Abstract
The constitutive androstane receptor (CAR, NR1i3) is a key regulator of CYP2B6, the enzyme predominantly responsible for the biotransformation of cyclophosphamide (CPA) to its pharmacologically active metabolite, 4-hydroxycyclophosphamide (4-OH-CPA). Previous studies from our laboratory illustrated that CAR activation increases the formation of 4-OH-CPA; however, CPA is rarely utilized clinically outside of combination therapies. Here, we hypothesize that including a selective human CAR activator with the CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen can improve the efficacy without exacerbating off-target toxicity of this regimen in non-Hodgkin lymphoma treatment. In this study, we have developed a novel multi-organ co-culture system containing human primary hepatocytes for hepatic metabolism, lymphoma cells as a model target for CHOP, and cardiomyocytes as a major site of off-target toxicity associated with this regimen. We found that a selective human CAR activator, CITCO (6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl)oxime), altered expression of key drug-metabolizing enzymes and transporters in human hepatocytes, which positively impacts the metabolic profile of CHOP. Co-administration of CITCO and CHOP in the co-culture model led to significantly enhanced cytotoxicity in lymphoma cells but not in cardiomyocytes. Moreover, the beneficial effects of CITCO were abrogated when CAR knockout HepaRG cells were used in the co-culture model. Importantly, synergistic anticancer effects were observed between CITCO and CHOP, in that inclusion of CITCO alongside the CHOP regimen offers comparable antineoplastic activity toward lymphoma cells at significantly reduced drug concentrations and the decreased CHOP load attenuates cardiotoxicity. Overall, these findings provide a potentially promising novel strategy for facilitating CHOP-based chemotherapy.
Keywords: CHOP, CAR, CYP2B6, NHL, multi-organ co-culture
Introduction
Non-Hodgkin lymphoma encompasses diverse cancers of the lymphatic system, including the nodes, spleen, and other organs that mediate immune responses. The most common such malignancy is diffuse large B-cell lymphoma. In the United States there were over 70,000 new cases of non-Hodgkin lymphoma and roughly 20,000 deaths due to the disease in 2014 (1). Many therapies, including combination chemotherapy regimens, have been utilized to treat non-Hodgkin lymphoma, yet more than 30% of patients diagnosed with the disease do not survive longer than five years (2).
CHOP chemotherapy, composed of cyclophosphamide (CPA), doxorubicin, vincristine, and prednisone, is the current front-line therapy for diffuse large B-cell lymphoma (3), frequently in combination with an anti-CD20 antibody, rituximab (4, 5). This combination therapy has provided significant improvement in the long-term survival of patients diagnosed in the early stages of the disease, with relapse-free survival achieved in 80–90% of patients (6, 7). However, patients diagnosed with stage III or IV disease have significantly lower survival rates (3, 7). Thus, there is a need to optimize the front-line therapy for advanced-stage lymphomas.
CPA, one of the primary components of the CHOP regimen, is an alkylating agent that has been used in combination therapies for the treatment of various cancers as well as disorders of the immune system for many years (8, 9). CPA is a prodrug that undergoes oxidation in the liver to form its therapeutically active metabolite 4-hydroxycyclophosphamide (4-OH-CPA) (10, 11). This oxidation reaction is primarily mediated by cytochrome P450 (CYP) 2B6, with a less significant contribution from CYP2C9 and CYP3A4, though CYP3A4-mediated metabolism of CPA may also yield a therapeutically inactive byproduct, dechloroethyl-CPA (12, 13). An important toxicity of CHOP arises from another important component of this regimen, doxorubicin, a widely used anthracycline with cumulative and severe cardiotoxicity, which most frequently manifests as congestive heart failure (14). Although the mechanism of doxorubicin cardiotoxicity has yet to be definitively elucidated, higher doses of doxorubicin result in increased DNA damage, apoptosis, and oxidative stress in cardiomyocytes (15, 16). For lymphoma patients with pre-existing heart conditions, limitation of exposure to doxorubicin mandates avoiding the use of the CHOP regimen (17).
The constitutive androstane receptor (CAR, NR1i3), a xenobiotic sensor expressed primarily in the liver, is the predominant modulator for the inductive expression of CYP2B6 (18, 19). Selective activation of CAR allows for the preferential induction of CYP2B6 over CYP3A4, promoting the formation of the 4-OH-CPA alkylating moiety in cultures of isolated human primary hepatocytes (HPH) (20). Previous investigation in our laboratory has illustrated that selective induction of CYP2B6 by CAR activation in HPH enhances the metabolism of CPA to 4-OH-CPA and increases the cytotoxicity of CPA toward HL-60 human leukemia cells (21). Although these proof-of-concept findings are intriguing, in clinical application CPA is often co-administered with other chemotherapeutic agents in combination treatment, including the CHOP regimen for non-Hodgkin lymphoma. Thus, it is critical to investigate whether inclusion of a selective human CAR activator in the full CHOP regimen would increase the therapeutic index of CHOP-based treatment of lymphoma.
In the present studies, we sought to elucidate the effects of a selective CAR activator, 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl)oxime (CITCO), on the anticancer activity of the leading treatment for non-Hodgkin lymphoma, the CHOP regimen. To examine this scenario, we have employed a novel multi-organ co-culture model providing a cellular environment that incorporates metabolism-capable HPH, lymphoma cells, which are the drug targets, and cardiomyocytes, which are a target for toxicity. This unique model offers an in vitro system that resembles the in vivo cellular environment and allows for examination of the impact of hepatic drug-drug interactions on CHOP anticancer activity and extrahepatic targets concurrently. We provide experimental evidence that inclusion of CITCO significantly enhances the cytotoxicity of CHOP-based treatment in lymphoma cells, but not in co-cultured cardiomyocytes. These results also indicate that co-administration of a CAR agonist with low toxicity in conjunction with CHOP may lower the overall load of chemotherapeutic agents and alleviate toxicities without jeopardizing antineoplastic activity.
Methods & Materials
Chemicals and Reagents
CPA, doxorubicin, vincristine, prednisone, and 2’,7’-dichlorofluorescein diacetate were purchased from Sigma-Aldrich (St. Louis, MO). CITCO was acquired from BIOMOL research laboratories (Plymouth Meeting, PA). Oligoniclueotide primers were synthesized by and purchased from Integrated DNA Technologies. Insulin, Matrigel, and ITS+ culture supplies were obtained from BD Biosciences (Bedford, MA).
Human Primary Hepatocytes and HepaRG Cells
HPH were obtained from Bioreclamation, IVT (Baltimore, MD). Hepatocytes with viability over 90% were seeded at 1.5 × 106 or 7.5 × 105 cells/well in 6- or 12-well collagen-coated plates. After 4 h of attachment, cells were overlaid with Matrigel (0.25 mg/ml) in serum-free Williams E medium to form the sandwich culture, as described previously (22). Following incubation for 36 h, HPH were treated with DMSO (0.1%) or CITCO (1 μM) for 24 or 72 h for mRNA and protein detection, respectively. In separate experiments, wild-type (WT) and CAR-knockout (KO) HepaRG cells obtained from Sigma-Aldrich were cultured in 6- or 12-well collagen-coated plates as described previously (23). Cells were further treated with DMSO or CITCO, as above.
Culture and Treatment of Lymphoma Cells
Immortalized lymphoma cell lines SU-DHL-4 and SU-DHL-6 were obtained from ATCC (Rockville, MD) between 2012 and 2013. The OCI-LY-3 cell line was kindly provided by Dr. Ronald Gartenhaus (Department of Medicine, University of Maryland) in 2012. All cell lines were cultured in RPMI-1640 medium containing 10% fetal bovine serum and 1% penicillin/streptomycin. The cell lines were used for less than 40 passages. The authenticity of the cell lines were confirmed by short tandem repeat polymorphism profiling (DDC Medical, Fairfield, OH). Cells were treated with DMSO (0.1%) or CITCO (1 μM) for 24 h before harvesting for total RNA extraction.
Quantitative PCR Analysis
Total RNA from HPH and lymphoma cells was isolated with TRIzol Reagent (Life Technologies) and reverse transcribed using a High Capacity cDNA Archive kit (Applied Biosystems) following the manufacturers’ instructions. mRNA expression of CYP2B6, CYP3A4, CYP3A5, carbonyl reductase 1 (CBR1), CBR3, and MDR1 was normalized against that of GAPDH. Real-time PCR assays were performed in 96-well optical plates on a StepOnePlus Real-Time PCR System with SYBR Green PCR Master Mix (Applied Biosystems). The primer sequences used for real-time PCR analyses were acquired from previous reports (24–27). These sequences included: CYP2B6: 5′-AGACGCCTTCAATCCTGACC-3’ and 5′-CCTTCACCAAGACAAATC-CGC-3’; CYP3A4: 5′-GTGGGGCTTTTATGATGGTCA-3’ and 5’GCGTCAGATTTCTCACCAACACA-3’; CYP3A5: 5′-GGGTCTCTGGAAATTTGACACA GAG-3’ and 5′-CTGTTCTGATCACGTCGATCT-3’; CBR1: 5′-CCCCTGACTGCCCTTTC TTA-3′ and 5′-TCACCAGCGCTACATGGAT-3′; CBR3: 5′-AACCTCATGGGAGAGTGGTG-3′ and 5′-TCCTCGATAAGACCGTGACC-3′; MDR1: 5′-CACGTGGTTGGAAGCTAACC-3′ and 5′-GAAGGCCAGAGCATAAGATGC-3′; and GAPDH, 5′-CCCATCACCATCTTCCAGGAG-3’ and 5′-GTTGTCATGGATGACCTTGGC-3’. Fold induction of genes over control was determined as 2ΔΔCt, where ΔCt is representative of the cycle threshold number difference between the target gene and GAPDH and ΔΔCt represents the relative change in the intergroup variations.
Hepatocyte/SU-DHL-4 co-culture
HPH were cultured in collagen-coated 12-well plates and pre-treated with vehicle control (0.1% DMSO) or CITCO (1 μM) for 24 h. The wells were then separated with 3.0 μm polycarbonate membrane inserts (Sigma-Aldrich). 0.5 × 106 SU-DHL-4 cells suspended in supplemented Williams’ Medium E were transferred into the insert chamber with a final volume of 2 mL/well. In separate co-culture experiments, HPH were replaced with either WT or CARCAR-KO HepaRG cells. The co-cultures were exposed to designated concentrations of the chemotherapy drugs in CHOP in the presence or absence of CITCO for various time intervals, as indicated.
Multi-organ Co-culture System
In addition to the co-culture system described above, H9c2 cells (a rat cardiomyoblast cell line obtained from ATCC in 2014) were plated on modified coverslips at 1 × 105 cells and allowed to attach overnight at 37°C and 5% CO2. Following attachment, the coverslips were inserted in each well between the sandwich-cultured HPH and the transwell insert, as depicted in Fig. 4A. The cultures were exposed to designated concentrations of the chemotherapy drugs in CHOP in the presence or absence of CITCO (1 μM). Both SU-DHL-4 and H9c2 cells were harvested at the indicated time points and assessed for viability, apoptosis, DNA damage, and oxidative stress, as detailed below.
Western Blotting Analysis
Homogenate proteins from treated HPH, H9c2 or HepaRG cells were resolved on NuPAGE Novex Bis-Tris 4–12% gels (Life Technologies) and electrophoretically transferred onto immobilon-P polyvinylidene difluoride membranes. Membranes were incubated with specific antibodies against human CAR (Perseus Proteomics), cleaved caspase-3 (Cell Signaling Technology), or γ-H2AX (Millipore), diluted 1:1000, 1:1000, and 1:400, respectively. β-actin was used to normalize protein loading. Following incubation with horseradish peroxidase goat anti-mouse or anti-rabbit IgG antibody, membranes were developed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific). Western blot signals were quantified by densitometry using ImageJ software from the National Institutes of Health.
Cell Viability Assays
Human primary hepatocytes and H9c2 rat cardiomyocytes were seeded at 7.5 × 104 cells/well in 96-well plates. HPH were cultured for 24 h before treatment with CITCO (1 μM) or vehicle control (0.1% DMSO), followed by treatment with the chemotherapy drugs included in CHOP at a range of concentrations. Culture medium containing CHOP drugs and metabolites was exchanged between the cell types at pre-determined time points. A typical 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) assay was carried out as described previously (21). Cell viability was expressed as percent of vehicle control (0.1% DMSO). Co-cultured cells were assembled and treated as described above. The viability of the SU-DHL-4 cells was determined at selected time points with a Cellometer Auto T4 (Nexcelom Biosciences) utilizing trypan blue exclusion.
Phosphorylated H2AX Imaging
The H9c2 cells plated on cover slips were removed from the previously described multiple-organ co-cultures after 24 h of treatment with CHOP chemotherapy drugs at a range of concentrations in the presence or absence of CITCO (1 μM). Immunofluorescence assays were performed as published (28). In brief, the cells were fixed with 4% paraformaldehyde in PBS at room temperature for 15 min. The fixed cells were then permeabilized with 0.5% Triton X-100 for 15 min, washed three times in PBS for 5 min, blocked using 15% fetal bovine serum in PBS, and then incubated with primary antibody to mouse anti-γ-H2AX (Ser139, clone JBW301, Millipore, 1:500 dilution) overnight. Subsequently, cells were incubated for 50 min with second antibody goat-anti-mouse IgG Alexa fluor 594 (1:500 dilution) for 1 h at room temperature. Nuclear DNA was stained using DAPI at 50 ng/ml. The slides were visualized at 1000× magnification on a NIKON 90i fluorescence microscope (photometric cooled mono CCD camera).
Oxidative Stress Imaging
Cover slips with H9c2 cells attached from the multiple-organ co-culture model were removed after 24 h of indicated treatments and placed in fresh 12-well plates and washed three times with PBS. Subsequently, 1 mL of PBS containing 10 μM 2’,7′-dichlorofluorescein diacetate (DFDA) was added to each well. The cells were incubated at 37°C and 5% CO2 for 40 min. The oxidation of DFDA to the fluorescent dichlorofluorescein was viewed via fluorescence microscopy (Nikon Eclipse Ti) with a FITC filter at 900 nm and quantified using NIH Image J.
Statistical Analysis
All data are presented as the mean of triplicate measurements ± S.D. unless noted otherwise. Comparisons were made via one-way analysis of variance (ANOVA) utilizing post-hoc Dunnett’s analysis. Significance was determined at P <0.05 (*).
Results
CAR activation enhances CHOP anticancer activity towards SU-DHL-4 cells co-cultured with HPH
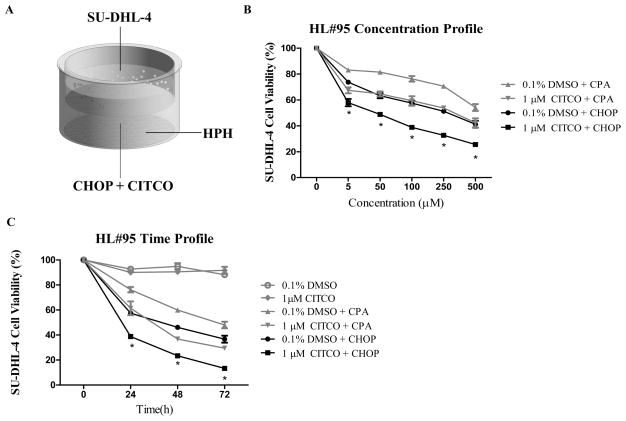
The chemotherapeutic benefit of combining the CHOP regimen with a selective CAR activator, CITCO, was initially examined using a HPH/SU-DHL-4 co-culture system as depicted in Fig. 1A. The composition of four drugs in CHOP was estimated based on literature and clinical instructions (Supplementary Table S1) (29). In the current study, 100 μM of CHOP indicates the combination of CPA (100 μM), doxorubicin (5 μM), vincristine (0.14 μM), and prednisone (10 μM). As demonstrated in Fig. 1B, both CPA and the CHOP regimen increased the cytotoxicity toward the target SU-DHL-4 cells in a concentration-dependent manner. Clearly, full CHOP achieved higher anticancer activity than CPA alone, as expected. Inclusion of CITCO with CHOP significantly enhanced cytotoxicity in SU-DHL-4 cells. In addition to the concentration-dependent cytotoxicity of CHOP with and without CITCO, time-dependent effects of this combination were observed. As shown in Figure 1C, similar beneficial effects were observed in the co-culture model 24 h after CITCO/CHOP treatment in a time-dependent manner. On the other hand, CITCO alone showed no effect on viability of SU-DHL-4 cells, indicating that the augmented cytotoxicity was not a direct result of the presence of CITCO. Importantly, 100 μM of CHOP in the presence of CITCO resulted in equal or greater anticancer activity than 500 μM of CHOP alone. These findings suggest the potential to lower the overall CHOP dosage by co-administration of CITCO in lymphoma treatment. It is worth noting that although the current study centers on CHOP-based lymphoma treatment, a similar CITCO-mediated augmentation of cytotoxicity of the FC (fludarabine, cyclophosphamide) regimen in leukemia cells was observed when co-cultured with HPH (Supplementary Fig. S1), suggesting this strategy may hold potential application in multiple hematological malignancies.
Figure 1. Activation of CAR improves the anticancer activity of the CHOP regimen in HPH/SU-DHL-4 co-culture.
Schematic representation of the HPH/SU-DHL-4 co-culture model utilized in the current studies (A). Concentration- (B) and time-dependent (C) anticancer activity of CPA and CHOP chemotherapy in the presence and absence of CITCO. Cells in co-culture were treated with CPA or CHOP (5, 50, 100, 250, 500 μM) in the presence of CITCO (1 μM) or vehicle control (0.1% DMSO) as detailed in Materials and Methods. Viability of SU-DHL-4 cells under these treatments was determined at 0, 24, 48, and 72 h. Data represent the mean ± S.D. of three independent measurements normalized as percent viability of vehicle control. Statistical significance between the treatment groups 0.1% DMSO/CHOP and CITCO/CHOP were analyzed (*, p < 0.05).
CITCO alters the expression of genes responsible for CHOP disposition in HPH, but not in target lymphoma cells
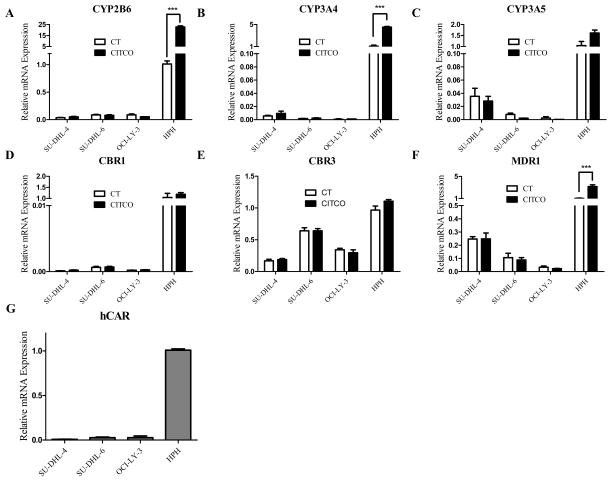
Key genes responsible for the disposition of the CHOP drugs, including CYP2B6, CYP3A4, CYP3A5, CBR1, CBR3, and MDR1 were measured in HPH and three lymphoma cell lines following incubation with CITCO, as detailed in the methods. As shown in Fig. 2, basal expression of CYP2B6, CYP3A4, CYP3A5, CBR1, CBR3, and MDR1 in lymphoma cells was negligible or significantly lower in comparison with that in HPH, supporting the notion that biotransformation of CHOP relies predominantly on hepatic metabolism. In HPH, CITCO treatment robustly induced the expression of CYP2B6 (17-fold) with moderate augmentation of CYP3A4 (4-fold), and MDR1 (4-fold), respectively (Fig. 2A, 2B, 2F). Expression of all tested genes in three lymphoma cell lines, on the other hand, was not altered significantly by CITCO, most likely due to the lack of CAR expression in these cells (Fig. 2G). Preferential induction of hepatic CYP2B6 over other tested genes favors CYP2B6-mediated oxidation of CPA to 4-OH-CPA. Additionally, based on lack of in situ increase of efflux transporter expression in lymphoma cells by CITCO, enhanced resistance to CHOP would not be expected.
Figure 2. Effects of CAR activation on the expression of genes responsible for CHOP disposition in HPH and lymphoma cell lines.
The expression of CYP2B6, CYP3A4, CYP3A5, CBR1, CBR3, MDR1, and CAR mRNA was measured in human primary hepatocytes as well as three representative lymphoma cell lines (SU-DHL-4, SU-DHL-6, and OCI-L-Y-3) following treatment with vehicle control (0.1% DMSO) or CITCO (1 μM) as described in the Materials and Methods. Expression of these genes was analyzed with RT-PCR. Data represent the mean ± S.D. of three independent experiments (***, p < 0.001).
The role of CITCO in CHOP-based treatment is CAR-dependent
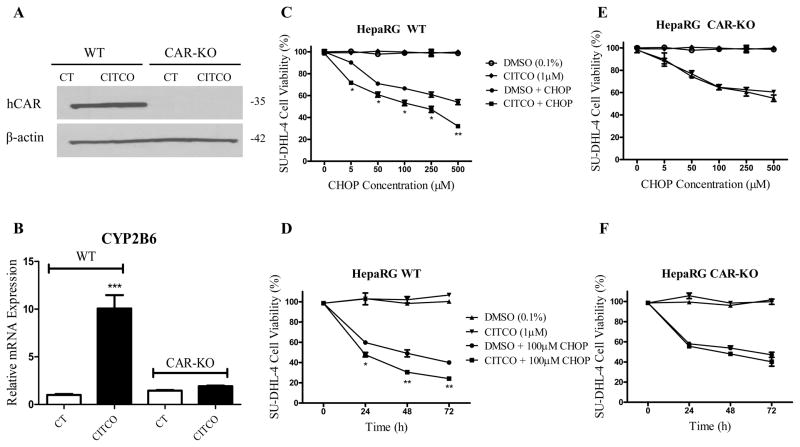
The HepaRG cell line has emerged as a promising alternative to HPH, exhibiting characteristic hepatic gene expression and metabolism capability (30). To further investigate whether the beneficial impact of CITCO on CHOP-based treatment is CAR-dependent, similar co-culture models were employed, with the replacement of HPH by WT or CAR-KO HepaRG cells. With the lack of functional CAR expression, induction of CYP2B6 by CITCO was completely abolished in CAR-KO HepaRG cells (Fig. 3A, 3B). CITCO significantly enhanced CHOP cytotoxicity in SU-DHL-4 cells co-cultured with WT-HepaRG cells in both concentration- and time-dependent manners (Fig. 3C, 3D), similarly to the beneficial response observed in the HPH-based model. In contrast, in the co-culture model containing CAR-KO HepaRG, CHOP-induced cytotoxicity of SU-DHL-4 cells was not altered regardless of the presence or absence of CITCO (Fig. 3E, 3F). These data clearly establish that the enhanced efficacy of the CHOP regimen in lymphoma cells induced by CITCO is primarily CAR-dependent.
Figure 3. CITCO enhanced CHOP anticancer activity is CAR-dependent.
Wild-type and CAR-KO HepaRG cells were treated with vehicle control (0.1% DMSO) or CITCO (1 μM) for 24 h for mRNA or 72 h for protein analysis. Expression of CAR protein (A) and CYP2B6 mRNA (B) was measured via western blot and RT-PCR, respectively. Co-culture and treatment of WT-HepaRG/SU-DHL-4 or CAR-KO-HepaRG/SU-DHL-4 were detailed in the Materials and Methods. Anticancer activity of CHOP in SU-DHL-4 cells was demonstrated in concentration-(C and E) and time (D and F)-dependent manners in the presence and absence of CITCO (1 μM). Data represent the mean ± S.D. of three independent experiments (*, p < 0.05; **, p < 0.01).
Activation of CAR selectively induces CHOP-mediated cytotoxicity in SU-DHL-4, but not in H9c2 cells
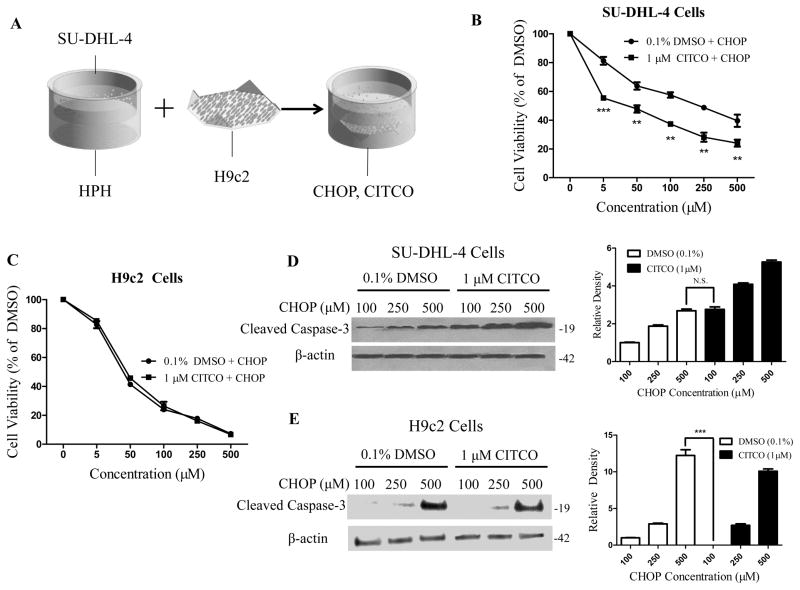
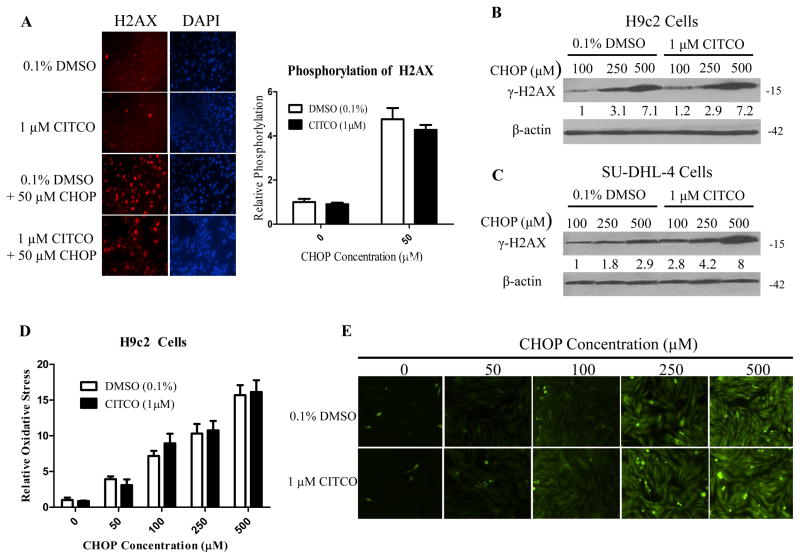
An important off-target side effect of the CHOP regimen is cardiotoxicity, which is attributed primarily to doxorubicin (Supplementary Fig. S2) (17). To examine the effects of CITCO on the cardiotoxicity associated with the CHOP treatment, we developed a novel multi-organ co-culture system, depicted in Fig. 4A, which incorporates cardiomyoblast H9c2 cells into the HPH/SU-DHL-4 co-culture, and in which the cells can be easily separated from one another and harvested for further analysis. Akin to other non-hepatic cells, expression of genes associated with CHOP disposition in H9c2 cells is generally low and non-responsive to CITCO treatment (Supplementary Fig. S3). Results from cell viability assays demonstrated that CITCO treatment selectively increased CHOP-mediated cytotoxicity in SU-DHL-4 cells, but not in H9c2 cells (Fig. 4B, 4C). Apoptosis is one of the major modes of action of the CHOP regimen (31). As such, we further sought to examine the large fragment of activated caspase 3, a well-accepted biomarker for apoptosis, in SU-DHL-4 and H9c2 cells. As shown in Fig. 4D, CITCO markedly increased CHOP-induced expression of cleaved caspase-3 protein in the targeted SU-DHL-4 cells, in that 100 μM CHOP + CITCO resulted in a similar apoptotic response as 500 μM CHOP alone. On the other hand, presence of CITCO had no measurable effects on cleaved caspase 3 expression in H9c2 cells treated with CHOP at comparable concentrations (Fig. 4E). Notably, in the off-target cells, cytotoxicity induced by 100 μM CHOP + CITCO was significantly lower than that induced by 500 μM CHOP.
Figure 4. CITCO enhances CHOP-mediated cytotoxicity in SU-DHL-4 but not in H9c2 cells.
Schematic illustration of the multi-organ co-culture model containing HPH, SU-DHL-4 and H9c2 cells (A). In the co-culture model, CHOP-induced cytotoxicity in SU-DHL-4 (B) or H9c2 (C) cells was measured as detailed in the Materials and Methods in the presence and absence of CITCO (1 μM). Western blotting assay was performed to measure cleaved caspase 3 protein in SU-DHL-4 (D) and H9c2 (E) cells harvested from the treated co-cultures. Densitometry of cleaved caspase 3 was normalized to that of β-actin. Data represent the mean ± S.D. of three independent experiments (**, p < 0.01, ***, p < 0.001).
Reduced CHOP load attenuates H2AX phosphorylation and oxidative stress in H9c2 cells
DNA double-strand breaks (DSB) caused by chemotherapeutic intervention result in rapid onset of phosphorylation of a variant of histone H2A, H2AX, and this phosphorylation has been shown to be representative of the extent of DSB in cells (32). Utilizing immunochemical staining and immunoblotting, we demonstrated that CHOP treatment markedly increased the phosphorylation of histone H2AX in H9c2 cells and that addition of CITCO did not further alter the induced H2AX expression (Fig. 5A, 5B). In treated SU-DHL-4 cells, however, CITCO effectively enhanced CHOP-induced DNA damage, measured by increasing histone H2AX expression (Fig. 5C). Oxidative stress and modulation of intracellular redox states can be important players in apoptotic signaling cascades (33). Doxorubicin has been shown to produce reactive oxygen species (ROS) in cardiac cells (34, 35). In our experiments, we aimed to examine the oxidative stress in H9c2 cells induced by the CHOP regimen. As shown in Fig. 5D and 5E, CHOP treatment resulted in a concentration-dependent increase in oxidative stress in H9c2 cells, inclusion of CITCO did not alter the oxidative stress in these cells, in that 100 μM CHOP + CITCO led to only marginal oxidative stress in comparison with 500 μM CHOP. Collectively, consistent with our earlier findings, these results support that inclusion of CITCO resulted in significantly greater DNA damage in target, but not off-target, cells.
Figure 5. CITCO didn’t enhance CHOP-induced H2AX phosphorylation and oxidative stress in H9c2 cells.
HPH/SU-DHL-4/H9c2 co-culture was treated with 0.1% DMSO, CITCO (1 μM), 0.1% DMSO/CHOP, or CITCO/CHOP at indicated concentrations for 24 h. H9c2 cells removed from the co-culture system were fixed with 4% paraformaldehyde, then immune-stained and imaged for phosphorylation at the serine-139 position of histone H2AX as detailed in the Materials and Methods. Relative H2AX phosphorylation was quantified using ImageJ Software from the National Institutes of Health and normalized to vehicle control (A). In separate experiments, western blot analysis was performed in H9c2 (B) and SU-DHL-4 (C) cells isolated from co-cultures following 24 h treatment of CHOP in the presence of absence of CITCO (1 μM). To assess oxidative stress in H9c2 cell under the same treatments, H9c2 cells were incubated with 10 μM 2’,7′-dichlorofluorescein diacetate (DFDA) at 37°C and 5% CO2 for 40 min. The oxidation of DFDA to the fluorescent dichlorofluorescein was quantified using NIH ImageJ Software (D). Representative images via fluorescence microscopy were shown in (E).
Discussion
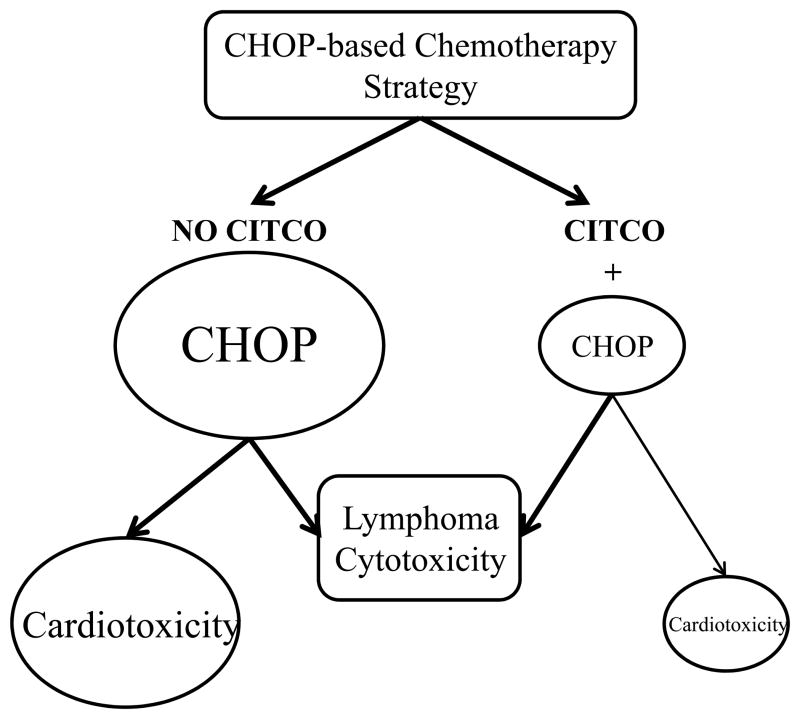
Currently, the CHOP regimen remains the standard first-line choice for the treatment of aggressive non-Hodgkin lymphoma, the most common hematological malignancy worldwide (1). Despite well-documented progress in the application of this regimen, significant numbers of patients are still not cured due to the development of drug resistance and/or intolerable toxicities that result in premature termination of the chemotherapy. As a primary component of this regimen and alkylating prodrug, CPA undergoes hepatic metabolism to its pharmacologically active form, 4-OH-CPA, via oxidation catalyzed primarily by CYP2B6. In addition, the anthracycline doxorubicin appears to be responsible for the risk of cardiotoxicity related to the CHOP regimen (17, 36). We previously showed that activation of the nuclear receptor CAR selectively induces CYP2B6 and increases the biotransformation of CPA to 4-OH-CPA (21). In the present study, we demonstrated that a selective human CAR activator, CITCO, synergistically enhanced CHOP-mediated cytotoxicity in therapeutically targeted lymphoma cells, but not in cardiomyocytes. Importantly, combination of CITCO with CHOP allows for comparable antineoplastic activity achieved at significantly lower concentrations of CHOP chemotherapy drugs, and alleviates the cardiotoxicity of the regimen (Fig. 6).
Figure 6.
Schematic illustration of CITCO-mediated enhancement of CHOP antineoplastic activity in targeted (SU-DHL-4) but not side-toxic (H9c2) cells.
The relationship between CAR and the key genes responsible for the disposition of the standard CHOP drugs remains incompletely understood. Interestingly, although targets of CHOP treatment are often extrahepatic, drug-metabolizing enzymes and transporters associated with CHOP disposition, including CYP2B6, CYP3A4 and CYP3A5, CBR1, CBR3, and MDR1, are expressed predominantly in the liver, where CAR is also highly expressed (37–39). In the CHOP regimen, CYP2B6 is primarily responsible for bioactivation of CPA to 4-OH-CPA, while CYP3A4 plays a key role in the formation of the undesirable inactive metabolite dechloroethyl-CPA, as well as the neurotoxic byproduct chloroacetaldehyde (24, 40). Additionally, CYP3A4 plays an important role in the metabolism of prednisone which, like CPA, is a prodrug requiring metabolic conversion to its active form, prednisolone (41). CYP3A5 is involved in the hepatic metabolism and clearance of vincristine (37). Metabolism of doxorubicin is mediated primarily by CBR1 and CBR3, though in the heart this reaction is catalyzed predominantly by aldo-keto reductase 1A (42). Conversely, lymphoma cells express negligible levels of CHOP-metabolizing enzymes and CAR (21). As a promiscuous xenobiotic receptor, CAR plays an important role in coordinating cellular responses to stimulation by xenobiotic chemicals by regulating the expression of numerous genes encoding drug-metabolizing enzymes and transporters in the liver, with CYP2B6 as its primary target (20, 38). The clinical importance of CYP2B6, in relation to other cytochrome P450 enzymes, was only recognized recently based on findings indicating that it plays a critical role in the biotransformation of several important drugs, such as CPA, efavirenz, ifosfamide, tamoxifen, and artemisinin (12, 43, 44). We found that CITCO, a known selective activator of human CAR, elicited a strong induction of hepatic CYP2B6 but moderate to no induction of other genes involved in CHOP disposition. Thus, combination of CITCO with CHOP enhances the bioactivation of CPA, while may only marginally influence the disposition of other drugs in the regimen. Moreover, CITCO did not alter the expression of any key CHOP-metabolizing enzymes nor drug transporters in target lymphoma cells. In line with these findings, addition of CITCO to CHOP combination therapy significantly enhanced cytotoxicity toward SU-DHL-4 cells, when co-cultured with functional HPH. Importantly, in a similar co-culture model containing HepaRG cells, a promising surrogate of HPH, the beneficial effects of CITCO on the CHOP regimen were abolished when CAR was knocked out, further confirming the pivotal role of CAR in this new therapeutic combination.
Although this initial hepatocyte/lymphoma co-culture model has demonstrated promising antineoplastic activity in SU-DHL-4 cells by inclusion of CITCO in the CHOP regimen, this rather simplified model does not include the major off-target toxic sites of this regimen. Clinically, CHOP-related late cardiotoxicity is a major concern for aggressive non-Hodgkin lymphoma patients (17). It was estimated that over 20% of patients experience cardiac events after CHOP chemotherapy (14, 17) and cardiac failure appeared to be the most important cause of death of patients in complete remission after CHOP (45). In this study, we have developed a multi-organ co-culture model incorporating HPH, SU-DHL-4, and H9c2 cells in different chambers, with shared culture medium. Building upon our previous co-culture model, this new design enabled synchronous monitoring of CHOP-mediated antineoplastic activity in SU-DHL-4 cells and cardiotoxicity in H9c2 cells in the presence of functional human hepatocytes. The H9c2 cell line, though originated from rat myocardium, has been broadly accepted as an in vitro cell model for investigating drug-induced cardiotoxicity (46). Notably, our results with this novel co-culture model revealed that co-treatment with CITCO and CHOP led to selectively enhanced cytotoxicity in SU-DHL-4, but not in H9c2, cells. Moreover, it appears that a synergistic effect between CITCO and CHOP exists, with 100 μM CHOP + CITCO achieving comparable cytotoxicity in SU-DHL-4 cells as was observed with 500 μM CHOP alone. Whereas this in vitro cell-based synergy cannot precisely predict clinical benefit, it indicates the potential that inclusion of CITCO may improve the therapeutic index of CHOP by lowering the doses of the regimen drugs required to achieve efficacy in non-Hodgkin lymphoma patients.
In accordance with these findings, the selective antineoplastic benefit of the CITCO/CHOP combination was illustrated further by examining apoptosis, DNA damage, and oxidative stress, which are associated with mechanisms of CHOP chemotherapy drug action (47). Cleavage of caspase 3 is a common mechanism of apoptosis induced by CPA, doxorubicin and vincristine (31, 48). The life-threatening cardiotoxicity related to doxorubicin is associated with its ability to intercalate into DNA, causing DNA damage and redox cycling activity, and thereby generating reactive oxidative species and oxidative stress (34, 35). In fact, doxorubicin-induced phosphorylation of histone H2AX, a marker for DNA double strand breaks, and oxidative stress in H9c2 cells have been extensively used to investigate cardiotoxicity of doxorubicin-containing regimens (15). We found that the CITCO/CHOP combination exhibited selective toxicity in SU-DHL-4 cells, manifested by increased caspase 3 cleavage, H2AX phosphorylation, and oxidative stress. Clearly, in the presence of CITCO, lower doses of CHOP chemotherapy drugs decrease its off-target cardiotoxicity in H9c2 cells.
Collectively, our results show that selective activation of CAR improves the overall cytotoxicity of CHOP chemotherapy toward its target non-Hodgkin lymphoma cells. The beneficial impact of CAR activation in CHOP-based treatment in hematopoietic malignancies derived primarily from enhanced formation of the active metabolite of CPA, the backbone of CHOP, via selective induction of CYP2B6 expression in human hepatocytes, and from the selective cytotoxicity toward lymphoma cells, in relation to cardiomyocyte cells. The newly established multi-organ co-culture model provides an excellent in vitro cellular environment allowing simultaneous investigation of hepatic metabolism, target therapeutic activity, and off-target toxicity of chemotherapeutic regimens. In the meantime, we do realize that cell-based in vitro models are associated with inherent limits and only reflect parts of the whole picture. Results from animal studies usually offer more physiologically relevant outcomes. However, it is important to point out that although hCAR exhibits several shared features with its rodent counterparts, marked differences between rodent and human CAR exist (38, 49). CITCO has been demonstrated to activate human but not mouse CAR. Importantly, phenobarbital, a known activator of CAR across multiple species, promotes liver tumor formation in mice but not humans (50). Overall, our current promising in vitro findings warrant future carefully designed in vivo and clinical studies to eventually establish the beneficial combination of CITCO or another selective human CAR activator with the CHOP regimen in the treatment of non-Hodgkin lymphoma. The results will also be applicable to other cyclophosphamide-containing combination regimens, including higher-intensity regimens that include high-dose cyclophosphamide. They may also be applicable to autologous and allogeneic hematopoietic stem cell transplant conditioning regimens.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by the National Institutes of Health R01 GM107058 and R01 DK061652 to H. Wang.
We thank Bioreclamation IVT for providing human primary hepatocytes for this study. The authors are also grateful to Dr. Douglas Ross (University of Maryland Medical Center, Baltimore, MD) for discussions and comments on the project. This work was supported by the National Institutes of Health (grants R01 GM107058 and DK061652 to H. Wang).
Footnotes
Conflict-of-interest disclosure: The authors declare no conflict of interest.
References
- 1.American Cancer Society. Cancer Facts and Figures. 2014. [Google Scholar]
- 2.American Cancer Society. Survival rates and factors that affect prognosis (outlook) for non-Hodgkin lymphoma. 2015. [Google Scholar]
- 3.Michallet AS, Coiffier B. Recent developments in the treatment of aggressive non-Hodgkin lymphoma. Blood reviews. 2009;23:11–23. doi: 10.1016/j.blre.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 4.Mounier N, Briere J, Gisselbrecht C, Emile JF, Lederlin P, Sebban C, et al. Rituximab plus CHOP (R-CHOP) overcomes bcl-2--associated resistance to chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL) Blood. 2003;101:4279–84. doi: 10.1182/blood-2002-11-3442. [DOI] [PubMed] [Google Scholar]
- 5.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–18. [PubMed] [Google Scholar]
- 6.Tomita N, Takasaki H, Miyashita K, Fujisawa S, Ogusa E, Matsuura S, et al. R-CHOP therapy alone in limited stage diffuse large B-cell lymphoma. British journal of haematology. 2013;161:383–8. doi: 10.1111/bjh.12281. [DOI] [PubMed] [Google Scholar]
- 7.Sehn LH. A decade of R-CHOP. Blood. 2010;116:2000–1. doi: 10.1182/blood-2010-07-293407. [DOI] [PubMed] [Google Scholar]
- 8.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. The New England journal of medicine. 1995;332:901–6. doi: 10.1056/NEJM199504063321401. [DOI] [PubMed] [Google Scholar]
- 9.Coiffier B. Treatment of diffuse large B-cell lymphoma. Current hematology reports. 2005;4:7–14. [PubMed] [Google Scholar]
- 10.Fenselau C, Kan MN, Rao SS, Myles A, Friedman OM, Colvin M. Identification of aldophosphamide as a metabolite of cyclophosphamide in vitro and in vivo in humans. Cancer research. 1977;37:2538–43. [PubMed] [Google Scholar]
- 11.Giraud B, Hebert G, Deroussent A, Veal GJ, Vassal G, Paci A. Oxazaphosphorines: new therapeutic strategies for an old class of drugs. Expert opinion on drug metabolism & toxicology. 2010;6:919–38. doi: 10.1517/17425255.2010.487861. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Roy P, Waxman DJ. Role of human liver microsomal CYP3A4 and CYP2B6 in catalyzing N-dechloroethylation of cyclophosphamide and ifosfamide. Biochemical pharmacology. 2000;59:961–72. doi: 10.1016/s0006-2952(99)00410-4. [DOI] [PubMed] [Google Scholar]
- 13.Code EL, Crespi CL, Penman BW, Gonzalez FJ, Chang TK, Waxman DJ. Human cytochrome P4502B6: interindividual hepatic expression, substrate specificity, and role in procarcinogen activation. Drug metabolism and disposition: the biological fate of chemicals. 1997;25:985–93. [PubMed] [Google Scholar]
- 14.Chlebowski RT. Adriamycin (doxorubicin) cardiotoxicity: a review. The Western journal of medicine. 1979;131:364–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Wang Y, Zheng D, Wei M, Xu H, Peng T. Rac1 signalling mediates doxorubicin-induced cardiotoxicity through both reactive oxygen species-dependent and -independent pathways. Cardiovascular research. 2013;97:77–87. doi: 10.1093/cvr/cvs309. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, Palmeira CM, Wallace KB. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicology letters. 2001;121:151–7. doi: 10.1016/s0378-4274(01)00329-0. [DOI] [PubMed] [Google Scholar]
- 17.Limat S, Demesmay K, Voillat L, Bernard Y, Deconinck E, Brion A, et al. Early cardiotoxicity of the CHOP regimen in aggressive non-Hodgkin's lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2003;14:277–81. doi: 10.1093/annonc/mdg070. [DOI] [PubMed] [Google Scholar]
- 18.Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. The Journal of biological chemistry. 1999;274:6043–6. doi: 10.1074/jbc.274.10.6043. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Negishi M. Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr Drug Metab. 2003;4:515–25. doi: 10.2174/1389200033489262. [DOI] [PubMed] [Google Scholar]
- 20.Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H. Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. The Journal of pharmacology and experimental therapeutics. 2006;317:1200–9. doi: 10.1124/jpet.105.098160. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Li L, Yang H, Ferguson SS, Baer MR, Gartenhaus RB, et al. The constitutive androstane receptor is a novel therapeutic target facilitating cyclophosphamide-based treatment of hematopoietic malignancies. Blood. 2013;121:329–38. doi: 10.1182/blood-2012-06-436691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, et al. Isolation and culture of primary human hepatocytes. Methods in molecular biology. 2005;290:207–29. doi: 10.1385/1-59259-838-2:207. [DOI] [PubMed] [Google Scholar]
- 23.Antherieu S, Chesne C, Li R, Camus S, Lahoz A, Picazo L, et al. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab Dispos. 2010;38:516–25. doi: 10.1124/dmd.109.030197. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Li L, Fuhrman J, Ferguson S, Wang H. The role of constitutive androstane receptor in oxazaphosphorine-mediated induction of drug-metabolizing enzymes in human hepatocytes. Pharmaceutical research. 2011;28:2034–44. doi: 10.1007/s11095-011-0429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin YS, Dowling AL, Quigley SD, Farin FM, Zhang J, Lamba J, et al. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Molecular pharmacology. 2002;62:162–72. doi: 10.1124/mol.62.1.162. [DOI] [PubMed] [Google Scholar]
- 26.Quinones-Lombrana A, Ferguson D, Hageman Blair R, Kalabus JL, Redzematovic A, Blanco JG. Interindividual variability in the cardiac expression of anthracycline reductases in donors with and without Down syndrome. Pharmaceutical research. 2014;31:1644–55. doi: 10.1007/s11095-013-1267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tada Y, Wada M, Kuroiwa K, Kinugawa N, Harada T, Nagayama J, et al. MDR1 gene overexpression and altered degree of methylation at the promoter region in bladder cancer during chemotherapeutic treatment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2000;6:4618–27. [PubMed] [Google Scholar]
- 28.Zhang J, Ma Z, Treszezamsky A, Powell SN. MDC1 interacts with Rad51 and facilitates homologous recombination. Nature structural & molecular biology. 2005;12:902–9. doi: 10.1038/nsmb991. [DOI] [PubMed] [Google Scholar]
- 29.Freedman A, Neuberg D, Mauch P, Gribben J, Soiffer R, Anderson K, et al. Cyclophosphamide, doxorubicin, vincristine, prednisone dose intensification with granulocyte colony-stimulating factor markedly depletes stem cell reserve for autologous bone marrow transplantation. Blood. 1997;90:4996–5001. [PubMed] [Google Scholar]
- 30.Marion MJ, Hantz O, Durantel D. The HepaRG cell line: biological properties and relevance as a tool for cell biology, drug metabolism, and virology studies. Methods in molecular biology. 2010;640:261–72. doi: 10.1007/978-1-60761-688-7_13. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz PS, Waxman DJ. Cyclophosphamide induces caspase 9-dependent apoptosis in 9L tumor cells. Molecular pharmacology. 2001;60:1268–79. doi: 10.1124/mol.60.6.1268. [DOI] [PubMed] [Google Scholar]
- 32.Kuo LJ, Yang LX. Gamma-H2AX - a novel biomarker for DNA double-strand breaks. In vivo. 2008;22:305–9. [PubMed] [Google Scholar]
- 33.Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology : the official journal of the International Society for Pathophysiology / ISP. 2000;7:153–63. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 34.Tsang WP, Chau SP, Kong SK, Fung KP, Kwok TT. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life sciences. 2003;73:2047–58. doi: 10.1016/s0024-3205(03)00566-6. [DOI] [PubMed] [Google Scholar]
- 35.Kim SY, Kim SJ, Kim BJ, Rah SY, Chung SM, Im MJ, et al. Doxorubicin-induced reactive oxygen species generation and intracellular Ca2+ increase are reciprocally modulated in rat cardiomyocytes. Experimental & molecular medicine. 2006;38:535–45. doi: 10.1038/emm.2006.63. [DOI] [PubMed] [Google Scholar]
- 36.Nickenig C, Dreyling M, Hoster E, Pfreundschuh M, Trumper L, Reiser M, et al. Combined cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP) improves response rates but not survival and has lower hematologic toxicity compared with combined mitoxantrone, chlorambucil, and prednisone (MCP) in follicular and mantle cell lymphomas: results of a prospective randomized trial of the German Low-Grade Lymphoma Study Group. Cancer. 2006;107:1014–22. doi: 10.1002/cncr.22093. [DOI] [PubMed] [Google Scholar]
- 37.Dennison JB, Jones DR, Renbarger JL, Hall SD. Effect of CYP3A5 expression on vincristine metabolism with human liver microsomes. The Journal of pharmacology and experimental therapeutics. 2007;321:553–63. doi: 10.1124/jpet.106.118471. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, LeCluyse EL. Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clinical pharmacokinetics. 2003;42:1331–57. doi: 10.2165/00003088-200342150-00003. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Covarrubias V, Zhang J, Kalabus JL, Relling MV, Blanco JG. Pharmacogenetics of human carbonyl reductase 1 (CBR1) in livers from black and white donors. Drug metabolism and disposition: the biological fate of chemicals. 2009;37:400–7. doi: 10.1124/dmd.108.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang TK, Weber GF, Crespi CL, Waxman DJ. Differential activation of cyclophosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer research. 1993;53:5629–37. [PubMed] [Google Scholar]
- 41.Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clinical pharmacology and therapeutics. 2012;92:414–7. doi: 10.1038/clpt.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pirolli D, Giardina B, Mordente A, Ficarra S, De Rosa MC. Understanding the binding of daunorubicin and doxorubicin to NADPH-dependent cytosolic reductases by computational methods. European journal of medicinal chemistry. 2012;56:145–54. doi: 10.1016/j.ejmech.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Ward BA, Gorski JC, Jones DR, Hall SD, Flockhart DA, Desta Z. The cytochrome P450 2B6 (CYP2B6) is the main catalyst of efavirenz primary and secondary metabolism: implication for HIV/AIDS therapy and utility of efavirenz as a substrate marker of CYP2B6 catalytic activity. The Journal of pharmacology and experimental therapeutics. 2003;306:287–300. doi: 10.1124/jpet.103.049601. [DOI] [PubMed] [Google Scholar]
- 44.Crewe HK, Notley LM, Wunsch RM, Lennard MS, Gillam EM. Metabolism of tamoxifen by recombinant human cytochrome P450 enzymes: formation of the 4-hydroxy, 4'-hydroxy and N-desmethyl metabolites and isomerization of trans-4-hydroxytamoxifen. Drug metabolism and disposition: the biological fate of chemicals. 2002;30:869–74. doi: 10.1124/dmd.30.8.869. [DOI] [PubMed] [Google Scholar]
- 45.Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:3121–7. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 46.Turakhia S, Venkatakrishnan CD, Dunsmore K, Wong H, Kuppusamy P, Zweier JL, et al. Doxorubicin-induced cardiotoxicity: direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. American journal of physiology Heart and circulatory physiology. 2007;293:H3111–21. doi: 10.1152/ajpheart.00328.2007. [DOI] [PubMed] [Google Scholar]
- 47.Mohammad RM, Al-Katib A, Aboukameel A, Doerge DR, Sarkar F, Kucuk O. Genistein sensitizes diffuse large cell lymphoma to CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. Molecular cancer therapeutics. 2003;2:1361–8. [PubMed] [Google Scholar]
- 48.Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22:2805–11. doi: 10.1038/sj.onc.1206366. [DOI] [PubMed] [Google Scholar]
- 49.Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, et al. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem. 2003;278:17277–83. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- 50.Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, et al. Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit Rev Toxicol. 2014;44:64–82. doi: 10.3109/10408444.2013.835786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.