Abstract
Cancer cells rewire their metabolism to promote growth, survival, proliferation, and long-term maintenance. The common feature of this altered metabolism is increased glucose uptake and fermentation of glucose to lactate. This phenomenon is observed even in the presence of completely functioning mitochondria and together is known as the Warburg Effect. The Warburg Effect has been documented for over 90 years and extensively studied over the past 10 years with thousands of papers reporting to have established either its causes or its functions. Despite this intense interest, the function of the Warburg Effect remains unclear. Here, we analyze several proposed biological explanations for the Warburg Effect, emphasize their rationale, and discuss their controversies.
Keywords: Warburg Effect, ATP synthesis, microenvironment acidification, ROS, chromatin remodeling
Glucose Metabolism and the Warburg Effect
The metabolism of glucose, the central macronutrient, allows for energy to be harnessed in the form of ATP through the oxidation of its carbon bonds. This process is essential for sustaining all mammalian life. In mammals, the end product can be lactate or, upon full oxidation of glucose via respiration in the mitochondria, CO2. In tumors and other proliferating or developing cells, the rate of glucose uptake dramatically increases and lactate is produced, even in the presence of oxygen and fully functioning mitochondria. This process, known as the Warburg Effect, has been studied extensively (Figure 1). However, after careful inspection, it becomes apparent that its benefits for cell growth and survival are not yet resolved. This analysis will focus on several proposals for its function, and in each case we discuss their appeal as well as their drawbacks. Before our discussion of each proposal, we first introduce the Warburg Effect in a historical context with an emphasis on lesser-appreciated aspects of its conceptual development. It is our hope that this retrospective brings additional context to current ideas in cancer metabolism.
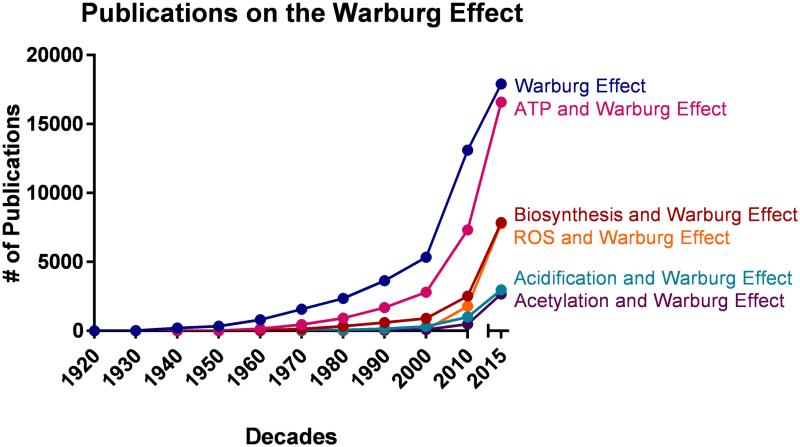
Figure 1. The frequency of publications on the Warburg Effect from the 1920s-2010s.
The Warburg Effect has been studied extensively since the 1920s with a surge in the number of publications from the 2000s to today. Many of the proposed functions of the Warburg Effect have also gained vastly renewed interest. Although energy (ATP), biosynthesis, and ROS have been intricately studied in the context of the Warburg Effect, acidification and acetylation have only recently gained attention.
Historical perspectives of the Warburg Effect
In the 1920s, Otto Warburg and colleagues made the observation that tumors were taking up enormous amounts of glucose compared to what was seen in the surrounding tissue. Additionally, glucose was fermented to produce lactate even in the presence of oxygen, thus the term aerobic glycolysis [1, 2]. However, it was also noted that respiration alone could maintain tumor viability. Therefore, it was concluded that in order to kill tumor cells by depriving them of energy, both glucose and oxygen had to be eliminated [3]. Subsequently, in 1929, an English biochemist, Herbert Crabtree, extended Warburg’s work and studied the heterogeneity of glycolysis in tumor types. He confirmed Warburg’s findings, but further discovered that the magnitude of respiration in tumors was variable with many tumors exhibiting a substantial amount of respiration [4]. Therefore, Crabtree concluded that not only do tumor cells exhibit aerobic glycolysis, but that there is also variability in fermentation presumably due to environmental or genetic influences.
Contrary to the findings of these previous works and for reasons unclear to these authors, Warburg later proposed that dysfunctional mitochondria is the root of aerobic glycolysis [5]. Warburg further hypothesized that this event is the primary cause of cancer. This phenomenon was then termed the Warburg Effect in the early 1970s by Efraim Racker, who also pointed out that previous data show respiratory capability of tumors. Racker developed his own theories about the origins of the Warburg Effect ranging from imbalances in intracellular pH to defects in ATPase activity [6]. It was later observed by Racker, Jeffrey Flier and Morris Birnbaum that aerobic glycolysis was a controllable process that can be directly regulated by growth factor signaling. By that time, the discovery of oncogenes led to the conclusion that aberrant regulation of growth factor signaling is an initiating event in oncogenesis. Thus, their observations brought newfound significance to Warburg’s hypothesis in cancer biology [7-10]. Nevertheless, it remained unclear whether the Warburg Effect was a bystander in cancer pathogenesis until more recently, when genetic and pharmacological studies conclusively showed that the Warburg Effect was required for tumor growth [11, 12]. Coming back to the original findings on tumor metabolism, it is now apparent that targeting both aerobic glycolysis and mitochondrial metabolism may be required [13-16]. Throughout this history, its functions have remained controversial. Here, we discuss several of the major proposals and argue that the functions of the Warburg Effect for tumor growth even today remain unknown.
Warburg Effect and rapid ATP synthesis
Per unit of glucose, aerobic glycolysis is an inefficient means of generating ATP compared to the amount obtained by mitochondrial respiration [17, 18]. However, the rate of glucose metabolism through aerobic glycolysis is higher such that the production of lactate from glucose occurs 10-100 times faster than the complete oxidation of glucose in the mitochondria. In fact, the amount of ATP synthesized over any given period of time is comparable when either form of glucose metabolism is utilized [19]. Thus, a reasonable hypothesis on the reason that cancer employs aerobic glycolysis should account for this inherent difference in kinetics.
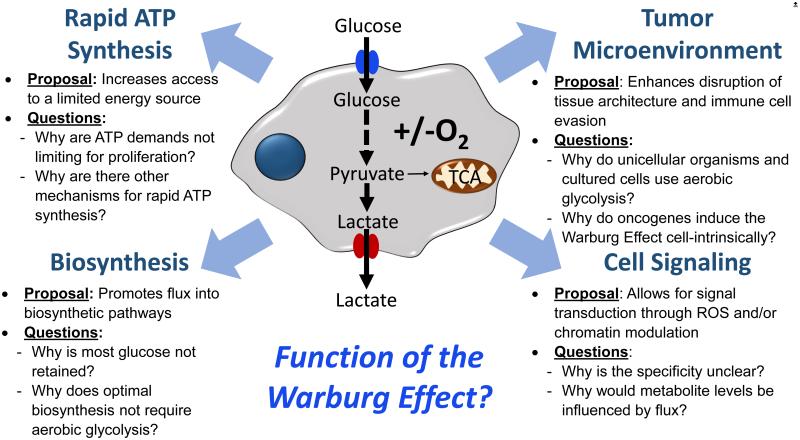
Theoretical calculations using evolutionary game theory support that cells with a higher rate, but lower yield, of ATP production may gain a selective advantage when competing for shared and limited energy resources (Figure 2, Key Figure) [20, 21]. In fact, tumor microenvironments have limited availability of glucose and undergo competition for nutrients with stromal cells and the immune compartment [22, 23]. Additional support is found in a recent study that showed when changes to the cellular environment were induced to greatly increase ATP demand by altering the demand of ATP-dependent membrane pumps, aerobic glycolysis increased rapidly and oxidative phosphorylation remained constant [24]. This finding provides additional rationale for the function of the Warburg Effect to be supporting the rapid production of ATP that can be rapidly tuned to support the demand for ATP synthesis.
Figure 2. Summary of the proposed functions of the Warburg Effect.
The Warburg Effect is defined as an increase in the rate of glucose uptake and preferential production of lactate, even in the presence of oxygen. Each of these functions have been hypothesized to be the function of the Warburg Effect.
Despite this attractive proposal, there are difficulties. Simple empirical calculations indicate that the amount of ATP required for cell growth and division may be much less than that required for normal cellular maintenance [18, 25]. Thus, ATP demand may never reach limiting values during tumor cell growth. Furthermore, the mechanisms that are available to other cell types in cases of rapid ATP demand are present in tumor cells as well. For example, rapid ATP synthesis from creatine kinases in exercised muscle or adenylate kinase under hormonal changes are present in most tumor cells and should be able to meet ATP demand. Thus further studies are needed to show whether this mechanism can account for the role of aerobic glycolysis.
Warburg Effect and biosynthesis
The Warburg Effect has been proposed to be an adaptation mechanism to support the biosynthetic requirements of uncontrolled proliferation (Figure 2, Key Figure). In this scenario, the increased glucose consumption is used as a carbon source for anabolic processes needed to support cell proliferation [17, 26-32]. This excess carbon is used for the de novo generation of nucleotides, lipids, and proteins and can be diverted into multiple branching pathways that emanate from glycolysis. One example is the diversion of glycolytic flux into de novo serine biosynthesis through the enzyme phosphoglycerate dehydrogenase (PHGDH) [18]. In addition to the usage of additional carbon from enhanced glucose metabolism for cellular building blocks, a now famous argument is that rather than having a rate-limiting demand for ATP, proliferating cells are in greater need of reducing equivalents in the form of NADPH. Increased glucose uptake allows for greater synthesis of these reducing equivalents in the oxidative branch of the pentose phosphate pathway, which are then used in reductive biosynthesis, most notably in de novo lipid synthesis [17, 33].
Another proposed mechanism to account for the biosynthetic function of the Warburg Effect is the regeneration of NAD+ from NADH in the pyruvate to lactate step that completes aerobic glycolysis. In this scenario, NADH that is produced by glyceraldehyde phosphate dehydrogenase (GAPDH) must be consumed to regenerate NAD+ to keep glycolysis active. This high rate of glycolysis allows for supply lines to remain open that can, for example, siphon 3-phosphoglycerate (3PG) to serine for one-carbon metabolism-mediated production of NADPH and nucleotides [17, 25]. These proposals together conclude that the Warburg Effect supports a metabolic environment that allows for the rapid biosynthesis to support growth and proliferation.
Furthermore, others have proposed that aerobic glycolysis is a tradeoff to support biosynthesis [34, 35]. In these scenarios, the inefficient way of making ATP occurs as a cost of maintaining high fluxes through anabolic pathways. These pathways require increased expression of biosynthesis genes such as those involved in nucleotide and lipid metabolism and the tradeoff occurs by limiting the use of mitochondria in order to preserve high expression of biosynthetic enzymes in the face of limited protein number that can be made. Another scenario of such a tradeoff comes from the idea that the physical volume available per cell may limit mitochondria number and thus any energy and biomass that exceed the limited mitochondrial capacity needs to be produced from aerobic glycolysis [36-38]. This concept has been termed the solvent capacity constraint. In both of these cases, the Warburg Effect is an adaptation to support biomass production in the face of limited options for ATP generation.
The attractiveness of this proposal in part comes from its ability to provide a simple explanation for the apparent correlation between aerobic glycolysis and cell growth and proliferation. Furthermore, it appears intuitive to some that the branching pathways from glycolysis would be used to a greater extent during the Warburg Effect since rate of glycolysis is larger and lactate production in this case would serve to regenerate NAD+ to allow for glycolysis to continue. Also, the requirements of NADPH for lipid generation can be summarized in a very simple chemical equation showing that the demand for NADPH is higher than that of ATP for biosynthesis [17].
However, there are major limitations for this proposed function of the Warburg Effect. First, during aerobic glycolysis, most of the carbon is not retained and is instead excreted as lactate [25]. In fact, the overall equation of 1 glucose molecule being converted into 2 lactate molecules with no overall gain or loss of NAD+ and NADH leaves no room for biomass. That is, due to the stoichiometry of glycolysis, biomass production is mutually exclusive with lactate generation and it is not possible for the regeneration of NAD+ by lactate alone to account for biosynthesis. Thus, the avenues that lead to the biosynthesis from glucose occur in the complete absence of making lactate which is the hallmark of the Warburg Effect. Also, it is now widely accepted that mitochondria are key components of the biosynthetic program whose substrates in the TCA cycle are used for nucleotide, amino acid, and lipid biosynthesis [39, 40]. In light of this evidence, it remains difficult to fathom how the Warburg Effect can directly promote biosynthesis.
Regarding proposals that define the Warburg Effect as a tradeoff to promote biosynthesis, recent estimates from quantitative proteomics show that the cost of protein production for conducting aerobic glycolysis is enormous. In fact, cells devote as much as 10% of their entire proteome and half of all of their metabolic genes to produce proteins involved in glycolysis [41]. In contrast, biosynthetic programs in cells require much lower amounts of protein. Thus, the cost of producing proteins for aerobic glycolysis is as large, if not larger, than the cost of producing proteins for biosynthesis. These proposals are further challenged by the evidence showing that mitochondrial functions occur concomitantly with the Warburg Effect and thus limiting mitochondrial activity appears not to occur during the Warburg Effect. Ultimately, further research is needed to elucidate whether the Warburg Effect functions to support biosynthetic programs.
Warburg Effect and the tumor microenvironment
In contrast to the cell-intrinsic functions described in the previous sections, the Warburg Effect may present an advantage for cell growth in a multicellular environment. Acidification of the microenvironment and other metabolic crosstalk are intriguing possibilities. Elevated glucose metabolism decreases the pH in the microenvironment due to lactate secretion (Figure 2, Key Figure) [42]. The potential benefits of acidosis to cancer cells are multifold. An acid-mediated invasion hypothesis suggests that H+ ions secreted from cancer cells diffuse into the surrounding environment and alter the tumorstroma interface allowing for enhanced invasiveness [42, 43]. A recent study showed that tumor-derived lactate is a contributor to M2 tissue-associated macrophage (TAM) polarization [44]. Also as briefly mentioned previously, the availability of glucose appears to be a result of direct competition between tumor and tumor infiltrating lymphocytes (TIL) [22, 23]. The high rates of glycolysis limit the availability of glucose for TILs that require sufficient glucose for their effector functions. Supporting this proposal is direct evidence indicating that targeting aerobic glycolysis in the tumor has the added benefit of increasing the supply of glucose to TILs and thus boosting their main function, which is to eradicate the tumor cells. Together, this body of evidence indicates that tumor cells can communicate with cells in the immune system to support pro-tumor immunity.
It is likely that the Warburg Effect provides an overall benefit that supports a tumor microenvironment conducive to cancer cell proliferation. However, the Warburg Effect is thought to be an early event in oncogenesis that is an immediate consequence of an initial oncogenic mutation, such as that of KRAS in pancreatic cancer or BRAF in melanoma thus occurring before cell invasion and in benign and early stage lesions as well [45, 46]. Another issue is that in conditions completely isolated from the environment such as in the growth phase of unicellular yeast, the Warburg Effect remains the choice of energy metabolism from glucose [38]. Altogether, these data suggest that non cell-intrinsic functions of the Warburg Effect are insufficient to entirely explain its functions.
The Warburg Effect and cell signaling
We and others have proposed that the Warburg Effect confers direct signaling functions to tumor cells [18, 39, 47-49]. This proposal is particularly attractive since it identifies a direct causal role of altered glucose metabolism in promoting tumorigenesis through this signal transduction affecting other cellular processes. Two areas of signaling function are the generation and modulation of reactive oxygen species (ROS) and the modulation of chromatin state. Other studies have identified additional possible signaling mechanisms [23, 50].
Maintaining the appropriate balance of ROS is essential [51]. Excessive ROS damages cell membranes, nucleic acids, and has other deleterious effects. Insufficient ROS disturbs signaling processes that are beneficial for cell proliferation, such as by inactivating phosphatase and tensin homolog (PTEN) and tyrosine phosphatases. The Warburg Effect causes alterations in mitochondrial redox potential, ultimately changing ROS generation [18].
An important determinant of redox potential in cells is the NADH that is available in the mitochondria for electron transport. Cellular mechanisms to maintain redox homeostasis are in place when glycolysis rates fluctuate. Up to a certain extent of glycolysis, the malate-aspartate shuttle through the mitochondria is able to restore the NADH imbalance [18]. However, when glycolysis rates are faster than what can be accommodated by the malate-aspartate shuttle, the conversion of pyruvate into lactate via lactate dehydrogenase (LDH) is able to regenerate NAD+. This process may also affect the homeostasis of ROS generation by affecting the concentration of reducing equivalents in the mitochondria (Figure 2, Key Figure) [18, 52]. This consequence of the Warburg Effect may be directly involved in oncogene-induced senescence (OIS) [53]. OIS has a tumor-suppressive cellular function and a recent study has reported that increased glucose oxidation through pyruvate dehydrogenase (PDH) can regulate OIS. This finding shows that the redox balance of NADH may contribute to direct signaling roles for the Warburg Effect.
In addition, metabolic pathways that stimulate redox homeostasis are upregulated alongside the Warburg Effect. For example, the pentose phosphate pathway coming from glycolysis generates NADPH. De novo serine metabolism, which feeds into the one-carbon metabolism, produces NADPH and glutathione, which modulate ROS levels [54, 55]. Together these findings provide direct biochemical links between aerobic glycolysis and ROS availability that could in turn affect myriad signaling processes.
In addition to cell signaling through ROS, a signaling link between glucose metabolism and histone acetylation has been well documented [56-59]. The status of chromatin structure is responsible for regulating different cellular functions including DNA repair and gene transcription. It has been established that acetyl-CoA, the substrate for histone acetylation can be regulated by glucose flux [59]. Studies have shown that there is a direct link between cellular metabolism and regulation of growth genes and that intracellular acetyl-CoA levels may represent a widely conserved mechanism that promotes this important link [60]. The activity of ATP-citrate lyase, the enzyme responsible for converting citrate into acetyl-coA can influence histone acetylation levels [47]. Elevated levels of acetyl-CoA may be enough to drive cells into growth phase via histone acetylation [56]. Removal of glucose or reduction of ATP-citrate lyase results in loss of acetylation on several histones and causes decreased transcription of genes involved in glucose metabolism. This indicates that there is some interplay between glucose metabolism and histone acetylation. Supporting this idea, glycolytic metabolism has been found to impact chromatin structure [58].
In addition to histone acetylation responding to glucose availability in cells, deacetylation can be influenced by nutrient availability as well [39]. Deacetylation plays an important role in nutrient sensing and signaling since the activity of multiple deacetylases are modulated by NAD+ levels. More specifically, the ratio of NAD+/NADH increases in nutrient deprived conditions [39, 56, 57]. Therefore, both acetylation and deacetylation can be influenced by nutrient availability, indicating that their statuses may be consequences of the Warburg Effect. These multiple lines of evidence point to glycolysis having cell signaling functions.
However, difficulties also limit this proposal from being the general mechanism that benefits cancer cells by undergoing aerobic glycolysis. One such limitation is that it is hard to imagine how molecular specificity arises through such a gross global signaling mechanism. In contrast to, for example, growth factor signaling in which ligand-binding to a substrate induces conformational and enzymatic activity changes that affect specific cellular processes, a mechanism whereby the state of glycolysis signals to other cellular processes lacks obvious sources of specificity. Another limitation is that such proposals typically lack falsifiability. This means it is extremely difficult to design experiments to conclusively show that a specific signaling mechanism, such as chromatin structure modulation, directly comes from the status of glucose metabolism as the key benefit for aerobic glycolysis. One reason for this is that the biochemical interaction occurs rapidly but the cellular phenotypic alterations evolve over much longer times resulting in many confounding factors that occur along the way. Genetic models that could test these hypotheses are difficult to conceive, and other experiments lack the ability to test whether specific cellular outcomes occur through such signaling mechanisms and not through indirect means. The extent to which these general features, such as ROS signaling homeostasis and chromatin structure organization, are key events in tumorigenesis also remains unclear [61]. In the future, such specificity and ability to experimentally test these hypotheses may come from observing quantitative aspects of the mechanism as has been shown in other studies of signal transduction. Experiments that can precisely control the levels of acetyl-CoA and ROS could allow for one to decouple many of the downstream effects of the Warburg Effect.
Concluding remarks
Extensive research on the Warburg Effect and its functions in cancer cells have advanced our understanding of its causes and requirements for tumor cell proliferation [29, 52]. However, we argue that it has left us with a surprising lack of clarity regarding its ontology. These uncertainties should challenge us to better understand its function in promoting tumor growth. It is likely we will require a better understanding of the biology of Warburg Effect if therapeutic advances are to be made in treating and preventing cancer using dietary and pharmacological intervention in metabolism, and in using glucose metabolism to manipulate the immune system, which are currently subjects of intense interest.
Outstanding Questions[NN1].
Does the Warburg Effect promote the development of cancer or is it a dependency imposed by other cancer-promoting processes?
How can experimental systems be devised that can conclusively test the proposals for the function of the Warburg Effect?
Does resolution of any given function of the Warburg Effect have immediate therapeutic consequences?
Does the function of the Warburg Effect provide insights into its role in tumor evolution?
Do the requirements of the Warburg Effect provide clues for its function?
Trends.
Both glycolytic and mitochondrial metabolism are essential for cell proliferation in both past and present conceptions of the Warburg Effect.
Numerous proposals of the Warburg Effect functions have emerged over the years.
Each of the proposed functions of the Warburg Effect are attractive, but also raise unanswered questions.
Signal transduction functions for the Warburg Effect appear likely, but are difficult to test experimentally.
Acknowledgements
This work was supported by awards from the National Institute of Health (R00CA168997, R01CA193256 and T32GM007273), the National Science Foundation, and the Sloan Foundation. JWL acknowledges Donald McDonnell and numerous other colleagues, notably Lew Cantley, for helpful discussions on the history of the Warburg Effect.
Glossary
- Aerobic glycolysis
enhanced rate of glycolysis and fermentation to lactate that occurs in the presence of functioning mitochondria.
- ATP
adenosine triphosphate. The source of energy in cells.
- Flux
the rate of flow in a metabolic pathway from one metabolite to another.
- NADH
reduced nicotinamide adenine dinucleotide. A reducing agent involved in redox reactions that is responsible for the transfer of electrons. NADH is a key reducing equivalent in glycolysis and the mitochondria.
- NADPH
reduced nicotinamide adenine dinucleotide phosphate. NADPH is most well-known for its use in reductive biosynthesis and regenerating reduced glutathione.
- ROS
reactive oxygen species. Chemically reactive molecules that contain oxygen radicals.
- Warburg Effect
another name for aerobic glycolysis. Coined by Efraim Racker in the early 1970s.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warburg O. The metabolism of carcinoma cells. The Journal of Cancer Research. 1925;9(1):148–163. [Google Scholar]
- 2.Warburg O, Posener K, Negelein E. Ueber den stoffwechsel der tumoren. Biochemische Zeitschrift. 1924;152(1):319–344. [Google Scholar]
- 3.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. The Journal of general physiology. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crabtree HG. Observations on the carbohydrate metabolism of tumours. Biochemical journal. 1929;23(3):536. doi: 10.1042/bj0230536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 6.Racker E. Bioenergetics and the Problem of Tumor Growth: An understanding of the mechanism of the generation and control of biological energy may shed light on the problem of tumor growth. American scientist. 1972:56–63. [PubMed] [Google Scholar]
- 7.Boerner P, Resnick RJ, Racker E. Stimulation of glycolysis and amino acid uptake in NRK-49F cells by transforming growth factor beta and epidermal growth factor. Proc Natl Acad Sci U S A. 1985;82(5):1350–3. doi: 10.1073/pnas.82.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flier JS, et al. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987;235(4795):1492–5. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- 9.Birnbaum MJ, Haspel HC, Rosen OM. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987;235(4795):1495–8. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- 10.Hiraki Y, Rosen OM, Birnbaum MJ. Growth factors rapidly induce expression of the glucose transporter gene. J Biol Chem. 1988;263(27):13655–62. [PubMed] [Google Scholar]
- 11.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9(6):425–34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 12.Shim H, et al. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proceedings of the National Academy of Sciences. 1998;95(4):1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birsoy K, et al. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell. 2015;162(3):540–51. doi: 10.1016/j.cell.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaveny CA, et al. Broad Anti-tumor Activity of a Small Molecule that Selectively Targets the Warburg Effect and Lipogenesis. Cancer Cell. 2015;28(1):42–56. doi: 10.1016/j.ccell.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sullivan LB, et al. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell. 2015;162(3):552–63. doi: 10.1016/j.cell.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viale A, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–32. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell metabolism. 2011;14(4):443–451. doi: 10.1016/j.cmet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shestov AA, et al. Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. Elife. 2014;3:e03342. doi: 10.7554/eLife.03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeiffer T, Schuster S, Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292(5516):504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 21.Slavov N, et al. Constant growth rate can be supported by decreasing energy flux and increasing aerobic glycolysis. Cell reports. 2014;7(3):705–714. doi: 10.1016/j.celrep.2014.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang C-H, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015 doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho P-C, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015 doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein T, et al. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab. 2014;2(7) doi: 10.1186/2049-3002-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annual review of cell and developmental biology. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 26.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330(6009):1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 27.DeBerardinis RJ, et al. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell metabolism. 2008;7(1):11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Dang CV. Links between metabolism and cancer. Genes & development. 2012;26(9):877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nature Reviews Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 30.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nature Reviews Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 31.Patra KC, et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell. 2013;24(2):213–28. doi: 10.1016/j.ccr.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nature cell biology. 2015;17(4):351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer cell. 2012;21(3):297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hermsen R, et al. A growth-rate composition formula for the growth of E.coli on co-utilized carbon substrates. Mol Syst Biol. 2015;11(4):801. doi: 10.15252/msb.20145537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hui S, et al. Quantitative proteomic analysis reveals a simple strategy of global resource allocation in bacteria. Mol Syst Biol. 2015;11(1):784. doi: 10.15252/msb.20145697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shlomi T, et al. Genome-scale metabolic modeling elucidates the role of proliferative adaptation in causing the Warburg effect. PLoS Comput Biol. 2011;7(3):e1002018. doi: 10.1371/journal.pcbi.1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vazquez A, et al. Catabolic efficiency of aerobic glycolysis: the Warburg effect revisited. BMC Syst Biol. 2010;4:58. doi: 10.1186/1752-0509-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molenaar D, et al. Shifts in growth strategies reflect tradeoffs in cellular economics. Mol Syst Biol. 2009;5:323. doi: 10.1038/msb.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wellen KE, Thompson CB. A two-way street: reciprocal regulation of metabolism and signalling. Nature reviews Molecular cell biology. 2012;13(4):270–276. doi: 10.1038/nrm3305. [DOI] [PubMed] [Google Scholar]
- 40.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nature reviews Cancer. 2013;13(4):227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madhukar NS, Warmoes MO, Locasale JW. Organization of enzyme concentration across the metabolic network in cancer cells. PLoS One. 2015;10(1):e0117131. doi: 10.1371/journal.pone.0117131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estrella V, et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer research. 2013;73(5):1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatenby RA, Gawlinski ET. A reaction-diffusion model of cancer invasion. Cancer research. 1996;56(24):5745–5753. [PubMed] [Google Scholar]
- 44.Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014 doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shain AH, et al. The Genetic Evolution of Melanoma from Precursor Lesions. New England Journal of Medicine. 2015;373(20):1926–1936. doi: 10.1056/NEJMoa1502583. [DOI] [PubMed] [Google Scholar]
- 47.Wellen KE, et al. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wellen KE, Thompson CB. Cellular metabolic stress: considering how cells respond to nutrient excess. Molecular cell. 2010;40(2):323–332. doi: 10.1016/j.molcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hamanaka RB, Chandel NS. Warburg effect and redox balance. Science. 2011;334(6060):1219–1220. doi: 10.1126/science.1215637. [DOI] [PubMed] [Google Scholar]
- 50.Chang C-H, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153(6):1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48(2):158–67. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Locasale JW. The consequences of enhanced cell-autonomous glucose metabolism. Trends in Endocrinology & Metabolism. 2012;23(11):545–551. doi: 10.1016/j.tem.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 53.Kaplon J, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498(7452):109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 54.Mehrmohamadi M, et al. Characterization of the usage of the serine metabolic network in human cancer. Cell reports. 2014;9(4):1507–1519. doi: 10.1016/j.celrep.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fan J, et al. Human Phosphoglycerate Dehydrogenase Produces the Oncometabolite d-2-Hydroxyglutarate. ACS chemical biology. 2014 doi: 10.1021/cb500683c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu C, Thompson CB. Metabolic regulation of epigenetics. Cell metabolism. 2012;16(1):9–17. doi: 10.1016/j.cmet.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cluntun AA, et al. The rate of glycolysis quantitatively mediates specific histone acetylation sites. Cancer & metabolism. 2015;3(1):1–12. doi: 10.1186/s40170-015-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu X-S, Little JB, Yuan Z-M. Glycolytic metabolism influences global chromatin structure. Oncotarget. 2015;6(6):4214. doi: 10.18632/oncotarget.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Evertts AG, et al. Quantitative dynamics of the link between cellular metabolism and histone acetylation. Journal of Biological Chemistry. 2013;288(17):12142–12151. doi: 10.1074/jbc.M112.428318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cai L, et al. Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Molecular cell. 2011;42(4):426–437. doi: 10.1016/j.molcel.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeNicola GM, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475(7354):106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]