Abstract
Cues (conditioned stimuli; CSs) associated with rewards can come to motivate behavior, but there is considerable individual variation in their ability to do so. For example, a lever-CS that predicts food reward becomes attractive, wanted, and elicits reward-seeking behavior to a greater extent in some rats (“sign-trackers”; STs), than others (“goal-trackers”; GTs). Variation in dopamine (DA) neurotransmission in the nucleus accumbens (NAc) core is thought to contribute to such individual variation. Given that the DA transporter (DAT) exerts powerful regulation over DA signaling, we characterized the expression and function of the DAT in the accumbens of STs and GTs. STs showed greater DAT surface expression in ventral striatal synaptosomes than GTs, and ex vivo fast-scan cyclic voltammetry recordings of electrically-evoked DA release confirmed enhanced DAT function in STs, as indicated by faster DA uptake, specifically in the NAc core. Consistent with this, systemic amphetamine (AMPH) produced greater inhibition of DA uptake in STs than in GTs. Furthermore, injection of AMPH directly into the NAc core enhanced lever-directed approach in STs, presumably by amplifying the incentive value of the CS, but had no effect on goal tracking behavior. On the other hand, there were no differences between STs and GTs in electrically-evoked DA release in slices, or in total ventral striatal DA content. We conclude that greater DAT surface expression may facilitate the attribution of incentive salience to discrete reward cues. Investigating this variability in animal sub-populations may help explain why some people abuse drugs, while others do not.
Keywords: Conditioning, Sign-Tracking, Amphetamine, Voltammetry, Sprague-Dawley Rat
Introduction
People with substance abuse disorders show an attentional bias towards drug cues (conditioned stimuli, CSs), and such cues acquire strong motivational control over behavior (Thewissen et al., 2007; Field et al., 2008; Franklin et al., 2011). Individual variation in the propensity to attribute motivational value (incentive salience) to reward cues can be studied in animals using a Pavlovian Conditioned Approach (PCA) procedure. Some rats, “sign-trackers” (STs) are attracted to reward cues to a much greater extent than others (“goal-trackers”, GTs), which instead direct their responding toward the location of reward delivery (Flagel et al., 2009; Robinson et al., 2014). In addition, reward cues function as more effective conditioned reinforcers (Robinson & Flagel, 2009) and are more effective in instigating reward-seeking behavior (Yager & Robinson, 2012; Yager et al., 2015) in STs than GTs. Importantly, these different behavioral responses to food-associated cues predict the extent to which drug cues come to motivate approach and drug-seeking behavior (Saunders & Robinson, 2012; Yager et al., 2015).
Dopamine (DA) neurotransmission, especially in the core of the nucleus accumbens (NAc), is known to be important in conferring motivational properties to reward cues (Robinson & Berridge, 1993; Cardinal et al., 2002). In both humans and non-human animals, food- or drug-related stimuli evoke DA release in the NAc (Boileau et al., 2007; Day et al., 2007; Flagel et al., 2011; Fotros et al., 2013), and, reward cues are more effective in evoking DA release in the NAc core in STs than GTs (Flagel et al., 2011). Although differential rates of spontaneous DA release events have been documented in selectively bred lines of rats that either sign- or goal-track (Flagel et al., 2010), this has not been described in outbred STs and GTs. However, both systemic and intra-accumbens injections of DA antagonists disrupt ST conditioned responses (CRs) to a greater extent than GT CRs (Danna & Elmer, 2010; Flagel et al., 2011; Saunders & Robinson, 2012; Saunders et al., 2013; Yager et al., 2015), and enhancing DA release increases sign-tracking, but not goal-tracking, behavior (Danna et al., 2013).
Amphetamine (AMPH) also augments the motivational value of CSs (Taylor & Robbins, 1984; Kelley & Delfs, 1991; Fletcher et al., 1998; Wyvell & Berridge, 2000; Peciña & Berridge, 2013). Interestingly, AMPH given directly into the NAc core selectively reinstates cue-induced drug seeking in STs (Saunders et al., 2013). AMPH is thought to exert effects on motivated behavior primarily by increasing DA release, in part due to reversal of the DA transporter (DAT; Giros et al., 1996). It is possible, therefore, that differential levels of DAT expression and/or function contribute to the greater effect of AMPH in STs compared to GTs (Saunders et al., 2013). Thus, we sought to characterize the expression and function of the DAT in rats that vary in their propensity to attribute incentive salience to reward cues.
Materials & Methods
Subjects & Pavlovian Training
Outbred male Sprague-Dawley rats (n=99; from both Harlan & Charles River Laboratories) were either housed individually (Exp. 3) or housed in pairs (Exp. 1–2) in a temperature- and humidity-controlled vivarium with a reverse light cycle. Food and water were available ad libitum. Starting 1-week after arrival, all procedures were performed during the dark phase of the light cycle.
Pavlovian training used operant chambers and procedures described previously (Saunders & Robinson, 2012). Briefly, conditioning (5 days) consisted of 25 CS-US pairings/day on a variable time 90s schedule. Each CS presentation (extension and illumination of a lever) lasted for 8s. Retraction of the lever was followed immediately by delivery of 1 banana-flavored pellet (US) into the food cup. The degree to which rats displayed lever (ST) or food cup (GT) directed behavior was quantified using a PCA index, as described previously (Meyer et al., 2012; Saunders & Robinson, 2012). Factors included in the index consisted of (1) response probability [P(lever) − P(food cup)], (2) response bias [(lever deflections − food cup entries)/(lever deflections + food cup entries)], and (3) the latency [(lever deflection latency − food cup entry latency)/8] for making either a lever-directed or food cup-directed CR during the CS. The PCA index was calculated from the average of their Day 4 and Day 5 data according to the following formula: [(probability difference + response bias + latency score)/3]. Based on this index, STs were defined as animals that had a score ranging from +0.4 to 1.0. In contrast, GTs had a score ranging from −1.0 to −0.4. Although rats with Intermediate scores (INs) were classified (PCA score −0.4 to +0.4), they were not used in the present experiments because we wanted to concentrate on differential neurochemistry between rats that varied markedly in their propensity to attribute incentive salience to the lever-CS (i.e., STs vs GTs). All procedures were conducted according to a protocol approved by the University of Michigan Committee on Use and Care of Animals (UCUCA).
Experiment 1: Presynaptic Regulation of DA Signaling in the NAc core
Stimulated DA Release in Brain Slices
A subset of rats (ST, n=6; GT, n=5) were left undisturbed in their home cages for ~2–5 weeks after PCA training, and were subsequently used for ex vivo measurement of stimulated DA release using fast-scan cyclic voltammetry (FSCV). ST and GT rats were sacrificed via rapid decapitation and brains were immediately extracted. 400μm-thick coronal slices of brain tissue were collected and bathed in oxygenated artificial cerebral spinal fluid (aCSF) at 32°C. During FSCV, a glass-encased carbon fiber microelectrode was lowered into the NAc core. Calibration of the carbon fiber FSCV electrode allows for current measurements to be converted to DA concentration ([DA]; 1.9–5.5 nA/μM DA). A bipolar stimulating electrode was placed nearby and positioned to evoke optimal DA release. Following stabilization, alternating 1-pulse (300μA, 4ms) and 5-pulse (300μA, 20Hz) electrical stimulations were delivered to the slice, once every 5-minutes. Since both stimulations resulted in similar DA responses, and because the kinetics of 1-pulse stimulations may be more accurately modeled using Michaelis–Menten parameters, only the 1-pulse stimulation data are presented in the results. Current changes recorded on the electrode are shown and plotted against the applied voltage (Eapp) and time. The holding potential applied to the carbon-fiber electrode (−0.4 V) was ramped to +1.3 V and back to −0.4 V at a rate of 10 Hz.
Following stimulation, the peak [DA] represents the total amount of DA released into the extracellular space ([DA]c). This measurement, calculated at the peak [DA] following stimulation, may be impacted by differences in both DA release and uptake (Wightman et al., 1988; Wu et al., 2001; Ramsson, Howard, et al., 2011), and therefore was modeled using Michaelis–Menten parameters, with the help of Demon Voltammetry software provided courtesy of Dr. Sara R. Jones at Wake Forest University (Yorgason et al., 2011). DA uptake was quantified using Vmax and the apparent Km. Vmax, the maximum speed of DA uptake ([DA] per second), is associated with DAT surface expression and turnover. Km is inversely related to the affinity for DA to bind DAT. As has been described elsewhere (Wu et al., 2001; Calipari et al., 2015), at baseline Km was set to 160 nM and Vmax was calculated as a value of DA uptake. In order to determine the amount of DA released per single stimulation pulse ([DA]p), the amount of DA removed by uptake (calculated using Vmax and Km values) was subtracted from [DA]c following stimulation, using Demon Voltammetry.
Tissue DA Content
Ventral striatal dopamine content was analyzed 3 weeks after cessation of conditioning. STs and GTs (n=6/group) were sacrificed via rapid decapitation. Brains were removed quickly and the ventral striatum was dissected on ice. Synaptosomes were then prepared and dopamine content was analyzed as described previously (Johnson et al., 2005; Chen et al., 2009). Briefly, bilateral tissue from single rats were first homogenized in 0.32 M sucrose containing 1 mM EDTA and Complete Mini protease inhibitor cocktail (Roche, Basel, Switzerland). Homogenates were then centrifuged at 4 °C (800 × g, 10 min) to remove cellular debris. The supernatant was next centrifuged again (12,000 × g, 15 min, 4 °C) and the pellet was used as crude synaptosomes. The pellet containing crude synaptosomes was resuspended in 100 μl of oxygenated Kreb-Ringer’s Buffer (KRB) (145 mM NaCl, 2.7 mM KCl, 1.2 mM KH2PO4, 1.0 mM MgCl2, 10 mM glucose, 24.9 mM NaHCO3, 0.05 mM ascorbic acid, 0.05 mM pargyline, pH 7.4). Synaptosomes (10 μl) were lysed using an internal standard solution composed of 50 mM perchloric acid, 25 μM EDTA, and 10 nM 2-aminophenol. Samples were diluted in KRB. Dopamine content in each sample was measured using high-performance liquid chromatography (HPLC) with electrochemical detection.
DAT Expression
After having been left undisturbed in their home cages for 3–5 weeks, rats [ST (n=30) and GT (n=22)] were rapidly decapitated and brains were harvested for measurement of DAT surface expression in synaptosomes. On ice, bilateral ventral striatal tissue (including the NAc core and shell) was obtained through hand-dissection. Due to low levels of DAT expression in the ventral striatum, each sample contained tissue pooled from 2–4 rats with similar PCA scores (ST, 10 samples; GT, 8 samples). On average, the difference between the strongest and weakest ST/GT in each pooled sample was a PCA index of 0.11 ± 0.046.
Synaptosome preparation was conducted as described above. Synaptosomal membrane proteins were subsequently biotinylated with 2 mg/ml sulfo-NHS-SS-biotin (Thermo Scientific, Waltham, MA, USA) in PBS/Ca-Mg (1 mM CaCl2 and MgCl2) overnight at 4 °C. Afterward, the biotinylation reaction was quenched by adding 100 mM glycine and incubating for 15 min. Synaptosomes were then washed twice with 100 mM glycine in PBS/Ca-Mg and subsequently lysed in RIPAE buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton X 100 and Complete Mini Protease inhibitor; Roche, Basel, Switzerland) for 1 hr at 4 °C. The obtained lysate was cleared by centrifugation at 20,000 g for 20 min at 4 °C and measured for protein concentration using a Bio-Rad Dc protein assay kit (Hercules, CA, USA). Samples (1 mg) were incubated with monomeric avidin beads (50 μl) overnight at 4 °C. The next day the beads were washed three times with RIPAE buffer and eluted in 2 X Laemmli sample buffer. The eluates and 50 μg of total synaptosomal lysate were resolved by SDS-PAGE (10% Tris-glycine) and immunoblotted using anti-DAT antibody (mouse monoclonal; 1:500 in 5% milk in TBST (Tris buffered saline with 0.05% Tween 20); courtesy of Dr. Vaughan, Department of Biochemistry, University of Nebraska) followed by horseradish peroxidase-conjugated secondary antibody (goat anti-mouse, SC-2055, RRID: AB 631738; 1:2000 in 5% milk in TBST; Santa Cruz Biotechnology, Dallas, TX, USA) and detected with enhanced chemiluminiscence reagent (Pierce, Thermo Scientific, Waltham, MA, USA).
Experiment 2: DA Response to AMPH
ST (n= 6) and GT (n=7) rats were left undisturbed in their home cages for 3–9 weeks. After induction of anesthesia (urethane, 1.5 g/kg, IP), a guide cannula (Bioanalytical Systems, West Lafayette, IN) was implanted dorsal to the NAc core (AP, +1.3; ML, ±1.3; DV, −2.5 mm relative to bregma) and an Ag/AgCl reference electrode was secured in the contralateral cortex (AP, −0.8; ML, ±4.0; DV, −2.0 mm relative to bregma). A bipolar stimulating electrode (AP, −5.2; ML, ±0.8 mm relative to bregma; Plastics One, Roanoke, VA) was lowered into the ventral tegmental area (VTA) until electrically-evoked DA release was able to be measured in the striatum, as previously described (Vander Weele et al., 2014). DA release was recorded using glass-encased cylindrical carbon-fiber electrodes. FSCV relied on the oxidation and reduction of the analyte of interest (i.e. DA) in response to the application of a triangular waveform (oxidative scan, −0.4 to 1.3 V; reductive scan, 1.3 V to −0.4 V; 400V/s). This waveform was cycled for 20 min at 60 Hz, followed by another 20 min at 10 Hz.
After the bipolar stimulating electrode was lowered into the VTA and cemented in place, stimulations of the VTA (60 Hz, 40 pulse) were applied once every 10 minutes until a stable DA response in the NAc core was achieved. Rats then received a saline injection (1 ml/kg, IP) followed by an AMPH injection (5 mg/kg, IP) 40 minutes later. VTA stimulations continued throughout this period, and finished 40 minutes after the final post-drug stimulation. At that time, rats were sacrificed via sodium pentobarbital overdose (60 mg/kg, IP) and electrolytic lesions (tungsten electrodes, 20 μA for 15 s) of the recording site were made. Brains were then removed and sliced to confirm NAc core electrode placements. Data were recorded for 40 minutes after AMPH administration in order to mirror the amount of time of a single conditioning session.
Analysis of stimulated DA release and uptake was performed using Demon Voltammetry software, in a similar fashion to Experiment 1. Vmax (the speed of DA uptake), Km (the ability of the treatment to inhibit DA uptake), and [DA]p (the ability of a single stimulation to evoke DA release) were first analyzed following the saline injection. The Vmax value determined for each rat following saline was then held constant when analyzing post-AMPH data, allowing for drug-induced changes in both Km and [DA]p to be modeled (Ramsson, Covey, et al., 2011; Daberkow et al., 2013; Calipari et al., 2015). “Fixing” Vmax at pre-drug levels is used because, as a competitive inhibitor of DA uptake at DATs, AMPH does not alter Vmax (Jones et al., 1999; Schmitz et al., 2001; John & Jones, 2007; Ramsson, Covey, et al., 2011; Ferris et al., 2015). Although AMPH has been reported to alter surface DAT in vitro (Zahniser & Sorkin, 2009), a recent study found that administration of AMPH in vivo or to striatal slices did not change DAT distribution (Block et al., 2015). Due to individual variation in the DA responses following both saline and AMPH, calculations of Km, [DA]p, and [DA]c were analyzed as a percent change from saline measurements.
Experiment 3: Behavioral Response to AMPH
A subset of ST/GT rats underwent stereotaxic surgery for implantation of chronic 22 gauge bilateral guide cannulae (Plastics One) aimed at the NAc core (A/P, +1.8; M/L, ±1.6; D/V, −5; mm from bregma and skull), as described previously (Saunders et al., 2013; Singer et al., 2014). After 1-week recovery from surgery, rats underwent 2 additional days of PCA training to confirm that conditioned responding was consistent with pre-surgical behavior.
The experiment assessed the effect of bilateral (2, 10, or 20μg; Wyvell and Berridge, 2000) microinjections of AMPH into the NAc core on PCA. On the first testing day all rats received saline (vehicle) microinjections. Thereafter, once every third day rats received an AMPH microinjection. The order in which doses were administered was counterbalanced across rats. After microinjection, rats were returned to their home cages for 15 minutes before being transported to operant chambers for PCA assessment. Rats then completed 25 conditioning trials using identical procedures as during initial PCA training. Rats were left undisturbed on days between microinjections. At the conclusion of experiment rats were deeply anaesthetized (sodium pentobarbital; 60 mg/kg, IP), brains were removed, and coronal brain sections were obtained to verify cannula tip placements. Only rats with bilateral cannula tips placed correctly in the NAc core were included in the behavioral analyses.
Statistical Analyses
For initial Pavlovian training, independent t-tests were used to analyze the number of food cup contacts during inter-stimulus-intervals in ST and GT rats. Independent t-tests were also used to analyze all voltammetry ([DA]c, [DA]p, and Vmax) and DAT expression results described in Experiment 1. For Experiment 2, the effect of AMPH on stimulated DA release and uptake ([DA]c, [DA]p, and Km) was analyzed using repeated measures ANOVAs, with group (ST or GT) as the between factor and time after AMPH (treatment) as the within-factor. Post hoc Bonferroni tests were used to determine group differences at each post-injection time point. Similar statistical procedures were used in Experiment 2; a repeated measures ANOVA was used to determine the effect of multiple doses of AMPH on lever (ST) and food cup (GT) contacts. For this analysis, behavioral group (ST/GT) was the between factor and AMPH dose was the within-factor. Again, Bonferroni tests were used to assess whether AMPH caused different dose-specific responses in STs and GTs. Next, separate 1-way ANOVAs were conducted to analyze ST and GT conditioned response contacts after AMPH, relative to vehicle. Bonferroni tests were then used for multiple comparisons across doses.
Results
Pavlovian Training
As described previously (Flagel et al., 2007; Robinson et al., 2014), patterns of conditioned responding were measured in order to identify sub-populations of rats. Over the course of 5 training days, STs increasingly made contact with the lever CS, whereas GTs failed to engage in this behavior (Figure 1A; STs, PCA index +1.0 to +0.4, 38% of rats trained; GTs, PCA index −0.4 to −1.0, 36% of rats trained). Rather, GTs showed increased food cup contacts (i.e., the CR characteristic of this sub-population) while STs showed decreases in this behavior (Figure 1B). Additionally, similar to a previous report (Meyer et al., 2012), GTs tended to contact the food cup more during inter-stimulus-intervals than STs (t97=1.868, p=0.0765). Although “intermediate” rats were classified (neither ST nor GT, PCA score −0.4 to +0.4), they were not used in the present experiments because we wanted to compare rats that differed maximally in their propensity to attribute incentive salience to reward cues; i.e., STs and GTs.
Figure 1. Conditioned approach.
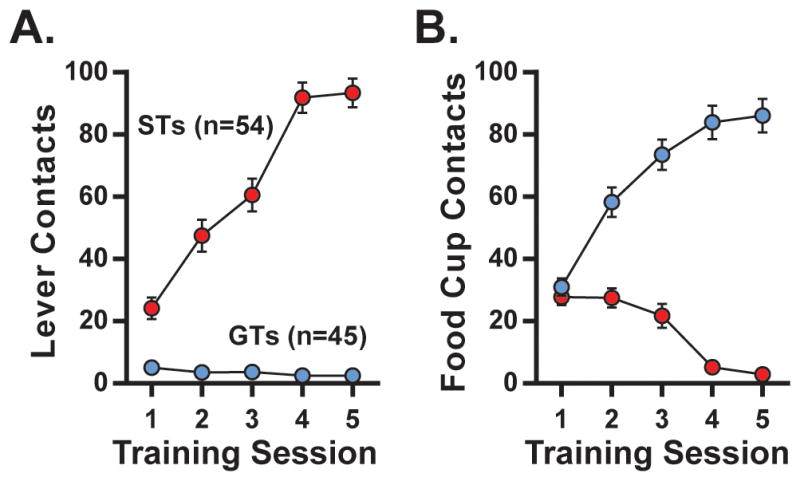
For all rats tested, average lever-contacts (“sign-tracking,” ST, PCA Index +0.4 to +1.0, n=54; A) and food cup contacts (“goal-tracking,” GT, PCA Index −0.4 to −1.0, n=45; B) across 5 training days.
Experiment 1: Presynaptic Regulation of DA Signaling in the NAc core
FSCV was used to determine whether STs and GTs displayed differences in DA release and/or uptake capability (Figure 2). Representative color plots (Figure 2A,B) and mean [DA] traces (Figure 2C,D) show that, following electrical stimulation, GTs had greater extracellular DA and slower DA uptake than STs. Quantification of these data demonstrate that the peak stimulated [DA]c was indeed greater for GTs than STs (Figure 2E; t9=2.559, *p=0.0307). Despite GTs showing greater extracellular DA than STs, GTs and STs did not differ in the amount of DA released per stimulation pulse ([DA]p; Figure 2F; t9=0.04010, p=0.9689, ns). Furthermore, STs and GTs did not differ in the total content of DA in synaptosomes prepared from ventral striatal tissue (Figure 2G; t10=0.7880, p=0.4490, ns). While there is need for further investigation, these latter findings suggest that STs and GTs may have the same amount of ventral striatal DA available for release.
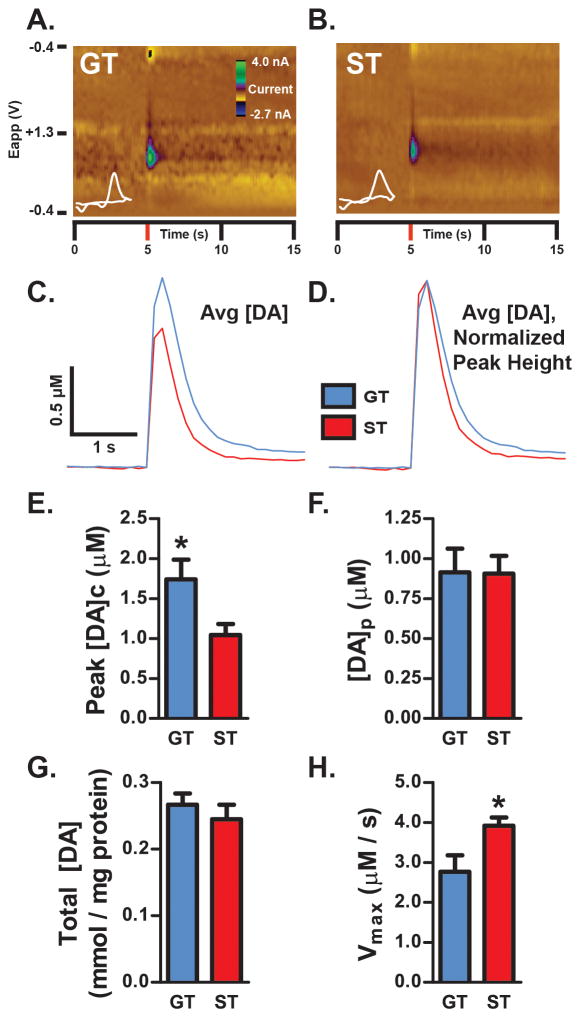
Figure 2. DA signaling.
DA release evoked by electrical stimulation of the NAc core was recorded using ex vivo FSCV. Representative color plots from a single 1-pulse stimulation are shown for a GT (A) and ST (B) rat. Stimulation occurred at 5-s into the recording. Plots show current changes recorded on the electrode, plotted against the applied voltage (Eapp) and time. The holding potential applied to the carbon-fiber electrode (−0.4 V) was ramped to +1.3 V and back to −0.4 V at a rate of 10 Hz. Cyclic voltammograms plotting current versus voltage at the peak DA response are shown in white. (C) Calibrated average [DA] traces for GT (n=5) and ST (n=6) rats following 1-pulse stimulation are shown. (D) In order to better illustrate differences in DA uptake between STs and GTs, the average [DA] traces in (C) were normalized to peak height following stimulation. The resulting [DA] traces show that STs return to baseline faster than GTs. (E) GTs displayed significantly greater peak stimulated [DA]c compared to ST (p<0.05). There were no significant differences in either the amount of DA released per stimulation pulse (F; [DA]p) or in total tissue [DA] measured in ventral striatal synaptosomes using HPLC (G). (H) STs displayed significantly faster DA uptake relative to GTs following electrical stimulation (*p<0.05). Results plotted as mean ± SEM.
A key factor that contributes to [DA]c levels following stimulation is DA uptake (Wightman et al., 1988; Wu et al., 2001; Ramsson, Howard, et al., 2011). Therefore, the speed of DA uptake (Vmax) in STs and GTs was investigated (Figure 2H). Relative to GTs, STs displayed faster clearance of extracellular DA in the NAc core (t9=2.616, *p=0.0280). Together, these findings suggest that, compared to STs, greater stimulated [DA]c in GTs (Figure 2E) results from relatively slow DA uptake, and not from differences in their ability to release DA ([DA]p). In other words, faster DA uptake in STs restricts the ability of electrical stimulation to increase [DA]c.
Increased speed of DA uptake in STs may reflect greater DAT expression compared to GTs. This was examined by measuring DAT expression in synaptosomes prepared from ventral striatal tissue. Indeed, we found that STs had greater surface expression of DATs than GTs (t16=2.718, *p=0.0152; Figure 3A), although, there was no difference between STs and GTs in total DAT expression in synaptosomal lysates (t16=1.386, p=0.1848, ns; Figure 3B). Thus, greater DAT surface expression in STs may account for greater DA uptake in STs following ex vivo stimulation of terminals.
Figure 3. DAT expression.
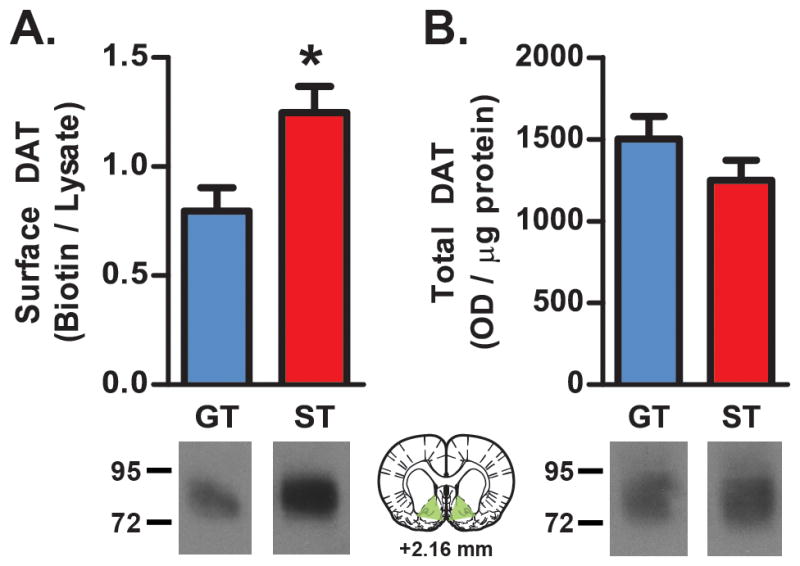
DAT expression was assessed in ventral striatal synaptosomes (ST, n=10; GT, n=8). (A) STs displayed greater DAT surface expression (biotinylated/lysate) compared to GTs (*p<0.05). (B) There was no difference in total (lysate) DAT expression. Representative western blots showing DAT expression are displayed below graphs. The green-shaded region on the drawing of a coronal brain section (adapted from Paxinos and Watson 2004) shows the region dissected for DAT measurements. Results plotted as mean ± SEM.
Experiment 2: DA Response to AMPH
Since 1) STs have more functional surface DATs than GTs, and 2) AMPH promotes increases in extracellular [DA] through binding to and reversal of DATs, we hypothesized that STs would also show a greater reduction in DA uptake following AMPH than GTs. To test this, urethane-anesthetized rats were first administered saline and then AMPH, and [DA] changes in the NAc core produced by electrical stimulation of the VTA were quantified. Representative color plots (Figure 4A,B) and mean peak-normalized [DA] traces (Figure 4C) show that, 30 minutes after AMPH administration, there was greater inhibition of DA uptake in STs than GTs compared to baseline (saline) conditions.
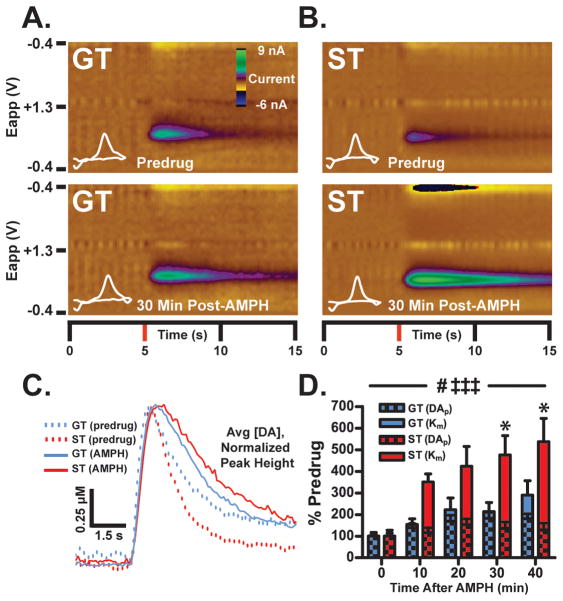
Figure 4. The influence of AMPH on electrically-stimulated release and uptake.
In urethane-anesthetized rats (ST, n=6; GT, n=7), the VTA was stimulated (60Hz/40-pulse biphasic) every 10 min and [DA] in the NAc core was recorded using FSCV following saline (1 ml/kg, IP) and AMPH (5 mg/kg, IP) injections. Representative color plots from single GT (A) and ST (B) rats, showing stimulated DA release 30-min after saline (top) and AMPH (bottom) injections. Stimulation occurred at 5-s into the recording and plots show current changes recorded on the electrode, plotted against the applied voltage (Eapp) and time. Cyclic voltammograms plotting current versus voltage at the peak DA response are shown in white. (C) Average stimulated [DA] traces (normalized to peak height) are shown at both 30-min post-saline and post-AMPH time-points for STs and GTs. While AMPH slowed uptake in both STs and GTs, the effect was exaggerated in STs since they had faster DA uptake under predrug conditions. These effects were analyzed in (D), where Vmax was held constant according to saline values and the “apparent” (app.) Km was used to determine AMPH-induced changes in DA uptake inhibition. While AMPH increased the app. Km in both STs and GTs (‡‡‡, p<0.001), this increase was significantly greater in STs than GTs at 30- and 40-minutes post-drug (*, p<0.05). In addition, the amount of DA release after stimulation ([DA]p) was enhanced after AMPH compared to saline (#, p<0.05), but did not differ between STs and GTs. Data are shown as means (±SEM).
Differences in DA uptake following AMPH, relative to saline, were determined from apparent Km values calculated using Michaelis-Menten modeling. Increases in Km values after AMPH represent enhanced inhibition of DA uptake. As expected, systemic AMPH injection reduced DA uptake in both STs and GTs (Figure 4D; time effect F4,44=19.43, ***p=0.0001). However, AMPH produced greater inhibition of DA uptake in STs than in GTs (group effect, F1,11=6.55, *p=0.0266; group x time interaction, F4,44=3.81, **p=0.0097). Bonferroni tests revealed that this reduction in uptake in STs progressed over time, reaching significant difference from GTs by 30 minutes post-injection. These data suggest that greater DAT surface expression in STs than in GTs may facilitate the ability of AMPH to inhibit DA uptake in STs.
In addition to reducing DA uptake, AMPH also increases synaptic DA levels by promoting vesicular release and efflux through DATs. As measured at the peak [DA] in the NAc core following VTA stimulation, AMPH enhanced the ability of stimulation to increase extracellular DA levels in both ST and GT rats ([DA]c time effect, F4,44=5.52, **p=0.0011; data not shown). Since changes in peak [DA]c may reflect both DA release and uptake (Wightman et al., 1988; Wu et al., 2001; Ramsson, Howard, et al., 2011), Michaelis-Menten modeling was used to determine the ability of AMPH to enhance DA release following a single electrical pulse ([DA]p). AMPH enhanced [DA]p responses in both STs and GTs (Figure 4D; [DA]p time effect, F4,44=3.25, *p=0.0201). Interestingly, relative to saline, there was no difference between STs and GTs in either [DA]c (group effect, F1,11=1.03, p=0.3315, ns; group X treatment interaction, F4,44=0.73, p=0.5762, ns) or [DA]p (group effect, F1,11=1.26, p=0.2864, ns; group X treatment interaction, F4,44=0.57, p=0.6872, ns).
We found this surprising, because greater inhibition of DA uptake after AMPH in STs than GTs would be expected to result in greater [DA]c, even if [DA]p were not changed. It is possible that inhibition of DA uptake by AMPH does not contribute to [DA]c. [DA]c is an instantaneous measurement taken at the peak [DA] following stimulation. Thus, [DA]c is not a measurement of the long-lasting tonic increase of DA in the synapse following stimulation, an effect that is exaggerated by AMPH to a greater degree in STs than in GTs. In addition, while NAc core DA responses measured using FSCV following electrical stimulation of the VTA allow for detection of differences in vesicular release, and for quantifying differences in reuptake, this technique may not be suitable for determining differences in DA efflux resulting from the depletion of vesicular stores and reversal of DATs produced by AMPH (Ramsson, Howard, et al., 2011). Furthermore, AMPH may enhance DA release through mechanisms that do not involve DATs (Dela Peña et al., 2015). Alternatively, the lack of a ST/GT difference in [DA]c and [DA]p may indicate a “ceiling effect”. This dose of AMPH would produce an extremely large and non-physiological increase in [DA] (Camp et al., 1994), and the combination of stimulation parameters and AMPH dose may result in a maximal amount of DA being released. Indeed, STs and GTs have similar synaptosomal DA levels in the ventral striatum (Figure 2G), as well as ability to release DA in the NAc core (Figure 2F).
Experiment 3: Behavioral Response to AMPH
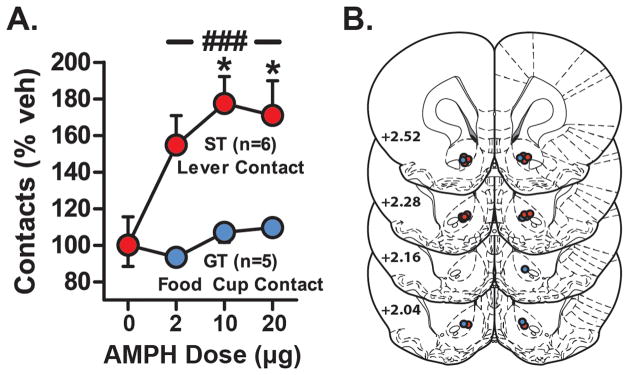
Given the ability of AMPH to reduce DA uptake to a greater degree in STs than in GTs, we next wanted to determine if this might have a functional consequence at the behavioral level. Thus, we examined the effect of an injection of AMPH into the NAc core on ‘contacts’ with either the lever (STs) or food cup (GTs) during performance on the PCA task. As expected, control infusions (saline) into the NAc core had no impact on the number of contacts made (Figure 5A). However, AMPH produced a dose-dependent increase in lever contacts made by STs. This robust increase was detected using a 2-way repeated measures ANOVA (group effect, F1,9=27.82, ***p=0.0005; treatment effect, F3,27=3.14, *p=0.0416; treatment X group interaction, F3,27=3.48, *p=0.0296). STs showed a greater increase in contacts at all doses tested compared to GTs (*p<0.01–0.05, Bonferroni tests). To better understand this change and demonstrate how conditioned responding compared to vehicle, STs and GTs were analyzed separately using 1-way ANOVAs. Indeed, AMPH microinjections significantly increased lever contacts in STs (F3,20=4.615, *p=0.0130) at both 10 and 20 μg doses of AMPH (*p<0.05, Bonferroni tests). In contrast, AMPH did not affect the number of food cup contacts made by GTs (one-way ANOVA, F3,16=1.117, p=0.3713, ns). Finally, AMPH had no effect of food cup contacts in STs (one-way ANOVA, F3,20=2.373, p=0.1007, ns) or lever presses in GTs (one-way ANOVA, F3,16=0.5294, p=0.6685, ns) during the CS period. Therefore, the effect of AMPH was not to increase sign-tracking in every rat, but only in rats that already attributed incentive salience to the cue (STs). While we hypothesize this may be due to the ability of AMPH to inhibit DA uptake in the NAc core to a greater degree in STs than in GTs, this interpretation may be complicated by the different routes of drug administration (IP vs intra-NAc).
Figure 5. AMPH amplifies conditioned approach in STs.
(A) After training, a subset of rats received saline and AMPH microinjections into the NAc core, 15 min before additional conditioning sessions (ST, n=6; GT, n=5). Data are plotted as percent behavioral changes relative to vehicle (saline, veh) infusion for lever contacts (STs) or food cup contacts (GTs). Relative to GTs, in STs AMPH increased conditioned approach at all doses tested (###, p<0.001). More specifically, both 10 & 20 μg of AMPH significantly increased lever contacts in STs (*, p<0.05) compared to their response to vehicle. AMPH had no effect on lever contacts in GTs or food cup contacts in STs (data not shown). (B) Microinjection locations in the NAc core for STs (red) and GTs (blue) (adapted from Paxinos and Watson 2004). Results plotted as mean ± SEM.
STs may have engaged with the lever more because AMPH enhanced non-specific motor output to a greater degree in STs (Fletcher et al., 1998). If this were the case one might expect an increase in food cup contacts during inter-stimulus-intervals. However, AMPH had no effect on food cup contacts during inter-stimulus-intervals in either STs or GTs (treatment effect, F3,27=0.08, p=0.9723, ns; group effect, F1,9=2.12, p=0.1795, ns; group X treatment interaction, F3,27=0.93, p=0.4408, ns; data expressed as % control; data not shown). We hypothesize, therefore, that increased lever contacts in STs reflects the ability of AMPH to amplify the incentive properties of the CS (Wyvell & Berridge, 2000), and it appears to do so preferentially in rats that already attribute incentive salience to the CS (STs).
Discussion
There is considerable individual variation in the extent to which predictive reward cues are attributed with incentive salience, in both humans and non-human animals (Mahler & de Wit, 2010; Robinson et al., 2014; Garofalo & di Pellegrino, 2015). For some rats (STs) discrete reward cues elicit approach into close proximity with them, and they work avidly to get them. In contrast, other rats (GTs) attribute less incentive value to discrete CSs, approaching the location of reward delivery instead of the CS itself, and they work less avidly for them. Previous studies have shown that DA transmission in the NAc core is more important for both the acquisition (Flagel et al., 2011) and performance (Saunders & Robinson, 2012) of ST than GT CRs. Here we further extend our understanding of how variation in the dopaminergic system may contribute to variation in the motivational control over behavior by reward cues.
Microinfusion of AMPH into the NAc core of STs increased the vigor with which they interacted with the lever-CS, without influencing conditioned behavior in GTs. This difference may be related to variation in the effect of AMPH on DA neurotransmission, as AMPH produced greater inhibition of DA uptake in STs than in GTs. In addition, STs had greater DAT surface expression in ventral striatal synaptosomes than GTs. In summary, the present results further support the idea that variation in DA neurotransmission may contribute to variation in the propensity to attribute incentive salience to reward cues (Flagel et al., 2011; Robinson et al., 2014), and in particular, STs and GTs vary in the expression and function of the DAT in the ventral striatum.
Behavioral Response to AMPH
Several studies have shown that inhibiting DA signaling by using DA antagonists, given either systemically (Danna and Elmer, 2010) or just in the NAc core (Saunders and Robinson, 2012; Yager et al., 2015), decreases sign-tracking, but not goal-tracking. Conversely, a lesion of the habenula, which is thought to disinhibit VTA DA neurons and lead to greater DA neurotransmission, enhances sign-tracking, but not goal-tracking (Danna et al., 2013). Infusion of psychostimulants directly into the NAc increases [DA]c (Heidbreder & Feldon, 1998), conditioned motivation (Wyvell & Berridge, 2000; Peciña & Berridge, 2013), and conditioned reinforcement (Fletcher et al., 1998). In STs, AMPH injected into the NAc core facilitates discrete cue-evoked drug seeking (Saunders et al., 2013). Consistent with this, the present results provide the first evidence that an AMPH injection into the NAc core is sufficient to facilitate sign-tracking (but not goal-tracking), presumably because of its ability to preferentially inhibit DA uptake in STs.
DA Signaling
The ex vivo FSCV results indicated greater clearance of DA in STs compared to GTs, potentially a reflection of increased DAT surface expression in STs. It is possible that in STs greater control over DA overflow could allow for more precise time-locked DA release events during CS presentation, influencing lever approach (Flagel et al., 2011). Indeed, the temporal precision of DA signaling may be very important to the encoding of information regarding salient stimuli (Wieland et al., 2015). Furthermore, low doses of AMPH may enhance CS-evoked [DA]c (Daberkow et al., 2013) and AMPH can increase [DA]c through inhibition of DAT alone (Siciliano et al., 2014). While the dose of AMPH used may have been too high to detect ST/GT differences in [DA]c (Camp et al., 1994), AMPH also did not produce differential effects on stimulated release ([DA]p). We therefore hypothesize that, under more physiological conditions, enhanced inhibition of DA uptake in STs (Km) following AMPH may allow the CS to elevate [DA]c to a greater degree than in GTs, enhancing conditioned approach.
In contrast, slower DA uptake in GTs is consistent with results from DAT knock-down (KD) or knock-out mice (Giros et al., 1996; Zhuang et al., 2001). Interestingly, both GTs (Meyer et al., 2012) and DAT KD mice (Yin et al., 2006) show increased food cup contacts during inter-stimulus-intervals. Thus, relative to STs, lower DAT surface expression in GTs may degrade the temporal control CSs exert over behavior. It is possible that reduced DAT expression may help elevate “tonic” levels of ventral striatal DA in GTs, potentially enhancing the probability of them entering the food cup during inter-stimulus-intervals. Alternatively, lower DAT surface expression in GTs may support different forms of conditioned responding compared to STs. For example, unlike STs who attribute incentive value to discrete CS, GTs are more motivated by contextual CSs (Saunders et al., 2014). It is possible that lower DAT expression in GTs results in greater “tonic” DA levels in the NAc compared to STs, and this different modality of DA signaling may more readily support contextual conditioning.
In brain slices, stimulation-evoked [DA]c was greater in GTs than STs. However, there were no group differences in total synaptosomal tissue [DA] content between STs and GTs, nor in the amount of DA released per stimulation pulse ([DA]p) in either brain slices or in urethane-anesthetized rats, before or after AMPH. It seems unlikely, therefore, that STs and GTs simply differ in the amount of DA available for release. Instead, the greater peak evoked [DA]c seen in slices may reflect reduced availability of (i.e. saturated) DAT binding in GTs (Wightman et al., 1988; Wu et al., 2001; Garris et al., 2003; Ramsson, Howard, et al., 2011). Of course there are other modulators of DA transmission that could contribute and require investigation (Garris et al., 2003; Zhang et al., 2009; Melchior et al., 2015).
DAT Expression
Greater surface DAT in STs may provide more binding sites for AMPH, potentially leading to exaggerated DA responses (Calipari et al., 2015). It is hypothesized that this drug-induced reduction in DA uptake contributes to the increase in sign-tracking behavior observed. Furthermore, AMPH microinjected into the NAc core also increases conditioned motivation to a greater degree in STs than in GTs (Saunders et al., 2013). Thus, greater DAT surface expression in STs may facilitate the ability of AMPH to enhance the incentive value of CSs.
Given that STs and GTs have similar total DAT levels, it is possible that these rats differ in the ability to traffic and retain DAT on the synaptic membrane. Increased DAT surface expression could reflect differential activation of proteins that regulate DAT trafficking, including PKCβ (Chen et al., 2009). In addition, STs and GTs could also differ in movement of DAT into and out of the membrane after administration of AMPH (Johnson et al., 2005; Yamamoto et al., 2013). However, a single AMPH injection does not alter the maximal rate of DA uptake (Vmax, a measure of DAT expression) within 1 hour of administration (Ferris et al., 2015). It remains to be explored whether psychostimulant exposure differently alters DAT expression in STs and GTs.
One caveat to the interpretation of the present findings is that it is not possible to tell if ST/GT differences in DAT expression and function are constitutive, as we hypothesize, or are a result of the initial training experience used to identify the phenotypes. DAT measures were obtained at different time-points weeks after training in an attempt to decrease any recent learning effects. Furthermore, it seems unlikely any such memories would be represented by a change in DAT, especially since in humans constitutive upregulation of DAT due to genetics also influences attraction to cues (Erblich et al., 2005; van Dyck et al., 2005). Even if DAT surface expression is not elevated before conditioning in STs, it is still likely that their neurophysiology supports up-regulation. Future experiments using micro positron emission tomography imaging of DAT expression in rats before conditioning may address this question.
Conclusion
Tight regulation over DA signaling in the NAc by DATs may facilitate the attribution of incentive salience to the reward-paired CS. Compared to GTs, upregulation of DAT surface expression and function in STs may enhance the ability of AMPH to increase DA signaling in the NAc and amplify sign-tracking. AMPH had no effect on conditioned approach in GTs, and thus presumably did not enhance the incentive value of the lever-CS in GTs. Importantly, individual variation in DAT expression may also produce differences in attraction to reward paired cues in humans. People with a variable number of tandem repeat (VNTR) polymorphism in the DAT gene (SLC6A3; 9-repeat allele) show greater cue-induced cigarette cravings than non-carriers (Vandenbergh et al., 1992; Erblich et al., 2005; Wetherill et al., 2014). These individuals show enhanced ventral striatal activation to nicotine cues (Franklin et al., 2009, 2011) and elevated smoking-induced DA signaling in the NAc (Brody et al., 2006). Furthermore, healthy adults with this polymorphism show greater striatal DAT expression compared to other individuals (Jacobsen et al., 2000; van Dyck et al., 2005; van de Giessen et al., 2009; but see the following references, Heinz et al., 2000; Martinez et al., 2001; Lynch et al., 2003). Therefore, both ST rats and people with the 9-repeat SLC6A3 polymorphism exhibit greater DAT expression and attraction to reward-paired cues relative to GTs and non-carriers, respectively. These findings thus support translational research using variation in Pavlovian conditioned approach behavior to investigate how DAT function contributes to addiction.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse to B.F.S. (F32 DA038383-01, T32 DA007268-21), T.E.R./B.J.A (P01 DA031656), and M.E.G. (5R01DA011697-13). We thank Myranda Bryan, Pavlo Popov, Erin Wright, Cody Carter, Melanie Schweir, Ali Rahim, and Ahmed Ibrahim for their assistance. We would also like to thank Dr. Sara R. Jones, Dr. Mark J. Ferris, and Dr. Jordan Yorgason for their advice regarding modeling with the Demon Voltammetry software.
Abbreviations
- AMPH
Amphetamine
- DA
Dopamine
- DAT
Dopamine transporter
- CR
Conditioned Response
- CS
Conditioned Stimulus
- FSCV
Fast-scan cyclic voltammetry
- GT
Goal-Tracker
- HPLC
High-performance liquid chromatography
- NAc
Nucleus Accumbens
- PCA
Pavlovian Conditioned Approach
- ST
Sign-Tracker
- US
Unconditioned stimulus
- VTA
Ventral tegmental area
References
- Block ER, Nuttle J, Balcita-Pedicino JJ, Caltagarone J, Watkins SC, Sesack SR, Sorkin A. Brain Region-Specific Trafficking of the Dopamine Transporter. J Neurosci. 2015;35:12845–12858. doi: 10.1523/JNEUROSCI.1391-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Welfeld K, Booij L, Diksic M, Benkelfat C. Conditioned dopamine release in humans: a positron emission tomography [11C]raclopride study with amphetamine. J Neurosci. 2007;27:3998–4003. doi: 10.1523/JNEUROSCI.4370-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S, Zamora-Paja E, Farahi J, Saxena S, London ED, McCracken JT. Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry. 2006;63:808–816. doi: 10.1001/archpsyc.63.7.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Siciliano CA, Jones SR. Differential influence of dopamine transport rate on the potencies of cocaine, amphetamine, and methylphenidate. ACS Chem Neurosci. 2015;6:155–162. doi: 10.1021/cn500262x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp DM, Browman KE, Robinson TE. The effects of methamphetamine and cocaine on motor behavior and extracellular dopamine in the ventral striatum of Lewis versus Fischer 344 rats. Brain Res. 1994;668:180–193. doi: 10.1016/0006-8993(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson Ja, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Chen R, Furman CA, Zhang M, Kim MN, Gereau RW, Leitges M, Gnegy ME. Protein kinase Cbeta is a critical regulator of dopamine transporter trafficking and regulates the behavioral response to amphetamine in mice. J Pharmacol Exp Ther. 2009;328:912–920. doi: 10.1124/jpet.108.147959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Garris PA, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna CL, Elmer GI. Disruption of conditioned reward association by typical and atypical antipsychotics. Pharmacol Biochem Behav. 2010;96:40–47. doi: 10.1016/j.pbb.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna CL, Shepard PD, Elmer GI. The habenula governs the attribution of incentive salience to reward predictive cues. Front Hum Neurosci. 2013;7:781. doi: 10.3389/fnhum.2013.00781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Dela Peña I, Gevorkiana R, Shi WX. Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms. Eur J Pharmacol. 2015;764:562–570. doi: 10.1016/j.ejphar.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich J, Lerman C, Self DW, Diaz GA, Bovbjerg DH. Effects of dopamine D2 receptor (DRD2) and transporter (SLC6A3) polymorphisms on smoking cue-induced cigarette craving among African-American smokers. Mol Psychiatry. 2005;10:407–414. doi: 10.1038/sj.mp.4001588. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Rose JH, Siciliano Ca, Sun H, Chen R, Jones SR. A Single Amphetamine Infusion Reverses Deficits in Dopamine Nerve-Terminal Function Caused by a History of Cocaine Self-Administration. Neuropsychopharmacology. 2015;40:1–11. doi: 10.1038/npp.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Kiernan A, Eastwood B, Child R. Rapid approach responses to alcohol cues in heavy drinkers. J Behav Ther Exp Psychiatry. 2008;39:209–218. doi: 10.1016/j.jbtep.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PEM, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Watson SJ, Robinson TE, Akil H. Individual differences in the propensity to approach signals vs goals promote different adaptations in the dopamine system of rats. Psychopharmacology (Berl) 2007;191:599–607. doi: 10.1007/s00213-006-0535-8. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM, Sabijan MS, DeSousa NJ. Injections of D-amphetamine into the ventral pallidum increase locomotor activity and responding for conditioned reward: a comparison with injections into the nucleus accumbens. Brain Res. 1998;805:29–40. doi: 10.1016/s0006-8993(98)00633-7. [DOI] [PubMed] [Google Scholar]
- Fotros A, Casey KF, Larcher K, Verhaeghe JA, Cox SM, Gravel P, Reader AJ, Dagher A, Benkelfat C, Leyton M. Cocaine Cue-Induced Dopamine Release in Amygdala and Hippocampus: A High-Resolution PET [(18)F]Fallypride Study in Cocaine Dependent Participants. Neuropsychopharmacology. 2013;38:1780–1788. doi: 10.1038/npp.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Lohoff FW, Wang Z, Sciortino N, Harper D, Li Y, Jens W, Cruz J, Kampman K, Ehrman R, Berrettini W, Detre JA, O’Brien CP, Childress AR. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Li Y, Suh JJ, Goldman M, Lohoff FW, Cruz J, Hazan R, Jens W, Detre JA, Berrettini W, O’Brien CP, Childress AR. Dopamine transporter genotype modulation of neural responses to smoking cues: confirmation in a new cohort. Addict Biol. 2011;16:308–322. doi: 10.1111/j.1369-1600.2010.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo S, di Pellegrino G. Individual differences in the influence of task-irrelevant Pavlovian cues on human behavior. Front Behav Neurosci. 2015;9:163. doi: 10.3389/fnbeh.2015.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PEM, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Heidbreder C, Feldon J. Amphetamine-induced neurochemical and locomotor responses are expressed differentially across the anteroposterior axis of the core and shell subterritories of the nucleus accumbens. Synapse. 1998;29:310–322. doi: 10.1002/(SICI)1098-2396(199808)29:4<310::AID-SYN3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB, Gelernter J. Prediction of dopamine transporter binding availability by genotype: A preliminary report. Am J Psychiatry. 2000;157:1700–1703. doi: 10.1176/appi.ajp.157.10.1700. [DOI] [PubMed] [Google Scholar]
- John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME. Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology. 2005;49:750–758. doi: 10.1016/j.neuropharm.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Jones SR, Joseph JD, Barak LS, Caron MG, Wightman RM. Dopamine neuronal transport kinetics and effects of amphetamine. J Neurochem. 1999;73:2406–2414. doi: 10.1046/j.1471-4159.1999.0732406.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. I Differential effects of amphetamine microinjections into striatal subregions. Psychopharmacology (Berl) 1991;103:187–196. doi: 10.1007/BF02244202. [DOI] [PubMed] [Google Scholar]
- Lynch DR, Mozley PD, Sokol S, Maas NMC, Balcer LJ, Siderowf AD. Lack of effect of polymorphisms in dopamine metabolism related genes on imaging of TRODAT-1 in striatum of asymptomatic volunteers and patients with Parkinson’s disease. Mov Disord. 2003;18:804–812. doi: 10.1002/mds.10430. [DOI] [PubMed] [Google Scholar]
- Mahler SV, de Wit H. Cue-reactors: Individual differences in cue-induced craving after food or smoking abstinence. PLoS One. 2010;5:1–3. doi: 10.1371/journal.pone.0015475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gelernter J, Abi-Dargham A, van Dyck CH, Kegeles L, Innis RB, Laruelle M. The variable number of tandem repeats polymorphism of the dopamine transporter gene is not associated with significant change in dopamine transporter phenotype in humans. Neuropsychopharmacology. 2001;24:553–560. doi: 10.1016/S0893-133X(00)00216-5. [DOI] [PubMed] [Google Scholar]
- Melchior JR, Ferris MJ, Stuber GD, Riddle DR, Jones SR. Optogenetic versus electrical stimulation of dopamine terminals in the Nucleus accumbens elicits local modulation of presynaptic release. J Neurochem. 2015 doi: 10.1111/jnc.13177. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, Robinson TE. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates - The New Coronal Set. Academic Press; 2004. English. [Google Scholar]
- Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered “wanting” for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci. 2013;37:1529–1540. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsson ES, Covey DP, Daberkow DP, Litherland MT, Juliano SA, Garris PA. Amphetamine augments action potential-dependent dopaminergic signaling in the striatum in vivo. J Neurochem. 2011;117:937–948. doi: 10.1111/j.1471-4159.2011.07258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsson ES, Howard CD, Covey DP, Garris PA. High doses of amphetamine augment, rather than disrupt, exocytotic dopamine release in the dorsal and ventral striatum of the anesthetized rat. J Neurochem. 2011;119:1162–1172. doi: 10.1111/j.1471-4159.2011.07407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: Individual differences. Neuropharmacology. 2014;76B:450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, O’Donnell EG, Aurbach EL, Robinson TE. A cocaine context renews drug seeking preferentially in a subset of individuals. Neuropsychopharmacology. 2014;39:2816–2823. doi: 10.1038/npp.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y, Lee CJ, Schmauss C, Gonon F, Sulzer D. Amphetamine distorts stimulation-dependent dopamine overflow: effects on D2 autoreceptors, transporters, and synaptic vesicle stores. J Neurosci. 2001;21:5916–5924. doi: 10.1523/JNEUROSCI.21-16-05916.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Ferris MJ, Jones SR. Biphasic mechanisms of amphetamine action at the dopamine terminal. J Neurosci. 2014;34:5575–5582. doi: 10.1523/JNEUROSCI.4050-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BF, Forneris J, Vezina P. Inhibiting cyclin-dependent kinase 5 in the nucleus accumbens enhances the expression of amphetamine-induced locomotor conditioning. Behav Brain Res. 2014;275:96–100. doi: 10.1016/j.bbr.2014.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology (Berl) 1984;84:405–412. doi: 10.1007/BF00555222. [DOI] [PubMed] [Google Scholar]
- Thewissen R, Havermans RC, Geschwind N, van den Hout M, Jansen A. Pavlovian conditioning of an approach bias in low-dependent smokers. Psychopharmacology (Berl) 2007;194:33–39. doi: 10.1007/s00213-007-0819-7. [DOI] [PubMed] [Google Scholar]
- van de Giessen EM, de Win MML, Tanck MWT, van den Brink W, Baas F, Booij J. Striatal dopamine transporter availability associated with polymorphisms in the dopamine transporter gene SLC6A3. J Nucl Med. 2009;50:45–52. doi: 10.2967/jnumed.108.053652. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. J Nucl Med. 2005;46:745–751. [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, Uhl GR. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Vander Weele CM, Porter-Stransky Ka, Mabrouk OS, Lovic V, Singer BF, Kennedy RT, Aragona BJ. Rapid dopamine transmission within the nucleus accumbens: Dramatic difference between morphine and oxycodone delivery. Eur J Neurosci. 2014:1–14. doi: 10.1111/ejn.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Jagannathan K, Lohoff FW, Ehrman R, O’Brien CP, Childress AR, Franklin TR. Neural correlates of attentional bias for smoking cues: Modulation by variance in the dopamine transporter gene. Addict Biol. 2014;19:294–304. doi: 10.1111/j.1369-1600.2012.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland S, Schindler S, Huber C, Köhr G, Oswald MJ, Kelsch W. Phasic Dopamine Modifies Sensory-Driven Output of Striatal Neurons through Synaptic Plasticity. J Neurosci. 2015;35:9946–9956. doi: 10.1523/JNEUROSCI.0127-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Pitchers KK, Flagel SB, Robinson TE. Individual variation in the motivational and neurobiological effects of an opioid cue. Neuropsychopharmacology. 2015;40:1269–1277. doi: 10.1038/npp.2014.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology (Berl) 2012;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DJ, Nelson AM, Mandt BH, Larson GA, Rorabaugh JM, Ng CMC, Barcomb KM, Richards TL, Allen RM, Zahniser NR. Rats classified as low or high cocaine locomotor responders: A unique model involving striatal dopamine transporters that predicts cocaine addiction-like behaviors. Neurosci Biobehav Rev. 2013;37:1738–1753. doi: 10.1016/j.neubiorev.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Zhuang X, Balleine BW. Instrumental learning in hyperdopaminergic mice. Neurobiol Learn Mem. 2006;85:283–288. doi: 10.1016/j.nlm.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR, Sorkin A. Trafficking of dopamine transporters in psychostimulant actions. Semin Cell Dev Biol. 2009;20:411–417. doi: 10.1016/j.semcdb.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Doyon WM, Clark JJ, Phillips PEM, Dani JA. Controls of tonic and phasic dopamine transmission in the dorsal and ventral striatum. Mol Pharmacol. 2009;76:396–404. doi: 10.1124/mol.109.056317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]