Abstract
Cranial placodes are thickenings in the ectoderm that give rise to sensory organs and peripheral ganglia of the vertebrate head. At gastrula and neurula stages, placodal precursors are intermingled in the neural plate border with future neural and neural crest cells. Here, we show that the epigenetic modifier, DNA methyl transferase (DNMT) 3A, expressed in the neural plate border region, influences development of the otic placode which will contribute to the ear. DNMT3A is expressed in the presumptive otic region at gastrula through neurula stages and later in the otic placode itself. Whereas neural plate border and non-neural ectoderm markers Erni, Dlx5, Msx1 and Six1 are unaltered, DNMT3A loss of function leads to early reduction in the expression of the key otic placode specifier genes Pax2 and Gbx2 and later otic markers Sox10 and Soho1. Reduction of Gbx2 was first observed at HH7, well before loss of other otic markers. Later, this translates to significant reduction in the size of the otic vesicle. Based on these results, we propose that DNMT3A is important for enabling the activation of Gbx2 expression, necessary for normal development of the inner ear.
Keywords: otic, placode, DNMT3A, Gbx2, Pax2
Introduction
Cranial ectodermal placodes are thickened epithelial structures in the head ectoderm of the early embryo that contribute to sensory organs (ear, eye and olfactory epithelium) and major ganglia of the head (trigeminal, epibranchial). All placode precursors arise at gastrula and neurula stages from the neural plate border region, between prospective neural and non-neural ectoderm, where they are intermingled with future neural and neural crest cells (Bhattacharyya and Bronner-Fraser, 2004; Meulemans and Bronner-Fraser, 2004; Streit and Stern, 1999).
Temporal and spatial integration of different signals and transcription factors defines the neural plate border territory. At neurula stages, mesenchymal FGF signaling induces Wnt8a and FGF signaling in neural ectoderm that defines the pre-placodal region in the non-neural ectoderm. Several transcription factors characteristic of this region are induced, including Dlx family genes, Erni, Sox3, Msx1 and Foxi genes (Ekker et al., 1992; Groves and Bronner-Fraser, 2000; Liu et al., 2003; Solomon and Fritz, 2002). At late neurula stages, FGF, BMP and WNT signaling are thought to segregate fates within the border area by regulating a set of transcription factors including Pax7, Snai2, FoxD3, Sox10, Six1/4 and Eya1/2. Subsequently, on the basal side, FGF induces otic invagination (Ladher et al., 2010; Streit, 2007).
This complex network of signaling and transcription factors requires tight spatiotemporal regulation, as might be provided by epigenetic modifiers. Recently, we showed that DNA methylation by DNMT3A promotes neural crest fate while inhibiting neural fate (Hu et al., 2012), suggesting a possible role for these epigenetic factors in regulating cell fate decisions at the neural plate border. DNA methyltransferases function by recognizing CpG islands and catalyzing the transfer of a methyl group to DNA on the C5 position of cytosine (Cheng and Blumenthal, 2008). Methylation of CpG sites in the promoter region of a gene is thought to inhibit its expression, as shown in cancer and stem cells (Altun et al., 2010; Miranda and Jones, 2007; Momparler and Bovenzi, 2000). In some cases, DNMTs are also thought to activate gene expression by directly methylating gene bodies (Ball et al., 2009; Lister et al., 2009) or by inhibiting binding of repressors via methylation (Bahar Halpern et al., 2014).
Since DNMT3A is broadly expressed in the neural plate border territory from which not only neural crest but also placodal precursors arise, this raised the possibility that it may be required for normal placode development. Here, we test the function of DNMT3A on formation of the otic placode and early development of the ear. The results show that loss of DNMT3A causes loss of early ear markers Gbx2 and Pax2 as well as later otic markers Sox10 and Soho1. This leads to later defects in the otic placode, suggesting that epigenetic regulation is critical for normal ear development.
Results
DNMT3A is expressed in presumptive otic region and otic placode
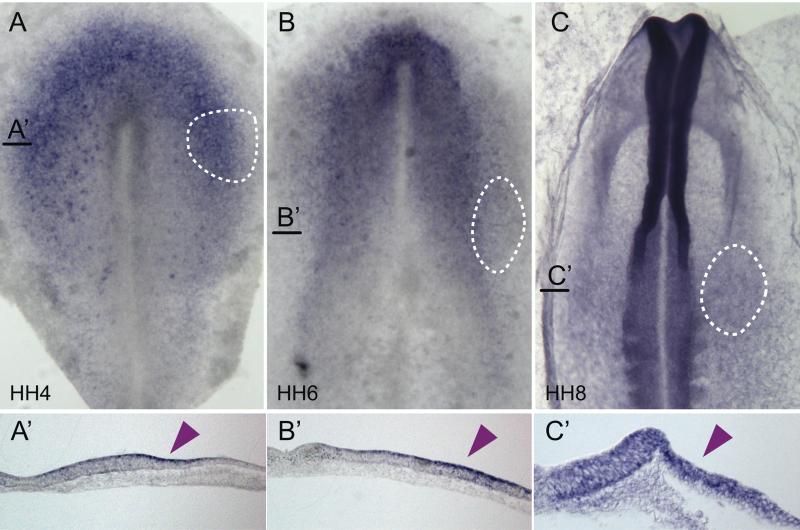
As a first step in examining the possible relationship between DNMT3A and placode development, we performed in situ hybridization at gastrula through neurula stages to determine its expression pattern relative to that of ear precursors (dashed lines in Fig. 1). We find that DNMT3A is expressed in the neural plate border at HH4 (Fig. 1A). While high expression continues in the neural plate, as previously shown (Hu et al., 2012), DNMT3A also is expressed laterally in the presumptive ear territory at HH6 (Fig. 1B) and HH8 (Fig. 1C). Transverse sections reveal that expression is elevated in the ectodermal layer at the stages examined (Fig. 1A’, B’, C’).
Fig. 1.
DNMT3A is expressed at sites of otic placode formation. DNMT3A transcripts are expressed (A) in the neural plate border region (HH 4), (B, C) the dorsal neural folds and otic placode (HH 6, 8). Dashed circles indicate position of otic precursors/placode. Black lines indicate position of section (A’, B’, C’). Right half of embryo is shown for sections. Arrowheads mark the position of the otic placode/vesicle. HH, staging after Hamburger and Hamilton.
Inhibition of DNMT3A reduces the expression of otic placode markers and the size of the otic vesicle
To assess the functional importance of DNMT3A, we performed loss of function experiments using a translational blocking morpholino (MO) (Hu et al., 2012). The morpholino was electroporated into the gastrula stage embryo at HH4 either targeting one half of the head region or the lateral placode-forming region of the embryo. The effects on placode development were examined at subsequent developmental stages by in situ hybridization to determine effects on otic marker genes.
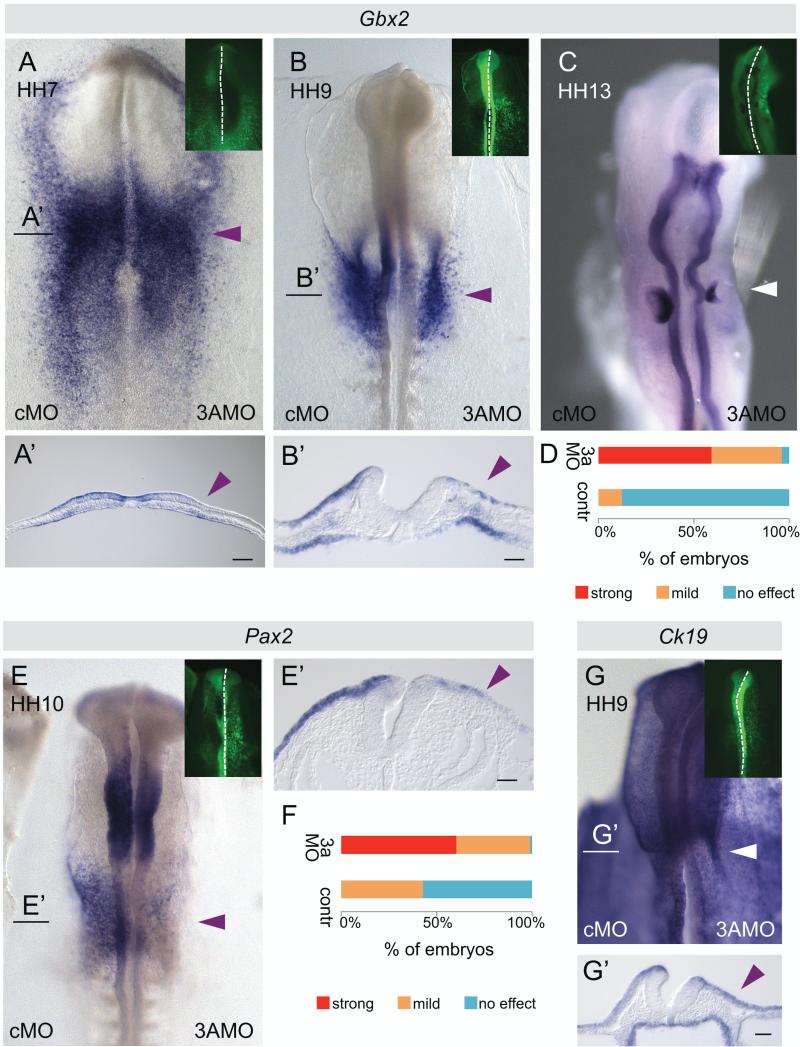
Knock down of DNMT3A caused a down-regulation of the otic placode marker Gbx2 very early in the process of placode specification, by HH7 (Fig. 2A, A’). At HH9 Gbx2 was markedly down-regulated in the DNMT3A MO treated side (Fig. 2B, B’, D). Similar results were obtained for Pax2, another otic placode marker, at HH10 (Fig. 2E, E’, F).
Fig. 2.
Morpholino-mediated knock down of DNMT3A reduces otic marker expression and otic vesicle size. DNMT3A MO or control MO were electroporated into the right or left half of the embryo, respectively, at HH4. Morpholinos were FITC-labeled (green, small insets). (A, A’) Gbx2 RNA expression was reduced in the pre-placodal domain at HH7 on the 3A MO electroporated side. Black line indicates level of section (A’). (B) At HH9 Gbx2 RNA expression was reduced in the otic placode on the 3A MO electroporated side. Black line indicates level of section (B’). (C) At HH13 otic vesicle size was reduced on the 3A MO injected side. (D) Graph shows quantification of Gbx2 loss of expression phenotype comparing 3A MO injected sides (n=18) to control MO or uninjected sides (n=26) at HH9-10. (E) DNMT3A morpholino knock down causes reduced Pax2 RNA expression in the otic area. Black line indicates level of section (E’). (F) The graph shows a quantification of the Pax2 loss of expression phenotype. Knock down, n=24; control, n=14. Arrowheads mark the position of the otic placode/vesicle on the 3AMO electroporated side. (G, G’) Expression of epidermal marker Ck19 is unaltered upon DNMT3A MO knockdown (n=9). Bar, 50 μm.
We also noted down-regulation of Sox10 at HH11 after initiation of its expression in the otic placode (Fig. S1A, B), similar to its effects on Sox10 expression in the neural crest (Hu et al., 2012), and otic expression of Soho1 at HH12 (Fig S1C) upon DNMT3A MO treatment. Interestingly, DNMT3A MO knockdown did not alter the expression of the epidermal marker Ck19 in the otic region (Fig 2G, G’).
The early effects on gene expression led to subsequent defects in otic development as evidenced by a reduced size of the otic vesicle (Fig. 2C). By the time that the morpholino was introduced at HH4, DNMT3A is already expressed. Thus, it is likely that some DNMT3A activity persists in the treated embryos due to perdurance of already translated protein and stability of resulting DNA methylation, likely explaining the semi-penetrant effects on otic vesicle formation.
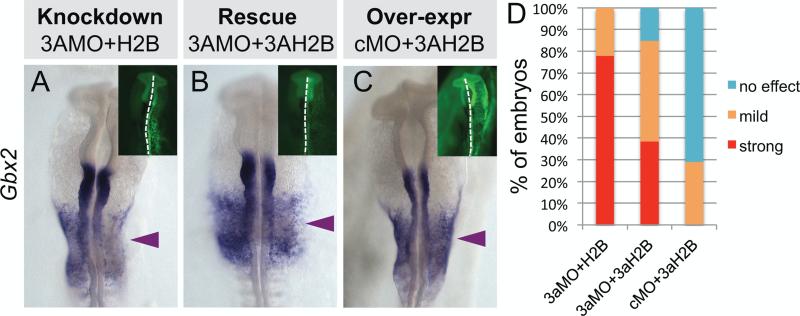
To control for the specificity of the DNMT3A morpholino and demonstrate that the observed phenotype was not due to off-target effects, we coelectroporated the DNMT3A MO with a construct encoding ubiquitously expressed DNMT3A. This resulted in a marked rescue of the loss of function phenotype and restored Gbx2 expression (Fig. 3A, B, D). Over-expression of the DNMT3A rescue construct alone did not alter Gbx2 expression (Fig. 3C).
Fig. 3.
Loss of Gbx2 is rescued by exogenous DNMT3A. Embryos were electroporated with DNMT3A MO in combination with (A) empty vector or (B) DNMT3A expression construct or (C) embryos were electroporated with control MO and DNMT3A expression vector. (D) Graph represents a quantification of embryos with a strong, mild or no effect on Gbx2 expression phenotype upon DNMT3A MO knock down compared to rescue and overexpression experiments. Knockdown, n=9; rescue, n=13; overexpression, n=7.
Altogether these results suggest that DNMT3A is critical for epigenetic regulation of the otic placode formation and normal early development of the inner ear.
DNMT3A knockdown does not alter expression of early neural plate border and non-neural ectoderm markers
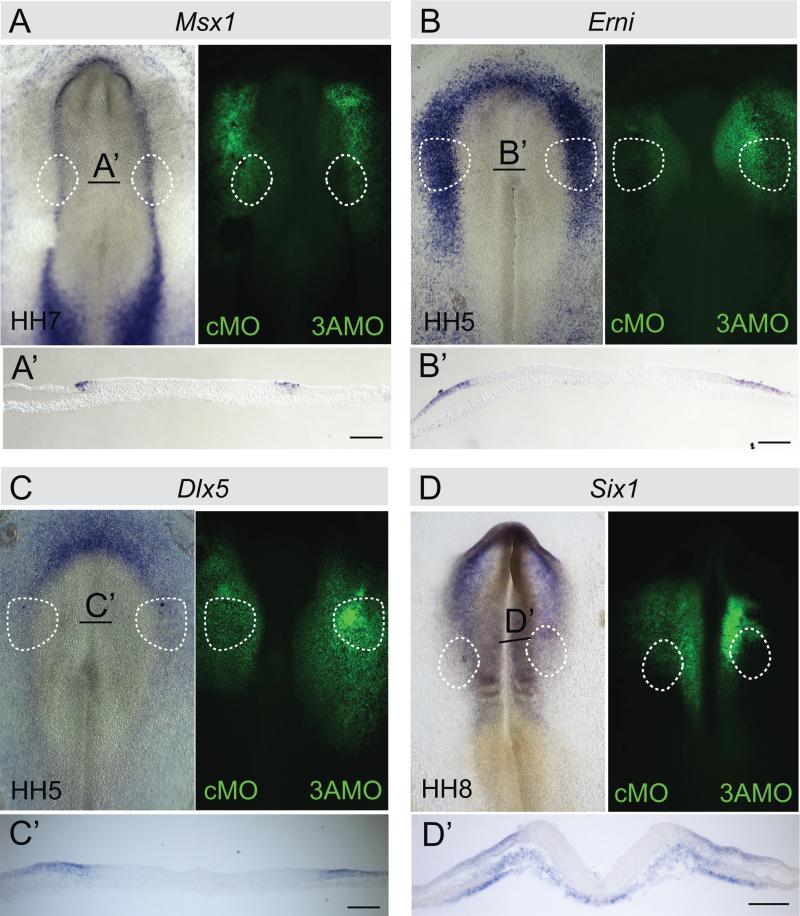
Because DNMT3A expression is already present at HH4, it is possible that loss of Gbx2 and Pax2 in the otic domain might be secondary to abnormalities in the formation of the neural plate border. To examine this possibility, we tested whether loss of DNMT3A influenced expression of genes important for neural plate border specification. The results show that expression of key early neural plate border markers Erni and Msx1 (Fig. 4 A, B) and non-neural ectoderm markers Dlx5 and Six1 (Fig. 4 C, D) was unaltered when comparing the DNMT3A-MO injected side with the contralateral control MO injected side of the same embryo. This suggests that loss of the otic fate is not due to a secondary effect on neural plate border formation.
Fig. 4.
Knock down of DNMT3A does not affect expression of early neural plate border and non-neural ectoderm markers Msx1, Erni, Dlx5 and Six1. HH4 embryos were electroporated with FITC labeled control MO (left side) or DNMT3A MO (right side). RNA levels of neural plate border markers (A) Msx1 (n=8/9), (B) Erni (8/8) and the non-neural ectoderm markers (C) Dlx5 (n=8/8) and (D) Six1 (n=6/7) were not reduced on the DNMT3A MO injected side. Black line indicates level of section (A’–D’). Bar, 50 μm.
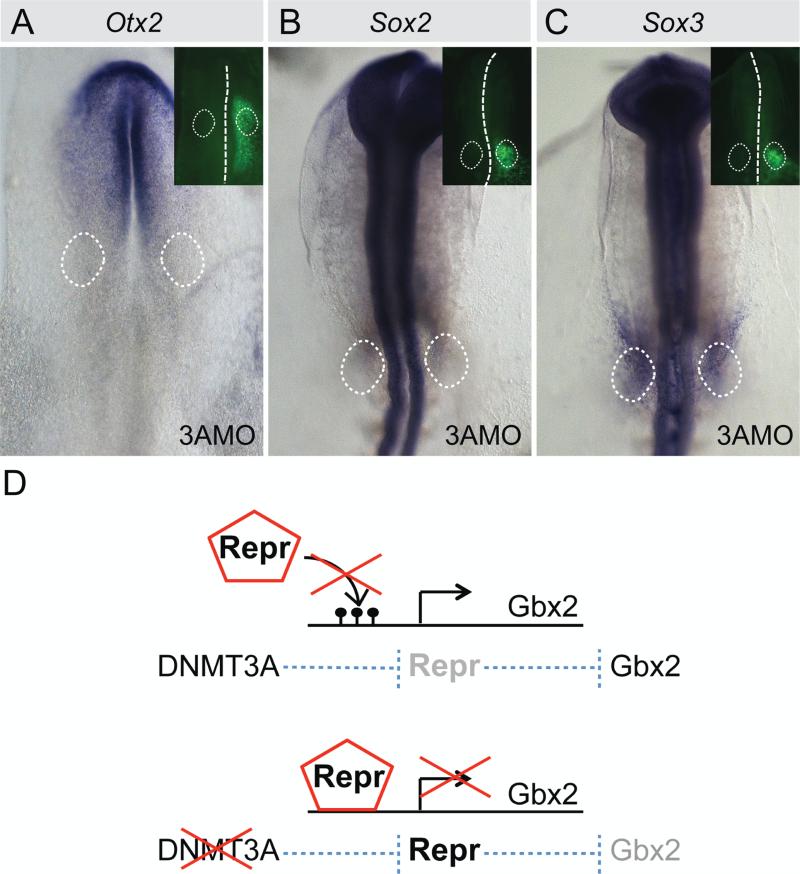
Mutual repression between Gbx2 and Otx2 has been reported in sensory placode formation (Steventon et al., 2012). Therefore we also tested whether DNMT3A knock down causes an expansion of Otx2 expression at the expense of Gbx2. However, the results revealed no change in Otx2 expression (Fig. 5A). Similarly, expression of Sox2 and Sox3, previously shown to be direct targets of DNMT3A during neural crest formation (Hu et al., 2012), were unaffected in the otic placodal domain at HH9 (Fig. 5B, C).
Fig. 5.
Otx2, Sox2 and Sox3 expression remains unchanged upon DNMT3A knock down. Embryos were injected with 3A MO on the embryonic right side. Morpholinos were FITC-labeled (green, small insets). DNMT3A knock down leaves (A) Otx2 (n=13/13), (B) Sox2 (n=7/7) and (C) Sox3 (n=7/8) expression unaltered. Dashed circles indicate position of otic domain. (D) Model of function of DNMT3A during otic development. In presence of DNMT3A, CpG islands in the putative repressor binding sites are methylated, preventing repressor binding to the target gene. When DNMT3A is decreased, methylation of repressor binding sites is reduced or not present. This allows repressors to bind and prevents target gene expression.
FGF signaling from tissues basal to the otic area has been shown to be important for proper otic invagination (Ladher et al., 2010; Streit, 2007). Therefore we checked the expression of a downstream effector of FGF signaling, Erm, to investigate whether the pre-placodal ectoderm is receiving FGF signals in the morphant embryos. Additionally, since canonical WNT signaling is important for Gbx2 expression (Li et al., 2009), we checked expression of a downstream repressor, Tcf3 (Cole et al., 2008; Kim et al., 2000; Merrill et al., 2004; Yi et al., 2011). We picked these markers, because they are both expressed in the region of interest at the time when we observe reduced Gbx2 expression upon DNMT3A knock down. However, both markers, Tcf3 and Erm, were unaltered upon DNMT3A knock down (Fig. S2).
Taken together, these results show that loss of DNMT3A selectively effects formation of the otic placode domain without influencing neural plate border formation.
Discussion
DNMT3s are essential for de novo methylation during early developmental stages (Okano et al., 1999). They play important roles in cancer and disease, but also normal aspects of mammalian development (Ehrlich et al., 2008; Linhart et al., 2007). Many tumor suppressor genes are silenced in a variety of human cancer cells by promoter methylation whereas some oncogenes are activated by hypomethylation. In tumor cells, excessive levels of DNMT3B bind to the promoter region of E-cadherin, reducing adhesion and aggregation, which correlates with increased metastasis (Kwon et al., 2010). Several studies demonstrate a role of DNMT3 methyltransferases in development. Single DNMT3B null embryos have rostral neural tube defects and growth impairment, whereas DNMT3A and 3B double mutants are embryonic lethal (Okano et al., 1999).
Interestingly, alterations in DNA methylation have also been associated with several syndromes that cause hearing related problems (Provenzano and Domann, 2007). For instance DNA hypomethylation has been related with hearing loss in patients with ICF syndrome (immunodeficiency, centromere instability, facial anomalies) (Robertson et al., 1999; Xie et al., 1999). In this disease, large parts of several chromosomes remain unmethylated, which leads to chromosomal rearrangements, thereby effecting gene expression (Jeanpierre et al., 1993; Stacey et al., 1995). Hearing loss has also been reported in patients with facioscapulohumeral muscular dystrophy (FSHD1). Deletions and hypomethylation in the D4Z4 array on chromosome 4 seem key events in this disease (van Overveld et al., 2003).
These data suggest that DNMT3 methyltransferases have specificity to certain genes to influence particular developmental processes during ear development. Here we show for the first time an influence of DNMT3A on very early stages of inner ear development, during the development of the otic placode.
Previous work from our lab has shown that DNMT3A is highly expressed in the neural plate border at gastrula stages and, later on, is expressed in neural folds and neural crest cells (Hu et al., 2012). Loss of functions experiments further revealed that epigenetic modifications were crucial for neural versus neural crest cell fate decisions in the neural tube (Hu et al., 2012), such that DNA methyltransferase DNMT3A promotes neural crest specification by directly binding to and repressing the neural genes Sox2 and Sox3. In contrast, in the developing ear, we find that Sox2 and Sox3 are neither expressed earlier nor is their expression altered after DNMT3A loss in the otic area. This difference might be explained by the possibility that DNMT3A cooperates with different cofactors in different tissues, which would go in line with the late onset of Sox2, and Sox3 expression in the otic region compared to the early neural tube expression of these two genes.
The present results show that, in addition to the neural plate border region, DNMT3A also is expressed in the pre-placodal domain at gastrula stages and later at lower levels in the chick otic placode domain. Selective loss of DNMT3A in the otic domain leads to loss of several otic markers, Gbx2, Pax2, Sox10 and Soho1, and subsequent reduction of the otic vesicle. This is in agreement with previous findings in mouse (Okano et al., 1999). Although the authors did not focus on otic placode development in DNMT3A knockout mice, their data suggest a reduction in otic vesicle size in DNMT3A+/−, but not DNMT3B+/− mice (Okano et al. 1999, Fig. 3, compare D and H).
In general, DNMTs are thought to act via methylation of CpG islands in the promoter region of genes, resulting in inhibition of gene expression. In contrast, the present data suggest that DNMT3A acts as a positive regulator of Gbx2 and Pax2, either directly or indirectly. A similar mechanism has been described for FoxA2 activation during endoderm development (Bahar Halpern et al., 2014). This study hypothesized that in undifferentiated hESC-derived early endoderm-stage cells, low DNA methylation of certain CpG islands enabled binding of repressive proteins. Upon differentiation, DNA methylation of these CpG islands caused loss of repressor binding, thus leading to activation of FoxA2.
We propose that a similar mechanism may be invoked in the otic placode, such that DNMT3A methylation of CpG islands prevents binding of yet unknown repressors, allowing the activation of otic marker expression. Accordingly to this model, in the presence of DNMT3A, CpG islands in the putative repressor binding sites become methylated, thus preventing repressor binding to the target gene. Loss of DNMT3A results in demethylation of these repressor binding sites. In this state, the repressors bind and block target gene expression (Fig. 5D).
In summary, we have interrogated the function of DNMT3A in early ear development. Our results place DNMT3A upstream of one of the earliest otic genes Gbx2 (Hidalgo-Sanchez et al., 2000; Paxton et al., 2010). Establishing epigenetic regulation as a driver of otic specifier expression provides important insights into how the chick inner ear is induced.
Methods
Embryos
Fertilized chicken eggs were incubated at 37°C to the desired stages.
In situ hybridization
Embryos were fixed with 4% paraformaldehyde, washed with PBS/0.1% Tween, dehydrated in MeOH, and stored at −20°C. Whole-mount in situ hybridization was performed as described (Acloque et al., 2008; Wilkinson, 1992). Dioxigenin-labeled RNA probes were made from DNA plasmids or ESTs of DNMT3A, Dlx5, Erm, Erni, Gbx2, Msx1, Otx2, Pax2, Six1, Sox2, Sox3, Sox10, Soho1, and. Tcf3. Embryos were cryo-sectioned at 20 mm.
Electroporation
Embryos were electroporated at HH 4–5 as described (Sauka-Spengler and Barembaum, 2008) using DNMT3AMO1 (over ATG codon) (TGGGTGTGTCACTGCTTTCCACCAT) or DNMT3AMO2 (95 nucleotides upstream of ATG) (CAGTGTCCCCACGGCGCTTCCTGCT) (Hu et al., 2012). The coding region of DNMT3A (NM001024832.1) (Hu et al., 2012) was cloned into the pCI H2B-RFP vector (Betancur et al., 2010) as rescue construct. For each embryo, 0.6-0.7 mM MO + 0.5 mg/mL DNA was used for knockdown experiments, and 0.6 mM MO + 1-1.5 mg/mL DNA was used for rescue experiments, except if stated otherwise in the text. The MO was targeted to either half of the anterior side of the embryo or the presumptive otic region with five 50-msec pulses of 5.2 V at 100-msec intervals. No phenotypic difference in the otic area was observed between one and the other injection method. Electroporated embryos were maintained in culture dishes with 1 mL of albumen at 37°C and then fixed.
Supplementary Material
Highlights.
epigenetic modifier DNMT3A influences development of the otic placode
DNMT3A knock down reduces expression of early otic placode specifiers Pax2 and Gbx2
DNMT3A knock down causes a reduction in the size of the otic vesicle
DNMT3A acts as a positive regulator of Gbx2 expression during inner ear development
Acknowledgements
We thank Bertrand Bénazéraf for helpful discussions and critical reading of the manuscript. This work was funded by grants DC011577 and DE16459 from the NIH. DR was funded by the Deutsche Forschungsgemeinschaft (DFG; RO 4334/1-1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement. None declared.
References
- Acloque H, Wilkinson DG, Nieto MA. In situ hybridization analysis of chick embryos in whole-mount and tissue sections. Methods in cell biology. 2008;87:169–185. doi: 10.1016/S0091-679X(08)00209-4. [DOI] [PubMed] [Google Scholar]
- Altun G, Loring JF, Laurent LC. DNA methylation in embryonic stem cells. Journal of cellular biochemistry. 2010;109:1–6. doi: 10.1002/jcb.22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar Halpern K, Vana T, Walker MD. Paradoxical role of DNA methylation in activation of FoxA2 gene expression during endoderm development. The Journal of biological chemistry. 2014;289:23882–23892. doi: 10.1074/jbc.M114.573469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball MP, Li JB, Gao Y, Lee JH, LeProust EM, Park IH, Xie B, Daley GQ, Church GM. Targeted and genome-scale strategies reveal gene-body methylation signatures in human cells. Nature biotechnology. 2009;27:361–368. doi: 10.1038/nbt.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancur P, Bronner-Fraser M, Sauka-Spengler T. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3570–3575. doi: 10.1073/pnas.0906596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Bronner-Fraser M. Hierarchy of regulatory events in sensory placode development. Current opinion in genetics & development. 2004;14:520–526. doi: 10.1016/j.gde.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Cheng X, Blumenthal RM. Mammalian DNA methyltransferases: a structural perspective. Structure. 2008;16:341–350. doi: 10.1016/j.str.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes & development. 2008;22:746–755. doi: 10.1101/gad.1642408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M, Sanchez C, Shao C, Nishiyama R, Kehrl J, Kuick R, Kubota T, Hanash SM. ICF, an immunodeficiency syndrome: DNA methyltransferase 3B involvement, chromosome anomalies, and gene dysregulation. Autoimmunity. 2008;41:253–271. doi: 10.1080/08916930802024202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker M, Akimenko MA, Bremiller R, Westerfield M. Regional expression of three homeobox transcripts in the inner ear of zebrafish embryos. Neuron. 1992;9:27–35. doi: 10.1016/0896-6273(92)90217-2. [DOI] [PubMed] [Google Scholar]
- Groves AK, Bronner-Fraser M. Competence, specification and commitment in otic placode induction. Development. 2000;127:3489–3499. doi: 10.1242/dev.127.16.3489. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Sanchez M, Alvarado-Mallart R, Alvarez IS. Pax2, Otx2, Gbx2 and Fgf8 expression in early otic vesicle development. Mechanisms of development. 2000;95:225–229. doi: 10.1016/s0925-4773(00)00332-4. [DOI] [PubMed] [Google Scholar]
- Hu N, Strobl-Mazzulla P, Sauka-Spengler T, Bronner ME. DNA methyltransferase3A as a molecular switch mediating the neural tube-to-neural crest fate transition. Genes & development. 2012;26:2380–2385. doi: 10.1101/gad.198747.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanpierre M, Turleau C, Aurias A, Prieur M, Ledeist F, Fischer A, Viegas- Pequignot E. An embryonic-like methylation pattern of classical satellite DNA is observed in ICF syndrome. Human molecular genetics. 1993;2:731–735. doi: 10.1093/hmg/2.6.731. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O, Jeong SJ, Kim SO, He L, Lee HG, Jang KL, Osada H, Jung M, Kim BY, Ahn JS. Modulation of E-cadherin expression by K-Ras; involvement of DNA methyltransferase-3b. Carcinogenesis. 2010;31:1194–1201. doi: 10.1093/carcin/bgq071. [DOI] [PubMed] [Google Scholar]
- Ladher RK, O'Neill P, Begbie J. From shared lineage to distinct functions: the development of the inner ear and epibranchial placodes. Development. 2010;137:1777–1785. doi: 10.1242/dev.040055. [DOI] [PubMed] [Google Scholar]
- Li B, Kuriyama S, Moreno M, Mayor R. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development. 2009;136:3267–3278. doi: 10.1242/dev.036954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart HG, Lin H, Yamada Y, Moran E, Steine EJ, Gokhale S, Lo G, Cantu E, Ehrich M, He T, Meissner A, Jaenisch R. Dnmt3b promotes tumorigenesis in vivo by gene-specific de novo methylation and transcriptional silencing. Genes & development. 2007;21:3110–3122. doi: 10.1101/gad.1594007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Chu H, Maves L, Yan YL, Morcos PA, Postlethwait JH, Westerfield M. Fgf3 and Fgf8 dependent and independent transcription factors are required for otic placode specification. Development. 2003;130:2213–2224. doi: 10.1242/dev.00445. [DOI] [PubMed] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E. Tcf3: a transcriptional regulator of axis induction in the early embryo. Development. 2004;131:263–274. doi: 10.1242/dev.00935. [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Developmental cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Miranda TB, Jones PA. DNA methylation: the nuts and bolts of repression. Journal of cellular physiology. 2007;213:384–390. doi: 10.1002/jcp.21224. [DOI] [PubMed] [Google Scholar]
- Momparler RL, Bovenzi V. DNA methylation and cancer. Journal of cellular physiology. 2000;183:145–154. doi: 10.1002/(SICI)1097-4652(200005)183:2<145::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- Paxton CN, Bleyl SB, Chapman SC, Schoenwolf GC. Identification of differentially expressed genes in early inner ear development. Gene Expr Patterns. 2010;10:31–43. doi: 10.1016/j.gep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provenzano MJ, Domann FE. A role for epigenetics in hearing: Establishment and maintenance of auditory specific gene expression patterns. Hear Res. 2007;233:1–13. doi: 10.1016/j.heares.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KD, Uzvolgyi E, Liang G, Talmadge C, Sumegi J, Gonzales FA, Jones PA. The human DNA methyltransferases (DNMTs) 1, 3a and 3b: coordinate mRNA expression in normal tissues and overexpression in tumors. Nucleic acids research. 1999;27:2291–2298. doi: 10.1093/nar/27.11.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauka-Spengler T, Barembaum M. Gain- and loss-of-function approaches in the chick embryo. Methods in cell biology. 2008;87:237–256. doi: 10.1016/S0091-679X(08)00212-4. [DOI] [PubMed] [Google Scholar]
- Solomon KS, Fritz A. Concerted action of two dlx paralogs in sensory placode formation. Development. 2002;129:3127–3136. doi: 10.1242/dev.129.13.3127. [DOI] [PubMed] [Google Scholar]
- Stacey M, Bennett MS, Hulten M. FISH analysis on spontaneously arising micronuclei in the ICF syndrome. J Med Genet. 1995;32:502–508. doi: 10.1136/jmg.32.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon B, Mayor R, Streit A. Mutual repression between Gbx2 and Otx2 in sensory placodes reveals a general mechanism for ectodermal patterning. Developmental biology. 2012;367:55–65. doi: 10.1016/j.ydbio.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A. The preplacodal region: an ectodermal domain with multipotential progenitors that contribute to sense organs and cranial sensory ganglia. The International journal of developmental biology. 2007;51:447–461. doi: 10.1387/ijdb.072327as. [DOI] [PubMed] [Google Scholar]
- Streit A, Stern CD. Establishment and maintenance of the border of the neural plate in the chick: involvement of FGF and BMP activity. Mechanisms of development. 1999;82:51–66. doi: 10.1016/s0925-4773(99)00013-1. [DOI] [PubMed] [Google Scholar]
- van Overveld PG, Lemmers RJ, Sandkuijl LA, Enthoven L, Winokur ST, Bakels F, Padberg GW, van Ommen GJ, Frants RR, van der Maarel SM. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nat Genet. 2003;35:315–317. doi: 10.1038/ng1262. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. situ hybridization: A practical approach. IRL Press; Oxford, UK: 1992. Whole mount in situ hybridization of vertebrate embryos. pp. 75–83. [Google Scholar]
- Xie S, Wang Z, Okano M, Nogami M, Li Y, He WW, Okumura K, Li E. Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene. 1999;236:87–95. doi: 10.1016/s0378-1119(99)00252-8. [DOI] [PubMed] [Google Scholar]
- Yi F, Pereira L, Hoffman JA, Shy BR, Yuen CM, Liu DR, Merrill BJ. Opposing effects of Tcf3 and Tcf1 control Wnt stimulation of embryonic stem cell self-renewal. Nat Cell Biol. 2011;13:762–770. doi: 10.1038/ncb2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.