Abstract
OBJECTIVES
To determine the relationship between chronic kidney disease (measured by cystatin C-based eGFR) and abnormal ambulatory blood pressure (including nocturnal dipping) in healthy older adults. Further, to assess agreement between clinic and ambulatory blood pressure monitoring.
METHODS
Serum cystatin C levels were measured to calculate eGFR. Participants underwent clinic and 24-hour ambulatory blood pressure measurement. Multiple linear regression, was performed to examine the association between reduced cystatin C-based eGFR (CKDcys) and blood pressure parameters. Bland-Altman analysis was performed to evaluate agreement between clinic and ambulatory measurements.
RESULTS
Average age was 72. There were 60 individuals with CKDcys (eGFR < 60 ml/min/1.73m2). Compared to those without CKDcys, individuals with CKDcys were older, more likely to have hypertension and less likely to have normal dipping patterns. After multivariate analysis, the presence of CKDcys was significantly associated with lower mean ambulatory diastolic blood pressure (DBP) (−2 mm Hg, p = 0.048), but not with nocturnal dipping or other blood pressure parameters. Clinic systolic blood pressure (SBP) significantly overestimated mean wake time ambulatory SBP; mean difference was 11 mmHg for those without CKDcys (95% limits of agreement −14 to 35 mmHg) and 14 mmHg for those with CKDcys (95% limits of agreement −13 to 41 mmHg); there was no statistically significant effect modification by CKD status.
CONCLUSION
In older, seemingly healthy adults, mild CKD was associated with lower ambulatory DBP. The presence of CKD did not affect interpretation of clinic vs. ambulatory blood pressure monitoring, although accuracy of clinic SBP was poor.
Keywords: Hypertension, ambulatory blood pressure monitoring (ABPM), chronic kidney disease (CKD), cystatin C
INTRODUCTION
Ambulatory blood pressure monitoring (ABPM) provides data on average blood pressure over 24 hours, including wake and sleep values and diurnal patterns. It is considered by many to be the diagnostic standard against which other blood pressure modalities should be compared.1,2 Individuals with confirmed out-of-office hypertension, and those with <10% diurnal variation (“non-dippers”) suffer increased morbidity and mortality.3,4 A recent systematic review on the improved accuracy of hypertension diagnosis has confirmed that ABPM is more closely linked to cardiovascular disease outcomes than standard clinic blood pressure measurements, and the US Preventive Services Task Force has released a draft recommendation to use ABPM routinely in all patients with a positive screen for hypertension in clinic blood pressure measurements.5,6,7 While this change would vastly increase the frequency with which this measure is utilized and interpreted in clinical practice, populations with comorbid conditions including chronic kidney disease (CKD) were excluded from the data used to produce this analysis.
Those with CKD are more likely to have clinically apparent hypertension, either as the causative factor for CKD or as a consequence of longstanding CKD. Further, advanced CKD (stages IV and V) is associated with abnormal nocturnal dipping.8 While mild decrements in kidney function as measured by cystatin C-based eGFR (eGFRcys) have been associated with increased rates of hypertension,9 to our knowledge few studies have examined whether mild decrements in kidney function are associated with abnormal 24-hour blood pressure patterns. Cystatin-C based eGFR is an important metric for these studies, because of its increased sensitivity at higher ranges of eGFR10 and because of its utility in older adults, for whom creatinine may overestimate kidney function in the setting of lower muscle mass.11
Furthermore, agreement between ABPM and clinic blood pressure measurements is variable and may differ in general populations12 as well as in those with advanced CKD,13 but this question has not been examined closely among older adults nor has it been investigated in the context of cystatin C-based eGFR measures. In older adults, cystatin C has the advantage of being less influenced by muscle mass or overall health status, and serves as an early marker of decreased eGFR.14,10 More work is needed, then, to understand the role ABPM plays in identifying hypertension among individuals with mild kidney disease.
In the present cross-sectional study, we examined the relationship between eGFRcys and ambulatory blood pressure parameters in a cohort of generally healthy, community-dwelling older adults in San Diego. We hypothesized that lower eGFRcys would be associated with abnormalities in blood pressure parameters, including non-dipping and ambulatory hypertension, given the sodium and water retention seen in CKD and the known higher prevalence of non-dipping in those with advanced CKD. We hypothesized that this association would be stronger using eGFRcys than for kidney dysfunction as measured by creatinine (eGFRcr). Finally, because the diagnosis of hypertension is overwhelmingly established based on clinic blood pressure measurement at present, we evaluated the agreement between ambulatory and clinic blood pressure measurements to determine the precision and accuracy of this approach in this population, and tested whether it differed by eGFRcys –based CKD status.
METHODS
Study Participants
Data were collected from a subset of participants originally enrolled in the San Diego Population Study (SDPS). A description of recruitment and study design for the original SDPS has been published previously.15 In brief, the SDPS is an ongoing observational study designed to examine the prevalence and incidence of chronic peripheral venous and arterial disease in a population of healthy, asymptomatic adults. Subjects enrolled in the original 1994-1998 study (n=2408) were current and former employees of the University of California, San Diego; 1103 returned for another visit between 2006-2011. We sent invitation letters to participants who had San Diego County addresses on file and had indicated willingness to be contacted for future studies (N=944); 354 responded and agreed to participate in the current study between January 2012 and June 2013. Participants were at least 55 years of age, living independently, and able to provide their own consent for the study. During a single study visit we obtained relevant measures including 24-hour ABPM, measurement of kidney function using cystatin C, creatinine, and albuminuria, and assessment of physical and cognitive function. Participants were excluded from the current analysis if they lacked serum cystatin C measurements (n=6), or did not undergo full 24-hour ambulatory monitoring (n=14).
Kidney Function
Serum specimens were collected from all participants at the time of the study visit. Serum creatinine was measured immediately at the University of California, San Diego Center for Advanced Laboratory Medicine using a standard, calibrated creatinine assay. Serum specimens were subsequently stored at −70°C. Serum cystatin C was measured at the University of Minnesota Advanced Research and Diagnostic laboratory using a Gentian assay on a Roche COBAS 6000 analyzer. Glomerular filtration rates were estimated (eGFR) using the 3 recently developed CKD-EPI equations for creatinine, cystatin C, and the combination of the two.10 Participants were categorized by the presence of CKD based on an eGFRcys < 60 mL/min per 1.73 m2 or an eGFRcr < 60 mL/min per 1.73 m2.
Covariates
Participant characteristics were obtained by self-reported questionnaire and included information on age, gender, race, smoking and alcohol use and medical conditions. Alcohol use was defined as current use; tobacco use was defined as current, former, or never. Personal history of hypertension was defined by self report, or active use of an antihypertensive agent without alternative indication. Diabetes was defined by self report, or active use of insulin or hypoglycemic agents. Prevalent cardiovascular disease was defined as history of myocardial infarction, congestive heart failure, stroke or transient ischemic attack (TIA). Height and weight were measured during the study visit, and were used to calculate body mass index (BMI, reported in kilograms per square meter). An abnormal Epworth Sleepiness Score was defined as any score ≥ 10 or 24 possible points based on previous literature identifying such a score as predictive of excessive daytime sleepiness.16
Blood Pressure
Blood pressure was initially measured during the study visit by performing three seated measurements using an automated blood pressure cuff (Dynapulse, Vista, CA), after 5 minutes of seated rest. The average of these three measurements was used for analysis; the first measurement was not eliminated from analysis as recent studies have found no statistically significant difference between this and subsequent measurement.17 Ambulatory blood pressure monitoring was then initiated using a SpaceLabs 90217 monitor. The first ambulatory measurement was obtained during the study visit to confirm proper cuff placement and machine accuracy. Blood pressure was measured every 20 minutes while awake and every 60 minutes while asleep for a total duration of 24 hours; measurement intervals were pre-programmed based on subject-anticipated sleep periods and sleep and wake intervals were confirmed by in-person interviews on the morning when overnight monitoring ended. Daytime nap, if it occurred, was not considered as part of a sleep period. If there were discrepancies between anticipated and actual sleep periods, analysis was performed based on self-reported sleep period during in-person interviews after completion of overnight monitoring. Values collected during ambulatory monitoring were then averaged and reported as overall, wake, and sleep mean systolic blood pressure (SBP), diastolic blood pressure (DBP) and pulse pressure. Blood pressure values collected during the sleep period were compared to those collected during the wake period to calculate the percent change between the two, known as “dipping,” for both SBP and DBP. We considered 14 daytime and 6 nighttime readings to ABPM readings to be an adequate ABPM report. BP values were also used to calculate ambulatory arterial stiffness index and average real variability, reflecting arterial stiffness and reading-to-reading variability, respectively.18,19
Statistical Analysis
Baseline characteristics of subjects were summarized by eGFRcys status. Differences between CKD status were determined using t-tests for continuous variables and chi-square tests for categorical variables. Pearson correlation coefficients were calculated for eGFRcys and eGFRcr versus clinic and ambulatory blood pressure measurements. Simple linear regression was performed to examine the relationship between eGFRcys and eGFRcr and various blood pressure measurements (clinic SBP; DBP; and pulse pressure, and mean ambulatory SBP; DBP; pulse pressure; and dipping). We chose covariates based on biological plausibility or statistical significance in univariate modeling. Staged multivariable linear regression was subsequently performed to account for 1) age and 2) then gender race, BMI, history of hypertension treatment, diabetes, cardiovascular disease, smoking history, and abnormal Epworth score. We used stepwise regression to identify individual confounders with particularly notable effects on the models. Similarly, we used linear regression to examine the associations of CKDcys and CKDcr with in-clinic and ABPM metrics, with a parallel set of nested models. The prevalence of “dippers”, again defined as individuals with diurnal variation ≥10%, was calculated for 10-unit incremental increases in eGFRcys and eGFRcr to ascertain prevalence rate ratios. This method was chosen given the relatively high percentage of non-dipping in our cohort and consequent concern that odds ratios would not accurately estimate relative risks.
We performed sensitivity analyses testing for differences between those taking blood pressure medication and those who were not, as well as testing whether the presence of CKD defined by both eGFR and the presence of microalbuminuria was associated with dipping. We also considered whether albuminuria and eGFRcys might contribute separately to dipping behavior. In order to evaluate this, we explored linear models of systolic dipping with albuminuria (expressed as log of albumin/creatinine ratio) and eGFR included separately and then together, first in univariate analysis and then adjusted for demographic and clinical factors as in our other models. We also examined associations between CKDcys and nocturnal blood pressure as a linear outcome, as opposed to dipping percentage, since some studies have shown nocturnal blood pressure to be most important for cardiovascular outcomes. We also compared separate cystatin and creatinine based CKD-EPI equations to the combined equation.
Finally, to examine agreement between ambulatory blood pressure monitoring and in-clinic blood pressure measurement, we created Bland-Altman plots stratified by CKD status, from which mean differences and 95% confidence intervals were identified. All analysis was performed using SAS statistical software (release 9.3, SAS Institute Inc., Cary, NC); p values of < 0.05 were considered significant.
RESULTS
Baseline Characteristics
There were 334 participants with a mean age of 72 ± 6 years for whom ambulatory blood pressure data and cystatin C measurements were collected; 225 (67%) were female (Table 1). Overall, average eGFRcys was 78 ± 20; sixty (18%) were classified as having CKD by the cystatin C equation. Compared to participants without CKDcys, those with CKDcys were older, more likely to be female and to have hypertension and diabetes. On average, participants with CKDcys had higher BMI (29.9 vs 26.7 kg/m2). Mean in-clinic SBP and pulse pressure were significantly higher in those with CKD but mean in-clinic DBP was significantly lower. Similarly, mean ambulatory pulse pressure was significantly higher in those with CKD but mean ambulatory DBP was significantly lower; mean ambulatory SBP did not differ significantly between those with and without CKD.
TABLE 1.
Characteristics of Participants by CKD status
| No CKD* | CKD* | P-value | |
|---|---|---|---|
| n | 274 | 60 | |
| Demographics | |||
| Age | 71 (6) | 78 (7) | <0.001 |
| Female | 177 (65%) | 48 (80%) | 0.02 |
| Race | 0.09 | ||
| White | 169 (62%) | 34 (57%) | - |
| Black | 30 (11%) | 13 (22%) | - |
| Hispanic | 37 (14%) | 10 (17%) | - |
| Asian | 29 (12%) | 2 (3%) | - |
| Medical History | |||
| Hypertension | 124 (45%) | 45 (75%) | <0.001 |
| Taking blood pressure medication(s) | 136 (50%) | 47 (78%) | 0.002 |
| Diabetes | 25 (9%) | 12 (20%) | 0.02 |
| Cardiovascular disease § | 24 (9%) | 10 (17%) | 0.07 |
| Family history of cardiovascular disease § | 221 (81%) | 55 (92%) | 0.04 |
| Alcohol use ^ | 198 (73%) | 30 (50%) | 0.001 |
| Tobacco use ^ | 85 (31%) | 23 (38%) | 0.3 |
| Measurements | |||
| estimated GFR (CKD-EPI cystatin) | 85 (14) | 47 (10) | <0.001 |
| estimated GFR (CKD-EPI creatinine) | 78 (13) | 58 (16) | <0.001 |
| Body mass index (kg/m2) | 26.7 (4.9) | 29.9 (6.6) | <0.001 |
| Mean in-clinic systolic blood pressure (mmHg) | 140 (16) | 145 (16) | 0.02 |
| Mean in-clinic diastolic blood pressure (mmHg) | 76 (9) | 71 (10) | 0.002 |
| Mean in-clinic pulse pressure (mmHg) | 64 (12) | 74 (14) | <0.001 |
| Mean 24-hour systolic blood pressure (mmHg) | 126 (12) | 129 (14) | 0.1 |
| Mean 24-hour diastolic blood pressure (mmHg) | 74 (7) | 69 (9) | <0.001 |
| Mean 24-hour pulse pressure (mmHg) | 53 (10) | 61 (12) | <0.001 |
| Systolic dipping (%) | 11 (7) | 9 (10) | 0.03 |
| Dipper± | 168 (61%) | 27 (45%) | 0.02 |
| Abnormal Epworth Sleep Score | 26 (9%) | 11 (18%) | 0.05 |
| AASI | 0.5 (0.13) | 0.55 (0.15) | 0.01 |
| ARV | 10.6 (2) | 11.2 (1.9) | 0.02 |
| Urine microalbumin:creatinine (mg/mmol), median | 18 (11, 34) | 23 (11, 32) | 0.3 |
Values are the means (SD) or n (%) unless otherwise stated
CKD = eGFR < 60 mL/min using CKD-EPIcys
history of myocardial infarction, heart failure or stroke ***
history of any alcohol or tobacco use, respectively ***
diurnal variation in SBP ≥ 10%
Those with CKDcys had slightly increased AASI, indicating increased stiffness, and more variable BP than those who did not have CKDcys. Albumin-creatinine ratios were relatively low in both groups.
Correlations
Correlation coefficients for eGFR and blood pressure measurements stratified by CKD status are provided in Table 2. In participants without CKDcys, eGFRcys was weakly correlated with systolic dipping but not with other ambulatory parameters or any clinic parameters. In participants with CKDcys, eGFRcys did not correlate with systolic dipping, but was moderately directly correlated with both mean ambulatory DBP and clinic DBP.
Table 2.
Pearson Correlation Coefficients(p for correlation) for eGFR and Blood Pressure by Kidney Function
| No CKD | ||||||
|---|---|---|---|---|---|---|
| eGFRcys | 24h systolic | 24h diastolic | systolic dipping | clinic systolic | clinic diastolic | |
| eGFRcys | X | 0.03 (0.6) | 0.12 (0.05) | 0.12 (0.05) | −0.0007 (0.9) | 0.09 (0.14) |
| 24h systolic | X | 0.54 (<0.001) | −0.02 (0.8) | 0.63 (<0.001) | 0.38 (<0.001) | |
| 24h diastolic | X | 0.03 (0.6) | 0.31 (<0.001) | 0.66 (<0.001) | ||
| systolic dipping | X | 0.08 (0.2) | 0.1 (0.1) | |||
| clinic systolic | X | 0.65 (<0.001) | ||||
| clinic diastolic | X |
| CKD | ||||||
|---|---|---|---|---|---|---|
| eGFRcys | 24h systolic | 24h diastolic | systolic dipping | clinic systolic | clinic diastolic | |
| eGFRcys | X | 0.09 (0.5) | 0.39 (0.002) | −0.02 (0.8) | 0.08 (0.5) | 0.38 (0.003) |
| 24h systolic | X | 0.5 (<0.001) | −0.18 (0.2) | 0.6 (<0.001) | 0.28 (0.03) | |
| 24h diastolic | X | −0.09 (0.5) | 0.23 (0.07) | 0.71 (<0.001) | ||
| systolic dipping | X | 0.02 (0.9) | 0.05 (0.7) | |||
| clinic systolic | X | 0.55 (<0.001) | ||||
| clinic diastolic | X |
Associations Between Kidney Function and Blood Pressure Parameters
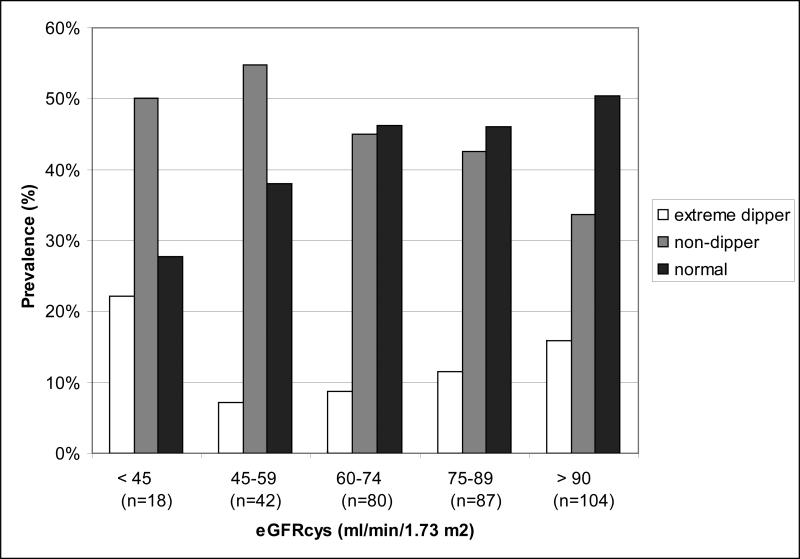
In unadjusted analysis, those with lower eGFRcys had lower prevalence of normal dipping patterns (Figure 1). Before adjustment, the presence of CKDcys but not CKDcr was associated with less dipping (per 1-percentage point), lower mean ambulatory DBP and higher mean ambulatory pulse pressure (Table 3a). Associations were also identified between the presence of CKDcys and higher mean clinic systolic and diastolic blood pressure. However, these effects were almost uniformly attenuated after full adjustment. In multivariate analysis, only an association between CKDcys status and mean ambulatory DBP remained (−2 mmHg, p = 0.048). We performed stepwise regression and determined that age and BMI were the primary confounders responsible for the attenuating effects on the multiple blood pressure parameters.
Figure 1.
Prevalence of dipping patterns across kidney function categories
Table 3a.
Association Between GFR and Blood Pressure Measurements, all participants
| Ambulatory Blood Pressure Monitoring | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Systolic dipping (%) |
|||||||||
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present | −3 | −5 to −1 | 0.007 | −2 | −5 to 0 | 0.06 | −1 | −4 to 1 | 0.3 |
| CKDcr present | −2 | −4 to 0 | 0.08 | −1 | −3 to 1 | 0.3 | −1 | −3 to 2 | 0.5 |
| Mean 24-hour DBP (mmHg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present | −5 | −7 to −3 | <0.001 | −3 | −5 to 0 | 0.02 | −2 | −5 to −0.02 | 0.05 |
| CKDcr present | −4 | −6 to −2 | <0.001 | −2 | −4 to 0 | 0.07 | −2 | −4 to 0 | 0.1 |
| Mean 24-hour SBP (mmHg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present | 3 | −1 to 6 | 0.1 | 2 | −2 to 6 | 0.3 | 0 | −4 to 4 | 0.99 |
| CKDcr present | −1 | −5 to 2 | 0.5 | −3 | −6 to 1 | 0.2 | −4 | −7 to 0 | 0.069 |
| Mean 24-hour pulse pressure (mmHg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present | 8 | 5 to 11 | <0.001 | 5 | 2 to 8 | 0.003 | 2 | −1 to 6 | 0.2 |
| CKDcr present | 3 | 0 to 6 | 0.08 | −1 | −4 to 3 | 0.8 | −2 | −5 to 1 | 0.3 |
| In-Clinic Measurements | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean clinic DBP (mmHg) |
|||||||||
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present | −5 | −7 to −2 | 0.001 | −2 | −5 to 1 | 0.2 | −3 | −6 to 0 | 0.06 |
| CKDcr present | −3 | −6 to 0 | 0.03 | −1 | −4 to 2 | 0.5 | −1 | −4 to 2 | 0.5 |
| Mean clinic SBP (mmHg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present | 5 | 1 to 10 | 0.016 | 4 | −2 to 8 | 0.2 | 0 | −5 to 5 | 0.9 |
| CKDcr present | 3 | −1 to 8 | 0.16 | 1 | −4 to 6 | 0.7 | 0 | −5 to 5 | 0.9 |
Kidney Function and Normal Dipping Pattern Prevalence
In the unadjusted model, the prevalence of normal dipping pattern significantly increased for every 10-ml/min increment in either eGFRcys and eGFRcr (Table 4). This effect was attenuated to non-significance after adjustment for age and other confounders.
Table 4.
Prevalence of normal dipping (> 10%) as a Function of eGFR
| Prevalence Rate Ratio (PRR) | Confidence Interval | p-value | |
|---|---|---|---|
| eGFR by CKD-EPI cystatin equation, per 10 ml/min/1.73 m2 | |||
| Unadjusted | 1.08 | 1.03 to 1.13 | 0.002 |
| Age-adjusted | 1.06 | 1.01 to 1.12 | 0.03 |
| Model 1* | 1.04 | 0.98 to 1.1 | 0.2 |
| eGFR by CKD-EPI creatinine equation, per 10 ml/min/1.73 m2 | |||
| Unadjusted | 1.09 | 1.03 to 1.17 | 0.006 |
| Age-adjusted | 1.07 | 1 to 1.15 | 0.07 |
| Model 1* | 1.06 | 0.99 to 1.14 | 0.09 |
Adjusted for age, sex, race, BMI and the presence of: diabetes, cardiovascular disease and smoking history
Agreement Between Ambulatory and Clinic Blood Pressure
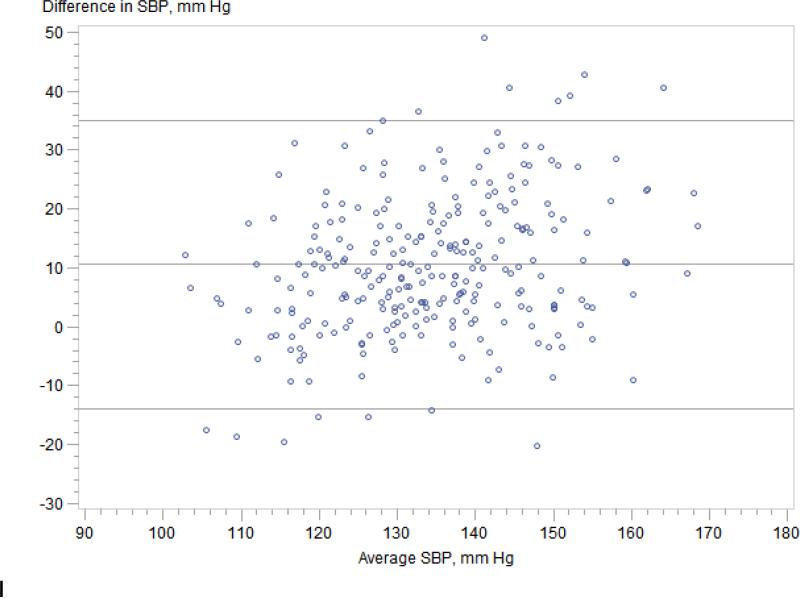
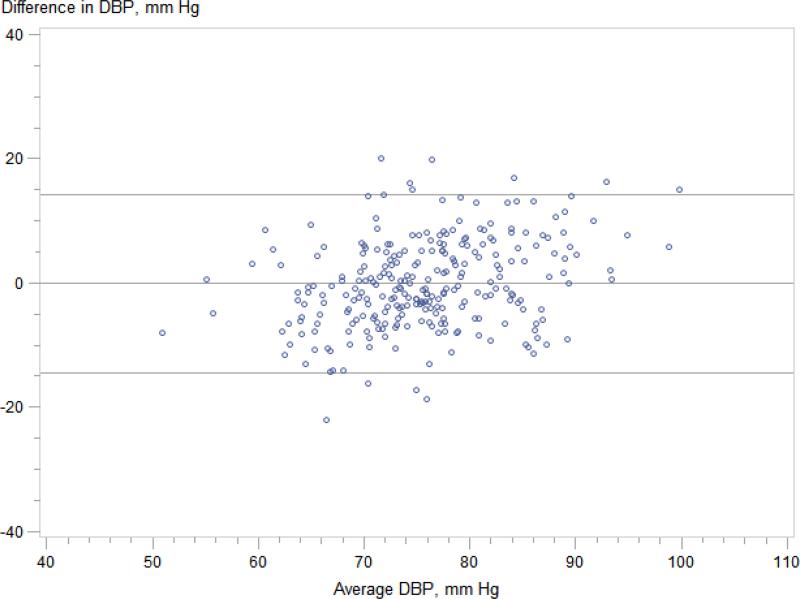
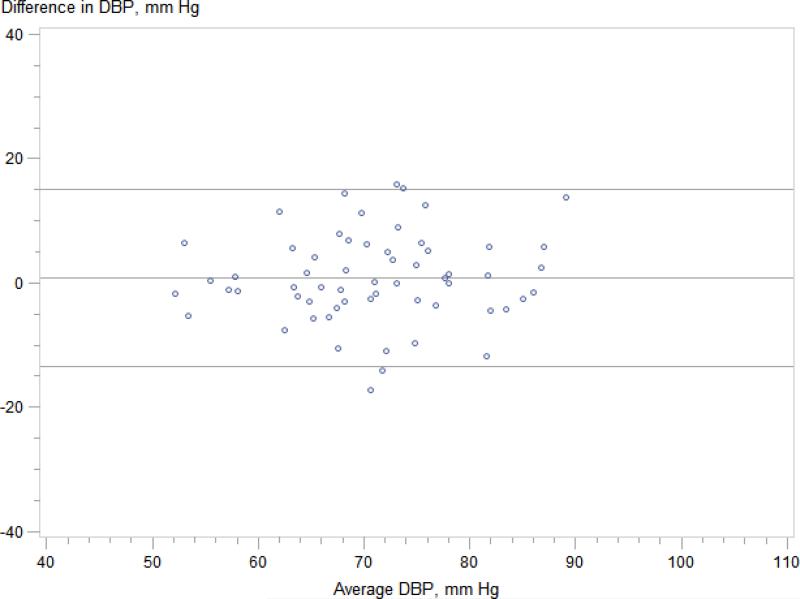
Regardless of CKDcys status, clinic systolic blood pressure significantly overestimated mean wake time ambulatory SBP (Figure 2); mean difference was 11 mmHg for those without CKDcys (95% limits of agreement −14 to 35 mmHg) and 14 mmHg for those with CKDcys (95% limits of agreement −13 mmHg to 41 mmHg). In contrast, clinic diastolic blood pressure accurately estimated mean wake time ambulatory DBP in both groups; mean difference was 0 mmHg for those without CKDcys (95% limits of agreement −14 to 14 mmHg) and 1 mmHg for those with CKDcys (95% limits of agreement -14 to 15 mmHg). We identified 67 individuals in our cohort (36 of whom were taking anti-hypertensive therapy) who met criteria for white coat hypertension defined by the European Society of Hypertension20 as a clinic blood pressure of ≥ 140/90 mmHg and 24-hour ambulatory blood pressure of < 130/80 mmHg; the prevalence of white-coat hypertension did not differ by CKDcys status.
Figure 2.
Figure 2A. Ambulatory wake time SBP vs. clinic SBP -- participants without CKD. Mean difference 14 (95% limits of agreement −14 to 35) mmHg.
Figure 2B. Ambulatory wake time SBP vs. clinic SBP -- participants with CKD Mean difference 14 (95% limits of agreement -13 to 41) mmHg.
Figure 2C. Ambulatory wake time DBP vs. clinic DBP -- participants without CKD Mean difference 0 (95% limits of agreement -14 to 14) mmHg.
Figure 2D. Ambulatory wake time DBP vs. clinic DBP -- participants with CKD Mean difference 1 (95% limits of agreement -14 to 15) mmHg.
Sensitivity Analyses
To examine whether the utilization of antihypertensive medications affected the relationship between kidney function and blood pressure, we repeated linear regression and prevalence rate ratio analysis comparing those on antihypertensive therapy to those who were not (Table 3b). No significant associations existed for either antihypertensive therapy group between blood pressure and CKDcys status.
Table 3b.
Association Between CKDcys and Blood Pressure Measurements, by antihypertensive medication use
| Ambulatory Blood Pressure Monitoring | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Systolic dipping (%) | |||||||||
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present, med use − | −5 | −9 to −1 | 0.02 | −3 | −8 to 1 | 0.2 | −2 | −8 to 2 | 0.2 |
| CKDcys present, med use + | −2 | −5 to 0 | 0.08 | −2 | −5 to 1 | 0.1 | −1 | −4 to 2 | 0.4 |
| Mean 24-hour DBP (mmHg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present, med use − | −4 | −8 to 1 | 0.09 | −1 | −5 to 3 | 0.7 | −2 | −7 to 2 | 0.3 |
| CKDcys present, med use + | −5 | −8 to −3 | <0.001 | −3 | −5 to 0 | 0.05 | −2 | −5 to 1 | 0.2 |
| Mean 24-hour SBP (mmHg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present, med use − | 5 | −2 to 13 | 0.2 | 4 | −4 to 12 | 0.37 | 0 | −8 to 7 | 0.9 |
| CKDcys present, med use + | 1 | −3 to 5 | 0.5 | 1 | −3 to 6 | 0.66 | 0.35 | −4 to 5 | 0.9 |
| Mean 24-hour pulse pressure (mmHg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present, med use − | 9 | 3 to 15 | 0.004 | 5 | −2 to 11 | 0.1 | 2 | −4 to 8 | 0.6 |
| CKDcys present, med use + | 7 | 3 to 10 | <0.001 | 4 | 0 to 8 | 0.06 | 2 | −2 to 6 | 0.2 |
| In-Clinic Measurements | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean clinic DBP (mm Hg) | |||||||||
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present, med use − | −3 | −9 to 2 | 0.2 | −2 | −9 to 4 | 0.5 | −5 | −11 to 1 | 0.1 |
| CKDcys present, med use + | −5 | −8 to −2 | 0.002 | −1 | −5 to 2 | 0.36 | −1 | −5 to 2 | 0.4 |
| Mean clinic SBP (mm Hg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Age-Adjusted | Model 1* | |||||||
| β | 95% CI | p-value | β | 95% CI | p-value | β | 95% CI | p-value | |
| CKDcys present, med use − | 7 | −3 to 17 | 0.2 | 1 | −9 to 12 | 0.8 | −3 | −13 to 7 | 0.6 |
| CKDcys present, med use + | 4 | −1 to 9 | 0.1 | 3 | −2 to 9 | 0.3 | 2 | −4 to 7 | 0.6 |
Adjusted for age, sex, race, BMI and the presence of: diabetes, cardiovascular disease and smoking history
We performed separate analysis that included the presence of microalbuminuria in the definition of CKD. Findings were similar to those obtained for the eGFR-based definition of CKDcys that did not include presence or absence of microalbuminuria.
In analyses considering albuminuria and eGFRcys as separate factors, we found that even in univariate models albumin/creatinine ratio had no association with systolic dipping (beta value for natural log of ACR, 0.62 (−0.41, 1.66), p 0.23). This remained the case in multivariate models, and adding ACR to a model with eGFRcys did not modify the beta coefficient for eGFRcys in unadjusted or adjusted models.
Sensitivity analysis was also performed using the combined CKD-EPI equation for cystatin C and creatinine (CKDcrcys); results (not shown) were similar to those obtained using the cystatin based equation.
Finally, we examined nocturnal blood pressure as a continuous variable rather than binary dipping or nondipping, with results again showing a statistically significant increase in nocturnal blood pressure that was attenuated by both age and BMI.
DISCUSSION
Chronic kidney disease and hypertension are highly prevalent conditions that are frequently comorbid in older adults. In our study of community-dwelling older adults with predominantly mild CKD, we found that those with CKD had higher in-clinic systolic blood pressures but only modest correlations between kidney function and ambulatory blood pressure parameters. After adjusting for multiple covariates, only a lower mean ambulatory DBP was significantly associated with CKD status; nocturnal dipping was not greater in those with normal vs. abnormal kidney function. Finally, clinic SBP – but not clinic DBP – was significantly higher in comparison to ambulatory wake time monitoring, and this difference was not affected by CKD status.
The finding that individuals with CKD had significantly lower 24-hour mean DBP is consistent with previous work demonstrating associations between CKD and lower inclinic DBP.21 Importantly, our study was performed in relatively healthy older adults without major illness, and so generalizes this finding outside a purely hypertensive population and to 24-hour measures rather than in-clinic measures.
We found it somewhat surprising that CKD in this cohort was not independently associated with dipping status after adjustment for age and other confounders. This finding may be because of the mild degree of CKD in our cohort (average eGFRcys in those with CKD was 47 ml/min and only 4 had eGFRcys < 30 ml/min/1.73 m2). In cohorts with greater degrees of CKD, or ESRD, non-dipping is common; Agarwal et al found a prevalence of 75% non-dipping among participants in a CKD clinic.22 It may be that only in more advanced disease are perturbations in blood pressure patterns observed.
We found that, after age adjustment, BMI was largely responsible for attenuating the relationship between CKD and abnormal blood pressure measurements, including dipping. One hypothesis to explain this observation may be that participants with higher BMI had both higher rates of CKD and of subclinical obstructive sleep apnea (OSA). OSA is well established to be associated with non-dipping.23,24 The prevalence of abnormal Epworth Sleepiness Scores (ESS) completed by participants at time of enrollment was modestly higher among individuals classified as having CKD, although adjusting for these did not change the associations beyond the contribution from BMI. However, many OSA patients do not report daytime sleepiness,25,26 and the correlation between OSA severity and ESS is weak at best27 suggesting that OSA could still be a confounder in our study. Thus, further work is clearly needed.
We did not find an association between albuminuria, either when included as part of the CKD definition, or when modeled alone, and systolic dipping; we believe this may be because the levels of albuminuria in our cohort were very low. Although 25% of the cohort technically met the > 30 mg/g definition of microalbuminuria, 95% had microalbumin/creatinine ratios less than 70 mg/g, and only 4 individuals had macroalbuminuria defined as > 300 mg/g.
Compared to mean ambulatory wake time DBP, clinic DBP was a relatively accurate measurement, whereas clinic SBP significantly overestimated ambulatory wake-time SBP in comparison to ABPM findings. This observation did not differ by CKD status. Previous studies have described bias in in-clinic BP determinations, including white coat hypertension28 and the lack of a standardized approach to blood pressure measurement.29 Our study shows this issue to be a problem both in those with and without CKD, and across the age range in our cohort. Bias in SBP, and lack of precision in either SBP or DBP is more likely to lead to overtreatment based on in-clinic BP, as those who are labeled ‘hypertensive’ are likely to receive medication. Our data add to the recognition that in-clinic SBP tends to overestimate out-of-office BP, and demonstrates that this effect is independent of age or of mild CKD. At least in this population, the use of ABPM as may be recommended by USPHTF would tend to decrease the number of individuals meeting current criteria for hypertension treatment.
Our study has limitations. We do not have repeated measures of ABPM, nor do we have objective data on sleep or sleep quality. Moreover, our study was cross-sectional, which precluded the ability to study the longitudinal relationships between CKD and blood pressure. Despite these limitations, our study also has several strengths. First, our participants were extensively characterized in regard to clinic and ambulatory blood pressure, kidney function, and medication use concurrently, which allowed exploration of the potential role of multiple covariates. This is the first study to our knowledge to evaluate the relationship between kidney disease defined by cystatin C and ABPM in community living older adults and to examine precision and accuracy of clinic versus ABPM in those with mild kidney disease defined by cystatin C. If ABPM comes into widespread clinical practice, as suggested by USPSTF, these insights will be important to inform clinical interpretation of ABPM in patients across the range of CKD.
In conclusion, the presence of CKD was independently associated with low ambulatory DBP only, and not other blood pressure parameters, including dipping, in our cohort. Clinic DBP but not SBP was in agreement with wake-time ABPM, and this was similar irrespective of CKD. Nonetheless, both clinic parameters lacked precision. Further studies may help clarify the role of ABPM in older individuals, including the longitudinal relationship between low DBP, kidney function and adverse outcomes.
Acknowledgements
This work was supported by NIH K23 DK091521 (DER).
Footnotes
Conflicts of interest: none declared
REFERENCES
- 1.Gorostidi M, et al. Spanish Society of Hypertension ABPM Registry investigators. Ambulatory blood pressure monitoring in hypertensive patients with high cardiovascular risk: a cross-sectional analysis of a 20,000-patient database in Spain. J. Hypertens. 2007;25:977–984. doi: 10.1097/HJH.0b013e32809874a2. [DOI] [PubMed] [Google Scholar]
- 2.Hermida RC, Smolensky MH, Ayala DE, Portaluppi F, with International Society for Chronobiology American Association of Medical Chronobiology and Chronotherapeutics, Spanish Society of Applied Chronobiology, Chronotherapy, and Vascular Risk, Spanish Society of Atherosclerosis, and Romanian Society of Internal Medicine. 2013 ambulatory blood pressure monitoring recommendations for the diagnosis of adult hypertension, assessment of cardiovascular and other hypertension-associated risk, and attainment of therapeutic goals. Chronobiol. Int. 2013;30:355–410. doi: 10.3109/07420528.2013.750490. [DOI] [PubMed] [Google Scholar]
- 3.Brotman DJ, Davidson MB, Boumitri M, Vidt DG. Impaired diurnal blood pressure variation and all-cause mortality. Am. J. Hypertens. 2008;21:92–97. doi: 10.1038/ajh.2007.7. [DOI] [PubMed] [Google Scholar]
- 4.Ohkubo T, et al. Relation Between Nocturnal Decline in Blood Pressure and Mortality* The Ohasama Study. Am. J. Hypertens. 1997;10:1201–1207. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 5.Piper MA, et al. Diagnostic and Predictive Accuracy of Blood Pressure Screening Methods With Consideration of Rescreening Intervals: An Updated Systematic Review for the U.S. Preventive Services Task ForceBlood Pressure Screening Methods and Consideration of Rescreening Intervals. Ann. Intern. Med. 2014 doi: 10.7326/M14-1539. N/A, N/A–N/A. [DOI] [PubMed] [Google Scholar]
- 6.Wright JT., Jr. The Benefits of Detecting and Treating Mild Hypertension: What We Know, and What We Need to LearnThe Benefits of Detecting and Treating Mild Hypertension. Ann. Intern. Med. 2014 doi: 10.7326/M14-2836. N/A, N/A–N/A. [DOI] [PubMed] [Google Scholar]
- 7.US Preventive Services Task Force High Blood Pressure in Adults: Screening. http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementDraft/hypertension-in-adults-screening-and-home-monitoring.
- 8.Bangash F, Agarwal R. Masked hypertension and white-coat hypertension in chronic kidney disease: a meta-analysis. Clin. J. Am. Soc. Nephrol. CJASN. 2009;4:656–664. doi: 10.2215/CJN.05391008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang M, et al. Association of Kidney Function and Albuminuria With Prevalent and Incident Hypertension: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014 doi: 10.1053/j.ajkd.2014.06.025. doi:10.1053/j.ajkd.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inker LA, et al. CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shlipak MG, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 12.Little P, et al. Comparison of agreement between different measures of blood pressure in primary care and daytime ambulatory blood pressure. BMJ. 2002;325:254. doi: 10.1136/bmj.325.7358.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal R. Home and ambulatory blood pressure monitoring in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2009;18:507–512. doi: 10.1097/MNH.0b013e3283319b9d. [DOI] [PubMed] [Google Scholar]
- 14.Kyhse-Andersen J, et al. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin. Chem. 1994;40:1921–1926. [PubMed] [Google Scholar]
- 15.Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J. Vasc. Surg. 2003;37:1047–1053. doi: 10.1067/mva.2003.168. [DOI] [PubMed] [Google Scholar]
- 16.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 17.Graves JW, Grossardt BR. Discarding the first of three nurse-auscultatory or oscillometric blood pressure measurements does not improve the association of office blood pressure with ABPM. Blood Press. Monit. 2010;15:146–151. doi: 10.1097/MBP.0b013e328337ce76. [DOI] [PubMed] [Google Scholar]
- 18.Dolan E, et al. Ambulatory Arterial Stiffness Index as a Predictor of Cardiovascular Mortality in the Dublin Outcome Study. Hypertension. 2006;47:365–370. doi: 10.1161/01.HYP.0000200699.74641.c5. [DOI] [PubMed] [Google Scholar]
- 19.Leoncini G, Viazzi F, Storace G, Deferrari G, Pontremoli R. Blood pressure variability and multiple organ damage in primary hypertension. J. Hum. Hypertens. 2013 doi: 10.1038/jhh.2013.45. doi:10.1038/jhh.2013.45. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien E, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. European society of hypertension position paper on ambulatory blood pressure monitoring. J. Hypertens. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 21.Peralta CA, et al. Control of Hypertension in Adults With Chronic Kidney Disease in the United States. Hypertension. 2005;45:1119–1124. doi: 10.1161/01.HYP.0000164577.81087.70. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal R, Kariyanna SS, Light RP. Circadian blood pressure classification scheme and the health of patients with chronic kidney disease. Am. J. Nephrol. 2009;30:536–546. doi: 10.1159/000252774. [DOI] [PubMed] [Google Scholar]
- 23.Loredo JS, Ancoli-Israel S, Dimsdale JE. Sleep quality and blood pressure dipping in obstructive sleep apnea. Am. J. Hypertens. 2001;14:887–892. doi: 10.1016/s0895-7061(01)02143-4. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M, Guilleminault C, Otsuka K, Shiomi T. Blood pressure ‘dipping' and ‘non-dipping' in obstructive sleep apnea syndrome patients. Sleep. 1996;19:382–387. doi: 10.1093/sleep/19.5.382. [DOI] [PubMed] [Google Scholar]
- 25.Young T, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 26.Peppard PE, et al. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chami HA, et al. Sleepiness, quality of life, and sleep maintenance in REM versus non-REM sleep-disordered breathing. Am. J. Respir. Crit. Care Med. 2010;181:997–1002. doi: 10.1164/rccm.200908-1304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franklin SS, Thijs L, Hansen TW, O'Brien E, Staessen JA. White-Coat Hypertension New Insights From Recent Studies. Hypertension. 2013;62:982–987. doi: 10.1161/HYPERTENSIONAHA.113.01275. [DOI] [PubMed] [Google Scholar]
- 29.Edwards C, Hiremath S, Gupta A, McCormick BB, Ruzicka M. BpTRUth: do automated blood pressure monitors outperform mercury? J. Am. Soc. Hypertens. JASH. 2013;7:448–453. doi: 10.1016/j.jash.2013.07.002. [DOI] [PubMed] [Google Scholar]