Summary
Objective
The pathophysiology of sudden unexpected death in epilepsy (SUDEP) remains undetermined. Seizures are accompanied by respiratory dysfunction (RD). Postictal generalized EEG suppression (PGES) may follow generalized tonic-clonic seizures (GTCS). Following GTCS patients have impaired arousal and may be motionless. Patients with SUDEP are usually prone. Postictal immobility (PI) may contribute to SUDEP by not permitting repositioning of the head to allow unimpeded ventilation. To determine whether RD and/or ictal characteristics are associated with PI, we analyzed patients with GTCS in the Epilepsy Monitoring Unit.
Method
We investigated for associations between PI duration and: PGES, ictal/postictal oxygen saturation (SpO2), end-tidal CO2 (ETCO2), seizure localization, duration, and tonic and total convulsive phase duration. We investigated for linkage between PGES and these measures.
Results
70 patients with 181 GTCS and available SpO2 and/or ETCO2 data were studied.
Simple linear regression analysis by seizures showed that PI duration was associated with peak peri-ictal ETCO2 (p=0.03), duration of oxygen desaturation (p=0.005) and with SpO2 nadir (p=0.02). PI duration was not associated with tonic, convulsive phase or total seizure duration. Analysis by patients also showed significant association of PI with RD.
Duration of PI was longer following seizures with PGES (p<0.001). PGES was not associated with the tonic, convulsive phase or total seizure duration. SpO2 nadir was lower in seizures with PGES (p=0.046), ETCO2 peak change (p=0.003) was higher and duration of ETCO2 elevation (p=0.03) was longer. Multivariable regression analysis showed that PGES and severe RD were associated with PI duration.
Significance
The duration of PI and presence of PGES are associated with peri-ictal RD. The duration of PI is also associated with the presence of PGES. Seizure duration or duration of the convulsive phase is not associated with PI or PGES. Interventions aimed at reversing impaired arousal and PI may reduce SUDEP risk.
Keywords: Postictal immobility, respiratory dysfunction, seizure, convulsion, postictal generalized EEG suppression, sudden unexpected death in epilepsy
Introduction
The pathophysiological mechanisms underlying sudden unexpected death in epilepsy (SUDEP) remain undetermined. Seizures recorded in the EMU are frequently accompanied by ictal/postictal hypoxemia and hypercapnia 1–5. A landmark study of ten SUDEP cases occurring in epilepsy monitoring units (EMU) has added considerable knowledge regarding the pathophysiology of SUDEP 6. All cases of SUDEP occurred following a generalized tonic-clonic seizure (GTCS). Assessment of respiratory function in this study was indirect and determined from observation of the available video data 6. Postictal tachypnea was observed followed by transient or terminal cardiorespiratory dysfunction. Terminal apnea preceded cardiac arrest in all patients 6.
Postictal generalized EEG suppression (PGES) is often present following GTCS 7–9. A possible association between PGES and SUDEP remains controversial. In one study, PGES duration greater than 20 sec was found to be associated with increased risk of SUDEP following GTCS 10. However, another study failed to demonstrate an association between PGES and SUDEP 7. In one study, where direct respiratory information was not available, the tonic phase of a GTCS was found to be associated with PGES 11. It has been suggested that PGES may represent a “switch-off” inhibitory mechanism that involves the brainstem and associated with apnea 10. On the other hand, EMU recordings demonstrate tachypnea in the immediate postictal period with increased amplitude of the nasal airflow signal during periods of PGES suggesting that respiratory brainstem mechanisms may not be impaired 12. PGES is associated with the severity of respiratory dysfunction 9. Despite the presence of postictal tachypnea, hypercapnia can persist for many minutes following a seizure indicating impaired ventilation and the possibility that intrinsic pulmonary dysfunction may come into play following a GTCS 12.
Patients may be motionless following a seizure, this lack of motion is associated with the presence of PGES 8. However, in that study, data from direct monitoring of respiratory parameters were not available 8. Seizure characteristics that may be associated with PI have not been adequately studied. Postictal immobility (PI) may be relevant in the pathophysiology of SUDEP. Most patients with SUDEP have been found in the prone position 6, 13. Following GTCS a patient who ends up prone and unable to reposition the head may be at risk for SUDEP by suffocation.
In a group of 39 patients, we previously showed that the duration of oxygen desaturation was associated with PI duration 14. In that study, statistical analysis of the multiple variables recorded was limited by the relatively small number of observations 14.To determine whether respiratory dysfunction and/or ictal characteristics are associated with PI and PGES, we have now analyzed a larger group of patients with GTCS undergoing VET in the EMU. These patients had concurrent synchronized recordings of peripheral oxygen saturation (SpO2) and end-tidal CO2 (ETCO2). We investigated associations between seizure characteristics, PI, PGES, and respiratory dysfunction. The larger number of observations now available, of ictal-related SpO2, ETCO2, PGES and PI, permit statistical analysis of the multiple variables investigated.
Materials and Methods
Patients undergoing VET in the EMU who had GTCS were studied at the University of California Davis Medical Center following approval by the UC Davis institutional review board. All patients admitted to this EMU also undergo concurrent recording of peripheral oxygen saturation (SpO2) using digital pulse oximetry and measurement of ETCO2 using a nasal cannula. Details of the recording techniques have been published previously 2. Consecutive patients with GTCS who had usable SpO2 and/or ETCO2 were selected for analysis. The present group of patients includes the 39 patients reported previously. The following primary parameters were recorded. The total duration of each seizure, duration of the tonic phase and that of convulsive component (tonic plus clonic) were determined. Total seizure duration was determined from the electrographic onset to end of seizure. The tonic phase was defined as bilateral extension of the arms whether symmetric or asymmetric. The tonic phase gradually merged into clonic activity with seizures. The onset of the clonic phase was defined as the presence of clonic activity on review of the VET with accompanying pauses in electromyographic activity. All seizures were reviewed by a board-certified epileptologist. The ictal/postictal SpO2 nadir and duration of oxygen desaturation below 90%, the peak ictal/postictal ETCO2 and duration of ETCO2 elevation above pre-ictal baseline were recorded. PGES of 2 seconds or longer was determined as previously described 10. The duration of PI was defined as the time from the end of the seizure determined electrographically, to the onset of the first postictal active non-respiratory movement detected on review of the video recording.
From these primary data, four major possible predictors for duration of PI were chosen for analysis using multivariable linear regression. The choice of these potential predictors was based on findings from previously published data 8, 9, 11, 14. The four predictors studied included (1) severe respiratory disturbance defined as a drop in SpO2 ≤ 70% or an elevation of ETCO2 ≥ 40 mm Hg above preictal baseline, (2) the presence of PGES ≥ 2 seconds, (3) the duration of the tonic component of the preceding seizure, (4) nursing intervention prior to the onset of hypoxemia defined as a drop in SpO2 below 90%.
Statistical analysis
Simple linear regression was first used to analyze for possible significant relationships between the parameters recorded and PI. A two-sided Wilcoxon rank-sum test was used to compare the group of seizures with PGES and that without PGES for total seizure duration, duration of convulsion, duration of the tonic phase of the seizure, ETCO2 change from preictal baseline, duration of ETCO2 elevation above baseline, SpO2 nadir, duration of oxygen desaturation below 90%, and the duration of PI. Simple logistic regression was used to determine possible relationships with the presence or absence of PGES of any duration that was 2 seconds or longer.
To account for correlation among seizures in each patient, a multivariable linear mixed-effects model was used to study the relation between possible predictors and duration of PI. Finally, multivariable linear regression analysis was performed for the four major potential predictors listed above on the duration of PI (outcome variable). A p-value <0.05 was considered significant. All analyses were performed with SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
Results
70 patients (37 female) were studied. The mean age was 35.1 ± 12.0 years. A total of 181 generalized convulsions were recorded. Seizures were of temporal onset in 129, frontal onset in 18, generalized at onset in 5, occipital in 1 and centroparietal in 1. Seizure onset could not be localized in the remainder.
The data are presented as mean ± standard deviation (median, range). For the entire group, the total seizure duration was 133 ± 78.8 sec (114, 38–516). The duration of the convulsive phase (tonic plus clonic) was 73.9 ± 36.9 sec (67, 16–324). The duration of the tonic phase was 21.3 ± 9.3 sec (20, 4–60).
The mean preictal baseline SpO2 was 97 ± 2.5% (98, 88–100). The ictal/postictal SpO2 nadir was 74 ± 11% (76, 42–98). The duration of oxygen desaturation was 100.6 ± 91.2 sec (82, 4–712). The preictal baseline ETCO2 was 37.0 ± 7.1 mmHg (37, 14.5–56). The peak ictal/postictal ETCO2 was 58.9 ± 14.5 mmHg (56.5, 36–98). The duration of ETCO2 elevation from peak to return to baseline was 288.1 ± 427.9 sec (102, 2–2298). The apnea duration was 75.7 ± 34.9 sec (77, 11–185). The preictal heart rate was 80.9 ± 17.5 beats per minute (80, 44–144). The peak postictal heart rate 149.2 ± 19.1 beats per minute (150, 96–210). The preictal respiratory rate was 18.3 ± 4.3 breaths per minute (18, 11–33). The postictal peak respiratory rate was 29.6 ± 4.3 breaths per minute (27, 18–60).
The duration of PGES was 33.7 ± 28.9 sec (28, 2–165). 68 seizures were followed by PGES, 94 seizures were not followed by PGES and artifact precluded a determination in the remaining seizures. The mean duration of postictal immobility was 166.9 ± 366.5 sec (53, −11–3156).
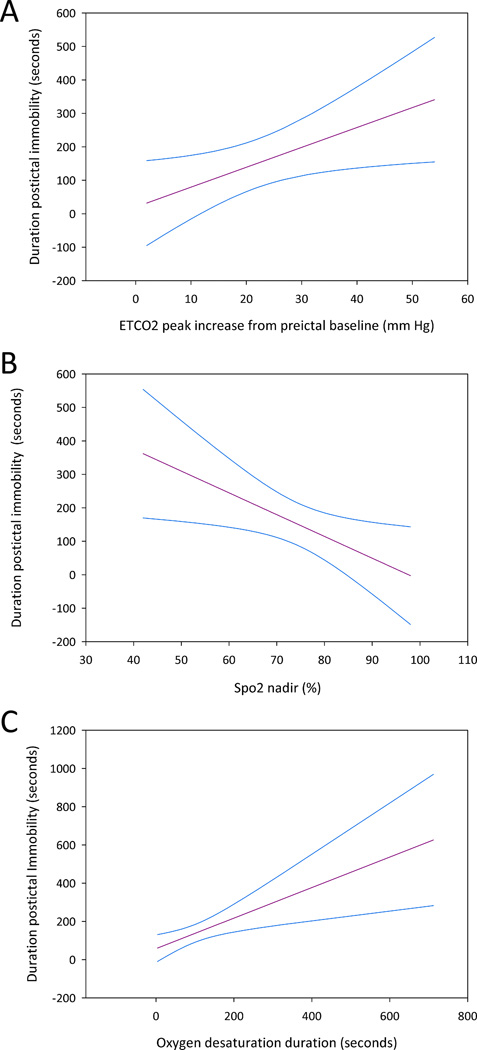
On simple linear regression analysis, the total duration of the preceding seizure, duration of the convulsive phase, and the duration of the tonic phase of the seizure were not significantly associated with the duration of PI. Seizure localization (temporal versus extratemporal) was not significantly associated with duration of PI. Respiratory changes were however significantly associated with the duration of PI. ETCO2 peak change from preictal baseline (p=0.03) and duration of oxygen desaturation (p=0.005) were positively associated with the duration of PI (Figure 1). The SpO2 nadir in the ictal/postictal period had a significant negative relationship with the duration of PI (p=0.02) (Figure 1).
Figure 1.
The linear regression lines and the 95% confidence intervals are shown.
A. The abscissa is the peak increase in ETCO2 (mm Hg) from the preictal baseline following a GTCS. The ordinate is the duration of postictal immobility.
B. The abscissa is the SpO2 nadir (%) following a GTCS. The ordinate is the duration of postictal immobility.
C. The abscissa is the duration of oxygen desaturation of <90% following a GTCS. The ordinate is the duration of postictal immobility.
On multivariable linear regression analysis of the four possible predictors, the presence of PGES (p=0.045) and severe respiratory disturbance (p=0.028) had significant effects on the duration of postictal immobility. The duration of the tonic seizure (p=0.07) and nursing intervention prior to onset of hypoxemia (p=0.208) were not associated with the duration of PI.
To account for correlation among seizures in a given patient that might have affected the overall results, a multivariable linear mixed-effects model was used that indicated a significant effect of severe respiratory distress on the duration of PI (p=0.044).
Summary statistics (two-sided Wilcoxon rank-sum test) for the various variables by whether PGES was present or absent are shown in Table 1 and indicate significant relationships between PGES and the presence of respiratory disturbance and PI. On simple logistic regression analysis, the presence of PGES of any duration was significantly associated with ETCO2 change above preictal baseline (p=0.007) and with SpO2 nadir (p=0.038). On simple linear regression analysis, there was no significant association of any of the measures with the total duration of PGES.
Table 1.
Two-sided Wilcoxon rank-sum test for seizures with and without PGES.
| Parameter | No PGES mean ± standard deviation (median, range) |
PGES mean ± standard deviation (median, range) |
p-value |
|---|---|---|---|
| Total seizure duration (seconds) | 143.1 ± 84.0 (123, 38–455) | 128.0 ± 78.5 (109.5, 57–516) | 0.136 |
| Duration of convulsion (seconds) | 76.6 ± 44.1 (67, 16–324) | 73.94 ± 27.6 (69, 38–216) | 0.544 |
| Duration of tonic phase (seconds) | 21.5 ± 9.1 (19, 6–60) | 21.9 ± 10.0 (20, 4–56) | 0.892 |
| Ictal/postictal ETCO2 peak (mm Hg) | 55.7 ± 13.8 (54, 36–94) | 64.4 ± 15.3 (61, 44–98) | 0.011 |
| ETCO2 change from preictal baseline (mm Hg) | 17.6 ± 12.8 (15, 2–50) | 28.4 ± 14.1 (25, 5–54) | 0.003 |
| Duration of ETCO2 elevation above preictal baseline (seconds) | 188.3 ± 432.4 (72, 2–2298) | 353.3 ± 390.6 (181, 11–1473) | 0.031 |
| Oxygen saturation nadir (%) | 76.3 ± 9.9 (77, 50–98) | 72.3 ± 11.5 (75, 43–93) | 0.046 |
| Duration of oxygen desaturation (seconds) | 93.7 ± 82.2 (79, 4–620) | 115.8 ± 110.1 (93, 8–712) | 0.186 |
| Duration of postictal immobility (seconds) | 139.1 ± 411.4 (46, 0–3156) | 200.5 ± 295.7 (76.5, -11–1192) | < 0.001 |
Discussion
We have shown that both the duration of PI and the presence of PGES of any duration have a significant association with postictal respiratory disturbance. There is also a significant association between the duration of PI and the presence of PGES. Seizure duration, duration of the convulsive component and that of the tonic phase are not significantly associated with PI or PGES. These findings may be relevant to the pathophysiology in SUDEP.
In a study of 48 patients with GTCS recorded in the EMU, 13 had PGES and 12 of these 13 patients were motionless 15. These patients were more likely to have nursing interventions. In contrast, spontaneous movements occurred in the immediate postictal period in 9 of 12 patients without PGES 15. Another study of GTCS found that the duration of the tonic phase of the seizure was longer in patients with PGES than in those without PGES 11. This study also found that postictal immobility was more likely in patients with PGES than in those without 11. Data from more direct monitoring of respiratory function were however not available in either of these two studies. A severe disturbance of respiratory function may occur following GTCS irrespective of the body position during the seizure 2, 12. Early administration of oxygen was found to prevent the occurrence of PGES in the EMU 16 and this finding is in agreement with our observation that the presence of PGES is associated with respiratory dysfunction.
Patients with SUDEP are usually found dead in the prone position following a GTCS 6, 13. In patients who are motionless and prone following a GTCS, with their face in a pillow, further worsening of respiratory compromise may occur and lead to SUDEP. We chose the timing of the first active non-respiratory movement as indicating the end of the period of PI. This measure may, in some patients, underestimate the postictal duration before the patient can effectively change body position to one where ventilation is not compromised. None of our patients were prone at seizure onset and nursing interventions precluded the patients from being prone postictally.
Experimental observations in primates determined that post-epileptic paralysis was the consequence of transient neuronal anoxia 17. This study also showed that postictal hypoxia led to flattening of the EEG and unresponsivity of the EEG to auditory stimuli that reversed once cortical oxygen tension rose 17. Gowers found “no relation between either the duration or degree of convulsion or both, and the subsequent weakness” 18. Our study confirms a lack of association between PI and seizure duration.
In the present study, we have demonstrated an association between PI, the presence of PGES, and a linkage to respiratory dysfunction. The contention that with PGES there is cessation of brainstem function affecting respiratory drive 10, 19 does not appear to be supported by current, albeit limited, human data. Ictal apnea does not typically persist postictally 9. There is tachypnea and increased amplitude of the airflow signal in the immediate postictal period despite the presence of PGES and persistent hypercapnia 12. The relatively short duration of ictal apnea may account in part for the postictal hypoxemia and hypercapnia but probably cannot fully account for the persistent respiratory disturbance that can last many minutes after the end of a seizure 12. Other factors, such as increased metabolic activity with the convulsion and seizure-related intrinsic pulmonary dysfunction likely play a role in the persistent hypercapnia. A caveat of these EMU studies is that direct measurements of minute-ventilation in the postictal period were not available. Thus it is possible that there is some postictal degradation of brainstem respiratory function, such that the increased ventilatory drive is submaximal and inadequate to rapidly reverse the consequences of increased ictal metabolic activity. PGES is also not associated with cardiac autonomic instability 20 providing additional evidence that any postictal derangement of brainstem control of respiratory and cardiac function does not represent a “shutdown” of brainstem function. These observations suggest that the cerebral shutdown reflected in PGES may only partially disrupt brainstem function and also raises the possibility that seizure-related intrinsic pulmonary dysfunction such as shunting or pulmonary edema may, in part, account for persistent postictal hypoxemia and hypercapnia 12, 21.
Although we have found that respiratory disturbance was associated with the occurrence of PGES of any duration, there was no direct association between the total duration of PGES and potential predictors that were analyzed. Other mechanisms, unrelated to respiratory impairment or seizure duration, may come into play and account for the persistence of PGES after its initiation. There were seizures where because of artifact we could not make a determination of the presence or absence of PGES. Therefore, it is possible that in patients without PI there would be a higher rate of EEG artifacts hampering analysis of PGES, and introducing a selection bias that would promote an apparent association between PGES and PI.
We do not know whether PI is solely related to decreased postictal arousal or whether there is also additional impairment of cortical and subcortical motor circuits independent of impaired arousal. During seizures there is decreased cholinergic transmission from subcortical arousal structures to the thalamus and frontal cortex with slow wave activity and decreased metabolism of frontal cortex 22, 23. Activation of subcortical arousal systems by stimulation of intralaminar nuclei following a seizure resulted in increased EEG desynchronization and resumption of exploratory behaviors 24.
We propose that the following sequence may be important in SUDEP. In the postictal period a patient who is prone, with the face in a pillow, there is a terminal positive feedback between respiratory dysfunction and persistent immobility. The patient remains unable to reposition the head to permit air exchange. During this time, other factors such as seizure-related pulmonary edema 21 may also come into play exacerbating postictal respiratory dysfunction. These factors result in increasing hypoxemia and hypercapnia eventually leading to asystole and death.
Findings indicating lower SUDEP rates in supervised environments outside the hospital setting suggest that stimulation rather than oxygenation may be more relevant 25. It remains to be determined whether there is possible role for supplemental oxygenation in SUDEP risk reduction. In one study, early nursing interventions reduced the period of respiratory dysfunction and PGES however it could not be determined whether oxygenation or other nursing maneuvers were crucial 14. In patients at high risk of SUDEP, interventions aimed at reversing impaired arousal and PI may reduce SUDEP risk by non-invasive stimulation of the patient in the postictal period 14. Such interventions could be automated using closed-loop systems and employing secondary markers for seizures 26 or alternatively with direct stimulation of arousal circuits with responsive neurostimulation 24.
Key Points.
Postictal immobility duration was significantly associated with severe respiratory dysfunction.
Postictal immobility duration was not associated with the duration of the tonic phase, convulsive phase or with total seizure duration.
The duration of postictal immobility was significantly longer with seizures followed by generalized EEG suppression (PGES) of any duration.
The presence of PGES was also associated with the severity of respiratory distress but not with the duration of the preceding seizure.
In patients at high risk of SUDEP, interventions aimed at reversing impaired arousal and postictal immobility may reduce SUDEP risk.
Acknowledgement
The project described was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through grant #UL1 TR000002.
Footnotes
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Disclosures
None of the authors have any conflicts of interest to disclose
References
- 1.Nashef L, Walker F, Allen P, et al. Apnoea and bradycardia during epileptic seizures: relation to sudden death in epilepsy. J Neurol Neurosurg Psychiatry. 1996;60:297–300. doi: 10.1136/jnnp.60.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131:3239–3245. doi: 10.1093/brain/awn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moseley BD, Nickels K, Britton J, et al. How common is ictal hypoxemia and bradycardia in children with partial complex and generalized convulsive seizures? Epilepsia. 2010;51:1219–1224. doi: 10.1111/j.1528-1167.2009.02490.x. [DOI] [PubMed] [Google Scholar]
- 4.Blum AS, Ives JR, Goldberger AL, et al. Oxygen desaturations triggered by partial seizures: implications for cardiopulmonary instability in epilepsy. Epilepsia. 2000;41:536–541. doi: 10.1111/j.1528-1157.2000.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 5.Hewertson J, Boyd SG, Samuels MP, et al. Hypoxaemia and cardiorespiratory changes during epileptic seizures in young children. Dev Med Child Neurol. 1996;38:511–522. doi: 10.1111/j.1469-8749.1996.tb12112.x. [DOI] [PubMed] [Google Scholar]
- 6.Ryvlin P, Nashef L, Lhatoo SD, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet Neurol. 2013;12:966–977. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 7.Surges R, Strzelczyk A, Scott CA, et al. Postictal generalized electroencephalographic suppression is associated with generalized seizures. Epilepsy Behav. 2011;21:271–274. doi: 10.1016/j.yebeh.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Semmelroch M, Elwes RD, Lozsadi DA, et al. Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia. 2012;53:e21–f24. doi: 10.1111/j.1528-1167.2011.03296.x. [DOI] [PubMed] [Google Scholar]
- 9.Seyal M, Hardin KA, Bateman LM. Postictal generalized EEG suppression is linked to seizure-associated respiratory dysfunction but not postictal apnea. Epilepsia. 2012;53:825–831. doi: 10.1111/j.1528-1167.2012.03443.x. [DOI] [PubMed] [Google Scholar]
- 10.Lhatoo SD, Faulkner HJ, Dembny K, et al. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68:787–796. doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- 11.Tao JX, Yung I, Lee A, et al. Tonic phase of a generalized convulsive seizure is an independent predictor of postictal generalized EEG suppression. Epilepsia. 2013;54:858–865. doi: 10.1111/epi.12094. [DOI] [PubMed] [Google Scholar]
- 12.Seyal M, Bateman LM, Albertson TE, et al. Respiratory changes with seizures in localization-related epilepsy: analysis of periictal hypercapnia and airflow patterns. Epilepsia. 2010;51:1359–1364. doi: 10.1111/j.1528-1167.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 13.Liebenthal JA, Wu S, Rose S, et al. Association of prone position with sudden unexpected death in epilepsy. Neurology. 2015;84:703–709. doi: 10.1212/WNL.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 14.Seyal M, Bateman LM, Li CS. Impact of periictal interventions on respiratory dysfunction, postictal EEG suppression, and postictal immobility. Epilepsia. 2013;54:377–382. doi: 10.1111/j.1528-1167.2012.03691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semmelroch M, Elwes RD, Lozsadi DA, et al. Retrospective audit of postictal generalized EEG suppression in telemetry. Epilepsia. 2011 doi: 10.1111/j.1528-1167.2011.03296.x. [DOI] [PubMed] [Google Scholar]
- 16.Alexandre V, Mercedes B, Valton L, et al. Risk factors of postictal generalized EEG suppression in generalized convulsive seizures. Neurology. 2015;85:1–6. doi: 10.1212/WNL.0000000000001949. [DOI] [PubMed] [Google Scholar]
- 17.Meyer JS, Portnoy HD. Post-epileptic paralysis A clinical and experimental study. Brain. 1959;82:162–185. [Google Scholar]
- 18.Gowers WR. Epilepsy and other chronic convulsive diseases. London: Churchill; 1881. [Google Scholar]
- 19.Massey CA, Sowers LP, Dlouhy BJ, et al. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014;10:271–282. doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamberts RJ, Laranjo S, Kalitzin SN, et al. Postictal generalized EEG suppression is not associated with periictal cardiac autonomic instability in people with convulsive seizures. Epilepsia. 2013;54:523–529. doi: 10.1111/epi.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy JD, Hardin KA, Parikh P, et al. Pulmonary edema following generalized tonic clonic seizures is directly associated with seizure duration. Seizure. 2015;27:19–24. doi: 10.1016/j.seizure.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motelow JE, Li W, Zhan Q, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85:561–572. doi: 10.1016/j.neuron.2014.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farzampour Z, Huguenard J. Seizing upon mechanisms for impaired consciousness. Neuron. 2015;85:453–455. doi: 10.1016/j.neuron.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gummadavelli A, Motelow JE, Smith N, et al. Thalamic stimulation to improve level of consciousness after seizures: evaluation of electrophysiology and behavior. Epilepsia. 2015;56:114–124. doi: 10.1111/epi.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Langan Y, Nashef L, Sander JW. Case-control study of SUDEP. Neurology. 2005;64:1131–1133. doi: 10.1212/01.WNL.0000156352.61328.CB. [DOI] [PubMed] [Google Scholar]
- 26.Hirsch M, Altenmuller DM, Schulze-Bonhage A. Latencies from intracranial seizure onset to ictal tachycardia: A comparison to surface EEG patterns and other clinical signs. Epilepsia. 2015 doi: 10.1111/epi.13117. [DOI] [PubMed] [Google Scholar]