Abstract
Objective
Purkinje cell loss has been documented in some although not all postmortem studies of essential tremor. Hence, there is considerable controversy concerning the presence of Purkinje cell loss in this disease. To date, few studies have been performed.
Methods
Over the past eight years, we have assembled 50 prospectively-studied cases and 25 age-matched controls; none were reported in our prior large series of 33 essential tremor and 21 controls. In addition to methods used in prior studies, the current study used a random sampling approach to quantify Purkinje cells along the Purkinje cell layer with a mean of 217 sites examined in each specimen, allowing for extensive sampling of the Purkinje cell layer within the section. For the first time, we also quantified the distance between Purkinje cell bodies - a nearest neighbor analysis.
Results
In the Purkinje cell count data collected from fifteen 100x-fields, cases had lower counts than controls in all three counting criteria (cell bodies, nuclei, nucleoli, all p<0.001). Purkinje cell linear density was also lower in cases than controls (all p<0.001). Purkinje cell linear density obtained by random sampling was similarly lower in cases than controls in all three counting criteria (cell bodies, nuclei, nucleoli, all p≤0.005). In agreement with the quantitative Purkinje cell counts, the mean distance from one Purkinje cell body to another Purkinje cell body along the Purkinje cell layer was greater in cases than controls (p=0.002).
Conclusions
These data provide support for the neurodegeneration of cerebellar Purkinje cells in essential tremor.
Keywords: essential tremor, cerebellum, Purkinje cell, neurodegeneration, pathology
Introduction
Essential tremor (ET) is a chronic, progressive neurological disease1,2 that seems to involve the cerebellar system.3–5 Controlled postmortem studies in recent years have documented a growing number of structural changes in the ET cerebellum, involving the Purkinje cell and neighboring neuronal populations.6–9 In addition to these changes, Purkinje cell loss has been documented in some10–12 although not all studies.13,14 Hence, there are conflicting data,15 resulting in considerable controversy over the issue of Purkinje cell loss in this disease.14,16 Overall, however, there have been few studies and there is a paucity of data. All but one of the previous studies selected microscopic fields with a straight Purkinje cell layer, thereby introducing the possibility of field selection bias. By contrast, one study13 quantified Purkinje cells using random field selection, although a small number of fields (i.e., 10) were quantified in each ET and control cerebellar section.
In our prior large series, we reported data collected between 2003 and 2007 on 33 ET cases and 21 controls.10 Over the past eight years, we have assembled a larger replicate sample of 50 prospectively studied ET cases and 25 age-matched controls and now report the results. In this study, we used quantification methods employed in our prior study (i.e., quantification of Purkinje cells in fifteen fields),10 but also added additional, unique approaches. First, we now use a random sampling approach to eliminate potential field-selection bias in the quantification of Purkinje cells; a mean of 217 sites were examined in each specimen, allowing for extensive sampling of the Purkinje cell layer within the section. Second, for the first time, using a nearest neighbor analysis,17 we quantify the distance (i.e., the length of the gap) between Purkinje cells; this is another potentially useful marker of Purkinje cell loss. The study specifically tests the hypothesis that there is a reduction in the number of Purkinje cells and an increase in the distance between Purkinje cells in ET cases compared with age-matched controls.
Methods
Cases and Controls
This study was conducted at the Essential Tremor Centralized Brain Repository,6 an effort that involves the prospective collection of ET brains from study participants who reside throughout the United States and have agreed to brain donation. All ET cases self-referred to the Repository rather than being referred by their physician. ET cases were diagnosed as described below.18 Controls were normal elderly subjects from the New York Brain Bank (Columbia University Medical Center, New York, NY), Harvard Brain Tissue Resource Center (McLean Hospital, Belmont, MA) and University of Kentucky Alzheimer’s Disease Center (Sanders-Brown Center on Aging, Lexington, KY). The controls were free of clinical diagnoses of Alzheimer’s disease, ET or Parkinson’s disease and without neuropathological diagnoses of neurodegenerative disease.6 All study subjects signed informed consent approved by these University Ethics Boards.
Data were available on 67 ET cases; however, 17 (25.4%) with Lewy bodies were excluded as prior research has indicated that these may represent a distinct and separable sub-group of ET cases, which may have a different patho-mechanistic basis than the majority of cases, in whom the most distinctive postmortem changes are in the cerebellum.10 This left 50 for analyses. Controls were frequency-matched to cases using a 2:1 scheme, reflecting the greater availability of ET tissue. The final sample comprised 50 ET cases and 25 age-matched controls. None of these had been included in our previously-reported extensive series of cases and controls.10
Clinical Evaluation
ET diagnoses were assigned using three sequential methods, as described in detail.6 First, the majority (>95%) of cases were diagnosed clinically with ET by their treating physician. Second, the cases completed a series of semi-structured clinical questionnaires (demographic data, general medical data including medications, tremor-specific data), which included data on age of onset (i.e., age at first symptoms and signs of ET). Each case then submitted four standardized hand-drawn Archimedes spirals (two right and two left hand, each drawn on an 8.5 × 11 inch sheet of white paper). These drawings were supplemented with additional clinical information from clinical records, treating physicians, and family members. ET diagnoses were then confirmed by a senior neurologist specializing in movement disorders (E.D.L.) who used the following criteria: (i) moderate or greater amplitude arm tremor (rating of 2 or higher using the Washington Heights Inwood Genetic Study of Essential Tremor Rating Scale)18 in at least one of the submitted Archimedes spirals; (ii) no history of Parkinson’s disease or dystonia; and (iii) no other etiology for tremor (e.g. medications, hyperthyroidism). Third, ET cases then underwent a standardized, videotaped neurological examination, including a detailed assessment of tremor. The videotape protocol included assessments of postural tremor (two positions), kinetic tremor (five activities with each arm), and intention tremor of the arms, as well as neck, voice and jaw tremors. The videotaped examination also included the motor portion of the Unified Parkinson’s Disease Rating Scale,19 including assessments of speech, facial expression, rest tremor (with arms in four positions: resting in the lap, relaxed at sides while standing, while walking, and while lying down), bradykinesia, posture, arising from a chair, and gait while walking and turning. Each videotape was reviewed (E.D.L.) and, based on the questionnaire and videotape data, the diagnosis of ET was re-examined in each case using published diagnostic criteria (moderate or greater amplitude kinetic tremor [tremor rating ≥2] during three or more activities or a head tremor in the absence of Parkinson’s disease or other known causes).20 Based on the videotape, ET cases were also assigned a total tremor score (range = 0 – 36), using the 0 – 3 ratings of postural and kinetic tremors on examination. Intention tremor during the finger-nose-finger maneuver and dysmetria during the heel-to-shin maneuver, which were videotaped, were rated using the International Cooperative Ataxia Rating Scale.21
In ET cases, data on lifetime exposure to medications (e.g., lithium, diphenylhydantoin, chemotherapeutic agents) known to cause cerebellar damage were collected and the average number of daily drinks of beer, liquor, or wine was collected. Heavy ethanol use was previously defined as consumption of an average of 4 or more standard drinks (15 mL of absolute ethanol) per day for a man or 3 or more per day for a woman at any point in their lives.6,10,22 Every 6 months, a follow-up semi-structured telephone evaluation was performed on ET cases and hand-drawn spirals were collected.
Neuropathological Assessment
At the time of death, the brain was removed by a local pathologist, and then the intact, fresh brain was tightly double bagged, placed in a pail containing wet-ice and water, and immediately shipped to the New York Brain Bank according to the Bank’s protocol.23 As previously described in detail,6 brains underwent a complete neuropathological assessment and all evaluations reported here were performed at the New York Brain Bank. Standardized measurements of brain weight (in grams) and postmortem interval (hours between death and placement of brain in a cold room or on ice) were recorded. Paraffin embedded blocks from standardized brain regions were sectioned at 7-μm and stained respectively with Luxol fast blue and hematoxylin and eosin (LH&E) for general tissue survey and assessment of myelin, and the modified Bielschowsky silver stain for axons and neurofibrils. Additional sections were processed with peroxidase-antiperoxidase methods for α-synuclein-, β–amyloid-, and for tauopathic-burdens. Brains underwent Braak and Braak Alzheimer’s Disease staging for neurofibrillary tangles,24 Consortium to Establish a Registry for Alzheimer’s Disease rating for neuritic plaques,25 and Braak Parkinson’s disease staging for Lewy bodies.26
Quantification of Purkinje Cells Using Fifteen 100x Fields (Method 1)
A standard 3 × 20 × 25 mm parasagittal, formalin-fixed, tissue block was harvested from the neocerebellum; the block included the cerebellar cortex, white matter, and dentate nucleus. The block contained the anterior quadrangulate lobules in the anterior lobe of the cerebellar cortex, which are involved in motor control.27 Using a LH&E stained 7-μm thick section,12 a senior neuropathologist (P.L.F.) blinded to clinical information quantified torpedoes in the entire section. Using the same section, a trained research technician (M.C.) blinded to clinical information counted Purkinje cells using three counting criteria (cell bodies, nuclei, and nucleoli) in fifteen 100x fields. The fields were selected as follows: (i) non-adjacent fields representing different regions of the section, and (ii) fields that were between but not inclusive of the base of the fissure and the apex of the folium. In an initial test sample of 35 fields in five cases, the correlation between the two investigators was high - for cell body counts, Pearson’s r = 0.99, p <0.001; for nuclei counts, Pearson’s r = 0.98, p <0.001; for nucleoli counts, Pearson’s r = 1.00, p <0.001.
Quantification of Purkinje Cell Linear Density (Method 2)
The counts obtained above, from fifteen 100x fields, were divided by the length of the Purkinje cell layer centered in the microscopic field (cells/mm). This resulted in a measure of Purkinje cell linear density.
Quantification of Purkinje Cells Using a Random Sampling Approach (Method 3)
To eliminate potential field-selection bias, we used Stereo Investigator software, version 11 (MBF Biosciences, Williston, Vermont). Using the same standard LH&E stained cerebellar section, the boundaries of the cerebellar cortex were initially outlined at low magnification (2x magnification) using the closed contour function of Stereo Investigator. A uniformly random sampling grid (1000 × 1000 μm2) was placed over the outlined cortex, and within each sampling site at 100x magnification an optical disector counting frame (600 × 600 μm2) defined the area for quantification. At each disector site, the blinded technician manually focused on the tissue surface and quantified Purkinje cells using either cell body, nucleus or nucleolus as counting criterion; optical magnification was increased to 200x or 400x within the disector frame to clearly visualize the presence of nucleus or nucleolus, as needed. There was a 2.6-times increase in the total number of Purkinje cells counted per section by the random sampling approach (mean = 358 ± 93) in comparison to the 15-field approach (mean = 135 ± 29). The software, based on each criterion, then estimated the total number of Purkinje cells in the cerebellar cortex in the section. A line was drawn along the entire Purkinje cell layer in the section, and the linear density of Purkinje cells was determined (total Purkinje cells/Purkinje cell layer length).
Quantification of the Distance between Purkinje Cells
The distance between Purkinje cells (i.e., the length of the gap) was determined using a nearest neighbor analysis.17 Using the same standard LH&E stained cerebellar section, each of the individual gyri within the Purkinje cell layer was assigned a number in sequential order (approximately 40 – 50 gyri per section). A random number generator was used to select ten gyri per section for quantification. These ten gyri were centered in a 5x objective microscopic field and imaged using a Zeiss Axioplan 2 microscope. The distance between each Purkinje cell body was measured in Open Lab software, version 5 (Improvision, Waltham, MA) by drawing a freehand line between adjacent Purkinje cell bodies along the entirety of the Purkinje cell layer within a given image. The average distance between Purkinje cell bodies was determined from each of the ten images and then averaged together to determine the mean nearest neighbor distance (μm) for each ET case and control.
Statistical Analyses
Statistical analyses were performed in SPSS (version 21.0). Clinical and pathological data were compared in ET cases vs. controls using Student’s t-tests and chi-square tests. When a continuous variable was not normally distributed, a non-parametric statistical test (Mann-Whitney test) was used. We used chi-square tests to assess the proportion of ET cases whose inter-Purkinje cell distance was greater than the median inter-Purkinje cell distance of controls, as well as greater than the 90th percentile inter-Purkinje cell distance of controls. Correlations between continuous variables were assessed using Pearson’s correlation coefficients and differences across groups were assessed using analysis of variance.
Results
The 50 ET cases and 25 controls were similar in age at death, gender, brain weight, Consortium to Establish a Registry for Alzheimer’s Disease score, and Braak and Braak stage (Table 1). The postmortem interval was shorter in ET cases than controls. No cases were heavy ethanol users and none had lifetime exposure to cerebellar toxic medications. Age of onset was ≤ 65 years in 82% of ET cases and the mean tremor duration was 43.7 ± 23.1 years; the mean total tremor score was 24.4 ± 6.4. Lewy bodies (alpha-synuclein: dorsal vagal nucleus, locus ceruleus, pars compacta of substantia nigra) were detected in none.
Table 1.
Clinical and Pathological Data on Essential Tremor Cases and Controls
| Controls (n = 25) | Essential Tremor Cases (n = 50) | Significance | |
|---|---|---|---|
|
| |||
| Age (years) | 84.9 ± 5.4 | 86.8 ± 5.8 | p = 0.17 a |
|
| |||
| Women | 15 (60.0) | 31 (62.0) | p = 0.87 b |
|
| |||
| Post-mortem interval (hours) | 10.8 ± 12.8 [5.6] | 2.7 ± 3.1 [1.8] | p < 0.001c |
|
| |||
| Brain weight (grams) | 1183 ± 150 | 1187 ± 154 | p = 0.92 a |
|
| |||
| CERAD plaque score * | p = 0.32 b | ||
| 0, A or B | 16 (100) | 42 (87.5) | |
| C | 0 (0) | 6 (12.5) | |
|
| |||
| Braak and Braak neuronal tangle stage * | p = 1.00 a | ||
| 0 – 4 | 22 (95.7) | 46 (97.9) | |
| 5 or 6 | 1 (4.3) | 1 (2.1) | |
|
| |||
| Torpedo count | 4.2 ± 2.8 [3.0] | 18.5 ± 15.0 [15.0] | p < 0.001 c |
|
| |||
| 15-Field Purkinje cell body count (per 100x field) | 10.6 ± 1.4 | 7.8 ± 1.2 8.0 ± 1.0 |
p < 0.001 a p < 0.001 a |
|
| |||
| 15-Field Purkinje cell nucleus count (per 100x field) | 8.5 ± 1.1 | 6.5 ± 0.9 6.6 ± 0.8 |
p < 0.001 a p < 0.001 a |
|
| |||
| 15-Field Purkinje cell nucleolus count (per 100x field) | 4.2 ± 0.7 | 3.1 ± 0.8 3.3 ± 0.7 |
p < 0.001 a p < 0.001 a |
|
| |||
| 15-Field approach – Purkinje cell body linear density (cells/mm) | 4.9 ± 0.7 | 3.6 ± 0.5 3.7 ± 0.5 |
p < 0.001 a p < 0.001 a |
|
| |||
| 15-Field approach - Purkinje cell nucleus linear density (cells/mm) | 3.9 ± 0.5 | 3.0 ± 0.4 3.1 ± 0.4 |
p < 0.001 a p < 0.001 a |
|
| |||
| 15-Field approach - Purkinje cell nucleolus linear density (cells/mm) | 2.0 ± 0.3 | 1.45 ± 0.4 1.5 ± 0.3 |
p < 0.001 a p < 0.001 a |
|
| |||
| Random sampling approach - Purkinje cell body linear density (cells/mm) | 4.8 ± 0.9 | 3.8 ± 0.8 4.0 ± 0.9 |
p < 0.001 a p = 0.002 a |
|
| |||
| Random sampling approach - Purkinje cell nucleus linear density (cells/mm) | 4.5 ± 0.9 | 3.6 ± 0.8 3.8 ± 0.9 |
p < 0.001 a p = 0.005 a |
|
| |||
| Random sampling approach - Purkinje cell nucleolus linear density (cells/mm) | 1.7 ± 0.4 | 1.4 ± 0.4 1.5 ± 0.4 |
p = 0.005 a p = 0.13 a |
|
| |||
| Purkinje cell nearest neighbor distance (μm) | 174.6 ± 41.4 | 209.3 ± 46.9 202.8 ± 45.6 |
p = 0.002 a p = 0.03 a |
All values represent mean ± standard deviation [median] or number (percentage).
For Purkinje cell counts and Purkinje cell nearest neighbor distance in ET cases, the upper value represents the data for the entire sample of 50 ET cases and the lower value represents the data for the sample which only included the 50% of ET cases with the lowest daily ethanol consumption.
Student’s t test.
Chi-square test or Fisher’s Exact test.
Mann-Whitney test.
Data not available for some brains.
Consortium to Establish a Registry for Alzheimer’s Disease (CERAD), μm = microns
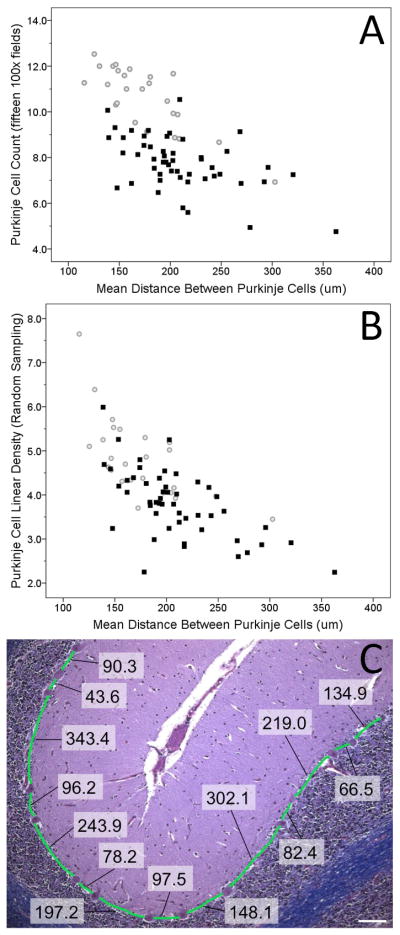
For the Purkinje cell count data collected from fifteen 100x-fields, ET cases had lower counts than controls in all three counting criteria (cell bodies [Figure 1A], nuclei, nucleoli, all p values <0.001, Table 1). Similarly, Purkinje cell linear density obtained from the 15 field approach was lower in ET cases than controls in all three counting criteria (all p values <0.001). Randomly-sampled Purkinje cell linear densities were similarly lower in ET cases than controls in all three measures (cell bodies [Figure 1B], nuclei, nucleoli, all p values ≤0.005). Indeed, 47 (94%) of 50 ET cases had a value (cell body) that was below the central tendency for the controls (i.e., below the norm for the norm [Figure 1B]).
Figure 1.
Reduced Mean Purkinje Cell Counts quantified in fifteen100x fields (A) or by randomly-sampled Purkinje cell linear density (B) in 50 Essential Tremor Cases (black squares) versus 25 Age-Matched Controls (gray circles) and Correlation with Mean Nearest Neighbor Distance. (C) Purkinje cell nearest neighbor quantification in LH&E- stained cerebellum cortical section. The nearest neighbor measurements between Purkinje cell bodies in a control are displayed and measured based on the Purkinje cell layer shown in green (100x magnification). All measurements were taken in micrometers (μm), scale bar in white is 100 μm.
The mean Purkinje cell nearest neighbor distances (distance from one Purkinje cell body to another Purkinje cell body along the Purkinje cell layer) was greater in ET cases than controls (p = 0.002, Table 1, Figure 1). The median inter-Purkinje cell distance for controls was 165.5 μm. The proportion of ET cases whose inter-Purkinje cell distance was greater than this value was 84% - this proportion differed from that seen in controls (i.e., 50%) (chi-square test = 8.73, p = 0.003). The proportion of ET cases whose inter-Purkinje cell distance was greater than 207.3 μm (the 90% percentile of the inter-Purkinje cell distance for controls) was 42.0% - this proportion differed from that seen in controls (i.e., 10%) (chi-square test = 6.89, p = 0.009).
The Purkinje cell quantitative data derived from the different methods (15-field method and randomly-sampled method) were highly correlated (Pearson’s r values ranging from 0.49 – 0.98, all p values <0.001) and was highly inversely correlated with the Purkinje cell nearest neighbor distance, as would be expected. Brains with the fewest Purkinje cells had the greatest distance between Purkinje cells (Pearson’s r values ranging from −0.62 to −0.69, all p values <0.001), and had the highest torpedo counts (Pearson’s r values ranging from −0.26 to −0.46, all p values ≤0.03).
The Purkinje cell counts and Purkinje cell nearest neighbor distances were not correlated with age or postmortem interval, either in cases or in controls (all Pearson’s correlation coefficients >0.05). The Purkinje cell counts and Purkinje cell nearest neighbor distances were not correlated with Consortium to Establish a Registry for Alzheimer’s Disease plaque score or Braak and Braak stage for neurofibrillary tangles, in either cases or controls (analysis of variance, all p values >0.05). Therefore, these variables could not have been confounders in our analysis. Nonetheless, Consortium to Establish a Registry for Alzheimer’s Disease plaque scores were missing in 36% of controls, raising the issue of residual confounding. Therefore, we performed a series of secondary analysis in which we stratified the sample by Consortium to Establish a Registry for Alzheimer’s Disease scores, comparing ET cases to controls within stratum that were defined by these scores. This was designed to further remove the possible confounding effects of this co-morbid Alzheimer’s-type pathology. These analyses continued to show the same within-strata ET case vs. control differences in Purkinje cell counts and nearest neighbor Purkinje cell distances.
In ET cases, the average number of daily drinks (beer, wine and liquor combined) was not associated with any of the Purkinje cell counts or with the Purkinje cell distances (all Pearson’s correlation coefficient p values > 0.05), which strongly suggests that differences between ET cases and controls in these counts were not confounded by any potential case-control differences in ethanol intake. In an additional analysis, we also compared controls to ET cases with the lowest daily ethanol consumption, excluding a full 50% of ET cases whose daily ethanol consumption was above the mean value for ET cases. None of these ET cases reported ethanol consumption of more than 2 – 4 drinks per week. In this analysis, the case-control differences remained significant for nine of ten comparisons (Table 1).
The controls were free from clinical diagnoses of Alzheimer’s disease. The more recently enrolled ET cases (n = 20) had a cognitive screen (Folstein Mini-Mental State Examination),28 although none were excluded based on the results. In 19 of these 20 ET cases, the Mini-Mental State Examination score was ≥25 (mean in these 19 = 26.1 ± 1.7), and hence, according to published guidelines, they were deemed cognitively normal.29,30 In an analysis in which we compared these 19 cognitively normal ET cases to our 25 controls, the case-control differences in Purkinje cell counts and the Purkinje cell distances remained significant for all ten comparisons (all p values ≤0.01).
In ET cases, the total tremor score did not correlate with tremor duration (Pearson’s r = 0.20, p = 0.19) or with any of the Purkinje cell counts (all p values > 0.05). Tremor duration did not correlate with Purkinje cell counts (all p values > 0.05).
Discussion
Controlled postmortem studies employing a range of quantitative assessments have documented a large number of structural changes in the ET cerebellum, involving the Purkinje cell and neighboring neuronal populations.6–9 Aside from these many changes, Purkinje cell loss has been documented in some10–12 although not all studies.13–14 Hence, there remains considerable controversy over the issue of Purkinje cell loss in this disease.14–16
Given the small number of studies and the paucity of data, we carefully assembled another large prospective replicate sample of ET cases over the past eight years, and used a variety of methods, including a random sampling approach, to quantify Purkinje cells in ET cases vs. controls. In this study, we demonstrated a significant reduction in Purkinje cells in ET cases using all counting methods employed as well as a greater distance between Purkinje cell bodies in a nearest neighbor analysis. The quantitative results on Purkinje cell counts that we now report corroborate those we had reported previously. In our cohort in 2007,10 we reported that the mean number of Purkinje cells per 100x field was reduced in ET cases vs. controls (6.6 ± 2.4 vs. 9.6 ± 3.4), values that are similar to those we now report in this replicate cohort (7.9 ± 1.3 vs. 10.7 ± 1.4). One methodological improvement over the prior study is that fifteen rather than five fields were sampled, resulting in a more precise measure and a lower standard deviation. The random sampling approach we now employed further eliminates potential field selection bias, with equal chance to sample areas of curvature (e.g., base and gyral crest) and intervening straight portions of each gyrus, and also samples a large number of fields and greater number of Purkinje cells per section. There was still a significant reduction of Purkinje cells in ET cases compared to controls. In a small study of Purkinje cell linear density in 7-μm sections on 8 ET cases and 11 controls,11 the Purkinje cell linear density was 2.1 ± 0.8 vs. 3.5 ± 1.3 cells/mm, values that are within but on the lower end of the range of what we report in this replicate study.
In this study, we also employed a new method to measure the distance between Purkinje cell bodies along the Purkinje cell layer. Here, too, we demonstrated a case-control difference; importantly the increase in mean distance between Purkinje cells in ET cases is consistent with a reduction in Purkinje cells in ET. We were able to show that our various methods, though different, yielded results that were robustly correlated with one another. This provides significant construct validity for these findings.
Although the mean numbers of Purkinje cells differed between ET cases and controls, and 94% of the ET cases had a value (cell body) that was below the central tendency for the controls (i.e., the norm for the norm [Figure 1B]), we recognize that the two distribution curves overlapped. It is important to note, however, that even in patients with spinocerebellar ataxia, who have marked and indisputable Purkinje cell loss, Purkinje counts in individual cases have been shown to fall within the range of normal controls.31 The presence of overlap in distribution is not used as an argument that Purkinje cell loss is of no pathomechanistic importance in spinocerebellar ataxia. Indeed, as shown in most biomarker studies, ranges overlap considerably and separation is rarely complete. Two additional points are worthy of reiteration. As discussed in detail previously,12 a modest and partial depletion of the Purkinje cell population, in conjunction with a variety of Purkinje cell axonal and dendritic changes and remodeling of basket cell processes around surviving Purkinje cells in ET, all suggest that the as-yet unknown underlying molecular abnormality in ET is one that leads to cellular injury and dysfunction of Purkinje cells. In some cases, the underlying abnormality probably overwhelms the cell, leading to Purkinje cell death. Thus, the likely primary pathophysiology of ET, occurring on a molecular and then cellular-physiological level, seems to involve to some extent a partial loss of Purkinje cells, with this being a gross and possibly imperfect cellular bio-marker of a larger underlying systems problem.12 As further discussed previously,12 postmortem data show cell counts at only one point in time (i.e., after death). Therefore, in the strictest sense, it is not possible using postmortem data to show a reduction in cells in one individual patient over time. While some cases and controls may have similar cell counts at the time of death, giving the impression of the absence of cell loss in some cases, this impression does not take into consideration the fact that cell counts may have started at different places at baseline.12 There is a range of cell counts even in normal individuals of the same age.12 Thus, as noted previously, a case who began with 1.50 cells/mm−1 prior to disease onset (e.g., age 50) and who had 1.00 cells/mm−1 at death (e.g., age 80), experienced a 33% loss of Purkinje cells, whereas a control who may have begun with 1.20 cells/mm−1 at age 50 and had 1.00 cells/mm−1 at death (e.g., age 80), experienced only a 20% loss of Purkinje cells.12
Two studies have not detected Purkinje cell loss in patients with ET,13–14 although a number of valid methodological concerns have been raised about both studies.16,32 Several plausible explanations could account for the differences across studies, and more specifically, the null and/or inconclusive findings reported by the Arizona and Canada studies.13,14 The first issue is case selection. In the Arizona study,13 tremor was defined simply as “action tremor of the hand, voice, or isolated head tremor.” This liberal definition does little to exclude enhanced physiological tremor, which is an action tremor of the hands and is very common in the elderly. Although the authors note that tremor had to have a rating of 2 or higher, for their cases who had had tremor for 3 or more years (i.e., most of their cases), a low-amplitude tremor (<2) was deemed acceptable. Furthermore, the specific examination maneuvers that they performed to assess kinetic tremor were not explicitly delineated, further raising questions about the extent of the clinical assessment and the stringency of the requirements for the ET diagnosis (i.e., whether it required moderate or greater amplitude kinetic tremor on several examination maneuvers). Indeed, in the Arizona study,13 this liberal case definition is a considerable problem - 177 of 753 enrolled subjects were diagnosed by study personnel as “ET” (i.e., 23.5%). The mean age of the subjects enrolled and evaluated in their study was 75 years. In other population-based studies, the expected prevalence of ET in this age group is on the order of 6 – 8%,33 which is 1/3 of that in their study. This raises the distinct possibility that the large majority of their ET cases, as many as 2/3, likely had other conditions such as enhanced physiological tremor. The possibility of such extensive diagnostic mis-classification could account for the null finding. Furthermore, the short duration of tremor in their ET cases could also have contributed to the null finding. The second issue relates to postmortem methods. Our standard tissue block was harvested from a specific region of the neocerebellum; the block contained the anterior quadrangulate lobules in the anterior lobe of the cerebellar cortex, which are involved in motor control.27 Neither of the other studies13,14 noted the precise location of their tissue block or whether it was derived from the motor rather than cognitive cerebellum. The third issue relates to unaccounted confounding as neither of the other studies excluded individuals exposed to chemotherapeutic agents;13,14 these agents are known to result in Purkinje cell loss. Furthermore, neither of the other studies considered the possible confounding effects of Alzheimer-type changes.13,14 The final issue is study interpretation. The Canadian study14 has often been misinterpreted as a null finding. However, as noted previously, that study was underpowered to detect a case-control difference, making this an inconclusive study rather than a null study.16
One issue is that our results reflect a decreased Purkinje cell density in ET cases. In theory, this could be the result of decreased Purkinje cell numbers, or, alternately, of a reduction in neuropil volume relative to Purkinje cell perikaryal volume in controls or conversely, and expansion of neuropil volume relative to Purkinje cell perikaryal volume in ET cases. This theoretic scenario could occur in the setting of a reduction of the Purkinje cell dendritic arborization in controls or expansion of the Purkinje cell dendritic arborization in ET cases. However, our prior study has actually demonstrated the converse; namely, that there is a statistically significant reduction in the Purkinje cell dendritic arbor in ET cases.7
The total tremor score was not correlated with any of the Purkinje cell counts and also did not correlate with tremor duration. Hence, one must wonder about the utility of the total tremor score as a measure of underlying disease duration or disease severity. This finding also raises the possibility that Purkinje cell loss is an epiphenomenon and not the direct and proximate cause of the tremor in ET. The following data should be considered. We have previously reported significantly increased Purkinje cell dendritic pruning and loss of dendritic architecture in ET, which are likely to be earlier postmortem findings.7 Importantly, there was a robust correlation of pathologic and clinical findings, where total tremor score was inversely associated with a variety of measures of dendritic arbor complexity including branch length (Spearman’s r = −0.44), maximum branch order (Spearman’s r = −0.41), and number of terminations (Spearman’s r = −0.43) (i.e. more severe tremor was associated with more marked dendritic pruning). By contrast, Purkinje cell loss is likely to be a later (i.e., more mechanistically downstream) and less constant finding in ET, which could account for the fact that it does not correlate with total tremor score. In a recent study,34 we compared postmortem changes in the right vs. left cerebellar hemisphere in 25 ET cases, including Purkinje cell linear density, torpedo counts, and a group of changes in Purkinje cell axonal shape and connectivity, and examined how these correlated with asymmetry of tremor on neurological examination. In 18 (72.0 %) of 25 ET cases, clinical and pathological features were concordant, providing additional evidence that the pathological changes in the cerebellum in ET are of patho-mechanistic importance.
Even though our study utilized several different methods to quantify Purkinje cells, it should be interpreted within the context of an important limitation; namely, the entire cerebellum was not available for these analyses and therefore a stereological approach was not used. Although stereological studies allow for an unbiased approach to tissue sampling, there are real issues of feasibility for stereological studies of the cerebellum, as the entire cerebellum or even a hemi-cerebellum are rarely if ever available, as is required for such analyses. Indeed, the number of stereological studies of the human cerebellum during the past decade have been limited,35–38 and the number of brains examined per study quite limited (4 – 10 per study group rather than the 50 and 25 per study group in this study).35–38 As an alternative, the non-stereological approach that we used has and continues to be used in numerous other studies, and furthermore, the sample sizes in these studies are considerable (up to 148 subjects in one study).39–42 Finally, in a small pilot study, we have previously shown that Purkinje cell counts that we obtain using the method we report in this study are highly correlated with stereologically-based Purkinje cell counts (r = 0.781, p < 0.05),31 demonstrating the validity of our findings.
Another limitation of the study was that we restricted our sampling to one region in the cerebellar hemisphere, and it would be of considerable interest in future studies to sample additional cerebellar regions. In a prior study,43 we showed that the increase in torpedoes observed in the cerebellar cortex of ET cases was also present in the vermis; however, that study did not quantify Purkinje cells. Both motor and cognitive regions occur in the cerebellar hemispheres.44 Whether differences in sampling, across studies, could account for the discrepant findings across these studies is worth considering but is not known.
This study provides additional evidence for the neurodegenerative hypothesis of ET. These findings may be added to the growing catalog of structural changes in the Purkinje cell population in ET.12 This study focused on more accurately quantifying Purkinje cell counts in an unbiased manner, and on validating our previous studies involving Purkinje cell counts.10,12 Our findings indicate that there is a significant reduction in Purkinje cells in ET cases compared to controls. This is likely to be important in the events involving the cerebellar cortex in ET.7
Acknowledgments
We thank the Harvard Brain Tissue Resource Center (supported in part by PHS grant number R24 MH068855) for providing control tissue. We similarly thank the University of Kentucky Alzheimer’s Disease Center Biobank (P30 AG028383) for providing control tissue, and the patients, staff, and clinicians who have contributed to their efforts.
Funding Sources: National Institutes of Health: NINDS #R01 NS042859, NINDS #R01 NS086736, NINDS #R01 NS085136 and NINDS #R01 NS088257.
Footnotes
Financial Disclosure: None of the listed authors has any conflicts of interest to declare.
Author Roles
1) Research project: A. Conception, B. Organization, C. Execution;
2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique;
3) Manuscript: A. Writing of the first draft, B. Review and Critique.
Matthew Choe, BA: 1C, 2B, 3A
Etty Cortés, MD: 1C, 2C, 3B
Jean-Paul G. Vonsattel, MD: 1B, 1C, 2C, 3B
Sheng-Han Kuo, MD: 1B, 2C, 3B
Phyllis L. Faust, MD, PhD: 1A, 1B, 1C, 2C, 3B
Elan D. Louis, MD, MSc: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Financial Disclosures
Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS086736 (principal investigator), NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). Dr. Faust has received funding from NINDS NINDS #R01 NS042859 (co-investigator), NINDS #R01 NS085136 (co-investigator) and NINDS #R01 NS088257 (co-investigator). Dr. Vonsattel has received funding from NINDS #R01 NS042859 (co-investigator), NINDS #R01 NS086736 (co-investigator), NINDS #R01 NS085136 (co-investigator) and NINDS #R01 NS088257 (co-investigator). Dr. Kuo has received funding from International Essential Tremor Foundation, NINDS #K08 NS08738 (principal investigator), Louis V. Gerstner Jr. Scholar Award (principal investigator), Parkinson’s Disease Foundation (principal investigator), American Parkinson’s Disease Association (principal investigator), NIEHS pilot grant #ES009089 (principal investigator), NINDS #R01NS084948 (co-investigator), and NINDS #R01NS088257 (co-investigator).
References
- 1.Putzke JD, Whaley NR, Baba Y, et al. Essential tremor: predictors of disease progression in a clinical cohort. J Neurol Neurosurg Psychiatry. 2006;77:1235–1237. doi: 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis ED, Agnew A, Gillman A, et al. Estimating annual rate of decline: prospective, longitudinal data on arm tremor severity in two groups of essential tremor cases. J Neurol Neurosurg Psychiatry. 2011;82:761–765. doi: 10.1136/jnnp.2010.229740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bares M, Husarova I, Lungu OV. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov (N Y) 2012 doi: 10.7916/D89G5KH9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28:1759–1761. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- 5.Passamonti L, Cerasa A, Quattrone A. Neuroimaging of Essential Tremor: What is the Evidence for Cerebellar Involvement? Tremor Other Hyperkinet Mov (N Y) 2012 doi: 10.7916/D8F76B8G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babij R, Lee M, Cortes E, et al. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–3061. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis ED, Lee M, Babij R, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. 2014;137:3142–3148. doi: 10.1093/brain/awu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erickson-Davis CR, Faust PL, Vonsattel JP, et al. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CY, Louis ED, Faust PL, et al. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137:3149–3159. doi: 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 11.Axelrad JE, Louis ED, Honig LS, et al. Reduced purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65:101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Louis ED, Babij R, Lee M, et al. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28:1854–1859. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Symanski C, Shill HA, Dugger B, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29:496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- 14.Rajput AH, Robinson CA, Rajput ML, et al. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–628. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Jellinger KA. Is there cerebellar pathology in essential tremor? Mov Disord. 2014;29:435–436. doi: 10.1002/mds.25852. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: Towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18:1003–1004. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Hamling KR, Tobias ZJ, Weissman TA. Mapping the development of cerebellar Purkinje cells in zebrafish. Dev Neurobiol 2015. 2015 Feb 15; doi: 10.1002/dneu.22275. [DOI] [PubMed] [Google Scholar]
- 18.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16:124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 19.Fahn S, Elton RL Members of the UPDRS Development Committee. In: Recent Developments in Parkinson’s Disease. Fahn S, Marsden CD, Goldstein M, Calne CD, editors. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 20.Louis ED, Ford B, Lee H, et al. Diagnostic criteria for essential tremor: a population perspective. Arch Neurol. 1998;55:823–828. doi: 10.1001/archneur.55.6.823. [DOI] [PubMed] [Google Scholar]
- 21.Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. 1997;145:205–211. doi: 10.1016/s0022-510x(96)00231-6. [DOI] [PubMed] [Google Scholar]
- 22.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25:228–235. [PubMed] [Google Scholar]
- 23.Vonsattel JP, Amaya MD, Cortes EP, Mancevska K, Keller CE. Twenty-first century brain banking: practical prerequisites and lessons from the past: the experience of New York Brain Bank, Taub Institute, Columbia University. Cell Tissue Bank. 2008;9:247–258. doi: 10.1007/s10561-008-9079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirra SS. The CERAD neuropathology protocol and consensus recommendations for the postmortem diagnosis of Alzheimer’s disease: a commentary. Neurobiol Aging. 1997;18:S91–S94. doi: 10.1016/s0197-4580(97)00058-4. [DOI] [PubMed] [Google Scholar]
- 26.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 27.Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59:1560–1570. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Pan JJ, Lee M, Honig LS, Vonsattel JP, Faust PL, Louis ED. Alzheimer’s-related changes in non-demented essential tremor patients vs. controls: Links between tau and tremor? Parkinsonism Relat Disord. 2014;20:655–658. doi: 10.1016/j.parkreldis.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013;9:529–537. doi: 10.1016/j.jalz.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louis ED, Kuo SH, Vonsattel JP, Faust PL. Torpedo formation and Purkinje cell loss: modeling their relationship in cerebellar disease. Cerebellum. 2014;13:433–439. doi: 10.1007/s12311-014-0556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louis ED, Faust PL. Purkinje cell loss in essential tremor. Mov Disord. 2014;29:1329–1330. doi: 10.1002/mds.25965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–41. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 34.Louis ED, Lee M, Cortes E, et al. Matching asymmetry of tremor with asymmetry of postmortem cerebellar hemispheric changes in essential tremor. Cerebellum. 2014;13:462–470. doi: 10.1007/s12311-014-0560-9. [DOI] [PubMed] [Google Scholar]
- 35.Andersen K, Andersen BB, Pakkenberg B. Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol Aging. 2012;33:197 e11–20. doi: 10.1016/j.neurobiolaging.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje cells are reduced in a subpopulation of autistic brains: a stereological experiment using calbindin-D28k. Cerebellum. 2008;7:406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- 37.Andersen BB, Korbo L, Pakkenberg B. A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol. 1992;326:549–560. doi: 10.1002/cne.903260405. [DOI] [PubMed] [Google Scholar]
- 38.Kiessling MC, Büttner A, Butti C, et al. Intact numbers of cerebellar purkinje and granule cells in sudden infant death syndrome: a stereologic analysis and critical review of neuropathologic evidence. J Neuropathol Exp Neurol. 2013;72:861–870. doi: 10.1097/NEN.0b013e3182a31c31. [DOI] [PubMed] [Google Scholar]
- 39.Andrioli A, Alonso-Nanclares L, Arellano JI, DeFelipe J. Quantitative analysis of parvalbumin-immunoreactive cells in the human epileptic hippocampus. Neuroscience. 2007;149:131–143. doi: 10.1016/j.neuroscience.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 40.Thom M, Liagkouras I, Elliot KJ, et al. Reliability of patterns of hippocampal sclerosis as predictors of postsurgical outcome. Epilepsia. 2010;51:1801–1808. doi: 10.1111/j.1528-1167.2010.02681.x. [DOI] [PubMed] [Google Scholar]
- 41.Andrade-Valenca LP, Valenca MM, Velasco TR, et al. Mesial temporal lobe epilepsy: clinical and neuropathologic findings of familial and sporadic forms. Epilepsia. 2008;49:1046–1054. doi: 10.1111/j.1528-1167.2008.01551.x. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed Z, Asi YT, Lees AJ, Revesz T, Holton JL. Identification and quantification of oligodendrocyte precursor cells in multiple system atrophy, progressive supranuclear palsy and Parkinson’s disease. Brain Pathol. 2013;23:263–273. doi: 10.1111/j.1750-3639.2012.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Louis ED, Faust PL, Ma KJ, Yu M, Cortes E, Vonsattel JP. Torpedoes in the cerebellar vermis in essential tremor cases vs. controls. Cerebellum. 2011;10:812–819. doi: 10.1007/s12311-011-0291-0. [DOI] [PubMed] [Google Scholar]
- 44.Koziol LF, Budding D, Andreasen N, et al. Consensus Paper: The Cerebellum’s Role in Movement and Cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]