Abstract
Introduction
Olfactory loss affects a majority of patients with chronic rhinosinusitis (CRS). Traditional objective measures of disease severity, including endoscopy scales, focus upon the paranasal sinuses and often have weak correlation to olfaction.
Methods
Adults with CRS were prospectively evaluated by blinded reviewers with a novel Olfactory Cleft Endoscopy Scale (OCES) that evaluated discharge, polyps, edema, crusting and scarring of the olfactory cleft. Objective olfactory function was assessed using “Sniffin’ Sticks testing, including composite threshold-discrimination-identification (TDI) scores. Olfactory-specific quality-of-life was evaluated using the short modified version of the Questionnaire of Olfactory Disorders (QOD-NS). Inter- and intra-rater reliability was assessed among 3 reviewers for OCES grading. Multivariate linear regression was then used to test associations between OCES scores and measures of olfaction, controlling for potential confounding factors.
Results
The OCES score was evaluated in 38 patients and had a high overall reliability (ICC=0.92; 95% CI: 0.91–0.96). The OCES significantly correlated with objective olfaction as measured by TDI score (p<0.001), with TDI score falling by 1.13 points for every 1 point increase in OCES score. Similar significant associations were found for threshold, discrimination, and identification scores (p<0.003 for all) after controlling for age, gender, race, and reviewer/review. The OCES was also highly associated with patient-reported QOD-NS scores (p=0.009).
Conclusion
A novel olfactory cleft endoscopy scale shows high reliability and correlates with both objective and patient-reported olfaction in patients with CRS. Further studies to determine prognostic value and responsiveness to change are warranted.
Keywords: Endoscopy, quality of life, olfaction, chronic rhinosinusitis, patient reported outcome measure
Introduction
Chronic rhinosinusitis (CRS) is one of the most common causes of impaired olfaction, with a population prevalence estimated at 10–15% across North America and Europe. 1,2 Although often overlooked by patients and physicians alike, olfactory loss is considered a cardinal symptom of CRS and can be found in roughly 40–80% of patients.3–5 Olfactory dysfunction results in significant declines in health related quality-of-life (QOL), including both general and olfactory-specific measures. Patients experiencing olfactory loss commonly complain of a lack of interest in food, decreases in social interaction, and appear to be at a greater risk for clinical depression.6–8
Olfactory dysfunction in CRS likely results from the mucosal inflammation present in all forms of CRS. Mucosal inflammation could affect olfaction directly via a variety of inflammatory-mediated damage to the olfactory epithelium, by impeding delivery of odorant-containing air to the olfactory cleft or a combination of both mechanisms. 9–11 Sinonasal endoscopy is a commonly utilized procedure to determine whether mucosal inflammation is present in patients with CRS. A number of different endoscopic grading systems have been developed in order to quantify the severity of mucosal inflammation.12–15 The majority of proposed systems include measures of edema, polyps, and discharge, with some also including scarring and crusting. However, these systems often focus on the middle meatus or the nasal cavity in general without specific attention to the olfactory cleft.
One could hypothesize that some patients might have severe ongoing inflammation in the middle meatus visible on endoscopy, but with relative sparing of the olfactory cleft proper. In other patients, focal inflammation in the olfactory cleft may be present but the remainder of the nasal cavity or sinuses might be unaffected. In these scenarios, an endoscopy scale focused upon the olfactory cleft might be more specific with regard to olfactory dysfunction than a generalized scale.
Olfaction in the setting of CRS can be vexing clinically, as it is often difficult to predict which patients are affected, why they are affected, and whether they will improve after medical or surgical therapies. It is our assertion that an endoscopy scale specific to olfaction might be useful both clinically and in future research endeavors which seek to better understand olfaction in the setting of CRS. The goal of this study was to explore the inter-rater and intra-rater reliability of the Olfactory Cleft Endoscopy Scale (OCES) and to determine whether it is significantly associated with objective and patient-reported measures of olfaction.
Methods
Adult patients (≥18 years old) with CRS were prospectively enrolled into a cross-sectional study. All patients satisfied diagnostic criteria for CRS according to the current Clinical Practice Guideline of the American Academy of Otolaryngology-Head and Neck Surgery (AAOHNS), including the presence of at least 2 cardinal symptoms for at least 12 weeks and confirmatory evidence of sinus mucosal inflammation on sinonasal endoscopy or computed tomography (CT) scan.16 Patients receiving systemic corticosteroids were excluded, as well as those having undergone surgery in the preceding 6 months. Demographic and exposure information was collected from each patient, including age, gender, race/ethnicity, and active smoking status. Comorbidities of interest were recorded, including the presence of asthma, allergic rhinitis, diabetes mellitus, prior sinus surgery, and polyps. Allergic rhinitis was defined as those who carried a physician diagnosis and had confirmation with prior positive objective testing (skin prick test or allergen-specific IgE antibody test). For all other medical comorbidities, the patient was considered to have the condition if that had received a prior diagnosis by a physician.
CRS disease severity
Patient-reported disease severity was assessed using the SinoNasal Outcomes Test 22 (SNOT-22).17 The SNOT-22 is a sinus-specific quality-of-life (QOL) instrument with scores ranging from 0–110, with higher scores indicating more severe disease impact. The SNOT-22 includes a single question (Q21) regarding olfaction (0–5), accounting for only 4.5% of the overall score. Computed tomography (CT) scans done within the preceding 30 days were graded using the Lund-Mackay scoring system (0–24), with higher scores indicating a greater burden of disease on imaging.18
Olfactory testing
Quantitative olfactory testing was performed using the “Sniffin’ Sticks” test (Burghardt, Wedel, Germany).19 Testing was performed by a trained clinical research coordinator blinded to other clinical data. The testing battery performed included odor threshold (OT), odor discrimination (OD) and odor identification (OI). The OT test was performed using dilutions of n-butanol in a single-staircase, triple-forced choice procedure. The OD test used triplets of pens presented in random order with two containing the same odorant and the third a different odorant. The OI test involved 16 odorants presented at supra-threshold intensity using multiple choice procedures. All subjects were blindfolded to avoid visual identification of odorant-containing pens. Each of the 3 individual tests was scored from 0 to 16. The overall results combined and reported as a composite threshold-discrimination-identification score (TDI), with higher scores representing better olfaction (0–48).
Olfactory-specific QOL
Olfaction-specific quality-of-life (QOL) was evaluated using the short modified version of the Questionnaire of Olfactory Disorders (QOD).20 This version of the QOD is a 25 question survey which includes 17 negative statements (QOD-NS), 2 positive statements (QOD-PS), and 6 socially desired statements (QOD-SD). The QOD-NS was specifically utilized in this study since it assesses the degree to which olfactory dysfunction affects a subject’s daily functioning (range 0–51, with higher scores representing better QOL). The QOD-PS and QOD-SD were not utilized since they measure coping ability and personality attributes respectively, neither of which were felt to be relevant to the study aims.
Olfactory Cleft Endoscopy
Rigid sinonasal endoscopy was performed by the treating physician using a 3mm, 45-degree angled rigid endoscope (Karl Storz, Tuttlingen, Germany) after topical application of lidocaine/phenylephrine via atomizer spray to the anterior nares. Passes were performed on each side extending from the anterior nares to the nasopharynx, with the endoscope angled superiorly towards the olfactory cleft region. Examinations were digitally recorded, edited to eliminate facial features, and archived by coded number for later analysis.
Three reviewers (ZS, TK, RS) independently analyzed each recorded endoscopic examination in a blinded fashion on two separate occasions. Each reviewer was blinded to the other reviewer’s scores, as well as to all other clinical measures recorded in the study, including olfaction. The second review was separated from the first temporally by one month and archived endoscopic examinations were presented in a randomly re-arranged order, with reviewers unable to consult their earlier scores.
Reviewers graded the degree to which the olfactory cleft was affected by discharge, edema, polyps, crusting and scarring using a score from 0–2 for each measure (Table 1). Results for each side were recorded separately and combined for a final Olfactory Cleft Endoscopy Scale (OCES) that ranged from 0–20, with higher scores representing increased disease severity. The olfactory cleft was considered to be a 3-dimensional space that started at the anterior plane of middle turbinate and ended just anterior to the face of the sphenoid sinus. The lateral boundary of the olfactory cleft was the attachment of the middle and/or superior turbinate, with the septum representing the medial limit. The roof of the olfactory cleft was the cribriform plate and the floor was an imaginary line drawn roughly 1 cm inferior to the cribriform.
Table 1.
Olfactory Cleft Endoscopy Scale
| Endoscopic Feature | Right Olfactory Cleft | Left Olfactory Cleft |
|---|---|---|
| Discharge (0,1,2) | ||
| Polyps (0,1,2) | ||
| Edema (0,1,2) | ||
| Crusting (0,1,2) | ||
| Scarring (0,1,2) | ||
|
| ||
| Total | ||
|
| ||
| Discharge: | ||
| 0=None or scant clear/thin drainage (normal) | ||
| 1=More abundant, thicker clear/white drainage (not normal but not purulent) | ||
| 2=Thicker, more abundant, discolored/purulent drainage | ||
| Polyps: | ||
| 0=None | ||
| 1=Discrete polyps partially narrowing/blocking the OC (<50%) | ||
| 2=Discrete polyps completely narrowing/blocking the OC (≥50%) | ||
| Edema: | ||
| 0=None | ||
| 1=Swelling partially narrowing/blocking the OC (<50%) | ||
| 2=Swelling completely narrowing/blocking the OC (≥50%) | ||
| NOTE: Those patients with discrete polyps but otherwise non-inflamed mucosa would get a 0. Those patients with discrete polyps in the setting of diffuse swelling/inflammation would get a 1 or 2. | ||
| Crusting: | ||
| 0=None; 1=mild; 2=severe | ||
| Scarring: | ||
| 0=None; 1= mild; 2=severe | ||
Analytic plan
Characteristics of the study cohort were summarized using means +/− standard deviation (SD) or counts (percentages). Inter- and intra-rater reliability was assessed using intraclass correlation coefficients (ICCs). Additionally, to assess the overall stability of the OCES, an overall ICC, pooling inter- and intra-rater reliability, was produced. Moreover, to evaluate the contribution of each item in the OCES, an ICC of the OCES total minus one of the items was produced. For all ICCs, a 95% confidence interval was produced using the bootstrapping method with 1,000 iterations.
Mixed-effects linear models were generated to explore the association between total OCES scores with objective measures of olfaction, including total TDI score and OT, OD, and OI subscores. Similar models were used to test the association between the OCES and olfaction-specific QOL using the QOD-NS instrument and SNOT-22 Q21. Key variables (age, gender, race, and review) nested within reviewer were included in all models to eliminate potential confounding. Statistical significance was assessed at α-level = 0.05. There was no correction for multiple comparisons. All analyses were performed using SAS v9.4 ©.
Results
Study Cohort
A total of 38 patients with CRS were enrolled in the study, equally split between females and males, with an average age of 58.0 (range 18–80). Demographics, medical comorbidities, and disease severity measures are detailed in Table 2. Of note, 63% of patients had visible nasal polyps and overall there was a high incidence of allergic rhinitis (53%) and asthma (47%) but no current smokers.
Table 2.
Characteristics of the study cohort
| Patient characteristic | Mean (SD) | Range | N (%) | |
|---|---|---|---|---|
| Demographics | ||||
| Age | 58.0 (15.9) | 18–80 | ||
| Sex | Female | 19 (50) | ||
| Male | 19 (50) | |||
| Race | African-American | 12 (32) | ||
| Asian | 2 (5) | |||
| White | 24 (63) | |||
| Ethnicity | Non-Hispanic/Latino | 37 (97) | ||
| Hispanic/Latino | 1 (3) | |||
| Comorbidities | ||||
| Allergic rhinitis | No | 18 (47) | ||
| Yes | 20 (53) | |||
| Asthma | No | 20 (53) | ||
| Yes | 18 (47) | |||
| ASA intolerance | No | 33 (87) | ||
| Yes | 5 (13) | |||
| Smoker | No | 38 (100) | ||
| Yes | 0 (0) | |||
| Diabetes | No | 27 (71) | ||
| Yes | 11 (29) | |||
| Polyps | No | 14 (37) | ||
| Yes | 24 (63) | |||
| CRS Disease Severity | ||||
| SNOT-22 | 44.5 (23.2) | 3–99 | 37 | |
| CT Score | 12.0 (6.9) | 2–23 | 17 | |
| Prior sinus surgeries | 2.5 (1.9) | 0–6 | ||
| Olfactory Measure | ||||
| TDI Score | 20.4 (7.7) | 8–35 | 37 | |
| OT Score | 2.7 (2.6) | 1–11 | 37 | |
| OD Score | 8.3 (3.0) | 3–15 | 37 | |
| OI Score | 9.4 (4.0) | 1–16 | 37 | |
| QOD-NS Score | 36.2 (11.9) | 8–51 | 38 | |
Reliability
The reliability of total OCES scores was evaluated by comparing the 1st review with the 2nd review separated temporally. This intra-rater reliability was high with an ICC=0.79 (95% CI: 0.74–0.84). When comparing total OCES among the 3 reviewers, the inter-rater reliability was also high with an ICC=0.81 (95% CI: 0.75–0.87). The overall reliability, pooling inter- and intra-rater reliabilities, was similarly high with an ICC=0.92 (95% CI: 0.91–0.96). Overall reliability remained high after removing and replacing individual items from the OCES score, indicating the scale is not overly dependent on any one feature (Table 3).
Table 3.
Overall reliability estimates for the OCES with different combinations of endoscopic measures
| Measure removed | ICC | SD | 97.5% CI |
|---|---|---|---|
| None | 0.92 | 0.01 | 0.91–0.96 |
| Discharge | 0.78 | 0.03 | 0.72–0.83 |
| Polyps | 0.78 | 0.03 | 0.72–0.83 |
| Edema | 0.84 | 0.02 | 0.81–0.88 |
| Crusting | 0.83 | 0.03 | 0.79–0.87 |
| Scarring | 0.84 | 0.02 | 0.80–0.88 |
Overall reliability represents pooling of both intra-rater and inter-rater estimates. Specific endoscopic measures were removed one at a time. ICC = intraclass correlation coefficient; SD = standard deviation; CI = confidence interval.
Olfactory cleft endoscopy and measures of olfaction
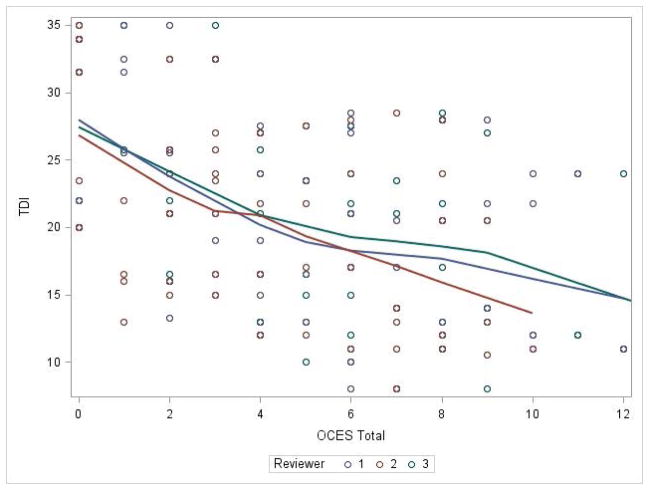
The OCES score was significantly correlated with objective olfaction as measured by the TDI score for each reviewer, with worse endoscopy scores predicting worse (lower) olfaction (Figure 1). This association remained significant on mixed effects linear modelling after controlling for age, gender, race, and reviewer/review (p<0.0001). Overall, for every 1 point increase in OCES score, the TDI score fell by 1.13 points (Table 4). Highly significant associations were also seen between OCES and threshold, discrimination, and identification scores.
Figure 1.
Olfactory cleft endoscopy correlates with TDI score for each reviewer (p<0.0001).
Table 4.
Associations between OCES and measures of olfactory function
| Olfactory Measure | Beta | SE | F value | P-value | Interpretation |
|---|---|---|---|---|---|
| Objective | |||||
| TDI Score | −1.13 | 0.15 | 57.41 | <0.0001 | For every 1 point increase in OCES score the TDI score falls by 1.13 points |
| OT Score | −0.21 | 0.06 | 13.89 | 0.0003 | For every 1 point increase in OCES score the OT score falls by 0.21 points |
| OD Score | −0.35 | 0.06 | 31.89 | <0.0001 | For every 1 point increase in OCES score the OD score falls by 0.35 points |
| OI Score | −0.57 | 0.08 | 55.81 | <0.0001 | For every 1 point increase in OCES score the OI score falls by 0.57 points |
| PROMs | |||||
| QOD-NS | −0.61 | 0.23 | 6.96 | 0.0092 | For every 1 point increase in OCES score the QOD-NS score falls by 0.61 points |
| SNOT-22 Q21 | 0.07 | 0.03 | 4.12 | 0.0440 | For every 1 point increase in OCES score the SNOT-22 Q21 score rises by 0.07 points |
Each association is based on a mixed-effects linear model which also includes age, gender, race, and review/reviewer. OCES = Olfactory Cleft Endoscopy Scale; TDI = threshold-discrimination-identification; OT = odor threshold; OD = odor discrimination; OI = odor identification; PROMs = patient reported outcome metrics; QOD-NS = Questionnaire of Olfactory Disorders-Negative Statements; SNOT-22 Q21 = Question 21 from the SinoNasal Outcomes Test 22.
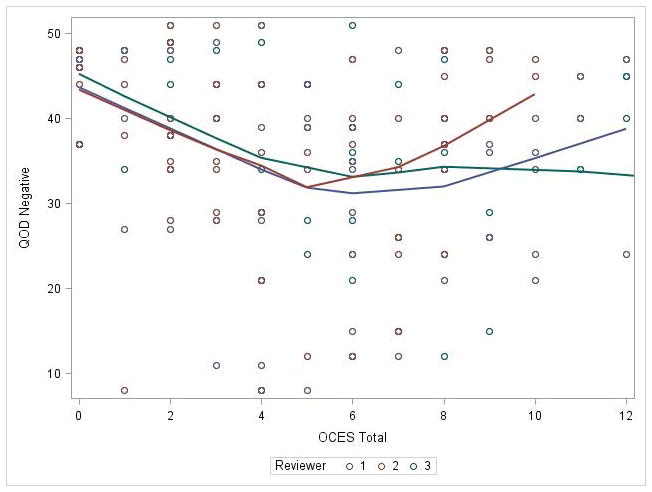
The OCES score was also significantly correlated with patient-reported, olfaction-specific QOL as measured by the QOD-NS, with worse endoscopy predicting worse QOL (Figure 2). This association remained significant on mixed effects linear modelling after controlling for age, gender, race, and reviewer/review (p<0.0092). Overall, for every 1 point increase in OCES score, the QOD-NS score fell by 0.61 points (Table 4). An association was also seen between OCES score and SNOT-22 Q21, although the strength of the association was weaker (p=0.0440).
Figure 2.
Olfactory cleft endoscopy correlates with olfaction specific quality of life as measured by Questionnaire of Olfactory Disorders - Negative Statements (p<0.0092).
Discussion
Nasal endoscopy is one of the most widely utilized methods to objectively measure disease severity in CRS. Endoscopy is less costly than computed tomography (CT) scan and does not require radiation exposure, allowing it to be utilized repeatedly to assess changes in the nose and sinuses over time. The OCES presented herein is unique in that it focuses on the olfactory cleft specifically, as opposed to existing instruments which typically concentrate on the middle meatus or individual sinuses.12,13 The OCES grading system might thus be useful for patients with CRS who are particularly bothered by olfactory loss, allowing longitudinal assessments in the clinic. Additionally, this system might be a useful objective measure for olfactory outcomes studies. To date, the treatment of olfactory loss in the setting of CRS has received relatively little attention, particularly with regard to medical therapies. Olfactory outcomes after endoscopic sinus surgery have been mixed and it remains difficult to predict which patients are likely to improve.21 The OCES could thus serve as an adjunct outcome measure for future medical and surgical trials focused on olfaction in the setting of CRS.
There are many steps involved in fully validating an instrument. In this study, face validity and content validity were presumed based on inclusion of five different endoscopic features commonly found in other nasal endoscopic scales. Predictive validity was confirmed, with independent associations found between OCES scores and objective olfaction, including threshold, discrimination, and identification. Additionally, the OCES predicted patient-reported, olfaction-specific QOL, which is notable as many objective measures of CRS disease severity often fail to correlate with patient-reported symptoms or QOL. Future studies might confirm these initial findings and explore other validity dimensions, such as responsiveness to change after treatment.
Reliability of the OCES grading system was high both for inter-rater and intra-rater assessments and was likely the result of several contributing factors. The first is the familiarity of the endoscopic features graded, which are similar across many other general sinonasal scoring systems. Additionally, gradations for each feature were kept to a minimum (0–2) and instructions were given to help guide the endoscopist as to what constitutes each score. However, it should be kept in mind that all three graders in this study were rhinologists highly experienced in endoscopic grading and it remains possible that less experienced reviewers would have lower reliability.
The goal of this paper was to evaluate the reliability and validity of an endoscopic grading system focusing on the olfactory cleft. Future studies might compare this scale to scoring systems which are focused on the middle meatus or sinuses specifically. Our hypothesis is that the current OCES system will more closely predict olfaction than a more generalized instrument that ignores the olfactory cleft. Studies examining CT scans have shown that opacification of the olfactory cleft is more predictive of olfaction than sinus-specific opacification, at least with respect to patients with polyps. 22–24 If similar findings are true with endoscopy, than the OCES might be an informative measure to include in future studies which aim to either predict olfaction in patients with CRS or explore mechanisms of olfactory loss.
There are several characteristics of the study cohort which are worth considering. Patients in the study overall had a high degree of atopy and asthma, with over 60% having nasal polyps. This combination of comorbidities likely contributed to the high degree of olfactory dysfunction seen and might not be typical of all patients with CRS. Additionally, patients in the cohort overall had severe disease, many had undergone prior sinus surgery and we did not enroll any smokers. All of these factors could potentially impact olfactory cleft endoscopy and should be taken into account before applying our endoscopy findings to other populations. Given the relatively small sample sizes, subgroup analyses exploring groups defined by polyp classification, revision surgical status, or disease severity was not possible, but would be worth exploring in future studies.
Conclusion
A novel olfactory cleft endoscopy scale shows high reliability and correlates with both objective and patient-reported olfaction in patients with CRS. Further studies to determine prognostic value and responsiveness to change are warranted.
Footnotes
Conflict(s) of Interest / Financial Disclosures: Zachary M. Soler is supported for this investigation by a grant from the National Institute on Deafness and Other Communication Disorders (NIDCD), one of the National Institutes of Health, Bethesda, MD (R03 DC013651-01; PI: ZM Soler). Dr. Soler is a consultant for Olympus, which is not affiliated with this manuscript. Rodney J. Schlosser is supported by grants from OptiNose and IntersectENT, neither are associated with this manuscript. Dr. Schlosser is also a consultant for Olympus and Arrinex which are not affiliated with this study.
References
- 1.Hastan D, Fokkens WJ, Bachert C, et al. Chronic rhinosinusitis in Europe--an underestimated disease. A GA(2)LEN study. Allergy. 2011;66:1216–1223. doi: 10.1111/j.1398-9995.2011.02646.x. [DOI] [PubMed] [Google Scholar]
- 2.Soler ZM, Mace JC, Litvack JR, Smith TL. Chronic rhinosinusitis, race, and ethnicity. Am J Rhinol Allergy. 2012;26:110–116. doi: 10.2500/ajra.2012.26.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvack JR, Fong K, Mace J, James KE, Smith TL. Predictors of olfactory dysfunction in patients with chronic rhinosinusitis. Laryngoscope. 2008;118:2225–2230. doi: 10.1097/MLG.0b013e318184e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Litvack JR, Mace JC, Smith TL. Olfactory function and disease severity in chronic rhinosinusitis. Am J Rhinol Allergy. 2009;23:139–144. doi: 10.2500/ajra.2009.23.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenfeld RM, Andes D, Bhattacharyya N, et al. Clinical practice guideline: adult sinusitis. Otolaryngol Head Neck Surg. 2007;137:S1–31. doi: 10.1016/j.otohns.2007.06.726. [DOI] [PubMed] [Google Scholar]
- 6.Blomqvist EH, Bramerson A, Stjarne P, Nordin S. Consequences of olfactory loss and adopted coping strategies. Rhinology. 2004;42:189–194. [PubMed] [Google Scholar]
- 7.Smeets MA, Veldhuizen MG, Galle S, et al. Sense of smell disorder and health-related quality of life. Rehabil Psychol. 2009;54:404–412. doi: 10.1037/a0017502. [DOI] [PubMed] [Google Scholar]
- 8.Neuland C, Bitter T, Marschner H, Gudziol H, Guntinas-Lichius O. Health-related and specific olfaction-related quality of life in patients with chronic functional anosmia or severe hyposmia. The Laryngoscope. 2011;121:867–872. doi: 10.1002/lary.21387. [DOI] [PubMed] [Google Scholar]
- 9.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. The Laryngoscope. 2000;110:1071–1077. doi: 10.1097/00005537-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Konstantinidis I, Witt M, Kaidoglou K, Constantinidis J, Gudziol V. Olfactory mucosa in nasal polyposis: implications for FESS outcome. Rhinology. 2010;48:47–53. doi: 10.4193/Rhin09.102. [DOI] [PubMed] [Google Scholar]
- 12.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1997;117:S35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 13.Wright ED, Agrawal S. Impact of perioperative systemic steroids on surgical outcomes in patients with chronic rhinosinusitis with polyposis: evaluation with the novel Perioperative Sinus Endoscopy (POSE) scoring system. Laryngoscope. 2007;117:1–28. doi: 10.1097/MLG.0b013e31814842f8. [DOI] [PubMed] [Google Scholar]
- 14.Durr ML, Pletcher SD, Goldberg AN, Murr AH. A novel sinonasal endoscopy scoring system: the discharge, inflammation, and polyps/edema (DIP) score. International forum of allergy & rhinology. 2013;3:66–72. doi: 10.1002/alr.21074. [DOI] [PubMed] [Google Scholar]
- 15.Psaltis AJ, Li G, Vaezeafshar R, Cho KS, Hwang PH. Modification of the Lund-Kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope. 2014;124:2216–2223. doi: 10.1002/lary.24654. [DOI] [PubMed] [Google Scholar]
- 16.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis executive summary. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;152:598–609. doi: 10.1177/0194599815574247. [DOI] [PubMed] [Google Scholar]
- 17.Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2009;34:447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 18.Lund VJ, Mackay IS. Staging in rhinosinusitus. Rhinology. 1993;31:183–184. [PubMed] [Google Scholar]
- 19.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 20.Simopoulos E, Katotomichelakis M, Gouveris H, Tripsianis G, Livaditis M, Danielides V. Olfaction-associated quality of life in chronic rhinosinusitis: adaptation and validation of an olfaction-specific questionnaire. The Laryngoscope. 2012;122:1450–1454. doi: 10.1002/lary.23349. [DOI] [PubMed] [Google Scholar]
- 21.Rudmik L, Smith TL. Olfactory improvement after endoscopic sinus surgery. Curr Opin Otolaryngol Head Neck Surg. 2012;20:29–32. doi: 10.1097/MOO.0b013e32834dfb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soler ZM, Pallanch JF, Sansoni ER, et al. Volumetric computed tomography analysis of the olfactory cleft in patients with chronic rhinosinusitis. International forum of allergy & rhinology. 2015 doi: 10.1002/alr.21552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenhende-Szymanski C, Hochet B, Chevalier D, Mortuaire G. Olfactory cleft opacity and CT score are predictive factors of smell recovery after surgery in nasal polyposis. Rhinology. 2015;53:29–34. doi: 10.4193/Rhino14.160. [DOI] [PubMed] [Google Scholar]
- 24.Kim DW, Kim JY, Jeon SY. The status of the olfactory cleft may predict postoperative olfactory function in chronic rhinosinusitis with nasal polyposis. American journal of rhinology & allergy. 2011;25:e90–94. doi: 10.2500/ajra.2011.25.3617. [DOI] [PubMed] [Google Scholar]