Abstract
In the era of combined anti-retroviral therapy (CART) many of the complications due to HIV-1 infection have diminished. One exception is HIV Associated Neurocognitive Disorders (HAND). HAND is a spectrum of disorders in cognitive function that ranges from asymptomatic disease to severe dementia (HAD). The milder form of HAND has actually remained the same or slightly increased in prevalence in the CART era. Even in individuals who have maintained undetectable HIV RNA loads, viral proteins such as Nef and Tat can continue to be expressed. In this report we show that Nef protein and nef mRNA are packaged into exosomes that remain in circulation in patients with HAD. Plasma-derived Nef exosomes from patients with HAD have the ability to interact with the neuroblastoma cell line SH-SY5Y and deliver nef mRNA. The mRNA can induce expression of Nef in target cells and subsequently increase expression and secretion of Aβ and Aβ peptides. Increase secretion of amyloid peptide could contribute to cognitive impairment seen in HAND.
Keywords: HIV, Nef, Combination antiretroviral therapy, HIV associated neurocognitive disorders, Amyloid, Nef
Introduction
HIV-associated neurocognitive disorders (HAND) are commonly observed in HIV-infected individuals (Antinori et al., 2007). HAND represents a broad spectrum of disorders that range from mild cognitive impairment to severe HIV associated dementia (HAD). With the advent of combined antiretroviral therapy (CART) the incidence of HAD has dramatically decreased (Dore et al., 2003). However, the incidence of milder forms of cognitive impairment appear to remain high (Robertson et al., 2007; Simioni et al., 2010; Tozzi et al., 2005). The exact reasons behind the continuing prevalence of milder forms of HAND are not clear. Several hypotheses have been formulated such as incomplete viral suppression in the CNS caused by poor penetration of antiretroviral drugs or toxic effect of therapy. Early in infections HIV penetrates the nervous system, most likely through perivascular macrophages and microglia in the CNS (Cosenza et al., 2002; Gonzalez-Scarano and Martin-Garcia, 2005; Williams et al., 2001). Astrocytes can also become latently infected, but do not appear to produce viable viral particles (Churchill et al., 2006; Gorry et al., 2003; Thompson et al., 2004). However, infection of astrocytes can lead to changes in gene expression (Wang et al., 2004) and induce apoptosis which correlates with the severity of HAD (Thompson et al., 2001). Other studies have suggested infection of astrocytes is extensive and plays a prominent role in HIV-associated dementia (Churchill et al., 2009). It has also been shown that overexpression of nef is a marker of latently infected astrocytes (Saito et al., 1994).
A role for beta-amyloid (Aβ) in HAND has also been suggested (Esiri et al., 1998; Green et al., 2005). In the normal brain, beta-amyloid is produced and rapidly degraded. It has been noted that deposition of beta-amyloid in the brain is a common pathologic feature in HIV infected individuals and that incidence of Alzheimer-like plaques are increased in infected individuals. In addition, the presence of biomarkers of Alzheimer’s disease such as Aβ1-40, Aβ1-42 and tau are increased in HAND patients (Clifford et al., 2009) although Ances et al. showed differences in plaque reactivity between HAND and Alzheimer’s disease using 11C-PiB imaging (Ances et al., 2012). Thus, it appears that HAND may be associated with amyloid deposition in a manner similar to that seen in Alzheimer’s disease.
HIV Tat protein has been previously reported to interact with and modulate the beta-amyloid pathway by inhibiting the major Aβ-degrading enzyme Neprilysin (Pulliam, 2009). The authors reported that soluble viral proteins such as gp120, Rev, and Nef did not increase beta-amyloid in a brain aggregate culture model. When intact HIV-1NL4-3 virus was added to the same cell aggregates there was an increase in secreted beta-amyloid production after 48-h treatment. Nef has been shown to be toxic to neural cells (Trillo-Pazos et al., 2000) and has been shown to have effects on the transcriptional levels of anaplastic lymphoma kinase (ALK) which has the ability to activate metalloproteinases (MMPs) and effect the mitogen-activated protein kinase (MAP-K; (Bergonzini et al., 2009). Structural analysis of Nef shows a correlation between HIV associated dementia (HAD) and particular structures of Nef and that patients with HAD had nef sequences that were more similar to subtype D structures (Lamers et al., 2011).
Nef is known to associate with exosomes and nef expression is sufficient to promote secretion of exosomes (Lenassi et al., 2010; Raymond et al., 2011). Nef exosomes carry RNA molecules that can be delivered to target cells (Aqil et al., 2014). Nef exosomes have also been shown to be present in the plasma of HIV infected individuals (Raymond et al., 2011). Therefore, Nef exosomes can be thought of as a means of communication between HIV infected cells and other cells of the body. Aβ peptides have also been shown to associate with exosomes (Rajendran et al., 2006). In the current study we show that Nef exosomes contain nef mRNA and Nef protein. We show evidence that it can deliver nef mRNA to SH-SY5Y neuroblastoma cells, which is translated into Nef protein. We show that production of Nef upregulates Aβ expression and secretion, which may play a role in the development of HAND.
Results
Characterization of Nef exosomes produced by transfected HEK293 cells
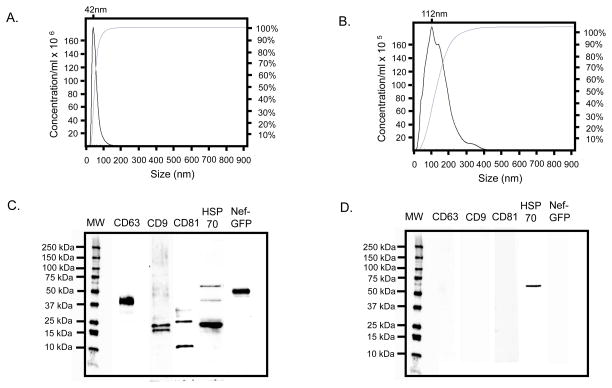
We performed several assays to characterize Nef exosomes produced from transfected HEK293 cells. Those assays are summarized in Fig. 1. First, we performed size analysis using the Nanosight nanoparticle tracking analysis (NTA). We measured eight preparations of Nef exosomes produced from transfected HEK293 cells (Campbell et al., 2008; Raymond et al., 2011) and eight preparations from mock-transfected cells. The mean diameter of the test preparations was 60nm ± 25nm and mean concentration was 6.0 x 109 ± 2.9 x 109. The mean diameter of the control preparations was 135nm ± 65nm and mean concentration was 6.7 x 108 ± 1.7 x 108. The preparation used in this study is shown in Fig. 1a. The median diameter of the vesicles in this preparation was 42nm, with the majority of vesicles in the range of 20nm to 100nm. This is consistent with the range normally expected for exosomes. Note the preparation also appears as a single peak. As a control for our assays we collected the same volume of conditioned media as used in the test preparation from mock transfected HEK293 cells. We then analyzed this control preparation with Nanosight (Fig. 1b). The mean diameter was 112nm with a range from 10nm to 400nm. Note that the material from the control had multiple peaks and was 10-fold lower in concentration. We have previously demonstrated by electron microscopy that conditioned medium from nef transfected HEK293 cells contains many vesicles consistent with the size and shape of exosomes while medium from mock transfected cells showed no visible vesicles (Campbell et al., 2008; Raymond et al., 2011).
Fig. 1.
Characterization of Nef exosomes produced by transfection of HEK293 cells. a Nanosight analysis of the isolated Nef exosomes used in this study showed a single peak of mean diameter of 42nm. Approximately 95% of the vesicles were in the range of 20nm – 100nm. The mean particle concentration was 6.0 ± 2.9 x 109. We have used Nanosight to measure average particle size of 8 independent Nef preparations. The mean particle size of these preparations was 60nm ± 25nm and the mean concentration was 6.0 x 109 ± 2.9 x 109. b Nanosight analysis was also performed on the control preparation from mock transfected cells used in this study. It showed a more heterogeneous population with a mean diameter of 112nm that ranged from 10 to 400nm in size. We have tested control preparations from 8 independent mock transfections. The mean diameter of the control preparations was 135nm ± 65nm. The mean particle concentration of these preparations was 6.7 x 108. c The same preparations used for Nanosight measurement were also tested by immunoblot using antibodies against CD63, CD9, CD81, HSP70 and Nef-GFP. d The control preparation from this study was also tested by immunoblot using the same panel of antibodies used for the test preparation. Note that both test and control preparations were run on the same gel and the molecular weight markers have been duplicated in this figure for easy comparison. e Test and control preparations were also analyzed for AChE activity. The mean AChE activity of 8 preparations was 375u ± 36.6u. In contrast, the mean AChE activity of 8 preparations from mock-transfected cells was approximately 25u ± 7u. f Test and control preparations were also tested using RT-PCR with primers specific for nef (550 bp product) and APP (300 bp product). Lane 1 conditioned medium from mock-transfected cells probed with nef primers. Lane 2 exosomes isolated from Nef-GFP transfected cells probed with nef primers. Lane 3 conditioned medium from mock-transfected cells probed with APP primers. Lane 4 Nef-GFP transfected probed with APP primers. Lanes 5 and 6 are no template control reactions.
We also probed our vesicle preparations for common exosome markers using a commercially available Western blot kit (SBI, cat# EXOAB-KIT-1) and antibody against Nef-GFP. The results are summarized in Fig. 1c. CD63, CD9, CD81, HSP70 and Nef-GFP were all present in the test preparation. Only a small amount of a high molecular weight band reactive with anti-HSP70 was present in mock-transfected HEK293 cells and no Nef-GFP was detected (Fig. 1d).
We performed AChE assays on the test preparation and mock-transfected control (Fig. 1e). The Nef-exosome preparation contained approximately 375μU/ml of AChE activity compared to approximately 25μU/ml of activity in the control.
Finally, we analyzed the Nef exosomes for the presence of mRNA for nef and APP (Fig. 1f). There was a single band present at 550bp, consistent with the expected size product for nef and a small amount of a 350bp product, consistent with the expected size of APP. Taken together, these data show that an exosome preparation isolated from conditioned medium produced by nef-transfected HEK293 cells contains material that is of the size range expected for exosomes, contains the common exosome markers CD63, CD9, CD81, and HSP70 as well as Nef-GFP, contains the exosome marker AChE and carries the mRNA for nef. In contrast, conditioned medium from mock-transfected HEK293 cells contains material that is more heterogeneous in size, does not contain detectable levels of CD63, CD9, CD81, or HSP70 and does not contain detectable levels of Nef-GFP. The control preparation contained only background levels of AChE and did not contain detectable levels of nef mRNA.
Expression of nef in SH-SY5Y cells enhances Aβ expression
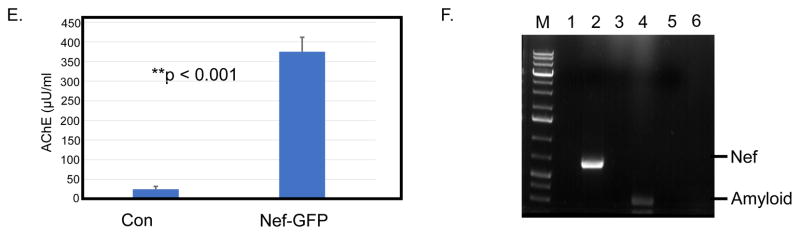
Since Nef has been associated with cell signaling (Bergonzini et al., 2009) and infection of brain cells (Lamers et al., 2011) we wanted to determine if expression of nef in brain cells might influence production or secretion of Aβ. To determine if nef expression has an effect on expression of Aβ, we transfected SH-SY5Y neuroblastoma cells with the nef expression vector pQBI-nefGFP (Quantum Biotechnologies) and harvested cells after 48h and performed an immunoblot against Nef-GFP and Aβ1-40. The results are summarized in Fig. 2a. Cells transfected with pQBI-nefGFP expressed a 55kDa protein that was detected using an anti-Nef antibody (Fig. 2a, upper bands). Cells transfected with the empty vector showed no Nef band. The same gel was stripped and re-probed with an anti-amyloid antibody that is reactive exclusively with Aβ1-40 (Fig. 2a, middle bands). Mock transfected cells showed a background level of expression of Aβ1-40 while the nef expressing cells showed a visible increase in Aβ1-40. The numbers below the gel show the average and standard deviation of three separate experiments. To provide a more quantitative means of comparison, we performed sandwich ELISA tests for Nef and Aβ1-42. The results are summarized in Fig. 2b. Mock transfected cells expressed 0.0pg/ml of Nef protein while nef-transfected cells expressed ~150 pg/ml (red line, red axis label). We tested for the presence of Aβ1-42 using the same cell lysate (blue bars, blue axis label). The mock transfected cells expressed 4.1pg/ml of Aβ1-42 while the nef-transfected cells expressed ~10 pg/ml of Aβ1-42. The results shown are the average of three independent experiments. Thus, it appears that expression of Nef in SH-SY5Y cells increases the production of Aβ1-42.
Fig. 2.
Transfection of SH-SY5Y cells with a Nef-GFP expression plasmid or treatment with Nef exosomes increases the amount of Aβ produced in target cells. a SH-SY5Y cells were transfected with 30μg of Nef-GFP expression plasmid or mock transfected with GFP vector. After 48 h cells were harvested and immunoblot was used to detect the amounts of Nef-GFP and Aβ1-40. b ELISA was also run to determine the amount of Nef-GFP (red line, red axis) and Aβ1-42 (blue bars, blue axis). c SH-SY5Y cells were treated with Nef exosomes isolated from transfected HEK293 cells or mock treated cells. 24 hours after treatment, the cells were harvested and lysed (P for cell pellet). We also collected conditioned medium and subjected it to ultra-centrifugation at 100,000 x g (S for supernatant) and run on a PAGE gel. The gel was blotted with antibody that reacts with intermediate forms of Aβ (see 36 kDa band and higher molecular weight forms). The blot was stripped and re-probed with antibody against the 87kDa APP, Nef-GFP and Tubulin. The numbers below the gel are the average and SD of three independent experiments. d The cell pellet (C) and conditioned medium (M) were also subjected to ELISA to quantitate Aβ1-42. The results shown are from three independent experiments.
Nef exosomes applied to SH-SY5Y cells increases production and secretion of Aβ1-42
Neural cells are not infectable by HIV-1. Therefore, it is not likely that nef would be expressed in neural cells as a consequence of infection by HIV-1. We have previously shown that transfection of HEK293 cells with a nef expression vector can induce the release of exosomes (Raymond et al., 2011) that we have termed “Nef exosomes”. Conditioned medium from HEK293 cells that is centrifuged at 100,000 x g does not contain visible numbers of vesicles by electron microscopy (Raymond et al., 2011). However, HEK293 cells transfected with a nef expression vector produce large quantities of vesicles consistent with the size and shape of exosomes (Raymond et al., 2011). HIV-1 infected cells also produce exosomes that contain Nef as the sole detectable viral protein. Nef exosomes are also present in the plasma of infected individuals (Ali et al., 2010; Campbell et al., 2008; Lenassi et al., 2010). We have observed that Nef exosomes have the ability to induce apoptosis in a variety of cells including vascular epithelial cells (unpublished observations). It is possible that Nef exosomes circulating in the blood could breach the blood-brain barrier (BBB) and ultimately come in contact with brain cells. Alternatively, Nef exosomes can also be produced from latently infected astrocytes present in the brain. Regardless of the source, there is reason to believe that brain cells could come in contact with Nef- exosomes. We wished to determine what effect Nef exosomes would potentially have if they came in contact with brain cells. As a model for brain cells we used the neuroblastoma cell line SH-SY5Y and as a model for Nef exosomes in circulation we used exosomes produced by transfection of HEK293 cells with a nef expression vector.
We treated SH-SY5Y cells with Nef exosomes isolated from transfected HEK293 cells and material isolated from mock transfected cells for 24h. We then lysed the cells and performed an immunoblot to detect the 87kDa APP and a 36kDa amyloid intermediate peptide and Nef-GFP (Fig. 2c; C for cells). We also isolated the conditioned medium, centrifuged it at 100,000 x g and subjected the pellet to the same analysis by immunoblot (Fig. 2c; M for medium). Mock-transfected cells showed a basal amount of 87 kDa amyloid and the 36kDa amyloid peptide and no detectable product in the conditioned medium. In contrast, the SH-SY5Y cells treated with Nef exosomes showed an increase in the amount of amyloid 87 kDa and 36kDa. Curiously, the amount of 36 kDa amyloid secreted from SH-SY5Y cells dramatically increased in Nef-exosome treated cells (Fig 2c, M). We also saw that Nef-GFP was present in the exosome treated cells (Fig 2c, C and M). The numbers below the gel image show the average and standard deviation of densitometry of three independent experiments. To more quantitatively determine the amount of Aβ1-42 produced and secreted we tested the cells and supernatant by ELISA (Fig. 2d). We also saw an increase in amyloid peptide produced in both cells and conditioned medium in Nef-exosome treated cells. The results shown are from three independent experiments. Together, these data suggest that SH-SY5Y cells treated with Nef exosomes make more APP and amyloid peptides and induce the secretion of amyloid peptides.
SH-SY5Y cells treated with Nef exosomes show an increase in the amount of nef and APP mRNAs
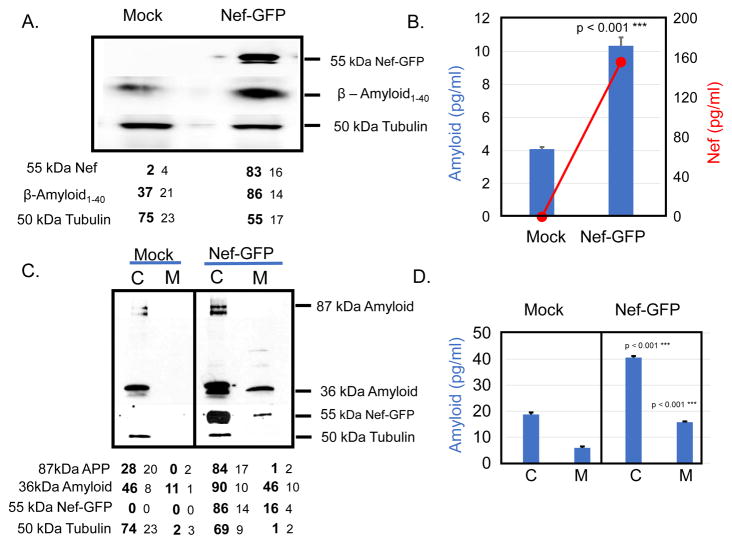
We wanted to determine how treatment of SH-SY5Y cells with Nef exosomes would affect the expression of APP. Our hypothesis was that nef mRNA present in the Nef exosomes could be delivered to SH-SY5Y cells. Since there is no gene for nef in SH-SY5Y cells the only way that nef mRNA could be present in these cells was uptake of mRNA from vesicles. We performed qRT-PCR to determine the relative amounts of nef mRNA present in SH-SY5Y cells (Fig. 3a; red line, red axis) at 2, 4, 8, 16, 24, and 48h. We saw that nef mRNA started at approximately 1.0 x 102 copies and increased to approximately 1.0 x 1012 over 24h before dropping back to 1.0 x 106 at 48h. Subsequent studies revealed that the relatively high level of nef mRNA present at 2h was due to incomplete removal of Nef exosomes from the surface of treated cells despite extensive washing. The number of copies of nef mRNA present immediately after addition of exosomes was very similar to the 2h time (~1x102). In contrast, the number of copies of nef mRNA present in the control treated cells remained relatively low (Fig. 3a, red dashed line). The results shown are the average of three independent experiments. We also determined the amount of APP mRNA present in SH-SY5Y cells after treatment with Nef exosomes (Fig. 3a; blue bars). APP mRNA increased from very low levels (<1 x 102 copies) to 1 x 1012 copies. The peak production of APP mRNA coincided with the peak presence of nef mRNA at 24h and fell to basal levels at 48h. The amount of APP mRNA present in control treated cells (Fig. 3a, hatched blue bars) increased to 1 x 106 copies concomitant with an increase in cell number. The results shown are the average of three independent experiments.
Fig 3.
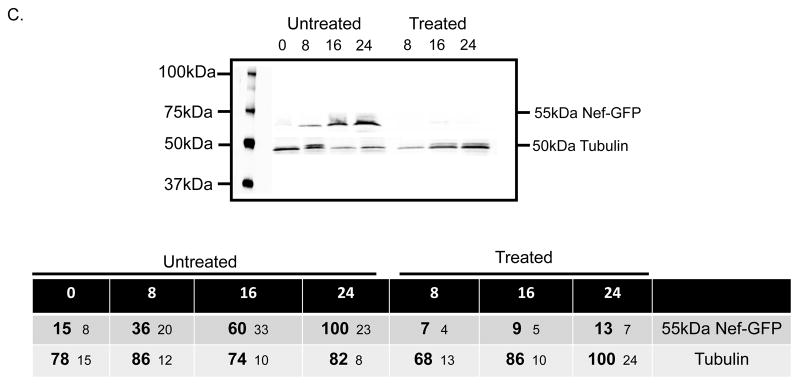
Treatment of SH-SY5Y cells with Nef exosomes from HEK293 cells induces production of Nef and increases expression of Amyloid. a SH-SY5Y cells were treated with Nef exosomes (red line, red axis) or the control preparation (red dashed line) for the times indicated and cells were harvested and tested by RT-PCR for the presence of nef mRNA. Cells treated with Nef exosomes (blue bars, blue axis) or mock control (blue hatched bars) were also tested for the presence of APP mRNA. b SH-SY5Y cells treated with Nef exosomes were also tested by ELISA for the presence of Nef protein (red line, red axis) and Aβ1-40 (blue bars). The dashed line and hatched bars were treated with exosomes from mock-transfected cells. The results shown are the average of three independent experiments. c SH-SY5Y cells were treated with 80μg/ml of cyclo-heximide at the same time as the addition of Nef exosomes or were untreated and tested for the presence of Nef-GFP at 0, 8, 16 and 24h. The table below the gel shows the results of densitometry from an average of three independent experiments.
We wanted to determine how the relative amounts of Nef protein and Aβ1-42 peptide would compare to levels of mRNA in Nef exosome treated SH-SY5Y cells. Therefore we performed sandwich ELISA tests for the presence of Nef (red line, red axis label) and Aβ1-42 peptide (blue bars, blue axis label). The results are shown in Fig. 3b. We saw an increase in the amount of Nef protein present in the cell pellet of Nef exosome treated SH-SY5Y cells. The amount of protein increased from ~300pg/ml at 2h to a peak of ~800pg/ml at 24h and fell to ~400pg/ml at 48h. As before, we saw relatively high background levels of Nef at 2h from incomplete removal of Nef exosomes. However, the amount of Nef increased in a manner similar to that seen for mRNA. In comparison, treatment of SH-SY5Y cells with exosomes isolated from mock-transfected cells showed only background levels of Nef (Fig. 3b; dashed red lines) and baseline (Fig. 3b; hatched blue bars). To demonstrate the specificity of the PCR, levels of Aβ1-40 we isolated the PCR product from the nef reaction and performed capillary sequencing, which confirmed the sequence was specific for the nef reading frame as published for pQBI-nefGFP. The results shown are the average of three independent experiments.
Nef exosomes contain both Nef protein and mRNA (Fig 1c and f). The Nef-GFP present in exosome treated cells in Fig 2c and increases in Nef seen over time in Fig 3b could represent protein delivered from Nef exosomes or could result from translation of the mRNA delivered to the target cells or a combination of both. To help determine whether increases in Nef were due to delivery of Nef by vesicles or translation of Nef from mRNAs that were delivered by exosomes we did an experiment in which the target cells were treated with cycloheximide. This treatment should block de novo protein synthesis from mRNAs but should not block delivery of protein by exosomes. The results are summarized in Fig. 3c. In cells not treated with cycloheximide Nef accumulates to a maximum at 24h. In cells treated with cycloheximide the amount of Nef present in SH-SY5Y cells treated with Nef exosomes was very low. The numbers in the table below the gel image show the average densitometry values of three independent experiments. These data suggest that de novo protein synthesis was necessary for Nef production and that mRNAs delivered by exosomes are responsible for a majority of Nef produced within the cell. Regardless of the source of the Nef induced in the target cells, the amount of Aβ1-42 peptide present increased in a manner that coincided with the peak presence of Nef in the cell.
Exosomes isolated from the plasma of HIV-infected individuals with HAD can modulate the secretion of Aβ in SH-SY5Y cells
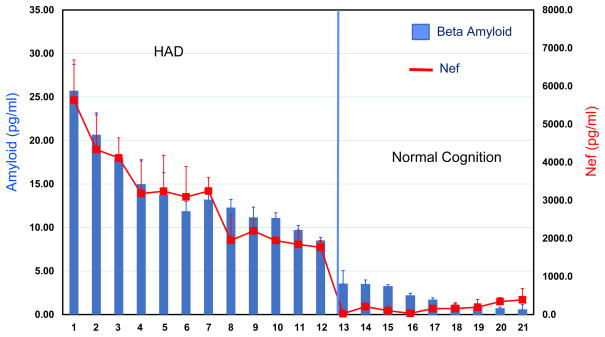
In order to determine if exosomes isolated from HIV-infected individuals would have effects similar to what we found with exosomes produced by transfection, we studied a small set (n= 21) of plasma samples obtained from the Hawaii Aging with HIV cohort as previously described (Valcour et al., 2004). The characteristics of this subset of the cohort are summarized in Table 1. In order to avoid the complication of needing to separate virions from vesicles from the limited amount (1.0ml) of plasma that was available, all of the specimens were from participants who had undetectable HIV RNA levels. AChE is incorporated into exosomes and serves as one measure of the relative number of exosomes present in the sample. Because of sample limitations we could only obtain a single measurement of AChE activity in plasma exosomes. In general, individuals with neurologic disorders appeared to have more exosomes in the 1ml of plasma (See Table 1). We isolated exosomes from plasma samples by differential centrifugation as previously described. The high speed pellet containing exosomes was re-suspended in 500μl of PBS. SH-SY5Y cells were grown to confluence and were then treated with the entire 500μl exosome pellet. After 48hours of incubation the conditioned medium was removed and tested using sandwich ELISA to detect Aβ1-42 peptide (Fig. 4; blue bars, blue axis label). We also tested the cell pellet for the presence of Nef by sandwich ELISA (Fig. 4; red line and axis label). The ELISA measurements were done in duplicate. The results are arranged from high to low Aβ1-42 peptide from left to right. HAND patients are on the left and normal cognition patients are on the right of the figure. In each case, SH-SY5Y cells treated with plasma exosomes isolated from those individuals with normal cognition had background levels of Nef (Avg = 109±112 pg/ml). In comparison, plasma exosomes from HAND patients were able to induce Nef production in SH-SY5Y cells (Avg = 2994±1000 pg/ml). The difference in the two groups was statistically significant (p < 0.001). In a similar fashion, levels of secreted Aβ1-42 peptide were below 5pg/ml in the normal cognition group (Avg = 2.2±1.0 pg/ml) while cells treated with plasma exosomes from HAD individuals showed a distinct increase in the amount of Aβ1-42 peptide (Avg 15.1±6 pg/ml). The difference in the two groups was statistically significant (p = 0.001). Taken together, these observations suggest that exosomes isolated from the plasma of HAND individuals was able to induce Nef production in target SH-SY5Y cells, while plasma exosomes from normal cognition patients was not able to induce Nef production or influence Aβ1-42 in a significant fashion. Consequently, the production of Nef in target SH-SY5Y cells induced secretion of Aβ1-42 peptide.
Table 1.
Characteristics of the cohort of patients in this study.
| Patient | Dx | VL | CD4 (cells/μl) | CD4 Nadir (cells/μl) | Gender | Age | AChE units |
|---|---|---|---|---|---|---|---|
| 1 | HAD | U | 496 | 434 | M | 68.9 | 1064 |
| 2 | HAD | U | 330 | 200 | M | 56.7 | 1177 |
| 3 | HAD | U | 428 | 320 | M | 52.8 | 1078 |
| 4 | HAD | U | 110 | 150 | M | 54 | 1152 |
| 5 | HAD | U | 325 | 220 | M | 57.9 | 944 |
| 6 | HAD | U | 186 | 200 | M | 57 | 1218 |
| 7 | HAD | U | 415 | 300 | M | 59.5 | 1181 |
| 8 | HAD | U | 512 | 370 | M | 54.3 | 1182 |
| 9 | HAD | U | 424 | 280 | M | 52.6 | 1212 |
| 10 | HAD | U | 565 | 585 | M | 53.8 | 1073 |
| 11 | HAD | U | 525 | 460 | F | 52 | 1199 |
| 12 | HAD | U | 146 | 52 | M | 73.7 | 805 |
| 13 | NC | U | 541 | 300 | M | 54.5 | 922 |
| 14 | NC | U | 588 | 350 | M | 58.5 | 333 |
| 15 | NC | U | 662 | 653 | M | 54.1 | 420 |
| 16 | NC | U | 718 | 485 | M | 56.7 | 195 |
| 17 | NC | U | 1023 | 869 | F | 40.4 | 567 |
| 18 | NC | U | 740 | 697 | M | 55 | 786 |
| 19 | NC | U | 521 | 323 | M | 33.9 | 608 |
| 20 | NC | U | 254 | 229 | M | 39.7 | 536 |
| 21 | NC | U | 744 | 744 | M | 42.7 | 611 |
HAD HIV-associated dementia, NC Normal cognition, VL viral load, U Undetectable HIV RNA level, AChE acetylcholinesterase, M Male, F Female
Fig. 4.
Exosomes isolated from the plasma of HAD patients can induced SH-SY5Y cells to produce Nef protein (red line, red axis) and induce secretion of Aβ1-42 (blue bars blue axis). Exosomes from the plasma of HAD and normal cognitive controls were isolated from 1ml of plasma by differential centrifugation. The isolated exosomes were placed on SH-SY5Y cells for 24h and the conditioned supernatant was analyzed for the presence Aβ1-42 using ELISA (blue bars, blue axis). The treated SH-SY5Y cells were also analyzed by Nef ELISA (red line, red axis). The mean measurement of Amyloid in the HAD group was 15.1 ± 6 pg/ml and of Nef 2994 ± 1000 pg/ml. The mean measurement of Amyloid in the normal cognition group was 2.2 ± 1 pg/ml and of Nef was 109 ± 112 pg/ml. The difference in both measurements were statistically significant (p <0.001).
Treatment of exosomes with anti-Nef antibody blocks uptake of Nef exosomes in SH-SY5Y cells
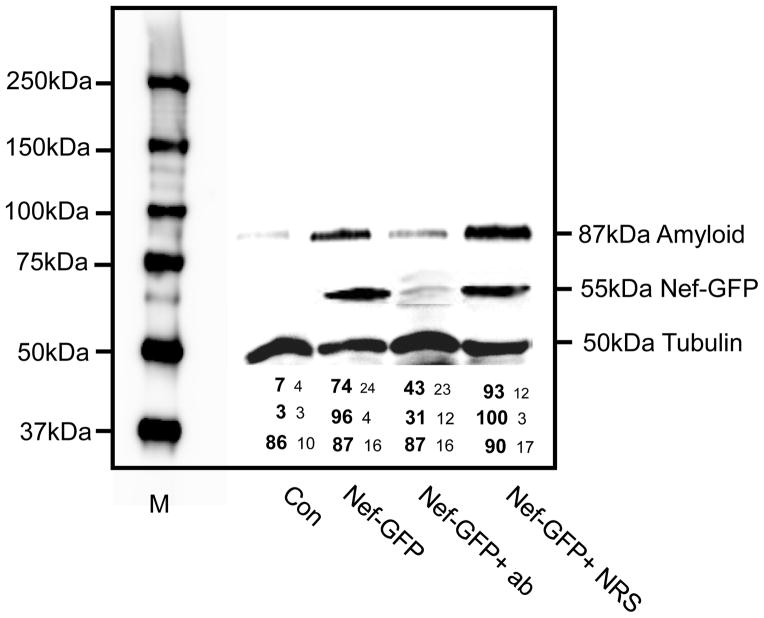
To determine if the presence of Nef was important for the uptake of Nef exosomes, we did a blocking experiment using polyclonal Nef antibody. We have previously shown that Nef exosomes carry most of the Nef protein in the lumen of the vesicle, but a small portion (less than 10%) is present on the outside of the vesicle (Raymond et al., 2011). To determine if Nef antibody could block the uptake of Nef vesicles into SH-SY5Y cells we pre-treated exosomes with antibody for 2h prior to adding them to target cells. The results are shown in Fig. 5. The pre-treated vesicles were able to substantially block production of Nef in target cells. Subsequently, the amount of the 87 kDa amyloid protein was not increased over basal levels. Treatment of exosomes with normal rabbit serum showed no significant blocking. The numbers below the image of the gel are the average of densitometry from three independent experiments. These data suggest that blocking of Nef on the surface of vesicles can influence the attachment and entry of Nef exosomes into SH-SY5Y cells.
Fig. 5.
Anti-Nef antibody can block the uptake of Nef exosomes into SH-SY5Y cells. SH-SY5Y cells were either untreated (Con) or treated with Nef-GFP exosomes (Nef-GFP), treated with Nef exosomes that were pre-incubated with Nef polyclonal antibody prior to treatment (Nef-GFP +ab) or pre-treated with normal rabbit serum prior to treatment (Nef-GFP+NRS). The presence of Nef or 87kDa APP was detected by immunoblot. The numbers below the gel are densitometry from three independent experiments.
Discussion
In this study we have shown that Nef exosomes may play a role in HAND by modulating expression of Aβ in brain cells. Previous studies have shown that exosome production is induced in HIV-1 infected cells and is present in the plasma of infected individuals (Lenassi et al., 2010; Raymond et al., 2011). We have previously shown that expression of nef in HEK293 cells is sufficient to induce exosome production (Raymond et al., 2011). In addition, Nef is the only viral protein we can detect in exosomes from virally infected cells and plasma from infected individuals. A recent publication (Luo et al., 2015) suggests that delivery of Nef via exosomes is not likely to occur. Their studies are fundamentally different in design than previous studies. They rely heavily on co-culture experiments to determine if Nef is being delivered by cell-to-cell contact or through extracellular means (e.g. exosomes). However, because they are ultimately unable to demonstrate that Jurkat cells infected with HIV-1NL4-3 produce exosomes that contain Nef, in contrast to previously published results (Lenassi et al., 2010; Raymond et al., 2011) and data in this publication. Thus, the co-culture design does not fundamentally test extracellular transfer of Nef. Transfer of Nef is monitored using flow cytometry to detect p24 negative Nef positive cells, although such cells only comprised ~1% of cells. The Nef-producing cells used in these experiments were from a previous study by Park, et al. (Park and He, 2009) which were described as tet-inducible Nef producing cells. In our hands, stably producing cells and in particular tet-inducible cells do not always produce detectable amounts of Nef exosomes after a time. In a second set of experiments the authors used Jurkat cells infected by HIV-1NL4-3 as the potential source of Nef. It is unclear exactly why Nef exosomes were not detected in these experiments. There are many potential sources of variation including multiplicity of infection, the variability in Jurkat clones, and the variability of antibodies used to detect Nef. Finally, the authors were able to detect a population of exosomes that contained AChE, TSG101, and Nef from HEK293T cells transfected with a nef expression plasmid, but attribute it to overexpression of nef.
It is thought that HIV virions can cross the blood-brain barrier early in infection and is present in the CSF (McArthur et al., 1988; Resnick et al., 1988). Although neural cells and astrocytes do not appear to be directly infected, a low level of virus is maintained by latent infection of brain monocyte/macrophages. We have demonstrated that cells of the macrophage/ monocyte lineage appear to produce large numbers of Nef exosomes (Campbell et al., 2008). Therefore infection of brain macrophages could supply a large number of Nef exosomes to the brain compartment. We have observed that Nef exosomes can be secreted into patient plasma in individuals long after virus has been suppressed by anti-retroviral therapy (unpublished observation). Alternatively, blood exosomes could cross a blood-brain barrier that has been compromised. In this work we show that expression of nef in SH-SY5Y cells upregulates the production of Aβ peptides. The question arises of how expression of nef in brain cells could actually occur if they are not directly infectable?
In addition to proteins, it has been shown that miRNAs and mRNAs can also be carried as exosome cargo (Valadi et al., 2007). These RNAs can be delivered to target cells where mRNAs can be directly translated into proteins. If exosomes produced in the blood were to cross over the blood-brain barrier or brain macrophages were to directly produce Nef exosomes into the CSF it is not difficult to imagine them coming in direct contact with brain cells. As a model of this dynamic we have used SH-SY5Y cells (a neuroblastoma cell line) that has been treated with Nef exosomes that have been produced by transfection of HEK293 cells. SH-SY5Y cells treated exogenously with Nef exosomes can upregulate the production and secretion of Aβ peptides (see Fig. 2). We demonstrated in this study that Nef exosomes contain full-length nef mRNA that can be delivered to SH-SY5Y cells and subsequently translated into Nef protein.
There is some precedence that Nef may play a role in the development of neurocognitive disorders. Recombinant Nef from HIV-IIIB has been shown to be toxic to primary human neurons (Trillo-Pazos et al., 2000). Nef has also been shown to have an effect on anaplastic lymphoma kinase (ALK), a protein preferentially expressed in the nervous system (Bergonzini et al., 2009). Nef and ALK can also activate matrix metalloproteinases (MMPs) which are thought to destabilize the BBB. It has also been shown that there are genetic determinants that influence the ability of particular viruses to replicate in the CNS. For example, subtype D isolates from Uganda had a rate of HAD of 89% while subtype A had a rate of 24% (Kanki et al., 1999). Lamers et al. suggested that a difference in Nef structure in these isolates actually correlates with dementia pathogenesis (Lamers et al., 2011). Taken together, these studies suggest that Nef could play a role in the pathogenesis leading to HAND.
Aβ secretion has also been linked with exosomes. It appears that Aβ peptides are released in association with exosomes (Rajendran et al., 2006) and that cleavage of APP occurs in early endosomes followed by routing to multivesicular bodies (MVBs) and subsequent secretion in exosomes. Exosomes are thought to play a role in viral infections of the CNS (for review see(Rajendran et al., 2006). We have seen that in primary cells expressing Nef the exosomal pathway is enhanced. In this study we saw that Nef vesicles could deliver mRNA to SH-SY5Y cells and induce them to produce Nef. If Aβ and Nef are secreted into exosomes it would make sense that expression of nef could alter the secretion of Aβ peptides.
In addition to altering secretion of Aβ peptides Nef also appears to enhance transcription of APP (see Fig.3a). It has previously been shown that HIV-1 Tat can inhibit neprilysin, a major source of degradation of Aβ. Nef is involved in a number of signaling pathways (Greenway et al., 2003). In astrocytes CCL2/MCP-1 was shown to be preferentially secreted and can attract monocytes/macrophages to the brain (Lehmann et al., 2006). While we have no information on the exact mechanism by which Nef can affect transcription of APP there is ample precedence that Nef can interact with a variety of promoter elements in the activation of CCL2/MCP-1 (Liu et al., 2014).
Exosomes isolated from the plasma of HIV infected individuals with HAD had a similar effect on SH-SY5Y cells (see Fig. 4). After treatment with plasma exosomes from HAD patients we saw a characteristic expression of Nef by both immunoblot and ELISA. The magnitude of the relative expression of Nef correlated somewhat with the amount of AChE that was detected in the exosome pellet (see Table 1). Although we were only able to obtain a single measurement from our limited clinical samples the range of AChE from HAD patients was a mean of 1040 units with a range from (944-1212). The normal cognition controls was a mean of 578 with a range from (195-922). These data suggest that the number of vesicles in the HAD group was almost twice that of the normal cognition group. Given that the potential to induce Nef expression was much lower in the normal cognition group it suggests there were much fewer Nef exosomes in the control group. Thus, the relative ability of the total exosomes isolated from 1ml of plasma to induce Nef expression in target SH-SY5Y cells was predictive of whether the patient showed symptoms of HAD.
If Nef exosomes play a role in the development of HAD/HAND how could this affect potential development of therapeutic strategies? Instead of a strategy to simply decrease the viral load in HAND it appears another goal would be to reduce the numbers of Nef exosomes in circulation or impair their ability to interact with neural cells. We show that blocking exosome effects on target cells can be gained by the presence of Nef antibodies. Prior treatment of Nef exosomes with a polyclonal Nef antibody dramatically reduced the increase in nef expression and subsequent increase in Aβ secretion in target cells. The recognition of Nef as a potential target for therapeutic strategies should aid in the development of new therapies targeted toward Nef exosomes.
Materials and Methods
Cell lines and plasmids
SH-SY5Y cells are a subline of the neuroblastoma cell line SK-N-SH that was purchased from ATCC (ATCC® CRL-2266) and were maintained according to the manufacturer’s recommendations. The base medium for this cell line is a 1:1 mixture of ATCC-formulated Eagle’s Minimum Essential Medium, Catalog No. 30-2003, and F12 Medium (Cellgro Cat# 10-025-CV). HEK 293 cells are a kidney cell line that was purchased from ATCC (CRL-1573). Exosome-free serum (Exo-FBS-250A-1, SBI) was used to supplement the media at 10%. Antibiotic-antimycotic solution 100X from Gibco as added to the medium to 1X. The nef expression plasmid pQBI-nefGFP (cat # AFP 3203) was purchased from Quantum Biology.
Antibodies
Anti-Aβ1-40 antibody (EP1876Y) ab76317 is a rabbit monoclonal to Aβ1-42 and was used for immunoblot at a 1:500 dilution. This antibody reacts with a band at 4–10kDa and was used in the experiment in Fig. 1a. Anti- Aβ ab97555 (Abcam) is a rabbit polyclonal antibody to a recombinant fragment, corresponding to a region within amino acids 602–757 of Human beta Amyloid (Aβ) and was used for immunoblot at a 1:500 dilution. It reacts with a band at 87 kDa on immunoblot and was used in the blots shown in Fig. 1c and Fig. 5. Anti-Aβ Antibody MABN10 (Millipore). Reacts with bands at 36kDa and 55kDa on immunoblot. This antibody was used in the blot shown in Fig. 1b. As a loading control we used anti-Tubulin (Sigma, T9026). Antibodies used to characterize Nef exosomes produced by HEK293 cells were from a kit (ExoAB kit from SBI) and consists of a panel of antibodies anti-CD63, anti-CD9, antiCD81, and HSP 70. We used a murine monoclonal antibody against Nef (Immunodiagnostics, cat #1108) in Nef immunoblots. We used a rabbit anti-Nef polyclonal antibody from the AIDS Reagent Program (cat #2949) in the blocking studies in Fig. 5. Normal Rabbit serum was used as a control.
Nanosight analysis
Nef exosomes isolated from HEK293 cells were analyzed using Nanoparticle Tracking Analysis (NTA 2.3). We have analyzed many preparations from HEK293 cells using this method. The chart in Fig. 1 shows the Nef-exosome preparation used in this study. Analysis settings: frames processed: 749 of 749, frame per second: 25, Calibration: 190nm/pixel, threshold: 10 multi, Temperature: 19.7°C, Viscosity: 1.01cP.
ELISA kits and AChE assay
Commercial sandwich ELISA kits were used to detect Nef (Imunodiagonostics; Nef ELISA; cat# 1108) and Aβ1-42 (IBL; cat # RE59791). The Aβ1-42 high sensitivity ELISA was obtained from IBL International. Both kits were used according to the manufacturer’s recommendations using the provided standards. For AChE we used the method of Ellman et al. as described (ELLMAN et al., 1961). Briefly, Dithibio-Nitrobenzoic (DTNB) was used as a color indicator and Acetylthiocholine Iodide was used as a substrate. A working stock was prepared by mixing 10ml PBS, 500μl DNTB and substrate in 100:2:5 ratios. A 50μl sample was added to each well followed by 200ul working stock. The absorbance was immediately measured using a spectrophotometer set at 450μm. In the case of plasma samples, 50μl of supernatant that was collected after treatment of SH-SY5Y cells was used.
Primers and PCR
For detection of APP mRNA we used forward and reverse primers: 5′-TCAGATCCG GTCCCAGGTTATG and 5′-AGAGTCAGCCCCAAAAGAATGC as described by Yoshida et al. (Yoshida et al., 2005). To detect Nef mRNA we used the following primers: 5′ ATGGGTGGCAAGTGGTCAAAA and 5′ TCAGCAGTTCTTGAAGTACTC as previously described (Ali et al., 2010). RNA was isolated from cells using Qiagen All Prep DNA/RNA mini kit Cat # 80204. 500ng RNA template was used in Q real time RT-PCR for each reaction. Real–time RT-PCR was done using the SensiFAST one-step kit from Bioline, USA. To calculate copy number we used serial dilutions of pQBI-nefGFP for nef and pEX-F0776-M02 (Gene Copoeia) for APP. We used an online gene copy calculator by URI Genomics and Sequencing Center (http://cels.uri.edu/gsc/cndna.html) to determine final copy number. The PCR product was isolated (Qiaquick; Qiagen cat # 28104) and sequenced using capillary sequencing (ABI).
Isolation of exosomes
Nef exosomes were isolated from HEK293 cells that were transfected with 30μg of nef expression vector pQBI- nefGFP by differential centrifugation as previously described (Raymond et al., 2011). As a control, we also transfected HEK293 cells with 30μg of empty vector expressing GFP alone. The transfections were done using lipofectamine 2000 (Invitrogen by Life Technology Ref # 11668-019) according to the manufacturer’s protocol. Transfections were carried out in T-75 flasks. The cell-free supernatants from four T-75 flasks (40ml of medium) were combined and subjected to a series of centrifugation steps to isolate exosomes as previously described (Thery et al., 2006). Briefly, the conditioned medium was first clarified by centrifugation at 300 x g for 10 min. and the supernatant was centrifuged at 2000 x g for 10 min. The supernatant was then centrifuged at 16,500 x g for 20 min. The supernatant was then filtered through a 0.2 μm filter before a final spin at 105,700 x g. The final pellets were re-suspended in 1ml of PBS. A portion of each pellet was analyzed using Nanosight Tracking Analysis (NTA), immunoblot, AChE assay and RT-PCR (see Fig. 1) before treating SH-SY5Y cells with the isolated exosomes. In each experiment 2 x 108 particles were added per well and the experiments were done in triplicate. Plasma was obtained from the HAHC, which was a prospective study examining the relationship between aging and neurocognitive impairment in HIV-infected individuals from October 22, 2001 to July 1, 2005. Details of enrollment and clinical characterization have been published elsewhere (Valcour et al., 2004). A convenience sample from available specimens from participants with normal cognition and HAD were used for this study. Plasma from the repository has been stored in liquid nitrogen and thawed for analyses. One ml of plasma was used to isolate exosomes by differential centrifugation as previously described (Raymond et al., 2011). The final pellet was re-suspended in 500μl of PBS before being placed on SH-SY5Y target cells.
Cycloheximide treatment of cells
SH-SY5Y cells were treated with 80μg/ml final concentration of cycloheximide (Sigma; cat # C 4859) at the time of addition of Nef exosomes. We then tested for Nef-GFP at 0, 8, 16 and 24h by immunoblot. Untreated cells transfected with Nef-GFP were used as a control.
Antibody blocking experiment
In brief, 40μg of anti-Nef polyclonal rabbit serum or normal rabbit serum were added to 500μl of exosomes and pre-incubated for 2h at 4°C prior to addition to SH-SY5Y cells and incubation for 24h. The cell lysates were then subjected to immunoblot using the 87kDa amyloid antibody and anti-Nef antibody. Tubulin was used as a loading control.
Acknowledgments
NIMHD G12 MD007602, U54MD008149 NIAID R21AI095150, CFAR P30AI050409 (MP, VB) NINDS/NIH U54NS43049, NIMH/NIH R21MH68173, and NIMHD/NIH U54MD007584 (BS)
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Reference List
- Ali SA, Huang MB, Campbell PE, Roth WW, Campbell T, Khan M, Newman G, Villinger F, Powell MD, Bond VC. Genetic characterization of HIV type 1 Nef-induced vesicle secretion. AIDS Res Hum Retroviruses. 2010;26:173–192. doi: 10.1089/aid.2009.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, Aldea P, Fagan AM, Holtzman DM, Morris JC, Clifford DB. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol. 2012;69:72–77. doi: 10.1001/archneurol.2011.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqil M, Naqvi AR, Mallik S, Bandyopadhyay S, Maulik U, Jameel S. The HIV Nef protein modulates cellular and exosomal miRNA profiles in human monocytic cells. J Extracell Vesicles. 2014;3 doi: 10.3402/jev.v3.23129. eCollection;%2014., 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergonzini V, Calistri A, Salata C, Del VC, Sartori E, Parolin C, Palu G. Nef and cell signaling transduction: a possible involvement in the pathogenesis of human immunodeficiency virus-associated dementia. J Neurovirol. 2009;15:238–248. doi: 10.1080/13550280902939748. [DOI] [PubMed] [Google Scholar]
- Campbell TD, Khan M, Huang MB, Bond VC, Powell MD. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn Dis. 2008;18:S2–S9. [PMC free article] [PubMed] [Google Scholar]
- Churchill MJ, Gorry PR, Cowley D, Lal L, Sonza S, Purcell DF, Thompson KA, Gabuzda D, McArthur JC, Pardo CA, Wesselingh SL. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146–152. doi: 10.1080/13550280600748946. [DOI] [PubMed] [Google Scholar]
- Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR. Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol. 2009;66:253–258. doi: 10.1002/ana.21697. [DOI] [PubMed] [Google Scholar]
- Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, Kauwe JS. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12:442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 2003;17:1539–1545. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. 88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res. 2003;1:463–473. doi: 10.2174/1570162033485122. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Greenway AL, Holloway G, McPhee DA, Ellis P, Cornall A, Lidman M. HIV-1 Nef control of cell signalling molecules: multiple strategies to promote virus replication. J Biosci. 2003;28:323–335. doi: 10.1007/BF02970151. [DOI] [PubMed] [Google Scholar]
- Kanki PJ, Hamel DJ, Sankale JL, Hsieh C, Thior I, Barin F, Woodcock SA, Gueye-Ndiaye A, Zhang E, Montano M, Siby T, Marlink R, NDoye I, Essex ME, MBoup S. Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis. 1999;179:68–73. doi: 10.1086/314557. [DOI] [PubMed] [Google Scholar]
- Lamers SL, Poon AF, McGrath MS. HIV-1 nef protein structures associated with brain infection and dementia pathogenesis. PLoS One. 2011;6:e16659. doi: 10.1371/journal.pone.0016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann MH, Masanetz S, Kramer S, Erfle V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J Cell Sci. 2006;119:4520–4530. doi: 10.1242/jcs.03231. [DOI] [PubMed] [Google Scholar]
- Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, Krogan NJ, Plemenitas A, Peterlin BM. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shah A, Gangwani MR, Silverstein PS, Fu M, Kumar A. HIV-1 Nef induces CCL5 production in astrocytes through p38-MAPK and PI3K/Akt pathway and utilizes NF-kB, CEBP and AP-1 transcription factors. Sci Rep. 2014;4:4450. doi: 10.1038/srep04450.,4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Fan Y, Park IW, He JJ. Exosomes are unlikely involved in intercellular Nef transfer. PLoS One. 2015;10:e0124436. doi: 10.1371/journal.pone.0124436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC, Cohen BA, Farzedegan H, Cornblath DR, Selnes OA, Ostrow D, Johnson RT, Phair J, Polk BF. Cerebrospinal fluid abnormalities in homosexual men with and without neuropsychiatric findings. Ann Neurol. 1988;23(Suppl):S34–7. S34–S37. doi: 10.1002/ana.410230712. [DOI] [PubMed] [Google Scholar]
- Park IW, He JJ. HIV-1 Nef-mediated inhibition of T cell migration and its molecular determinants. J Leukoc Biol. 2009;86:1171–1178. doi: 10.1189/jlb.0409261. [DOI] [PubMed] [Google Scholar]
- Pulliam L. HIV regulation of amyloid beta production. J Neuroimmune Pharmacol. 2009;4:213–217. doi: 10.1007/s11481-009-9151-9. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AD, Campbell-Sims TC, Khan M, Lang M, Huang MB, Bond VC, Powell MD. HIV Type 1 Nef is released from infected cells in CD45(+) microvesicles and is present in the plasma of HIV-infected individuals. AIDS Res Hum Retroviruses. 2011;27:167–178. doi: 10.1089/aid.2009.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick L, Berger JR, Shapshak P, Tourtellotte WW. Early penetration of the blood-brain-barrier by HIV. Neurology. 1988;38:9–14. doi: 10.1212/wnl.38.1.9. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault AA, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Thery C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3(Unit 3.22) doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- Thompson KA, Churchill MJ, Gorry PR, Sterjovski J, Oelrichs RB, Wesselingh SL, McLean CA. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56:873–877. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- Thompson KA, McArthur JC, Wesselingh SL. Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Ann Neurol. 2001;49:745–752. doi: 10.1002/ana.1011. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, Piselli P, Lorenzini P, Visco-Comandini U, Vlassi C, Quartuccio ME, Giulianelli M, Noto P, Galgani S, Ippolito G, Antinori A, Narciso P. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retroviruses. 2005;21:706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, McFarlane-Abdulla E, Campbell IC, Pilkington GJ, Everall IP. Recombinant nef HIV-IIIB protein is toxic to human neurons in culture. Brain Res. 2000;864:315–326. doi: 10.1016/s0006-8993(00)02213-7. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Valcour V, Shikuma C, Shiramizu B, Watters M, Poff P, Selnes O, Holck P, Grove J, Sacktor N. Higher frequency of dementia in older HIV-1 individuals: the Hawaii Aging with HIV-1 Cohort. Neurology. 2004;63:822–827. doi: 10.1212/01.wnl.0000134665.58343.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, Fisher PB, Volsky DJ. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):25–32. 25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I. The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest. 2005;115:2793–2800. doi: 10.1172/JCI24635. [DOI] [PMC free article] [PubMed] [Google Scholar]