Summary
The primary aim was to examine the preliminary efficacy of a family-tailored problem-solving intervention to improve antiepileptic drug (AED) adherence in families of children with new-onset epilepsy. Secondary aims were to assess changes in targeted mechanisms and treatment feasibility and acceptability. Fifty families (Mage=7.6±3.0; 80% Caucasian; 42% idiopathic localization related) completed baseline questionnaires and were given an electronic monitor to observe daily AED adherence. If adherence was <95% in the first 7 months of the study, families were randomized (Supporting Treatment Adherence Regimens (STAR): n=11; Treatment as Usual (TAU): n=12). Twenty-one families were not randomized due to adherence being >95%. The STAR intervention included four face-to-face and two telephone problem-solving sessions over 8 weeks. Significant group differences in adherence were found during active intervention (Weeks 4-6; TAU=−12.0 vs STAR=18.1, p < .01; and Weeks Session 6-8: TAU=−9.7 vs STAR=15.3, p < .05). Children who received the STAR intervention exhibited improved adherence compared to children in the TAU group during active treatment. Significant changes in epilepsy knowledge and management were noted for the STAR group. Families expressed benefitting from the STAR intervention. Future studies should include a larger sample size and booster intervention sessions to maintain treatment effects over time.
Keywords: pediatric epilepsy, self-management, problem-solving, intervention, compliance
Introduction
Non-adherence to antiepileptic drugs (AEDs) is a significant problem for children with epilepsy and their families, with approximately 60% demonstrating non-adherence to treatment1; 2. The impact of AED non-adherence is significant for children with epilepsy, including increased seizures2; 3, inaccurate clinical decision-making4, and poor health-related quality of life5. Despite the known impact of non-adherence on health outcomes for children with epilepsy, few adherence interventions have been developed.
A Cochrane review indicated that we lack well-designed randomized controlled clinical trials (RCT) with long follow-up periods to improve adherence in epilepsy6. Since the Cochrane review, our group conducted a small pilot study of 30 children with epilepsy to improve adherence to AED therapy7. Results from the pilot intervention were promising as families had a mean adherence improvement of 32% from baseline to post-intervention and they perceived the intervention as feasible and acceptable. Limitations of the pilot study included a small sample size, only targeting families demonstrating non-adherence soon after diagnosis (90% or less), lack of follow-up, and inability to examine mechanisms of change. An important next step in the intervention process was to use an enrichment design (e.g., randomization occurs at different time points when adherence declines) to capture families most in need of intervention and follow families past the post-intervention period.
The aim of the current study was to examine changes in adherence rates from baseline to post intervention and 3-month follow-up, between children and families randomized to the Supporting Treatment Adherence Regimen (STAR) intervention versus Treatment As Usual (TAU). Adherence data over 2-week periods were used to examine group differences. It was hypothesized that children and families in the STAR intervention group would demonstrate significant improvements in adherence compared to the TAU group and maintain these effects during the 3-month follow-up period. Secondary aims were to assess mechanisms of change targeted during the intervention and treatment feasibility and acceptability. The STAR intervention group was hypothesized to have greater changes in caregiver-reported epilepsy treatment knowledge, adherence barriers, problem-solving skills, and epilepsy management compared to the TAU group. The families in the STAR intervention were hypothesized to find the treatment feasible and acceptable, reflected by ratings in the ideal range.
Methods
Participants
Children and their caregivers were recruited from a New Onset Seizure Clinic in a Midwestern children’s hospital shortly after epilepsy diagnosis from January 2011 to October 2012. Inclusion/exclusion criteria were: 1) recent diagnosis of epilepsy (within 7 months), 2) aged 2-12 years, 3) no comorbid chronic illnesses requiring routine medications (e.g., diabetes), 4) AED medication in pill or sprinkle form, 5) family residing within 75 miles of the hospital, 6) no significant parent-reported developmental disorders (e.g., autism) and, 7) no prior AED treatment. We chose not to include children with major developmental disorders because these children typically cannot participate well in problem-solving sessions and there may be additional barriers to adherence for these families that our intervention does not address. A total of 50 families were consented and enrolled. The study was approved by the hospital’s Institutional Review Board.
Procedure
At study entry, participants completed questionnaires and received an electronic monitor to assess AED adherence, a MEMS™ TrackCap. After a 30-day monitoring period, the research coordinator conducted a home visit to download the adherence data. We used 30 days of data to determine eligibility for randomization to wash out any reactivity that may have occurred with the electronic monitoring8; 9. Based on this data, participants were either followed due to good adherence (≥ 95%; High Adherence group) or randomized into one of two groups (< 95%; TAU or STAR Intervention). In addition to the 30-day monitoring period, participants with good adherence had two additional opportunities to be randomized over the next 6-months. Similar to their 1-month run-in period, if adherence fell below 95% at the 3 or 6-month assessment period, the family was randomized to TAU or STAR. We chose to monitor adherence for 7-months because our prior data suggests that adherence patterns are relatively stable if they are high during this period of time1; 2. If patients were randomized during the 3 or 6-month assessment, their baseline data only included adherence in the one-month preceding the randomization.
Randomization procedures
Participants were randomly assigned to either STAR intervention or TAU using permuted block randomization with block size 2. Stratification occurred based on the one-month adherence data preceding study visits 2, 3, or 4 (e.g. adherence for 1-month ≥ 80% (48 doses of 60) or < 80%) to ensure equality across groups. The randomization list was generated by the first author and held by a research assistant independent of the study to reduce biases.
Treatment as Usual Group/High Adherence Families
Participation in the TAU and High Adherence groups involved 5 study visits. The majority of study visits coincided with clinic appointments and occurred approximately every 3 months with the exception of the 1-month home visit to download the MEMS™ TrackCap for the run-in period.
STAR Intervention Group
Participation in the STAR intervention involved 1-3 assessment visits, 4 face-to-face (Sessions 1, 2, 4, and 6) and 2 telephone intervention sessions (Sessions 3 and 5), and 1-3 follow-up visit (Supplemental Table 1) depending on when the family was randomized. Intervention sessions were led by a trained interventionist (e.g. psychology doctoral student, post-doctoral fellow) and caregivers and children participated in sessions
Description of the STAR Intervention
STAR Intervention Session 1 (Weeks 1-2)
The first session of the intervention focused on addressing deficits in epilepsy knowledge and providing education about the importance of AED adherence. We reviewed an adapted version of the Epilepsy Knowledge Questionnaire10 completed by each family and corrected errors with explanations. We also reviewed the participant’s prescribed treatment regimen and provided them with feedback on their own AED adherence over the past two weeks.
STAR Intervention Session 2-4 (Weeks 3-8)
The goals of the problem-solving approach were as follows: 1) The interventionist helped the caregiver/child identify an adherence barrier (Problem Definition); 2) The caregiver and child were taught to generate several creative solutions (Generating Alternative Solutions); 3) The potential solutions were written down and systematically evaluated by caregiver and child (Family Decision-Making); 4) The family selected one solution for implementation (Implementation of New Solution); 5) A detailed solution was written out with specifics regarding when, where, and how the new solution will be attempted. A behavioral contract was signed by all participants of the problem-solving session; and 6) Phone follow-ups were conducted one week after the problem-solving session to assist the family in either fine-tuning the solution or renegotiating a new solution (Evaluation and Re-Negotiation). Of note, even young children participated in problem-solving sessions with variable engagement depending on age. For example, toddlers and preschool children could provide examples of rewards/reinforcers they liked and whether they liked possible solutions. In contrast, older children were more likely to provide viable solutions that families could choose and would often be involved in helping write these down and choose the solution.
Three-month follow up visit
MEMS™ TrackCap data were downloaded and all caregivers completed questionnaires at this 3-month follow-up visit. A medical chart review also occurred at this visit. Due to patients who were in the STAR intervention having different levels of follow-up (e.g., those randomized early had more follow-up than those randomized later in the study), we used 3-month follow-up data to ensure consistency across all participants.
Measures
Background Information Form
Parents completed a form regarding information about the child’s age, gender, race/ethnicity, and caregiver occupational history.
Medical Chart Review
Chart reviews were conducted to collect information regarding the treatment regimen, seizure activity (absence/presence), seizure type, AED prescription, and changes to the regimen over time. Baseline side effects was also collected, which is assessed in routine care via the Pediatric Epilepsy Side Effects Questionnaire11. Total side effects scores can range from 0-100, with higher scores reflecting higher side effects.
MEMS® 6 TrackCap
The Medication Event Monitoring Systems (MEMS™; Aardex Corporation) TrackCap was used to measure daily AED adherence. Adherence rates were calculated using daily data over 2-week intervals. For example, if the patient missed 10/28 doses in 2-weeks, then the child’s adherence rate would be [(28-10)/28 × 100%= 64.3%].
Epilepsy Knowledge Questionnaire (EKQ10)
The adapted EKQ is a 55-item questionnaire regarding knowledge about medical and social aspects of epilepsy. This measure has adequate reliability and validity, with Cronbach’s alphas ranging from .49 to .63. Scores range from 0-100% correctness with higher scores representing greater knowledge.
Social Problem-Solving Inventory-Revised (SPSI-R: Short Form 12)
The SPSI-R: Short Form is a 25-item self-report measure of an individual’s ability to resolve problems in everyday life. The SPSI-R: Short Form generates five subscales: Positive Problem Orientation, Negative Problem Orientation, Rational Problem Solving, Impulsivity/Carelessness Style, and Avoidance Style. Raw scores are converted to standardized scores, using 100 as the mean and 15 as the standard deviation. Scores between 86-114 represent the normative group average. Measurement properties of the SPSI-R: Short Form are strong, with reliability ranging from 0.74 to 0.8912.
Pediatric Epilepsy Medication Self-Management Questionnaire (PEMSQ13)
The PEMSQ assesses critical aspects of medication management by caregivers of children with epilepsy. The PEMSQ is 27-items with four scales (Epilepsy and Treatment Knowledge and Expectations, Adherence to Medications and Clinic Appointments, Barriers to Medication Adherence, and Beliefs about Medication Efficacy). Cronbach’s alphas ranged from 0.68-0.85 for the scales. For this study, we examined the Epilepsy and Treatment Knowledge, Barriers to Medication Adherence (scores range from 8-40), and Total Self-Management (scores range 27-135) scores. Higher scores represent better medication self-management.
Caregiver Response to Child Illness (PRCI 14)
The PRCI is a 35-item questionnaire and assesses parents’ responses and perceptions related to seizures. It is comprised of five subscales: Child Support, Family Life/Leisure, Condition Management (i.e., Epilepsy management), Child Autonomy, and Child Discipline. Items for Child Support include cheering up the child when sad, while those for Family Life /Leisure discuss disruptions in activities as a result of seizures. The Epilepsy Management scale encompasses items regarding confidence to manage seizures and side effects and Child Autonomy includes items related to dependence/independence on parents. Finally the Child Discipline scale has items related to how parents feel about child discipline and their responses to various child behaviors. Internal consistencies ranged from 0.67-0.85 and strong test-retest reliability was demonstrated (ICC = 0.47-0.72). Higher scores reflect better functioning.
Treatment Acceptability and Feasibility Questionnaire
Caregivers participating in the STAR intervention completed a study-specific questionnaire assessing feasibility and acceptability of the STAR intervention, which has been used in prior studies 7.
Statistical Analyses
Our primary outcome was AED adherence rates across two week intervals (i.e., 2-4 weeks, 4-6 weeks, 6-8 weeks, 8-10 weeks, and 10-12 weeks). We chose to examine two week time periods instead of daily data because our intervention sessions occurred every two weeks, with the first session focused on education but subsequent sessions being more problem-solving focused. Thus, we were able to better identify the timing of adherence changes during intervention and follow-up. We examined group differences in change from baseline to each of these two week intervals across the eight weeks of intervention and 3-month follow-up. Notably, baseline data included 30 days of monitoring preceding the randomization, regardless of when the patient was randomized (e.g., Assessment 1, 2 or 3). These analyses were carried out using a repeated measures model based on maximum likelihood estimation15 with the MIXED procedure in SAS (version 9.3; SAS Institute, Cary, NC).
Secondary outcomes included potential mechanisms of change, such as epilepsy knowledge, adherence barriers, and social problem-solving skills. These were examined from baseline to post-intervention only. We employed the same statistical model to examine group differences in changes in treatment mechanisms (e.g., knowledge, barriers) from baseline to post-treatment. Statistical significance was defined as p < .05. Notably, because this was a pilot study with goals of evaluating preliminary effect size, power considerations were not considered in the design.
Results
Participants
Participant characteristics are contained in Table 1. A consort diagram describes participant data throughout the RCT (See Supplemental Figure 1). The recruitment rate for this intervention study was 66%.
Table 1.
Participant and Epilepsy-Specific Descriptive Statistics (N = 50)
| Characteristics | Total Samplea (n=50) |
STAR Intervention (n=11) |
Treatment as Usual (n=12) |
High Adherence (n=22) |
|---|---|---|---|---|
| Child Age (mean ± SD) | 7.6 ± 3.0 | 6.4 ± 3.5 | 8.4 ± 3.1 | 7.4 ± 2.9 |
| Child Male (%) | 66.0 | 55.0 | 75.0 | 72.7 |
| Child Race (%) | ||||
| Caucasian | 86.0 | 81.8 | 83.3 | 90.9 |
| African-American | 12.0 | 18.2 | 16.7 | 9.1 |
| Biracial | 2.0 | 0.0 | 0.0 | 0.0 |
| Caregiver Relation to Child (%) | ||||
| Mothers | 84.0 | 90.9 | 66.7 | 90.9 |
| Fathers | 10.0 | 0.0 | 33.3 | 4.5 |
| Other (e.g., Uncle, Grandmother) | 6.0 | 9.1 | 0.0 | 4.5 |
| Caregiver Marital Status (%) | ||||
| Married | 57.1 | 63.6 | 33.0 | 68.2 |
| Single | 24.5 | 18.2 | 41.7 | 22.7 |
| Divorced | 18.4 | 18.2 | 25.0 | 9.1 |
| Socioeconomic Status (mean ± SD) b | 51.7 ± 21.3 | 45.5 ± 19.6 | 54.5 ± 22.3 | 59.0 ± 22.4 |
| Epilepsy Diagnosis (%) | ||||
| Idiopathic Localization-related | 42.0 | 63.6 | 33.3 | 40.9 |
| Idiopathic Generalized | 24.0 | 18.2 | 25.0 | 31.8 |
| Idiopathic Unclassified | 28.0 | 9.1 | 33.3 | 27.3 |
| Cryptogenic Generalized | 2.0 | 9.1 | 0.0 | 0.0 |
| Symptomatic Localization-related | 4.0 | 0.0 | 8.3 | 0.0 |
| Initial Prescribed Anti-Epileptic Drug (%) | ||||
| Carbamazepine | 32.0 | 63.6 | 25.0 | 18.2 |
| Valproic Acid | 30.0 | 27.3 | 33.0 | 27.3 |
| Levetiracetam | 24.0 | 9.1 | 41.7 | 22.7 |
| Ethosuximide | 8.0 | 0.0 | 0.0 | 18.2 |
| Other | 6.0 | 0.0 | 0.0 | 13.6 |
| Baseline Total Side effects score 11 | 11.4 ± 12.1 | 13.2 ± 13.9 | 7.5 ± 7.5 | 12.9 ± 13.9 |
| Seizure Presence in past 3 months | 36% | 18% | 16% | 59% |
Note: Five patients withdrew prior to randomization.
Aim 1: Preliminary Efficacy of the STAR Intervention
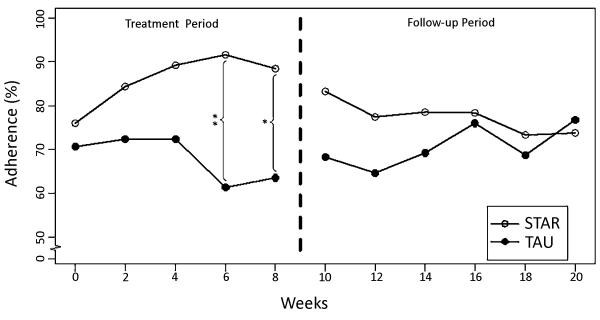
Overall, data indicated significant differences between groups following intervention session 2. Specifically, a trend for group differences was noted after the first problem-solving session (Weeks 2-4; STAR: Mchange=15.8 and TAU: Mchange=−0.5; p=0.053) and statistically significant differences were noted between groups following intervention session 3 (Weeks 4-6; STAR: Mchange=18.1 and TAU: Mchange=−12.0; p=0.002) and 4 (Weeks 6-8; STAR: Mchange=15.3 and TAU: Mchange=−9.7; p=.021) (See Figure 1). During the 3-month follow-up period, no significant group differences were found on AED adherence.
Figure 1.
Biweekly Adherence Rates for the STAR Intervention and Treatment as Usual (TAU) Groups Across Treatment and Follow-Up Periods
Aim 2. Mechanisms of Change
Group differences were examined on changes in the proposed mechanism of action to improve AED adherence (Supplemental Table 2). Significant group differences were found on changes in epilepsy knowledge: PEMSQ-Epilepsy Disease and Treatment Knowledge (p < .05) and the EPK: Epilepsy Knowledge (p < .01). Specifically, the STAR intervention group increased their score by 2.1 points and 6.6 point, respectively. Significant group differences were also noted on self-management: PEMSQ: Total Score (p < .01) and PRCI: Epilepsy management (p> .01). The STAR intervention group increased their epilepsy management score by 3.7 and 0.33, respectively. No significant group differences were found on change scores for social problem solving skills or adherence barriers.
Aim 3-Feasibility and Acceptability of the STAR Intervention
Eleven families were randomized to the STAR intervention. Of those, 2 withdrew prior to treatment initiation and 1 family completed the intervention sessions but was lost to follow-up. Of the nine families who completed STAR, a majority of families found the intervention to be feasible and acceptable (Supplemental Table 3).
Discussion
The current study expands findings from a previous pilot study demonstrating promising benefits of the STAR intervention. Families receiving the STAR intervention (i.e., education and problem-solving skills) had higher rates of AED adherence during active intervention. Improved epilepsy knowledge and self-management skills post-intervention appear to be mechanisms that increase daily AED adherence. Additionally, families in the STAR intervention felt it was feasible and acceptable. However, adherence improvements were not sustained at the 3-month follow-up indicating the need for booster sessions to sustain results detected during active treatment.
The short-term efficacy of the STAR intervention was supported by this study and validates that family-based problem-solving can improve AED adherence for children 2-12 years of age and their families who are newly managing epilepsy and establishing a daily AED regimen. The STAR intervention provides families with the opportunity to receive feedback on their AED adherence data and engage in active problem-solving discussions. Use of the patient’s own electronic monitoring of adherence behaviors for intervention was meaningful to children and their caregivers and is increasingly being used in clinical settings with other pediatric populations (e.g., blood glucose monitors16). This type of data can potentially increase dialogue in clinical practice around common adherence issues.
Adherence declined when active intervention ended, with comparable rates at the end of the 3-month follow-up for STAR and TAU groups. During active intervention, families developed a formal behavioral plan to address their adherence barrier and were then held accountable for implementing the plan via telephone contacts and subsequent sessions. Unfortunately, without these continued contacts and reinforcement, adherence declined for families. Booster sessions could extend the benefits of the STAR intervention by increasing accountability and provision of support.
Families who received the STAR intervention demonstrated greater epilepsy knowledge and epilepsy management skills from pre- to post-intervention compared to the TAU cohort. The first session of the STAR intervention provides educational content that extends beyond education provided during standard medical care (e.g., epilepsy restrictions, introduction of AED adherence), which normalizes discussion around AED adherence. While knowledge is recognized as necessary to improve adherence, the adherence literature suggests it is not sufficient to change adherence behaviors. The STAR cohort also demonstrated greater epilepsy self-management skills, including greater ability to recognize AED side effects, manage new symptoms (i.e., seizures) and when to solicit medical advice (i.e., call physician, visit the emergency department) compared to the TAU cohort.
The study is not without limitations. First, our small sample size may have limited detection of significant effects. Second, some families felt the location of the intervention was inconvenient. Additionally, the problem-solving measure used may not reliability assess the problem-solving skills delivered in the STAR intervention. Finally, only caregiver-reported questionnaires were used in the study and there were no child-reported outcomes. Child perspectives of perceived key mechanisms of change should be considered (i.e, epilepsy knowledge), particularly for older children. Future investigations may also consider a more heterogeneous (e.g., older, chronic epilepsy) and larger sample size and use of a problem-solving measures that better reflects the skills targeted in the STAR intervention. Clinic-based delivery or telehealth (e.g., Skype, mobile apps) would also address the issue of convenience for families, as well as the provision of booster sessions so AED adherence improvements are maintained over time. Furthermore, a large-scale RCT with an attention-control group would help disentangle the effects of attention, which could have improved adherence for the STAR group, on our primary adherence outcome.
Clinical implications gleaned from the current pilot study suggest the need to engage families with children with epilepsy in active adherence promotion efforts early in the disease course. Problem-solving and education strategies appear to be helpful initially and could be implemented in clinical settings by health care providers. However, the need for continued monitoring and engagement around adherence behaviors is critical to maintaining improvements in adherence and self-management. This is especially salient in pediatric epilepsy because variable adherence and early non-adherence are associated with worse seizure outcomes2; 3. The fact that families deemed the intervention as a feasible, beneficial and liked the treatment is promising.
Supplementary Material
Supplemental Figure 1. Consort Diagram
Acknowledgements
Sources of funding and support: This research was funded by a grant from the National Institutes of Health (NIH) awarded to the first author (K23HD057333: Novel Adherence Measurement and Intervention in Children with New-Onset Epilepsy).
Role of the Sponsors: The study sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Funding:
This research was supported by a grant from the National Institutes of Health (K23HD057333) awarded to A.M.
Footnotes
Disclosures, Conflicts of Interests
Financial Disclosures: Dr. Avani Modi, Dr. Shanna Guilfoyle, Krista Mann, and Dr. Joseph Rausch report no disclosures.
Additional contributions: We would like to extend our deepest appreciation to children, adolescents and their families who participated in this study. We would like to especially thank the research assistants, undergraduate and graduate students, pre-doctoral interns, and postdoctoral fellows (Cincinnati Children’s Hospital Medical Center) for recruiting study patients, providing the STAR intervention and data collection. We would also like to thank the New Onset Seizure and Advanced Therapies Teams at Cincinnati Children’s Hospital for their support of the study.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
First author summary statement: Dr. Modi is a pediatric psychologist with research interests in antiepileptic medication adherence.
References
- 1.Modi AC, Rausch JR, Glauser TA. Patterns of non-adherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305:1669–1676. doi: 10.1001/jama.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Modi AC, Wu YP, Rausch JR, et al. Antiepileptic drug nonadherence predicts pediatric epilepsy seizure outcomes. Neurology. 2014 doi: 10.1212/WNL.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modi AC, Rausch JR, Glauser TA. Early pediatric antiepileptic drug nonadherence is related to lower long-term seizure freedom. Neurology. 2014;82:671–673. doi: 10.1212/WNL.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modi AC, Wu YP, Guilfoyle SM, et al. Uninformed Clinical Decisions Resulting From Lack of Adherence Assessment in Children with New Onset Epilepsy. Epilepsy and Behavior. 2012;25:481–484. doi: 10.1016/j.yebeh.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu YP, Follansbee-Junger K, Rausch J, et al. Parent and family stress factors predict health-related quality in pediatric patients with new-onset epilepsy. Epilepsia. 2014 doi: 10.1111/epi.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-aqeel S, Al-sabhan J. Strategies for improving adherence to antiepileptic drug treatment in patients with epilepsy. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008312.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Modi AC, Guilfoyle SM, Rausch J. Preliminary Feasibility, Acceptability, and Efficacy of an Innovative Adherence Intervention for Children With Newly Diagnosed Epilepsy. J Pediatr Psychol. 2013;38:605–616. doi: 10.1093/jpepsy/jst021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riekert KA, Rand CS. Electronic monitoring of medication adherence: When is high-tech best? Journal of Clinical Psychology in Medical Settings. 2002;9:25–34. [Google Scholar]
- 9.Pai AL, Gray E, Kurivial K, et al. The Allocation of Treatment Responsibility scale: a novel tool for assessing patient and caregiver management of pediatric medical treatment regimens. Pediatr Transplant. 2010;14:993–999. doi: 10.1111/j.1399-3046.2010.01391.x. [DOI] [PubMed] [Google Scholar]
- 10.Jarvie S, Espie CA, Brodie MJ. The development of a questionnaire to assess knowledge of epilepsy: 1--General knowledge of epilepsy. Seizure. 1993;2:179–185. doi: 10.1016/s1059-1311(05)80125-6. [DOI] [PubMed] [Google Scholar]
- 11.Morita DA, Glauser TA, Modi AC. Development and Validation of the Pediatric Epilepsy Side Effects Questionnaire. Neurology. 2012;79:1252–1258. doi: 10.1212/WNL.0b013e3182635b87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Zurilla T, Nezu A, Maydeu-Olivares A. Social Problem-Solving Inventory-Revised: Technical Manual. Multi-Health Systems, Inc; Toronto: 2002. [Google Scholar]
- 13.Modi AC, Monahan S, Daniels D, et al. Development and validation of the Pediatric Epilepsy Medication Self-Management Questionnaire. Epilepsy Behav. 2010;18:94–99. doi: 10.1016/j.yebeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Austin JK, Shore CP, Dunn DW, et al. Development of the parent response to child illness (PRCI) scale. Epilepsy Behav. 2008;13:662–669. doi: 10.1016/j.yebeh.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winkens B, van Breukelen GJ, Schouten HJ, et al. Randomized clinical trials with a pre- and a post-treatment measurement: repeated measures versus ANCOVA models. Contemp Clin Trials. 2007;28:713–719. doi: 10.1016/j.cct.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Harris MA, Freeman KA, Duke DC. Seeing Is Believing: Using Skype to Improve Diabetes Outcomes in Youth. Diabetes Care. 2015 doi: 10.2337/dc14-2469. [DOI] [PubMed] [Google Scholar]
- 17.Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Soc Sci Res. 1981;10:364–395. [Google Scholar]
- 18.Hauser RM. Measuring socioeconomic status in studies of child development. Child Dev. 1994;65:1541–1545. doi: 10.1111/j.1467-8624.1994.tb00834.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Consort Diagram