Abstract
Most psychiatric and neurological diseases are exacerbated by stress. Because this may partially result from stress-induced inflammation, we examined factors involved in this stress response. After a paradigm of inescapable foot shock stress that causes learned helplessness depression-like behavior, eighteen cytokines and chemokines increased in mouse hippocampus, peaking 6 to 12 hr after stress. A 24 hr prior pre-conditioning stress accelerated the rate of stress-induced hippocampal cytokine and chemokine increases, with most reaching peak levels after 1 to 3 hr, often without altering the maximal levels. Toll-like receptor 4 (TLR4) was involved in this response because most stress-induced hippocampal cytokines and chemokines were attenuated in TLR4 knockout mice. Stress activated glycogen synthase kinase-3 (GSK3) in wild-type mouse hippocampus, but not in TLR4 knockout mice. Administration of the antidepressant fluoxetine or the GSK3 inhibitor TDZD-8 reduced the stress-induced increases of most hippocampal cytokines and chemokines. Stress increased hippocampal levels of the danger-associated molecular pattern (DAMP) protein high mobility group box 1 (HMGB1), activated the inflammatory transcription factor NF-κB, and the NLRP3 inflammasome. Knockdown of HMGB1 blocked the acceleration of cytokine and chemokine increases in the hippocampus caused by two successive stresses. Fluoxetine treatment blocked stress-induced up-regulation of HMGB1 and subsequent NF-κB activation, whereas TDZD-8 administration attenuated NF-κB activation downstream of HMGB1. To test if stress-induced cytokines and chemokines contribute to depression-like behavior, the learned helplessness model was assessed. Antagonism of TNFα modestly reduced susceptibility to learned helplessness induction, whereas TLR4 knockout mice were resistant to learned helplessness. Thus, stress-induces a broad inflammatory response in mouse hippocampus that involves TLR4, GSK3, and downstream inflammatory signaling, and these stress responses contribute to susceptibility to depression-like behavior in mice.
Keywords: stress, neuroinflammation, depression, Toll-like receptor 4, fluoxetine, glycogen synthase kinase-3
INTRODUCTION
Psychological stress activates the inflammatory system and exacerbates a diverse array of psychiatric and neurological diseases, which may be partly mediated by stress-induced neuroinflammation (Miller et al., 2009; Kubera et al., 2011). In particular, there is increasing evidence that inflammation increases susceptibility to depression, a progressive and debilitating disease that afflicts nearly 20% of people in the United States (Belmaker et al., 2008; Raison and Miller, 2015). This includes many reports of increased plasma levels in depressed patients of inflammatory cytokines, particularly tumor necrosis factor-α (TNFα), interleukin-6 (IL-6), and IL-1β (Dantzer et al., 2008; Zunszain et al., 2013). Rodents exhibiting depression-like behaviors also have elevated brain cytokine levels (Goshen et al., 2008; Kreisel et al., 2014), and administration of inflammatory cytokines causes depression-like behaviors in rodents (Bluthé et al., 2000; De la Garza et al., 2005; Dantzer and Kelley 2007; Palin et al., 2008; Fu et al., 2010). Acute inescapable tail shocks, acute or chronic restraint stress, and social defeat stress, all of which induce depressive-like behaviors in rodents, activate the inflammatory transcription factor nuclear factor-κB (NF-κB) and increase levels of the cytokines IL-1β, TNFα, IL-6 and IL-10 in rodent brains (Nguyen et al., 2000; Madrigal et al., 2002; O’Connor et al., 2003; Deak et al., 2003; Deak et al., 2005; Blandino et al., 2006; Blandino et al., 2009; Audet et al., 2011; Wohleb et al., 2011; You et al., 2011). In addition to inducing neuroinflammation, stress amplified the increases of inflammatory cytokines (e.g., IL-1β, TNFα) in rodent brains induced by peripheral administration of the inflammatory Toll-like receptor 4 (TLR4) agonist lipopolysaccharide (LPS) (Quan et al., 2001; Johnson et al., 2002; Johnson et al., 2003; Johnson et al., 2004; Munhoz et al., 2006; De Pablos et al., 2006; Frank et al., 2007; Espinosa-Oliva et al., 2009; Wohleb et al., 2012). Thus, stress increases rodent brain levels of several cytokines and primes the TLR4-mediated inflammatory response to LPS.
TLR4 is a pattern recognition receptor expressed not only in macrophages and other immune cells, but also in neurons, astrocytes, and microglia (Akira et al., 2006; Pandey and Agrawal, 2006; Crack and Bray, 2007; Hanke and Kielian, 2011). Besides being activated by pathogens, TLR4 is activated by endogenous molecules known as damage- or danger-associated molecular patterns (DAMPs) that are produced by host cells in response to stress or injury (Piccinini et al 2010; Schaefer, 2014). DAMPs include molecules normally stored intracellularly and released by insults (e.g., high mobility group box 1 (HMGB1) protein and heat shock proteins), proteolytic products of the extracellular matrix (e.g., hyaluronic acid), and a wide variety of other endogenous molecules (Schaefer, 2014). Despite the structural heterogeneity of DAMPs, they frequently stimulate TLR4 (Schaefer, 2014). TR4 may mediate some components of stress-induced inflammation since restraint stress increased TLR4 expression in rodent brain and blocking TLR4 activity attenuated stress-induced NF-κB activation and the ensuing up-regulation of IL-1β and IL-6 in the prefrontal cortex (Gárate et al., 2011; Gárate et al., 2013; Gárate et al., 2014). Several DAMPs have been shown to be increased after psychological stressors in a variety of tissues, suggesting that increased DAMPs induced by stress may activate the inflammatory response (Campisi et al., 2003; Fleshner et al., 2004; Campisi et al., 2012; Maslanik et al., 2013; Beninson et al., 2014; Weber et al., 2015). Particular interesting are the findings that acute inescapable tail shock stress increased HMGB1 levels in rodent hippocampus, and central administration of an HMGB1 antagonist diminished the potentiation by stress of LPS-induced increases of IL-1β by hippocampal microglia, suggesting that HMGB1 mediates the priming effect of stress on LPS-stimulated microglia (Weber et al., 2015).
In both the periphery and the CNS, TLR4 signaling depends on active glycogen synthase kinase-3 (GSK3), a kinase linked to depression (Beurel, 2011; Beurel et al., 2015). GSK3 inhibition reduced the production of pro-inflammatory cytokines after TLR4 stimulation in human monocytes (Martin et al 2005) and in mouse microglia (Yuskaitis et al 2009) and astrocytes (Beurel et al 2009). However, it is not known if GSK3 promotes stress-induced inflammatory responses, which may be particularly important in disorders involving inflammation in which GSK3 is known to promote the disease, such as depression (Jope, 2011; Beurel et al., 2015).
Although stress causes neuroinflammation, there remains limited information about the breadth, timing, magnitude and signaling of the neuroinflammatory response to stress. The present study examined (1) the extent of neuroinflammatory cytokine and chemokine induction in mouse hippocampus induced by a paradigm of foot shock stress that causes learned helplessness depression-like behavior, (2) the effects of a prior stress on cytokine and chemokine responses to a second stress in the brain, (3) the inflammatory molecules involved in stress-induced neuroinflammation, (4) the effects of TLR4 deficiency, the antidepressant fluoxetine, and a specific GSK3 inhibitor on the neuroinflammatory response to stress, and (5) the effects of abrogating stress-induced neuroinflammation by TLR4 deficiency on susceptibility to learned helplessness depression-like behavior.
MATERIALS AND METHODS
Mice and drug administration
These studies used 8–12 week old male wild-type C57Bl/6 mice obtained from the National Cancer Institute and from Charles River Laboratories when the National Cancer Institute terminated their mouse production program, 8–12 week old male TLR4−/− C57Bl/6 mice (generously provided by Dr. Sue Michalek, University of Alabama at Birmingham) bred with wild-type mice from the National Cancer Institute, and 8–12 week old female wild-type mice were used for the experiments reported in Figure 9A. All mice were housed and treated in accordance with NIH and the University of Miami Institutional Animal Care and Use Committee regulations. Where indicated, mice were pre-treated by intraperitoneal (i.p.) injection daily for 2 weeks prior to stress with the antidepressant fluoxetine (20 mg/kg; National Institute of Mental Health Chemical Synthesis and Drug Supply Program) or the GSK3-specific inhibitor TDZD-8 (5 mg/kg; produced in the Martinez laboratory). Other mice were treated with the TNFα antagonist etanercept (100 μg/mouse, i.p.; every other day for a week; Enbrel® Amgen), a newly developed small molecule TNFα inhibitor, Vax-ATC1 (5 mg/kg, i.p.; produced in the Zagury laboratory) or vehicle (5% DMSO/Tween 80/saline) every other day for a week. HMGB1 siRNA( 10 μg/mouse; 5 μg/nostril; ON-TARGETplus Mouse Hmgb1 (15289) siRNA-SMARTpool, Dharmacon, a combination of four pre-validated sequences specifically targeting HMGB1) or control scrambled siRNA were administered intranasally 1 hr after one stress session of inescapable foot shocks.
Stress and learned helplessness
Inescapable foot shocks were used to stress mice by using a protocol that induces learned helplessness depression-like behavior (Beurel et al 2013). Mice were placed in one side of a Gemini Avoidance system shuttle box (San Diego Instruments, San Diego, CA, USA) with the gate between chambers closed, and 180 inescapable foot shocks (IES) were delivered at an amplitude of 0.3 mA, a duration of 6 sec, and a randomized inter-shock interval of 5–45 sec. Some groups of mice were subjected to the identical protocol 24 hr after the first stress in order to compare the effects of a single stress and two stresses on cytokine and chemokine levels. For measuring learned helplessness, 24 hr after one session of inescapable foot shocks, mice were returned to the shuttle box and 30 escape trials were carried out with a 0.3 mA foot shock for a maximum duration of 24 sec with door of the chamber opening at the beginning of the foot shock administration to allow mice to escape. Latency to escape the shock was recorded using Gemini software, and trials in which the mouse did not escape within the 24 sec time limit were counted as escape failures. Mice with greater than 15 escape failures were defined as learned helpless.
Cytokine and chemokine measurements
The prefrontal cortex and hippocampus were rapidly dissected in ice-cold phosphate-buffered saline, snap-frozen, and stored at −80°C. Brain regions were homogenized in ice-cold lysis buffer containing 20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton-100, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 5 μg/ml pepstatin, 1 mM phenylmethanesulfonyl fluoride, 1 mM sodium vanadate, 50 mM sodium fluoride, and 100 nM okadaic acid. The lysates were centrifuged at 14,000 rpm for 10 min to remove insoluble debris, and protein concentrations in the supernatants were determined using the Bradford protein assay. Enzyme-linked immunosorbent assays (ELISA) were carried out according to the manufacturer’s instructions (eBioscience) using 100–150 μg protein. For multiplex measurements, 50 μg protein from brain region extracts from 3–4 mice were combined and the cytokines and chemokines IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17A, GM-CSF, IFN-κ, CXCL1, CCL11, CCL3, CCL4, CCL2, CCL5, and TNFα were measured using the Bio-Plex mouse cytokine and chemokine multiplex assay according to the manufacturer’s instructions (Bio-Rad, Hercules, CA, USA) with the same control samples loaded on multiple plates to control for inter-plate variability. Cytokine and chemokine concentrations were determined by the multiplex assay reader (Magpix; Luminex) and Bio-Plex manager software (Bio-Rad).
Western blotting
Proteins (5–20 μg) in brain region extracts prepared as described above were resolved with SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antibodies to HMGB1 (ab18256, Abcam), IκBα (#9242, Cell Signaling Technology), active NF-κB (MAB3026, Millipore), NLRP3 (AG-20B-0014, Adipogen), active p20 caspase-1 (AG-20B-0042, Adipogen), phospho-Ser9-GSK3β (#9322, Cell Signaling Technology), phospho-Ser21-GSK3α (#9316, Cell Signaling Technology), total GSK3α/β (05–412, Millipore), and reblotted with β-actin (Sigma Aldrich) to ensure loading consistency.
Quantitative real-time polymerase chain reaction
Total RNA from brain regions was isolated by TRIzol extraction (Invitrogen). 500 ng of RNA was converted to cDNA using ImProvII reverse transcriptase (Promega) according to the manufacturer’s instructions. Quantitative changes in the mRNA levels were determined by the QuantStudio 6 Flex real-time PCR system (Applied Biosystems) using Taqman gene expression assay according to the manufacturer’s instructions using 100 ng of cDNA. Quantification was made with 2−ΔΔCt method using 18s as the housekeeping gene. The primers used for TLR4 were: (Forward: AAACTTGCCTTCAAAACCTGGC; Reverse: ACCTGAACTCATCAATGGTCACATC Taqman probe: CACGTCCATCGGTTGATCTTGGGAGAA) (Tanga et al., 2005); 18s primers were purchased from Applied Biosystems.
Statistical analysis
Statistical significance was analyzed with Student’s t test when one treatment group was compared with a control group, with the Mann Whitney test for the data in Figure 9A, or with one-way ANOVA with Bonferroni post-hoc test, using Prism software, and p<0.05 was considered significant.
RESULTS
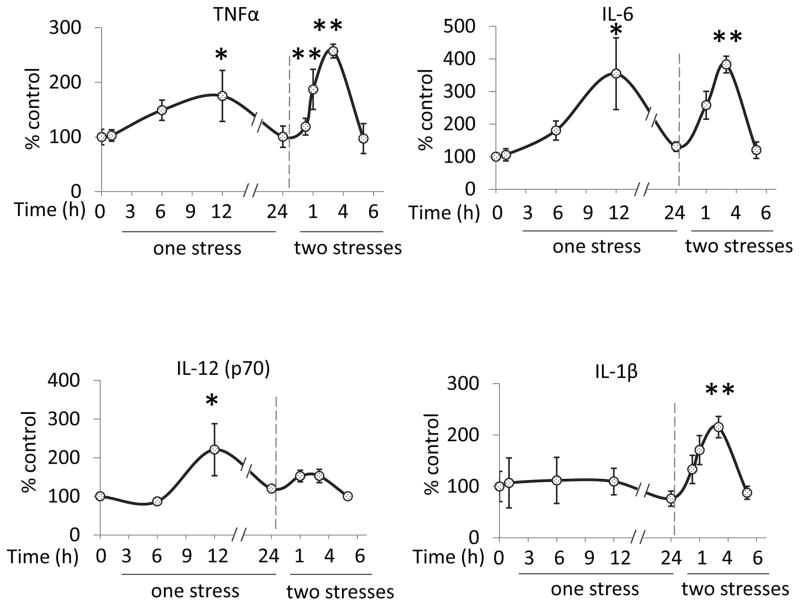
Stress increases cytokine and chemokine levels in the hippocampus and prefrontal cortex of wild-type mice. TNFα, IL-6, IL-12(p70), and IL-1β were measured by ELISA in mouse hippocampus from 1 to 24 hr after administration of a single stress session consisting of inescapable foot shocks that induces learned helplessness depression-like behavior (Beurel et al, 2013). There were significant increases 12 hr after stress in the hippocampal levels of TNFα (one-way ANOVA; F(4,35) = 2.884, p < 0.05)β, IL-6 (F(4, 20) = 3.888, p < 0.05), and IL-12(p70) (F (3,17) = 4.639, p < 0.05), but not IL-1β (Figure 1). These results demonstrate that 1, 6, 12, and 24 hr would be appropriate times to evaluate stress-induced changes in cytokines and chemokines measured by multiplex.
Figure 1. Cytokines and chemokines in the brain after one and two stresses.
Mice were subjected to one or two daily sessions of inescapable foot shocks. Hippocampi were collected 1, 6, 12, and 24 hr after a single session of inescapable foot shocks, or 0.25, 1, 3, and 6 hr after two daily sessions of inescapable foot shocks, followed by measurements of TNFα (n = 6–10), IL-6 (n = 4–7), IL-12(p70) (n= 4–7), and IL-1β (n = 3–4) in the hippocampus by ELISA. Values are means±SEM; Vertical dashed line separates the mice subjected to one stress (left) and those subjected to two stresses (right); *p < 0.05 compared to non-stressed control (0 hr) group; **p < 0.05 compared to 24 hr after the first stress (one-way ANOVA).
Evaluation of multiple cytokines and chemokines by multiplex revealed that a single stress session increased the levels of most cytokines and chemokines measured in the hippocampus, with peak values occurring after 6 hr for IL-2, IL-3, IL-10, IL-13, IL-12(p40), CXCL1 and CCL11 (Supplementary Figure 1A), or 12 hr for TNFα, IL-12(p70), IL-5, IL-17A, CCL2, CCL3, CCL4, CCL5, IFNγ, and GM-CSF (Supplementary Figure 1B and Supplementary Table 1), whereas the levels of IL-1β, IL-1α, and IL-4 did not increase (Supplementary Figure 1C). Table 1 reports the absolute values of each cytokine and chemokine. In contrast to the hippocampus, none of these cytokines and chemokines, except for CXCL1, were elevated in the prefrontal cortex after a single stress (Supplementary Figure 2). These results demonstrate that a single stress that causes a model of depression in mice is sufficient to induce increases of most cytokines and chemokines in the hippocampus.
Table 1. Absolute values of each cytokine and chemokine in untreated mice.
Cytokine and chemokine concentrations in control non-stressed hippocampus
| Cytokines and chemokines | Means ± SEM (pg/ml/100 μg protein) |
|---|---|
| TNFα | 23.1 ± 6.5 |
| IL-6 | 2.7 ± 0.5 |
| IL-12 (p70) | 21.5 ± 3.9 |
| IL-1β | 22.1 ± 9.4 |
| IL-10 | 43.3 ± 3.1 |
| IL-17A | 33.1 ± 3.0 |
| GM-CSF | 152.2 ± 20.5 |
| CCL2 | 98.8 ± 12.0 |
| IL-12 (p40) | 11.8 ± 0.7 |
| IL-5 | 3.8 ± 0.2 |
| CXCL1 | 12.1 ± 0.7 |
| CCL11 | 669.8 ± 8.5 |
| IL-3 | 11.0 ± 2.0 |
| CCL5 | 9.5 ± 0.8 |
| IFNκ | 32.3 ± 2.6 |
| CCL3 | 27.1 ± 9.5 |
| IL-13 | 292.1 ± 20.5 |
| CCL4 | 73.5 ± 0.7 |
| IL-2 | 135.6 ± 21.8 |
| IL-1α | 15.7 ± 0.9 |
| IL-4 | 4.1 ± 1.0 |
A preconditioning stress accelerates the rate of increases in hippocampal cytokines and chemokines after stress
To determine the effects of a prior stress on cytokine and chemokine increases induced by a second stress, mice were exposed to two identical inescapable foot shock sessions with a 24 hr interval. Stress pretreatment accelerated the rate of increases, but often did not alter the magnitudes, of many cytokines and chemokines. As opposed to maximal increases at 6 or 12 hr after a single stress, there were maximal increased levels of cytokines and chemokines in the hippocampus 1 or 3 hr following two stresses. Thus, TNFα (one-way ANOVA; F (4, 23) = 7.842, p < 0.05 compared to 24 hr after the first stress), IL-6 (F (3, 11) = 10.83, p < 0.05), IL-1β (F (4, 10) = 6.326, p < 0.05), IL-2, IL-3, IL-13, CXCL1, IL-5, IL-17A, and GM-CSF increased to peak levels 3 hr following two stresses, whereas peak levels were evident 1 hr after two stresses of IL-12(p70), IL-10, IL-12 (p40), CCL11, CCL2, CCL3, CCL4, CCL5, and IFNκ(Figure 1, Supplementary Figure 1, and Supplementary Table 1). Cytokines and chemokines with similar magnitudes of increases after two stresses as after a single stress include IL-6, IL-2, IL-13, IL-12 (p40), CXCL1, CCL11, IL-5, CCL2, CCL3, CCL4, CCL5 and GM-CSF, whereas greater increases after two stresses were evident with TNFα and IL-1β, and lower increases occurred with IL-12(p70), IL-3, IL-10, IL-17A, and IFNκafter two stresses than a single stress (Figure 1 and Supplementary Figure 1). These results demonstrate that in the hippocampus two daily stresses induce more rapid cytokine and chemokine increases than a single stress. In contrast to the hippocampus, most cytokines and chemokines in the prefrontal cortex did not change after two stresses, except the levels of IL-1β increased 3 hr after two stresses (Supplementary Figure 2). These results demonstrate that the hippocampus is more susceptible to cytokine and chemokine increases induced by the stress of inescapable foot shocks than the prefrontal cortex, and that two stresses accelerates the accumulation of most cytokines and chemokines tested.
Effects of TLR4 deficiency on stress-induced increases of hippocampal cytokines and chemokines
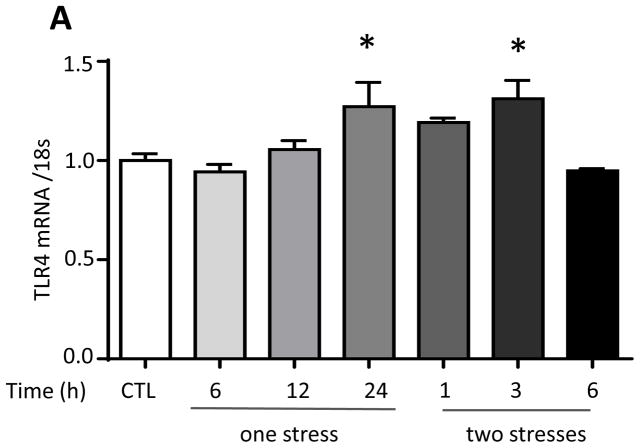
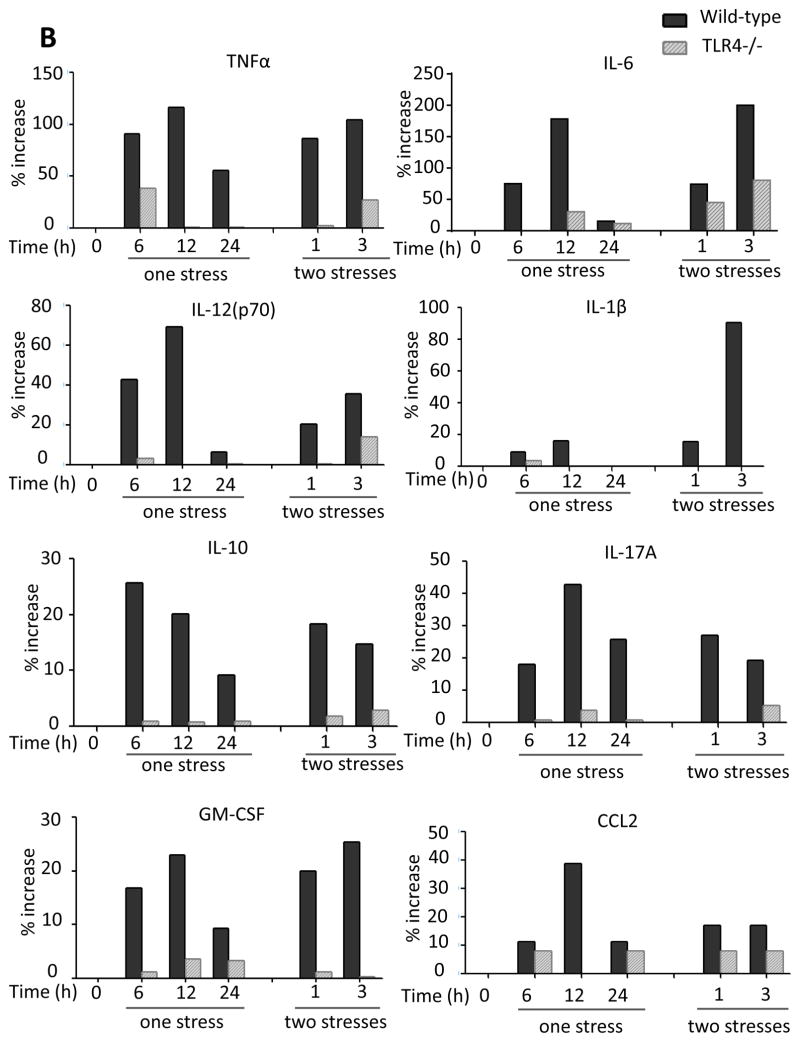
Since TLR4 is a major mediator of pathogen-induced cytokine production in the periphery and has been linked to stress-induced inflammation, we first tested if stress modulates TLR4 expression in the hippocampus. TLR4 mRNA levels significantly increased 24 hr after stress by 22% (one-way ANOVA; F (6, 20) = 5.042, p < 0.05; Figure 2A), similar to the 20% increase previously reported to occur in mouse cortex following acute or chronic restraint stress (Gárate et al 2013; Gárate et al. 2014). We tested if stress-induced hippocampal cytokines and chemokines were dependent on TLR4 by comparing cytokines and chemokines in TLR4 knockout mice and wild-type mice after stress. TLR4 deficiency resulted in a blunted cytokine and chemokine response to stress, with lower than wild-type hippocampal levels of TNFα, IL-6, IL-12 (p70), IL-1β, IL-10, IL-17A, GM-CSF, CCL2 (Figure 2B), IL-12 (p40), IL-5, CXCL1, CCL11, IL-3, CCL5, IFNγ and CCL3 (Supplementary Figure 3A). However, the stress-induced hippocampal levels of IL-13 indicated a complex relationship with TLR4, and CCL4, were similar in TLR4−/− and wild-type mice. Thus, hippocampal TLR4 expression is induced after one stress and TLR4 deficiency blocked the elevations of many cytokines and chemokines in the hippocampus after stress.
Figure 2. Role of TLR4 in the response to stress.
(A) TLR4 mRNA levels 6, 12, and 24 hr following administration of a single session of inescapable foot shocks. 18s mRNA was used as an internal control. Bars represent means±SEM; n = 3–5 in each group; *p < 0.05 compared to non-stressed control (CTL) group (one-way ANOVA). (B) Wild-type and TLR4−/− mice were subjected to one or two daily sessions of inescapable foot shocks followed by measurements of cytokines and chemokines in the hippocampus by multiplex analysis. Hippocampi were collected 6, 12, and 24 hr after a single session of inescapable foot shocks, or 1 and 3 hr after two sessions of inescapable foot shocks. Hippocampi from 3–4 mice in each group were pooled for multiplex analysis.
Pretreatment of mice with the antidepressant fluoxetine or the GSK3-specific inhibitor TDZD-8 attenuate stress-induced cytokine and chemokine increases in the hippocampus
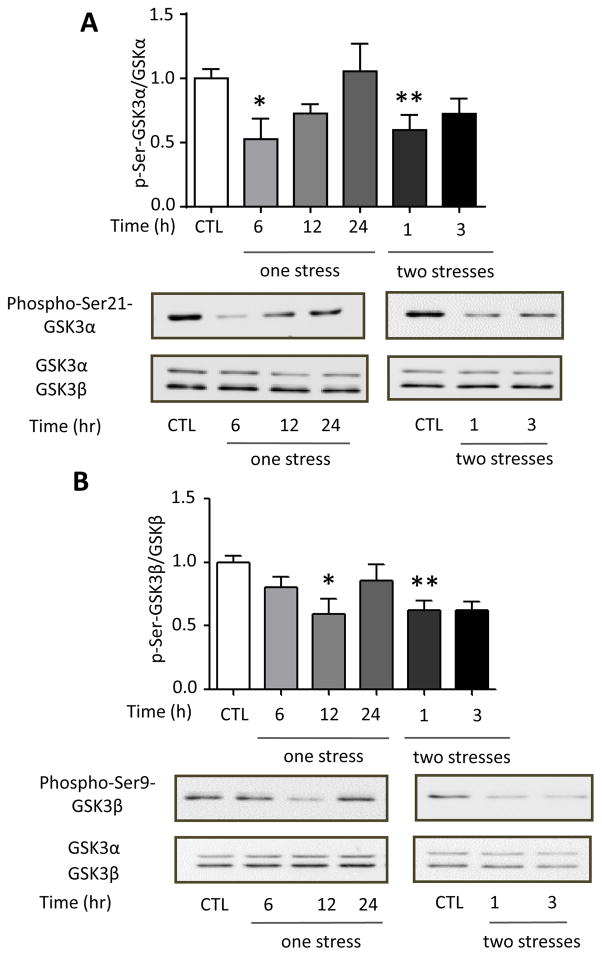
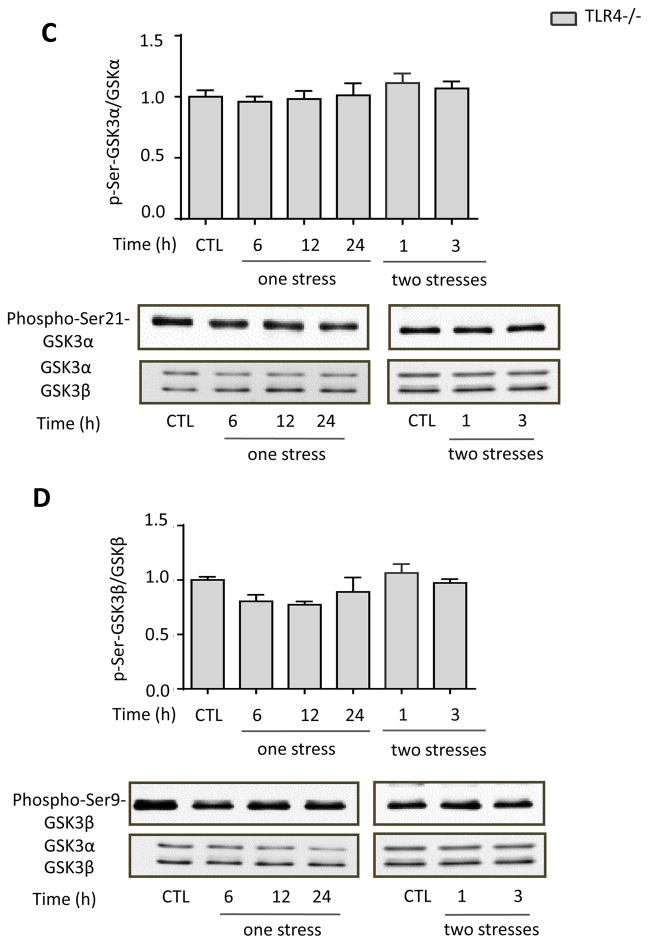
We tested if stress activates GSK3 and if this is altered by TLR4 deficiency because active GSK3 is necessary for LPS-induced TLR4-mediated inflammation (Jope et al., 2007). The two isoforms of GSK3 are inhibited by phosphorylation on a regulatory serine residue, serine-21 in GSK3α and serine-9 in GSK3β. Immunoblotting showed that the inhibitory serine-phosphorylation of GSK3α and of GSK3β in the hippocampus were significantly reduced 6 or 12 hr after a single stress (one-way ANOVA; GSK3α: F(3, 16) = 4.601, p < 0.05; GSK3β: F (3, 29) = 3.754, p < 0.05), and 1 hr after two stresses (GSK3α: F (2, 13) = 4.986, p < 0.05 compared to 24 hr after the first stress; GSK3β: F (2,17) = 4.079, p < 0.05; Figure 3A and B). This demonstrates that inescapable foot shock stress activates hippocampal GSK3 and stress pretreatment accelerates the rate of GSK3 activation. Remarkably, the stress-induced activation of hippocampal GSK3 was dependent on TLR4, as TLR4−/− mice exhibited no activation of either GSK3 isoform (Figure 3C and 3D) after one stress (one-way ANOVA; GSK3α: F (3, 10) = 0.0909, p > 0.05; GSK3β: F (3, 12) = 0.9734, p > 0.05) or two stresses (GSK3α: F (2, 9) = 0.3937, p > 0.05 compared to 24 hr after the first stress; GSK3β: F (2, 9) = 0.8844, p > 0.05).
Figure 3. Stress activates GSK3 in mouse hippocampus.
Serine-phosphorylated (A, C) GSK3α and (B, D) GSK3β, and total GSK3α/β (GSK3) were measured in mouse hippocampus at the indicated times after one or two daily inescapable foot shock stresses in wild-type (A: n = 4–7; B: n = 7–9) and TLR4−/− mice (C and D: n = 3–4). Quantitation of GSK3β includes both the main isoform and the slightly slower migrating alternatively spliced GSK3β2 variant. Bars represent means±SEM; *p < 0.05 compared to non-stressed control (CTL) group; **p < 0.05 compared to 24 hr after the first stress (one-way ANOVA).
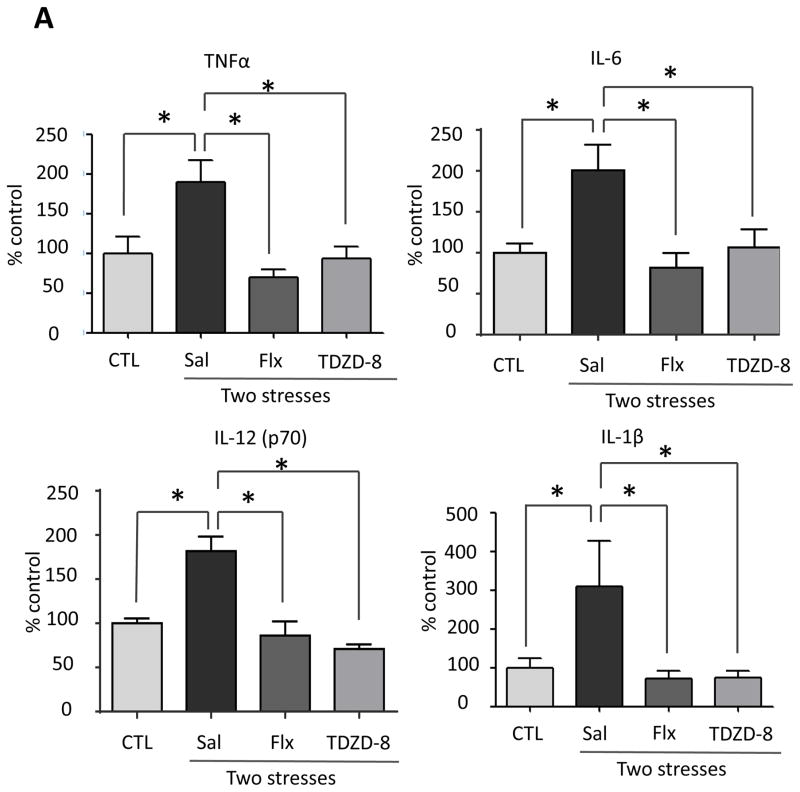
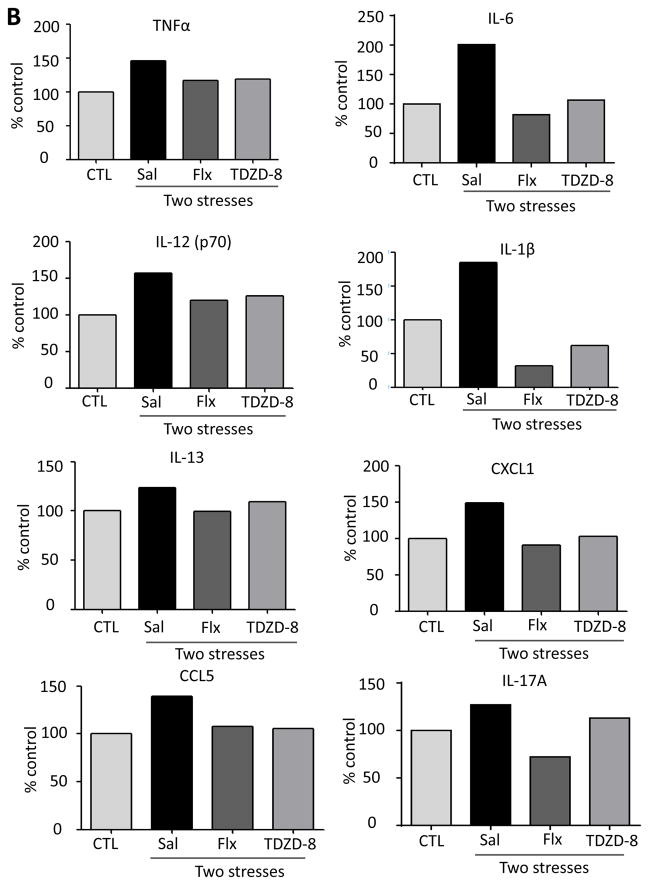
Since stress activated GSK3 and GSK3 promotes pathogen-induced TLR4-mediated cytokine production (Martin et al., 2005; Yuskaitis et al 2009; Beurel et al 2009; Beurel, 2011), we tested if active GSK3 also promotes stress-induced cytokine production. Prior to administration of two daily stresses, mice were treated for two weeks with the antidepressant fluoxetine (20 mg/kg; i.p.), a serotonin-selective reuptake inhibitor that inhibits GSK3 in mouse brain in vivo (Li et al., 2004), or with the direct GSK3-specific inhibitor TDZD-8 (5 mg/kg; i.p.) (Martinez et al., 2002). Treatment with fluoxetine reduced stress-induced increases of ELISA-detected TNFα (one-way ANOVA; F (2, 15) = 9.091, p < 0.05 ), IL-6 (F (2, 11) = 9.346, p < 0.05 ), IL-12 (p70) (F (2, 14) = 14.98, p < 0.05 ) and IL-1β (F (2, 15) = 5.505, p < 0.05 ) (Figure 4A), and multiplex-detected IL-13, CXCL1, CCL5, IL-17A, IL-2, IL-3, IL-5, CCL2 and CCL11 in the hippocampus 3 hr after stress (Figure 4B and Supplementary Figure 4), but did not affect the levels of IL-1α, IL-4, IL-10, IL-12 (p40), IFNκ, GM-CSF, CCL3 and CCL4 (data not shown). Pretreatment with TDZD-8 reduced stress-induced increases of ELISA-detected TNFα(one-way ANOVA; F (2, 15) = 6.157, p < 0.05), IL-6 (F (2, 15) = 6.212, p < 0.05), IL-12 (p70) (F(2, 14) = 28.27, p < 0.05), and IL-1β (F (2, 15) = 5.439, p < 0.05) (Figure 4A), and multiplex-detected IL-13, CXCL1, CCL5, IL-17A, and IL-5, but did not affect the levels of IL-2, IL-3, CCL2, CCL11 (Figure 4B and Supplementary Figure 4), IL-1α, IL-4, IL-10, IL-12 (p40), IFNκ, GM-CSF, CCL3 and CCL4 (data not shown). These results demonstrate that administration of an antidepressant or a selective inhibitor of GSK3 attenuate the induction of several stress-induced cytokines and chemokines in mouse hippocampus.
Figure 4. Pretreatment with fluoxetine or TDZD-8 attenuates stress-induced cytokines and chemokines in mouse hippocampus.
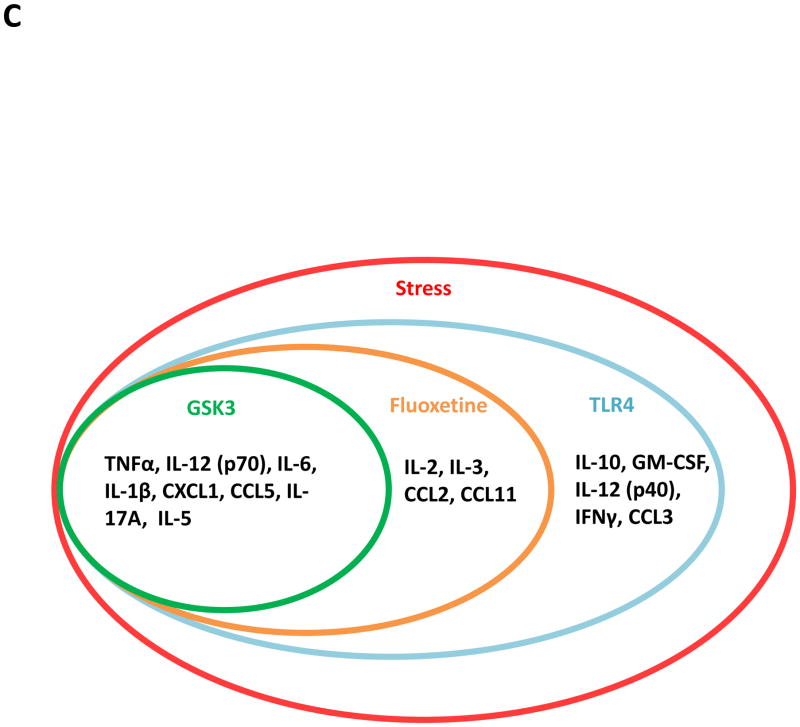
Following two weeks of daily treatment with fluoxetine (Flx), TDZD-8, or saline (Sal), mice were subjected to two daily sessions of inescapable foot shocks and after 3 hr cytokines and chemokines in the hippocampus were measured by (A) ELISA or (B) multiplex. ELISA values are means±SEM; n = 5–6 mice in each group; *p < 0.05 compared to non-stressed control (CTL) mice or to mice treated with saline (one-way ANOVA). Hippocampi from 3–4 mice in each group were pooled for multiplex analysis. (C) A diagram summarizing hippocampal cytokine and chemokine changes affected by two daily stresses stress. Cytokines and chemokines within the outer circle were increased by stress, and the labeled circles show the cytokines and chemokines that were decreased by TLR4 deficiency, fluoxetine treatment, and GSK3 inhibition.
In summary (Figure 4C), hippocampal TNFα, IL-12 (p70), IL-6, IL-1β, (Figure 1) CXCL1, CCL5, IL-17A, and IL-5 (Supplementary Figure 1) were induced by two stresses by a pathway involving TLR4 (Figure 2B and Supplementary Figure 3A) and reduced by treatment with fluoxetine or a GSK3 inhibitor (Figure 4 and Supplementary Figure 4). Stress-induced increases in hippocampal IL-2, IL-3, CCL2, and CCL11 were also dependent on TLR4 (Figure 2B and Supplementary Figure 3) and reduced by fluoxetine but not by TDZD-8 (Supplementary Figure 4), and IL-10, GM-CSF, IL-12 (p40), CCL3, and IFNκwere dependent on TLR4 (Figure 2B and Supplementary Figure 3A), but not diminished by fluoxetine or GSK3 inhibition (Figure 4 and Supplementary Figure 4). Altogether this data indicates that multiple mechanisms contribute to stress-induced cytokine and chemokine increases in the hippocampus although TLR4 signaling and GSK3 is a predominant pathway.
Inflammatory signaling in the hippocampus induced by stress
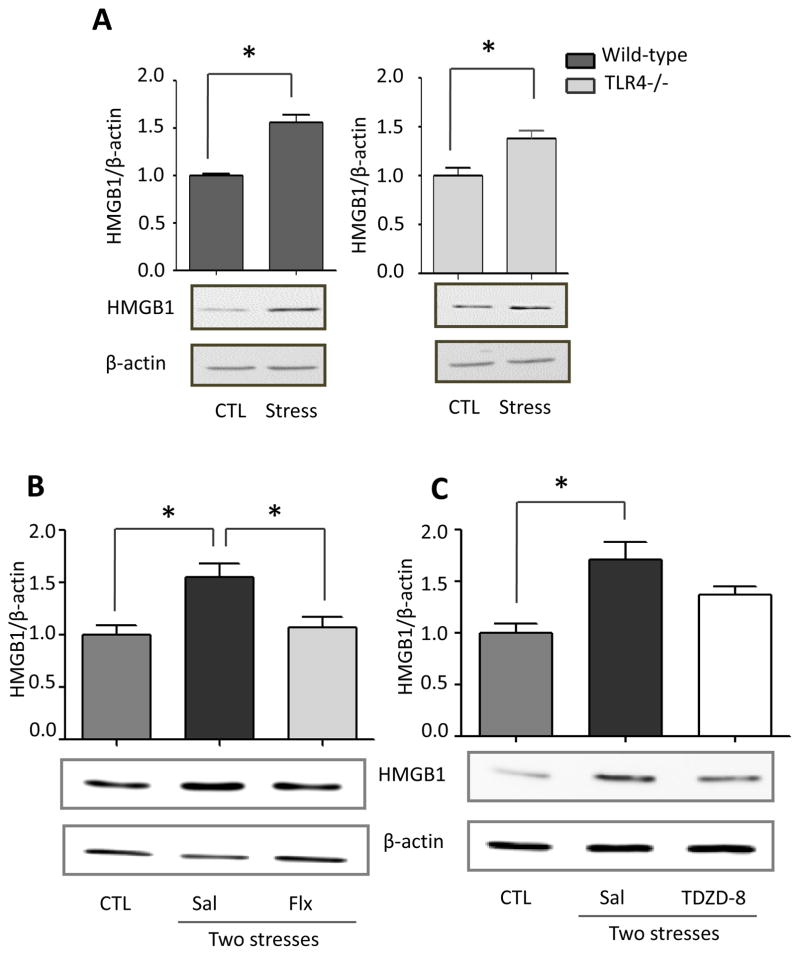
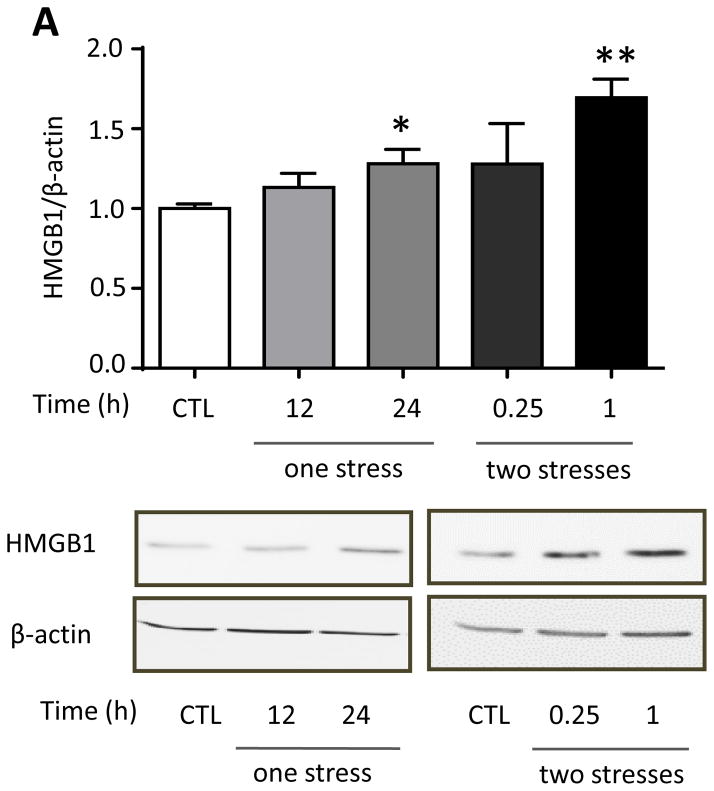
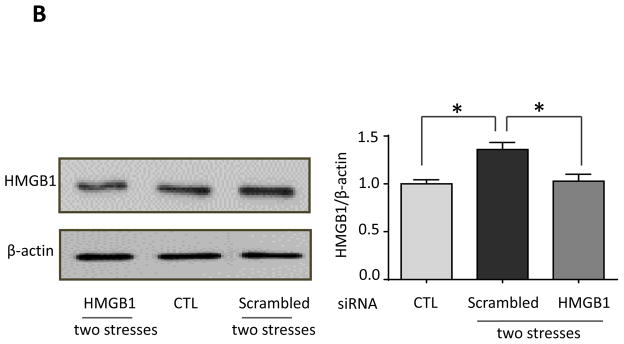
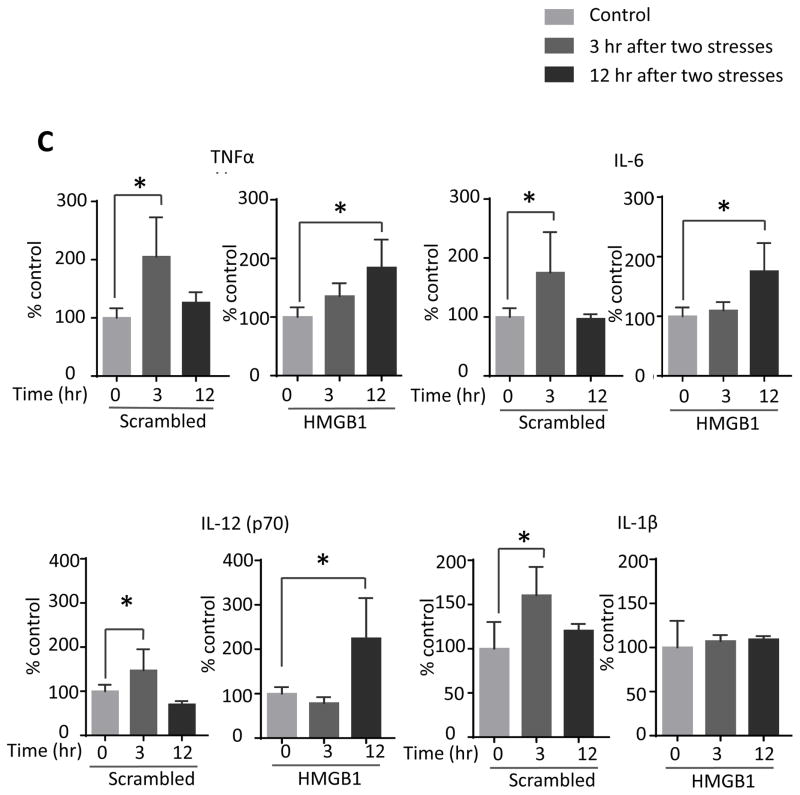
We tested if hippocampal HMGB1 levels were increased by inescapable foot shocks since HMGB1 stimulates TLR4 (Schaefer 2014) and HMGB1 was reported to be increased by inescapable tail shocks (Weber et al., 2015). Hippocampal HMGB1 levels were significantly increased 24 hr after a single stress (one-way ANOVA; F (2, 18) = 4.590, p < 0.05) and further increased 1 hr after two daily stresses (F (2, 14) = 3.751, p < 0.05 compared to 24 hr after the first stress) (Figure 6A). This suggests that HMGB1 is not required for hippocampal cytokine and chemokine increases induced by a single stress, which increased more rapidly, but HMGB1 may contribute to the priming effects of stress-pretreatment, leading to the accelerated rate of induction of most hippocampal cytokines and chemokines after two stresses. Since previously intranasal administration has been shown to allow modification of hippocampal functions and intranasal or intracerebroventricular administration of a variety of siRNA constructs have been shown to knockdown levels of proteins in the hippocampus (Bortolozzi et al., 2012; Kim et al., 2012; Ferres-Coy et al., 2013; Kanazawa et al., 2014; Ferres-Coy et al., 2015; Mohanty et al., 2015), we tested the effects of knocking down HMGB1 by intranasal siRNA administration, followed by measurements of hippocampal cytokines and chemokines that were increased at 3 hr after two stresses. Intranasal administration of HMGB1 siRNA eliminated the stress-induced increase in the hippocampal HMGB1 level at 3 hr after two stresses (one-way ANOVA; F (2, 12) = 9.957, p < 0.05: Figure 5B). We examined the effects of intranasal administration of HMGB1 siRNA on the levels of cytokines and chemokines with peak values 3 hr after two stresses to test if the peak reverted to the 12 hr time that occurred after a single stress. Remarkably, intranasal administration of HMGB1 siRNA blocked the accelerated rate of induction of hippocampal cytokines and chemokines induced by a prior stress (Figure 5C and Supplementary Figure 5), delaying increases after two stresses of TNFα (one-way ANOVA; scrambled siRNA 3 hr: F (2, 12) = 8.316, p < 0.05; HMGB1 siRNA 12 hr: F (2, 12) = 9.645, p < 0.05), IL-6 (scrambled siRNA 3 hr: F (2, 9) = 4.628, p < 0.05; HMGB1 siRNA 12 hr: F (2, 12) = 7.919, p < 0.05), and IL-12 (p70) (scrambled siRNA 3 hr: F (2, 12) = 7.858, p < 0.05; HMGB1 siRNA 12 hr: F (2, 12) = 10.79, p < 0.05) measured by ELISA, and multiplex-measured IL-2, IL-5, IL-13, CXCL1 and IL-17A, but not GM-CSF. Administration of HMGB1 siRNA also inhibited the increase of hippocampal IL-1β (scrambled siRNA 3 hr: F (2, 10) = 6.18, p < 0.05; HMGB1 siRNA 12 hr: F (2, 10) = 0.246, p > 0.05) and IL-3 that were otherwise induced 3 hr after administration of two stresses (Figure 5C and Supplementary Figure 5). This data indicates that increases in HMGB1 induced by stress contribute to the accelerated rate of increases of hippocampal cytokines and chemokines occurring after two stresses, and the increases of IL-1β and IL-3.
Figure 6. Stress-induced increases in hippocampal HMGB1 levels is blocked by fluoxetine treatment.
(A) Hippocampal HMGB1 levels 1 hr after two sessions of inescapable foot shocks in wild-type and TLR4−/− mice. n = 4–6 mice in each group; *p < 0.05 compared with control (CTL) mice that were not stressed (Student’s t test). Following two weeks of daily treatment with saline (Sal), (B) fluoxetine (Flx), or (C) TDZD-8, mice were subjected to two daily sessions of inescapable foot shocks and HMGB1 levels were measured by immunoblotting hippocampal extracts using β-actin as a loading control. n = 5–6 mice in each group; *p < 0.05 (one-way ANOVA).
Figure 5. Stress increases hippocampal HMGB1 that accelerates increases in stress-induced cytokines and chemokines.
(A) Hippocampal HMGB1 levels in wild-type mice after administration of a single or two sessions of inescapable foot shocks at the indicated times. n = 5–8 mice in each group; *p<0.05 compared with control (CTL) mice that were not stressed; **p<0.05 compared to 24 hr after the first stress (one-way ANOVA). (B, C) HMGB1 siRNA or scrambled siRNA was administered intranasally to mice 1 hr after the first of two daily sessions of inescapable foot shocks, and hippocampi were collected 3 and 12 hr after the second session. (B) Hippocampal HMGB1 levels were measured 3 hr after two stresses by immunoblotting hippocampal extracts using β-actin as a loading control. Bars represent means±SEM; n = 4–6 mice in each group; *p<0.05 compared with control (CTL) mice that were not stressed or mice treated with scrambled siRNA (one-way ANOVA). (C) Measurements of hippocampal TNFα, IL-6, IL-12(p70), and IL-1β by ELISA at 3 and 12 hr after two stresses. Values are means±SEM; n = 4–6 mice per group; *p < 0.05 compared to non-stressed control (0 hr) mice (one-way ANOVA).
TLR4 deficiency did not affect the increases in hippocampal HMGB1 induced by stress (Figure 6A), as HMGB1 levels were also significantly increased in TLR4−/− mice 1 hr after two stresses (t (11) = 5.899, p < 0.05 for wild-type mice; t (6) = 3.370, p < 0.05 for TLR4−/− mice), indicating that HMGB1 is upstream of TLR4 signaling. Remarkably, pretreatment with fluoxetine (20 mg/kg) for two weeks completely blocked the stress-induced increase of hippocampal HMGB1 (one-way ANOVA; F (2, 13) = 7.521, p < 0.05; Figure 6B), indicating that fluoxetine acts on an upstream target to reduce the stress-induced induction of this DAMP. In contrast, two weeks of pretreatment with TDZD-8 (5 mg/kg) did not significantly reduce stress-induced hippocampal HMGB1 (Figure 6C). HMGB1 levels in the prefrontal cortex did not change 24 hr after one stress (t (5) = 0.6167, p > 0.05; Supplementary Figure 5A), or 3 hr after two stresses, and were unaffected by treatment with fluoxetine or TDZD-8 (Supplementary Figure 6B and 6C), further indicating that the prefrontal cortex is less susceptible to stress-induced inflammatory responses than the hippocampus.
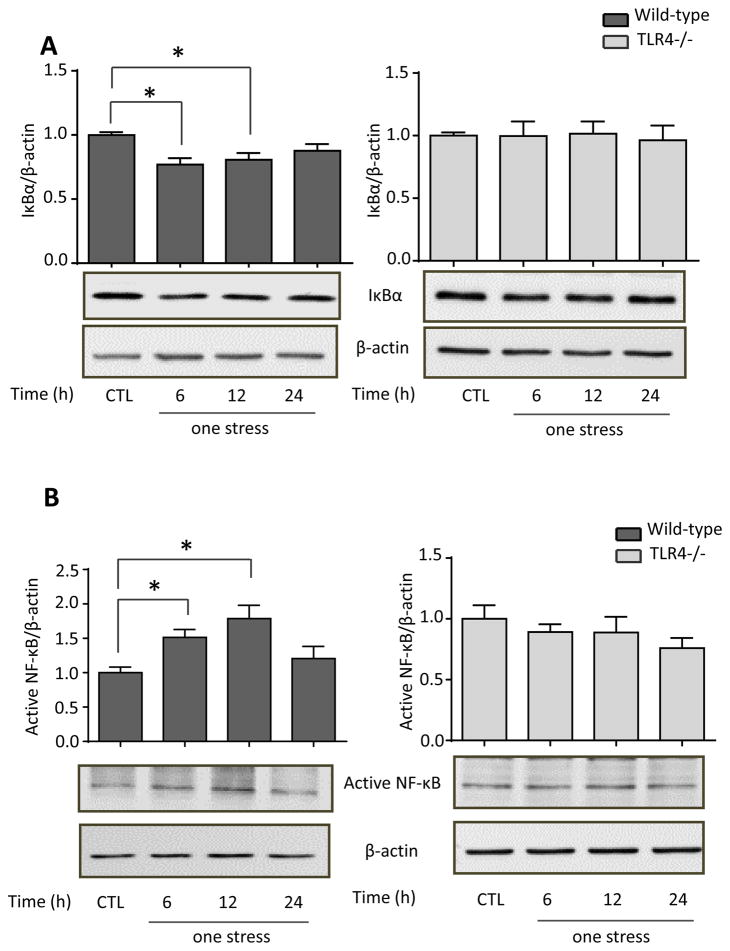
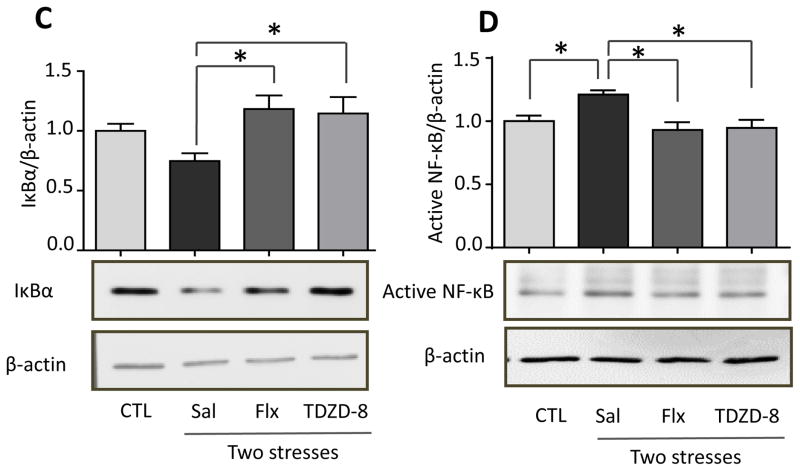
The activation of TLR4 signaling induces degradation of IκBα to release active NF-κB, which induces the expression of many cytokines and chemokines (Akira et al., 2006). Therefore, following stress we measured IκBα levels and active p65-NF-κB, using an antibody that binds to an epitope of the p65 subunit of NF-κB that is exposed after release from IκBα (Blondea et al., 2001; Weber et al., 2015). Hippocampal IκBα levels were significantly reduced (one-way ANOVA; F (3, 20) = 5.399, p < 0.05) and active p65-NF-κB levels were significantly increased (F (3, 19) = 6.757, p < 0.05) 6 and 12 hr after stress (Figure 7A and B). In TLR4−/− mice, stress did not significantly decrease IκBα (F (3, 12) = 0.365, p > 0.05; Figure 7A) or activate p65-NF-κB (F (3, 12) = 0.96, p > 0.05; Figure 7B). Furthermore, pretreatment with either fluoxetine (F (2, 14) = 9.714, p < 0.05) or TDZD-8 (F (2, 14) = 6.137, p < 0.05) prevented the stress-induced degradation of IκBα (Figure 7C) and activation of p65-NF-κB (Fluoxetine: F (2, 14) = 5.436, p < 0.05; TDZD-8: F (2, 14) = 7.636, p < 0.05; Figure 7D). These data demonstrate that acute stress is sufficient to activate NF-κB by a TLR4-mediated signaling pathway in the hippocampus that is attenuated by treatment with fluoxetine or TDZD-8.
Figure 7. Effects of TLR4 deficiency and pretreatment with fluoxetine or TDZD-8 on stress-induced hippocampal IκBα and p65-NF-κB levels.
Hippocampal (A) IκBα and (B) p65-NF-κB levels 6, 12 and 24 hr after administration of a single session of inescapable foot shocks in wild-type and TLR4−/− mice. n = 4–6 mice in each group; *p < 0.05 compared with control (CTL) mice that were not stressed (one-way ANOVA). Mice were pretreated for two weeks with fluoxetine (Flx), TDZD-8, or saline (Sal) and subjected to two daily sessions of inescapable foot shocks followed by measuring hippocampal (C) IκBα and (D) active p65-NF-κB levels. n = 5–6 in each group; *p < 0.05 compared with saline-treated mice (one-way ANOVA).
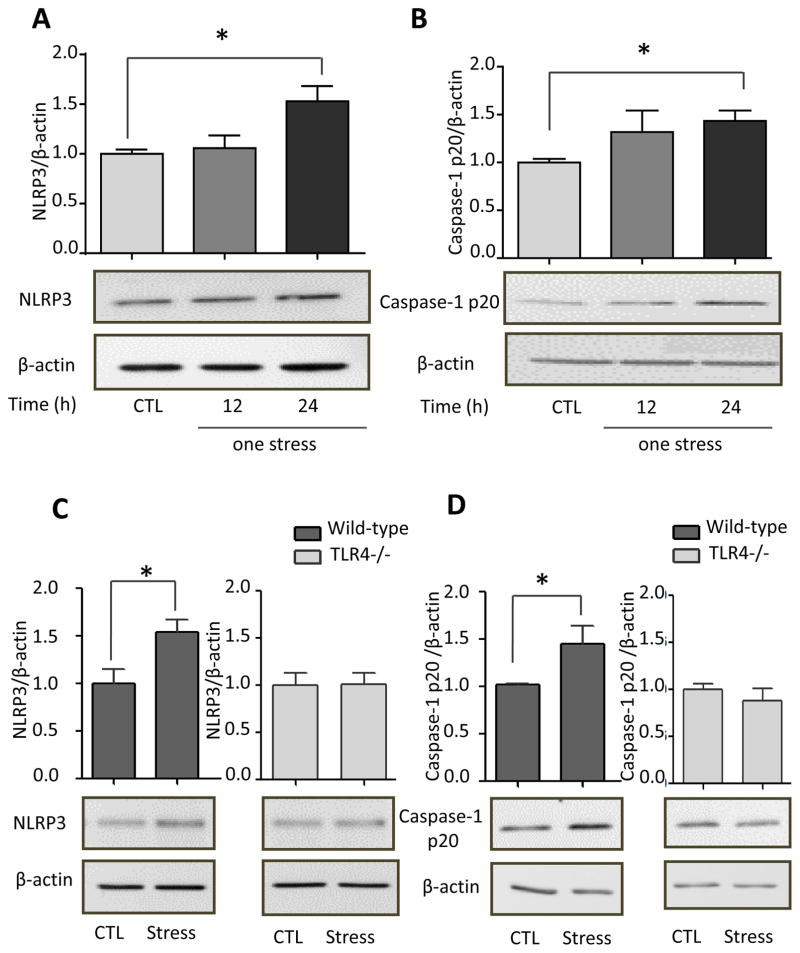
Previous reports have shown that HMGB1 increases the levels of NLRP3, a component of the NLRP3 inflammasome (Xiang et al., 2011; Weber et al., 2015) that activates caspase-1, leading to the production of IL-1β (Tschopp and Schroder, 2010). Since IL-1β levels in the hippocampus did not increase after a single stress but increased after two stresses, we examined if stress-induced IL-1β is associated with activated NLRP3 inflammasome. The hippocampal levels of NLRP3 and the p20 active form of caspase-1 were up-regulated 24 hr after stress (one-way ANOVA; F(2, 20) = 3.036, p < 0.05 for NLRP3; F (2, 19) = 2.213, p < 0.05 for caspase-1 p20; Figure 8A and B), indicating a prior stress primes the NLRP3 inflammasome for IL-1β increases in the hippocampus after a second stress. This is similar to previous reports that NLRP3 is primed in response to TLR4 activation by LPS, priming that includes increased levels of NLRP3 (Bauernfeind et al., 2009; Guo et al., 2015; Weber et al., 2015). Indeed, TLR4 deficiency attenuated stress-induced increases in NLRP3 (t (6) = 2.705, p < 0.05 for wild-type mice; t (6) = 0.074, p > 0.05 for TLR4−/− mice; Figure 8C), and the p20 active form of caspase-1 in the hippocampus 1 hr after two stresses (t (10) = 2.351, p < 0.05 for wild-type mice ; t (6) = 0.8121, p > 0.05 for TLR4−/− mice; Figure 8D), suggesting that TLR4 mediates stress-induced activation of the NLRP3 inflammasome.
Figure 8. Effects of stress on NLRP3 inflammasome activation in the hippocampus.
Hippocampal (A) NLRP3 and (B) active p20 caspase-1 levels were measured 12 and 24 hr after administration of a single session of inescapable foot shocks in wild-type mice. n = 6–8 mice in each group; *p < 0.05 compared with mice that were not stressed (one-way ANOVA). Hippocampal (A) NLRP3 and (B) active p20 caspase-1 levels were measured 1 hr after two daily sessions of inescapable foot shocks in wild-type and TLR4−/− mice. n = 4–6 mice p group; *p < 0.05 compared with mice that were not stressed (Student’s t test).
TLR4−/− mice display resistance to learned helplessness depression-like behavior
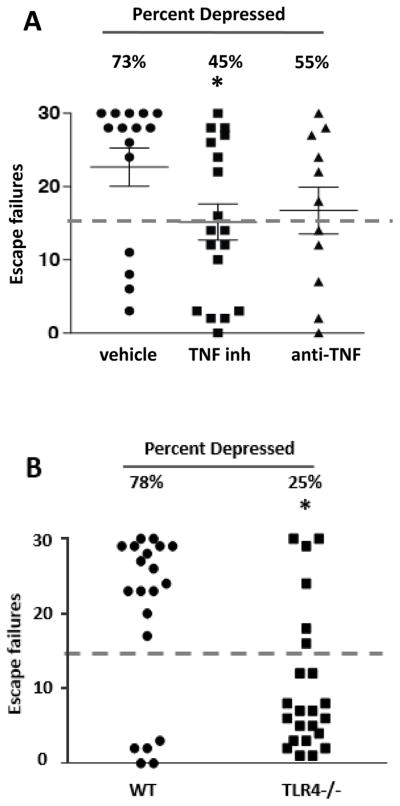
Since hippocampal TNFα was increased after stress, many studies have linked TNFα to depression (Dantzer and Kelley 2007; Palin et al., 2008; Tonelli et al., 2008; Dean, 2011; Kaster et al., 2012; Pandey et al., 2012), and TNFα production was blocked by the antidepressant fluoxetine, we tested if blocking TNFα is sufficient to provide resistance to learned helplessness. TNFα was inhibited using either a newly developed small molecule TNFα inhibitor, Vax-ATC1, or the classical TNFα antagonist etanercept. 73% of vehicle-treated mice exhibited learned helplessness depression-like behavior (Figure 9A). Administration of TNFα inhibitors tended to reduce the number of mice that developed learned helplessness, as 45% of the TNFα inhibitor-treated mice and 55% of the etanercept-treated mice exhibited learned helplessness (Figure 9A). This supports previous reports that inhibiting TNFα exerts antidepressant properties in mouse models of depression (Karson et al., 2013; Simen et al., 2006: Kaster et al., 2012), but also demonstrates that inhibiting TNFα only modestly reduces the induction of learned helplessness depression-like behavior.
Figure 9. TLR4−/− mice are resistant to learned helplessness.
(A) Learned helplessness was evaluated in wild-type mice following treatment with a small molecule TNFα inhibitor, Vax-ATC1, the anti-TNFα etanercept, or vehicle every other day for a week. n = 11–18 in each group. *p < 0.05 compared with mice treated with vehicle (Mann Whitney test). (B) Wild-type (WT) and TLR4−/− mice were subjected to the learned helplessness paradigm. Each point represents the number of failures to escape for a single mouse. Bars represent means ± SEM. n = 20–23 mice in each group. *p < 0.05 compared with wild-type mice (Student’s t test).
The limited success of anti-TNFα interventions suggested that simultaneously targeting multiple cytokines and chemokines may provide more a powerful antidepressant effect. Therefore, we tested if TLR4 deficiency, which reduces stress-induced increases of TNFα, IL-6, IL-12 (p70), IL-1β, IL-10, IL-17A, GM-CSF, CCL2 (Figure 2B), IL-12 (p40), IL-5, CXCL1, CCL11, IL-3, CCL5, IFNγ and CCL3, increased resistance to learned helplessness. TLR4−/− mice did not differ from wild-type mice in locomotor activity in a novel open field (Supplementary Figure 7A) or in sensitivity to foot shocks in an escapable foot shock paradigm (without previous inescapable foot shocks) (Supplementary Figure 7B). However, TLR4−/− mice exhibited resistance to learned helplessness compared with wild-type mice. Most wild-type mice (78%) exhibited learned helplessness (Figure 9B). In contrast, TLR4−/− mice were largely resistant to the induction of learned helplessness, with only 25% displaying learned helplessness (t (41) = 2.921, p < 0.05; Figure 9B). This demonstrates that blocking a major pathway that mediates stress-induced cytokine and chemokine production is sufficient to reduce susceptibility to this depression-like behavior.
DISCUSSION
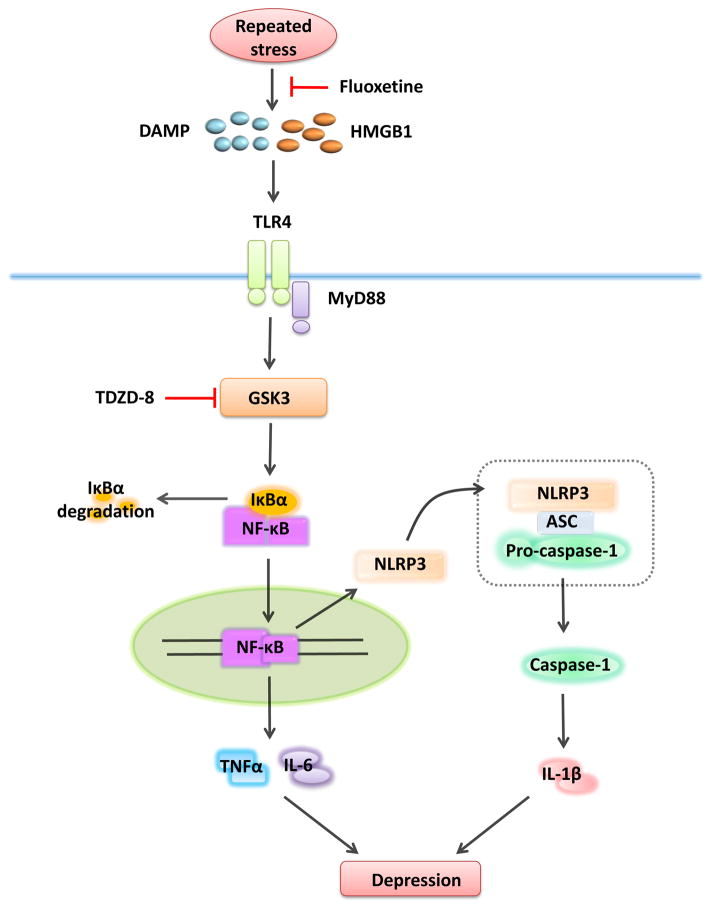
Stress is a pervasive condition that exacerbates numerous psychiatric and neurological conditions, such as depression. Increasing evidence demonstrates that stress activates inflammatory signaling (Dantzer et al., 2008; Miller et al., 2009; Kubera et al., 2011; Zunszain et al., 2013), raising the possibility that inflammation contributes to the deleterious health effects of stress. Here we report a comprehensive evaluation of the cytokine and chemokine increases after stress in mouse hippocampus, which increased more rapidly after a pre-conditioning stress, were largely dependent on TLR4, and many cytokine and chemokine increases were attenuated by administration of the antidepressant fluoxetine or the GSK3 inhibitor TDZD-8 (Figure 10). Furthermore, the attenuation of stress-induced inflammation in TLR4−/− mice reduced vulnerability to learned helplessness depression-like behavior.
Figure 10. Hypothetical scheme of the signaling activated by stress.
Stress activates TLR4, possibly through DAMP molecules, leading to the degradation of IκBα and the activation of NF-κB, which induces increased levels of several cytokines and chemokines including TNFα and IL-6. A pre-conditioning stress activates TLR4 signaling and increases expression of NLRP3, possibly through NF-κB activation (Bauernfeind et al., 2009; Guo et al., 2015), followed by assembly of the NLRP3 inflammasome and activation of caspase-1 leading to increased IL-1β production. A prior stress also increases the DAMP HMGB1, which may activate TLR4 to accelerate the rate of cytokine and chemokine increases after the second stress. Fluoxetine reduces stress-induced neuroinflammation and dampens stress-induced increases in HMGB1 and activation of NF-κB, and inhibition of GSK3 by TDZD-8 reduces stress-induced cytokines and chemokines and inhibits activation of NF-κB. MyD88 = Myeloid differentiation primary response gene 88; ASC = Apoptosis-associated speck-like protein containing a CARD.
Acute stress induced by a single session of inescapable foot shocks that induces learned helplessness increased the levels of 18 cytokines and chemokines in the hippocampus. Stress was previously reported to increase the levels of certain cytokines in rodent brain, as summarized in Supplementary Table 2. We found that a single stress of inescapable foot shocks increased the hippocampal levels of TNFα, IL-12(p70), IL-6, IL-2, IL-3, IL-10, IL-13, IL-12(p40), CXCL1, CCL11, IL-5, IL-17A, CCL2, CCL3, CCL4, CCL5, IFNκ, and GM-CSF. It is interesting to note that there was a wide range in the magnitude of the stress-induced increases in cytokines and chemokines in the hippocampus, spanning from an approximately 300% increase of IL-6 to increases near 20% of CCL3, CCL4, and CCL5. These variable increases emphasize the limited understanding of mechanisms that differentially regulate the responses of hippocampal cytokines and chemokines to stress, and suggest that those displaying the largest increases may have the greatest impact on behavioral responses to stress. The lack of increase of hippocampal IL-1β after a single session of inescapable foot shocks is in agreement with previous studies showing that hippocampal IL-1β levels were not increased at several times after stress (Nguyen et al., 2000; O’Connor et al., 2003; Deak et al., 2003; Deak et al., 2005; Blandino et al., 2006; Blandino et al., 2009). The same stress that increased hippocampal cytokine and chemokine levels did not increase cytokines and chemokines in the prefrontal cortex. While there is no information regarding the cause of this differential inflammatory response to stress, previous studies have reported that inescapable foot or tail shocks do not increase IL-6 or IL-1 in the prefrontal cortex (Nguyen et al., 2000; Deak et al., 2003; Blandino et al., 2009). On the other hand, cytokines in the prefrontal cortex or cortex were reported to increase after acute social defeat stress (Audet et al., 2011), chronic restraint stress (Garate et al., 2013), and chronic mild stress (Garate et al., 2011; You et al., 2011). These disparate findings raise the possibility that stress induced by shock paradigms preferentially affect hippocampal cytokines whereas stress induced by non-shock paradigms affect cytokines in both the hippocampus and the prefrontal cortex. Other differential responses of the hippocampus and prefrontal cortex to stress have been reported previously, notably a variety of stresses have been reported to increase brain-derived neurotropic factor mRNA and or protein levels within the prefrontal cortex (Bland et al., 2007; Fanous et al., 2010), but they were unchanged or decreased in subregions of the hippocampus (Smith et al., 1995; Barrientos et al., 2004; Pizarro et al., 2004). The greater sensitivity of hippocampal cytokine increases to shock stresses may be related to the reportedly larger density of microglia in the hippocampus than in the prefrontal cortex (Lawson et al., 1990), although potential mechanisms underlying the differential inflammatory responses to stress in the hippocampus and the prefrontal cortex remain to be clarified. Here we found that a single stress that causes depression-like behaviors increases the levels of many cytokines and chemokines in the hippocampus. In the future it will be important to examine the responses to stress of cytokines and chemokines that were not measured in this study to gain a more complete understanding of the influence of stress on cytokines and chemokines in the brain.
We tested if a prior stress affects neuroinflammatory responses to a second stress because stress is often encountered repeatedly and stress pretreatment amplified cytokine increases in the brain induced by LPS administration. For example, LPS-induced increases in hippocampal TNFα and IL-1β levels were potentiated by prior inescapable tail shocks, repeated social defeat stress or chronic unpredictable stress (Quan et al., 2001; Johnson et al., 2002; Johnson et al., 2003; Johnson et al., 2004; Munhoz et al., 2006; De Pablos et al., 2006; Frank et al., 2007; Espinosa-Oliva et al., 2011; Wohleb et al., 2012) (Supplementary Table 3). In addition, previous studies have reported that acute administration of IL-1β sensitized rodents to stress-induced increases in corticosterone and adrenocorticotropin hormone (Tilders and Schmidt, 1999; Schmidt et al., 2003), TNFα pretreatment sensitized rodents to TNFα-induced changes in corticosterone, corticotropin-releasing hormone, and sickness behaviors (Hayley et al., 1999; Hayley et al., 2001; Hayley et al., 2003; Anisman et al., 2003), repeated social defeat stress increased mouse IL-1β and TNF-α mRNA levels in the prefrontal cortex in response to a single episode of social defeat stress (Audet et al., 2011), and pre-exposure to three days of variable stress increased IL-1β mRNA levels in the hippocampus and the prefrontal cortex induced by acute social defeat stress in male mice (Hudson et al., 2014). Extending those results to stress interactions, we found that a prior stress increased subsequent stress-induced hippocampal levels of TNFα and IL-1β. However, the magnitudes of increases for most cytokines and chemokines after two stresses were similar to after a single stress, but the rates of increases were faster. Therefore, the major effect of a preconditioning stress is to accelerate the induction of hippocampal cytokines and chemokines after a subsequent stress.
TLR4 may play a central role in stress-induced neuroinflammation because it is activated by many types of DAMPs, some of which are induced by stress, and TLR4 activation can often increase TLR4 expression (Dantzer et al., 2008; Miller et al., 2009; Kubera et al., 2011; Zunszain et al., 2013; Schaefer, 2014). For example, acute or chronic restraint stress increased TLR4 mRNA expression by 20% in rodent frontal cortex and increased the activation of NF-κB, a transcription factor downstream of TLR4 signaling that induces the production of many cytokines and chemokines (Madrigal et al., 2002; Gárate et al., 2013; Gárate et al., 2014). Additionally, administration of an antagonist to block TLR4 prevented stress-induced potentiation of LPS-induced increases in hippocampal IL-1β (Weber et al., 2013). We found that acute inescapable foot shock stress increased TLR4 mRNA levels by 22%, similar to previous findings (Gárate et al., 2013; Gárate et al., 2014), increased IκBα degradation, and increased active p65-NF-κB in the hippocampus. Notably, stress-induced increases of most hippocampal cytokines and chemokines and activation of hippocampal NF-κB were attenuated in TLR4−/− mice. This data indicates that TLR4 activation plays a central role in a substantial portion of stress-induced inflammatory responses in the hippocampus.
The NLRP3 inflammasome has been reported to mediate some components of stress-induced neuroinflammation, particularly increases in IL-1β. For example, chronic mild stress increased NLRP3 levels, active caspase-1, and hippocampal IL-1β levels, and a NLRP3 inflammasome inhibitor reduced stress-induced IL-1β increases (Pan et al., 2014; Zhang et al., 2015). In addition, chronic corticosterone treatment that mimics stress increased NLRP3 levels in the hippocampus (Frank et al., 2014). The recent findings of increased levels of NLRP3 and active caspase-1 in mononuclear blood cells from patients with major depression (Alcocer-Gómez et al., 2014) indicate that activation of the NLRP3 inflammasome contributes to inflammation that is known to be associated with depression (Dantzer et al., 2008; Miller et al., 2009; Kubera et al., 2011; Zunszain et al., 2013). We found that NLRP3 and p20 active caspase-1 increased 24 hr after acute stress in the hippocampus. Since hippocampal IL-1β did not increase after a single stress, similar to previous findings (Nguyen et al., 2000; O’Connor et al., 2003; Deak et al., 2003; Deak et al., 2005), but increased after two stresses, and the NLRP3 inflammasome was not activated until 24 hr after the first stress, we hypothesize that a prior stress primes the NLRP3 inflammasome for IL-1β increases induced by a second stress. Interestingly, stress-induced increases in hippocampal NLRP3 and p20 caspase-1 were blocked in TLR4−/− mice, suggesting that TLR4 plays a role in stress-induced activation of the NLRP3 inflammasome for IL-1β production.
Stress activates HMGB1, a DAMP protein that is an agonist of TLR4 (Schaefer 2014). A previous study showed that acute inescapable tail shock stress increased HMGB1 in rodent hippocampus, and its release from isolated hippocampal microglia, and central administration of an HMGB1 antagonist diminished the potentiation by stress of LPS-induced increases of IL-1β by hippocampal microglia, suggesting HMGB1 mediates the priming effects of stress on microglia stimulated by LPS (Weber et al., 2015). In addition, HMGB1 increased NLRP3 levels in hippocampal microglia and lung epithelial cells (Xiang et al., 2011; Weber et al., 2015). We found that the hippocampal HMGB1 levels increased 24 hr after acute stress of inescapable foot shocks, indicating that HMGB1 does not mediate cytokine and chemokine increases induced by one stress. We also found that intranasal injection of HMGB1 siRNA blocked the accelerated rate of cytokine and chemokine increases induced by a prior stress, delaying the increases in several hippocampal cytokines and chemokines after two stresses. These findings indicate that HMGB1 contributes to the priming effects of stress-pretreatment, along with up-regulated expression of TLR4, leading to the rapid activation of TLR4 and the NLRP3 inflammasome for faster induction of most hippocampal cytokines and chemokines after two stresses. However, the mechanism by which stress increases HMGB1 is unknown. Frank et al (2015) recently proposed that the stress-induced increase in HMGB1 is mediated by glucocorticoids because glucocorticoids mediate the potentiation by stress of neuroinflammation induced by a subsequent immune challenge. Thus, it is possible that stress-induced glucocorticoids increase HMGB1, which then activates TLR4 and NLRP3 to increase cytokines and chemokines.
Fluoxetine is a selective serotonin reuptake inhibitor that is widely used as an antidepressant and has previously been reported to attenuate inflammation. For example, fluoxetine reduced TNFα and IL-6 levels in microglia stimulated with LPS (Liu et al., 2011), and pretreatment with fluoxetine reduced peripheral cytokines in animal models of inflammatory diseases, such as rheumatoid arthritis, LPS-induced septic shock, and allergic asthma (Abdel-Salam et al., 2004; Roumestan et al., 2007; Sacre et al., 2010). However, the effects of antidepressants on inflammation are controversial, as variable changes, or no changes, in cytokines have been reported in some studies following antidepressant administration, particularly in the serum of depressed patients (reviewed in Baumeister et al., 2015), and anti-inflammatory agents were reported to impair the behavioral responses to antidepressants (Warner-Schmidt et al., 2011). We found that pretreatment with fluoxetine reduced many stress-induced hippocampal cytokines and chemokines, including TNFα and IL-6. In addition, fluoxetine pretreatment reduced stress-induced increases in HMGB1 levels, and prevented stress-induced degradation of IκBα. These findings indicate that fluoxetine acts early in the response to stress, blocking the increase in HMGB1, which binds and activates TLR4, inhibited activation of NF-κB, and reduced stress-induced cytokines and chemokines.
Substantial evidence has shown that LPS-induced TLR4 signaling depends on active GSK3. GSK3 inhibitors reduced the levels of pro-inflammatory cytokines after stimulation with the TLR4 agonist LPS in human monocytes (Martin et al, 2005) and mouse microglia and astrocytes (Yuskaitis et al 2009; Beurel et al 2009). We found that stress activates GSK3α and GSKβ in the hippocampus and that this was dependent on TLR4. Pretreatment with the selective GSK3 inhibitor TDZD-8 reduced stress-induced increases in many hippocampal cytokines and chemokines, indicating that active GSK3 is required for maximal increases in multiple cytokines and chemokines following stress. Inhibition of GSK3 with TDZD-8 administration did not reduce stress-induced hippocampal HMGB1 increases, but prevented stress-induced IκBα degradation in the hippocampus. This indicates that active GSK3 is required for stress-induced activation of NF-κB, as previously shown for LPS-induced activation of NF-κB (Martin et al., 2005), thus promoting stress-induced cytokine increases in the hippocampus.
Inflammation, and in particular TNFα, have previously been linked to depression (Dantzer et al., 2008; Miller et al., 2009; Kubera et al., 2011; Zunszain et al., 2013). For example, central administration of TNFα induced depression-like behaviors in the forced swimming test and tail suspension test (Kaster et al., 2012), while treatment with the TNFα inhibitor infliximab and targeted deletion of TNF receptors had antidepressant effects in mice (Simen et al., 2006; Karson et al., 2013). In agreement with previous studies, we found that administration of TNFα inhibitors reduced by ~20~30% the number of mice that developed learned helplessness, suggesting that stress-induced TNFα contributes to learned helplessness. However, since depression is associated with elevations of many cytokines and chemokines, we hypothesized that blocking the production of multiple cytokines and chemokines may be more efficacious than targeting a single cytokine in treating depression. Furthermore, stress responses, particularly after acute stress, are not only deleterious but also in part provide support for adaptation to the environment. In order to test if the TLR4-mediated inflammatory response to stress is healthy or deleterious for mood regulation, susceptibility to learned helplessness was measured in TLR4-deficient mice and compared to wild-type mice. TLR4−/− mice were resistant to learned helplessness behavior, with only 25% of TLR4−/− mice developing learned helplessness compared with 78% of wild-type mice. This demonstrates the important role of TLR4 in modulating the susceptibility to depression-like behaviors in mice.
In conclusion, this study found that inescapable foot shock stress induced increases in eighteen cytokines and chemokines in mouse hippocampus, and a pre-conditioning stress accelerated the rate of induction of stress-induced cytokines and chemokines, often without changing the maximal levels. These responses were mediated by TLR4, GSK3, and inflammatory molecules that promote susceptibility to learned helplessness. These findings suggest that interventions blocking the molecules that mediate stress-induced neuroinflammatory responses may provide possible treatments for depression.
Supplementary Material
Foot shock stress increased mouse hippocampal levels of 18 cytokines
Prior stress accelerated the rate of increase of stress-induced hippocampal cytokines
Knockdown of HMGB1 reduced acceleration caused by prior stress on cytokine increases
TLR4 knockout reduced stress-induced increases of many hippocampal cytokines
Fluoxetine or GSK3 inhibitor pretreatment reduced stress-induced cytokine increases
Acknowledgments
This research was supported by grants from the NIMH (MH095380, MH038752, MH090236, MH104656), and the Expedition Inspiration Foundation. We thank the National Institute of Mental Health Chemical Synthesis and Drug Supply Program for providing fluoxetine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Salam OM, Baiuomy AR, Arbid MS. Studies on the anti-inflammatory effect of fluoxetine in the rat. Pharmacological Research. 2004;49:119–131. doi: 10.1016/j.phrs.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Alcocer-Gómez E, de Miguel M, Casas-Barquero N, Núñez-Vasco J, Sánchez-Alcazar JA, Fernández-Rodríguez A, Cordero MD. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav Immun. 2014;36:111–117. doi: 10.1016/j.bbi.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Anisman H, Merali Z, Hayley S. Sensitization associated with stressors and cytokine treatments. Brain Behav Immun. 2003;17:86–93. doi: 10.1016/s0889-1591(02)00100-9. [DOI] [PubMed] [Google Scholar]
- Audet M, Jacobson-Pick S, Wann BP, Anisman H. Social defeat promotes specific cytokine variations within the prefrontal cortex upon subsequent aggressive or endotoxin challenges. Brain Behav Immun. 2011;25:1197–1205. doi: 10.1016/j.bbi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1β administration. J Neuroimmunology. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Latz E. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunology. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Ciufolini S, Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4044-5. In press. [DOI] [PubMed] [Google Scholar]
- Belmaker RH, Agam G. Major depressive disorder. N Engl J Med. 2008;358:55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- Beninson LA, Brown PN, Loughridge AB, Saludes JP, Maslanik T, Hills AK, Fleshner M. Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (hsp72) and microRNA (miR-142–5p and miR-203) PloS One. 2014;9:e108748. doi: 10.1371/journal.pone.0108748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E. Regulation by glycogen synthase kinase-3 of inflammation and T cells in CNS diseases. Front Mol Neurosci. 2011;31:4–18. doi: 10.3389/fnmol.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Lipopolysaccharide-induced interleukin-6 production is controlled by glycogen synthase kinase-3 and STAT3 in the brain. J Neuroinflammation. 2009;6:9–20. doi: 10.1186/1742-2094-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73:622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, Day HE, Maier SF. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;144:1219–1228. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Deak T. The involvement of norepinephrine and microglia in hypothalamic and splenic IL-1β responses to stress. J Neuroimmunology. 2006;173:87–95. doi: 10.1016/j.jneuroim.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Blandino P, Jr, Barnum CJ, Solomon LG, Larish Y, Lankow BS, Deak T. Gene expression changes in the hypothalamus provide evidence for regionally-selective changes in IL-1 and microglial markers after acute stress. Brain Behav Immun. 2009;23:958–968. doi: 10.1016/j.bbi.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Blondea N, Widmann C, Lazdunski M, Heurteaux C. Activation of the nuclear factor-κB is a key event in brain tolerance. J Neurosci. 2001;21:4668–4677. doi: 10.1523/JNEUROSCI.21-13-04668.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthé RM, Michaud B, Poli V, Dantzer R. Role of IL-6 in cytokine-induced sickness behavior: a study with IL-6 deficient mice. Physiology & Behavior. 2000;70:367–373. doi: 10.1016/s0031-9384(00)00269-9. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Castañé A, Semakova J, Santana N, Alvarado G, Cortés R, Ferrés-Coy A, Fernández G, Carmona MC, Toth M, Perales JC, Montefeltro A, Artigas F. Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol Psychiatry. 2012;17:612–623. doi: 10.1038/mp.2011.92. [DOI] [PubMed] [Google Scholar]
- Campisi J, Leem TH, Fleshner M. Stress-induced extracellular Hsp72 is a functionally significant danger signal to the immune system. Cell stress & Chaperones. 2003;8:272–286. doi: 10.1379/1466-1268(2003)008<0272:sehiaf>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Sharkey C, Johnson JD, Asea A, Maslanik T, Bernstein-Hanley I, Fleshner M. Stress-induced facilitation of host response to bacterial challenge in F344 rats is dependent on extracellular heat shock protein 72 and independent of alpha beta T cells. Stress. 2012;15:637–646. doi: 10.3109/10253890.2011.653596. [DOI] [PubMed] [Google Scholar]
- Crack PJ, Bray PJ. Toll-like receptors in the brain and their potential roles in neuropathology. Immunol Cell Biol. 2007;85:476–480. doi: 10.1038/sj.icb.7100103. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Garza R., 2nd Endotoxin- or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neurosci Biobehav Rev. 2005;29:761–70. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- De Pablos RM, Villaran RF, Argüelles S, Herrera AJ, Venero JL, Ayala A, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neuroscience. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B. Understanding the role of inflammatory-related pathways in the pathophysiology and treatment of psychiatric disorders: evidence from human peripheral studies and CNS studies. International J Neuropsychopharmacology. 2011;14:997–1012. doi: 10.1017/S1461145710001410. [DOI] [PubMed] [Google Scholar]
- Deak T, Bellamy C, D’Agostino LG. Exposure to forced swim stress does not alter central production of IL-1. Brain Res. 2003;972:53–63. doi: 10.1016/s0006-8993(03)02485-5. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bulletin. 2005;64:541–556. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Espinosa-Oliva AM, Espinosa-Oliva RM, Villarán RF, Argüelles S, Venero JL, Machado A, Cano J. Stress is critical for LPS-induced activation of microglia and damage in the rat hippocampus. Neurobiology Aging. 2009;32:85–102. doi: 10.1016/j.neurobiolaging.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Fanous S, Hammer RP, Jr, Nikulina EM. Short- and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience. 2010;167:598–607. doi: 10.1016/j.neuroscience.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrés-Coy A, Pilar-Cuellar F, Vidal R, Paz V, Masana M, Cortés R, Carmona MC, Campa L, Pazos A, Montefeltro A, Valdizán EM, Artigas F, Bortolozzi A. RNAi-mediated serotonin transporter suppression rapidly increases serotonergic neurotransmission and hippocampal neurogenesis. Transl Psychiatry. 2013;3:e211. doi: 10.1038/tp.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrés-Coy A, Galofré M, Pilar-Cuéllar F, Vidal R, Paz V, Ruiz-Bronchal E, Campa L, Pazos Á, Caso JR, Leza JC, Alvarado G, Montefeltro A, Valdizán EM, Artigas F, Bortolozzi A. Therapeutic antidepressant potential of a conjugated siRNA silencing the serotonin transporter after intranasal administration. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Campisi J, Amiri L, Diamond DM. Cat exposure induces both intra-and extracellular Hsp72: the role of adrenal hormones. Psychoneuroendocrinology. 2004;29:1142–1152. doi: 10.1016/j.psyneuen.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun. 2015;48:1–7. doi: 10.1016/j.bbi.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zunich SM, O’Connor JC, Kavelaars A, Dantzer R, Kelley KW. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2, 3 dioxygenase in murine organotypic hippocampal slice cultures. J Neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gárate I, García-Bueno B, Madrigal JL, Bravo L, Berrocoso E, Caso JR, Micó JA, Leza JC. Origin and consequences of brain Toll-like receptor 4 pathway stimulation in an experimental model of depression. J Neuroinflammation. 2011;8:151. doi: 10.1186/1742-2094-8-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gárate I, Garcia-Bueno B, Madrigal JL, Caso JR, Alou L, Gomez-Lus ML, Micó JA, Leza JC. Stress-induced neuroinflammation: role of the Toll-like receptor-4 pathway. Biol Psychiatry. 2013;73:32–43. doi: 10.1016/j.biopsych.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Gárate I, García-Bueno B, Madrigal JL, Caso JR, Alou L, Gómez-Lus ML, Leza JC. Toll-like 4 receptor inhibitor TAK-242 decreases neuroinflammation in rat brain frontal cortex after stress. J Neuroinflammation. 2014;11:8. doi: 10.1186/1742-2094-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Guo H, Callawa JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke ML, Kielian T. Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci. 2011;121:367–387. doi: 10.1042/CS20110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Brebner K, Lacosta S, Merali Z, Anisman H. Sensitization to the effects of tumor necrosis factor-α: neuroendocrine, central monoamine, and behavioral variations. J Neuroscience. 1999;19:5654–5665. doi: 10.1523/JNEUROSCI.19-13-05654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Staines W, Merali Z, Anisman H. Time-dependent sensitization of corticotropin-releasing hormone, arginine vasopressin and c-fos immunoreactivity within the mouse brain in response to tumor necrosis factor-α. Neuroscience. 2001;106:137–148. doi: 10.1016/s0306-4522(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Hayley S, Merali Z, Anisman H. Stress and cytokine-elicited neuroendocrine and neurotransmitter sensitization: implications for depressive illness. Stress. 2003;6:19–32. doi: 10.1080/1025389031000091167. [DOI] [PubMed] [Google Scholar]
- Hudson SP, Jacobson-Pick S, Anisman H. Sex differences in behavior and pro-inflammatory cytokine mRNA expression following stressor exposure and re-exposure. Neuroscience. 2014;277:239–249. doi: 10.1016/j.neuroscience.2014.07.007. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Stark M, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–577. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Jope RS, Yuskaitis CJ, Beurel E. Glycogen synthase kinase-3 (GSK3): inflammation, diseases, and therapeutics. Neurochem Res. 2007;32:577–595. doi: 10.1007/s11064-006-9128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16–26. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa T, Morisaki K, Suzuki S, Takashima Y. Prolongation of life in rats with malignant glioma by intranasal siRNA/drug codelivery to the brain with cell-penetrating peptide-modified micelles. Mol Pharmacol. 2014;11:1471–1478. doi: 10.1021/mp400644e. [DOI] [PubMed] [Google Scholar]
- Karson A, Demirtaş T, Bayramgürler D, Balci F, Utkan T. Chronic Administration of Infliximab (TNF-α Inhibitor) Decreases Depression and Anxiety-like Behaviour in Rat Model of Chronic Mild Stress. Basic & Clinical Pharmacology & Toxicology. 2013;112:335–340. doi: 10.1111/bcpt.12037. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Gadotti VM, Calixto JB, Santos AR, Rodrigues ALS. Depressive-like behavior induced by tumor necrosis factor-α in mice. Neuropharmacology. 2012;62:419–426. doi: 10.1016/j.neuropharm.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Kim ID, Shin JH, Kim SW, Choi S, Ahn J, Han PL, Park JS, Lee JK. Intranasal delivery of HMGB1 siRNA confers target gene knockdown and robust neuroprotection in the postischemic brain. Mol Ther. 2012;20:829–839. doi: 10.1038/mt.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Kubera M, Obuchowicz E, Goehler L, Brzeszcz J, Maes M. In animal models, psychosocial stress-induced (neuro) inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:744–759. doi: 10.1016/j.pnpbp.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3β (GSK3β) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Wang Z, Liu S, Wang F, Zhao S, Hao A. Anti-inflammatory effects of fluoxetine in lipopolysaccharide (LPS)-stimulated microglial cells. Neuropharmacology. 2011;61:592–599. doi: 10.1016/j.neuropharm.2011.04.033. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Hurtado O, Moro MA, Lizasoain I, Lorenzo P, Castrillo A, Boscá L, Leza JC. The increase in TNF-alpha levels is implicated in NF-κB activation and inducible nitric oxide synthase expression in brain cortex after immobilization stress. Neuropsychopharmacology. 2002;26:155–163. doi: 10.1016/S0893-133X(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Alonso M, Castro A, Pérez C, Moreno FJ. First non-ATP competitive glycogen synthase kinase 3β (GSK-3β) inhibitors: thiadiazolidinones (TDZD) as potential drugs for the treatment of Alzheimer’s disease. J Med Chem. 2002;45:1292–1299. doi: 10.1021/jm011020u. [DOI] [PubMed] [Google Scholar]
- Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain Behav Immun. 2013;28:54–62. doi: 10.1016/j.bbi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty C, Kundu P, Sahoo SK. Brain Targeting of siRNA via Intranasal Pathway. Curr Pharm Des. 2015;21:4606–4613. doi: 10.2174/138161282131151013191737. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, de Sá Lima L, Avellar MCW, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-κB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neuroscience. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Will MJ, Hansen MK, Hunsaker BN, Fleshner M, Watkins LR, Maier SF. Time course and corticosterone sensitivity of the brain, pituitary, and serum interleukin-1beta protein response to acute stress. Brain Res. 2000;859:193–201. doi: 10.1016/s0006-8993(99)02443-9. [DOI] [PubMed] [Google Scholar]
- O’Connor KA, Johnson JD, Hansen MK, Wieseler Frank JL, Maksimova E, Watkins LR, Maier SF. Peripheral and central proinflammatory cytokine response to a severe acute stressor. Brain Res. 2003;991:123–132. doi: 10.1016/j.brainres.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Palin K, McCusker RH, Strle K, Moos F, Dantzer R, Kelley KW. Tumor necrosis factor-α-induced sickness behavior is impaired by central administration of an inhibitor of c-jun N-terminal kinase. Psychopharmacology. 2008;197:629–635. doi: 10.1007/s00213-008-1086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Chen XY, Zhang QY, Kong LD. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatric Res. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Agrawal DK. Immunobiology of Toll-like receptors: emerging trends. Immunol Cell Biol. 2006;84:333–341. doi: 10.1111/j.1440-1711.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/672395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunology. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Anti-inflammatory agents as antidepressants: truth or dare? Psychiat Annals. 2015;45:255–261. [Google Scholar]
- Roumestan C, Michel A, Bichon F, Portet K, Detoc M, Henriquet C, Mathieu M. Anti-inflammatory properties of desipramine and fluoxetine. Respir Res. 2007;8:35–45. doi: 10.1186/1465-9921-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacre S, Medghalchi M, Gregory B, Brennan F, Williams R. Fluoxetine and citalopram exhibit potent antiinflammatory activity in human and murine models of rheumatoid arthritis and inhibit toll-like receptors. Arthritis & Rheumatism. 2010;62:683–693. doi: 10.1002/art.27304. [DOI] [PubMed] [Google Scholar]
- Schaefer L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ED, Aguilera G, Binnekade R, Tilders FJH. Single administration of interleukin-1 increased corticotropin releasing hormone and corticotropin releasing hormone-receptor mRNA in the hypothalamic paraventricular nucleus which paralleled long-lasting (weeks) sensitization to emotional stressors. Neuroscience. 2003;116:275–283. doi: 10.1016/s0306-4522(02)00555-9. [DOI] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilders FJ, Schmidt ED. Cross-sensitization between immune and non-immune stressors. A role in the etiology of depression? Adv Exp Med Biol. 1999;461:179–197. doi: 10.1007/978-0-585-37970-8_11. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatrica Scandinavica. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature Rev Immunology. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proc Natl Acad Sci U S A. 2011;108:9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress induces the danger-associated molecular pattern HMGB-1 in the hippocampus of male Sprague Dawley rats: a priming stimulus of microglia and the NLRP3 inflammasome. J Neurosci. 2015;35:316–24. doi: 10.1523/JNEUROSCI.3561-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, La’Tonia MS, Bailey MT, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neuroscience. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37:1491–1505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Shi X, Li Y, Xu J, Yin L, Xiao G, Fan J. Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunology. 2011;187:4809–4817. doi: 10.4049/jimmunol.1102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z, Luo C, Zhang W, Chen Y, He J, Zhao Q, Wu Y. Pro-and anti-inflammatory cytokines expression in rat’s brain and spleen exposed to chronic mild stress: involvement in depression. Behavioural Brain Res. 2011;225:135–141. doi: 10.1016/j.bbr.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Yuskaitis CJ, Jope RS. Glycogen synthase kinase-3 regulates microglial migration, inflammation, and inflammation-induced neurotoxicity. Cell Signal. 2009;21:264–273. doi: 10.1016/j.cellsig.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu L, Liu YZ, Shen XL, Wu TY, Zhang T, Jiang CL. NLRP3 Inflammasome Mediates Chronic Mild Stress-induced Depression in Mice via Neuroinflammation. Int J Neuropsychopharmacology. 2015 doi: 10.1093/ijnp/pyv006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Hepgul N, Pariante CM. Inflammation and depression. Curr Top Behav Neurosci. 2013;14:135–151. doi: 10.1007/7854_2012_211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.