Abstract
Several studies demonstrated that impairment in glutamatergic neurotransmission is linked to drug dependence and drug-seeking behavior. Increased extracellular glutamate concentration in mesocorticolimbic regions has been observed in animals developing nicotine dependence. Changes in glutamate release might be associated with stimulatory effect of nicotinic acetylcholine receptors (nAChRs) via nicotine exposure. We and others have shown increased extracellular glutamate concentration, which was associated with downregulation of the major glutamate transporter, glutamate transporter 1 (GLT-1), in brain reward regions of animals exposed to drug abuse, including nicotine and ethanol. Importantly, studies from our laboratory and others showed that upregulation of GLT-1 expression in the mesocorticolimbic brain regions may have potential therapeutic effects in drug dependence. In this review article, we discussed the effect of antagonizing presynaptic nAChRs in glutamate release, the upregulatory effect in GLT-1 expression and the role of glutamate receptors antagonists in the treatment of nicotine dependence.
Keywords: Nicotine, nAChRs, GLT-1, xCT, iGLURs, mGluRs
1. Introduction
Nicotine dependence is one of the most preventable causes of death in the world (Jacobs et al., 1999, Doll et al., 2004). Consumption of tobacco, a product containing nicotine, leads to premature death in developing countries and in the USA (Cosin-Aguilar et al., 1995, Holford et al., 2014). It is well known that chronic nicotine consumption increases the mortality and morbidity rates in the world (Perry et al., 1984, Slotkin et al., 1997, Thun et al., 2000). Nicotine acts on nicotinic receptors, which are distributed at both pre- and post-synaptic terminals in neurons of various brain regions (Albuquerque et al., 2009), and it regulates different signaling pathways, including reward system (Watkins et al., 2000). The role of nicotine in the brain’s reward neurocircuitry has been investigated extensively (Pontieri et al., 1996, Reid et al., 2000, Saellstroem Baum et al., 2006, Goriounova and Mansvelder, 2012). It has been shown that nicotine exposure is linked to dopamine and glutamate neurotransmission (Fu et al., 2000, Lambe et al., 2003, Saellstroem Baum et al., 2006, Kleijn et al., 2011). Nicotine stimulates dopaminergic neurons in the ventral tegmental area (VTA) via activation of nicotinic acetylcholine receptors (nAChRs) (Tizabi et al., 2002, Li et al., 2014). It is important to note that dopaminergic neurotransmission plays an important role in drug dependence (Fu et al., 2000, Tizabi et al., 2002, Dani, 2003). However, several studies demonstrated that glutamatergic neurotransmission is also involved in drug dependence (Cornish and Kalivas, 2000, Giorgetti et al., 2001, Christian et al., 2013). It has been reported that neuroadaptation of the glutamatergic system occurs in drug dependence (McFarland et al., 2003).
Glutamatergic projections from the prefrontal cortex (PFC) into nucleus accumbens (NAc) and ventral tegmental area (VTA) are very critical in drug dependence (Parsegian and See, 2014). In addition, dopaminergic inputs from NAc into VTA have been found to play an important role in drug dependence (Yun et al., 2004). Additionally, changes in glutamate release may affect dopamine release in the PFC and NAc (Markou, 2008) (Figure 1).
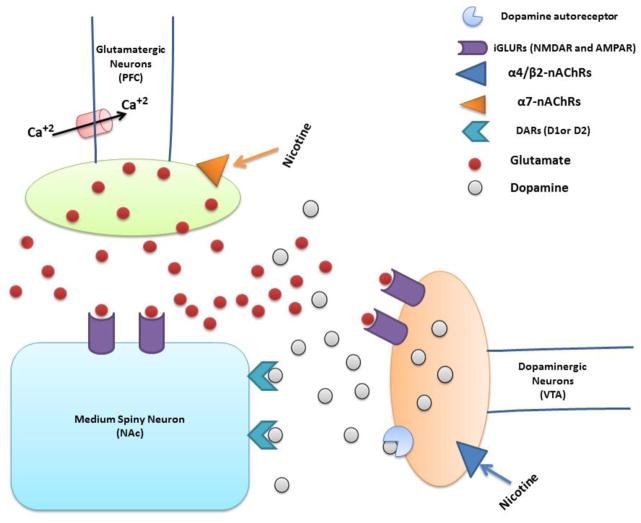
Figure 1.
Schematic diagram shows the effect of nicotine on presynaptic α7-nAChRs in glutamatergic terminals in the PFC. Glutamate released from glutamateregic neurons, binds to iGLURs in both striatal medium spiny neuron (MSN) in the NAc and dopaminergic terminals in the VTA. Glutamate activates dopamine release through stimulation of iGLURs in dopaminergic neurons. Dopamine then binds to dopamine receptor 1 (DAR1) or dopamine receptor 2 (DAR2) in the MSN, inducing dopamine actions.
Nucleus accumbens (NAc); Ventral tegmental area (VTA); Prefrontal cortex (PFC); Nicotinic acetylcholine receptors (nAChRs); Ionotropic glutamate receptors (iGLURs); N-methyl-D-aspartate (NMDA); α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA); Medium spiny neuron (MSN); Dopamine receptors (DARs).
Both dopamine and glutamate release are increased by nicotine via stimulation of presynaptic nicotinic acetylcholine receptors (nAChRs) in dopaminergic and glutamatergic neurons in the mesocorticolimbic brain regions (Markou, 2008, Parikh et al., 2010) (Figure 1). Varenicline, an nAChRs partial agonist, attenuated nicotine and ethanol interactions in the mesocorticolimbic dopaminergic system in rat models (Ericson et al., 2009, Bito-Onon et al., 2011). This compound was also found to have an analgesic effect in a mouse pain model (AlSharari et al., 2012). It has been shown that α4β2 nAChRs are present in two distinct stoichiometric arrangements, (α4) 2(β2) 3 nAChRs and (α4) 3 (β2) 3 nAChRs (Moroni et al., 2006). However, it has been found that exposure to nicotine can alter the stoichiometry of α4β2 nAChRs and consequently increase its expression (Nelson et al., 2003, Vallejo et al., 2005). Furthermore, upregulation of α4β2 nAChRs has been suggested to be the mechanism of nicotine -stimulated glutamate release (Garduno et al., 2012). Additionally, several studies found that nicotine has been found to bind to α7 nAChRs and increased glutamate and calcium release (Gray et al., 1996, Wang et al., 2006). Thus, modulation of glutamate release following exposure to nicotine might be mediated through stimulation of nAChRs expressed in glutamatergic neurons. Moreover, it has been reported that nicotine applied on medial prefrontal pyramidal cells can lead to increased extracellular glutamate concentration in rats (Lambe et al., 2003), which might be also a result of the downregulatory effect of nicotine on glutamate transporters.
Importantly, studies have demonstrated the important role of the major glutamate transporter, glutamate transporter 1 (GLT-1, its human homolog, excitatory amino acid transporter 2, EAAT2), in nicotine self-administration, nicotine dependence, nicotine withdrawal and nicotine-induced reinstatement of preference (Knackstedt et al., 2009, Alajaji et al., 2013). GLT-1 is known to regulate the majority of glutamate uptake (Danbolt, 2001). Glutamate transmission is also regulated by another glial transporter, cystine/glutamate exchanger (xCT). This transporter was also shown to play a critical role in nicotine dependence in rats and humans (Knackstedt et al., 2009). GLT-1 and xCT have suggested as targets for treatment of drug dependence, including nicotine and alcohol (Knackstedt et al., 2009, Alhaddad et al., 2014a). Therefore, it is important to find potential therapeutic compounds that upregulate GLT-1 and xCT, and consequently attenuate nicotine and drug dependence.
Additionally, several studies demonstrated the important role of glutamate receptors in attenuating nicotine dependence (Kenny et al., 2003b, Kenny et al., 2009). It is important to note that blocking glutamate receptors has been found to reduce nicotine self-administration (Kenny et al., 2003b, Sidique et al., 2012). Moreover, inhibiting glutamate receptors has been found to decrease nicotine-induced dopamine release in mesocorticolimbic area (Kenny et al., 2003b, Sidique et al., 2012).
It has been discussed extensively about the potential role of nicotine in glutamatergic system, particularly glutamate receptors (Li et al., 2014). In addition, effects of glutamate following exposure to nicotine on both dopaminergic system and medium spiny neuron (MSN) have been investigated recently (Pistillo et al., 2015). In this review article, we discussed the literature on the modulatory effect of nAChRs in glutamate release on nicotine dependence. Importantly, we further discussed the important role of GLT-1 and xCT, as well as the implications of glutamate receptors and their potential therapeutic role for the treatment of nicotine dependence.
2. Role of nicotinic acetylcholine receptors in the modulation of glutamate release
Several studies were conducted to demonstrate the role of presynaptic nAChRs in the release of glutamate following exposure to nicotine (Gray et al., 1996, Wang et al., 2006, Garduno et al., 2012). Glutamatergic terminals express presynaptic α7 nAChRs in the rat VTA and PFC (Jones and Wonnacott, 2004, Huang et al., 2014). As shown in Figure 1, glutamate release via stimulating presynaptic α7 nAChRs in glutamate terminals may have an indirect action in dopamine release by activating ionotropic glutamate receptors (iGLURs) in dopaminergic terminals (Desce et al., 1992, Fu et al., 2000, Kaiser and Wonnacott, 2000).
Studies have shown that chronic nicotine administration modulated glutamate concentration in the VTA (Changeux, 2010) and PFC (Shameem and Patel, 2012, Falasca et al., 2014). Additionally, it has been suggested that calcium influx is the main signal pathway for releasing glutamate after acute and chronic nicotine administration at different concentrations (McGehee et al., 1995, Gray et al., 1996, Wang et al., 2006, Dougherty et al., 2008). An influx of intracellular calcium in the PFC and hippocampus in presynaptic glutamate terminals, expressing α7 nAChRs, enhanced glutamate release after both acute and chronic exposure to nicotine (McGehee et al., 1995, Gray et al., 1996, Wang et al., 2006, Dougherty et al., 2008). Furthermore, it has been reported that the association of glutamate release and calcium influx might be blocked by methyllycaconitine, α7 nicotinic receptor antagonist (Wang et al., 2006). Moreover, α-bungarotoxin irreversibly binds to α7 nAChRs and inhibits nicotine-induced increased presynaptic calcium signaling in the central nervous system (McGehee et al., 1995). Additionally, pretreatment with α-bungarotoxin blocked choline-induced glutamate release in the PFC through inhibitory binding of choline to α7 -nAChRs (Konradsson-Geuken et al., 2009). Together, these findings suggest that presynaptic α7 nAChRs in glutamatergic terminals play an important role in the release of glutamate, and consequently release of dopamine following administration of nicotine.
Several studies demonstrated the role of α4β2 nAChRs in glutamate release after exposure to nicotine (Lambe et al., 2003, Parikh et al., 2010, Garduno et al., 2012). It has been demonstrated that acute nicotine administration activated glutamatergic synaptic transmission through stimulation of presynaptic α4β2 nAChRs in the dorsal raphe nucleus (Garduno et al., 2012). Moreover, chronic nicotine exposure has been found to upregulate α4β2 nAChRs in humans (Buisson and Bertrand, 2001). A lower dose of nicotine has been able to upregulate α4β2 nAChRs as compared to either α6β2 nAChRs or α3β2 nAChRs (Walsh et al., 2008). Moreover, amplitude of glutamate release induced by nicotine or α4β2 nAChRs agonists has been revealed to be decreased in β2 nAChRs knockout animal model (Lambe et al., 2003, Parikh et al., 2010). A study was performed to determine the morphological effects of nicotine on dendritic spines of α4β2 nAChRs showed that nicotine-induced lateral enlargement in the spine heads of α4β2 nAChRs can lead to glutamatergic synaptic plasticity, since glutamate receptors antagonists blocked the nicotine-induced spine remolding effect (Oda et al., 2014). It is important to note that α4β2 nAChRs antagonist also abolished this effect, which suggests the potential role of this receptor in glutamate release. The stoichiometry of α4β2 nAChRs was found to be altered after short and long term exposure to nicotine (Nelson et al., 2003, Vallejo et al., 2005, Srinivasan et al., 2011). It is well known that the increase in assembly of α4β2 nAChRs stoichiometry can be developed by acute and chronic nicotine administrations (Nelson et al., 2003, Kuryatov et al., 2005, Vallejo et al., 2005). This effect can lead to an increase in the expression of α4β2 nAChRs. Additionally, it has been shown that the stoichiometry of α4β2 nAChRs is an important mechanism of nicotine-induced upregulation of α4β2 nAChRs (Vallejo et al., 2005, Srinivasan et al., 2011). We suggest here that the upregulatory effects of nicotine on α4β2 nAChRs may induce the release of glutamate in the mesocorticolimbic regions. Moreover, presynaptic nAChRs antagonist in the glutamatergic terminals could be effective in reducing both nicotine-induced glutamate and dopamine releases.
3. Role of glutamate transporters in nicotine dependence
Several studies found that exposure to drugs of abuse induced a marked increase in extracellular glutamate concentration in the mesocorticolimbic regions (Smith et al., 1995, Del Arco et al., 1998, Reid et al., 2000, Williams and Steketee, 2004, Ward et al., 2009, Ding et al., 2012, Ding et al., 2013, Das et al., 2015). It has been reported that this effect can be associated with downregulation of glutamate transporters (Knackstedt et al., 2009, Changeux, 2010, Knackstedt et al., 2010, Alhaddad et al., 2014a, Alhaddad et al., 2014b). Several glutamate transporters regulate glutamate uptake in astrocytes (Su et al., 2003, Holtje et al., 2008). GLT-1 is responsible for the removal of the majority of extracellular glutamate concentration into astrocytes (Danbolt, 2001, Jensen et al., 2015). Additionally, xCT is co-expressed with GLT-1 in astrocytes regulating glutamate homeostasis [For review see (Reissner and Kalivas, 2010)]. Studies have demonstrated the potential implications of GLT-1 and xCT expression in central reward brain regions in cocaine-seeking behavior (Sari et al., 2009, Knackstedt et al., 2010). It has been revealed that GLT-1 and xCT are downregulated in NAc after cocaine exposure (Knackstedt et al., 2010). Similarly, GLT-1 and xCT were found downregulated in the NAc, amygdala and hippocampus but not in PFC in P rats exposed to ethanol as compared to ethanol naïve group (Alhaddad et al., 2014b, Aal-Aaboda et al., 2015). Importantly, it has been shown that chronic nicotine exposure can lead to downregulation of GLT-1 (Knackstedt et al., 2009). Acute exposure to nicotine increased extracellular glutamate concentration in NAc (Reid et al., 2000, Saellstroem Baum et al., 2006). A study was performed to determine the neuropharmacological cause of high extracellular glutamate concentration induced by chronic nicotine administration (Reid et al., 2000). This study found that mecamylamine and L-trans-pyrolidine-2,4 dicarboxylic acid, a non-selective glutamate transporter blocker, inhibited nicotine-induced increases in extracellular glutamate concentration in the NAc. In addition, denervation of dopamine by local injection of 6-hydroxydopamine enhanced nicotine-induced glutamate release in NAc (Reid et al., 2000). Moreover, local perfusion of artificial cerebrospinal fluid-calcium free did not affect nicotine-increased glutamate release (Reid et al., 2000). Together, this study found that nicotine-induced glutamate release in the NAc may not be calcium or dopamine dependent-related mechanisms, which suggest that glutamate transporters may have a critical role in nicotine-induced glutamate release in mesocorticolimbic regions (Reid et al., 2000).
Importantly, nicotine self-administration decreased GLT-1 and xCT expression in the NAc and VTA but not in PFC (Knackstedt et al., 2009) (Figure 2). Furthermore, reinstatement of nicotine-seeking behavior was found associated with increased extracellular glutamate concentration, decreased GLT-1 expression and increased behavioral reactions, suggesting the potential role of glutamate transporters in relapse-like nicotine seeking (Gipson et al., 2013). Recent studies from our lab and others have demonstrated the important role of glutamate transporters. GLT-1 and xCT have been suggested as key players in ethanol intake (Aal-Aaboda et al., 2015, Alasmari et al., 2015). Thus, upregulation of these transporters by ceftriaxone, a β-lactam antibiotic known to upregulate GLT-1, was associated with attenuation of relapse to ethanol and cocaine seeking (Knackstedt et al., 2010, Qrunfleh et al., 2013, Alhaddad et al., 2014a). Additionally, ceftriaxone reduced reinstatement of conditioned place preference induced by nicotine (Alajaji et al., 2013). It has been shown that ceftriaxone attenuated also tolerance developed by the analgesic effects of morphine and nicotine dependence (Rawls et al., 2010, Schroeder et al., 2011). These effects have been associated in part through upregulation of both GLT-1 and xCT expression. In clinics, it has been shown that N-acetylcysteine, a prodrug of L-cysteine involving xCT activation, can attenuate dependence to nicotine (Knackstedt et al., 2009, Schmaal et al., 2011). Additionally, glutamate transporter 3 type (excitatory amino acid transporter 3, EAAT3) transports glutamate at post-synaptic neurons. It has been reported that EAAT3 was found to be regulated through neuronal activity, mediating other signaling pathways like phosphatidylinositol-3-kinase (PI3K) and protein kinase C (PKC) (Nieoullon et al., 2006, Yoon et al., 2014). Moreover, P13K inhibitor, and PKC inhibitor have been found to decrease EAAT3 activity (Yoon et al., 2014). Importantly, it has been found that chronic exposure to nicotine- reduced EAAT3 activity, and this effect was found to be P13K- and PKC-dependent, since P13K- and PKC activators blocked the nicotine-induced decrease in EAAT3 activity (Yoon et al., 2014). Taken together, we suggest that GLT-1, xCT and EAAT3 may play an important role in nicotine dependence.
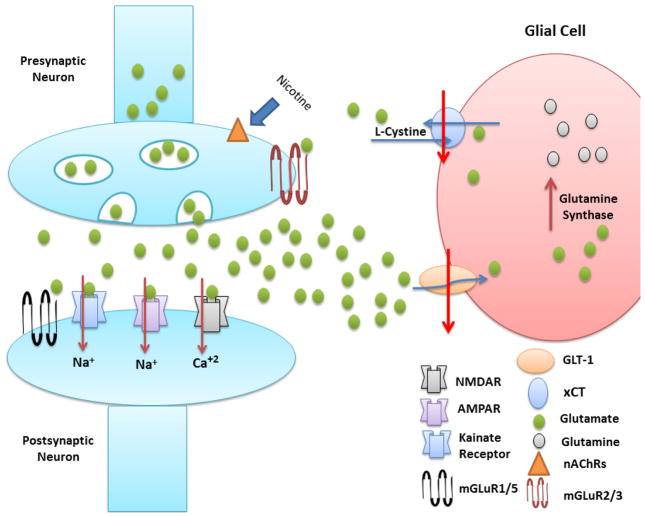
Figure 2.
Schematic diagram shows the effect of nicotine on glutamatergic system. Nicotine binds to nAChRs located at the glutamatergic terminal and elevates extracellular glutamate concentration. Moreover, decreased GLT-1 and xCT expression were associated with chronic exposure to nicotine. Glutamate is converted to glutamine by glutamine synthase enzyme in glial cells. Extracellular glutamate binds to iGLURs (NMDA and AMPA receptors) located in postsynaptic neurons. Negative feedback mechanism can occur due to binding of extracellular glutamate to mGlu2/3 receptor in presynaptic neurons of glutamatergic terminals, and consequently decreases extracellular glutamate concentration.
Glutamate transporter 1 (GLT-1); cystine glutamate exchanger (xCT); Nicotinic acetylcholine receptors (nAChRs); N-methyl-D-aspartate (NMDA); α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA); glutamate (GLU); metabotropic glutamate receptors (mGluRs).
4. Role of glutamate receptors in nicotine dependence
It has been shown extensively that ionotropic glutamate receptors (iGLURs) and metabotropic glutamate receptors (mGluRs) have a critical role in nicotine and drug dependence (Moran et al., 2005, Terry et al., 2012, Gipson et al., 2013). It is important to note that iGLURs such as N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors are found in dopamine neurons in the VTA (Wang and French, 1993, Gao and Wolf, 2007). Interestingly, NMDA receptor was found to be involved in nicotine-induced dopamine release in the NAc and VTA (Fu et al., 2000, Wang et al., 2010, Salamone et al., 2014) (Figure 1). Competitive NMDA receptor antagonist, CGS 19755, administration in the VTA blocked nicotine-induced dopamine release in the NAc (Fu et al., 2000). Furthermore, it has been found that glutamate release in the VTA mediated with high doses of nicotine increased the release of dopamine in the NAc (Fu et al., 2000). Alternatively, glycine may potentiate glutamate-activated NMDA receptors and consequently stimulate [3H] dopamine release in the striatum (Desce et al., 1992). It has been shown that using conditioned place preference, nicotine dependence was attenuated in mice lacking NMDA receptors in the dopaminergic axon terminals in the VTA (Wang et al., 2010). Furthermore, administration of NMDA receptor antagonists directly into the VTA inhibited nicotine-stimulated release of dopamine in the NAc (Schilstrom et al., 1998, Fu et al., 2000). Moreover, systemic administration of NMDA receptor antagonist also blocked nicotine-induced release of dopamine (Kosowski and Liljequist, 2004). It has been reported that 2-amino-5-phosphonopentanoic acid (AP-5), a competitive NMDA receptor antagonist, blocked nicotine-activated NMDA receptor and consequently reduced [3H] dopamine release in rat VTA (Jin and Fredholm, 1997, Kalivas, 2000).
Alternatively, chronic nicotine self-administration increased NMDA receptor NR2A and NR2B subunits’ expression in the PFC and increased AMPA receptor GluR2/GluR3 subunits’ expression in the VTA (Wang et al., 2007). Moreover, the NMDA receptor NR2A subunit expression in the VTA, PFC and amygdala was found to be increased after nicotine self-administration in rat models (Liechti and Markou, 2008, Kenny et al., 2009). Studies showed that chronic nicotine self-administration upregulated NMDA receptor NR2B subunit as well as AMPA receptor GluR2 subunit in the PFC and in the amygdala as compared to control group (Kenny et al., 2009). NMDA-increased release of glutamate has been found in cerebellar granule cells exposed to a subacute nicotine concentration (Lim et al., 2000). Furthermore, studies have shown that systemic administration or direct application of NMDA antagonists into the VTA reduced self-administration of nicotine in rats (Blokhina et al., 2005, Liechti and Markou, 2008, Kenny et al., 2009). Reinstatement to nicotine-seeking behavior can be inhibited by the NMDA receptor subunit antagonist, suggesting that glutamate neurotransmission has a crucial role in relapse to nicotine seeking (Gipson et al., 2013). Interestingly, cotinine, a metabolite of nicotine, attenuated the effects of NMDA receptor antagonist, MK-801, in rats (Terry et al., 2012).
In regards to AMPA receptors, studies demonstrated that these receptor antagonists blocked nicotine-increased dopamine release (Sziraki et al., 2002, Kosowski et al., 2004). Topiramate, a non-selective AMPA/kainate receptor antagonist, decreased the release of monoamine that is induced by nicotine in the NAc (Schiffer et al., 2001). In addition, it has been reported that the head diameter of the dendritic spine of the NAc core and AMPA to NMDA receptors ratio currents were increased within two weeks after starting nicotine self-administration in the NAc in rat model (Gipson et al., 2013). Moreover, microinjection of AMPA receptor antagonists directly into the VTA was reported to attenuate chronic nicotine and sucrose self-administration (Wang et al., 2008). However, conflicting data have been shown regarding the effects of AMPA receptor antagonists on nicotine self-administration (Wang et al., 2008, Kenny et al., 2009). Moreover, nicotine withdrawal effects have been shown to be increased precipitately in animal models injected with AMPA/kainate receptor antagonist (Kenny et al., 2003a). This suggests that AMPA/kainite receptors may play a role in nicotine dependence.
In addition to iGLURs, mGluRs have been also demonstrated to be involved in nicotine dependence (Bespalov et al., 2005, Dravolina et al., 2007, Liechti et al., 2007, Palmatier et al., 2008, Tronci et al., 2010, Tronci and Balfour, 2011, Akkus et al., 2013). Alternatively, it has been shown that mGluR5 antagonist, 6-methyl-2-(phenylethynyl)-pyridine (MPEP), decreased nicotine self-administration in rats and mice (Kenny et al., 2003b, Paterson et al., 2003, Tronci and Balfour, 2011). In addition, mGluR5 antagonist prevented relapse to nicotine-seeking behavior in rats (Tessari et al., 2004). Moreover, MPEP reduced nicotine-induced dopamine release into the NAc (Tronci and Balfour, 2011). Another study demonstrated that MPEP decreased nicotine seeking in rats (Palmatier et al., 2008). It is important to note that long-term use ex-smokers had higher mGluR5 binding as compared to recent use ex-smokers in thalamus and frontal cortex suggesting that mGluR5 is an important biomarker for nicotine dependence (Akkus et al., 2015). Furthermore, studies have demonstrated that mGluR5 or mGluR1 antagonists are able to reduce cue-induced reinstatement of nicotine self-administration in rats (Bespalov et al., 2005, Dravolina et al., 2007). In addition, nicotine self-administration decreased mGlu2/3 receptors’ function in the mesocorticolimbic area (Liechti et al., 2007). It has been suggested that presynaptic inhibitory mGluR2/3 regulates extracellular glutamate concentration (Moran et al., 2005) (Figure 2). Thus, blocking mGluR2/3 inhibits the efficacy of N-acetylcystine to reduce reinstatement of cocaine self-administration in rats, suggesting that mGluR2/3 has a role in the decrease of extracellular glutamate concentration in drug dependence (Moran et al., 2005). It has been reported that systemic or microinjection of mGluR2/3 agonist, LY379268, reduced nicotine-seeking behavior (Liechti et al., 2007). Moreover, stimulation of mGluR2 by positive receptor modulator reduced nicotine self-administration (Sidique et al., 2012). We suggest here that both iGLURs and mGluRs play a critical role in nicotine dependence. For example, iGLURs and mGluR1/5 antagonists attenuated nicotine seeking (Bespalov et al., 2005, Dravolina et al., 2007). However, mGluR2/3 functions as a negative regulatory role in glutamate neurotransmission, since mGluR2/3 agonists are able to attenuate nicotine-self-administration behavior (Liechti et al., 2007, Sidique et al., 2012).
5. Conclusion
Nicotine may be able to affect excitatory and inhibitory neurotransmitters in mesocorticolimbic brain regions. Dopamine has been long standing as target for the treatment of nicotine dependence through the use of bupropion as an FDA- approved drug, which is a dopamine transporter blocker. In addition to dopamine as a target, nicotine has been studied to have modulatory effects on glutamatergic system through multiple mechanisms in the mesocorticolimbic area. Nicotine dependence may result on changes in glutamatergic transmission mediated by smoking or tobacco use. Thus, studies clearly demonstrated that chronic exposure to nicotine has been linked to increase the release of glutamate through stimulatory effect in presynaptic nAChRs located in glutamatergic axon terminals. Furthermore, upregulating GLT-1 expression, antagonizing certain glutamate receptors or antagonizing presynaptic nAChRs may have modulatory effects in glutamate transmission, and consequently lead to attenuation of nicotine dependence. These suggest that targeting glutamatergic neurotransmission through different key proteins may have potential therapeutic effect in the treatment of nicotine dependence.
Highlights.
Nicotine stimulates glutamate release and calcium influx
NMDA and AMPA receptors antagonists decrease nicotine-induced dopamine release
GLT-1 and xCT upregulators reduce nicotine self-administration
iGLUR and mGluR1/5 antagonists and mGluR2/3 agonist attenuate nicotine seeking
Acknowledgments
The review article was written during the period of fund supported by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism.
Abbreviations
- GLT-1
Glutamate transporter 1
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- VTA
ventral tegmental area
- nAChRs
nicotinic acetylcholine receptors
- iGLURs
ionotropic glutamate receptors
- NMDA
N-methyl-D-aspartate
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- MSN
Medium spiny neuron
- DA
Dopamine
- EAAT3
Glutamate transporter 3
- PI3K
phosphatidylinositol-3-kinase
- alphaPKC
alpha protein kinase C
- xCT
cystine/glutamate exchanger
- mGluRs
metabotropic glutamate receptors
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aal-Aaboda M, Alhaddad H, Osowik F, Nauli SM, Sari Y. Effects of (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. Journal of neuroscience research. 2015 doi: 10.1002/jnr.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, Gomez Mancilla B, Sovago J, Buck A, Hasler G. Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:737–742. doi: 10.1073/pnas.1210984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus F, Treyer V, Johayem A, Ametamey SM, Mancilla BG, Sovago J, Buck A, Hasler G. Association of Long-Term Nicotine Abstinence with Normal Metabotropic Glutamate Receptor-5 Binding. Biological psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Alajaji M, Bowers MS, Knackstedt L, Damaj MI. Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology. 2013;228:419–426. doi: 10.1007/s00213-013-3047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari F, Abuhamdah S, Sari Y. Effects of ampicillin on cystine/glutamate antiporter and glutamate transporter 1 isoforms as well as ethanol drinking in male P rats. Neuroscience letters. 2015;600:148–152. doi: 10.1016/j.neulet.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiological reviews. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014a;231:4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SHS, Wei YJ, Sari Y. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci. 2014b:8. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlSharari SD, Carroll FI, McIntosh JM, Damaj MI. The antinociceptive effects of nicotinic partial agonists varenicline and sazetidine-A in murine acute and tonic pain models. The Journal of pharmacology and experimental therapeutics. 2012;342:742–749. doi: 10.1124/jpet.112.194506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, Danysz W, van Heeke G, Markou A. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(Suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Bito-Onon JJ, Simms JA, Chatterjee S, Holgate J, Bartlett SE. Varenicline, a partial agonist at neuronal nicotinic acetylcholine receptors, reduces nicotine-induced increases in 20% ethanol operant self-administration in Sprague-Dawley rats. Addiction biology. 2011;16:440–449. doi: 10.1111/j.1369-1600.2010.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina EA, Kashkin VA, Zvartau EE, Danysz W, Bespalov AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–225. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human (alpha)4((beta)2 nicotinic acetylcholine receptor function. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP. Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nature reviews Neuroscience. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Christian DT, Alexander NJ, Diaz MR, McCool BA. Thalamic glutamatergic afferents into the rat basolateral amygdala exhibit increased presynaptic glutamate function following withdrawal from chronic intermittent ethanol. Neuropharmacology. 2013;65:134–142. doi: 10.1016/j.neuropharm.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosin-Aguilar J, Andres-Conejos F, Hernandiz-Martinez A, Solaz-Minguez J, Marrugat J, Bayes-De-Luna A. Effect of smoking on sudden and premature death. Journal of cardiovascular risk. 1995;2:345–351. [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dani JA. Roles of dopamine signaling in nicotine addiction. Molecular psychiatry. 2003;8:255–256. doi: 10.1038/sj.mp.4001284. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Martinez R, Mora F. Amphetamine increases extracellular concentrations of glutamate in the prefrontal cortex of the awake rat: a microdialysis study. Neurochemical research. 1998;23:1153–1158. doi: 10.1023/a:1020769816332. [DOI] [PubMed] [Google Scholar]
- Desce JM, Godeheu G, Galli T, Artaud F, Cheramy A, Glowinski J. L-glutamate-evoked release of dopamine from synaptosomes of the rat striatum: involvement of AMPA and N-methyl-D-aspartate receptors. Neuroscience. 1992;47:333–339. doi: 10.1016/0306-4522(92)90249-2. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Engleman EA, Rodd ZA, McBride WJ. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcoholism, clinical and experimental research. 2012;36:633–640. doi: 10.1111/j.1530-0277.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years’ observations on male British doctors. Bmj. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty JJ, Wu J, Mehta TK, Brown B, Nichols RA. Chronic nicotine alters nicotinic receptor-induced presynaptic Ca2+ responses in isolated nerve terminals. Neurochemical research. 2008;33:1106–1112. doi: 10.1007/s11064-007-9557-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravolina OA, Zakharova ES, Shekunova EV, Zvartau EE, Danysz W, Bespalov AY. mGlu1 receptor blockade attenuates cue- and nicotine-induced reinstatement of extinguished nicotine self-administration behavior in rats. Neuropharmacology. 2007;52:263–269. doi: 10.1016/j.neuropharm.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stomberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. The Journal of pharmacology and experimental therapeutics. 2009;329:225–230. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Falasca S, Ranc V, Petruzziello F, Khani A, Kretz R, Zhang X, Rainer G. Altered neurochemical levels in the rat brain following chronic nicotine treatment. Journal of chemical neuroanatomy. 2014;59–60:29–35. doi: 10.1016/j.jchemneu.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Fu Y, Matta SG, Gao W, Brower VG, Sharp BM. Systemic nicotine stimulates dopamine release in nucleus accumbens: re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. The Journal of pharmacology and experimental therapeutics. 2000;294:458–465. [PubMed] [Google Scholar]
- Gao C, Wolf ME. Dopamine alters AMPA receptor synaptic expression and subunit composition in dopamine neurons of the ventral tegmental area cultured with prefrontal cortex neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:14275–14285. doi: 10.1523/JNEUROSCI.2925-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garduno J, Galindo-Charles L, Jimenez-Rodriguez J, Galarraga E, Tapia D, Mihailescu S, Hernandez-Lopez S. Presynaptic alpha4beta2 nicotinic acetylcholine receptors increase glutamate release and serotonin neuron excitability in the dorsal raphe nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15148–15157. doi: 10.1523/JNEUROSCI.0941-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetti M, Hotsenpiller G, Ward P, Teppen T, Wolf ME. Amphetamine-induced plasticity of AMPA receptors in the ventral tegmental area: effects on extracellular levels of dopamine and glutamate in freely moving rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6362–6369. doi: 10.1523/JNEUROSCI.21-16-06362.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD. Nicotine exposure during adolescence alters the rules for prefrontal cortical synaptic plasticity during adulthood. Frontiers in synaptic neuroscience. 2012;4:3. doi: 10.3389/fnsyn.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Holford TR, Meza R, Warner KE, Meernik C, Jeon J, Moolgavkar SH, Levy DT. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. Jama. 2014;311:164–171. doi: 10.1001/jama.2013.285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtje M, Hofmann F, Lux R, Veh RW, Just I, Ahnert-Hilger G. Glutamate uptake and release by astrocytes are enhanced by Clostridium botulinum C3 protein. The Journal of biological chemistry. 2008;283:9289–9299. doi: 10.1074/jbc.M706499200. [DOI] [PubMed] [Google Scholar]
- Huang M, Felix AR, Flood DG, Bhuvaneswaran C, Hilt D, Koenig G, Meltzer HY. The novel alpha7 nicotinic acetylcholine receptor agonist EVP-6124 enhances dopamine, acetylcholine, and glutamate efflux in rat cortex and nucleus accumbens. Psychopharmacology. 2014;231:4541–4551. doi: 10.1007/s00213-014-3596-0. [DOI] [PubMed] [Google Scholar]
- Jacobs DR, Jr, Adachi H, Mulder I, Kromhout D, Menotti A, Nissinen A, Blackburn H. Cigarette smoking and mortality risk: twenty-five-year follow-up of the Seven Countries Study. Archives of internal medicine. 1999;159:733–740. doi: 10.1001/archinte.159.7.733. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Fahlke C, Bjorn-Yoshimoto WE, Bunch L. Excitatory amino acid transporters: recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Current opinion in pharmacology. 2015;20C:116–123. doi: 10.1016/j.coph.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Jin S, Fredholm BB. Electrically-evoked dopamine and acetylcholine release from rat striatal slices perfused without magnesium: regulation by glutamate acting on NMDA receptors. British journal of pharmacology. 1997;121:1269–1276. doi: 10.1038/sj.bjp.0701267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Wonnacott S. Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Molecular pharmacology. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. A role for glutamate transmission in addiction to psychostimulants. Addict Biol. 2000;5:325–329. doi: 10.1111/j.1369-1600.2000.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chartoff E, Roberto M, Carlezon WA, Jr, Markou A. NMDA receptors regulate nicotine-enhanced brain reward function and intravenous nicotine self-administration: role of the ventral tegmental area and central nucleus of the amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:266–281. doi: 10.1038/npp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. The Journal of pharmacology and experimental therapeutics. 2003a;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, Koob GF, Skoubis PD, Markou A. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Annals of the New York Academy of Sciences. 2003b;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- Kleijn J, Folgering JH, van der Hart MC, Rollema H, Cremers TI, Westerink BH. Direct effect of nicotine on mesolimbic dopamine release in rat nucleus accumbens shell. Neuroscience letters. 2011;493:55–58. doi: 10.1016/j.neulet.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biological psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradsson-Geuken A, Gash CR, Alexander K, Pomerleau F, Huettl P, Gerhardt GA, Bruno JP. Second-by-second analysis of alpha 7 nicotine receptor regulation of glutamate release in the prefrontal cortex of awake rats. Synapse. 2009;63:1069–1082. doi: 10.1002/syn.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosowski AR, Cebers G, Cebere A, Swanhagen AC, Liljequist S. Nicotine-induced dopamine release in the nucleus accumbens is inhibited by the novel AMPA antagonist ZK200775 and the NMDA antagonist CGP39551. Psychopharmacology. 2004;175:114–123. doi: 10.1007/s00213-004-1797-7. [DOI] [PubMed] [Google Scholar]
- Kosowski AR, Liljequist S. The NR2B-selective N-methyl-D-aspartate receptor antagonist Ro 25-6981 [(+/−)-(R*, S*)-alpha-(4-hydroxyphenyl)-beta-methyl-4-(phenylmethyl)-1-piperidine propanol] potentiates the effect of nicotine on locomotor activity and dopamine release in the nucleus accumbens. The Journal of pharmacology and experimental therapeutics. 2004;311:560–567. doi: 10.1124/jpet.104.070235. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Molecular pharmacology. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2003;28:216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Li X, Semenova S, D’Souza MS, Stoker AK, Markou A. Involvement of glutamatergic and GABAergic systems in nicotine dependence: Implications for novel pharmacotherapies for smoking cessation. Neuropharmacology. 2014;76(Pt B):554–565. doi: 10.1016/j.neuropharm.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Markou A. Role of the glutamatergic system in nicotine dependence : implications for the discovery and development of new pharmacological smoking cessation therapies. CNS drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- Lim DK, Park SH, Choi WJ. Subacute nicotine exposure in cultured cerebellar cells increased the release and uptake of glutamate. Archives of pharmacal research. 2000;23:488–494. doi: 10.1007/BF02976578. [DOI] [PubMed] [Google Scholar]
- Markou A. Review. Neurobiology of nicotine dependence. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGehee DS, Heath MJ, Gelber S, Devay P, Role LW. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Molecular pharmacology. 2006;70:755–768. doi: 10.1124/mol.106.023044. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Molecular pharmacology. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S. The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? Journal of neurochemistry. 2006;98:1007–1018. doi: 10.1111/j.1471-4159.2006.03978.x. [DOI] [PubMed] [Google Scholar]
- Oda A, Yamagata K, Nakagomi S, Uejima H, Wiriyasermkul P, Ohgaki R, Nagamori S, Kanai Y, Tanaka H. Nicotine induces dendritic spine remodeling in cultured hippocampal neurons. Journal of neurochemistry. 2014;128:246–255. doi: 10.1111/jnc.12470. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Donny EC, Caggiula AR, Sved AF. Metabotropic glutamate 5 receptor (mGluR5) antagonists decrease nicotine seeking, but do not affect the reinforcement enhancing effects of nicotine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:2139–2147. doi: 10.1038/sj.npp.1301623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:811–822. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology. 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- Perry RJ, Griffiths W, Dextraze P, Solomon RJ, Trebbin WM. Elevated nicotine levels in patients undergoing hemodialysis. A role in cardiovascular mortality and morbidity? The American journal of medicine. 1984;76:241–246. doi: 10.1016/0002-9343(84)90780-0. [DOI] [PubMed] [Google Scholar]
- Pistillo F, Clementi F, Zoli M, Gotti C. Nicotinic, glutamatergic and dopaminergic synaptic transmission and plasticity in the mesocorticolimbic system: focus on nicotine effects. Prog Neurobiol. 2015;124:1–27. doi: 10.1016/j.pneurobio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. Journal of psychopharmacology. 2013;27:541–549. doi: 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Zielinski M, Patel H, Sacavage S, Baron DA, Patel D. Beta-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug Alcohol Depend. 2010;107:261–263. doi: 10.1016/j.drugalcdep.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Fox L, Ho LB, Berger SP. Nicotine stimulation of extracellular glutamate levels in the nucleus accumbens: neuropharmacological characterization. Synapse. 2000;35:129–136. doi: 10.1002/(SICI)1098-2396(200002)35:2<129::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW. Using glutamate homeostasis as a target for treating addictive disorders. Behavioural pharmacology. 2010;21:514–522. doi: 10.1097/FBP.0b013e32833d41b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saellstroem Baum S, Huebner A, Krimphove M, Morgenstern R, Badawy AA, Spies CD. Nicotine stimulation on extracellular glutamate levels in the nucleus accumbens of ethanol-withdrawn rats in vivo. Alcoholism, clinical and experimental research. 2006;30:1414–1421. doi: 10.1111/j.1530-0277.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Salamone A, Zappettini S, Grilli M, Olivero G, Agostinho P, Tome AR, Chen J, Pittaluga A, Cunha RA, Marchi M. Prolonged nicotine exposure down-regulates presynaptic NMDA receptors in dopaminergic terminals of the rat nucleus accumbens. Neuropharmacology. 2014;79:488–497. doi: 10.1016/j.neuropharm.2013.12.014. [DOI] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer WK, Gerasimov MR, Marsteller DA, Geiger J, Barnett C, Alexoff DL, Dewey SL. Topiramate selectively attenuates nicotine-induced increases in monoamine release. Synapse. 2001;42:196–198. doi: 10.1002/syn.10000. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Nomikos GG, Nisell M, Hertel P, Svensson TH. N-methyl-D-aspartate receptor antagonism in the ventral tegmental area diminishes the systemic nicotine-induced dopamine release in the nucleus accumbens. Neuroscience. 1998;82:781–789. doi: 10.1016/s0306-4522(97)00243-1. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, van den Brink W. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. European addiction research. 2011;17:211–216. doi: 10.1159/000327682. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Quick KF, Landry PM, Rawls SM. Glutamate transporter activation enhances nicotine antinociception and attenuates nicotine analgesic tolerance. Neuroreport. 2011;22:970–973. doi: 10.1097/WNR.0b013e32834d87eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shameem M, Patel AB. Glutamatergic and GABAergic metabolism in mouse brain under chronic nicotine exposure: implications for addiction. PloS one. 2012;7:e41824. doi: 10.1371/journal.pone.0041824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidique S, Dhanya RP, Sheffler DJ, Nickols HH, Yang L, Dahl R, Mangravita-Novo A, Smith LH, D’Souza MS, Semenova S, Conn PJ, Markou A, Cosford ND. Orally active metabotropic glutamate subtype 2 receptor positive allosteric modulators: structure-activity relationships and assessment in a rat model of nicotine dependence. Journal of medicinal chemistry. 2012;55:9434–9445. doi: 10.1021/jm3005306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Saleh JL, McCook EC, Seidler FJ. Impaired cardiac function during postnatal hypoxia in rats exposed to nicotine prenatally: implications for perinatal morbidity and mortality, and for sudden infant death syndrome. Teratology. 1997;55:177–184. doi: 10.1002/(SICI)1096-9926(199703)55:3<177::AID-TERA2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson SE. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain research. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA. Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. The Journal of general physiology. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sziraki I, Sershen H, Hashim A, Lajtha A. Receptors in the ventral tegmental area mediating nicotine-induced dopamine release in the nucleus accumbens. Neurochemical research. 2002;27:253–261. doi: 10.1023/a:1014844823534. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, Hutchings EJ, Chapman JM, Li P, Bartlett MG. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochemical pharmacology. 2012;83:941–951. doi: 10.1016/j.bcp.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. European journal of pharmacology. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- Thun MJ, Apicella LF, Henley SJ. Smoking vs other risk factors as the cause of smoking-attributable deaths: confounding in the courtroom. Jama. 2000;284:706–712. doi: 10.1001/jama.284.6.706. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcoholism, clinical and experimental research. 2002;26:394–399. [PubMed] [Google Scholar]
- Tronci V, Balfour DJ. The effects of the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) on the stimulation of dopamine release evoked by nicotine in the rat brain. Behavioural brain research. 2011;219:354–357. doi: 10.1016/j.bbr.2010.12.024. [DOI] [PubMed] [Google Scholar]
- Tronci V, Vronskaya S, Montgomery N, Mura D, Balfour DJ. The effects of the mGluR5 receptor antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) on behavioural responses to nicotine. Psychopharmacology. 2010;211:33–42. doi: 10.1007/s00213-010-1868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, Green WN. Up-regulation of nicotinic receptors by nicotine varies with receptor subtype. The Journal of biological chemistry. 2008;283:6022–6032. doi: 10.1074/jbc.M703432200. [DOI] [PubMed] [Google Scholar]
- Wang BW, Liao WN, Chang CT, Wang SJ. Facilitation of glutamate release by nicotine involves the activation of a Ca2+/calmodulin signaling pathway in rat prefrontal cortex nerve terminals. Synapse. 2006;59:491–501. doi: 10.1002/syn.20267. [DOI] [PubMed] [Google Scholar]
- Wang F, Chen H, Sharp BM. Neuroadaptive changes in the mesocortical glutamatergic system during chronic nicotine self-administration and after extinction in rats. Journal of neurochemistry. 2008;106:943–956. doi: 10.1111/j.1471-4159.2008.05456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Chen H, Steketee JD, Sharp BM. Upregulation of ionotropic glutamate receptor subunits within specific mesocorticolimbic regions during chronic nicotine self-administration. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32:103–109. doi: 10.1038/sj.npp.1301033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Li F, Shen X, Tsien JZ. Conditional knockout of NMDA receptors in dopamine neurons prevents nicotine-conditioned place preference. PloS one. 2010;5:e8616. doi: 10.1371/journal.pone.0008616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, French ED. Electrophysiological evidence for the existence of NMDA and non-NMDA receptors on rat ventral tegmental dopamine neurons. Synapse. 1993;13:270–277. doi: 10.1002/syn.890130310. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Colivicchi MA, Allen R, Schol F, Lallemand F, de Witte P, Ballini C, Corte LD, Dexter D. Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. Journal of neurochemistry. 2009;111:1119–1128. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Williams JM, Steketee JD. Cocaine increases medial prefrontal cortical glutamate overflow in cocaine-sensitized rats: a time course study. The European journal of neuroscience. 2004;20:1639–1646. doi: 10.1111/j.1460-9568.2004.03618.x. [DOI] [PubMed] [Google Scholar]
- Yoon HJ, Lim YJ, Zuo Z, Hur W, Do SH. Nicotine decreases the activity of glutamate transporter type 3. Toxicology letters. 2014;225:147–152. doi: 10.1016/j.toxlet.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Yun IA, Wakabayashi KT, Fields HL, Nicola SM. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. The Journal of neuroscience. 2004;24:2923–2933. doi: 10.1523/JNEUROSCI.5282-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]