Google Scholar]
- Jacobs SE, Lamson DM, George KS, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev. 2013;26(1):135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP. Multiplex bead array assays for detection of soluble cytokines: Comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin Cytom. 2004;61(1):35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362:9398, 1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- Petrovsky N, McNair P, Harrison LC. Diurnal rhythms of pro-inflammatory cytokines: Regulation by plasma cortisol and therapeutic implications. Cytokine. 1998;10(4):307–312. doi: 10.1006/cyto.1997.0289. [DOI] [PubMed] [Google Scholar]
- Proud D. Upper airway viral infections. Pulm Pharmacol Ther. 2008;21:468–473. doi: 10.1016/j.pupt.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28(7):916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin 1. J Clin Endocrinol Metab. 2000;85(1):42–47. doi: 10.1210/jcem.85.1.6294. doi: http://dx.doi.org/10.1210/jcem.85.1.6294. [DOI] [PubMed] [Google Scholar]
- Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann NY Acad Sci. 1998;840(1):803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RL, Kalman BA, Dhabhar FS. Role of endogenous glucocorticoids in immune system function: Regulation and counterregulation. Compr Physiol. 2011:381–423. doi: 10.1002/cphy.cp070418. [DOI] [Google Scholar]
- Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954–963. doi: 10.1093/clinids/11.6.954. [DOI] [PubMed] [Google Scholar]
- Thomson SP, McMahon LJ, Nugent CA. Endogenous cortisol: A regulator of the number of lymphocytes in peripheral blood. Clin Immunol Immunopathol. 1980;17(4):506–514. doi: 10.1016/0090-1229(80)90146-4. [DOI] [PubMed] [Google Scholar]
- Tseng RJ, Padgett DA, Dhabhar FS, Engler H, Sheridan JF. Stress-induced modulation of NK activity during influenza viral infection: Role of glucocorticoids and opioids. Brain Behav Immun. 2005;19(2):153–164. doi: 10.1016/j.bbi.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Turner RB. The epidemiology, pathogenesis, and treatment of the common cold. Ann Allergy Asthma Immunol. 1997;78:531–539. doi: 10.1016/S1045-1870(05)80052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]