Abstract
Peritoneal dissemination is the most frequent metastasis in gastric cancer and is associated with poor prognosis. The lack of particular target antigens in gastric cancer other than human epidermal growth factor receptor 2 (HER2) has hampered the development of treatments for peritoneal dissemination of gastric cancer. We hypothesized that HER2-extracellular domain (HER2-ECD) gene transduction combined with trastuzumab-based photoimmunotherapy (PIT) might provide excellent and selective anti-tumor effects for peritoneal dissemination of gastric cancer. In vitro, adenovirus/HER2-ECD (Ad/HER2-ECD) efficiently transduced HER2-ECD into HER2-negative gastric cancer cells. Trastuzumab-IR700 (Tra-IR700)-mediated PIT induced selective cell death of HER2-ECD–transduced tumor cells. Ad/HER2-ECD also induced homogenous expression of HER2 in heterogeneous gastric cancer cells, resulting in uniform sensitivity of the cells to Tra-IR700–mediated PIT. Anti-HER2 PIT integrated with adenoviral HER2-ECD gene transfer was applied in mice bearing peritoneal dissemination of HER2-negative gastric cancer. Intraperitoneal administration of Ad/HER2-ECD and Tra-IR700 with PIT inhibited peritoneal metastasis and prolonged the survival of mice bearing MKN45. Furthermore, minimal side effects allowed the integrated therapy to be used repeatedly, providing better control of peritoneal dissemination. In conclusion, the novel therapy of molecular-targeted PIT integrated with gene transfer technology is a promising approach for the treatment of peritoneal dissemination in gastric cancer.
Keywords: photoimmunotherapy, peritoneal metastasis, HER2, adenoviral gene transfer, gastric cancer
INTRODUCTION
Gastric cancer is one of the most common malignancies worldwide, making it the third leading cause of cancer-related death (1). Although the localized stage of gastric cancer can be treated curatively with surgical resection, it is frequently diagnosed as far advanced disease (2). Additionally, even after an optimal curative surgery, cancer recurrence is sometimes inevitable (3, 4).
Peritoneal dissemination, which is the most frequent mode of metastasis in gastric cancer (2, 4), is associated with poor prognosis (5, 6). The lack of treatment success is due to the difficulty of selectively delivering anti-cancer drugs to peritoneal lesions while sparing normal tissue. Therefore, a novel therapeutic strategy that can provide highly selective anti-tumor effects against peritoneal dissemination is desired.
Photoimmunotherapy (PIT) is a novel cancer therapy employing a monoclonal antibody (mAb) conjugated to a photosensitizer, IRDye700DX (mAb-IR700). PIT provides a highly specific cytotoxicity to tumor cells expressing particular antigens (7). PIT could work only where mAb-IR700 binds to the targeted cell membrane following the irradiation of near-infrared (NIR) light. Therefore, its ability to target tumors fully depends on the nature of antibody conjugated to IRDye700DX. Trastuzumab, a humanized monoclonal antibody for human epidermal growth factor receptor 2 (HER2) is the only molecular-targeting anti-cancer drug that improves the survival of patients with HER2-positive advanced gastric cancer (8, 9). Hence, trastuzumab-based PIT might be an ideal candidate as a novel targeted therapy even for peritoneally disseminated gastric cancer (10).
Mitsunaga et al. showed that Tra-IR700-mediated PIT has promising anti-tumor effects on HER2-positive cancer (7). However, before Tra-IR700–mediated PIT is widely applied to the treatment of peritoneal dissemination of gastric cancer, some problems remain to be addressed. Only 12.2–22.1% of gastric cancers are HER2 positive (9, 11–13). Moreover, peritoneal dissemination of gastric cancer has an extremely low HER2 expression rate of 2.9% as HER2-positive status is more common among intestinal-type cells than diffuse-type cells that often cause peritoneal dissemination (14, 15). In addition, gastric cancer itself exhibits intratumoral and intertumoral HER2 heterogeneity (16–18). Thus, even a HER2-postive tumor contains cells with different levels of HER2 expression, which leads to the inaccurate assessment of HER2 status, leading to therapeutic resistance of HER2-targeted therapy (19–21). The lack of particular target antigens in gastric cancer also hampers the success of molecular-targeted therapy (22). Consequently, cancer-targeted therapy cannot be simply applied as a therapy of choice for peritoneal dissemination of gastric cancer.
To overcome these limitations, we previously developed an adenoviral vector that expresses the HER2-extracellular domain (HER2-ECD) on the cancer cell membrane, Ad/HER2-ECD. Ad/HER2-ECD induced exogenous HER2-ECD overexpression on HER2-negative cancer cells and successfully sensitized them to trastuzumab (23). In addition, we demonstrated that the integrated therapy of Ad/HER2-ECD and Tra-IR700-mediated PIT effectively and selectively killed HER2-negative breast cancer cells in vitro, suggesting that the integration of gene transduction with PIT expands molecular-targeted therapy even for target-negative cancer(24).
Here, we investigated the therapeutic effects of this integration therapy for peritoneal dissemination of HER2-negative gastric cancer in vivo. The approach used in the present study overcomes the limitations of HER2-mediated therapy and provides a novel therapeutic strategy for the peritoneal dissemination of gastric cancer.
MATERIALS AND METHODS
Cell lines and cell cultures
The human gastric adenocarcinoma cell lines MKN1 and MKN45 were obtained from Human Science Research Resources Bank (Osaka, Japan). The HER2-expressing human gastric adenocarcinoma cell N87 was obtained from American Type Culture Collection. MKN45/Luc and SKOV-3/Luc, a human ovarian cancer cell line, both stably express luciferase and were purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan) and Cell Biolabs, Inc. (San Diego, CA, USA), respectively. The authentication was not performed by the authors. All gastric cancer cell lines were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Sigma-Aldrich, Tokyo, Japan), and the ovarian cancer cell line was cultured in Dulbecco's Modified Eagle's medium (high glucose) with 0.1 mM non-essential amino acids (MP Biomedicals, Santa Ana, CA, USA). The media were supplemented with 1% penicillin/streptomycin (Sigma-Aldrich) and 10% fetal bovine serum. Cells were maintained at 37°C in a humidified incubator at an atmosphere of 95% air and 5% CO2.
Recombinant adenovirus
A replication-deficient adenoviral vector expressing the extracellular and transmembrane domains of HER2 (Ad/HER2-ECD) was constructed, expanded, and purified as described previously (23). The titer of virus vector was determined by a plaque assay using HEK 293 cells. Adenovirus/GFP (Ad/GFP), which expressed green fluorescent protein (GFP), was used as control adenovirus (25).
Synthesis of IR700-conjugated trastuzumab
A water-soluble, IRDye 700DX NHS ester was obtained from LI-COR Bioscience (Lincoln, NE, USA). Trastuzumab was purchased from Chugai Pharmaceutical Co. (Tokyo, Japan). Conjugation of IR700 with trastuzumab was performed according to previous reports (7, 10). Briefly, trastuzumab was incubated with IR700 in Na2HPO4 at room temperature for 15 min. The mixture was purified with a Sephadex G50 column (PD-10; GE Healthcare UK Ltd., Buckinghamshire, UK). The protein concentration was determined with a BIO-RAD protein assay kit (Bio-Rad, Hercules, CA, USA).
Western blotting analysis
MKN1, MKN45 and N87 cells (2×105 cells) were seeded into 6-well plates. Cells were washed with cold phosphate buffered saline (PBS) and lysed with the sodium dodecyl sulfate (SDS) buffer. Equivalent amounts of protein from whole cell lysates were loaded into each lane of an 8% SDS-polyacrylamide gel and electrophoretically transferred to Hybond-polyvinylidene difluoride transfer membranes (GE Healthcare UK Ltd.). Membranes were incubated with primary antibodies against HER2-ECD (Thermo Scientific., Yokohama, Japan) overnight at 4°C and visualized using an Amersham ECL chemiluminescence system (GE Healthcare UK Ltd.) according to the manufacturer’s protocol. Equal loading of samples was confirmed by stripping each blot and reprobing with anti-β actin antibody (Sigma-Aldrich). In this experiment, MKN1 and MKN45 cells were infected with Ad/HER2-ECD at a different multiplicity of infection (MOI) (0, 20, 50 and/or 100) for 24 and/or 48 h. Ad/GFP was used as a control adenovirus.
Flow cytometric analysis
Cells were fixed with 4% para-formaldehyde for 10 min without permeabilization and labeled with the primary antibodies at room temperature for 45 min. APC-conjugated anti-human ErbB2 (HER2) monoclonal mouse antibody (R&D Systems Inc., Minneapolis, MN, USA) and PE-conjugated anti-human epithelial cell adhesion molecule (EpCAM) monoclonal mouse antibody (BD Biosciences, Sunnyvale, CA, USA) were used for the confirmation of HER2-ECD expression and the identification of MKN45 cells, respectively. APC-conjugated mouse IgG antibody (Miltenyi Biotec, K.K., Tokyo, Japan) and PE-conjugated mouse IgG antibody (BD Biosciences) were also used as each isotype control antibody. After cells were washed with PBS and trypsinized, flow cytometry was performed with a FACS instrument (BD Biosciences). The intensity of staining was calculated by using the BD-FACS Software (Flow Jo 7.6.1; BD Biosciences). MKN1 and MKN45 cells were infected with Ad/HER2-ECD at an MOI of 50 for 48 h.
Immunohistochemistry
Cells were fixed with 4% para-formaldehyde for 30 min and then washed with PBS. After blocking with Blocking One reagent (Nacalai Tesque, Inc. Kyoto, Japan), cells were labeled with the primary antibodies for 1 h. The secondary antibodies were also reacted for 1 h. To detect HER2 expression, the anti-HER2 extracellular domain monoclonal mouse antibody (R&D Systems Inc.) with FITC-conjugated polyclonal goat secondary antibody to mouse IgG (abcam, Tokyo, Japan) was used. APC-conjugated mouse monoclonal anti-human HER2-ECD antibody (R&D Systems Inc.) was also used for HER2 detection. PE-conjugated anti-human EpCAM monoclonal mouse antibody (BD Biosciences) was used to identify gastric cancer cells. DAPI was used for nuclear and chromosomal counterstaining. Tra-IR700 could be detected by IR700 fluorescence. Cells were subsequently photographed and the merged images were overlaid using a confocal laser scanning biological microscope (FV10i; Olympus, Tokyo, Japan).
In vitro photoimmunotherapy with adenovirus/HER2-ECD
MKN1 and MKN45 cells were seeded on plates (4 plates or wells per group) for 24 h. The cells were infected with Ad/HER2-ECD or Ad/GFP at an MOI of 50 for 48 h. Trastuzumab and Tra-IR700 (10 µg/ mL) were added for 6 h, and cells were irradiated with NIR light at 10 J/cm2 (20 mW/cm2, 500 s). The irradiation was performed by an irradiator using a light emitting diode (LED) light with a peak at 690 nm. The irradiation power density was measured by energy meter console, PM100D (Thorlabs, Inc. Tokyo, Japan). The morphological cell changes after treatment were observed under a fluorescence microscope (IX71; Olympus). The time-lapse movies were taken serially at hourly intervals for 80 hours just after PIT using a confocal laser scanning biological microscope with built-in culture incubator (FV10i; Olympus).
Cell death and viability assay
To assess the HER2 target selectivity cell death, N87 cells labeled with Cell Tracker® Blue CMAC dye (Life Technologies, Tokyo, Japan) were co-cultured with unlabeled MKN1 cells. Co-cultured cells were infected with Ad/HER2-ECD at an MOI of 50 for 48 h and incubated with Tra-IR700 (10 µg/mL) for 1 h. The irradiation of NIR light was performed at 5 J/cm2. After the irradiation, cells were stained with propidium iodide (PI) (1 µg/mL) to identify dead cells. Cell viability for quantitative evaluation was determined using an XTT Cell Proliferation Kit II (Roche Life Science, Indianapolis, IN, USA), according to the manufacturer’s protocol.
Ex vivo experiments
Athymic female BALB/c nu/nu nude mice were purchased from CLEA (Tokyo, Japan). The animal care and experimental procedures were conducted in accordance with the regulations of the Animal Care and Use Committee of Okayama University. Normal mouse peritoneal cells were collected from 6-week-old nude mice. Briefly, 5 cc of RPMI1640 medium (Sigma-Aldrich) was injected into the abdominal cavity and then the abdomen was massaged. The ascites was collected, and cells were isolated by a centrifugal separator. The cells collected from normal mice were seeded into two culture conditions, a single culture (normal mouse peritoneal cells, 2×105 cells) and a co-culture (mixed normal mouse peritoneal cells with MKN45 cells, 1×105 cells of each type). We analyzed the cells using two-color flow cytometry (BD Biosciences) with APC-conjugated anti-human HER2 monoclonal mouse antibody (R&D Systems Inc.) and PE-conjugated anti-human EpCAM monoclonal mouse antibody (BD Biosciences). To confirm the HER2 expression and Tra-IR700 conjugation on floating tumor cells in the abdominal cavity of peritoneal dissemination xenografted mice, immunohistochemistry was performed with APC-conjugated anti- HER2 antibody and PE-conjugated anti-EpCAM antibody, as mentioned above. Ad/HER2-ECD at a dose of 1×108 plaque-forming units (pfu) and Tra-IR700 (80 µg) were injected into the peritoneal cavity of mice on days 5 and 7 after tumor injection, respectively. The free floating cells in the peritoneal cavity were collected by lavage wash methods.
Evaluation of antitumor effects in the peritoneal dissemination mouse model
We established the peritoneal dissemination xenografted mouse model by intraperitoneal (IP) administration of MKN45 cells (1×107 cells) into 6- to 8-week-old nude mice using a 22-gauge catheter needle. To assess the efficiency of adenoviral gene transfer to the peritoneally disseminated tumors, mice were injected with Ad/HER2-ECD at a dose of 1×108 pfu in 500 µL PBS into the peritoneal cavity 14 days after injection of MKN45 cells. These mice were sacrificed 48 hours later. Immunohistochemical analysis of paraffin-embedded tissues was performed using HER2/ErbB2 (D8F12) XP™ rabbit monoclonal antibody (Cell Signaling Technology, Inc. Danvers, MA, USA), according to the manufacturer’s protocol.
To evaluate the antitumor effects, the mice were randomly divided into the following four groups (each group: n= 5): no treatment (control), treated with Tra-IR700-meditated PIT (IR+PIT), treated with Ad/HER2-ECD-mediated PIT (Ad+PIT) and treated with Ad/HER2-ECD with Tra-IR700-mediated PIT (Ad+IR+PIT). Ad/HER2-ECD was IP administered 5 days after tumor cell injection into the peritoneal cavity at a dose of 1×108 pfu in 500 µL PBS. Tra-IR700 was also IP administered at 80 µg in 500 µL PBS 48 h after Ad/HER2-ECD administration. One day after Tra-IR700 administration, irradiation was performed with 50 J/cm2 of NIR light using an LED light source (L690-66-60 with Lens550; EPITEX, Inc., Kyoto, Japan) at 690 nm as the peak wavelength. Subcutaneous anesthesia was used for all procedures. All mice were sacrificed, and the disseminated peritoneal tumors were resected. The total weight of tumors per mouse was measured on day 28 after tumor cell injection.
Evaluation of luciferase activity in peritoneal dissemination mouse model
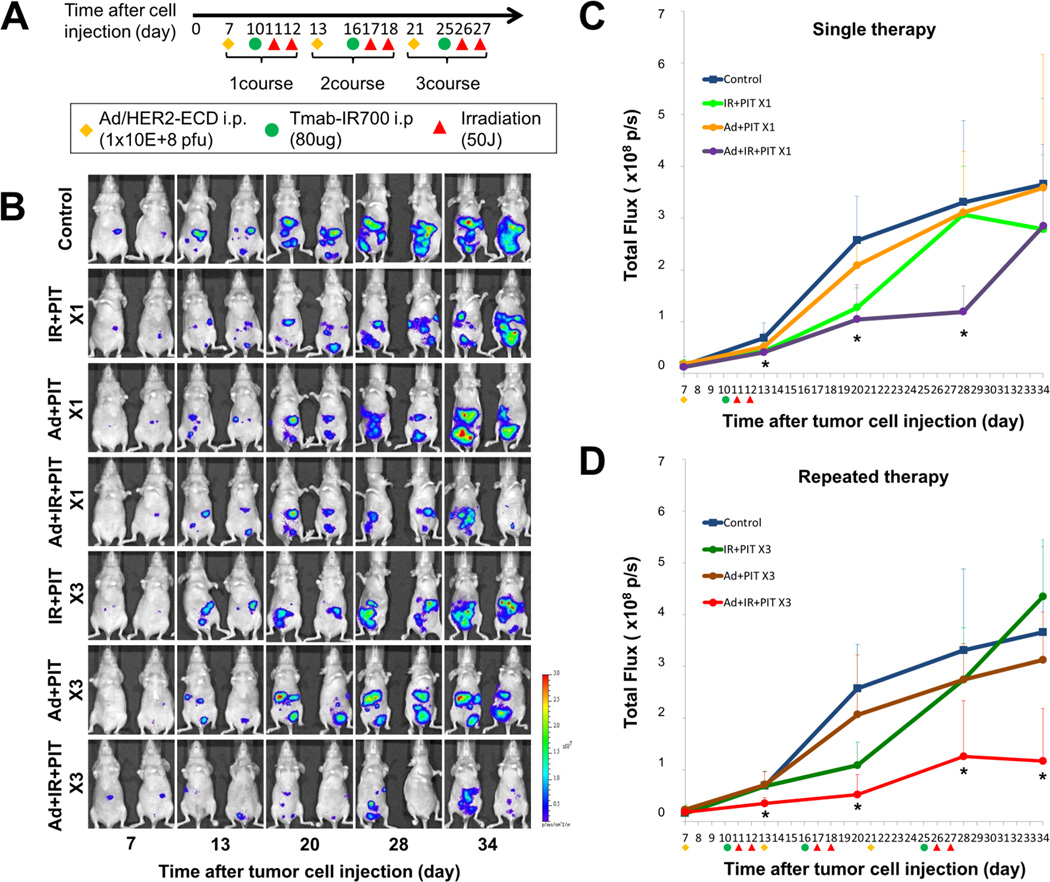
We assessed the tumor inhibitory effects of PIT by using the peritoneal dissemination mouse model bearing luciferase-expressing cells, SKOV-3/Luc cells and MKN45/Luc cells. SKOV-3/Luc and MKN45/Luc cells (5×106 of each cell type) were injected into the peritoneal cavity of 6- to 8-week-old nude mice. Seven days after tumor injection, the fluorescence intensities of the peritoneal dissemination in the mice were measured. The mice bearing SKOV3/Luc cells that exhibited fluorescence were randomly assigned to three groups: no treatment (control), only Tra-IR700 (IR) and Tra-IR700-mediated PIT (IR+PIT). After the randomization, Tra-IR700 (80 µg) was IP administered, and irradiation with NIR light was performed two days after Tra-IR700 administration. The mice bearing MKN45/Luc cells were also randomly assigned to one of 7 groups as follows: no treatment (control); a single treatment of Tra-IR700-meditated PIT (IR+PIT×1), Ad/HER2-ECD–mediated PIT (Ad+PIT×1) or Ad/HER2-ECD with Tra-IR700-mediated PIT (Ad+IR+PIT×1); and three repeated treatments of Tra-IR700–meditated PIT (IR+PIT×3), Ad/HER2-ECD-mediated PIT (Ad+PIT×3) or Ad/HER2-ECD with Tra-IR700-mediated PIT (Ad+IR+PIT×3). The schedule of the integrated therapy regimen is shown in Figure 4A. For analyzing fluorescence intensities, the mice were intraperitoneally injected with Xenolight Rediject D-luciferin (Caliper Life Sciences, Hopkinton, MA, USA) at 150 mg and imaged under isoflurane anesthesia after 4 min. The in vivo fluorescence and bioluminescence images were obtained with an IVIS Lumina imaging system (Xenogen IVIS® Lumina II; Caliper Life Sciences, Hopkinton, MA, USA), and the image analysis and bioluminescent quantification were performed by using Living Image software. In MKN45/Luc mice, the survival rate at 50 days after tumor injection was also assessed.
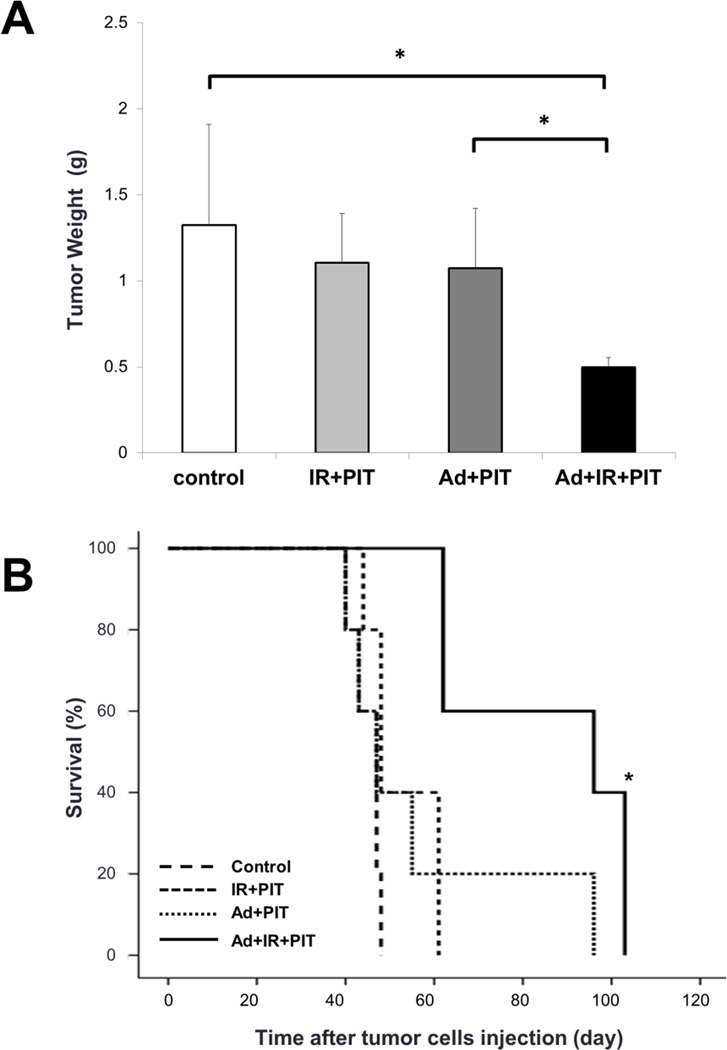
Figure 4.
Inhibitory effects by the integrated therapy of Ad/HER2-ECD and Tra-IR700–mediated PIT in vivo. A, mice peritoneally bearing MKN45 cells were treated with or without Ad/HER2-ECD (1×108 pfu) and/or Tra-IR700 (80 µg) on days 5 and 7 after tumor injection, respectively. All groups except for the control group were irradiated with NIR light at 50 J/cm2. All mice were sacrificed on day 28 after tumor injection, and the total weight of peritoneal tumors in each mouse were measured. The tumor weight of the integrated therapy group (n= 4, Ad+IR+PIT; 0.50±0.06 g) was significantly lower than that of the other condition groups (n= 4, control; 1.32±0.58 g, IR+PIT; 1.10±0.29 g, Ad+PIT; 1.07±0.35 g: Student’s t-test, *P< 0.05). B, the survival of mice with peritoneal dissemination after the integrated therapy. The survival curve showed that the integrated therapy led to the significantly prolonged survival of mice with peritoneal dissemination as compared with controls (n= 5 mice in each treatment group; *P< 0.05 vs the other control group using a log-rank test).
Statistical analysis
Data analysis was performed using the two-sided Student’s t test or one-way repeated measures analysis of variance (ANOVA) followed by a Dunnett’s test for multiple comparisons. Survival curves were estimated using the Kaplan-Meier method, and survival differences between subgroups were analyzed using the log-rank test. All statistical analyses were performed with SPSS for Windows, version 19.0 (SPSS, Inc., Chicago, IL, USA). P values <0.05 were considered to indicate a statistically significant difference.
RESULTS
HER2-ECD expression induced by Ad/HER2-ECD and the distribution of Tra-IR700 in gastric cancer cells
HER2 protein expression in the parental gastric cancer cell lines MKN1, MKN45 and N87 was evaluated. The expression of HER2-wild type (wt) protein (185 kDa) was undetectable in MKN1 and MKN45 cells, whereas N87 cells were HER2-positive. To examine functionality of HER2 protein, the sensitivity to trastuzumab was tested in these cells. MKN1 and MKN45 cells were resistant to trastuzumab, while the growth of N87 cells was efficiently inhibited by trastuzumab (Supplementary Fig. S1). These findings indicate that MKN1 and MKN45 cells were HER2-negative and did not express functional HER2.
To transduce extracellular HER2 protein in these MKN1 and MKN45 cells, they were infected with Ad/HER2-ECD. Infection with Ad/HER2-ECD markedly increased the expression of the HER2-ECD protein in MKN1 and MKN45 cells in a time- and dose-dependent manner (Fig.1A and 1B), and the expression of HER2-ECD proteins was specific to Ad/HER2-ECD (Fig. 1C). Based on these pilot experiments, infection with Ad/HER2-ECD at an MOI of 50 for 48 h was used as the optimal conditions for all subsequent experiments.
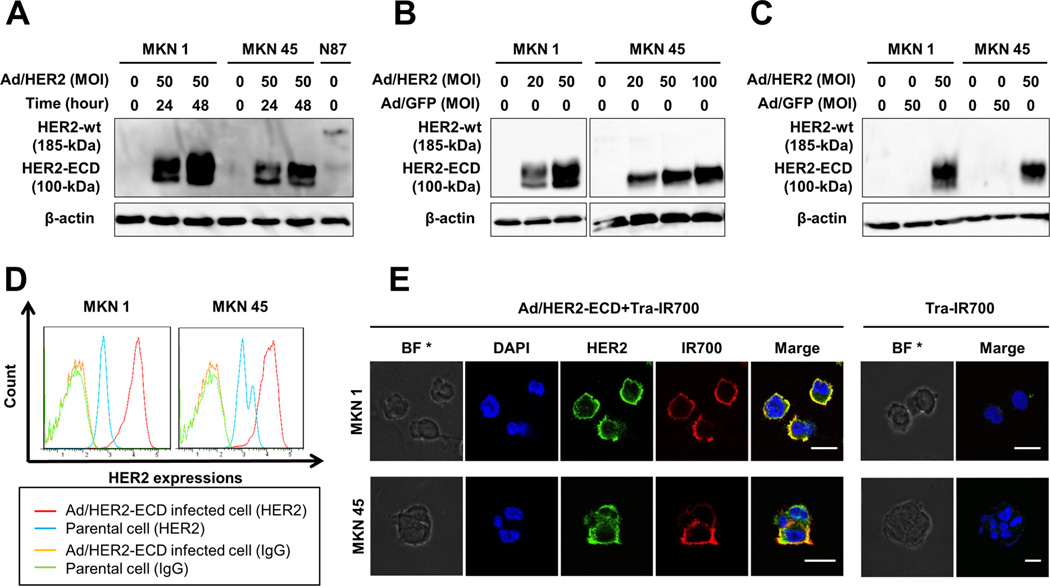
Figure 1.
HER2-ECD expression induced by Ad/HER2-ECD and the distribution of Tra-IR700 in gastric cancer cells. A, Western blot analysis revealed that MKN1 and MKN45 did not express wild-type (wt) HER2 (185 kDa), whereas N87 cells expressed it. Infection with Ad/HER2-ECD at a multiplicity of infection (MOI) of 50 for 24 and 48 h induced the expression of the 100 kDa HER2-ECD protein in MKN1 and MKN45 cells. β-actin was used as a loading control. B, MKN1 and MKN45 cells were infected with Ad/HER2-ECD at a different MOI for 48 h. Western blot analysis showed that the HER2-ECD protein levels were increased depending on the MOI of Ad/HER2-ECD. C, Ad/HER2-ECD induced HER2-ECD protein to be specifically expressed in gastric cancer cells as compared with Ad/GFP as a control adenovirus expressing green fluorescent protein. D, flow cytometric analysis showed HER2-ECD expression. Parental or Ad/HER2-ECD-infected cells were stained with APC-conjugated mouse monoclonal anti-HER2-ECD antibody (blue and red) and APC-conjugated mouse monoclonal IgG2b as a negative control (green and orange). The expression levels of HER2-ECD in Ad/HER2-ECD-infected cells were increased as compared to those of parental cells. E, immunocytochemistry of HER2-ECD and Tra-IR700 on gastric cancer cells. Cells were infected with Ad/HER2-ECD at an MOI of 50 for 48 h and then Tra-IR700 was added for 1 h. HER2-ECD expression was identified by APC-conjugated anti-HER2 antibody, and Tra-IR700 was confirmed by IR700 fluorescence signal. The yellow color on the cellular membrane in the merged image indicated the identical distribution of Tra-IR700 and HER2 induced by Ad/HER2-ECD. * Bright field. Scale bar, 20 µm.
To further examine whether transduced HER2-ECD was expressed on the cell surface, Ad/HER2-ECD–infected cells were subjected to flow cytometric analysis. The increased HER2 expression was confirmed in the infected cells, as compared with the parental cells (Fig. 1D). The transduced HER2-ECD was also detected on the cellular membrane by immunocytochemistry (Fig. 1E). In HER2-ECD-transduced and Tra-IR700–treated cells, the fluorescence signal of IR700 conjugated to trastuzumab was distributed in the same pattern of HER2-ECD expression. These findings indicated that Ad/HER2-ECD could successfully transduce the HER2-ECD proteins on the cellular membrane of HER2-negative gastric cancer cells, and Tra-IR700 could target and bind to it efficiently in vitro.
Microscopic observations of Ad/HER2-ECD-infected gastric cancer cells after treatment with Tra-IR700-mediated PIT
PIT provokes its cytotoxicity on Tra-IR700–bound cells through exposure to NIR light. Next, we irradiated gastric cancer cells with NIR light and observed the effects of PIT on the cells. After PIT, only the cells that were treated with Ad/HER2-ECD and Tra-IR700 showed morphological changes within a few minutes after irradiation with NIR light. The cells swelled and ballooned with bleb formations of the cell membranes (red arrows), while cells in the other conditions continued to proliferate without damage even after PIT (Fig. 2A). Furthermore, the time lapse imaging of the MKN45 cells confirmed that the morphological cell changes were irreversible (Supplementary Movie). These findings indicate that Tra-IR700-mediated PIT directly injures the membrane of cancer cells and that the morphological cell changes are rapid and crucial.
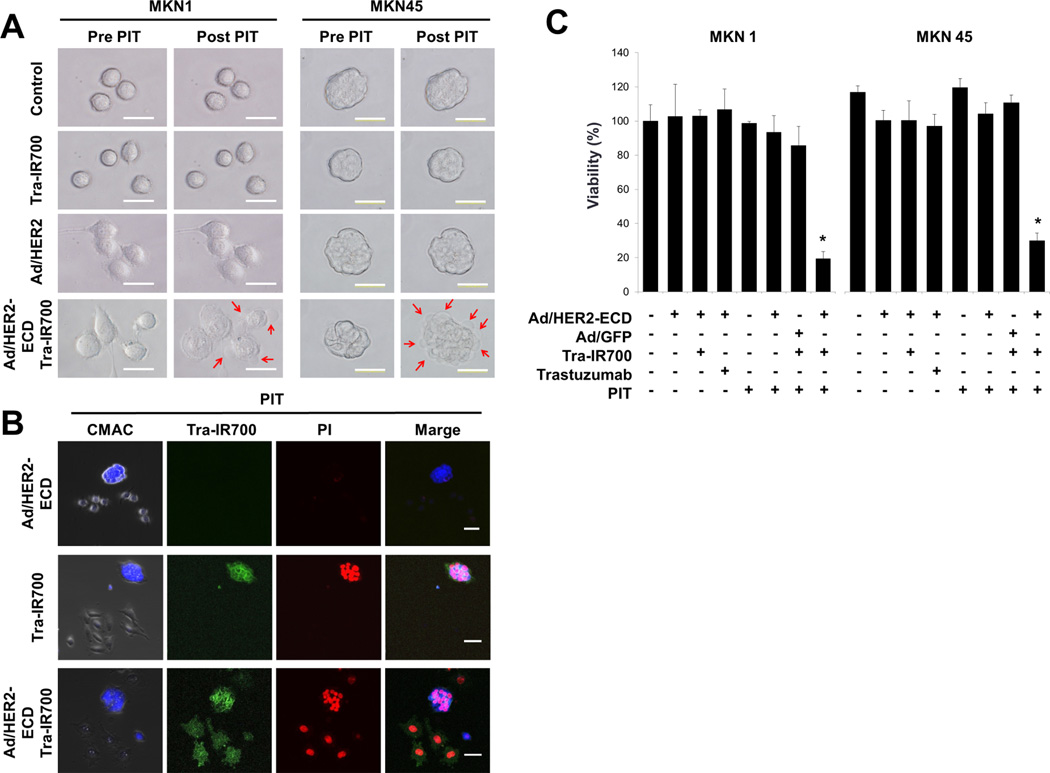
Figure 2.
Microscopic analysis of the HER2-ECD-induced HER2-negative cells treated with Tra-IR700-mediated PIT. A, morphological changes of the tumor cells after PIT. MKN1 and MKN45 were cultured with Ad/HER2-ECD for 48 h and were treated with Tra-IR700 for 6 h. Changes in microscopic morphology were observed before and after PIT with 5 J/cm2 at 690 nm. Treated cells were morphologically captured as swelling like a balloon after PIT. Bleb formations on cellular membranes are indicated with red arrows. B, Tra-IR700 mediated PIT-induced cell death. N87 cells were labeled with Cell Tracker® Blue CMAC dye and then co-cultured with unstained MKN1 cells. These cells were exposed to Tra-IR700 for 1 h and were irradiated with near infrared light at 5 J/cm2. After irradiation, the cells were stained with propidium iodide (PI) for identifying dead cells. Immunohistochemistry showed that PI stained only labeled N87 cells bound by Tra-IR700 (middle row). The co-cultured plate was infected with Ad/HER2-ECD at an MOI of 50 for 48 h and treated with Tra-IR700-mediated PIT. PI stained labeled N87 cells as well as unlabeled MKN1 cells (bottom row). C, a comparison of the cell viability after the treatment of the Ad/HER2-ECD with Tra-IR700-mediated PIT. MKN1 and MKN45 cells were seeded on 96-well plates (n= 4 per group) for 24 h. Ad/HER2-ECD were infected at an MOI of 50 for 48 h. After 24 h of treatment with trastuzumab and Tra-IR700, the cells were irradiated with NIR light (10 J/cm2). An XTT assay demonstrated the statistically significant loss of cell viability in only the integration of Ad/HER2-ECD to the Tra-IR700-mediated PIT group as compared to other groups (n= 4, * Cell viability, P status: MKN1; 19%, P< 0.01, MKN45; 26%, P< 0.01, using the Student’s t test).
To further verify the target specificity of cell death induced by Tra-IR700-mediated PIT, PIT was exposed on the N87 cells labeled with Cell Tracker dye together with unlabeled MKN1 cells co-cultured on the same plate. Staining the treated cells with PI, which stains dead cells with disrupted cell membranes, showed that only Tra-IR700-bound N87 cells were specifically killed (Fig. 2B, middle row). It can be said that co-culture of HER2-positive N87 cells with HER2-negative MKN1 cells mimics the HER2 antigen heterogeneity in gastric tumors. Next, we infected the cells with Ad/HER2-ECD and then treated them with Tra-IR700-mediated PIT. Unlabeled MKN1 cells as well as labeled N87 cells were bound by Tra-IR700 and efficiently killed by PIT (Fig. 2B, bottom row). These findings suggest that Ad/HER2-ECD could induce homogenous expression of HER2 in gastric cancer cells, resulting in uniform sensitivity of the cells to Tra-IR700-mediated PIT. Again, Tra-IR700 or Ad/HER2-ECD by itself did not damage the tumor cells.
Quantitative evaluations of treatment with Ad/ HER2-ECD and Tra-IR700-mediated PIT in vitro
To assess the effects of Ad/HER2-ECD with Tra-IR700-mediated PIT, we compared eight groups of cells. The XTT assay demonstrated a statistically significant loss of cell viability in only the MKN1 and MKN45 cell groups treated by the integration of Ad/HER2-ECD and Tra-IR700-mediated PIT, while cell viability was not affected in the other cell groups (Fig. 2C). These findings indicate that the integrated therapy of Ad/HER2-ECD and Tra-IR700–mediated PIT has the potential to eradicate HER2-negative gastric cancer cells with low cytotoxicity.
In vivo experiments with Ad/HER2-ECD and Tra-IR700
To apply the integrated therapy for the treatment of peritoneal dissemination in vivo, we assessed the influence of Ad/HER2-ECD on normal mouse peritoneal cells ex vivo. The normal mouse peritoneal cells including the mesothelium cells and lymphocytes were collected from the peritoneal cavities of mice. The single culture of normal mouse peritoneal cells and the co-culture of normal mouse peritoneal cells and MKN45 cells were infected with Ad/HER2-ECD. In flow cytometric analysis, the cells with low expression levels of anti-human EpCAM indicated normal mouse peritoneal cells, and the cells with high expression levels of anti-human EpCAM indicated MKN45 cells. The normal mouse peritoneal cells did not show a difference in HER2 expression levels between before and after Ad/HER2-ECD infection. On the other hand, the co-cultured cells showed increased HER2 expression levels after Ad/HER2-ECD infection, especially the cells with high expression levels of anti-human EpCAM; these results indicate that the MKN45 cells had increased HER2 expression levels (Fig. 3A).
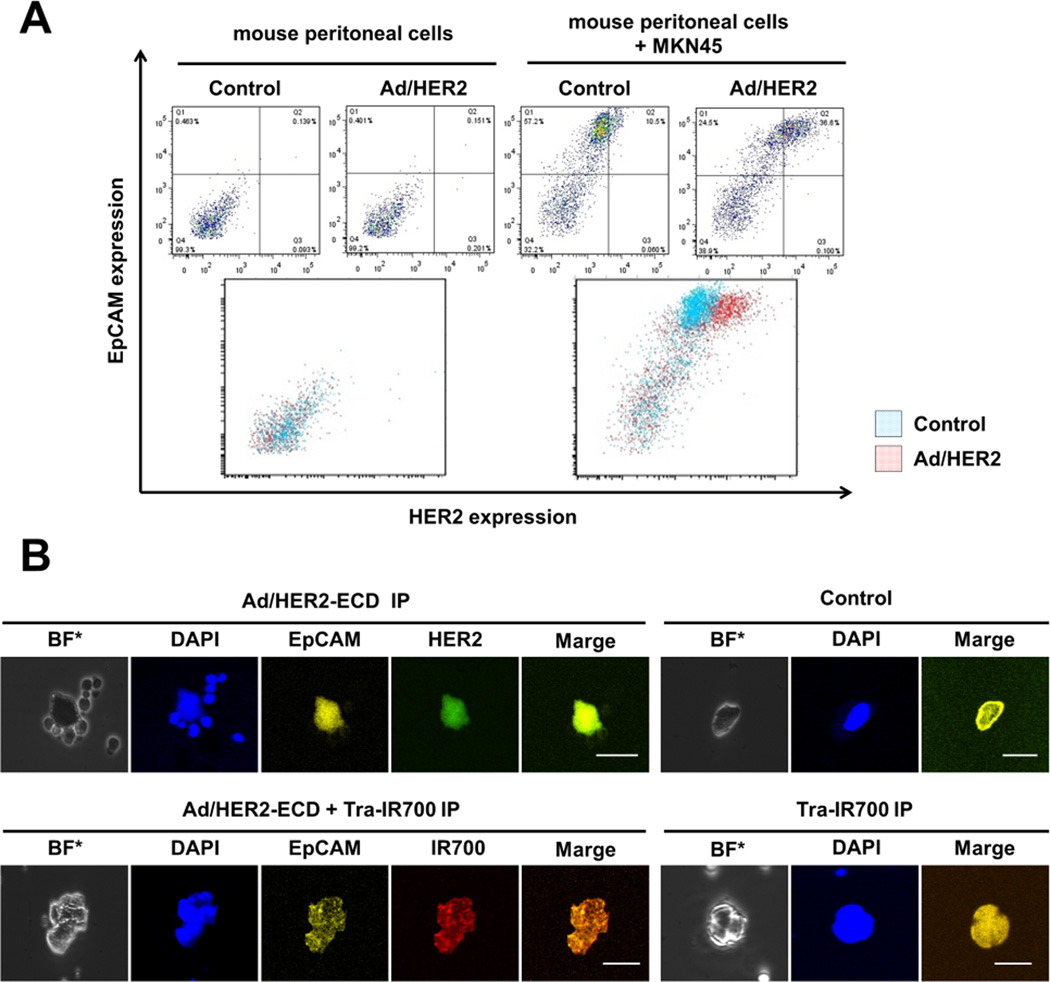
Figure 3.
Evaluation of the HER2 expression induced by Ad/HER2-ECD on the tumor cells and normal mice peritoneal cells ex vivo. A, a culture of normal mouse peritoneal cells (2×105 cells) and the co-culture of normal mouse peritoneal cells and MKN45 cells (1×105 cells of each type) were infected with Ad/HER2-ECD at an MOI of 50 for 48 h. The expression of HER2 and EpCAM was analyzed by FACS. EpCAM–positive cells (MKN45 cells) in co-culture showed increased HER2 expression after Ad/HER2-ECD infection. The normal mouse peritoneal cells showed no difference in HER2 expression before and after Ad/HER2-ECD infection. B, HER2 expression levels and Tra-IR700 conjugations on free floating cells in the peritoneal cavity of the peritoneal dissemination xenografted mouse models. Ad/HER2-ECD (1×108 pfu) and Tra-IR700 (80 µg) were injected into the peritoneal cavity of mice on days 5 and 7 after tumor injection, respectively. The free floating cells in the peritoneal cavity were collected by lavage. Immunohistochemistry demonstrated that the HER2 expression and Tra-IR700 conjugations were identified on only the EpCAM-positive cells.
We further assessed the efficacy of HER2-ECD gene transfer to the peritoneal dissemination of MKN45 cells in vivo. Ad/HER2-ECD was IP administered 14 days after tumor cell injection, and the peritoneal tumors and organs were subjected to HER2 staining 48 hours later. Immunohistochemistry revealed that the HER2-ECD gene was transferred diffusely into the peritoneal tumors and not normal organs, including the liver and the small intestine (Supplementary Fig. S2). Together with the ex vivo results, Ad/HER2-ECD appeared to transduce the HER2-ECD gene preferentially on cancer cells in the peritoneal cavity.
At last, we confirmed the HER2-ECD expression levels and the distribution of Tra-IR700 on the peritoneally disseminated cells in vivo. Ad/HER2-ECD and Tra-IR700 were IP administered. Peritoneally disseminated cells were collected by peritoneal lavage. Immunocytochemistry demonstrated that HER2 expression and Tra-IR700 conjugation could be identified in only the anti-human EpCAM-positive cells (MKN45 cells) (Fig. 3B). These findings indicated that Ad/HER2-ECD administered into the peritoneal cavity could infect the cancer cells and change them to HER2-ECD-positive cells. In addition, Tra-IR700 could diffuse in the peritoneal cavity and bind to cells that express HER2-ECD.
In vivo anti-tumor effects of the integrated therapy in a peritoneal dissemination mouse model
At first, to confirm the effect of Tra-IR700-mediated PIT in vivo, we also established a peritoneal dissemination mouse model bearing luciferase-expressing HER2-positive ovarian cancer cells, SKOV-3/Luc cells (Supplementary Fig. S3). Luciferase-expressing cells enable therapeutic evaluation with non-invasive bioluminescence imaging. The mice bearing SKOV-3/Luc were treated with Tra-IR700-mediated PIT, and the fluorescence intensities were measured. The bioluminescence imaging showed that total fluorescence of the mice treated with Tra-IR700–mediated PIT was lower than that of other mouse groups (Supplementary Fig. S4). These findings indicated that the NIR light applied by external LED light irradiation could reach the mouse peritoneal cavity and that Tra-IR700-mediated PIT could control the progression of the peritoneal dissemination of HER2-positive cells.
Next, we investigated the effect of the integrated therapy of Ad/HER2-ECD and Tra-IR700-mediated PIT in the peritoneal dissemination mouse model with MKN45 cells. We sacrificed the mice 28 days after treatment and resected the disseminated peritoneal tumors. The tumor weight of the integrated therapy group (n= 4, Ad+ IR+ PIT; 0.50±0.06 g) was significantly lower than that of the other groups (Fig. 4A). In addition, the survival curve showed that the integrated therapy significantly prolonged the survival of mice bearing peritoneal dissemination as compared with controls (Fig. 4B). We also recorded the weight of mice every week after treatment and calculated the median weight change based on the weight on treatment start day. No significant difference in weight change among the groups was identified (Data not shown).
In vivo luciferase activity in peritoneal dissemination mouse model
We monitored the bioluminescence intensity of the peritoneal dissemination after the treatment in a mouse model xenografted with luciferase-expressing gastric cancer cells, MKN45/Luc (Fig.5A and 5B). We compared the total fluorescence of the integrated treatment mice with that of untreated control mice in single and repeat integrated therapy groups, respectively (Fig.5C and 5D). Both the single and repeated integrated therapy groups had remarkably suppressed tumor growth as compared to the controls. In particular, the repeated integrated treatment group exhibited significantly lower fluorescence during the observation period. The survival rate on the last day of the observation period (50 days after tumor injection) was as follows: control, 0%; IR+ PIT×1, 0%; Ad+ PIT×1, 20%; Ad+ IR+ PIT×1, 60%; IR+ PIT×3, 25%; Ad+ PIT×3, 0% and Ad+ IR+ PIT×3, 60%. These results indicate that the repetitive therapy of Tra-IR700-mediated PIT integrated with Ad/HER2-ECD can provide better control of peritoneal dissemination and has the potential to improve the prognosis of gastric cancer.
Figure 5.
Evaluation of the treatment effects on luciferase activity in a peritoneal dissemination xenografted mouse model bearing MKN45/Luc cells. We monitored the bioluminescence intensity of the peritoneal dissemination and compared the total fluorescence in single or repeated integrated therapy groups with that of a control group. Before treatment, mice with approximately the same luciferase intensity were selected and randomized into 7 groups (at least 4 animals per group). A, the schedule of the integrated therapy regimen is shown. B, the time course of the bioluminescence images of tumor-bearing mice after treatment in vivo. C, D, the quantitative luciferase activity in mice after treatment. The total fluorescence of the integrated therapy group was remarkably suppressed compared with that of controls. The luciferase activity of the mice treated with repeated integrated therapy remained significantly lower than in other groups (n= 4 or 5 mice in each treatment group; *P< 0.05 vs control group using one-way repeated ANOVA).
DISCUSSION
Peritoneal dissemination of gastric cancer is an intractable disease entity to which no effective treatments have been established (26–28). Recent efforts to categorize gastric cancer into various molecular subtypes (29) have not yet elucidated specific therapeutic targets. In the present study, we described a novel strategy for peritoneally disseminated gastric cancer that integrates HER2-targeted PIT and virus-mediated HER2 gene transfer, exploiting the high specificity for target, the compartmentalized nature of the peritoneal disease, deeply reachable but harmless NIR light, and the efficient in vivo gene transfer capability of adenovirus vector. In a series of in vivo experiments using mouse models with xenografted human gastric cancer cells, HER2-targeted PIT integrated with Ad/HER2-ECD demonstrated robust therapeutic activity even in HER2-negative gastric cancer without overt adverse effects.
Among numerous molecular targeting therapies, thus far, the anti-HER2 strategy is the most successful in breast and gastric cancer (8, 9, 30); but, its usefulness is limited by low expression rates and the heterogeneous nature of HER2 expression (20, 21). A critical advantage of the present integrated strategy is that it addresses the general hurdles of cancer-targeting therapy including low frequency of cancer target antigen and its intra- or inter-tumor heterogeneity. The development of resistance due to the loss of antigen during the course of treatment and acquired mutations in its signal unit are problems that need to be addressed (31–33).
Against these issues, we employed gene transfer technology using adenovirus. HER2 antigen transduced by Ad/HER2-ECD consisted of extracellular and transmembrane domains that were successfully expressed on the surface membrane of gastric cancer cells without oncogenic activation, as previously proven (23). We have further shown that in co-culture of HER2-negative cells and HER2-positive cells, HER2-negative gastric cancer cells can be transformed to be HER2-positive cells by Ad/HER2-ECD. Transduced HER2-ECD can be engaged by Tra-IR700, and both HER2-negative and HER2-positive cells were evenly killed by PIT, indicating that any un-targeted cancer cells can be modified to be targeted cancer cells as long as viral vector can transduce a gene of interest, and the nature of heterogeneity might be overcome by this strategy.
Another advantage of PIT is its mode of action. In contrast to conventional photodynamic therapy (PDT) which requires the uptake of photosensitizer into tumor cells and the production of reactive oxygen species (ROS) (34), the phototoxic effect of PIT does not require the internalization of IR700 to the tumor cells. PIT directly provokes structural damage to the tumor cell membrane by exposure to NIR light and leads to necrotic cell death (7). Thus, any resistance due to the loss of antigen or acquired mutations in its signal unit are theoretically negligible in this PIT integrated with Ad/HER2-ECD. In addition, although PDT necessitates tissue oxygen for its action (35), the phototoxic phenomenon of PIT works even under hypoxia because the ROS formation is a minor cause of the cell death for PIT (36).
Although brisk and substantial tumor suppression was achieved with this integrated therapy, complete eradication was not observed in a series of in vivo experiments. There are two potential hurdles for this experimental therapy. One is uneven dispersion of Ad/HER2-ECD in the peritoneal cavity, and another is uneven distribution of extracorporeally irradiated NIR light to the disseminated tumors. Despite the complex shape of the peritoneal cavity, we have administered an adenovirus directly into it rather than into the blood vessels. Several studies demonstrated that IP administration of anticancer drugs is effective for peritoneal dissemination of gastric cancer, maintaining high concentrations of the drug in the peritoneal cavity (37). We and others also had previously demonstrated that gene therapy with IP administration of adenoviral vector expressing a therapeutic gene suppressed the growth of disseminated gastric cancer (38, 39). Although unintended, Ad/HER2-ECD worked mainly on tumor cells in the peritoneal cavity. Since adenoviral infection greatly depends on receptor-mediated integration, the preference for tumor cells is probably due to the difference in susceptibility to adenoviral infection between the implanted tumor cells and the other cells including lymphocytes and mesothelial cells. The report by Tomko et al. showing that the tissue distribution of adenovirus receptor was detected mainly in epithelial cells (40) might support this speculation, although the precise underlying mechanism is not fully understood.
On the other hand, there is concern that the IP administered mAb-IR700 might be taken up into the liver and that there is a risk of losing a large amount of its efficacy (41). However, because we targeted floating peritoneal tumor cells in our mouse model, IP administration of Tra-IR700 was chosen as a more optimal route than intravenous administration.
Since PIT necessitates sufficient NIR light to kill Tra-IR700-engaged cancer cells, the target lesions must be within its reach. NIR light travels at least several centimeters through tissues (42–44). Thus, extracorporeally irradiated NIR light was expected to penetrate the murine bodies, reach the whole peritoneal cavity, and elicit photo toxicity (10, 41). To apply this therapy to clinical situations, however, the source of NIR light needed to be devised to reach intra-visceral gastric cancer lesions. Nowadays, laparoscopy is widely used in gastric cancer surgery (45) as the peritoneal cavity can be fully inspected through the scope (46, 47). Accordingly, as long as the disseminated cancerous lesions are compartmented in the peritoneal cavity, NIR light can reach them though an endoscope.
Based on the advantages of HER2-targeted PIT integrated with viral gene transfer, including its tremendous specificity, fast-acting property, and minimal side effects, repetitive administration is expected to enhance the therapeutic activity of a single treatment. In the present study, we have tested up to three courses of treatment, which significantly improved the survival rate at 50 days after tumor inoculation. Although the treatment schedule may need more optimization, repetitive administration of PIT integrated with Ad/HER2-ECD appeared to be a feasible and effective option.
Even after multiple courses of PIT, however, eradication of peritoneally disseminated gastric cancer remained incomplete; thus, we still need further improvement of the system. In the present study, we have employed an adenoviral vector that was engineered not to replicate after infection. However, to address incomplete gene transduction by adenovirus, a conditionally replicating viral vector might be a solution as an alternative approach. We had previously developed a telomerase-targeted replicating adenovirus, which can replicate only in telomerase-active malignant cells (48). Kishimoto et al. previously demonstrated that, when intraperitoneally administered, replicating adenovirus carrying the GFP gene efficiently infected the disseminated cancer nodules and expressed GFP in the lesions in the peritoneal cavity of mice (49). These data suggested that viral vectors with cancer-specific replication would facilitate the expression of the transduced gene and improve transduction efficiency by further infection of viral progeny to the uninfected adjacent cancer cells in the peritoneal cavity.
In conclusion, our data demonstrated that PIT integrated with adenovirus-mediated HER2-ECD gene transfer could overcome the lack of tumor-targeted antigen and its heterogeneity and efficiently inhibit the growth of peritoneal dissemination of gastric cancer and prolong mouse survival. The novel integration therapy of gene transfer technology and antibody-based PIT is a promising approach for breaking the limitations of and resistance to cancer therapy.
Supplementary Material
Acknowledgements
We thank Tomoko Sueishi and Tae Yamanishi for her technical support.
Financial support: This study was supported by grants-in-aid from the Ministry of Education Culture, Sports, Science and Technology, Japan (T. Fujiwara, H. Tazawa, S. Tanabe, and S. Kagawa) and grants from the Ministry of Health, Labor and Welfare, Japan (T. Fujiwara).
Abbreviations
- HER2-ECD
human epidermal growth factor receptor 2-extracellular domain
- PIT
photoimmunotherapy
- Ad
Adenovirus
- Tra-IR700
Trastuzumab-IR700
- mAb
monoclonal antibody
- IR700
IRDye 700DX
- NIR
near-infrared
- RPMI
Roswell Park Memorial Institute
- GFP
green fluorescent protein
- PBS
phosphate buffered saline
- SDS
sodium dodecyl sulfate
- MOI
multiplicity of infection
- EpCAM
epithelial cell adhesion molecule
- LED
light emitting diode
- PI
propidium iodide
- pfu
plague-forming units
- IP
intraperitoneal
- ANOVA
analysis of variance
- wt
wild type
- PDT
photodynamic therapy
- ROS
reactive oxygen species
- BF
bright field
Footnotes
Conflict of interest: The authors disclose no potential conflicts of interest
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015 doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2013;16:1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 4.Katai H, Maruyama K, Sasako M, Sano T, Okajima K, Kinoshita T, et al. Mode of Recurrence after Gastric Cancer Surgery. Digestive Surgery. 1994;11:99–103. [Google Scholar]
- 5.Roviello F, Caruso S, Neri A, Marrelli D. Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: overview and rationale. Eur J Surg Oncol. 2013;39:1309–1316. doi: 10.1016/j.ejso.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Imano M, Okuno K. Treatment strategies for gastric cancer patients with peritoneal metastasis. Surg Today. 2014;44:399–404. doi: 10.1007/s00595-013-0603-8. [DOI] [PubMed] [Google Scholar]
- 7.Mitsunaga M, Ogawa M, Kosaka N, Rosenblum LT, Choyke PL, Kobayashi H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nature medicine. 2011;17:1685–1691. doi: 10.1038/nm.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holden J, Garrett Z, Stevens A. NICE guidance on trastuzumab for the treatment of HER2-positive metastatic gastric cancer. Lancet Oncol. 2011;12:16–17. doi: 10.1016/s1470-2045(10)70276-x. [DOI] [PubMed] [Google Scholar]
- 9.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 10.Sato K, Choyke PL, Kobayashi H. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PLoS One. 2014;9:e113276. doi: 10.1371/journal.pone.0113276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon MA, Gundacker HM, Benedetti J, Macdonald JS, Baranda JC, Levin WJ, et al. Assessment of HER2 gene amplification in adenocarcinomas of the stomach or gastroesophageal junction in the INT-0116/SWOG9008 clinical trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:1754–1761. doi: 10.1093/annonc/mdt106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KC, Koh YW, Chang HM, Kim TH, Yook JH, Kim BS, et al. Evaluation of HER2 protein expression in gastric carcinomas: comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Annals of surgical oncology. 2011;18:2833–2840. doi: 10.1245/s10434-011-1695-2. [DOI] [PubMed] [Google Scholar]
- 13.Katai H, Ishida M, Yamashita H, Ohashi M, Morita S, Katayama H, et al. HER2 expression in carcinomas of the true cardia (Siewert type II esophagogastric junction carcinoma) World journal of surgery. 2014;38:426–430. doi: 10.1007/s00268-013-2256-6. [DOI] [PubMed] [Google Scholar]
- 14.Imano M, Satou T, Itoh T, Yasuda A, Kato H, Shinkai M, et al. Peritoneal metastatic lesions of gastric cancer exhibit low expression of human epidermal growth factor receptor 2. Target Oncol. 2012;7:213–216. doi: 10.1007/s11523-012-0223-z. [DOI] [PubMed] [Google Scholar]
- 15.Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:2360–2364. doi: 10.1093/annonc/mdt232. [DOI] [PubMed] [Google Scholar]
- 16.Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, et al. HER2 testing in gastric cancer: a practical approach. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann M, Stoss O, Shi D, Buttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797–805. doi: 10.1111/j.1365-2559.2008.03028.x. [DOI] [PubMed] [Google Scholar]
- 18.Ishida M, Sekine S, Taniguchi H, Fukagawa T, Katai H, Kushima R. Consistent absence of HER2 expression, regardless of HER2 amplification status, in neuroendocrine carcinomas of the stomach. Histopathology. 2014;64:1027–1031. doi: 10.1111/his.12348. [DOI] [PubMed] [Google Scholar]
- 19.Warneke VS, Behrens HM, Boger C, Becker T, Lordick F, Ebert MP, et al. Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:725–733. doi: 10.1093/annonc/mds528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HE, Park KU, Yoo SB, Nam SK, Park do J, Kim HH, et al. Clinical significance of intratumoral HER2 heterogeneity in gastric cancer. European journal of cancer. 2013;49:1448–1457. doi: 10.1016/j.ejca.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Luo H, Li Y, Li J, Cai Z, Su X, et al. Intratumoral heterogeneity determines discordant results of diagnostic tests for human epidermal growth factor receptor (HER) 2 in gastric cancer specimens. Cell Biochem Biophys. 2012;62:221–228. doi: 10.1007/s12013-011-9286-1. [DOI] [PubMed] [Google Scholar]
- 22.Kothari N, Almhanna K. Current status of novel agents in advanced gastroesophageal adenocarcinoma. J Gastrointest Oncol. 2015;6:60–74. doi: 10.3978/j.issn.2078-6891.2014.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshida R, Tazawa H, Hashimoto Y, Yano S, Onishi T, Sasaki T, et al. Mechanism of resistance to trastuzumab and molecular sensitization via ADCC activation by exogenous expression of HER2-extracellular domain in human cancer cells. Cancer immunology, immunotherapy : CII. 2012;61:1905–1916. doi: 10.1007/s00262-012-1249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoyama K, Kagawa S, Ishida M, Watanabe S, Noma K, Takehara K, et al. Viral transduction of the HER2-extracellular domain expands trastuzumab-based photoimmunotherapy for HER2-negative breast cancer cells. Breast Cancer Res Treat. 2015;149:597–605. doi: 10.1007/s10549-015-3265-y. [DOI] [PubMed] [Google Scholar]
- 25.Umeoka T, Kawashima T, Kagawa S, Teraishi F, Taki M, Nishizaki M, et al. Visualization of intrathoracically disseminated solid tumors in mice with optical imaging by telomerase-specific amplification of a transferred green fluorescent protein gene. Cancer research. 2004;64:6259–6265. doi: 10.1158/0008-5472.CAN-04-1335. [DOI] [PubMed] [Google Scholar]
- 26.Isobe Y, Nashimoto A, Akazawa K, Oda I, Hayashi K, Miyashiro I, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2011;14:301–316. doi: 10.1007/s10120-011-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi H, Kitayama J, Ishigami H, Emoto S, Yamashita H, Watanabe T. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119:3354–3358. doi: 10.1002/cncr.28204. [DOI] [PubMed] [Google Scholar]
- 28.Imano M, Peng YF, Itoh T, Nishikawa M, Satou T, Yasuda A, et al. A preliminary study of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel for advanced gastric cancer with peritoneal metastasis. Anticancer Res. 2012;32:4071–4075. [PubMed] [Google Scholar]
- 29.Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573–582. doi: 10.1038/ng.2983. [DOI] [PubMed] [Google Scholar]
- 30.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Muthuswamy SK. Trastuzumab resistance: all roads lead to SRC. Nature medicine. 2011;17:416–418. doi: 10.1038/nm0411-416. [DOI] [PubMed] [Google Scholar]
- 33.Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nature medicine. 2011;17:461–469. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. J Natl Cancer Inst. 1998;90:889–905. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145–157. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 36.Shirasu N, Yamada H, Shibaguchi H, Kuroki M. Potent and specific antitumor effect of CEA-targeted photoimmunotherapy. International journal of cancer Journal international du cancer. 2014;135:2697–2710. doi: 10.1002/ijc.28907. [DOI] [PubMed] [Google Scholar]
- 37.Soma D, Kitayama J, Konno T, Ishihara K, Yamada J, Kamei T, et al. Intraperitoneal administration of paclitaxel solubilized with poly(2-methacryloxyethyl phosphorylcholine-co n-butyl methacrylate) for peritoneal dissemination of gastric cancer. Cancer Sci. 2009;100:1979–1985. doi: 10.1111/j.1349-7006.2009.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsunemitsu Y. Molecular therapy for peritoneal dissemination of xenotransplanted human MKN-45 gastric cancer cells with adenovirus mediated Bax gene transfer. Gut. 2004;53:554–560. doi: 10.1136/gut.2003.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueda K, Iwahashi M, Matsuura I, Nakamori M, Nakamura M, Ojima T, et al. Adenoviral-mediated gene transduction of the hepatocyte growth factor (HGF) antagonist, NK4, suppresses peritoneal metastases of gastric cancer in nude mice. European journal of cancer. 2004;40:2135–2142. doi: 10.1016/j.ejca.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Tomko RP, Johansson Cb Fau, Totrov M, Totrov M Fau, Abagyan R, Abagyan R Fau, Frisen J, Frisen J Fau, Philipson L, Philipson L. Expression of the adenovirus receptor and its interaction with the fiber knob. doi: 10.1006/excr.1999.4761. [DOI] [PubMed] [Google Scholar]
- 41.Sato K, Hanaoka H, Watanabe R, Nakajima T, Choyke PL, Kobayashi H. Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer. Mol Cancer Ther. 2015;14:141–150. doi: 10.1158/1535-7163.MCT-14-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MT, et al. Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg. 2003;21:67–74. doi: 10.1089/104454703765035484. [DOI] [PubMed] [Google Scholar]
- 43.Otberg N, Grone D, Meyer L, Schanzer S, Hoffmann G, Ackermann H, et al. Water-filtered infrared-A (wIRA) can act as a penetration enhancer for topically applied substances. Ger Med Sci. 2008;6:Doc08. [PMC free article] [PubMed] [Google Scholar]
- 44.Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura K, Katai H, Mizusawa J, Yoshikawa T, Ando M, Terashima M, et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer (JCOG0912) Japanese journal of clinical oncology. 2013;43:324–327. doi: 10.1093/jjco/hys220. [DOI] [PubMed] [Google Scholar]
- 46.de Graaf GW, Ayantunde AA, Parsons SL, Duffy JP, Welch NT. The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol. 2007;33:988–992. doi: 10.1016/j.ejso.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Muntean V, Mihailov A, Iancu C, Toganel R, Fabian O, Domsa I, et al. Staging laparoscopy in gastric cancer. Accuracy and impact on therapy. J Gastrointestin Liver Dis. 2009;18:189–195. [PubMed] [Google Scholar]
- 48.Kawashima T, Kagawa S, Kobayashi N, Shirakiya Y, Umeoka T, Teraishi F, et al. Telomerase-specific replication-selective virotherapy for human cancer. Clin Cancer Res. 2004;10:285–292. doi: 10.1158/1078-0432.ccr-1075-3. [DOI] [PubMed] [Google Scholar]
- 49.Kishimoto H, Zhao M, Hayashi K, Urata Y, Tanaka N, Fujiwara T, et al. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.