Abstract
High-fat diet (HFD) induced obesity is associated not only with metabolic dysregulation, e.g., impaired glucose homeostasis and insulin sensitivity, but also with neurological dysfunction manifested with aberrant behavior and/or neurotransmitter imbalance. Most studies have examined HFD's effects predominantly in male subjects, either in the periphery or on the brain, in isolation and after a finite feeding period. In this study, we evaluated the time-course of selected metabolic, behavioral, and neurochemical effects of HFD intake in parallel and at multiple time points in female (C57BL/6) mice. Peripheral effects were evaluated at three feeding intervals (short: 5–6 weeks, long: 20–22 weeks, and prolonged: 33–36 weeks). Central effects were evaluated only after long and prolonged feeding durations; we have previously reported those effects after the short (5–6 weeks) feeding duration) [1]. Ongoing HFD feeding resulted in an obese phenotype characterized by increased visceral adiposity and, after prolonged HFD intake, an increase in liver and kidney weights. Peripherally, 5 weeks of HFD intake was sufficient to impair glucose tolerance significantly, with the deleterious effects of HFD being greater with prolonged intake. Similarly, 5 weeks of HFD consumption was sufficient to impair insulin sensitivity. However, sensitivity to insulin after prolonged HFD intake was not different between control, low-fat diet (LFD) and HFD-fed mice, most likely due to age-dependent decrease in insulin sensitivity in the LFD-fed mice. HFD intake also induced bi-phasic hepatic inflammation and it increased gut permeability. Behaviorally, prolonged intake of HFD caused mice to be hypoactive and bury fewer marbles in a marble burying task; the latter was associated with significantly impaired hippocampal serotonin homeostasis. Cognitive (short-term recognition memory) function of mice was unaffected by chronic HFD feeding. Considering our prior findings of short-term (5–6 weeks) HFD-induced central (hyperactivity/anxiety and altered ventral hippocampal neurochemistry) effects and our current results, it seems that in female mice some metabolic/inflammatory dysregulations caused by HFD, such as gut permeability, appear early and persist, whereas others, such as glucose intolerance, are exaggerated with continuous HFD feeding; behaviorally, prolonged HFD consumption mainly affects locomotor activity and anxiety-like responses, likely due to the advanced obesity phenotype; neurochemically, the serotonergic system appears to be most sensitive to continued HFD feeding.
Keywords: High-fat diet, Glucose intolerance, Insulin sensitivity, Hepatic inflammation, Hypoactivity, Anxiety
1. Introduction
Obesity has reached epidemic proportions globally, affecting people of different gender, ethnicity, age, and socioeconomic status [2]; both genetic and environmental factors contribute to its development [3]. Among the environmental factors, consumption of energy-dense, high-fat diets (HFD), frequently paired with increasingly sedentary lifestyles, plays a key role in obesity pathogenesis [4, 5].
Detrimental effects on the brain of excess weight gain and/or obesity following intake of dietary fat have been reported. For example, emotional, i.e., anxiety/depression, and/or cognitive deficits have been observed in obese individuals, as well as in rodents fed HFD [6–10]. It is noteworthy that majority of these reports are reflective of neurological manifestations at a single time point post HFD consumption and in the case of laboratory studies, most of the data are on males. To begin to fill the female-specific data void, in a recently published study we demonstrated that HFD intake by female C57BL/6 mice, even for a relatively short period (5–6 weeks), can cause emotional (anxiety-like) and locomotor (hyperactivity) deficits and associated neurochemical changes in specific regions (ventral hippocampus [vHIP]) of the brain [1]. However, excessive intake of dietary fat in humans is typically of a long-term, chronic nature.
Besides neurological deficits, studies have demonstrated HFD-induced adverse peripheral effects, notably diabetes, a major obesity related co-morbidity that affects > 25 million people in the US alone. Obesity is characterized as a low-grade inflammatory disease [11] and obesity-associated inflammation originates, at least partially, from the gut microflora [12]. Obesity is associated with an increase in gut permeability and a leaky gut allows translocation to the circulation of bacterial pro-inflammatory factors, which can increase liver synthesis of inflammatory cytokines [13, 14]. These cytokines, through series of signaling events, can lead to insulin resistance [15, 16]. In rodents, administration of pro-inflammatory bacterial products results in inflammation and insulin resistance [12]. Inflammatory changes in response to HFD in key insulin-sensitive tissues, such as liver, have been reported in males [13, 17, 18]; the time-dependent progression of gut permeability, peripheral inflammation, and accompanying metabolic dysregulation is understudied.
In this paper, we aimed to (i) extend our previous work (on HFD-induced early central effects [1]) by looking at the concomitant peripheral effects (if any) following HFD consumption for a period of 5–6 weeks in female C57BL/6 mice and (ii) evaluate the time course of neurobehavioral/neurochemical and metabolic/inflammatory effects in female mice after long (20–22 weeks) and prolonged (32–36 weeks) HFD feeding. The extended feeding durations were selected based on data indicating that leptin signaling dysregulation, characterized by hyperleptinemia or leptin resistance, develops in male and female mice after 15–20 weeks on HFD [19–23] and that leptin status affects anxiety behavior and serotonin signaling [24]. In addition learning deficits caused by HFD appear to be age-dependent [25].
To determine the HFD-induced metabolic and inflammatory alterations in the periphery, metabolic tests such as glucose tolerance and insulin sensitivity tests (GTT and IST, respectively) were performed. Additionally, qPCR was conducted to analyze the HFD-induced alterations in the expression of key inflammatory markers in the liver; gut permeability was assessed using FITC-dextran. To assess the potential neurobehavioral consequences of continued HFD consumption, as we had done with mice fed HFD for shorter duration [1], we conducted several behavioral tests that could effectively assess locomotor (open field, grip strength and pole tests), emotional (forced swim and marble burying tests) and cognitive alterations (novel object recognition test) that might be affected by diet. To investigate potential HFD-induced neurochemical correlates to the measured behaviors, monoamines (dopamine [DA], serotonin [5-HT], norepinephrine [NE]) and their metabolites were measured in different brain regions implicated in the control of locomotor activity and/or regulation of emotional and/or cognitive functions (prefrontal cortex [ PFC], nucleus accumbens [NAc], striatum [STR], dorsal hippocampus [dHIP] and ventral hippocampus [vHIP]).
2. Materials and methods
2.1. Animals
Female C57BL/6 mice (Harlan, Indianapolis, IN) were housed (4–5 per cage) in an environmentally controlled room (22–24°C) with food and water available ad libitum on a 12 h light/dark cycle in an AAALAC accredited facility throughout the study. Mice were given one-week acclimatization period before initiation of the experiments. All procedures were in accord with the latest National Institutes of Health (NIH) guidelines and were approved in advance by the Institutional Animal Care and Use Committee (IACUC) of the University of Georgia.
2.2. Animal treatment
Mice (6–7 weeks old) weighing 16.0 ± 0.20 g (mean ± SEM) were randomly divided into two groups (n = 8/group/time point) and placed on either a low-fat diet (LFD; 10% kcal from fat, D12450J, Research Diets, Inc., New Brunswick, NJ) or a high-fat diet (HFD; 60% kcal from fat, D12492, Research Diets) for a period of 6, 22, or 36 weeks. The LFD diet provided 3.85 kcal/g of energy (70% carbohydrate, 20% protein, 10% fat, of which 22.7% saturated fatty acids [SFA], 29.9% monounsaturated fatty acids [MUFA], 47.4% polyunsaturated fatty acids [PUFA]). The HFD diet supplied 5.24 kcal/g of energy (20% carbohydrate, 20% protein, 60% fat, of which 32.0% SFA, 35.9% MUFA, 32% PUFA). Compared to normal regular chow, LFD is phytoestrogen-free and it is appropriately matched in composition (micronutrients and carbohydrate [sucrose]) with the purified HFD diet [26, 27]; hence, purified LFD is increasingly used over regular chow as a control diet [19, 28–30].
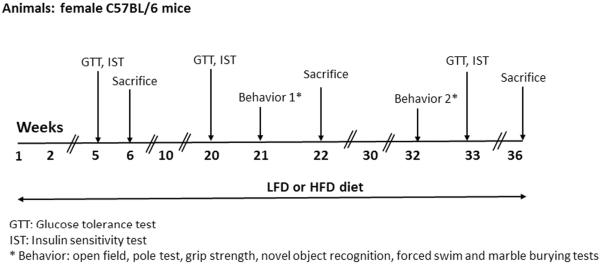
Glucose tolerance (GTT) and insulin sensitivity (IST) tests were performed after 5, 20 and 33 weeks of HFD treatment. Behavioral tests were carried out after 21 and 32 weeks of HFD consumption. GTT, IST and behavioral tests procedures are described in detail below. Mice were sacrificed at three time points (6, 22 and 36 weeks), body weights (BW) were recorded and organs (brain, liver, spleen, kidneys and thymus) were harvested, weighed, and quickly frozen at −80°C. Brain (22 and 26 weeks) and liver (6, 22, and 36 weeks) samples were used for, respectively, neurochemical and qPCR (described below) analysis. At all data collection time points, mice's estrous cycle stage was not monitored. The experimental design is presented in Fig. 1. We would like to note that the morphometric and liver qPCR data from the 6 week time point reported in this manuscript are from the same cohort of mice used in our recently published study [1].
Figure 1.
Detailed outline of the study's experimental design. For Abbreviations: see the text.
2.3. Glucose tolerance test (GTT) and insulin sensitivity test (IST)
After 5, 20 and 33 weeks (n = 8/group/time point) on respective diets, GTT and IST were performed in succession (3 days apart) on the same animals over a 2-h time window and after a 3-h fast [17]. Glucose (2 g/kg BW; oral) and insulin (0.5 IU/kg; i.p.), both from Sigma (Sigma-Aldrich, St. Louis, MO), were administered and blood glucose levels were determined with a glucometer (TRUEresult®, Nipro Diagnostics, Fort Lauderdale, FL) by serial tail bleeds at various time points (GTT: 0, 15, 30, 60, 90, and 120 min; IST: 0, 15, 30, 60, 75, 90, 105, and 120 min). During IST, those animals with blood glucose levels lower than 20 mg/dl (glucometer's low-end sensitivity) for two consecutive time points were given a single bolus of glucose to prevent them from developing severe signs of hypoglycemia; blood glucose value of 20 mg/dl was assigned to these animals.
2.4. Behavioral tests
Behavioral tests were performed in succession over 3 days after 21 and 32 weeks (n = 8/group) on respective diets as in [1]. Animals were naïve to the testing ambience at each time point of behavioral testing and all tests were performed by a treatment-blinded experimenter in a designated behavioral testing room located nearby, but separate, from that in which animals were housed.
2.4.1. Open field
Mouse activity was monitored for a period of 30 min in an open field arena (25 cm × 25 cm × 40 cm; Coulbourn Instruments, Whitehall, PA) with Limelight software (Actimetrics, Wilmette, IL) as described previously [1, 31]. Parameters evaluated included: (1) distance traveled (cm/5 min intervals); (2) time spent in defined regions, namely the center versus periphery of the square arena, analyzed per 5 min intervals; and (3) number of rearings during the first 5 min of open field testing.
2.4.2. Pole test
After 5 min resting period (following the open field test), mice were placed head-up on top of a vertical gauze-wrapped pole (1 × 55 cm; d × h) as detailed in [32]. A total of four trials were completed with a 3–5 min resting period between each trial. The average times to turn, to descend, and the total time spent on the pole from the four trials were used for statistical analysis. The maximum turning time allowed was 60 s and the maximum total time allowed per trial was 120 s [31].
2.4.3. Grip strength
Forelimb grip strength was measured using a strength gauge with an attached mouse specific square wire grid (6 cm × 6 cm; Bioseb, France) as previously described [31, 32]. The average grip force (in newtons [N]) of the four trials (with 1 min inter-trial interval) was used for statistical analysis.
2.4.4. Novel object recognition (NOR)
The NOR was conducted at the beginning of day 2 of behavioral testing with the previous day 30-min open field testing period used as a habituation phase, as described in [32]. Briefly, during the training phase, mice were placed in the open field arenas in the presence of two identical objects and were allowed to explore them for 5 min. After 1 h rest in their home cages, mice were placed back into the arenas with one familiar and one novel object and allowed to explore them for 5 min. Number of approaches towards the familiar or the novel object, as well as times spent exploring the familiar or the novel object were used to determine novelty preference as in [32].
2.4.5. Forced swim test (FST)
Following the NOR test and a 1.5-h rest period in the home cage, FST was carried out as detailed in [32]. Mice were placed in a large beaker filled with 3L of water (29±1 °C) for 15 min. The total times spent swimming, climbing, or immobile were scored using the Limelight video tracking software (Actimetrics) in a treatment-blind manner.
2.4.6. Marble burying test (MBT)
This test was performed on day 3 as described previously [1, 33, 34]. Clean testing cages were filled with a 4-5-cm layer of pine bedding (American Wood Fibers, Columbia, MD). Mice were individually placed in these cages for 10 min (habituation phase). After a 40-min home cage resting period, mice were placed back into the cages which now contained twenty glass marbles (diameter ~10 mm, Panacea Products Corp., Columbus, OH), evenly placed on the bedding arranged in a 4 × 5 matrix for a 10 min testing phase. The number of marbles buried (at least two-thirds covered by bedding material) was counted based on images collected at times 0 and 10 min of the testing phase [33, 34].
2.5. Neurochemistry
Regional brain concentration of monoamines and their metabolites was measured using HPLC with electrochemical detection as we have described it previously [35]. Briefly, micropunches (1.5-mm diameter) from PFC, NAc, STR, dHIP and vHIP were collected from 500-μm thick sections, placed in centrifuge tubes containing 100 μl of 0.2 N perchloric acid, sonicated, and centrifuged (13,200 g at 4°C for 10 min). An aliquot (20 μl) of the supernatant was injected into the HPLC for determination of: (1) DA and its metabolites: DOPAC (3, 4-dihydroxyphenylacetic acid) and HVA (homovanillic acid); (2) 5-HT and its metabolite 5-HIAA (5-hydroxyindoleacetic acid) and (3) NE (norepinephrine) and its metabolite MHPG (3-methoxy-4-hydroxyphenylglycol). Prior to statistical analysis, all neurochemistry data were normalized on per mg of protein basis. Protein digestion and concentration determination was done as previously described [35].
2.6. Intestinal permeability
Testing of intestinal wall integrity was based on [36], with some modifications. Briefly, after 6, 22 or 36 weeks on their respective diets, mice were fasted for 3 h and administered FITC-labeled dextran (4,000 kDa; Sigma) diluted in sterile saline (1000 mg/kg, 200 mg/ml) via oral gavage. After 1 h, mice were deeply anesthetized with CO2, blood was collected by cardiac puncture, centrifuged (10,000 rpm for 3 min at 4°C) and plasma FITC-dextran concentration was determined with a spectrophotometer (SpectraMax M3; Molecular Devices, Sunnyvale, CA) at 485/535 nm excitation/emission wavelengths and a standard curve (8.0–0.125 μg/ml) as in [36].
2.7. Real-time quantitative PCR (qPCR)
Total RNA from liver tissue (20 mg) was isolated using a GeneJET ™ RNA Purification Kit (Thermo Fisher Scientific, Pittsburgh, PA) and quantified using a Take 3 plate and Epoch microplate spectrophotometer (Bio-Tek, Winooski, VT). RNA was converted to cDNA using qScript cDNA SuperMix (Quanta Bioscience, Gaithersburg, MD) and a Peltier thermal cycler (Bio-Rad, Hercules, CA). Using 3 ng of cDNA per sample (with each sample run in duplicate), expression of key inflammation-related genes, such as tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6) and haptoglobin (Hp), was determined by qPCR using mouse-specific primers (Real Time Primers, Elkins Park, PA) and SYBR Green-based master mix (Qiagen, Valencia, CA). Amplifications were performed on a Mx3005P qPCR machine (Stratagene) and treatment differences were calculated as a fold change by the ΔΔ Ct method with 18S used as a house-keeping gene (HKG) as in [32].
2.8. Statistical analysis
Two-way ANOVA (duration × treatment) was used initially to analyze the morphometric data, gut permeability, neurochemical and behavioral endpoints (except as specified below). Student's T-test was used to analyze the effect of HFD on these endpoints within a time point. Two-way analysis of variance (ANOVA) was conducted to analyze the open field (treatment × time [interval]) and GTT/IST (treatment × time) data within a time point. Prior to statistical analysis, the blood glucose integrated areas under the curve (AUC) in the GTT and IST tests were calculated using the trapezoidal method [37]. These data were subjected to 2-way ANOVA (duration × treatment) analysis prior to post hoc. If ANOVA's overall main effects were found significant, treatment means were separated by Student-Newman-Keuls post hoc test. All results are presented as mean ± SEM and are considered significant at P ≤ 0.05.
3. Results
3.1. Body weight and organ weights
LFD and HFD groups did not differ with respect to the initial BW (LFD vs. HFD: 16.0 ± 0.13 vs. 16.2 ± 0.11, P > 0.30; Fig. 2). After 6 weeks, HFD-fed mice were significantly heavier than the LFD mice (LFD vs. HFD: 20.08 ± 0.38 vs. 25.00 ± 0.99, P ≤ 0.001; Fig. 2). After 22 weeks on respective diets, HFD-fed mice were also significantly heavier (P ≤ 0.001; Fig 2). Specifically, HFD feeding for 22 weeks resulted in a 29% greater increase in BW compared to mice fed LFD for 22 weeks (P ≤ 0.001; Fig 2). HFD consumption for another 14 weeks, i.e., a total of 36 weeks, resulted in an even greater difference (109%) between the LFD and HFD-fed mice (P ≤ 0.001; Fig 2). Also, the BW of 36-week HFD-fed mice was significantly higher than 22-week HFD-fed mice (P ≤ 0.001; Fig 2). Compared to a 69% weight increase in the LFD-fed mice over the 36 week period, HFD-fed mice's BW increased by 242% at the 36 week time point (Fig. 2).
Figure 2.
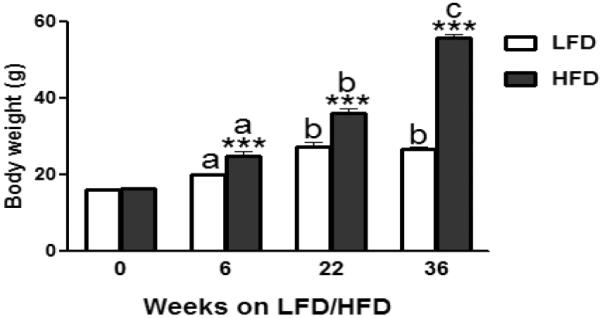
Effect of low-fat diet (LFD) or high-fat diet (HFD) consumption on mean body weights of female C57BL/6 mice after 6, 22, or 36 weeks on respective diets. Graphical representations are mean ± SEM (n = 8/group/time point). *** P ≤ 0.001 indicates significant difference from LFD within a time point. “a, b, c” indicate significant difference between time points within a dietary treatment (P ≤ 0.001).
HFD consumption for 6, 22 or 36 weeks did not affect the absolute weights (g) of brain, spleen and thymus (P ≥ 0.07; Fig. 3A, B and C). However, when normalized to total body weight, HFD feeding for 6, 22 or 36 weeks significantly decreased the relative weights (g/kg BW) of brain, liver, kidneys and spleen (P ≤ 0.05; data not shown); relative thymus weight was significantly decreased only after 36 weeks of HFD feeding (P ≤ 0.05; data not shown). Additionally, HFD intake for 36 weeks (Fig. 3C), but not 6 or 22 weeks, significantly increased the absolute liver and kidney weights (P ≤ 0.01; Fig. 3A and B).
Figure 3.
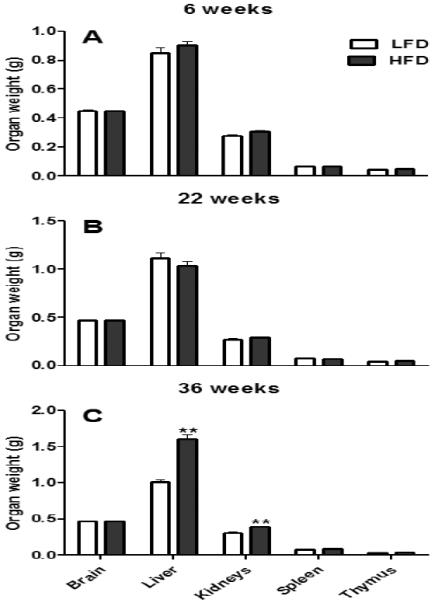
Absolute (g) organ weights of female C57BL/6 mice fed either a high fat diet (HFD) or a control, low fat diet (LFD) for 6 (A), 22 (B), or 36 (C) weeks. Graphical representations are mean ± SEM (n = 8/group/time point). ** P ≤ 0.01 represent effects within a time point.
3.2. Glucose tolerance test (GTT)
The fasting (0 min) baseline blood glucose level of HFD-fed mice was significantly (P ≤ 0.001) greater than LFD after 5 or 20 weeks (Fig. 4A and 4B), but not after 33 weeks on respective diets (P > 0.2; Fig. 4C). Following oral glucose challenge, the hyperglycemia in HFD-fed mice after 5, 20, or 33 weeks of feeding was significantly higher than in the LFD-fed mice at the remaining time points (15, 30, 60, 90 and 120 min) recorded (P ≤ 0.01; Fig. 4A, 4B and 4C); HFD-induced hyperglycemia after 33 weeks of feeding was the most prominent (P ≤ 0.001). Compared to LFD-fed mice within a time point, the integrated area under the curve (AUC) for GTT was 78%, 43% and 90% greater in mice fed HFD for 5, 20 and 33 weeks, respectively (P ≤ 0.001; Fig. 4D). Furthermore, the GTT AUC of 33-week HFD-fed mice was significantly greater than the GTT AUC after 5 or 20 weeks of HFD feeding (P ≤ 0.001; Fig. 4D).
Figure 4.
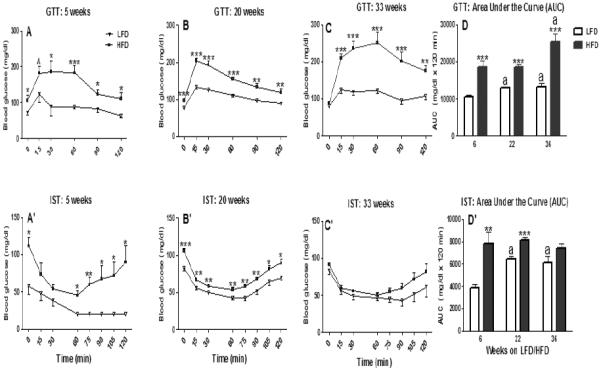
Glucose and test (GTT; 2 g/kg BW, oral; A, B, and C) and insulin sensitivity test (IST; 0.5 IU, i.p.: A', B', and C') of female C57BL/6 mice fed either a high fat diet (HFD) or a control (low fat diet; LFD) for 6 (A, A'), 22 (B, B'), or 36 (C, C') weeks. Integrated areas under the curve (AUC) of GTT and IST responses are presented, respectively, in Fig. 4D and Fig. 4D'. Graphical representations are mean ± SEM (n = 8/group/time point). * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001 represent effects within a time point (min, A, A', B, B', C and C'; weeks, D and D'). Note: for simplicity, only the differences between LFD and HFD groups within a time point are represented in A, A', B, B', C, and C' panels. “a” indicates significant difference between time points within treatment (P ≤ 0.001).
3.3. Insulin sensitivity test (IST)
Mice fed HFD for 5 or 20 weeks, but not 33 weeks, showed a significant (P ≤ 0.05) increase in the baseline blood glucose level (Fig. 4A′, 4B′ and 4C′). Both LFD and HFD-fed mice after 5, 20 or 33 weeks showed a similar response kinetics to insulin characterized by decreased blood glucose level at 15 and 30 min post insulin challenge (Fig. 4A′, B′ and C′). Starting at 60 min post insulin challenge, the 5 week HFD-fed mice showed an early blood sugar rebound with the blood glucose levels returning to almost normal level by 2 h (Fig. 4A′); their LFD-fed counterparts remained greatly hypoglycemic throughout the 2 h sampling period (Fig. 4A′). However, by 20 weeks, starting at 60 min post insulin challenge, both LFD and HFD fed mice showed a rebound in the blood glucose level; nonetheless, the blood glucose level of HFD mice remained consistently higher than the LFD group (P ≤ 0.05; Fig. 4B′). Similar to 20 weeks, we observed a rebound in the blood glucose level in the 33-week LFD and HFD-fed mice beginning 60 min post insulin injection (Fig. 4C′). However, their blood glucose (LFD vs. HFD) mice did not differ at any of the time points recorded from 60 min to 120 min post insulin challenge (P ≥ 0.10; Fig. 4C′). Compared to corresponding LFD mice, the AUC for IST was significantly higher in the HFD mice after 5 (101%, P ≤ 0.01; Fig. 4D′) or 20 weeks (26%, P ≤ 0.001; Fig. 4D′), but not after 33 weeks (P > 0.09; Fig. 4D′).
3.4. Intestinal permeability
Although 6 and 22 weeks of HFD feeding resulted in a similar fold (2.1) increase in the plasma FITC-dextran concentration, only the latter one reached significance (P ≤ 0.05; Fig. 5), likely due to the greater variability in the 6 week HFD group (P = 0.10; Fig. 5). Similar to the 22 week data, after 36 weeks on the diet, continued HFD intake increased the FITC-dextran plasma levels (P ≤ 0.05; Fig. 5), suggesting that HFD consumption by female C57BL/6 mice increases the gastrointestinal permeability, with the effect being more prominent during longer duration on a HFD.
Figure 5.
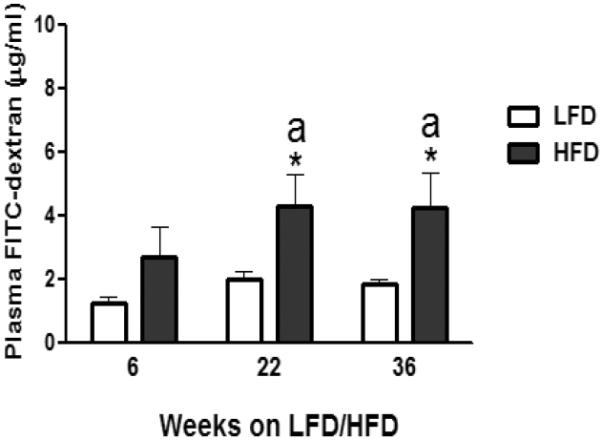
Effect of low-fat diet (LFD) or high-fat diet (HFD) consumption on gastrointestinal permeability (plasma FITC-dextran levels) of female C57BL/6 mice after 6, 22 or 36 weeks on respective diets. Graphical representations are mean ± SEM (n = 8/group/time point). * P ≤ 0.05 represents treatment effect within feeding duration. “a” indicates significant difference between time points within a dietary treatment.
3.5. Liver qPCR
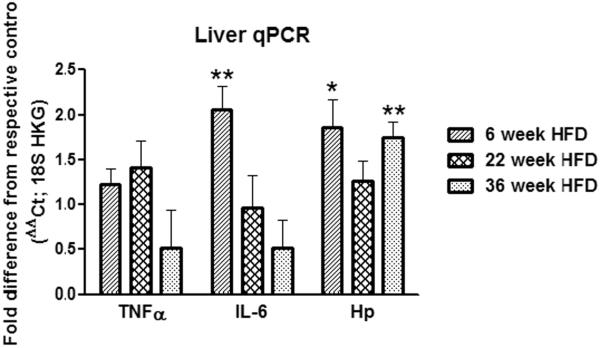
HFD feeding for 6, 22 or 36 weeks did not alter the levels of hepatic TNFα mRNA levels (P ≥ 0.20; Fig. 6). Hepatic mRNA expression of the other pro-inflammatory cytokine, IL-6, was significantly upregulated after 6 (2.1-fold, P ≤ 0.01; Fig. 9), but not after 22 or 36 (P ≥ 0.15; Fig. 6) weeks of HFD intake. Except for 22 weeks, the mRNA expression of the inflammatory marker Hp was significantly increased in the livers of 6- (1.9-fold, P ≤ 0.05; Fig. 9) and 36- week HFD-fed mice (1.8-fold, P ≤ 0.01; Fig. 6).
Figure 6.
Effect of 6, 22 or 36 weeks of high-fat diet (HFD) consumption by female C57BL/6 mice on liver mRNA levels of tumor-necrosis factor alpha (TNFα), interleukin 6 (IL-6) and haptoglobin (Hp). The house keeping gene (HKG) 18S was used to normalize the mRNA data, which are presented as fold change relative to respective low-fat diet (LFD) group at each time point. Graphical representations are means ± SEM (n = 6–8 per group). * P < 0.05, ** P < 0.01 indicate treatment effect within a feeding duration.
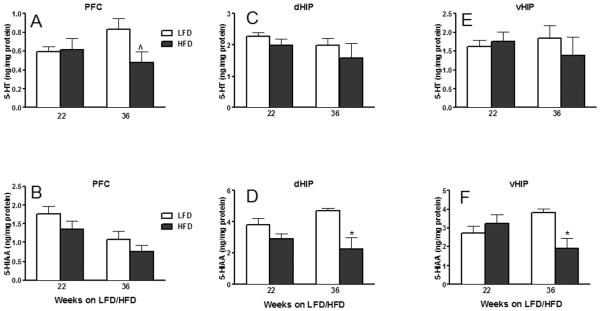
Figure 9.
Effect of 22 or 36 weeks of high-fat diet (HFD) consumption on concentrations of serotonin (5-HT; A, C and E) and its metabolite, 5-hydroxyindole acetic acid (5-HIAA; B, D and F) in the prefrontal cortex (PFC; A and B), dorsal hippocampus (dHIP, C and D) and ventral hippocampus (vHIP, E and F) of female C57BL/6 mice. 5-HT and 5-HIAA concentrations are normalized on a per mg protein basis and are presented as mean ± SEM (n = 8/group/time point). *P ≤ 0.05 indicates significant difference from low-fat diet (LFD) within a time point. (^) indicates trend towards significance (P = 0.06).
3.6. Behavior
3.6.1. Open field
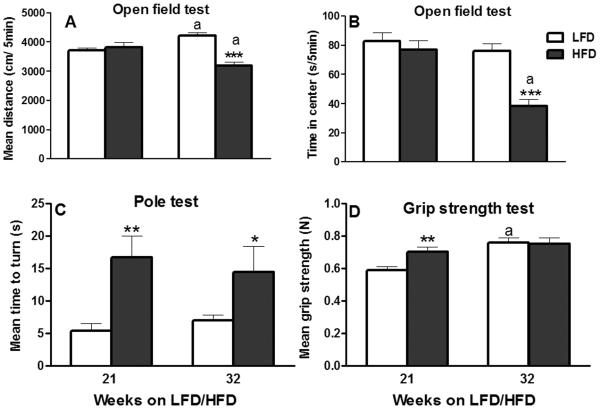
Mice's locomotor activity after 21 weeks of HFD intake did not differ from that of the LFD-fed mice (P ≥ 0.50; Fig. 7A). As expected, both LFD and HFD-fed mice habituated to the arenas over time and their overall activity decreased (distance traveled: 4322.77 ± 212.76 vs. 4761.72 ± 375.96 cm in first 5 min and 3455.85 ± 173.62 vs. 3604.58 ± 362.26 cm in last 5 min of LFD and HFD groups, respectively; data not shown). Vertical activity, measured by the number of rearings during the first 5 min of open field exploration was also unaffected after 21 weeks of HFD feeding (P > 0.35; data not shown). Similarly, the mean times spent in the center or periphery (data not shown) of the arena were unaffected by 21-week HFD intake (P ≥ 0.40; Fig. 7B).
Figure 7.
Effect of 21 or 32 weeks of high-fat diet (HFD) consumption on: (A) distance traveled (per 5 min interval); (B) time spent per 5 min interval in the center of the open field arena during open field testing; (C) average time to turn during the pole test; and (D) mean forelimb grip strength. Graphical representations are mean ± SEM (n = 8/group/time point). * P ≤ 0.05, ** P ≤ 0.01 and *** P ≤ 0.001 indicates significant difference from low-fat diet (LFD) within a time point. “a” indicates significant difference between time points within a dietary treatment (P ≤ 0.05).
After 32 weeks of HFD intake, two-way ANOVA demonstrated overall significant main effects of diet (P ≤ 0.001) and interval (5 min time period; P ≤ 0.05), but without any significant interaction between the two (P = 0.42) with respect to the distance traveled in the open field arena; the locomotor activity of HFD-fed mice was significantly lower (P ≤ 0.001; Fig. 7A). As expected, both LFD and HFD-fed mice habituated to the arenas over time, but HFD-fed mice were hypoactive throughout the 30-min testing (data not shown). For example, their locomotor activity during the 6th (LFD vs. HFD: 3882.11 ± 254.68 vs. 2919.32 ± 397.74 cm) 5-min interval compared to their activity during the 1st interval (first 5 min) of open field testing (LFD vs. HFD: 5237.61 ± 289.47 vs. 3584.66 ± 233.79 cm) was significantly (P ≤ 0.05) lower (data not shown). HFD-fed mice also showed a significant decrease in rearing (LFD vs. HFD: 21.6 ± 2.76 vs. 13.14 ± 1.35; P ≤ 0.05; data not shown). At 32 weeks, the mean distance travelled by the LFD mice was significantly greater than at 21 weeks; on the contrary, mice in HFD group were hypoactive at 32 weeks compared to their activity at 21 weeks (P ≤ 0.05; Fig. 7A). At this time point, the mean time spent in the center (LFD vs. HFD: 76.14 ± 5.01 vs. 38.28 ± 4.84 s per 5 min; Fig. 7B) and periphery (LFD vs. HFD: 223.76 ± 5.01 vs. 261.62 ± 4.84 s per 5 min; data not shown) was significantly (P ≤ 0.001) decreased and increased, respectively, by HFD.
3.6.2. Pole test
HFD intake for 21 or 32 weeks produced robust, significant (3.1 and 2.1-folds, respectively) increase in the mean turn time during the pole test (P ≤ 0.01; Fig. 7C). The other two pole test parameters, time to descend and total time, were not affected by HFD after either 21 or 32 weeks of HFD intake (P ≥ 0.15; data not shown).
3.6.3. Grip strength
The mean grip strength of LFD-fed mice was significantly increased at 32 weeks compared to the 21-week time point (P ≤ 0.05; Fig. 7D). HFD intake for 21 weeks significantly increased the average forelimb grip strength (P ≤ 0.01; Fig. 7D). However, after 32 weeks of HFD consumption, the mean grip strength was not different from that of mice fed LFD for 32 weeks (P > 0.90; Fig. 7D).
3.6.4. Novel object recognition (NOR)
Mice exhibited normal NOR performance after 21 or 32 weeks of HFD consumption evidenced by the significantly increased novel object preference of both LFD and HFD-fed mice in terms of time (P ≤ 0.05; Fig. 8A) or approaches (P ≤ 0.05; data not shown).
Figure 8.
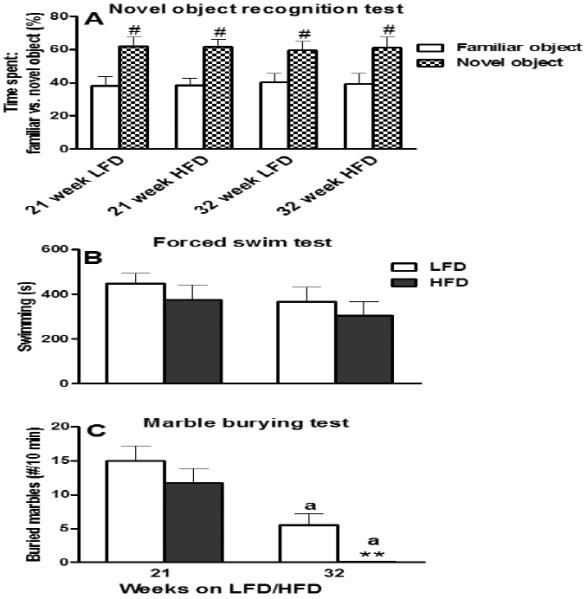
Effect of 21 or 32 weeks of high-fat diet (HFD) consumption on: (A) time spent with a familiar vs. a novel object (%) in a novel object recognition test (NOR); (B) total time spent swimming in a forced swim test (FST); and (C) number of marbles buried (≥ 70%) during a marble burying test (MBT). Graphical representations are mean ± SEM (n = 8/group/time point). “#” indicates significant (P ≤ 0.05) difference between the novel vs. familiar object within a dietary treatment group. ** P ≤ 0.01 indicates significant difference from low-fat diet (LFD) within a time point. “a” indicates significant difference between time points within a dietary treatment (P ≤ 0.001).
3.6.5. Forced swim test (FST)
Mice fed HFD for 21 or 32 weeks did not show any significant difference with respect to the swimming time compared to mice fed LFD (P ≥ 0.35; Fig. 8B); the total times spent climbing and immobile were also unaffected after either 21 or 32 weeks on HFD (P ≥ 0.10; data not shown).
3.6.6. Marble burying test (MBT)
Compared to LFD-fed mice, 21 weeks on HFD did not affect (P > 0.30) the number of buried marbles (Fig. 8C). By 32 weeks, mice from both LFD and HFD groups buried less marbles (P ≤ 0.01; Fig. 8C). However, the HFD-fed mice performed much worse than the LFD-fed controls and they buried significantly fewer marbles (P ≤ 0.01; Fig. 8C).
3.7. Neurochemistry
In the PFC, 22 or 36 weeks of HFD consumption did not affect DA or its metabolites (P ≥ 0.09; Table 1). Similarly, HFD feeding for 22 weeks did not change the PFC concentration of NE (P ≥ 0.50; Table 1), 5-HT (P ≥ 0.80; Fig. 7A) or their metabolites (P ≥ 0.15; Fig. 7B and Table 1). However, continued HFD feeding for 14 more weeks resulted in a significant increase in PFC NE concentration (P ≤ 0.01; Table 1) and an in apparent trend towards 5-HT decrease (P = 0.06; Fig. 9A), without affecting their metabolite levels (P ≥ 0.25; Fig. 9B and Table 1).
Table 1.
Monoamine or their metabolites concentrations in different brain regions of female C57BL/6 mice fed control low-fat diet (LFD) or a high-fat diet (HFD) for 22 or 36 weeks (n = 8/group/time point).
| Brain monoamine neurotransmitters and their metabolites a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Duration | 22 weeks | 36 weeks | ||||||||
|
| ||||||||||
| Diet | Brain region | PFC | ||||||||
| DA | DOPAC | HVA | NE | MHPG | DA | DOPAC | HVA | NE | MHPG | |
| LFD | 0.31 ± 0.09 | 3.73 ± 0.29 | 1.09 ± 0.10 | 4.78 ± 0.67 | 7.81 ± 0.49 | 0.11 ± 06 | 4.23 ± 0.28 | 0.97 ± 0.15 | 3.49 ± 0.36 | 9.81 ± 1.26 |
| HFD | 0.31 ± 0.07 | 3.96 ± 0.23 | 0.94 ± 0.16 | 5.34 ± 0.60 | 6.98 ± 0.34 | 0.10 ± 04 | 4.75 ± 0.25 | 1.36 ± 0.13 | 5.20 ± 0.30* | 10.04 ± 0.92 |
| NAc | ||||||||||
|
| ||||||||||
| LFD | 54.29 ± 8.50 | 31.53 ± 2.83 | 11.03 ± 0.89 | ND | ND | 56.09 ± 6.19 | 35.74 ± 1.97 | 15.36 ± 0.73 | ND | ND |
| HFD | 64.27 ± 7.06 | 30.73 ± 1.44 | 10.94 ± 0.79 | ND | ND | 58.25 ± 3.60 | 42.19± 3.09 | 18.22 ± 1.75 | ND | ND |
| STR | ||||||||||
|
| ||||||||||
| LFD | 121.62 ± 10.65 | 32.46 ± 2.09 | 13.69 ± 0.78 | ND | ND | 79.47 ± 2.64 | 35.35 ± 3.90 | 16.78 ± 0.47 | ND | ND |
| HFD | 120.30 ± 3.92 | 30.14 ± 2.28 | 14.74 ± 0.80 | ND | ND | 77.71 ± 6.28 | 33.85 ± 2.74 | 16.86 ± 1.10 | ND | ND |
| dHIP | ||||||||||
|
| ||||||||||
| LFD | 0.30 ± 0.10 | 5.99 ± 0.39 | 1.03 ± 0.17 | 6.13 ± 0.79 | 23.90 ± 1.21 | 0.57 ± 0.21 | 6.28 ± 0.16 | 1.07 ± 0.20 | 6.64 ± 1.06 | 24.45 ± 1.45 |
| HFD | 0.18 ± 0.05 | 6.87 ± 0.37 | 0.76 ± 0.24 | 6.98 ± 0.23 | 26.06 ± 2.46 | 0.43 ± 0.12 | 7.80 ± 0.39* | 1.82 ± 0.36 | 7.64 ± 0.88 | 25.12 ± 2.09 |
| vHIP | ||||||||||
|
| ||||||||||
| LFD | 0.20 ± 0.06 | 4.22 ± 0.38 | 0.70 ± 0.11 | 4.33 ± 0.35 | 16.98 ± 1.66 | 0.42 ± 0.15 | 5.06 ± 0.36 | 1.13 ± 0.28 | 4.47 ± 0.25 | 19.78 ± 1.62 |
| HFD | 0.20 ± 0.08 | 6.17 ± 0.59* | 0.78 ± 0.25 | 6.73 ± 0.74* | 25.25 ± 2.50* | 0.35 ± 0.10 | 6.05 ± 0.67 | 1.53 ± 0.34 | 5.52 ± 0.58 | 21.58 ± 3.00 |
Abbreviations: PFC: prefrontal cortex; NAc: nucleus accumbens; STR: striatum; dHIP: dorsal hippocampus; vHIP: ventral hippocampus; DA: dopamine; DOPAC: dihydroxyphenylacetic acid; HVA: homovanillic acid; NE: norepinephrine; MHPG: 3-methoxy-4-hydroxyphenylglycol; ND: not detected/determined.
Data are presented as mean ± SEM; unit: ng/mg protein
indicates significant difference from LFD within a time point (P ≤ 0.05).
In the NAc, 22 or 36 weeks of HFD feeding did not cause any alterations of DA or its metabolites (P ≥ 0.10; Table 1). Neither 22 (P ≤ 0.06; data not shown) nor 36 (P ≥ 0.15; data not shown) weeks of HFD intake significantly affected the NAc 5-HT and 5-HIAA levels.
Striatal concentrations of DA or its metabolites (P ≥ 0.25; Table 1), as well as of 5-HT or its metabolite (P ≥ 0.09; data not shown) were unaffected by HFD regardless of feeding duration.
Dorsal hippocampal neurochemistry remained unaffected after 22 weeks of HFD consumption (P ≥ 0.10; Fig. 9C, D and Table 1). However, continued HFD intake significantly increased and decreased, respectively, DOPAC (P ≤ 0.01; Table 1) and 5-HIAA (P ≤ 0.01; Fig. 9D) levels after 36 weeks on HFD; their parent (DA and 5-HT, respectively) neurotransmitters were not affected (P ≥ 0.55; Fig. 9C and Table 1).
Contrary to the lack of HFD effect on the dorsal hippocampal neurochemistry after 22 weeks on HFD, DA and NE monoamine homeostasis was noticeably affected in the vHIP at this time point (Table 1). Specifically, 22 week HFD intake significantly increased the concentrations of the DA metabolite DOPAC and of NE and its metabolite MHPG (P ≤ 0.05; Table 1); vHIP 5-HT homeostasis was unaltered after 22 weeks on HFD (P ≥ 0.40; Fig. 9E and F). By 36 weeks, similar to HFD's effect in the dHIP, vHIP 5-HT homeostasis (decreased 5-HIAA, P ≤ 0.01; Fig. 9F) was compromised.
4. Discussion
Epidemiological evidence has associated increased high-fat consumption and obesity with the development of metabolic syndrome and neurological deficits [38–40]. Despite the progressive and chronic nature of excessive dietary fat intake, most of the experimental studies have evaluated the adverse effects of HFD consumption after a single time point and have mostly done so in males. In this study, a continuum of our previous work which investigated the central effects of relatively short-term (5–6 weeks) HFD consumption in female mice [1], we sought to determine the associated/concomitant peripheral metabolic effects at this relatively early time point after HFD intake and to follow the progression of selected central and peripheral aberrations in the event of continued HFD consumption. The main findings from this work include: (i) continued HFD consumption caused an obese phenotype associated with early and sustained visceral (6, 22 and 36 weeks) and delayed intra-organ (liver and kidneys; 36 weeks) fat deposition; (ii) HFD-induced glucose intolerance was apparent as early as after 5 weeks on HFD, persisted throughout the feeding duration, and was greatest at the end of the study; (iii) HFD-induced insulin resistance at the early and intermediate time points was not apparent at the late stages, likely due to age-related decrease in insulin sensitivity seen in control, LFD-fed mice; (iv) chronic HFD feeding increased gastrointestinal permeability and induced bi-phasic hepatic inflammation; (v) behaviorally, the HFD-fed mice were hypoactive, less coordinated, and less anxious, but these effects were mostly seen at the end of the dietary study; (vi) neurochemically, hippocampal serotonergic dysfunction was the most prominent effect and it was observed after prolonged HFD intake.
HFD-induced accumulation of visceral fat (evidenced by the decrease in the relative organ weights) and subsequent increase in body weight has been demonstrated by multiple animal studies [41, 42]. Although, in the female mice, HFD's effect on body weight was apparent as early as after 6 weeks on the diet, it became more pronounced with continued HFD consumption. Prolonged HFD consumption resulted in an advanced obese phenotype characterized by exaggerated weight gain and increased absolute liver and kidney weights, suggesting that the weight gain is at this stage is not only due to intra-abdominal, but also to intra-organ (liver, kidneys) fat deposition as chronic HFD-induce increase in absolute liver weight correlates with increased intra-organ fat deposition [43]. Preferentially increased visceral fat accumulation has been observed in female, but not male mice, fed HFD for a chronic period, suggestive of the sex-specific differences in the visceral adiposity upon exposure to HFD [44]. Interestingly, similar sex-specific pattern of fat distribution has been observed in humans with obese females showing excess visceral deposition as compared to obese males [44] and the greater female susceptibility to high-carbohydrate diet induced non-alcoholic liver disease was attributed in part to visceral fat-specific signaling defects [45].
There appears to be a close association between obesity and inflammation; inflammation in insulin-sensitive tissues, such as liver and adipose tissue, is implicated in the development of insulin resistance [46]. Obesity-associated disruption in the intestinal epithelial barrier integrity and subsequent translocation of bacterial components from the gut into the circulation has been associated with the development of systemic metabolic abnormalities [12, 36], in part by enhanced inflammation and downregulation of insulin signaling proteins [15, 16]. In this study, HFD increased gastrointestinal permeability, evidenced by an increased plasma FITC-dextran level, with the effect being more prominent at the latter stages of the feeding duration. Hence, it is conceivable that the increased gut permeability might have contributed to the impaired glucose tolerance and insulin resistance we observed, but this needs to be studied in more detail in the future.
The glucose intolerance, which was observed as early as after 5 weeks of HFD feeding, persisted with continued HFD consumption, and was most pronounced towards the end of the study; this suggests that in female C57BL/6 mice impaired glucose tolerance appears early, persists, and is exacerbated with continued HFD intake. Besides being glucose intolerant, mice were insensitive to insulin after short-term (5 weeks) HFD feeding, which has been demonstrated elsewhere [17, 47]. Interestingly, we found that compared to matched LFD controls HFD-fed mice remained insulin-resistant after 20, but not after 33 weeks on HFD. The lack of HFD effect at 33 weeks is likely due to age-related, diet-independent loss of insulin sensitivity [48]. In support of this, we found that after 5 weeks, the control LFD-fed mice (young adults at the time) were highly insulin sensitive. However, by 33 weeks (middle-aged adults at this stage), the LFD-fed mice, while still glucose-tolerant, were relatively insulin-resistant, which is indicative of a normal non-insulin mediated uptake of glucose from the circulation [49]; on the contrary, the non-insulin dependent resolution of glycemia is highly susceptible to the effects of HFD. Central administration of hormones, such as leptin or fibroblast growth factor 19, restored glucose tolerance in insulin- or leptin-deficient diabetic rodent models; the anti-diabetic effect of these hormones was attributed to an increased glucose uptake in tissues such as skeletal muscle, heart and brown adipose tissue [50]. In this regard, the normal glucose tolerance (in the presence of impaired insulin sensitivity) exhibited by LFD mice at the later stage could be due to a compensatory increase in hormones that are implicated in glucose disposal, such as leptin. Overall, our data are suggestive of an age-dependent differential mechanism in the regulation of peripheral glucose uptake that allows for the maintenance of normal glucose tolerance in the face of some insulin resistance; this interesting finding needs to be investigated further.
In order to assess whether the observed HFD-induced alterations of glucose homeostasis were associated with peripheral tissue inflammation, we evaluated the expression of key inflammatory molecules in the liver. Inflammation and subsequent development of insulin resistance has been demonstrated before [51–53]. In this study, hepatic expression of TNFα was unchanged, but the expression of both IL-6 and Hp was significantly upregulated after 6 weeks of HFD feeding. Increased hepatic IL-6 levels, depending on timing could be viewed its pro- or anti-inflammatory [54], but based on the concurrent rise in the hepatic Hp and the general tight correlation between inflammation and inflammatory cytokine-induced Hp expression [55, 56], it could be inferred that the liver of the HFD-fed mice was inflamed at this stage of HFD intake. Compared to the early response, liver was relatively less vulnerable to HFD (evidenced by unaltered mRNA levels of TNFα, IL-6 and Hp) after 22 weeks of HFD feeding, suggesting the development of transient hepatic tolerance to HFD. Similar to 22 week data, levels of TNFα and IL-6 remained unchanged in the livers of 36-week HFD-fed mice, but Hp was upregulated. In humans, C-reactive protein and serum amyloid A (acute phase proteins linked to systemic insulin resistance) showed an initial rise and rapid decline within 24–72 h following an inflammatory stimulus, whereas Hp remained elevated 10 days after the onset of inflammation [57]. Of note, liver Hp levels that increased after 1 week of HFD feeding remained elevated after 16 weeks of HFD intake in male mice [58]. Put together, our female mice data showing recurrent increase in the hepatic Hp levels (even in the absence of a significant change in TNFα or IL-6) during the late stage of HFD feeding together with the consistent increase in the HFD-induced gut permeability (plasma FITC-dextran levels) is indicative of an ongoing chronic low-grade inflammation.
Sex-specific differences in the development of obesity and associated metabolic abnormalities have been reported by several animal and human studies. Specifically, studies suggest that males are more susceptible to obesity than females [59–61]; the protection against obesity in females is attributed to the direct effects of the circulating estrogen levels on insulin/glucose homeostasis, body fat distribution and pro-inflammatory markers [62, 63]. Estrogen also downregulates hepatic lipogenesis [64, 65], which could be a potential reason for why the hepatic fat deposition (evidenced by the increased absolute liver weight) in our female mice was delayed and was observed only during the later stages of HFD intake. However, it is worth noting that several animal studies, including ours, have demonstrated peripheral (adipose tissue/liver) inflammation, metabolic abnormalities and visceral fat accumulation even within relatively shorter duration of HFD intake in female subjects [66–68]. Importantly, several conflicting results have been reported regarding the anti- or pro-inflammatory actions of estrogens in both animals and humans [69–73]. Nevertheless, the modest effect of chronic HFD intake on hepatic inflammation (characterized by elevation only in Hp, but not TNFα or IL-6, levels after 36 weeks on HFD) and the delay in hepatic fat accumulation (evidenced by increased absolute liver weight after 36, but not 6 or 22, weeks on HFD) observed in our mice is in line with previous reports showing early onset of fat infiltration or inflammation in the liver in males and relative lesser susceptibility to HFD-induced liver damage and non-alcoholic fatty liver disease (NAFLD) in females [74–77]. However, additional studies conducting side-by-side comparisons of males and females over a range of feeding durations, which also monitor, among others, circulating estrogen levels in both sexes, are needed to investigate this apparent sex-specific difference in susceptibility to NAFLD in more detail.
Several animal studies exist on HFD-induced alteration in the circulating estrogen levels and associated changes in reproductive functions of female subjects [78–80]. We did not investigate the hormonal effects of HFD feeding in the current study as it was not our primary goal. However, in our prior neurobehavioral and electrophysiological study we found that the distribution of mice across the stages of the estrus cycle was not different between the LFD and HFD groups after 6 or 11–12 weeks on respective diets [1], suggesting that the data at the early time point in the current study are not affected by differential cyclicity, which is estrogen-dependent [81]. While earlier research from Balasubramanian et al. [79] demonstrated estrus cycle irregularities following HFD consumption it did so in diet induced obese (DIO) rats (generated by selective breeding of outbred Sprague-Dawley rats over generations to retain their propensity to gain body weight), which are genetically predisposed to diet-induced obesity. We cannot exclude the possibility of HFD-fed female mice showing altered estrus cyclicity as a consequence of chronic (22 or 36 weeks) intake of dietary fat in our study, but age-dependent, diet-independent loss of normal cyclicity [81], might have mitigated dietary influences in the oldest (36 weeks on the diet) mice. With regard to the HFD-induced estrus cycle irregularities, earlier studies have demonstrated obese female mice spending more days in estrus phase [60], while others have shown prolongation of diestrus in HFD-fed mice [78]. Hence, it is possible that following chronic HFD intake, the female mice in our study might cycle irregularly and hence their estrus cycle stage could confound the behavioral endpoints measured. Intriguingly, it has been demonstrated that the phase of the estrus cycle per se has no significant effect on the behavior of C57BL/6 mice in open field or in anxiety-related tests and that their behavior remains stable across different phases of the estrus cycle [82]. This, together with the lack of increased variability of the behavioral data from the HFD-fed mice and the fact all mice used in this study were housed in the same room and hence the likelihood of them cycling through the same estrus phase is high [83], that suggests that HFD intake is less likely to have had a significant impact on the behavioral endpoints we measured in the current study.
Prior to our study, information on the time-course of behavioral changes in the face of continued HFD feeding and in females was lacking. Earlier, we demonstrated that 5 weeks of HFD intake increases locomotor activity in female mice [1]. Here, we found that the HFD's effect on locomotor activity was no longer evident by 21 weeks which is in line with a previous report showing absence of locomotor deficits in mice fed HFD for similar duration [7]. Interestingly, mice with advanced obese phenotype caused by continued HFD consumption for another 11 weeks (total 32 weeks) displayed locomotor deficits, characterized by decreased locomotor activity in the open field test. Taken together, our previous [1] and current findings demonstrate a biphasic effect of HFD consumption on locomotor activity in the female with the initial increased locomotor activity dissipating over time and further following an opposite trend (decreased locomotion) with continued HFD consumption, likely due to an advanced obese phenotype. Besides diet, age is another factor that could influence the locomotor activity of mice in this study. Generally, locomotion decreases with advancing age in humans [84]; however, animal studies have reported inconsistent results regarding the age-related changes in the locomotor behavior. For example, some studies have demonstrated age-related decline in locomotor activity in WT mice [85–87], while others have reported no significant age-related changes in locomotion [88, 89]. We found a significant increase in locomotion in the control LFD mice at 32 weeks compared to the 21 week time point, but our mice were not aged as the mice in [85–87].
Previously we also found that 5 weeks of HFD feeding to female mice resulted in a trend towards increased grip strength [1]. The significant increase in forelimb grip strength after 21 weeks on HFD observed in this study is indicative of an increased skeletal muscle mass and muscle strength in addition to the increase in HFD-induced body fat mass. This phenomenon has been observed in humans when overweight individuals' absolute muscle strength and power are increased compared to that of lean counterparts [90, 91]. However, given the unaltered grip strength after the late stage (32 weeks) of HFD intake, it seems that after a certain time point the increase in body fat content does not translate into further increase of muscle mass and strength; this has also been observed in humans [92, 93]. While some studies have demonstrated a decrease in muscle strength with advancing age [85–87], we found a significant increase in the forelimb grip strength of the control LFD mice at 32 weeks compared to the 21 week time point. However, even after prolonged LFD/HFD feeding our mice were less than 1 year old, whereas the decreased muscle strength is observed in much older (≥ 20 months) mice [85–87].
Several studies with male rodents have demonstrated emotional disturbances, characterized by increased anxiety and/or depression following HFD consumption [7, 94]. Our prior work showed elevated anxiety-like behavior in female mice fed a HFD for 5 weeks [1]. Here, we found that HFD consumption for 21 weeks did not induce anxiety-like behavior in the open field, FST or MBT tests. Increasing evidence has shown the existence of a hippocampal regional dissociation, with the dHIP and vHIP showing, respectively, preferential roles in the modulation of spatial memory and anxiety-related behaviors [95–97]. Besides vHIP, PFC is another forebrain region that plays an important role in emotional regulation [98]. Specifically, PFC acts as a downstream target in the modulation of anxiety-like behaviors (because of the direct efferent projections to it from the vHIP) and thus, not surprisingly, a functional interaction between these two brain regions during anxiety has been reported [95]. Importantly, it has been recently shown that a synchronized interaction between both PFC and vHIP and not necessarily activity alone in either brain region, is crucial to drive anxiety-like behaviors [99]. Interestingly, the increased anxiety exhibited by the HFD-fed female mice in our prior study was associated with significantly altered monoamine homeostasis in both PFC and vHIP [1]. That said, our findings of a lack of an apparent change in PFC monoamine homeostasis (even in the presence of a significantly altered ventral hippocampal monoamine [DA and NE] balance) in conjunction with the unaltered anxiety level exhibited by mice at 21 weeks suggest that a monoamine imbalance in both prefrontocortical and ventral hippocampal (and not necessarily vHIP alone) brain regions is necessary for an altered anxiety response to be observed.
By 32 weeks, the HFD-fed mice spent decreased time in the center and increased time in the corner of the open field arena, which is typically indicative of an increased anxiety level [100, 101]. On the contrary, the observed drastic reduction in the number of marbles buried by the 32-week HFD-fed mice as compared to the age-matched LFD controls generally suggests decreased anxiety [102]. Our neurochemical data offer an explanation of this apparent discordant interpretation of the open field and MBT tests. We found that the serotonergic, but not dopaminergic or noradrenergic signaling was significantly altered; 32-week HFD-fed mice had decreased hippocampal 5-HT utilization or 5-HT turnover (evidenced by the decreased 5-HT metabolite concentration). Information on the mechanisms underlying HFD-induced serotonergic dyshomeostasis and how it translates to an altered mood response is limited. Recently, the anxiogenic/depressive-like phenotype of mice fed a HFD was attributed to an increased sensitivity of the dorsal raphe 5-HT1A autoreceptor and subsequent reduction in hippocampal 5-HT level [103]. Additionally, the involvement of HFD-induced neuroinflammatory changes in brain regions associated with mood regulation, such as hippocampus has been reported [104]. Specifically, enhanced brain cytokine expression associated with high-fat consumption and obesity has been related to an increased activation of indoleamine 2,3 dioxygenase (IDO) that can lead to decreased tryptophan (the primary amino acid precursor of 5-HT), which in turn can contribute to decreased 5-HT synthesis [104, 105]. In this regard, the impaired serotonergic homeostasis we observed in chronically HFD-fed obese female mice in our study might be associated with an increased state of neuroinflammation that is in turn stemming from HFD-induced peripheral (hepatic) inflammation and metabolic dysregulation. Unlike the dietary effect, the brain serotonin levels remained relatively unaffected with advancing age in this study as demonstrated previously [106].
Interestingly, earlier studies have reported sex-specific differences in serotonergic activity with female rats showing greater serotonin metabolism or serotonin utilization (evidenced by much higher 5-HIAA/5-HT ratio in the dorsal raphe nucleus (midbrain structure involved in anxiety regulation) compared to male rats [107]. Importantly, it was shown that the observed increase in serotonergic activity of female rats was independent of their estrus cycle stage [107]. Similarly, another study showed that although the 5-HT level in the auditory midbrain changed in response to changes in the behavioral context, the observed effect was independent of the estrus phase of the female mice tested [108]. Hence, in light of these findings, we posit that even if there is any variation in the estrus cycle stage induced by chronic dietary fat intake in this study, the chances of it affecting the 5-HT metabolism is remote.
Different forebrain regions, such as PFC, dHIP and vHIP, regulate mood and anxiety [95, 109, 110]; dopaminergic, noradrenergic and/or serotonergic dysregulations have been implicated in emotional disorders, such as anxiety and/or depression [109, 111, 112]. For example, several animal studies have associated increased dopaminergic or serotonergic utilization and/or turnover with increased anxiety-like behavior [113–115]. That said, the decreased forebrain serotonergic turnover observed in this study after 36 weeks of HFD feeding is suggestive of reduced anxiety; hence, the behavior exhibited by the mice in the MBT, and not the open field test, could be viewed as a more accurate reflection of their anxiety-like response. Taking into account the decreased locomotor activity of the mice in the open field test, their increased corner time or decreased center time could merely be a result of their overall decreased locomotion and not necessarily a manifestation of altered anxiety level [116]. Collectively, our previous 5-week neurobehavioral data [1] combined with the present data suggest that in female mice, the HFD-induced locomotor and emotional deficits are fluid, with the initially exhibited hyperactivity and increased anxiety [1] transitioning to hypoactivity and reduced anxiety in advanced obese phenotypes. Studies in male rodents have reported that HFD can act as an anxiogenic or as an anxiolytic agent depending on the duration of HFD exposure [8]. Specifically, in male rodents, short-term HFD feeding has been found to decrease anxiety [117, 118], whereas long-term HFD consumption increases it [8, 119]. Our data suggest that the female response to HFD in terms of anxiety may differ from the one in males, but this needs to be investigated.
Previously, we also found that relatively short (5 weeks) HFD feeding did not affect the short-term non-spatial object recognition memory [1]. Here, we show that mice displayed intact short-term NOR memory after 21 or 32 weeks of HFD feeding, which suggests that HFD consumption by female mice, regardless of the duration, does not affect their short-term non-spatial memory. These findings are consistent with earlier studies in male rodents that demonstrated intact recognition memory after short- or long-term HFD feeding [7, 120, 121]. However, the decreased NOR performance observed in middle-aged (9-month) male mice fed HFD for a chronic period (4 months) [122] indicates that the HFD's effect on non-spatial recognition memory might have a different outcome when HFD diet is initiated at a later stage of life.
Chronic low-grade central/peripheral inflammation is implicated in the development of neurodegenerative disorders and metabolic dysfunctions [123–125]. In this study, prolonged (36 weeks) HFD consumption resulted in an advanced obese phenotype in female mice with an associated increase in hepatic inflammation (increased Hp levels) and development of metabolic dysfunction, such as glucose intolerance and insulin resistance. Interestingly, metabolic dysregulation, including impaired glucose metabolism and abnormal appetite regulation, exhibits comorbidity with neurodegenerative disorders [126, 127]; restoration of metabolic homeostasis improves motor deficits in neurodegenerative diseases [126]. Intriguingly, chronic liver diseases, such as hepatitis and cirrhosis, are comorbid with several neurological deficits in humans, with neuroinflammation induced by inflammatory changes in the periphery implicated as the culprit [128]. Hence, the neurological deficits we observed in chronically HFD-fed obese female mice might be associated with an increased state of neuroinflammation, resulting from HFD-induced peripheral (hepatic) inflammation and metabolic dysfunction; studies focusing on the chronic HFD-induced inflammatory changes in the female brain are thus warranted.
In summary, after taking into account the findings from our prior study [1] and the current results, it appears that in female mice, some of the HFD-induced behavioral effects (locomotor activity and anxiety) change over time, whereas certain metabolic effects (glucose intolerance) appear early and persist and/or are exaggerated with continuous HFD feeding. Age-related alterations may obscure some effects of chronic HFD (i.e. insulin insensitivity). Collectively, our findings imply that in female mice, a constant intake of high-fat products over a chronic period leads to an exaggerated obese state which is associated with increased peripheral inflammatory tone, metabolic dysregulation and motor/emotional disturbances.
Highlights.
-
■
HFD-induced glucose intolerance increases with continued HFD intake in female mice.
-
■
HFD induces insulin resistance that is masked by age-dependent insulin sensitivity loss.
-
■
Chronic HFD intake causes bi-phasic increase in the liver inflammatory marker Hp.
-
■
Chronic HFD intake results in reduced locomotor activity and anxiety-like behavior.
-
■
Prolonged HFD intake compromises hippocampal serotonin homeostasis.
Acknowledgements
This project was supported in part with funds from the University of Georgia's Obesity Initiative (http://obesity.ovpr.uga.edu/), by University of Georgia Research Foundation (UGARF) and Georgia Research Alliance (GRA) start-up funds provided to DAH, and by T35 OD010433 (NIH), which provided summer research support to LMP and DMJ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement The authors declare that there are no conflicts of interest.
References
- [1].Krishna S, Keralapurath MM, Lin Z, Wagner JJ, de La Serre CB, Harn DA, et al. Neurochemical and electrophysiological deficits in the ventral hippocampus and selective behavioral alterations caused by high-fat diet in female C57BL/6 mice. Neuroscience. 2015;297:170–81. doi: 10.1016/j.neuroscience.2015.03.068. [DOI] [PubMed] [Google Scholar]
- [2].James PT, Leach R, Kalamara E, Shayeghi M. The worldwide obesity epidemic. Obes Res. 2001;9(Suppl 4):228S–33S. doi: 10.1038/oby.2001.123. [DOI] [PubMed] [Google Scholar]
- [3].Hebebrand J, Hinney A. Environmental and genetic risk factors in obesity. Child Adolesc Psychiatr Clin N Am. 2009;18:83–94. doi: 10.1016/j.chc.2008.07.006. [DOI] [PubMed] [Google Scholar]
- [4].Jacobs DR., Jr. Fast food and sedentary lifestyle: a combination that leads to obesity. Am J Clin Nutr. 2006;83:189–90. doi: 10.1093/ajcn/83.2.189. [DOI] [PubMed] [Google Scholar]
- [5].Swinburn BA, Caterson I, Seidell JC, James WP. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004;7:123–46. doi: 10.1079/phn2003585. [DOI] [PubMed] [Google Scholar]
- [6].Kang SS, Kurti A, Fair DA, Fryer JD. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J Neuroinflammation. 2014;11:156. doi: 10.1186/s12974-014-0156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Heyward FD, Walton RG, Carle MS, Coleman MA, Garvey WT, Sweatt JD. Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiol Learn Mem. 2012;98:25–32. doi: 10.1016/j.nlm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Del Rosario A, McDermott MM, Panee J. Effects of a high-fat diet and bamboo extract supplement on anxiety- and depression-like neurobehaviours in mice. Br J Nutr. 2012;108:1143–9. doi: 10.1017/S0007114511006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond) 2013;37:382–9. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- [10].Mizunoya W, Ohnuki K, Baba K, Miyahara H, Shimizu N, Tabata K, et al. Effect of dietary fat type on anxiety-like and depression-like behavior in mice. Springerplus. 2013;2:165. doi: 10.1186/2193-1801-2-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- [12].Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- [13].Gao X, Liu X, Xu J, Xue C, Xue Y, Wang Y. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118:476–81. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- [14].Backhed F, Normark S, Schweda EK, Oscarson S, Richter-Dahlfors A. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 2003;5:1057–63. doi: 10.1016/s1286-4579(03)00207-7. [DOI] [PubMed] [Google Scholar]
- [15].Jager J, Gremeaux T, Cormont M, Le Marchand-Brustel Y, Tanti JF. Interleukin-1beta-induced insulin resistance in adipocytes through down-regulation of insulin receptor substrate-1 expression. Endocrinology. 2007;148:241–51. doi: 10.1210/en.2006-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–74. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cano PG, Santacruz A, Trejo FM, Sanz Y. Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring) 2013;21:2310–21. doi: 10.1002/oby.20330. [DOI] [PubMed] [Google Scholar]
- [18].McDonald SD, Pesarchuk E, Don-Wauchope A, El Zimaity H, Holloway AC. Adverse metabolic effects of a hypercaloric, high-fat diet in rodents precede observable changes in body weight. Nutr Res. 2011;31:707–14. doi: 10.1016/j.nutres.2011.08.009. [DOI] [PubMed] [Google Scholar]
- [19].El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–32. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–94. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- [21].Lin S, Thomas TC, Storlien LH, Huang XF. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int J Obes Relat Metab Disord. 2000;24:639–46. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- [22].Gallou-Kabani C, Vige A, Gross MS, Rabes JP, Boileau C, Larue-Achagiotis C, et al. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity (Silver Spring) 2007;15:1996–2005. doi: 10.1038/oby.2007.238. [DOI] [PubMed] [Google Scholar]
- [23].Matyskova R, Maletinska L, Maixnerova J, Pirnik Z, Kiss A, Zelezna B. Comparison of the obesity phenotypes related to monosodium glutamate effect on arcuate nucleus and/or the high fat diet feeding in C57BL/6 and NMRI mice. Physiol Res. 2008;57:727–34. doi: 10.33549/physiolres.931274. [DOI] [PubMed] [Google Scholar]
- [24].Haleem DJ, Haque Z, Inam QU, Ikram H, Haleem MA. Behavioral, hormonal and central serotonin modulating effects of injected leptin. Peptides. 2015;74:1–8. doi: 10.1016/j.peptides.2015.10.002. [DOI] [PubMed] [Google Scholar]
- [25].Kesby JP, Kim JJ, Scadeng M, Woods G, Kado DM, Olefsky JM, et al. Spatial Cognition in Adult and Aged Mice Exposed to High-Fat Diet. PLoS One. 2015;10:e0140034. doi: 10.1371/journal.pone.0140034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Warden CH, Fisler JS. Comparisons of diets used in animal models of high-fat feeding. Cell Metab. 2008;7:277. doi: 10.1016/j.cmet.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sasidharan SR, Joseph JA, Anandakumar S, Venkatesan V, Ariyattu Madhavan CN, Agarwal A. An experimental approach for selecting appropriate rodent diets for research studies on metabolic disorders. Biomed Res Int. 2013;2013:752870. doi: 10.1155/2013/752870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Masuzaki H, Paterson J, Shinyama H, Morton NM, Mullins JJ, Seckl JR, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–70. doi: 10.1126/science.1066285. [DOI] [PubMed] [Google Scholar]
- [29].Hong Q, Xia C, Xiangying H, Quan Y. Capsinoids suppress fat accumulation via lipid metabolism. Mol Med Rep. 2015;11:1669–74. doi: 10.3892/mmr.2014.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–12. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Krishna S, Dodd CA, Hekmatyar SK, Filipov NM. Brain deposition and neurotoxicity of manganese in adult mice exposed via the drinking water. Arch Toxicol. 2014;88:47–64. doi: 10.1007/s00204-013-1088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lin Z, Dodd CA, Filipov NM. Short-term atrazine exposure causes behavioral deficits and disrupts monoaminergic systems in male C57BL/6 mice. Neurotoxicol Teratol. 2013;39:26–35. doi: 10.1016/j.ntt.2013.06.002. [DOI] [PubMed] [Google Scholar]
- [33].Gaikwad U, Parle M, Kumar A, Gaikwad D. Effect of ritanserin and leuprolide alone and combined on marble-burying behavior of mice. Acta Pol Pharm. 2010;67:523–7. [PubMed] [Google Scholar]
- [34].Lin Z, Dodd CA, Xiao S, Krishna S, Ye X, Filipov NM. Gestational and lactational exposure to atrazine via the drinking water causes specific behavioral deficits and selectively alters monoaminergic systems in C57BL/6 mouse dams, juvenile and adult offspring. Toxicol Sci. 2014;141:90–102. doi: 10.1093/toxsci/kfu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Coban A, Filipov NM. Dopaminergic toxicity associated with oral exposure to the herbicide atrazine in juvenile male C57BL/6 mice. J Neurochem. 2007;100:1177–87. doi: 10.1111/j.1471-4159.2006.04294.x. [DOI] [PubMed] [Google Scholar]
- [36].de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299:G440–8. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rodriguez-Rivera J, Denner L, Dineley KT. Rosiglitazone reversal of Tg2576 cognitive deficits is independent of peripheral gluco-regulatory status. Behav Brain Res. 2011;216:255–61. doi: 10.1016/j.bbr.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gadalla TM. Association of obesity with mood and anxiety disorders in the adult general population. Chronic Dis Can. 2009;30:29–36. [PubMed] [Google Scholar]
- [39].Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70:288–97. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- [40].Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- [41].Chung YM, Hyun Lee J, Youl Kim D, Hwang SH, Hong YH, Kim SB, et al. Dietary D-psicose reduced visceral fat mass in high-fat diet-induced obese rats. J Food Sci. 2012;77:H53–8. doi: 10.1111/j.1750-3841.2011.02571.x. [DOI] [PubMed] [Google Scholar]
- [42].Murase T, Mizuno T, Omachi T, Onizawa K, Komine Y, Kondo H, et al. Dietary diacylglycerol suppresses high fat and high sucrose diet-induced body fat accumulation in C57BL/6J mice. J Lipid Res. 2001;42:372–8. [PubMed] [Google Scholar]
- [43].Cao M, Pan Q, Dong H, Yuan X, Li Y, Sun Z, et al. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res Ther. 2015;6:208. doi: 10.1186/s13287-015-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yasmeen R, Reichert B, Deiuliis J, Yang F, Lynch A, Meyers J, et al. Autocrine function of aldehyde dehydrogenase 1 as a determinant of diet- and sex-specific differences in visceral adiposity. Diabetes. 2013;62:124–36. doi: 10.2337/db11-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Spruss A, Henkel J, Kanuri G, Blank D, Puschel GP, Bischoff SC, et al. Female mice are more susceptible to nonalcoholic fatty liver disease: sex-specific regulation of the hepatic AMP-activated protein kinase-plasminogen activator inhibitor 1 cascade, but not the hepatic endotoxin response. Mol Med. 2012;18:1346–55. doi: 10.2119/molmed.2012.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zeyda M, Stulnig TM. Obesity, inflammation, and insulin resistance--a mini-review. Gerontology. 2009;55:379–86. doi: 10.1159/000212758. [DOI] [PubMed] [Google Scholar]
- [47].Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16:180–8. doi: 10.1016/j.cmet.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Escriva F, Gavete ML, Fermin Y, Perez C, Gallardo N, Alvarez C, et al. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol. 2007;194:131–41. doi: 10.1677/joe.1.07043. [DOI] [PubMed] [Google Scholar]
- [49].Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–74. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- [50].Schwartz MW, Seeley RJ, Tschop MH, Woods SC, Morton GJ, Myers MG, et al. Cooperation between brain and islet in glucose homeostasis and diabetes. Nature. 2013;503:59–66. doi: 10.1038/nature12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lanthier N, Molendi-Coste O, Horsmans Y, van Rooijen N, Cani PD, Leclercq IA. Kupffer cell activation is a causal factor for hepatic insulin resistance. Am J Physiol Gastrointest Liver Physiol. 2010;298:G107–16. doi: 10.1152/ajpgi.00391.2009. [DOI] [PubMed] [Google Scholar]
- [52].Ma Y, Gao M, Liu D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm Res. 2015;32:1200–9. doi: 10.1007/s11095-014-1526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liu Z, Patil IY, Jiang T, Sancheti H, Walsh JP, Stiles BL, et al. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS One. 2015;10:e0128274. doi: 10.1371/journal.pone.0128274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- [55].Huntoon KM, Wang Y, Eppolito CA, Barbour KW, Berger FG, Shrikant PA, et al. The acute phase protein haptoglobin regulates host immunity. J Leukoc Biol. 2008;84:170–81. doi: 10.1189/jlb.0208100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sharpe-Timms KL, Nabli H, Zimmer RL, Birt JA, Davis JW. Inflammatory cytokines differentially up-regulate human endometrial haptoglobin production in women with endometriosis. Hum Reprod. 2010;25:1241–50. doi: 10.1093/humrep/deq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- [58].Williams LM, Campbell FM, Drew JE, Koch C, Hoggard N, Rees WD, et al. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PLoS One. 2014;9:e106159. doi: 10.1371/journal.pone.0106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hong J, Stubbins RE, Smith RR, Harvey AE, Nunez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutr J. 2009;8:11. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Litwak SA, Wilson JL, Chen W, Garcia-Rudaz C, Khaksari M, Cowley MA, et al. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology. 2014;155:4447–60. doi: 10.1210/en.2014-1342. [DOI] [PubMed] [Google Scholar]
- [61].Pettersson US, Walden TB, Carlsson PO, Jansson L, Phillipson M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One. 2012;7:e46057. doi: 10.1371/journal.pone.0046057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gend Med. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Gupte AA, Pownall HJ, Hamilton DJ. Estrogen: an emerging regulator of insulin action and mitochondrial function. J Diabetes Res. 2015;2015:916585. doi: 10.1155/2015/916585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Gao H, Bryzgalova G, Hedman E, Khan A, Efendic S, Gustafsson JA, et al. Long-term administration of estradiol decreases expression of hepatic lipogenic genes and improves insulin sensitivity in ob/ob mice: a possible mechanism is through direct regulation of signal transducer and activator of transcription 3. Mol Endocrinol. 2006;20:1287–99. doi: 10.1210/me.2006-0012. [DOI] [PubMed] [Google Scholar]
- [65].Hewitt KN, Pratis K, Jones ME, Simpson ER. Estrogen replacement reverses the hepatic steatosis phenotype in the male aromatase knockout mouse. Endocrinology. 2004;145:1842–8. doi: 10.1210/en.2003-1369. [DOI] [PubMed] [Google Scholar]
- [66].Gao M, Ma Y, Liu D. High-fat diet-induced adiposity, adipose inflammation, hepatic steatosis and hyperinsulinemia in outbred CD-1 mice. PLoS One. 2015;10:e0119784. doi: 10.1371/journal.pone.0119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(Suppl 3):S215–9. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- [68].Altunkaynak BZ. Effects of high fat diet induced obesity onfemale rat livers (a histochemical study) Eur J Gen Med. 2005;2:100–9. [Google Scholar]
- [69].Payette C, Blackburn P, Lamarche B, Tremblay A, Bergeron J, Lemieux I, et al. Sex differences in postprandial plasma tumor necrosis factor-alpha, interleukin-6, and C-reactive protein concentrations. Metabolism. 2009;58:1593–601. doi: 10.1016/j.metabol.2009.05.011. [DOI] [PubMed] [Google Scholar]
- [70].Kocak H, Oner-Iyidogan Y, Gurdol F, Oner P, Suzme R, Esin D, et al. Advanced oxidation protein products in obese women: its relation to insulin resistance and resistin. Clin Exp Med. 2007;7:173–8. doi: 10.1007/s10238-007-0143-x. [DOI] [PubMed] [Google Scholar]
- [71].Nilsson BO. Modulation of the inflammatory response by estrogens with focus on the endothelium and its interactions with leukocytes. Inflamm Res. 2007;56:269–73. doi: 10.1007/s00011-007-6198-z. [DOI] [PubMed] [Google Scholar]
- [72].Cushman M, Legault C, Barrett-Connor E, Stefanick ML, Kessler C, Judd HL, et al. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation. 1999;100:717–22. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- [73].Miller CN, Morton HP, Cooney PT, Winters TG, Ramseur KR, Rayalam S, et al. Acute exposure to high-fat diets increases hepatic expression of genes related to cell repair and remodeling in female rats. Nutr Res. 2014;34:85–93. doi: 10.1016/j.nutres.2013.10.010. [DOI] [PubMed] [Google Scholar]
- [74].Acedo SC, Caria CR, Gotardo EM, Pereira JA, Pedrazzoli J, Ribeiro ML, et al. Role of pentoxifylline in non-alcoholic fatty liver disease in high-fat diet-induced obesity in mice. World J Hepatol. 2015;7:2551–8. doi: 10.4254/wjh.v7.i24.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Safwat GM, Pisano S, D'Amore E, Borioni G, Napolitano M, Kamal AA, et al. Induction of non-alcoholic fatty liver disease and insulin resistance by feeding a high-fat diet in rats: does coenzyme Q monomethyl ether have a modulatory effect? Nutrition. 2009;25:1157–68. doi: 10.1016/j.nut.2009.02.009. [DOI] [PubMed] [Google Scholar]
- [76].Senthil Kumar SP, Shen M, Spicer EG, Goudjo-Ako AJ, Stumph JD, Zhang J, et al. Distinct metabolic effects following short-term exposure of different high-fat diets in male and female mice. Endocr J. 2014;61:457–70. doi: 10.1507/endocrj.ej13-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pan JJ, Fallon MB. Gender and racial differences in nonalcoholic fatty liver disease. World J Hepatol. 2014;6:274–83. doi: 10.4254/wjh.v6.i5.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Akamine EH, Marcal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, et al. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. 2010;206:65–74. doi: 10.1677/JOE-09-0461. [DOI] [PubMed] [Google Scholar]
- [79].Balasubramanian P, Jagannathan L, Mahaley RE, Subramanian M, Gilbreath ET, Mohankumar PS, et al. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol. 2012;24:748–55. doi: 10.1111/j.1365-2826.2011.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hilakivi-Clarke L, Cho E, Onojafe I. High-fat diet induces aggressive behavior in male mice and rats. Life Sci. 1996;58:1653–60. doi: 10.1016/0024-3205(96)00140-3. [DOI] [PubMed] [Google Scholar]
- [81].Nelson JF, Karelus K, Bergman MD, Felicio LS. Neuroendocrine involvement in aging: evidence from studies of reproductive aging and caloric restriction. Neurobiol Aging. 1995;16:837–43. doi: 10.1016/0197-4580(95)00072-m. discussion 55–6. [DOI] [PubMed] [Google Scholar]
- [82].Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- [83].Hayes AW, Kruger CL. Hayes' Principles and Methods of Toxicology. 6th Ed. CRC Press; New York: 2014. 2014. [Google Scholar]
- [84].Lexell J. Evidence for nervous system degeneration with advancing age. J Nutr. 1997;127:1011S–3S. doi: 10.1093/jn/127.5.1011S. [DOI] [PubMed] [Google Scholar]
- [85].Fahlstrom A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging. 2011;32:1868–80. doi: 10.1016/j.neurobiolaging.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [86].Fahlstrom A, Zeberg H, Ulfhake B. Changes in behaviors of male C57BL/6J mice across adult life span and effects of dietary restriction. Age. 2012;34:1435–52. doi: 10.1007/s11357-011-9320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Justice JN, Carter CS, Beck HJ, Gioscia-Ryan RA, McQueen M, Enoka RM, et al. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age. 2014;36:583–92. doi: 10.1007/s11357-013-9589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Li Y, Abdourahman A, Tamm JA, Pehrson AL, Sanchez C, Gulinello M. Reversal of age-associated cognitive deficits is accompanied by increased plasticity-related gene expression after chronic antidepressant administration in middle-aged mice. Pharmacol Biochem Behav. 2015;135:70–82. doi: 10.1016/j.pbb.2015.05.013. [DOI] [PubMed] [Google Scholar]
- [89].Weber M, Wu T, Hanson JE, Alam NM, Solanoy H, Ngu H, et al. Cognitive Deficits, Changes in Synaptic Function, and Brain Pathology in a Mouse Model of Normal Aging(1,2,3) eNeuro. 2015;2 doi: 10.1523/ENEURO.0047-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Hulens M, Vansant G, Lysens R, Claessens AL, Muls E, Brumagne S. Study of differences in peripheral muscle strength of lean versus obese women: an allometric approach. Int J Obes Relat Metab Disord. 2001;25:676–81. doi: 10.1038/sj.ijo.0801560. [DOI] [PubMed] [Google Scholar]
- [91].Hulens M, Vansant G, Lysens R, Claessens AL, Muls E. Assessment of isokinetic muscle strength in women who are obese. J Orthop Sports Phys Ther. 2002;32:347–56. doi: 10.2519/jospt.2002.32.7.347. [DOI] [PubMed] [Google Scholar]
- [92].Jackson AW, Lee DC, Sui X, Morrow JR, Jr., Church TS, Maslow AL, et al. Muscular strength is inversely related to prevalence and incidence of obesity in adult men. Obesity (Silver Spring) 2010;18:1988–95. doi: 10.1038/oby.2009.422. [DOI] [PubMed] [Google Scholar]
- [93].Stenholm S, Mehta NK, Elo IT, Heliovaara M, Koskinen S, Aromaa A. Obesity and muscle strength as long-term determinants of all-cause mortality--a 33-year follow-up of the Mini-Finland Health Examination Survey. Int J Obes (Lond) 2014;38:1126–32. doi: 10.1038/ijo.2013.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sharma S, Zhuang Y, Gomez-Pinilla F. High-fat diet transition reduces brain DHA levels associated with altered brain plasticity and behaviour. Sci Rep. 2012;2:431. doi: 10.1038/srep00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–69. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Bannerman DM, Grubb M, Deacon RM, Yee BK, Feldon J, Rawlins JN. Ventral hippocampal lesions affect anxiety but not spatial learning. Behav Brain Res. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- [97].Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [98].Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/s0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- [99].Schoenfeld TJ, Kloth AD, Hsueh B, Runkle MB, Kane GA, Wang SS, et al. Gap junctions in the ventral hippocampal-medial prefrontal pathway are involved in anxiety regulation. J Neurosci. 2014;34:15679–88. doi: 10.1523/JNEUROSCI.3234-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bailey KR, Crawley JN. Methods of Behavioral Analysis in Neuroscience. 2nd Ed. CRC Press; Florida: 2009. Anxiety-Related Behaviors in Mice. [PubMed] [Google Scholar]
- [101].Nguyen LN, Ma D, Shui G, Wong P, Cazenave-Gassiot A, Zhang X, et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature. 2014;509:503–6. doi: 10.1038/nature13241. [DOI] [PubMed] [Google Scholar]
- [102].de Almeida AA, de Carvalho RB, Silva OA, de Sousa DP, de Freitas RM. Potential antioxidant and anxiolytic effects of (+)-limonene epoxide in mice after marble-burying test. Pharmacol Biochem Behav. 2014;118:69–78. doi: 10.1016/j.pbb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- [103].Zemdegs J, Quesseveur G, Jarriault D, Penicaud L, Fioramonti X, Guiard BP. High fat diet-induced metabolic disorders impairs serotonergic function and anxiety-like behaviours in mice. Br J Pharmacol. 2015 doi: 10.1111/bph.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Castanon N, Luheshi G, Laye S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front Neurosci. 2015;9:229. doi: 10.3389/fnins.2015.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Miller AH. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23:149–58. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Morgan DG. The dopamine and serotonin systems during aging in human and rodent brain. A brief review. Prog Neuropsychopharmacol Biol Psychiatry. 1987;11:153–7. doi: 10.1016/0278-5846(87)90053-4. [DOI] [PubMed] [Google Scholar]
- [107].Dominguez R, Cruz-Morales SE, Carvalho MC, Xavier M, Brandao ML. Sex differences in serotonergic activity in dorsal and median raphe nucleus. Physiol Behav. 2003;80:203–10. doi: 10.1016/j.physbeh.2003.07.012. [DOI] [PubMed] [Google Scholar]
- [108].Hanson JL, Hurley LM. Context-dependent fluctuation of serotonin in the auditory midbrain: the influence of sex, reproductive state and experience. J Exp Biol. 2014;217:526–35. doi: 10.1242/jeb.087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Solati J, Zarrindast MR, Salari AA. Dorsal hippocampal opioidergic system modulates anxiety-like behaviors in adult male Wistar rats. Psychiatry Clin Neurosci. 2010;64:634–41. doi: 10.1111/j.1440-1819.2010.02143.x. [DOI] [PubMed] [Google Scholar]
- [111].Baskerville TA, Douglas AJ. Dopamine and oxytocin interactions underlying behaviors: potential contributions to behavioral disorders. CNS Neurosci Ther. 2010;16:e92–123. doi: 10.1111/j.1755-5949.2010.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7:9–13. doi: 10.2147/NDT.S19619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013;37:1022–31. doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–81. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Richardson-Jones JW, Craige CP, Nguyen TH, Kung HF, Gardier AM, Dranovsky A, et al. Serotonin-1A autoreceptors are necessary and sufficient for the normal formation of circuits underlying innate anxiety. J Neurosci. 2011;31:6008–18. doi: 10.1523/JNEUROSCI.5836-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hiroi R, Neumaier JF. Estrogen decreases 5-HT1B autoreceptor mRNA in selective subregion of rat dorsal raphe nucleus: inverse association between gene expression and anxiety behavior in the open field. Neuroscience. 2009;158:456–64. doi: 10.1016/j.neuroscience.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Alsio J, Roman E, Olszewski PK, Jonsson P, Fredriksson R, Levine AS, et al. Inverse association of high-fat diet preference and anxiety-like behavior: a putative role for urocortin 2. Genes Brain Behav. 2009;8:193–202. doi: 10.1111/j.1601-183X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- [118].Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60:1039–42. doi: 10.1016/0031-9384(96)00135-7. [DOI] [PubMed] [Google Scholar]
- [119].Buchenauer T, Behrendt P, Bode FJ, Horn R, Brabant G, Stephan M, et al. Diet-induced obesity alters behavior as well as serum levels of corticosterone in F344 rats. Physiol Behav. 2009;98:563–9. doi: 10.1016/j.physbeh.2009.09.003. [DOI] [PubMed] [Google Scholar]
- [120].Jurdak N, Kanarek RB. Sucrose-induced obesity impairs novel object recognition learning in young rats. Physiol Behav. 2009;96:1–5. doi: 10.1016/j.physbeh.2008.07.023. [DOI] [PubMed] [Google Scholar]
- [121].Tucker KR, Godbey SJ, Thiebaud N, Fadool DA. Olfactory ability and object memory in three mouse models of varying body weight, metabolic hormones, and adiposity. Physiol Behav. 2012;107:424–32. doi: 10.1016/j.physbeh.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Carey AN, Gomes SM, Shukitt-Hale B. Blueberry Supplementation Improves Memory in Middle-Aged Mice Fed a High-Fat Diet. J Agric Food Chem. 2014 doi: 10.1021/jf404565s. [DOI] [PubMed] [Google Scholar]
- [123].Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–69. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Ferrari CC, Tarelli R. Parkinson's disease and systemic inflammation. Parkinsons Dis. 2011;2011:436813. doi: 10.4061/2011/436813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- [126].Cai H, Cong WN, Ji S, Rothman S, Maudsley S, Martin B. Metabolic dysfunction in Alzheimer's disease and related neurodegenerative disorders. Curr Alzheimer Res. 2012;9:5–17. doi: 10.2174/156720512799015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Dunn L, Allen GF, Mamais A, Ling H, Li A, Duberley KE, et al. Dysregulation of glucose metabolism is an early event in sporadic Parkinson's disease. Neurobiol Aging. 2014;35:1111–5. doi: 10.1016/j.neurobiolaging.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Montoliu C, Llansola M, Felipo V. Neuroinflammation and neurological alterations in chronic liver diseases. Neuroimmunol Neuroinflammation. 2015;2:138–44. [Google Scholar]