Abstract
Objectives
Diabetes mellitus (DM) is a frequent consequence of chronic pancreatitis (CP). Little is known about pancreatic endocrine function before the development of DM in CP, particularly in females, or those without calcific and/or alcoholic pancreatitis.
Methods
Twenty-five non-diabetic adult CP patients (19 female, age 34.2 ± 2.4 yrs) were compared to 25 healthy controls matched for age, gender, and BMI. Subjects underwent frequent sample intravenous glucose tolerance testing (FSIVGTT) and mixed meal tolerance testing (MMTT).
Results
Mean fasting glucose was higher in CP patients (89.5 ±2.3 mg/dL) than in controls (84.4 ±1.2 mg/dL, p=0.04). On MMTT, CP patients had a higher area under the curve (AUC) glucose and AUC glucagon compared to controls (p≤0.01). AUC C-peptide was equivalent (p=0.6) but stimulated C-peptide at 30 minutes was lower in CP patients (p=0.04). Mean insulin sensitivity index calculated from the FSIVGTT was lower in CP group, indicating reduced insulin sensitivity (p≤0.01). Disposition index (insulin secretion adjusted for insulin sensitivity on FSIVGTT) was lower in CP patients (p=0.01).
Conclusions
CP patients had higher fasting and MMTT glucose levels, without a compensatory increase in insulin secretion suggesting subtle early islet dysfunction. Our cohort had relative hyperglucagonemia and were less insulin sensitive than controls.
Keywords: beta cell, pancreatitis, diabetes, total pancreatectomy and islet autotransplantation, pancreatectomy, islet
INTRODUCTION
Chronic pancreatitis is an inflammatory condition that causes permanent structural damage to the pancreas. While it is severe abdominal pain that generally brings the disease to clinical attention, diabetes mellitus is an important and frequent consequence (1–5). The lifelong risk of developing diabetes mellitus in the setting of chronic pancreatitis is estimated at 25–75% (6–9), and depends in part on prior surgical resection, etiology of disease, and extent of pancreatic damage, with exocrine insufficiency and calcifications as important markers of the latter (7–11).
Compared to type 1 and type 2 diabetes mellitus, very few clinical studies exist to characterize the pathophysiology and natural history of diabetes mellitus in chronic pancreatitis. Impaired insulin responses to oral and intravenous secretagogues have been observed in those with diabetes mellitus and chronic pancreatitis (6, 10, 12), and in a handful of patients with normal glucose tolerance and chronic pancreatitis (10). However, these studies included nearly all male patients, usually with alcohol-induced pancreatitis, and with late calcific chronic pancreatitis. More than half of chronic pancreatitis cases are due to causes other than alcohol, and women are frequently affected (13). This important demographic has been overlooked. Moreover, glucose homeostasis is regulated by multiple other factors; mixed data exist on the role of glucagon and prandial incretin responses, and insulin sensitivity in metabolic control in this population (4, 6, 14–20).
Little is known about the natural history of pancreatic endocrine function in chronic pancreatitis prior to the onset of diabetes mellitus. It has been proposed that inflammation and an altered pancreatic milieu may cause beta cell dysfunction early in the disease course, with progressive loss of beta cells leading to frank diabetic mellitus in a subset of patients (21, 22). The aim of this study was to advance our current understanding of the development of endocrine insufficiency in chronic pancreatitis. Insulin secretion in response to oral and intravenous secretagogues, post-prandial glucagon suppression, and the incretin axis were assessed in non-diabetic patients with chronic pancreatitis, and compared to well-matched healthy controls. Moreover, we enriched our CP cohort with patients who have been under-represented in earlier studies, including those with non-alcoholic disease, females, and patients who had not yet developed pancreatic calcifications. Better understanding of the progression from endocrine dysfunction to diabetes mellitus is critical to develop screening tools for early detection and intervention.
MATERIALS AND METHODS
Subjects
Twenty-five non-diabetic patients with chronic pancreatitis and 25 healthy volunteers were studied in a matched case-control cross-sectional analysis. Chronic pancreatitis patients were recruited from a cohort of adult patients, age ≥18 years, scheduled for total pancreatectomy and islet autotransplantation (TP-IAT) at the University of Minnesota from 2010 to 2012. All chronic pancreatitis patients were participants in a clinical trial, which included metabolic testing performed within 2 weeks prior to TP-IAT. Patients were considered ineligible for study participation if they had a prior diagnosis of diabetes mellitus, were using any anti-diabetic medications at the time of study enrollment, if fasting blood glucose was >115 mg/dL, or if hemoglobin A1c level was >6.0%.
All patients with chronic pancreatitis were seen in consultation and clinical history was reviewed by a multi-disciplinary team. Chronic pancreatitis diagnosis prior to TPIAT was based on at least one of the following, as previously described: (1) documented episodes of recurrent acute pancreatitis (amylase/lipase >3x ULN) with progression to characteristic chronic abdominal pain; (2) imaging or functional studies supporting chronic pancreatitis (at least two tests abnormal among abnormal MRCP, ≥4 criteria on EUS, or abnormal secretin-stimulated pancreatic function tests); (3) pancreatic calcifications on CT scan; or (4) histopathology-confirmed CP at time of prior surgery (if done).
Healthy control subjects were recruited from 2012 to 2013 through fliers and advertisements displayed around the community. Interested subjects were screened by phone or through e-mail for eligibility. Eligible healthy controls were matched to chronic pancreatitis patients based on gender, age (±3 years), and BMI (±3 kg/m2). Subjects were excluded if they had a history of any of the following: past or present use of insulin or oral anti-diabetic mediation, diabetes mellitus, diagnosis of acute or chronic pancreatitis, chronic unexplained abdominal pain, or use of corticosteroids in the past 6 months. Screening fasting glucose was obtained at time of informed consent.
The University of Minnesota Institutional Review Board reviewed and approved the protocols (IRB # 1006M83756 for CP group, IRB # 1203M11602 for healthy control group). Informed consent was obtained from all participants.
Subject demographics and medical history
Basic demographic characteristics were collected for all participants. Additional data elements were obtained for all chronic pancreatitis patients including etiology of disease, duration of disease, presence of calcifications on imaging, prior pancreatic surgeries, prior ERCP procedures, pancreatic enzyme supplementation status (as prescribed by managing physician). Height and weight were measured at time of enrollment of healthy controls to confirm BMI match before performing metabolic testing.
Metabolic testing
Chronic pancreatitis patients and healthy controls underwent frequent sample IV glucose tolerance testing (FSIVGTT) and mixed meal tolerance testing (MMTT) on 2 mornings following an overnight fast of ≥ 10 hours. Subjects’ weight and height (averaged based on 3 measurements) were determined, and BMI (kg/m2) was calculated.
Protocols for metabolic testing have been previously described (23). On the morning of the FSIVGTT, two peripheral IVs were placed in antecubital fossa or large forearm vein 30 minutes prior to blood draws. Glucose, insulin, and C-peptide levels were measured at baseline fasting (in triplicate, with mean value calculated for “time 0”), and then measured at predefined intervals over 180 minutes. At time 0, dextrose 0.3g/kg was given via intravenous push over 30 seconds. Immediately following the 20 minute draw, insulin, 0.025 units/kg, was given via IV push to augment glucose disposal. Insulin and C-peptide levels were then measured at 1, 2, 3, 4, 5, 7, and 10 minutes after dextrose injection. The acute C-peptide response to glucose (ACRglu) was calculated by the trapezoidal area under the curve for the first 10 min after dextrose. The acute insulin response to glucose (AIRglu), insulin sensitivity index (SI), and disposition index (AIRglu x SI) were calculated from insulin and glucose levels over the 180 minute period using MINMOD software (24, 25). Because adequate intravenous access was difficult to maintain in some patients with CP, and hemolysis can compromise the accuracy of insulin immunoassay measures, we excluded CP patients and their matched controls for respective analyses of AIRglu and/or SI if lacking adequate samples for insulin measures during necessary time points (resulting in matched-pairs, n=20 for AIRglu, n=21 for SI).
The mixed meal tolerance test required placement of one peripheral IV 30 minutes prior to the first blood draw. At time 0, the patient consumed 6 mL/kg Boost HP to a maximum of 360 mL within 5 minutes. Patients on pancreatic enzyme therapy were instructed to take their usual dose with the Boost HP. Glucose, insulin, C-peptide, glucagon, gastric inhibitory peptide (GIP), and active glucagon-like peptide 1 (GLP-1) were measured at −1 min, +30, +60, +90, and +120 min. Area under the curve for C-peptide (AUC C-peptide) and glucose (AUC glucose) were calculated using the trapezoidal method. One patient in the CP group was unable to complete the MMTT due to vomiting and was excluded from the MMTT analyses. The earliest 5 patients in our CP cohort did not have glucagon collected (n= 20 case-control pairs for AUC glucagon).
Plasma glucose levels were measured by glucose oxidase assay, and plasma C-peptide levels were measured by Siemen’s immunolite 2000 chemiluminescent immunoassay. Insulin levels were measured by two-site immuno-enzymometric assay on a TOSOH 2000 autoanalyzer (26, 27). Glucagon was measured by radioimmunoassay, active GLP-1 and GIP by ELISA fluorescent sandwich immunoassay (EMD Millipore Corp, Billerica, MA). HbA1c was obtained on day 1 of testing and analyzed by high performance liquid chromatography.
Data analysis
Data are presented as mean ± standard deviation. Statistical analysis was performed with SAS software version 9.2 (Cary, NC). Two-tailed paired student t-tests were used to compare matched case-control pairs. Disposition index was log-adjusted to normalize for wide distribution of values in each group (46-fold difference in minimum vs maximal value). A subgroup analysis was performed for those with calcific vs non-calcific chronic pancreatitis; non-parametric Wilcoxin rank-sum approach was utilized for subgroup analysis due the small number of patients per group and non-paired nature of the data. P-values ≤0.05 were considered statistically significant.
RESULTS
Patient Characteristics
Characteristics of the chronic pancreatitis and control cohorts are displayed in table 1. As expected by study design, gender, mean age, and mean BMI were similar in the two cohorts. Although BMI was equivalent, the chronic pancreatitis patients were on average shorter (165.7 ± 1.8 vs 170.9 ± 1.9 cm, p=0.02) and weighed less (68.3 ± 3.2 vs 72.5 ± 3.0 kg, p=0.04) compared to healthy controls. Seven patients (28%) with chronic pancreatitis had calcific pancreatitis; the remaining patients were diagnosed with non-calcific chronic pancreatitis based on a combination of recurrent acute pancreatitis with clinical progression and/or supportive studies as described above. Fourteen (56%) were on pancreatic enzyme replacement therapy (PERT).
Table 1.
Characteristics of the 25 non-diabetic patients with chronic pancreatitis and the 25 healthy control participants, matched based on age, gender, and body mass index (BMI). Data are displayed as mean ± standard error.
| Chronic Pancreatitis (n=25) | Healthy Control (n=25) | p-value | |
|---|---|---|---|
| Gender, n Female | 19 (76%) | 19 (76%) | 1.00 |
| Age | 34.2 ± 2.4 | 34.3 ± 2.5 | 0.74 |
| BMI (kg/m2) | 24.7 ± 0.9 | 24.8 ± 0.9 | 0.71 |
| Weight (kg) | 68.3 ± 3.2 | 72.5 ± 3.0 | 0.04 |
| Height (cm) | 165.7 ± 1.8 | 170.9 ± 1.9 | 0.02 |
| Fasting Blood Glucose (mg/dL) | 89.5 ± 2.3 | 84.4 ± 1.2 | 0.04 |
| HbA1c (%) | 5.15 ± 0.06 | 5.09 ± 0.04 | 0.41 |
| HbA1c (mmol/mol) | 32.8 ± 0.66 | 32.1 ± 0.44 | 0.41 |
Causes of pancreatitis were genetic (n=10), idiopathic (n=6), or obstructive (n=9; congenital abnormality or sphincter of Oddi dysfunction). The patients with calcific CP all had genetic risk factors: 2 with protease serine 1 (PRSS1), 3 with serine protease inhibitor Kazal-type 1 (SPINK1), and 2 with Cystic fibrosis transmembrane conductance regulator (CFTR) mutations. Four patients (16%) had prior surgery, Puestow drainage procedure in 3, and pancreatic debridement and pseudocyst drainage in 1.
Histopathology at the time of TPIAT showed evidence of CP in 23/25 patients; in the remaining two cases, one had well-documented recurrent acute pancreatitis with progression to daily chronic pain and imaging findings suggesting CP (but normal pathology from head and tail biopsy), and the second had a grossly abnormal pancreas with pancreatic pseudocyst at surgery but pancreatic tissue was not captured in the biopsy sample for histopathology. Five patients had impaired fasting glucose by current American Diabetes Association criteria (≥100 mg/dL) but only 1 had a fasting glucose >110 mg/dL (111 mg/dL on day of testing). As per study design, none had diabetes mellitus.
Mixed meal tolerance tests
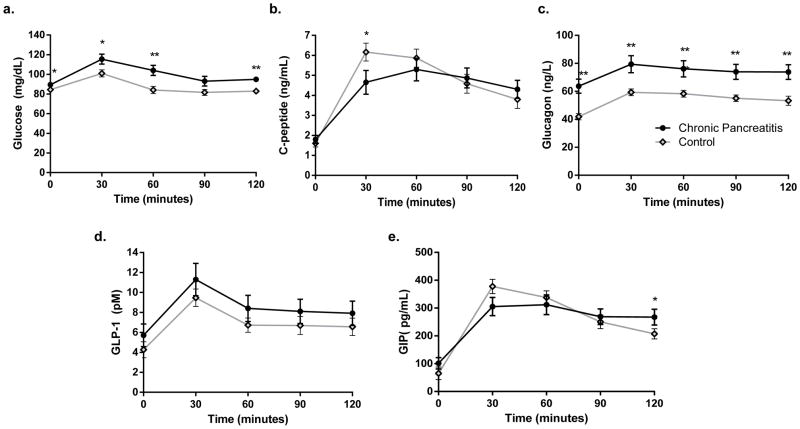
Although mean glucose levels were in the non-diabetic range in the chronic pancreatitis cohort, the area under the curve (AUC) glucose from MMTT was significantly higher in the chronic pancreatitis patients compared to healthy controls (12,144 ± 412 vs 10,511 ± 306 mg/dL*min, p=0.009). Mean glucose levels were higher than in controls at fasting baseline, 30, 60, and 120 minutes during testing (figure 1). In contrast, AUC C-peptide was no different in the chronic pancreatitis cohort compared to controls (536 ± 53 vs 579 ±45, p=0.6). However, there was a notably lower stimulated C-peptide value at 30 minutes in the chronic pancreatitis patients (4.6 ±0.6 ng/mL vs 6.2 ± 0.5 ng/mL, p=0.04, figure 1).
Figure 1.
From mixed meal tolerance tests in chronic pancreatitis (dark circle) and healthy control (grey diamonds): (a) glucose; (b) C-peptide; (c) glucagon levels, (d) GLP-1, and (e) GIP. AUC glucose and AUC glucagon were significantly higher in the CP cohort, but AUC C-peptide, GLP-1, and GIP did not differ between groups. * indicates p-value ≤0.05, ** p ≤0.01.
While the change in glucagon (peak-baseline on MMTT) did not differ between the two groups (21.5 ± 5.1 vs 22.9 ± 2.2 ng/L, p=0.84), overall glucagon levels were elevated both basally and throughout the MMTT in the chronic pancreatitis patients (figure 2), resulting in a greater AUC glucagon (including baseline) in the chronic pancreatitis cohort (8,956 ± 627 vs 6,376 ± 263 ng/L*min in controls, p= 0.0009).
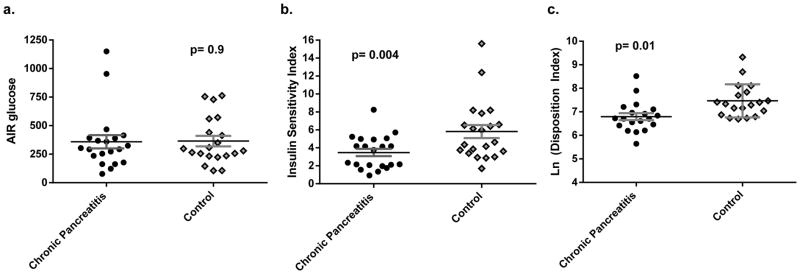
Figure 2.
Results from the frequent sample intravenous glucose tolerance test in chronic pancreatitis and matched control patients: (a) Acute insulin response to glucose (n=20 pairs); (b) insulin sensitivity index (n=21 pairs); and (c) natural log of the disposition index (n=19 pairs).
The incretin hormones GLP-1 and GIP did not differ overall in the chronic pancreatitis and control cohorts. Mean AUC GLP-1 was 1247 ± 263 pM*min in chronic pancreatitis and 860 ± 91 pM*min in controls (p=0.2). Mean AUC GIP was 32,121 ± 3,197 pg/mL*min in chronic pancreatitis and 31,918 ± 1,970 pg/mL*min in controls (p=0.9, figure 1).
Frequent Sample Intravenous Glucose Tolerance Tests
FSIVGTT were performed to assess the AIRglu, insulin sensitivity index (SI), and disposition index (DI) (figure 2). There was no difference in AIRglu in the chronic pancreatitis group and matched controls (340 ± 55 vs 359 ± 58 mIU/mL*min, p=0.9, and supplemental figure 1). However, mean SI was lower in the chronic pancreatitis group (3.48 ± 0.04 vs 5.8 ± 0.73 in controls, p=0.004) indicating that these patients were, on average, less insulin sensitive than healthy control patients. When insulin secretion was adjusted for insulin sensitivity, the mean DI was lower in chronic pancreatitis compared to healthy controls (log-adjusted DI: 6.79 ± 0.15 vs 7.47 ± 0.16, p=0.01) suggesting a defect in insulin secretion. For all parameters, there was a significant overlap between the two groups (figure 2).
We also calculated the acute C-peptide response to glucose (ACRglu) using C-peptide levels measured in the first 10 minutes after the intravenous dextrose bolus. The ACRglu did not differ between chronic pancreatitis and control patients. However, when normalizing stimulated C-peptide to fasting baseline, only 63% of chronic pancreatitis patients had an at least a 2.5 fold increase in C-peptide (defined as peak divided by fasting baseline), compared to 84% of controls (p=0.08).
Differences in patients with non-calcific and calcific pancreatitis suggest disease progression
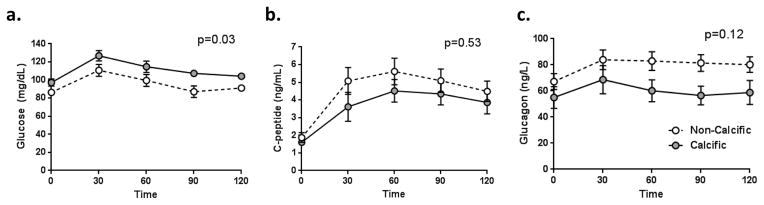
We performed a subgroup analysis to compare those patients with non-calcific pancreatitis (n=18) to those with pancreatic calcifications, presuming that the latter represents a more severe stage of disease with potentially greater pancreatic damage. Notably, the patients with calcific pancreatitis, on average, exhibited higher hemoglobin A1c levels, greater AUC glucose on MMTT, and lower AIRg, ACRg, and DI from FSIVGTT (Table 2, on-line supplementary figure 1). The mean disposition index was nearly 70% reduced for the calcific chronic pancreatitis group compared to the group with non-calcific pancreatic disease. Although there was a trend towards lower AUC glucagon in the calcific pancreatitis group, this difference was not statistically significant in this small cohort.
Table 2.
Fasting basal, mixed meal tolerance test, and frequent sample intravenous glucose tolerance test parameters in those with calcific vs non-calcific chronic pancreatitis. Data are displayed as mean ± standard error. The healthy control data are provided for reference.
| Healthy Controls (for reference) | Non-calcific pancreatitis (n=18) | Calcific pancreatitis (n=7) | p-value for non-calcific vs calcific CP | |
|---|---|---|---|---|
| HbA1c (%) | 5.09 ± 0.04 | 5.03 ± 0.06 | 5.45 ± 0.04 | 0.002 |
| HbA1c (mmol/mol) | 32.1 ± 0.44 | 31.5 ± 0.66 | 36.1 ± 0.44 | 0.002 |
| Fasting Blood Glucose (mg/dL) | 84.4 ± 1.2 | 86.4 ± 2.5 | 97.3 ± 3.9 | 0.07 |
| N with IFG (%) | 0 | 2 (11%) | 4 (43%) | 0.07 |
| Fasting C-peptide (ng/mL) | 1.60 ± 0.19 | 1.87 ± 0.27 | 1.60 ± 0.16 | 0.81 |
| Fasting glucagon (ng/L) | 41.8 ± 2.2 | 67.2 ± 5.9 | 55.0 ± 8.3 | 0.25 |
| AIRglu | 359 ± 58 | 339 ± 60 | 187 ± 45 | 0.02 |
| ACRglu | 44.8 ± 4.2 | 50.5 ± 4.5 | 28.2 ± 3.9 | 0.006 |
| Disposition Index | 2349 ± 568 | 1434 ± 283 | 465 ± 65 | 0.003 |
| SI | 5.8 ± 0.7 | 3.7 ± 0.4 | 3.5 ± 2.8 | 0.49 |
| AUC glucose (MMTT, mg/dL*min) | 10511 ± 306 | 11588 ± 513 | 13493 ± 321 | 0.03 |
| AUC C-peptide (MMTT, ng/mL*min) | 579 ± 45 | 569 ± 68 | 456 ± 67 | 0.53 |
| AUC glucagon (MMTT, ng/L*min) | 6379 ± 263 | 9680 ± 723 | 7267 ± 996 | 0.12 |
Abbreviations: IFG= impaired fasting glucose, AIRglu = acute insulin response to glucoses; ACRglu = acute C-peptide response to glucose; SI= insulin sensitivity index; AUC = area under the curve; MMTT= mixed meal tolerance test.
DISCUSSION
In chronic pancreatitis, progressive replacement of the pancreatic parenchyme with fibrosis can result in irreversible damage to the pancreatic islets and risk of diabetes mellitus (3). This form of pancreatic diabetes (also called type 3c diabetes mellitus) may occur in more than half of patients with long-standing severe chronic pancreatitis (7–9, 28), and is characterized clinically by loss of islet mass (6, 20, 22). However, most research to date has focused on small cohorts (often 10 or fewer patients per study group) with late-stage disease, often with diabetes mellitus already present or at least severe calcific chronic pancreatitis on imaging (10, 12, 20). Thus, our understanding of the natural history of progression from early stage chronic pancreatitis to late disease with diabetes mellitus remains incomplete. In the current analysis, we performed metabolic testing in a cohort of patients with largely non-calcific “earlier” stages of pancreatic damage who were scheduled for total pancreatectomy and islet autotransplant, and compared pancreatic endocrine function in these patients with healthy controls matched for important attributes of age, gender, and body mass index. In our cohort of non-diabetic patients with CP due to obstructive, genetic, or idiopathic causes, we observed small but significant elevations in glucose fasting and after mixed meal challenge, a reduced early insulin secretory response to a meal, insulin resistance, and a state of relative hyperglucagonemia compared to healthy individuals. Those with more advanced disease, as represented by pancreatic calcifications, exhibited a more severe defect in insulin production.
In patients with pancreatogenous diabetes mellitus, insulin secretion in response to intravenous and oral secretagogues is abnormal (6, 10, 12). We observed modestly higher glucose levels in the patients in our cohort afflicted with chronic pancreatitis, with a blunted C-peptide response at 30 minutes after ingestion of the mixed meal. The first phase insulin response (AIRglu) was not in and of itself different in the chronic pancreatitis patients but when adjusted for insulin sensitivity using the disposition index was lower on average in the chronic pancreatitis patients. These results taken together suggest that the earliest abnormalities in chronic pancreatitis are subtle changes in glycemic control with blunted early insulin secretory response to a meal.
Unexpectedly, we found an elevation of basal and post-meal glucagon levels in patients with chronic pancreatitis. This, on the surface, seems in contrast to the classic picture of glucagon deficiency in advanced chronic pancreatitis complicated by diabetes mellitus (6, 20). It also differs from another disease with partial fibrotic destruction of islets, cystic fibrosis, where both insulin and glucagon secretion are diminished (29, 30). Because this result was unanticipated, we first questioned whether this could be a technical or assay problem. However, all specimens were collected in aprotinin-containing tubes prepared by the same technician, were subjected to the same processing procedures, and run with an identical glucagon assay. In addition, there was overlap in the timeframes during which the chronic pancreatitis and control glucagon assays were run making assay drift an unlikely cause.
Our data suggest that hypoglucagonemia may be a late finding which is preceded by a period of hyperglucagonemia early in the course of the disease. Elevations in basal and alanine-stimulated glucagon has been previously observed in acute pancreatitis (20) and chronic pancreatitis (17, 31). Kannan et al reported a similar elevation in basal glucagon and following stimulation with oral glucose or IV arginine in 10 patients with chronic pancreatitis (31). This latter cohort of patients was similar to ours in that only 20% (2 of 10 patients) had calcific pancreatitis, with strikingly similar differences in basal and post-prandial glucagon levels compared to healthy controls to those we observed in the present study. We postulate that elevations in glucagon may result from chronic stress and inflammation, until the pancreas reaches a state where too many alpha cells have been lost to maintain high levels. Alternatively, slowly dying alpha cells may release stored glucagon leading to chronic elevation, or beta cell dysfunction may alter the normal beta cell- alpha cell signaling for glucagon suppression and exaggerate such responses, as is observed in those with type 1 diabetes (32, 33). A progressive loss of glucose suppression of glucagon in response to an oral glucose load has been reported in chronic pancreatitis patients, as severity of disease progressed from normal to impaired glucose tolerance to diabetes (17). Interestingly, in our cohort, the elevation in glucagon was present in the basal and post-meal measures, but the increment of change (ie basal to post-meal) was similar between groups, suggesting a different “set point” in the glucagon secretion rather than a truly exaggerated meal response. Interestingly, it has been proposed that patients with chronic pancreatitis have increased hepatic insulin resistance based on clinical and pre-clinical models (4, 34–36); thus, one could postulate that hyperglucagonemia is contributing to the phenotype of hepatic insulin resistance. Regardless of the cause, the observed differences in glucagon levels between normal and chronic pancreatitis patients may be contributing to the higher glucose levels we observed in the latter during mixed meal testing.
In contrast to prior publications by Knop et al., we did not observe any abnormalities in the incretin response to a meal (14–16). It has been previously postulated that malabsorption in chronic pancreatitis may lead to abnormal secretion of small bowel incretin hormones GLP-1 and GIP, thereby contributing to insulin dysregulation and hyperglycemia in chronic pancreatitis. However, our patients were instructed to take their pancreatic enzymes as they would with a meal if prescribed by their gastroenterologist (14 of our 25 participants with CP); thus, we were likely to avoid malabsorption in those who might have been at risk.
Insulin sensitivity was measured by FSIVGTT. Peripheral insulin sensitivity has been postulated to be enhanced in chronic pancreatitis, although the data from hyperinsulinemic euglycemic clamp studies are mixed (6, 19, 37). We observed that our cohort of patients with chronic pancreatitis, who were clinically well at the time of testing, were less insulin sensitive than the healthy controls. One might hypothesize that the chronic illness, chronic inflammation, and reduced physical activity state of these patients with chronic pancreatitis leads to a state of reduced insulin sensitivity. However, cystic fibrosis patients, who also have lost islets to fibrosis and who experience chronic inflammation, compensate for insulin insufficiency with normal to increased insulin sensitivity, unless they are acutely ill (which leads to transient insulin resistance) (29, 38). It is possible, however, that inflammation within the pancreas itself is greater in chronic pancreatitis than in CF, since most of the exocrine tissue is destroyed and replaced by fibrosis and fat very early in the course of CF in the majority of patients. Furthermore, the Minmod model using FSIVGTT does not distinguish between total body or hepatic insulin resistance, so it is possible that there is a relative hepatic insulin resistance while retaining whole body insulin sensitivity, as has been proposed in this population previously (4).
A potential important contributor to insulin resistance that is unexplored in our current study is pancreatic polypeptide (PP) deficiency. PP is absent in patients with type 3c (pancreatogenous) diabetes mellitus and those with pancreatic head resection (1,4). Current data from clamp studies suggest that this deficiency of PP contributes to hepatic insuiln resistance, and infusion of PP can reverse this insulin resistance (4). Thus, future analyses in this population should include PP measures to determine progression to PP loss, if prior to diabetes onset, and the importance of PP deficiency in modulating the observed changes.
While this is not the first study to evaluate endocrine function in patients with chronic pancreatitis, we believe there are several features of this study that distinguish it from previously published work. First, much of the early literature on chronic pancreatitis focuses on those with diabetes or impaired glucose tolerance, or with no diabetes but advanced pancreatic parenchymal damage as evidenced by calcifications on CT. Our data from the calcific pancreatitis subgroup (7 patients) suggest that this represents a more severe stage of disease progression. Second, early studies generally focused on a specific population of patients—men, with alcoholic-induced pancreatitis. Newer epidemiologic data in fact suggests that alcohol is not the causative factor in the majority of patients with chronic pancreatitis. Our cohort, in contrast, included more female participants, and included idiopathic, anatomic, and genetic disease etiologies. We had histopathology from the TPIAT procedure to aid in confirming the clinical/imaging diagnosis for the majority of our participants, to validate this cohort. Lastly, and importantly, prior studies have largely not controlled for the healthy population. Glucose regulation and insulin secretion can depend on important attributes such as body mass index and age. We attempted to better control for the variation in the normal population by matching our healthy control cohort to the chronic pancreatitis cohort for gender, age, and BMI.
Chronic pancreatitis is a heterogeneous disease, with varying etiologies, but a similar endpoint of pancreatic fibrosis and loss of pancreatic function. Given the variability in disease course and causes, generalized conclusions are limited by our small cohort. However, our results do suggest that early changes may be detectable before frank diabetes onset. Future studies might consider using other measures of beta cell mass such arginine stimulation, and repeating studies on a routine basis to assess disease progression (39). Progressive loss of pancreatic endocrine function has implications, particularly with when to intervene in the process of disease with a major procedure such as TPIAT. Interestingly, our group of patients with calcific pancreatitis largely had genetic etiologies of disease; it is feasible that the progressive decrements in beta cell function that were observed with the calcific pancreatitis group were attributable to genetic disease more so that the calcific changes themselves. This would require further study with prospective longitudinal assessments.
In summary, the progression to diabetes in chronic pancreatitis is poorly characterized, particularly within populations other than men with alcoholic disease. In our population of patients with chronic pancreatitis due to non-alcohol etiologies, including both men and women, patients with chronic pancreatitis had a reduced disposition index on FSIVGTT, modest but significant elevations in their glucose response to a meal, a lower early C-peptide response to mixed meal testing, basal and post-meal hyperglucagonemia, and reduced insulin sensitivity compared to healthy controls. Insulin deficiency is more pronounced in those with calcific chronic pancreatitis, presumably representing a more advanced stage of fibrosis and islet destruction. Early deficits in glucose regulation in chronic pancreatitis appear to result from small deficits in insulin production and abnormal glucagon elevations before the classic glucagon deficiency of severe late-stage chronic pancreatitis complicated by “brittle” diabetes occurs.
Supplementary Material
Insulin and C-peptide levels by time point during intravenous glucose tolerance test for chronic pancreatitis (blue diamonds) and healthy controls (grey circles).
Figure 3.
Mixed meal tolerance test glucose (A), C-peptide (B), and glucagon levels (C) in patients with non-calcific chronic pancreatitis (n=18, open circles with dashed line) and calcific pancreatitis (n=7, grey circles, solid grey line). The AUCglucose was significantly higher in calcific chronic pancreatitis, while differences in C-peptide and glucagon were not statistically significant. Data are graphically represented as mean ± standard error.
Acknowledgments
Financial Support:
This study was funded in part by the American Diabetes Association (#1-11-CT-06) and the National Pancreas Foundation. Dr. Bellin receives support from the National Institute for Diabetes, Digestive, and Kidney diseases (1K23DK084315). Dr. Robertson receives support from the National Institute for Diabetes, Digestive, and Kidney diseases (R01-39994).
The authors would like to thank the Clinical Translational Sciences Institute and their staff for their contributions to conducting the study. The CTSI is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114.
Footnotes
Conflict of Interest Disclosure: The authors declare no conflict of interest.
Author contributions: R.L. and M.D.B. constructed the design of the study, collected and analyzed data, and wrote the manuscript. G.J.B. constructed the design of the study and revised the manuscript. T.B.D., T.L.P., K.L.B., and P.P. participated in participant care, data collection for the study, and approval of final manuscript. M.L.F., R.P.R., and A.M. contributed to data interpretation, revised and approved the manuscript. R.L. and M.D.B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Contributor Information
Rachel Lundberg, Department of Pediatrics, University of Minnesota, Minneapolis, Minnesota.
Gregory J. Beilman, Department of Surgery, University of Minnesota, Minneapolis, Minnesota.
Ty B. Dunn, Department of Surgery, University of Minnesota, Minneapolis, Minnesota.
Tim L. Pruett, Department of Surgery, University of Minnesota, Minneapolis, Minnesota.
Martin L. Freeman, Department of Medicine, University of Minnesota, Minneapolis, Minnesota.
Peggy E. Ptacek, Department of Pediatrics, University of Minnesota, Minneapolis, Minnesota
K. Louise Berry, Department of Surgery, University of Minnesota, Minneapolis, Minnesota.
R. Paul Robertson, Department of Medicine, University of Minnesota, Minneapolis, Minnesota.
Antoinette Moran, Department of Pediatrics, University of Minnesota, Minneapolis, Minnesota.
Melena D. Bellin, Department of Pediatrics, University of Minnesota, Minneapolis, Minnesota.
References
- 1.Rickels MR, Bellin M, Toledo FG, et al. Detection, evaluation and treatment of diabetes mellitus in chronic pancreatitis: recommendations from PancreasFest 2012. Pancreatology. 2013;13:336–42. doi: 10.1016/j.pan.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammann RW. Diagnosis and management of chronic pancreatitis: current knowledge. Swiss medical weekly: official journal of the Swiss Society of Infectious Diseases, the Swiss Society of Internal Medicine, the Swiss Society of Pneumology. 2006;136:166–74. doi: 10.4414/smw.2006.11182. [DOI] [PubMed] [Google Scholar]
- 3.Braganza JM, Lee SH, McCloy RF, et al. Chronic pancreatitis. Lancet. 2011;377:1184–97. doi: 10.1016/S0140-6736(10)61852-1. [DOI] [PubMed] [Google Scholar]
- 4.Cui Y, Andersen DK. Pancreatogenic diabetes: special considerations for management. Pancreatology. 2011;11:279–94. doi: 10.1159/000329188. [DOI] [PubMed] [Google Scholar]
- 5.Ewald N, Hardt PD. Diagnosis and treatment of diabetes mellitus in chronic pancreatitis. World J Gastroenterol. 2013;19:7276–81. doi: 10.3748/wjg.v19.i42.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen S. Diabetes mellitus secondary to chronic pancreatitis. Dan Med Bull. 1993;40:153–62. [PubMed] [Google Scholar]
- 7.Malka D, Hammel P, Sauvanet A, et al. Risk factors for diabetes mellitus in chronic pancreatitis. Gastroenterology. 2000;119:1324–32. doi: 10.1053/gast.2000.19286. [DOI] [PubMed] [Google Scholar]
- 8.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252–61. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 9.Rebours V, Boutron-Ruault MC, Schnee M, et al. The natural history of hereditary pancreatitis: a national series. Gut. 2009;58:97–103. doi: 10.1136/gut.2008.149179. [DOI] [PubMed] [Google Scholar]
- 10.Domschke S, Stock KP, Pichl J, et al. Beta-cell reserve capacity in chronic pancreatitis. Hepato-gastroenterology. 1985;32:27–30. [PubMed] [Google Scholar]
- 11.Wakasugi H, Funakoshi A, Iguchi H. Clinical assessment of pancreatic diabetes caused by chronic pancreatitis. J gastroenterol. 1998;33:254–9. doi: 10.1007/s005350050079. [DOI] [PubMed] [Google Scholar]
- 12.Nyboe Andersen B, Krarup T, Thorsgaard Pedersen NT, et al. B cell function in patients with chronic pancreatitis and its relation to exocrine pancreatic function. Diabetologia. 1982;23:86–9. doi: 10.1007/BF01271165. [DOI] [PubMed] [Google Scholar]
- 13.Cote GA, Yadav D, Slivka A, et al. Alcohol and smoking as risk factors in an epidemiology study of patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2011;9:266–73. doi: 10.1016/j.cgh.2010.10.015. quiz e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knop FK. Incretin hormones and beta cell function in chronic pancreatitis. Dan Med Bull. 2010;57:B4163. [PubMed] [Google Scholar]
- 15.Knop FK, Vilsboll T, Hojberg PV, et al. The insulinotropic effect of GIP is impaired in patients with chronic pancreatitis and secondary diabetes mellitus as compared to patients with chronic pancreatitis and normal glucose tolerance. Regul Pept. 2007;144:123–30. doi: 10.1016/j.regpep.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Knop FK, Vilsboll T, Larsen S, et al. Increased postprandial responses of GLP-1 and GIP in patients with chronic pancreatitis and steatorrhea following pancreatic enzyme substitution. Am J Physiol Endocrinol Metab. 2007;292:E324–30. doi: 10.1152/ajpendo.00059.2006. [DOI] [PubMed] [Google Scholar]
- 17.Knop FK, Vilsboll T, Larsen S, et al. Glucagon suppression during OGTT worsens while suppression during IVGTT sustains alongside development of glucose intolerance in patients with chronic pancreatitis. Regul Pept. 2010;164:144–50. doi: 10.1016/j.regpep.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Yasuda H, Harano Y, Ohgaku S, et al. Insulin sensitivity in pancreatitis, liver diseases, steroid treatment and hyperthyroidism assessed by glucose, insulin and somatostatin infusion. Horm Metab Res. 1984;16:3–6. doi: 10.1055/s-2007-1014681. [DOI] [PubMed] [Google Scholar]
- 19.Nosadini R, del Prato S, Tiengo A, et al. Insulin sensitivity, binding, and kinetics in pancreatogenic and type I diabetes. Diabetes. 1982;31:346–55. doi: 10.2337/diab.31.4.346. [DOI] [PubMed] [Google Scholar]
- 20.Donowitz M, Hendler R, Spiro HM, et al. Glucagon secretion in acute and chronic pancreatitis. Ann Intern Med. 1975;83:778–81. doi: 10.7326/0003-4819-83-6-778. [DOI] [PubMed] [Google Scholar]
- 21.Sasikala M, Talukdar R, Pavan kumar P, et al. beta-Cell dysfunction in chronic pancreatitis. Dig Dis Sci. 2012;57:1764–72. doi: 10.1007/s10620-012-2086-7. [DOI] [PubMed] [Google Scholar]
- 22.Schrader H, Menge BA, Zeidler C, et al. Determinants of glucose control in patients with chronic pancreatitis. Diabetologia. 2010;53:1062–9. doi: 10.1007/s00125-010-1705-0. [DOI] [PubMed] [Google Scholar]
- 23.Lundberg R, Beilman GJ, Dunn TB, et al. Metabolic Assessment Prior to Total Pancreatectomy and Islet Autotransplant: Utility, Limitations and Potential. Am J Transplant. 2013 doi: 10.1111/ajt.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boston RC, Stefanovski D, Moate PJ, et al. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–15. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 25.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23:113–22. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 26.Mahon JL, Beam CA, Marcovina SM, et al. Comparison of two insulin assays for first-phase insulin release in type 1 diabetes prediction and prevention studies. Clinica chimica acta. 2011;412:2128–31. doi: 10.1016/j.cca.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcovina S, Bowsher RR, Miller WG, et al. Standardization of insulin immunoassays: report of the American Diabetes Association Workgroup. Clin chem. 2007;53:711–6. doi: 10.1373/clinchem.2006.082214. [DOI] [PubMed] [Google Scholar]
- 28.De Bruijn KM, van Eijck CH. New-onset Diabetes After Distal Pancreatectomy: A Systematic Review. Ann Surg. 2014 doi: 10.1097/SLA.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 29.Moran A, Becker D, Casella SJ, et al. Epidemiology, pathophysiology, and prognostic implications of cystic fibrosis-related diabetes: a technical review. Diabetes care. 2010;33:2677–83. doi: 10.2337/dc10-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran A, Diem P, Klein DJ, et al. Pancreatic endocrine function in cystic fibrosis. J Pediatr. 1991;118:715–23. doi: 10.1016/s0022-3476(05)80032-0. [DOI] [PubMed] [Google Scholar]
- 31.Kannan V, Nabarro JD, Cotton PB. Glucagon secretion in chronic pancreatitis. Horm Res. 1979;11:203–12. doi: 10.1159/000179055. [DOI] [PubMed] [Google Scholar]
- 32.Sherr J, Tsalikian E, Fox L, et al. Evolution of abnormal plasma glucagon responses to mixed-meal feedings in youth with type 1 diabetes during the first 2 years after diagnosis. Diabetes care. 2014;37:1741–4. doi: 10.2337/dc13-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer CK, Borgono CA, Van Nostrand P, et al. Glucagon response to oral glucose challenge in type 1 diabetes: lack of impact of euglycemia. Diabetes care. 2014;37:1076–82. doi: 10.2337/dc13-2339. [DOI] [PubMed] [Google Scholar]
- 34.Andersen DK, Ruiz CL, Burant CF. Insulin regulation of hepatic glucose transporter protein is impaired in chronic pancreatitis. Ann Surg. 1994;219:679–86. doi: 10.1097/00000658-199406000-00011. discussion 86–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nathan JD, Zdankiewicz PD, Wang J, et al. Impaired hepatocyte glucose transport protein (GLUT2) internalization in chronic pancreatitis. Pancreas. 2001;22:172–8. doi: 10.1097/00006676-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Seymour NE, Turk JB, Laster MK, et al. In vitro hepatic insulin resistance in chronic pancreatitis in the rat. J Surg Res. 1989;46:450–6. doi: 10.1016/0022-4804(89)90159-5. [DOI] [PubMed] [Google Scholar]
- 37.Niebisz-Cieslak AB, Karnafel W. Insulin sensitivity in chronic pancreatitis and features of insulin resistance syndrome. Polskie Archiwum Medycyny Wewnetrznej. 2010;120:255–63. [PubMed] [Google Scholar]
- 38.Moran A, Pyzdrowski KL, Weinreb J, et al. Insulin sensitivity in cystic fibrosis. Diabetes. 1994;43:1020–6. doi: 10.2337/diab.43.8.1020. [DOI] [PubMed] [Google Scholar]
- 39.Robertson RP. Estimation of beta-cell mass by metabolic tests: necessary, but how sufficient? Diabetes. 2007;56:2420–4. doi: 10.2337/db07-0742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Insulin and C-peptide levels by time point during intravenous glucose tolerance test for chronic pancreatitis (blue diamonds) and healthy controls (grey circles).