Abstract
The circadian system regulates biological rhythmicity in the human body. The role of the circadian system in neurological disorders is a theme that is attracting an increasing amount of interest from the scientific community. This has arisen, in part, from emerging evidence that disorders such as Parkinson’s disease (PD) are multifactorial with many features exhibiting diurnal fluctuations, thereby suggestive of circadian involvement. While the importance of fluctuating motor and non-motor manifestations in PD have been well acknowledged, the role of the circadian system has received little attention until recently. It is proposed that intervening with circadian function provides a novel research avenue down which new strategies for improving symptomatic treatment and slowing of the progressive degenerative process can be approached to lessen the burden of PD. In this manuscript we review the literature describing existing circadian research in PD and its’ experimental models.
Keywords: Parkinson’s, circadian, sleep, clock genes, chronobiology
Introduction
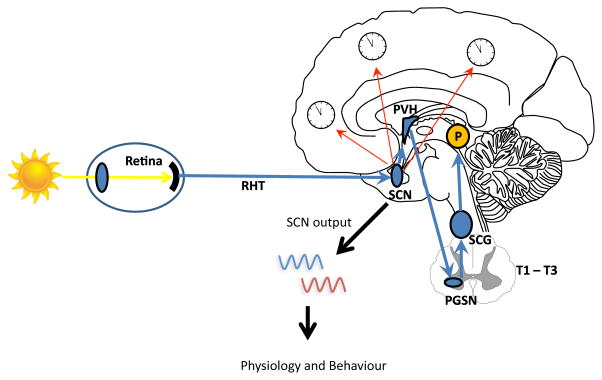
Circadian rhythms are physiological and behavioural cycles with a periodicity of approximately 24 hours, generated by the endogenous biological clock in the suprachiasmatic nucleus (SCN), located in the anterior hypothalamus.1–3 The SCN receives input from external “zeitgebers” (German for “time-giver”) and uses that information to synchronize physiology and behaviour with 24-hour rotation of Earth. Light is the most important environmental zeitgeber. 3 Other exogenous signals, such as physical activity and timing of meals, also facilitate synchronization of circadian rhythms with the environment.
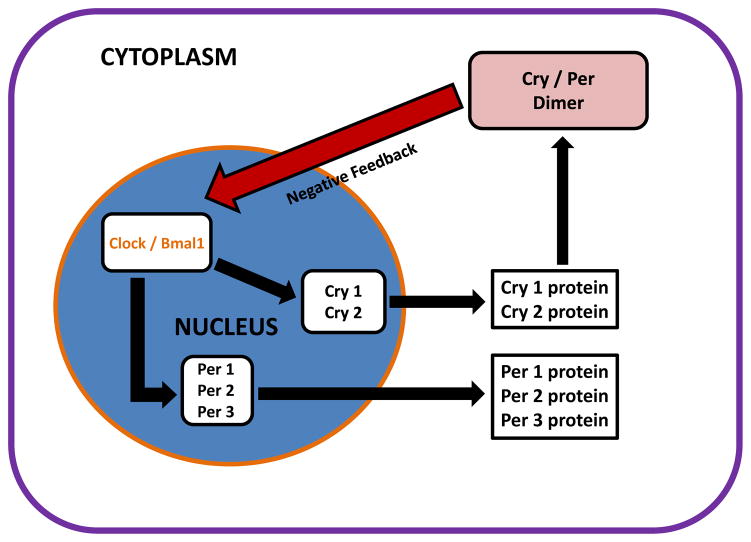
Circadian rhythms regulate many physiological and behavioural functions, and play an important role in the preservation of homeostasis.4–6 A core set of clock genes, including three Per genes (Per1, Per2, Per3), Clock, Bmal1 and two cryptochrome gene homologues (Cry1 and Cry2) interact to form transcription-translation feedback loops that provide the molecular basis of circadian rhythmicity.7
Ageing is associated with changes in the circadian system. These changes result in a reduced amplitude and period length of circadian rhythms, an increased intra-daily variability, and a decreased inter-daily stability of a rhythm.8–14 The timing of the rhythms can be disturbed with aging, leading to changes in the time relationship of rhythms to each other, known as internal desynchronization. This loss of coordination has negative consequences on rest-activity cycles and other physiological and behavioural functions.15
Disruption of circadian rhythmicity is common in some neurodegenerative disorders. While the pathophysiological mechanisms which underlie disruption of circadian rhythmicity are observed in common neurodegenerative disorders such as Alzheimer’s disease, the magnitude and impact of circadian timekeeping in Parkinson’s disease (PD) is much less understood. Emerging evidence, however, suggests that a disruption of circadian function in PD may not only have negative consequences on the primary symptom expression, but may also influence the biology of PD-associated neurodegeneration. In this manuscript we review the current understanding of circadian function in experimental models of PD and in the clinical expression of this disorder.
Methods
This study involved an analysis of published articles related to circadian system in PD. We searched the PubMed electronic database of the National Library of Medicine using the terms “circadian” and “Parkinson’s disease”. We also examined the reference lists of relevant publications dealing with this theme while including only original studies published in English. Clinical research studies and basic preclinical studies employing animal models of PD are reported separately.
Circadian Rhythms Disruption in the PD population
Diurnal rhythms of physiologic functions in Parkinson’s Disease
The intensity and frequency of many motor and non-motor manifestations of PD exhibit diurnal oscillations. Examples include disrupted rest-activity cycles, changes in blood pressure and heart rate rhythms, impaired sleep and alertness, and oscillations in mood and behaviour. It is therefore reasonable to examine to what degree these fluctuations in PD are mediated by chronobiological function.
Spontaneous circadian fluctuations of motor symptoms in PD are very common. Actigraphy studies in PD patients demonstrate lower peak activity levels and lower amplitude of the rest-activity cycle compared to healthy older adults.16–18 Increased levels of physical activity and shorter periods of immobility during the night are reported to result in an almost flat diurnal pattern of motor activity in these patients.19,20 Examination of the circadian pattern of motor symptoms in PD also reveals progressive deterioration in motor function as the day progresses: a pattern reported in patients for which medication-induced stability has been achieved and for patients with motor complications.21,22 This daily activity pattern occurs independently of the timing of dopaminergic medications, and may be more closely related to circadian regulation of dopaminergic systems. Similarly, responsiveness of PD motor symptoms to dopaminergic medications declines throughout the day, in the absence of significant changes in levodopa pharmacokinetics, suggesting circadian influences may come into play.21,23
Similarly to circadian fluctuations in motor performance, visual performance, as assessed by contrast sensitivity, also fluctuates in PD.24 Impairment of retinal dopamine is the likely culprit of such fluctuations.25 While it has been proposed that circadian changes in contrast sensitivity may occur independently of oscillations in motor symptoms, it is possible that various anatomical networks (retina, striatum, cortex) may have differential thresholds for governing the circadian signal of dopamine in different anatomical locations.26
Blood pressure (BP) and heart rate (HR) alterations are common in PD and have been attributed to dysautonomia intrinsic to PD. These parameters have a distinct diurnal rhythm, with elevation of BP and HR occurring during the light phase and decreases in these measures reported during the dark phase of the light/dark cycle.27 On this basis, HR and BP are important parameters for investigating the interface of circadian system and autonomic function in PD. Ambulatory BP monitoring is a tool suitable for the characterization of the circadian profile of HR and BP. In a case controlled study of 139 newly diagnosed PD patients and 55 age-matched controls, 24-hour ambulatory BP monitoring revealed significant differences in the rhythm of non-dipping, the percent of nocturnal BP decrease, night-time BP level, and nocturnal decrease of HR in PD patients.28 These changes, however, were not related to the disease severity or phenotype, which supports the hypothesis that these alterations may stem from intrinsic circadian dysregulation. Ejaz and colleagues reported reversal of circadian BP rhythm in PD patients, manifesting as postprandial hypotension and nocturnal hypertension using 24-hour ambulatory pressure monitoring.29 Holter electrocardiographic monitoring in these PD patients reveals a decrease of sympathetic activity during the day with a loss of the circadian HR variability and a disappearance of the sympathetic morning peak in melatonin secretion. 30 A power spectral analysis of HR variability using 24-hour ambulatory ECG reveals that the total frequency component and low frequency/high frequency ratio is also diminished.31 Furthermore, low frequency components of heart rate variability and low/high frequency ratio tend to be more reduced in patients with MSA compared to PD patients.32,33 While observed abnormalities in circadian rhythms of HR and BP in PD may arise from the peripheral autonomic ganglia involvement, the influence of the central autonomic networks, such as those traversing the hypothalamus and affected by the neurodegenerative process of PD, may be significant.34–36
In addition to the possible influence of compromised circadian function on the manifestation of motor and sleep-wake events in PD, circadian disruption has been associated with neuropsychiatric disturbances in these patients. PD patients with hallucinations demonstrate diminished day to day stability of rest-activity cycle, reduced amplitude of activity and increased night-time activity compared to non-hallucinators.16 These changes appear to be independent of the severity of motor impairment. Depressed PD patients have lower amplitudes of core body temperature and higher minimum rectal temperature relative to PD patients without depression.37 Further, studies examining diurnal hypothalamic function after stimulation of the subthalamic nucleus (STN) using deep brain stimulation (DBS) demonstrate intact diurnal variability of cortisol secretion postoperatively, and decreased mean 24-hour cortisol levels, which correlates with improved postoperative depression.38 These findings suggest that circadian markers may be a useful tool in assessment of mood and therapeutic interventions directed to these non-motor manifestations of PD.
Sleep-wake cycle is the intricately tied to circadian function. Disturbed sleep-wake cycles are common non-motor manifestation of PD, affecting up to 90% of the patient throughout the course of the disease.39–41 Sleep fragmentation and excessive daytime sleepiness are the most common impairments of the sleep-wake cycle in the PD that interfere with effective therapeutic interventions. Objective measures of sleep quality demonstrate alterations in sleep-wake cycles in early, de novo patients with PD. 42 Disrupted sleep and alertness has been associated with more than a three-fold increase in the risk of developing PD.43 The exact pathophysiology of sleep/wake disturbances in PD remains largely unknown but it might be fairly surmised that the underlying aetiology is likely to be affected by adverse effects of antiparkinsonian medications and primary neurodegeneration of central sleep regulatory areas.44–50 While detailed studies on circadian involvement in the disruption of sleep-wake cycles has not been systematically studied in PD, recent investigations link weak circadian signal with impaired sleep and alertness in these patients.51
Endogenous markers of circadian system in Parkinson’s disease
Circadian rhythms can be characterized by analysing well established circadian markers such as melatonin, cortisol and core body temperature. These markers are being increasingly investigated in PD. Initial investigations of circadian secretion of melatonin in PD found no significant differences in the amplitude of the melatonin rhythm and its phase advance relative to healthy controls.52–54 These findings are in contrast with recent investigations. Using salivary dim light melatonin onset (DLMO) in 29 PD patients and 27 healthy controls, Bolitho et al. demonstrated prolongation of the phase angle of melatonin rhythm compared to the PD un-medicated and normal controls.55 This prompted the conclusion that dopaminergic treatment increases melatonin secretion in PD. Further, observed changes in the phase angle provide the rationale to suggest uncoupling of circadian and sleep regulation by dopaminergic therapy. Similarly, two other recent studies did not show alterations in the circadian phase of melatonin secretion in PD.51,56 Both studies, however, reported decreased amplitudes of melatonin secretion. The amplitude of melatonin circadian rhythm and the 24-hour area under the curve (AUC) for circulating melatonin was significantly lower in 20 PD patients with moderate PD compared with 15 age-matched controls.51 Further, compared with PD patients without excessive daytime sleepiness, patients with excessive sleepiness had significantly lower amplitudes and 24-hour melatonin AUC. These finding raise interesting questions about the role of circadian dysregulation in the pathophysiology of excessive sleepiness frequently associated with PD. Breen and colleagues examined circadian profiles of melatonin, cortisol, and clock genes in a cohort of 30 patients with early PD and 15 matched controls.56 PD patients had elevated cortisol levels, reduced melatonin levels, and altered expression of the core clock genes, Bmal1. The significance of these recent investigations is enhanced by the rigorous circadian experimental design employed, controlling for the intervening exogenous signals such as light exposure, feeding schedules, ambient temperature, and physical activity. Furthermore, these methodological aspects may explain opposing results from early studies of melatonin rhythms in PD.53,54 These recent investigations further suggest alterations of the endogenous circadian rhythmicity in PD.
The core body temperature and secretory rhythm of cortisol are also sensitive markers of circadian function. The core-body temperature, rest-activity rhythms, and sleep were simultaneously studied in 12 PD patients and 11 healthy controls.57 The mesor and the nocturnal fall in the core-body temperature were lower in PD patients while changes in the core-body temperature correlated strongly with the severity of self-reported RBD symptoms, a reduction in percentage of REM sleep, and prolonged sleep latency. Cortisol rhythm also appears to be impaired in PD. In a recent study of 30 patients with early PD, the daily rhythm of cortisol was preserved in PD and the total amount of cortisol secreted was elevated.56 Somatotrophic, thyrotrophic and lactotrophic axes appear to be intact in early-stage PD. In eight de novo, medication-free PD patients and eight age-, sex-and body-mass index matched controls, no differences in total 24-hour secretion as well as in circadian profile of growth hormone, thyrotropin and prolactin secretion were observed.58 Adipokines are endocrine factors released by fat cells and have an important role in feeding, body weight regulation and metabolism. Since patients with PD frequently experience weight loss, a recent study by Aziz et al examined the circadian aspects of adipokines, measuring leptin, adiponectin and resistin levels in these patients.59 In a cohort of eight de novo un-medicated PD patients and eight age-, gender- and fat mass matched controls, no differences were observed in the levels of these adipokines between two study groups.
Chronophramacology and effects of “zeitgeibers” on Parkinson’s disease
Chronopharmacology examines the effects of drugs on the timing of biologic rhythms, and the relation of timing to the effects of drugs. Chronopharmacological principles may have significant implications in the treatment of PD. In a study examining circadian rhythmicity in levodopa pharmacokinetics of PD, a slower absorption rate of levodopa during the night-time was observed, while the extent of absorption and bioavailability were not affected by daytime or night-time administration.23 When patients were tested in supine position during daytime, levodopa plasma pharmacokinetics were in between daytime and nighttime values. These observations suggest that circadian rhythmicity has a profound effect on gastric emptying and absorption of levodopa, but also that body position may be an important factor for administration of levodopa.
Circadian rhythms are synchronized with the solar day by “zeitgebers” of which light is the most important and potent stimulus.60,61 This synchronization ensures that behavioural, physiologic and genetic rhythms are timed appropriately with daily changes in the environment and with each other. Exposure to light facilitates recovery of motor function in a chronic experimental models of PD.62 Few exploratory studies examined the effects of bright light in PD, and documented significant improvements in depression, bradykinesia, rigidity, and dyskinesias.63,64 Ocular light transmission may be impaired in PD given age-related deficiencies in the eyes and the specific retinal dysfunction associated with dopaminergic deficits in PD.65 Together, these observations suggest that augmenting bright light exposure may result in improved sleep quality and daytime somnolence, and that this improvement may be mediated by improved circadian functioning.
Circadian rhythms in experimental models of PD
Investigations of the circadian system in the PD population are somewhat limited due to challenges that complex circadian experimental designs pose to PD patients. Animal models of PD provide a unique opportunity to overcome these limitations and provide a platform from which the neuroanatomical, neurophysiological and behavioural aspects of PD can be examined in a circadian context.
Circadian function in 6-OHDA model of PD
6-Hydroxydopamine (6-OHDA) model has been frequently implemented for studying circadian function in PD. Bilateral striatal injection of 6-OHDA differentially affect circadian rhythms of heart rate, temperature and locomotor activity of 6-OHDA treated rats. While a significant decrease of the mesor and the phase advance of all three parameters have been observed, the amplitude of only heart rate rhythm was significantly decreased in 6-OHDA-lesioned animals. These alterations of circadian rhythmicity did not recover spontaneously after 4 weeks of observation.66 Subsequent administration of levodopa resulted in the recovery of the abolished circadian rhythms but without simultaneous improvements in the mesor of temperature and locomotion rhythms.67 Similarly, employing the unilateral 6-OHDA PD model, lesioned animals had reduced locomotor activity, while the overall diurnal distribution of motor activity remained unchanged. Some 6-OHDA lesioning effects cause more pronounced changes during the resting phase than in the activity phase.68
Of a particular interest are experiments examining the interface of dopaminergic retinal function, circadian system, and experimental PD. Willis and colleagues administered levodopa into the vitreous humour adjacent to retina of 6-OHDA-lesioned rats and reported improved motor function.69 Similarly, administration of the melatonin receptor antagonist ML-23 resulted in improved motor function early in the neurodegenerative process and during the dark phase of the light/dark cycle.70 In a separate experiment, unilateral lesions of lateral hypothalamus were combined with enucleation ipsilateral or contralateral to the hemisphere where 6-OHDA was applied. Hemi-enucleation ipsilateral to the side of 6-OHDA administration resulted in impaired vertical movement, limb retraction, ambulation and spontaneous levodopa-induced turning.71 Furthermore, hemi-enucleation contralateral to the side of 6-OHDA lesion resulted in improved motor performance. These experiments illustrate the importance of the visual system and its connection for circadian and motor function in PD.
Molecular regulation of circadian timekeeping has been explored using 6-OHDA model of PD. Unilateral depletion of dopamine by 6-OHDA injection into the medial forebrain results in blunting of daily expression of the clock protein, PERIOD2 (PER2) in the dorsal striatum of rat.72 Timed daily activation of D2 receptors restores and entrains the PER2 rhythms in the DA-depleted striatum. 6-OHDA-lesioned rats housed in constant darkness were less active and had disorganized patter of wheel-running activity.73 None of the above manipulations, however, affected the PER2 rhythm in the SCN.72,73 These experiments demonstrate a prominent role played by dopamine in regulating circadian rhythmicity both at molecular and behavioural levels.
Circadian function in MPTP model of PD
One of the earliest circadian experiments in animal models of PD employed administration of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) to dogs. While several hormonal circadian markers were tested, only the rhythms of urine volume and vasopressin were altered.74 The urine volume disappeared two weeks after MPTP treatment, and the rhythm of vasopressin remained severely blunted. Levodopa administration was associated with reappearance of both rhythms.74 This is consistent with the study that examined the effects of MPTP on adrenocorticotropin and cortisol concentrations prompting the conclusion that the hypothalamic-pituitary-adrenal axis is modulated by circadian system and can be restored by promoting normal dopamine function.75
Other studies have shown that MPTP-treated mice exhibit alterations in body temperature, locomotor activity and mRNA levels of several clock genes in a dose dependent manner. The addition of ATP to the drinking water of MPTP-mice attenuated neurodegeneration in dopaminergic neurons while preventing circadian disruption.76 This point to the potential role of ATP in regulation of the molecular clock in PD. Not all studies, however, reported alterations in locomotor activity in MPTP-treated models of PD.77
Circadian function in rotenone model of PD
Similarly to other toxin-induced PD animal models, rotenone-treated rats exhibit reduced daily locomotor activity, decreased amplitudes, lower the inter-daily stability of circadian rhythms with higher fragmentation of locomotion and body temperature rhythms.78 Significant decreases in the mean 24 h levels of tryptophan, 5-hydroxytryptophan, serotonin and melatonin have been recently reported in rotenone-treated rat model of PD.79 Similarly, levels of Per1, Cry1 and Bmal1 were decreased. Subsequent restoration of melatonin levels by systemic administration of this hormone resulted in restoration of Per1 daily rhythm and no effects on other clock components.79
Circadian function in other models of PD
Using the alpha-synuclein overexpressing (ASO) transgenic mouse model of PD, Kudo and colleagues demonstrated impaired circadian locomotion rhythm with lower night-time activity, greater fragmentation in the wheel-running activity, and impaired sleep.80 These changes progressively worsened with age. While the expression of PER2 was normal in the SCN, the daytime firing rate of the SCN was reduced in ASO transgenic mice. In their subsequent work, circadian deficits of PD were hypothesized to result from altered signalling output from the SCN, which facilitates the pathology underlying PD.81 This hypothesis provides the rationale for treating circadian misalignment and sleep problems early in the disease to prevent the disease progression.80 Significance of downstream processes of clock genes has also been emphasized in the MPTP non-human primate model of PD.82
A transgenic MITOPARK mouse model of PD exhibits a slow and progressive degeneration of dopaminergic midbrain neurons. In this model of PD, a gradual age-dependent decline in the amplitude and the stability of rest-activity rhythms starts around 20 weeks of age.83 Exposure to constant darkness or light challenges the circadian system and results in severe disturbance or complete loss of locomotion rhythms in MITOPARK mice, while the re-exposure to light-dark conditions restores daily locomotion rhythms. These results indicated the impact of dopaminergic midbrain degeneration in circadian dysregulation of rest-activity cycles in PD.
Conclusions and Future Directions
Basic and clinical investigations of circadian system point to significant alterations of circadian rhythmicity in PD. Circadian disruption associated with PD manifests itself on physiological and behavioural levels. Disruption of clock genes that influence function of almost 10% of active genome may also be significantly altered in PD. Collectively, these changes of circadian homeostasis likely adversely affect sleep-wake cycles, autonomic and sensory functions, and motor performance in PD.
A major question which arises is whether circadian disruption represents a primary process with the downstream consequences on PD, or whether the biology of PD related neurodegeneration at some point starts to negatively affects circadian homeostasis. Systematic study of the circadian system as a whole will enable us to pinpoint to the site(s) of dysregulation within the circadian system in PD. This research will need to examine the interactions between the circadian system and other neuroanatomical networks of relevance for PD. These interactions are critical to understand as the relationship between the circadian system and other systems involved in PD may be of a bidirectional nature. Alterations within other systems involved in the pathophysiology of PD, such as autonomic or motor dysfunction may affect markers of circadian timekeeping; for example, autonomic dysfunction may cause changes in melatonin secretion, and motor disability of PD may affect metrics of rest-activity cycles that are frequently assessed in circadian studies. Future investigations that employ longitudinal assessments of circadian function throughout the course of the disease, along with simultaneous assessments of PD genotype and phenotype, will be required to understand these complex interactions. This will necessitate a collaborative effort of basic scientist and clinical researchers.
A very important direction and challenge for circadian research in PD and other neurodegenerative disorders will be to implement rigorous experimental protocols that allow to precisely and comprehensively define endogenous circadian signals while controlling for the effects of exogenous circadian modulators. This will be especially important in clinical investigations, as only few circadian studies up to date employed controlled experimental conditions with rigor in the PD population.51,55,56 A dedicated attention to these methodological aspects in this area of PD research will allow us to determine if changes in circadian markers such as melatonin, rest-activity cycles, and temperature are driven by primary circadian dysregulation or are influenced significantly by behavioural and environmental factors.
Improved understanding of circadian biology of PD may lead to development of novel, circadian-based interventions for motor and non-motor manifestations of PD. Further exploration of bidirectional relationship between circadian disruption and neurodegenerative process of PD therefore positions circadian system as a novel diagnostic and therapeutic target in PD.
Figure 1.
Figure 2.
Table 1.
Clinical studies that examined diurnal and/or circadian rhythms of motor and non-motor functions in PD
| Study Author / Date | Study population | Study methods | Main findings of the study |
|---|---|---|---|
| Fluctuations of motor symptoms | |||
| Van Hilten et al. / 1991 17 | 9 PD patients 10 controls |
Actigraphy (6 days) |
|
| Van Hilten et al. / 1993 18 | 15 PD patients with and without dyskinesias 10 ontrols |
Actigraphy (5 days) |
|
| Van Hilten et al. / 1993 20 | 68 PD patients 68 controls |
Actigraphy |
|
| Van Hilten et al. / 1994 19 | 89 PD patients 83 controls |
Actigraphy (6 nights) |
|
| Bonuccelli et al. / 2000 21 | 52 PD patients: 19 de novo 20 stable 13 wearing-off |
Mean motor scores and pharmacokinetic data, evaluated for a period of 3 hours after each levodopa dose |
|
| Whitehead et al. / 2008 16 | 50 PD patients with and without hallucinations Controls |
Actigraphy (5 days) |
|
| Nyholm et al. / 2010 23 | 8 PD patients | 4-hour levodopa profiles tested at daytime and before bedtime |
|
| Fluctuations of cardiovascular functions | |||
| Devos et al. / 2003 30 | 30 PD patients with variable degree of PD severity Controls |
Continuous hear rate recording |
|
| Ejaz et al. / 2006 29 | 13 PD patients | 24-hour ambulatory blood pressure monitoring |
|
| Niwa et al. / 2011 31 | 37 PD patients 30 controls |
Actigraphy (7 days) 24-hour ambulatory ECG recording |
|
| Oh et al. / 2014 28 | 225 de novo PD patients (36 with co- existent RLS) | 24-hour ambulatory blood pressure monitoring |
|
| Fluctuations of sleep-wake cycles | |||
| Lees et al. / 1988 40 | 220 PD patients | Nation-wide survey in UK |
|
| Tandberg et al. / 1998 41 | 245 PD patients 100 patients with diabetes mellitus 100 healthy controls |
Community-based survey |
|
| Rye et al. / 2000 49 | 27 PD patients | Multiple Sleep Latency Test |
|
| Fabbrini et al. / 2002 45 | 25 de novo PD patients 50 PD patients on dopaminergic therapy 25 controls |
Epworth Sleepiness Scale Pittsburg Sleep Quality Index |
|
| Abbott et al. / 2005 43 | 3078 participants in the Honolulu-Asia Aging Study | Sleep questionnaire |
|
| Fluctuations of sensory functions | |||
| Struck et al. / 2010 24 | 23 PD patients Controls |
Contrast sensitivity (CS) assessments at 2- hour intervals |
|
| Fluctuations of body temperature | |||
| Suzuki et al. / 2007 37 | 30 PD patients with and without depression | 48-hour assessment of rectal temperature |
|
Acknowledgments
Funding Sources for Study:
Dr. Videnovic – NIH/NINDS - K23 NS072283
Dr. Willis - The Bronowski Institute of Behavioural Neuroscience
Footnotes
Financial Disclosure/Conflicts of Interest: none
Documentation of Author Roles:
- Research project: A. Conception, B. Organization, C. Execution.
- Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
Dr. Willis:
- Research project: A. Conception, B. Organization, C. Execution.
- Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
- Employment: Massachusetts General Hospital (AV); The Bronowski Institute of Behavioural Neuroscience (GLW)
- Honoraria: Acorda Therapeutics and Wilsons Therapeutics for serving on a Data Safety Monitoring Beoards (AV)
- Grants: NIH/NINDS - K23 NS072283
References
- 1.Dijk DJ, Lockley SW. Integration of human sleep-wake regulation and circadian rhythmicity. J Appl Physiol. 2002;92:852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 2.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- 3.Weaver DR. The suprachiasmatic nucleus: a 25-year retrospective. J Biol Rhythms. 1998;13:100–112. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 4.Hastings M, O’Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- 5.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 6.Wijnen H, Young MW. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet. 2006;40:409–448. doi: 10.1146/annurev.genet.40.110405.090603. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 9.Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]
- 10.Hofman MA. The human circadian clock and aging. Chronobiol Int. 2000;17:245–259. doi: 10.1081/cbi-100101047. [DOI] [PubMed] [Google Scholar]
- 11.Touitou Y, Haus E. Alterations with aging of the endocrine and neuroendocrine circadian system in humans. Chronobiol Int. 2000;17:369–390. doi: 10.1081/cbi-100101052. [DOI] [PubMed] [Google Scholar]
- 12.Turek FW, Penev P, Zhang Y, van Reeth O, Zee P. Effects of age on the circadian system. Neurosci Biobehav Rev. 1995;19:53–58. doi: 10.1016/0149-7634(94)00030-5. [DOI] [PubMed] [Google Scholar]
- 13.van Coevorden A, Mockel J, Laurent E, et al. Neuroendocrine rhythms and sleep in aging men. Am J Physiol. 1991;260:E651–661. doi: 10.1152/ajpendo.1991.260.4.E651. [DOI] [PubMed] [Google Scholar]
- 14.Drug therapy for Parkinson’s disease. Med Lett Drugs Ther. 1975;17:33–34. [PubMed] [Google Scholar]
- 15.Harper DG, Volicer L, Stopa EG, McKee AC, Nitta M, Satlin A. Disturbance of endogenous circadian rhythm in aging and Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:359–368. doi: 10.1176/appi.ajgp.13.5.359. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead DL, Davies AD, Playfer JR, Turnbull CJ. Circadian rest-activity rhythm is altered in Parkinson’s disease patients with hallucinations. Mov Disord. 2008;23:1137–1145. doi: 10.1002/mds.22057. [DOI] [PubMed] [Google Scholar]
- 17.van Hilten JJ, Middelkoop HA, Kerkhof GA, Roos RA. A new approach in the assessment of motor activity in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1991;54:976–979. doi: 10.1136/jnnp.54.11.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Hilten JJ, Kabel JF, Middelkoop HA, Kramer CG, Kerkhof GA, Roos RA. Assessment of response fluctuations in Parkinson’s disease by ambulatory wrist activity monitoring. Acta Neurol Scand. 1993;87:171–177. doi: 10.1111/j.1600-0404.1993.tb04096.x. [DOI] [PubMed] [Google Scholar]
- 19.van Hilten B, Hoff JI, Middelkoop HA, et al. Sleep disruption in Parkinson’s disease. Assessment by continuous activity monitoring. Arch Neurol. 1994;51:922–928. doi: 10.1001/archneur.1994.00540210094018. [DOI] [PubMed] [Google Scholar]
- 20.van Hilten JJ, Hoogland G, van der Velde EA, Middelkoop HA, Kerkhof GA, Roos RA. Diurnal effects of motor activity and fatigue in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1993;56:874–877. doi: 10.1136/jnnp.56.8.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonuccelli U, Del Dotto P, Lucetti C, et al. Diurnal motor variations to repeated doses of levodopa in Parkinson’s disease. Clin Neuropharmacol. 2000;23:28–33. doi: 10.1097/00002826-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Piccini P, Del Dotto P, Pardini C, D’Antonio P, Rossi G, Bonuccelli U. Diurnal worsening in Parkinson patients treated with levodopa. Riv Neurol. 1991;61:219–224. [PubMed] [Google Scholar]
- 23.Nyholm D, Lennernas H, Johansson A, Estrada M, Aquilonius SM. Circadian rhythmicity in levodopa pharmacokinetics in patients with Parkinson disease. Clin Neuropharmacol. 2010;33:181–185. doi: 10.1097/WNF.0b013e3181e70f7a. [DOI] [PubMed] [Google Scholar]
- 24.Struck LK, Rodnitzky RL, Dobson JK. Circadian fluctuations of contrast sensitivity in Parkinson’s disease. Neurology. 1990;40:467–470. doi: 10.1212/wnl.40.3_part_1.467. [DOI] [PubMed] [Google Scholar]
- 25.Wirz-Justice A, Da Prada M, Reme C. Circadian rhythm in rat retinal dopamine. Neurosci Lett. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- 26.Dearry A, Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas: I. Induction of cone contraction is mediated by D2 receptors. J Neurochem. 1986;46:1006–1021. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Yang G. Recent advances in circadian rhythms in cardiovascular system. Front Pharmacol. 2015;6:71. doi: 10.3389/fphar.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh YS, Kim JS, Park IS, et al. Association between nocturnal/supine hypertension and restless legs syndrome in patients with Parkinson’s disease. J Neurol Sci. 2014;344:186–189. doi: 10.1016/j.jns.2014.06.056. [DOI] [PubMed] [Google Scholar]
- 29.Ejaz AA, Sekhon IS, Munjal S. Characteristic findings on 24-h ambulatory blood pressure monitoring in a series of patients with Parkinson’s disease. Eur J Intern Med. 2006;17:417–420. doi: 10.1016/j.ejim.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Devos D, Kroumova M, Bordet R, et al. Heart rate variability and Parkinson’s disease severity. J Neural Transm. 2003;110:997–1011. doi: 10.1007/s00702-003-0016-8. [DOI] [PubMed] [Google Scholar]
- 31.Niwa F, Kuriyama N, Nakagawa M, Imanishi J. Circadian rhythm of rest activity and autonomic nervous system activity at different stages in Parkinson’s disease. Auton Neurosci. 2011;165:195–200. doi: 10.1016/j.autneu.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Harada T, Ishizaki F, Nitta Y, et al. Circadian rhythm of cardiovascular autonomic nervous function in Parkinson’s disease, early-onset Parkinsonism and multiple system atrophy. International Medical Journal. 2006;13:131–133. [Google Scholar]
- 33.Harada T, Ishizaki F, Tachiki N, et al. Circadian rhythm of cradiovascular autonomic function in Parkinson’s disease and multiple system atrophy. International Medican Journal. 2005;12:37–39. [Google Scholar]
- 34.Mochizuki A, Komatsuzaki Y, Shoji S. Association of Lewy bodies and glial cytoplasmic inclusions in the brain of Parkinson’s disease. Acta Neuropathol. 2002;104:534–537. doi: 10.1007/s00401-002-0582-0. [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi K, Takahashi H. Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol. 1997;38(Suppl 2):2–7. doi: 10.1159/000113469. [DOI] [PubMed] [Google Scholar]
- 36.Langston J. The hypothalamus in Parkinson’s disease. Ann Neurol. 1978;3:129–133. doi: 10.1002/ana.410030207. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki K, Miyamoto T, Miyamoto M, Kaji Y, Takekawa H, Hirata K. Circadian variation of core body temperature in Parkinson disease patients with depression: a potential biological marker for depression in Parkinson disease. Neuropsychobiology. 2007;56:172–179. doi: 10.1159/000119735. [DOI] [PubMed] [Google Scholar]
- 38.Seifried C, Boehncke S, Heinzmann J, et al. Diurnal variation of hypothalamic function and chronic subthalamic nucleus stimulation in Parkinson’s disease. Neuroendocrinology. 2013;97:283–290. doi: 10.1159/000343808. [DOI] [PubMed] [Google Scholar]
- 39.Factor SA, McAlarney T, Sanchez-Ramos JR, Weiner WJ. Sleep disorders and sleep effect in Parkinson’s disease. Mov Disord. 1990;5:280–285. doi: 10.1002/mds.870050404. [DOI] [PubMed] [Google Scholar]
- 40.Lees AJ, Blackburn NA, Campbell VL. The nighttime problems of Parkinson’s disease. Clin Neuropharmacol. 1988;11:512–519. doi: 10.1097/00002826-198812000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Tandberg E, Larsen JP, Karlsen K. A community-based study of sleep disorders in patients with Parkinson’s disease. Mov Disord. 1998;13:895–899. doi: 10.1002/mds.870130606. [DOI] [PubMed] [Google Scholar]
- 42.Placidi F, Izzi F, Romigi A, et al. Sleep-wake cycle and effects of cabergoline monotherapy in de novo Parkinson’s disease patients. An ambulatory polysomnographic study. J Neurol. 2008;255:1032–1037. doi: 10.1007/s00415-008-0836-4. [DOI] [PubMed] [Google Scholar]
- 43.Abbott RD, Ross GW, White LR, et al. Excessive daytime sleepiness and subsequent development of Parkinson disease. Neurology. 2005;65:1442–1446. doi: 10.1212/01.wnl.0000183056.89590.0d. [DOI] [PubMed] [Google Scholar]
- 44.Fabbrini G, Barbanti P, Aurilia C, Pauletti C, Vanacore N, Meco G. Excessive daytime somnolence in Parkinson’s disease. Follow-up after 1 year of treatment. Neurol Sci. 2003;24:178–179. doi: 10.1007/s10072-003-0118-y. [DOI] [PubMed] [Google Scholar]
- 45.Fabbrini G, Barbanti P, Aurilia C, Vanacore N, Pauletti C, Meco G. Excessive daytime sleepiness in de novo and treated Parkinson’s disease. Mov Disord. 2002;17:1026–1030. doi: 10.1002/mds.10193. [DOI] [PubMed] [Google Scholar]
- 46.Fronczek R, Overeem S, Lee SY, et al. Hypocretin (orexin) loss in Parkinson’s disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 47.Linazasoro G, Marti Masso JF, Suarez JA. Nocturnal akathisia in Parkinson’s disease: treatment with clozapine. Mov Disord. 1993;8:171–174. doi: 10.1002/mds.870080209. [DOI] [PubMed] [Google Scholar]
- 48.Rye DB. Sleepiness and Unintended Sleep in Parkinson’s Disease. Curr Treat Options Neurol. 2003;5:231–239. doi: 10.1007/s11940-003-0014-z. [DOI] [PubMed] [Google Scholar]
- 49.Rye DB, Bliwise DL, Dihenia B, Gurecki P. FAST TRACK: daytime sleepiness in Parkinson’s disease. J Sleep Res. 2000;9:63–69. doi: 10.1046/j.1365-2869.2000.00201.x. [DOI] [PubMed] [Google Scholar]
- 50.Stack EL, Ashburn AM. Impaired bed mobility and disordered sleep in Parkinson’s disease. Mov Disord. 2006;21:1340–1342. doi: 10.1002/mds.20944. [DOI] [PubMed] [Google Scholar]
- 51.Videnovic A, Noble C, Reid KJ, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014;71:463–469. doi: 10.1001/jamaneurol.2013.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bordet R, Devos D, Brique S, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol. 2003;26:65–72. doi: 10.1097/00002826-200303000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in Parkinson’s disease. J Neural Transm Park Dis Dement Sect. 1991;3:41–47. doi: 10.1007/BF02251135. [DOI] [PubMed] [Google Scholar]
- 54.Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase-shifting properties of l-dopa. J Neural Transm Park Dis Dement Sect. 1993;5:227–234. doi: 10.1007/BF02257677. [DOI] [PubMed] [Google Scholar]
- 55.Bolitho SJ, Naismith SL, Rajaratnam SM, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med. 2014;15:342–347. doi: 10.1016/j.sleep.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Breen DP, Vuono R, Nawarathna U, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014;71:589–595. doi: 10.1001/jamaneurol.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong G, Bolitho S, Grunstein R, Naismith SL, Lewis SJ. The relationship between thermoregulation and REM sleep behaviour disorder in Parkinson’s disease. PLoS One. 2013;8:e72661. doi: 10.1371/journal.pone.0072661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aziz NA, Pijl H, Frolich M, Roelfsema F, Roos RA. Diurnal secretion profiles of growth hormone, thyrotrophin and prolactin in Parkinson’s disease. J Neuroendocrinol. 2011;23:519–524. doi: 10.1111/j.1365-2826.2011.02134.x. [DOI] [PubMed] [Google Scholar]
- 59.Aziz NA, Pijl H, Frolich M, Roelfsema F, Roos RA. Leptin, adiponectin, and resistin secretion and diurnal rhythmicity are unaltered in Parkinson’s disease. Mov Disord. 2011;26(4):760–761. doi: 10.1002/mds.23463. [DOI] [PubMed] [Google Scholar]
- 60.Czeisler CA, Allan JS, Strogatz SH, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 61.Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF, 3rd, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;274:R991–996. doi: 10.1152/ajpregu.1998.274.4.r991. [DOI] [PubMed] [Google Scholar]
- 62.Harrell LE, Balagura S. The effects of dark and light on the functional recovery following lateral hypothalamic lesions. Life Sci. 1974;15:2079–2087. doi: 10.1016/0024-3205(74)90024-1. [DOI] [PubMed] [Google Scholar]
- 63.Willis GL, Turner EJ. Primary and secondary features of Parkinson’s disease improve with strategic exposure to bright light: a case series study. Chronobiol Int. 2007;24:521–537. doi: 10.1080/07420520701420717. [DOI] [PubMed] [Google Scholar]
- 64.Paus S, Schmitz-Hubsch T, Wullner U, Vogel A, Klockgether T, Abele M. Bright light therapy in Parkinson’s disease: a pilot study. Mov Disord. 2007;22:1495–1498. doi: 10.1002/mds.21542. [DOI] [PubMed] [Google Scholar]
- 65.Archibald NK, Clarke MP, Mosimann UP, Burn DJ. The retina in Parkinson’s disease. Brain. 2009;132:1128–1145. doi: 10.1093/brain/awp068. [DOI] [PubMed] [Google Scholar]
- 66.Ben V, Bruguerolle B. Effects of bilateral striatal 6-OHDA lesions on circadian rhythms in the rat: a radiotelemetric study. Life Sci. 2000;67:1549–1558. doi: 10.1016/s0024-3205(00)00751-7. [DOI] [PubMed] [Google Scholar]
- 67.Boulamery A, Simon N, Vidal J, Bruguerolle B. Effects of L-Dopa on circadian rhythms of 6-OHDA striatal lesioned rats: a radiotelemetric study. Chronobiol Int. 2010;27:251–264. doi: 10.3109/07420521003664213. [DOI] [PubMed] [Google Scholar]
- 68.Baier PC, Branisa P, Koch R, Schindehutte J, Paulus W, Trenkwalder C. Circadian distribution of motor-activity in unilaterally 6-hydroxy-dopamine lesioned rats. Exp Brain Res. 2006;169:283–288. doi: 10.1007/s00221-005-0343-0. [DOI] [PubMed] [Google Scholar]
- 69.Willis GL. Intraocular microinjections repair experimental Parkinson’s disease. Brain Res. 2008;1217:119–131. doi: 10.1016/j.brainres.2008.03.083. [DOI] [PubMed] [Google Scholar]
- 70.Willis GL, Robertson AD. Recovery of experimental Parkinson’s disease with the melatonin analogues ML-23 and S-20928 in a chronic, bilateral 6-OHDA model: a new mechanism involving antagonism of the melatonin receptor. Pharmacol Biochem Behav. 2004;79:413–429. doi: 10.1016/j.pbb.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Willis GL, Kelly AM, Kennedy GA. Compromised circadian function in Parkinson’s disease: enucleation augments disease severity in the unilateral model. Behav Brain Res. 2008;193:37–47. doi: 10.1016/j.bbr.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 72.Hood S, Cassidy P, Cossette MP, et al. Endogenous dopamine regulates the rhythm of expression of the clock protein PER2 in the rat dorsal striatum via daily activation of D2 dopamine receptors. J Neurosci. 2010;30:14046–14058. doi: 10.1523/JNEUROSCI.2128-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gravotta L, Gavrila AM, Hood S, Amir S. Global depletion of dopamine using intracerebroventricular 6-hydroxydopamine injection disrupts normal circadian wheel-running patterns and PERIOD2 expression in the rat forebrain. J Mol Neurosci. 2011;45:162–171. doi: 10.1007/s12031-011-9520-8. [DOI] [PubMed] [Google Scholar]
- 74.Hineno T, Mizobuchi M, Hiratani K, Inami Y, Kakimoto Y. Disappearance of circadian rhythms in Parkinson’s disease model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in dogs. Brain Res. 1992;580:92–99. doi: 10.1016/0006-8993(92)90930-8. [DOI] [PubMed] [Google Scholar]
- 75.Mizobuchi M, Hineno T, Kakimoto Y, Hiratani K. Increase of plasma adrenocorticotrophin and cortisol in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated dogs. Brain Res. 1993;612:319–321. doi: 10.1016/0006-8993(93)91678-l. [DOI] [PubMed] [Google Scholar]
- 76.Hayashi A, Matsunaga N, Okazaki H, et al. A disruption mechanism of the molecular clock in a MPTP mouse model of Parkinson’s disease. Neuromolecular Med. 2013;15:238–251. doi: 10.1007/s12017-012-8214-x. [DOI] [PubMed] [Google Scholar]
- 77.Fifel K, Dkhissi-Benyahya O, Cooper HM. Lack of long-term changes in circadian, locomotor, and cognitive functions in acute and chronic MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse models of Parkinson’s disease. Chronobiol Int. 2013;30:741–755. doi: 10.3109/07420528.2012.762011. [DOI] [PubMed] [Google Scholar]
- 78.Lax P, Esquiva G, Esteve-Rudd J, Otalora BB, Madrid JA, Cuenca N. Circadian dysfunction in a rotenone-induced parkinsonian rodent model. Chronobiol Int. 2012;29:147–156. doi: 10.3109/07420528.2011.649870. [DOI] [PubMed] [Google Scholar]
- 79.Mattam U, Jagota A. Daily rhythms of serotonin metabolism and the expression of clock genes in suprachiasmatic nucleus of rotenone-induced Parkinson’s disease male Wistar rat model and effect of melatonin administration. Biogerontology. 2015;16:109–123. doi: 10.1007/s10522-014-9541-0. [DOI] [PubMed] [Google Scholar]
- 80.Kudo T, Loh DH, Truong D, Wu Y, Colwell CS. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp Neurol. 2011;232:66–75. doi: 10.1016/j.expneurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Willison LD, Kudo T, Loh DH, Kuljis D, Colwell CS. Circadian dysfunction may be a key component of the non-motor symptoms of Parkinson’s disease: insights from a transgenic mouse model. Exp Neurol. 2013;243:57–66. doi: 10.1016/j.expneurol.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fifel K, Vezoli J, Dzahini K, et al. Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human primates. PLoS One. 2014;9:e86240. doi: 10.1371/journal.pone.0086240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fifel K, Cooper HM. Loss of dopamine disrupts circadian rhythms in a mouse model of Parkinson’s disease. Neurobiol Dis. 2014;71:359–369. doi: 10.1016/j.nbd.2014.08.024. [DOI] [PubMed] [Google Scholar]