Abstract
Management of patients with glioblastoma (GBM) often includes radiation (RT) and temozolomide (TMZ). The association between severe treatment-related lymphopenia (TRL) after the standard chemoradiation and reduced survival has been reported in GBM patients with the median age of 57. Similar findings were described in patients with head and neck, non-small cell lung, and pancreatic cancers. This retrospective study is designed to evaluate whether elderly GBM patients (age ≥65) develop similar TRL after RT/TMZ and whether such TRL is associated with decreased survival. Serial total lymphocyte counts (TLC) were retrospectively reviewed in patients (age ≥65) with newly diagnosed GBM undergoing RT/ TMZ and associated with treatment outcomes. Seventy-two patients were eligible: median KPS 70, median age 71 years (range 65–86) with 56 % of patients >70 years, 53 % female, 31 % received RT ≤45 Gy. Baseline median TLC was 1100 cells/mm3 which fell by 41 % to 650 cells/ mm3 2 months after initiating RT/TMZ (p < 0.0001). Patients with TLC <500 cells/mm3 at 2 months had a shorter survival than those with higher TLCs with a median overall survival of 4.6 versus 11.6 months, respectively. Multivariate analysis revealed a significant association between TRL and survival (HR 2.76, 95 % CI 1.30–5.86, p = 0.008). Treatment-related lymphopenia is frequent, severe, and an independent predictor for survival in elderly patients with GBM. These findings add to the body of evidence that immunosuppression induced by chemoradiation is associated with inferior clinical outcomes. Prospective studies are needed to confirm these findings suggesting that immune preservation is important in this cancer.
Keywords: Lymphopenia, Glioblastoma, Radiation, Chemotherapy, Treatment-related toxicities
Introduction
The concept that the immune system plays a pivotal role in the prevention and control of cancer is not new to the literature. This has led to an explosion in immunotherapy research and the FDA approval of sipuleucel-T, nivolumab, pembrolizumab and ipilimumab. Lymphocytes are one of the main components of human immune system and they are the essential effector cells in the immune response to cancer [1]. The presence of adequate circulating lymphocytes appears to be correlated with the pharmacological efficacy of the immune checkpoint blocking agent ipilimumab and cancer vaccine sipuleucel-T [2–4]. Furthermore, the clinical consequences of lymphopenia have been well described. Pre-treatment lymphopenia has been found to be a poor prognostic indicator in many cancers [5, 6]. The extent of lymphocytic infiltration of solid tumors on pathology has also been correlated with overall survival (OS) [7–10].
More recently, lymphopenia following initiation of treatment with chemoradiation has been explored in various cancers with findings showing inferior OS. Most notably, a prospective study consisting of 96 patients with high grade glioma examined the association between CD4 cell counts and clinical outcomes. These patients received three highly lymphotoxic therapies: radiation (RT), glucocorticoids, and temozolomide (TMZ). Total lymphocyte counts (TLC) and CD4 cell counts were normal prior to initiation of antineoplastic therapy. However, 2 months following RT and TMZ, 40 % of these patients suffered from severe grade III-IV lymphopenia with a TLC <500 cells/mm3 and CD4 count <200 cells/mm3 with persistence of severe lymphopenia for more than a year. Multivariate analysis revealed these significant reductions in CD4 counts at 2 months was independently associated with inferior OS due primarily to tumor progression [11]. The association of treatment related lymphopenia (TRL) and decreased OS has also been observed in non-small cell lung cancer, head and neck cancer, and pancreatic cancer [12–17].
Recent studies suggest that RT may play a major role in lymphopenia. Yovino et al. used a mathematical model to estimate the RT exposure of circulating lymphocytes during RT to the brain. They found that over 95 % of circulating cells received a lymphocyte toxic dose after the standard 30 fractions of RT treatment [18]. In addition, a study of 183 patients with glioblastoma (GBM) evaluated for predictors of lymphopenia, and found that older age, female sex, lower baseline TLC, and higher brain volume receiving 25 Gy were predictors of severe lymphopenia. In this study, 29 % of patients developed severe TRL with TLC less than 500 cell/mm3 [19].
With the exploration of immunotherapy in the treatment of GBM, the literature on TRL becomes particularly relevant. However, the current literature on TRL only sheds light on patients with a median age of 57, and does not address elderly patients (age ≥70 years) with GBM [11]. The current standard treatment for GBM is surgery followed by concurrent RT/TMZ and adjuvant monthly TMZ for patients age 18–70 years old [20]. At this time, there is no standard treatment for elderly patients with GBM. Most elderly patients with a good KPS haven been treated with 6 weeks standard RT/TMZ, but others have received 3–4 weeks short course of RT/TMZ, TMZ alone, or hypofractionated RT due to the morbidity of standard RT/ TMZ. A recent study suggested that survival was not inferior with TMZ and hypofractionated RT than with standard RT in patients over age 70 [21]. Considering the toxicities of standard RT and TMZ in the elderly, and the potential for treatment with immunotherapy it is imperative that the degree of TRL in the elderly be explored, along with the associated effects on OS.
This retrospective study was performed to determine if elderly patients with newly diagnosed GBM also develop significant lymphopenia after chemoradiation and if this TRL is associated with reduced OS in this elderly patient population.
Patient and methods
Study population
This study was reviewed and approved by the Institutional Review Board of Washington University. Patients were retrospectively identified using a database of consecutive GBM patients at Washington University School of Medicine/Barnes-Jewish Hospital. The following eligibility criteria were required: (1) newly diagnosed GBM between 2000 and 2013, (2) ≥65 years of age, (3) received at least RT therapy at our institution during their course of treatment, and (4) baseline and follow-up complete blood counts (CBC) performed at Washington University School of Medicine/Barnes-Jewish Hospital and thus accessible through the electronic medical record.
Treatment and total lymphocyte count examination
Prognostic factors in GBM including KPS, MGMT methylation status, and extent of surgical resection were obtained from the patient’s medical record. More specifically, the surgical procedure performed was categorized as biopsy, subtotal resection, or gross total resection. In addition, baseline steroid use, anticonvulsant use, chemotherapy treatments, and total RT doses were collected. Baseline steroid use was defined as the use of any glucocorticoid before initiation of therapy. Monthly CBC that were routinely performed after initiation of RT and chemotherapy were collected.
Total lymphocyte counts were collected before beginning chemoradiation and monthly thereafter for a total of 12 months. Baseline TLCs were obtained for all patients, which was defined as the most recent TLC obtained just prior to initiation of RT therapy. Baseline TLCs were classified as normal (≥1000 cells/mm3) or abnormal (<1000 cell/mm3). All subsequent TLCs were obtained during treatment and/or observation in one-month intervals as available in the patient’s record. Considering the literature has provided evidence that the nadir of TLCs during chemoradiation treatment is around 2 months, in patients without a 2-month TLC the TLC at 1 month was utilized [11]. Severity of TRL following initiation of antineoplastic therapy was classified based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE—Version 4.03).
The TLCs at 2 months after the initiation of RT and chemotherapy were dichotomized to CTCAE grade 0-II versus grade III-V for the relevant analyses. Dichotomization between grade 0-II and III-V was performed as grade III (TLC <500 cells/mm3) and above adverse events are defined as severe or medically significant (CTCAE—Version 4.03). Furthermore, the literature on TRL in high grade gliomas and other solid tumors have found an association with TLC <500 cells/mm3 at 2 months and OS [11–17]. Overall survival time was measured from the date of diagnosis to the date of death due to any cause, and those subjects alive at the time of last follow-up were censored.
Statistical Analysis
Patient baseline characteristics were summarized using descriptive statistics, and the difference between lymphopenia groups were compared using Chi square test, Fisher’s exact test, Student’s t test or Mann–Whitney rank-sum test as appropriate. Survival probability was estimated using the Kaplan–Meier product limit method and compared using log-rank test. The confidence interval of median survival time was constructed by the method of Brookmeyer–Crowley. A univariate proportional hazard Cox model was used to assess an association between potential prognostic factors and OS. To assess whether lymphopenia was an independent predictor of survival, a multivariate Cox model was constructed using all other prognostic factors that had attained a p value less than 0.1 in the univariate analyses. All analyses were two-sided and significance was set at a p value of 0.05. Statistical analyses were performed using SAS 9.3 (SAS Institutes, Cary, NC).
Results
Baseline characteristics of patients
Seventy-two patients met the required eligibility criteria. Baseline demographic information for these patients is provided in Table 1. The median age of the patients was 71 years (range 65–86) with 56 % of patients greater than 70 years of age. Fifty-three percent of the patients were female and 97 % were Caucasian with a median KPS of 70. Forty-two percent of patients underwent a gross total resection and 15 patients were found to be positive for MGMT methylation. Thirty-one percent of patients received a RT dose less than 45 Gy and 90 % of patients received concurrent TMZ. Prior to the initiation of chemoradiation therapy, 85 % of patients were taking glucocorticoids (Table 1).
Table 1.
Baseline characteristics of all patients and those with lymphocyte counts above and below 500 cells/mm3 at 2 months
| All patients (N = 72) | Patients with lymphocyte counts ≥500 at 2 months (N = 57) | Patients with lymphocyte counts <500 at 2 months (N = 15) | p Value | |
|---|---|---|---|---|
| Demographic data | ||||
| Age: median (range) | 71 (65–86) | 71 (65–86) | 71 (66–79) | 0.62 |
| Age: >70 years (%) | 40 (55.56) | 29 (50.88) | 11 (73.33) | 0.119 |
| Male: # (%) | 34 (47.22) | 28 (49.12) | 6 (440) | 0.529 |
| Caucasian: # (%) | 70 (97.22) | 55 (96.49) | 15 (100) | 0.462 |
| KPS ≤70: # (%) | 41 (56.94) | 33 (57.89) | 8 (53.33) | 0.751 |
| Baseline laboratory data | ||||
| Total lymphocyte count: median (range) | 1100 (300–3200) | 1100 (300–3200) | 800 (400–3200) | 0.057 |
| Lymphocyte ≥ 1000:# (%) | 41 (56.94) | 35 (61.4) | 6 (40) | 0.136 |
| WBC: median (range) | 9.9 (4.1–26.2) | 10.2 (4.1–26.2) | 9.5 (6.1–15.2) | 0.782 |
| Hematocrit: median (range) | 38.95 (25–49.9) | 39.2 (28.5–49.9) | 37.4 (25–44.4) | 0.398 |
| Platelet: median (range) | 209.5 (101–466) | 210 (101–466) | 205 (132–388) | 0.835 |
| Clinical data | ||||
| MGMT methylated: # (%) | 15 (20.83) | 15 (26.32) | 0 (0) | 0.057 |
| Gross total resection: # (%) | 30 (41.67) | 26 (45.61) | 4 (26.67) | 0.059 |
| Use of dexamethasone: # (%) | 61 (84.72) | 47 (82.46) | 13 (92.86) | 0.335 |
| Use of anticonvulsant: # (%) | 68 (94.44) | 54 (94.74) | 14 (93.33) | 0.833 |
| Radiation dose ≤ 45 Gy: # (%) | 22 (30.56) | 18 (31.58) | 4 (26.67) | 0.713 |
| Concurrent chemotherapy (TMZ): # (%) | 65 (90.28) | 50 (87.72) | 15 (100) | 0.15 |
Total lymphocyte counts over time
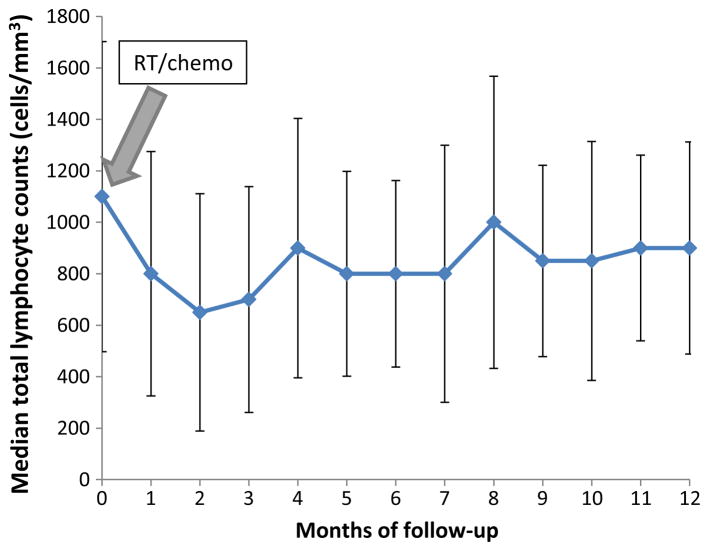
The median TLC for all patients prior to chemoradiation was 1100 cells/mm3 (range 300–3200 cells/mm3) with 57 % of patients having a baseline TLC ≥1000 cells/mm3 and 97 % with a baseline TLC ≥500 cells/mm3. Two months after beginning RT with TMZ (n = 65) or no TMZ (n = 7), the median TLC fell by 41 % (median 650 cells/ mm3, range 200–2200 cells/mm3) with 21 % of patients with a TLC<500 cells/mm3 (median 400 cells/mm3, range 200–400 cells/mm3) (Fig. 1). In addition, as shown in Fig. 1, the median TLC remained below 1000 cells/mm3 for the entire one-year observation period. Twenty patients had missing lymphocyte counts at 2 months and in these patients the TLCs at 1 month were used. As shown in Table 1, there were no significant differences appreciated in the baseline demographics, laboratory data, MGMT methylation status, extent of resection, RT dose, baseline steroid use, anticonvulsant use, or concurrent TMZ when comparing patients with TLCs ≥500 cells/mm3 to those with TLCs <500 cells/mm3 at 2 months.
Fig. 1.
Treatment related lymphopenia is common, severe, and long-lasting in elderly GBM patients treated with chemo/RT. Median TLC over a 12-month period after initiation of chemo/RT (n = 72). The median TLCs were normal prior to chemo/RT. However, 2 months after initiation of concurrent chemo/RT, the median TLC fell rapidly by 41 % (p < 0.0001)
Associations between patient characteristics and overall survival
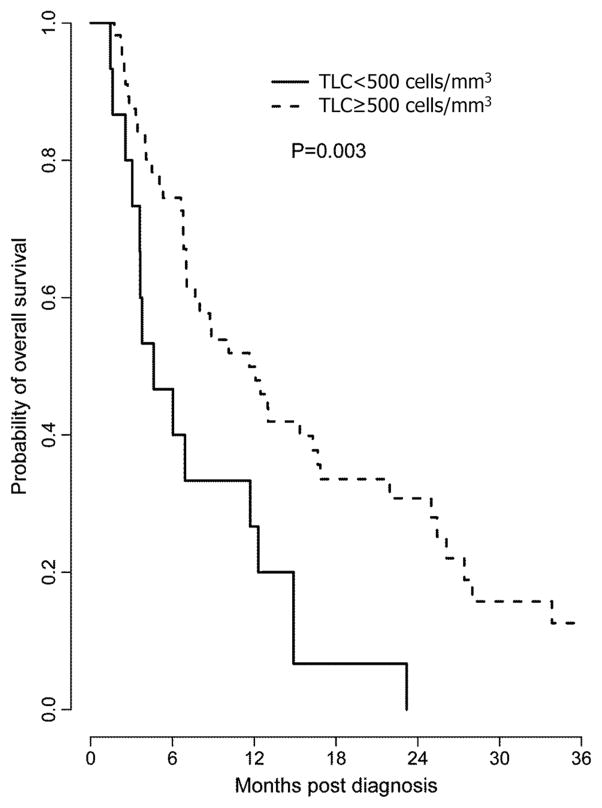
Table 2 presents univariate and multivariate associations between patient characteristics and OS in this elderly patient population. Not surprisingly, MGMT methylation and gross total resection were associated with better prognosis in both univariate and multivariate analyses. Interestingly, the median OS of patients who developed grade III–IV TRL (TLC <500 cells/mm3) 2 months after beginning RT and chemotherapy was only 4.6 months compared to 11.6 months in those patients who maintained their lymphocytes (TLC ≥500 cells/mm3) at 2 months with a p value of 0.003 in the univariate analysis. This association remained valid in the multivariate analysis with a hazard ratio of 2.76 and a p value of 0.008 after adjusting for extent of surgery, MGMT status, steroid use, and RT dose (HR 2.76, 95 %CI 1.30–5.86; p = 0.008) (Table 2). Kaplan–Meier survival curves of patients with TLC <500 and ≥500 cells/mm3 at 2 months after beginning chemoradiation are presented in Fig. 2. Tumor progression was the cause of death in the vast majority of patients. Interestingly, a RT dose ≤45 Gy was found to be associated with inferior outcomes. This may represent a selection bias given most elderly patients with poor KPS or multiple comorbidities are often treated with a shorter course of RT therapy.
Table 2.
Univariate and multivariate associations between patient characteristics and overall survival in elderly patients with newly diagnosed glioblastoma
| Characteristic | Hazard ratio (95 % CI) | p value |
|---|---|---|
| Univariate associations | ||
| Age: >70 versus ≤70 | 1.34 (0.79–2.26) | 0.275 |
| KPS: ≤70 versus > 70 | 1.30 (0.77–2.19) | 0.327 |
| Sex: female versus male | 1.22 (0.73–2.05) | 0.457 |
| MGMT: methylated versus unmethylated | 0.07 (0.02–0.27) | <.001 |
| Extent of surgery: gross total resection versus subtotal resection or biopsy only | 0.21 (0.11–0.38) | <.001 |
| Steroid use: yes versus no | 2.29 (0.98–5.36) | 0.049 |
| Radiation dose: >45 versus ≤45 Gy | 0.22 (0.12–0.40) | <.001 |
| Baseline total lymphocyte counts <1000 versus ≥1000 | 0.99 (0.58–1.67) | 0.959 |
| Total lymph count at 2 months post chemoradiation: <500 versus ≥500* | 2.46 (1.34–4.53) | 0.003 |
| Multivariate associations | ||
| Total lymph count at 2 months: < 500 versus ≥500 | 2.76 (1.30–5.86) | 0.008 |
| MGMT: methylated versus unmethylated | 0.21 (0.07–0.61) | 0.004 |
| Extent of surgery: gross total resection versus subtotal resection or biopsy only | 0.23 (0.11–0.484) | <.001 |
| Steroid use: yes versus no | 1.17 (0.44–3.11) | 0.747 |
| Radiation dose: >45 versus ≤45 Gy | 0.25 (0.11–0.53) | <.001 |
Lymphocyte count at 2 months is dichotomized at 500 cells/mm3 (per the CTCAE NIH grade III-IV treatment induced lymphopenia)
Fig. 2.
Severe treatment-related lymphopenia in elderly patients with GBM is associated with reduced overall survival. Kaplan–Meier survival curves for elderly patients with GBM who have undergone chemo/RT are depicted below. Patients who developed severe treatment-related lymphopenia (TLC<500 cells/mm3) 2 months after beginning chemo/RT had a significant shorter median overall survival than those whose TLC were ≥500 cells/mm3 (multivariate analysis HR 2.76, 95 %CI 1.30–5.86, p = 0.008)
Discussion
The landmark Stupp study demonstrated a survival advantage with concurrent and adjuvant TMZ with standard 6 weeks of radiotherapy in GBM patients but excluded those older than 70 years [20]. Grossman et al. reported that severe TRL with TLC <500 cell/mm3 and CD4 counts <200 cell/mm3 at 2 months following initiation of chemoradiation was associated with reduced OS in GBM patients who were treated with the Stupp regimen with RT and TMZ [11]. When considering this TRL and the theory of age-related lymphopenia, it is prudent to consider the degree of TRL in the elderly and its effects on OS. To our knowledge, this is the first study to explore lymphopenia in the elderly patients following initiation of RT therapy and TMZ.
This retrospective analysis of elderly patients 65 years or older with GBM demonstrates that despite a normal median TLC, 2 months following the initiation of RT and TMZ, TLCs fell by 41 %. Severe grade III–IV lymphopenia with TLCs<500 cells/mm3 were found in 21 % of patients after 2 months. Furthermore, this lymphopenia did not return to baseline levels during 12 months of observation. The timing, severity, and duration of this treatment-induced lymphopenia is comparable to that recently reported in patients with newly diagnosed high grade gliomas, resected and unresected pancreatic cancer, head and neck squamous cell carcinoma, and stage III non-small cell lung cancers after treatment with chemoradiation [11–17].
More importantly, elderly patients who experienced a TLC of <500 cells/mm3 at 2 months had a decreased OS by 7 months that was statistically significant (4.6 vs. 11.6 months, p = 0.003). This finding was further supported on multivariable analysis with a significant hazard rate of 2.76 in patients with TLC <500 cells/mm3 at 2 months following initiation of chemoradation (HR 2.76, 95 %CI 1. 30–5.86, p = 0.008). These findings are concordant with the literature in multiple solid tumors showing an association between TRL and reduced OS [11–17].
Our findings and recent publications have suggested that RT might play a major role in TRL. A recent meta-analysis study explored the association among TRL and OS in various solid tumors [17]. The study found that the association between TRL and OS was independent of pre-treatment prognostic factors, tumor histology, chemotherapy regimen, or corticosteroid use, and that RT likely played an important role considering it was the one common variable in all of the studies. As acknowledged by the authors, this hypothesis is supported by a study of patients with stage III non-small cell lung cancer who, despite receiving neoadjuvant chemotherapy, did not develop lymphopenia until RT was delivered [13]. A mathematical model has been used to estimate the RT exposure of circulating lymphocytes during RT to the brain, considering the radio-sensitivity of lymphocytes. Receiving 60 Gy in 30 fractions exposes nearly all circulating cells to potentially lymphotoxic doses of RT [18]. Most recently, a study of 183 patients with high grade gliomas evaluated for predictors of lymphopenia, and found that higher brain volume receiving 25 Gy was one of the predictors of severe lymphopenia [19]. Together this evidence suggests that RT might play a major role in TRL.
There are a few interesting but unique findings in this elderly group of patients. First, in Grossman et al’s report of 96 GBM patients with a median age of 57 years old, over 90 % of patients had a normal baseline TLC (≥1000 cells/mm3) [11]. On the contrary, in our study of elderly patients with a median age of 71 years, only 57 % patients had a normal baseline TLC. This is likely due to advanced age, relative poor KPS (90 vs. 70), and nutrition status compared to younger patients [11, 19, 22, 23]. Second, both univariate and multivariate analyses suggested that patients receiving >45 Gy of RT were associated with longer OS than those receiving ≤45 Gy of RT. This was an unexpected finding. Previous publications demonstrated that a 3-week short course of RT was at least equivalent to the standard 6-week course of RT in older GBM patients [24, 25]. This discrepancy is likely the result of patient selection bias within our study, as elderly patients with a good KPS are more likely to be offered the standard 6-week course of RT with concurrent TMZ. Interestingly, when comparing TLCs over time in patients who received RT doses >45 Gy versus RT doses ≤45 Gy, patients developed similar TRL regardless of the RT dose they received. TLC dropped significantly in both short course (≤45 Gy) and longer course (>45 Gy) RT groups. Baseline median TLC was 800 cells/mm3 which fell by 25 % to 600 cells/mm3 2 months after initiating RT/TMZ in 22 patients who received RT doses ≤45 Gy (p = 0.002). Baseline median TLC was 1200 cells/mm3 which fell by 37 % to 750 cells/mm3 2 months after initiating RT/TMZ in 50 patients who received RT doses >45 Gy (p = 0.0002). The lower level of baseline TLC in patients who received short course of RT may be a poor prognostic characteristic which might contribute to the shorter survival in these patients. This was another unexpected finding, as a previous report from Huang et al. demonstrated that higher brain volume receiving 25 Gy was one of the predictors of acute severe lymphopenia [19]. Another group showed the percentage of circulating lymphocyte damaged was proportionally related to the fractions of RT treatment [18]. Based on this literature, we were expecting more TRL in patients who received a RT dose >45 Gy. Our unexpected result is likely due to the small sample size, and a fragile patient population with the potential to have a higher risk of developing treatment related toxicity even with a short course of RT. Third, the utilization of 1-month TLCs in patients where a 2-month TLC was not available is a limitation of the study as this potentially underestimates the degree of lymphopenia experienced at 2 months. Lastly, in this elderly patient population only 21 % patients had MGMT promoter methylation and 42 % achieved a gross total resection. Consistent with the literature, both MGMT promoter methylation and gross total resection were associated with improved clinical outcomes in elderly GBM patients [26, 27].
Ongoing clinical trial NRG-BN001 is a phase II randomized trial investigating the OS in patients who receive dose escalation and intensified proton therapy or IMRT with concurrent and adjuvant TMZ as compared to standard RT therapy with concurrent and adjuvant TMZ in patients with newly diagnosed GBM. This study has included a secondary analysis of CD4 counts at baseline and overtime based on literature showing CD4 counts at 2 months aid in prognostication. Considering the inclusion of elderly patients in this study with an age criteria of 18 years or older, a secondary analysis of this study will contribute significantly to the literature on TRL in the elderly as it would be the first analysis of a prospective, randomized patient population. If the association of TRL and reduced survival is validated in larger studies, TLC should be routinely monitored in this patient population and therapy could be adjusted per TLC levels. For instance, RT and/or TMZ could be reduced in patients who experienced a significant drop in TLC.
Conclusion
In summary, our findings strongly suggest that TRL is common, severe and long-lasting in elderly patients with GBM, and this severe lymphopenia is associated with reduced OS in this patient population. These findings add to the body of evidence that immunosuppression induced by chemoradiation is associated with inferior clinical outcomes. However, there are several substantial limitations to our study which should be carefully considered. This is a small, retrospective study with a limited number of eligible patients and a heterogeneic patient population. As a result, larger prospective studies are needed to confirm these findings. In addition, an improved understanding of the biology behind TRL is critical to developing novel approaches to prevent inadvertent lymphocyte depletion or to restore lymphocytes during or after RT and chemotherapy. These approaches may have the potential to improve survival in selected patients with deadly cancers such as GBM.
Acknowledgments
Funding This study was supported by National Cancer Institute K12 Paul Calabresi Career Development Award for Clinical Oncology Program.
Footnotes
Compliance with ethical standards
Conflict of Interest None.
References
- 1.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ku GY, Yuan J, Page DB, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116:1767–1775. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Huber ML, Haynes L, Parker C, et al. Interdisciplinary critique of sipuleucel-T as immunotherapy in castration-resistant prostate cancer. J Natl Cancer Inst. 2012;104:273–279. doi: 10.1093/jnci/djr514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lissoni P, Brivio F, Fumagalli L, et al. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers. 2004;19:135–140. doi: 10.1177/172460080401900208. [DOI] [PubMed] [Google Scholar]
- 6.Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69:5383–5391. doi: 10.1158/0008-5472.CAN-08-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8 + T cells and CD4 + T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer. 2006;94:275–280. doi: 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 9.Dahlin AM, Henriksson ML, Van Guelpen B, et al. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod Pathol. 2011;24:671–682. doi: 10.1038/modpathol.2010.234. [DOI] [PubMed] [Google Scholar]
- 10.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17:5473–5480. doi: 10.1158/1078-0432.CCR-11-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balmanoukian A, Ye X, Herman J, et al. The association between treatment-related lymphopenia and survival in newly diagnosed patients with resected adenocarcinoma of the pancreas. Cancer Invest. 2012;30:571–576. doi: 10.3109/07357907.2012.700987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campian JL, Ye X, Brock M, et al. Treatment-related lymphopenia in patients with stage III non-small cell lung cancer. Cancer Invest. 2013;31:183–188. doi: 10.3109/07357907.2013.767342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wild AT, Ye X, Ellsworth S, et al. The association between chemoradiation-related lymphopenia and clinical outcomes in patients with locally advanced pancreatic adenocarcinoma. Am J Clin Oncol. 2015;38(3):259–265. doi: 10.1097/COC.0b013e3182940ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campian JL, Ye X, Sarai G, et al. The association between severe treatment-related lymphopenia and progression free survival in patients with newly diagnosed squamous cell head and neck cancer. Head Neck. 2014;36:1747–1753. doi: 10.1002/hed.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campian JL, Ye X, Gladstone DE, et al. Pre-radiation lymphocyte harvesting and post-radiation reinfusion in patients with newly diagnosed high grade gliomas. J Neurooncol. 2015;124(2):307–316. doi: 10.1007/s11060-015-1841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossman SA, Ellsworth S, Campian J, et al. Survival in patients with severe lymphopenia following treatment with radiation and chemotherapy for newly diagnosed solid tumors. J Natl Compr Cancer Netw. 2015;13(10):1225–1231. doi: 10.6004/jnccn.2015.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yovino S, Kleinberg L, Grossman SA, et al. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31:140–144. doi: 10.3109/07357907.2012.762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang J, DeWees TA, Badiyan SN, et al. Clinical and dosimetric predictors of acute severe lymphopenia during radiation therapy and concurrent temozolomide for high-grade glioma. Int J Radiat Oncol Biol Phys. 2015;92(5):1000–1007. doi: 10.1016/j.ijrobp.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Stupp R, Mason WP, van den Bent MJ European Organisation for Research and Treatment of Cancer Brain Tumor and Radio-therapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 21.Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 22.Dennis MS, Nicolson A, Lehmann AB, et al. Clinical associations of lymphopenia in elderly persons admitted to acute medical and psychiatric wards. Gerontology. 1998;44(3):168–171. doi: 10.1159/000022003. [DOI] [PubMed] [Google Scholar]
- 23.Numeroso F, Barilli AL, Delsignore R. Prevalence and significance of hypoalbuminemia in an internal medicine department. Eur J Intern Med. 2008;19(8):587–591. doi: 10.1016/j.ejim.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 24.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 25.Cao JQ, Fisher BJ, Bauman GS, et al. Hypofractionated radiotherapy with or without concurrent temozolomide in elderly patients with glioblastoma multiforme: a review of ten-year single institutional experience. J Neurooncol. 2012;107(2):395–405. doi: 10.1007/s11060-011-0766-3. [DOI] [PubMed] [Google Scholar]
- 26.Minniti G, Salvati M, Arcella A, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in elderly patients with glioblastoma treated with radiotherapy plus concomitant and adjuvant temozolomide. J Neurooncol. 2011;102(2):311–316. doi: 10.1007/s11060-010-0324-4. [DOI] [PubMed] [Google Scholar]
- 27.Babu R, Komisarow JM, Agarwal VJ, et al. Glioblastoma in the elderly: the effect of aggressive and modern therapies on survival. J Neurosurg. 2015;9:1–10. doi: 10.3171/2015.4.JNS142200. [DOI] [PubMed] [Google Scholar]