Abstract
Both HIV disease and advanced age have been associated with alterations to cerebral white matter, as measured with white matter hyperintensities (WMH) on fluid attenuated inversion recovery (FLAIR) magnetic resonance imaging (MRI), and more recently with diffusion tensor imaging (DTI). This study investigates the combined effects of age and HIV serostatus on WMH and DTI measures, as well as the relationships between these white matter measures, in 88 HIV seropositive (HIV+) and 49 seronegative (HIV-) individuals aged 23–79 years. A whole-brain volumetric measure of WMH was quantified from FLAIR images using a semi-automated process, while fractional anisotropy (FA) was calculated for 15 regions of a whole-brain white matter skeleton generated using tract-based spatial statistics (TBSS). An age by HIV interaction was found indicating a significant association between WMH and older age in HIV+ participants only. Similarly, significant age by HIV interactions were found indicating stronger associations between older age and decreased FA in the posterior limbs of the internal capsules, cerebral peduncles, and anterior corona radiata in HIV+ vs. HIV- participants. The interactive effects of HIV and age were stronger with respect to whole-brain WMH than for any of the FA measures. Among HIV+ participants, greater WMH and lower anterior corona radiata FA were associated with active hepatitis C virus infection, a history of AIDS, and higher current CD4 cell count. Results indicate that age exacerbates HIV associated abnormalities of whole-brain WMH and fronto-subcortical white matter integrity.
Keywords: HIV, aging, white matter, white matter hyperintensities, diffusion tensor imaging, fractional anisotropy
Introduction
The advent of combination antiretroviral therapies (cART) dramatically reduced morbidity and mortality in people with the Human Immunodeficiency Virus-1 (HIV) (Detels et al. 1998). Since that time, the life expectancy of HIV infected people has been approaching that of the general population (Manfredi et al. 2002; van Sighem et al. 2010), and the number of older adults living with chronic HIV infection has significantly increased (Mack & Ory 2003; Effros et al. 2008). Despite improvements in cognitive function following cART initiation (Cohen et al. 2001), neurocognitive dysfunction remains common, even among cART-treated individuals (Simioni et al. 2010), and risk for cognitive symptoms increases significantly with advanced age (Becker et al. 2004). This suggests that neurological damage continues to occur in spite of successful antiretroviral medication, and, in fact, it has been shown that HIV can persist in latent form in the brain despite suppression of viral replication (Finzi et al. 1997). Given that people are living longer with HIV, it is important to study the effects of HIV on the brain in the context of aging.
Cerebral white matter damage can lead to cognitive and functional decline (Bendlin et al. 2010; Grueter & Schulz 2012) and is common in both HIV and older age, especially in frontal and subcortical regions (Good et al. 2001; Pomara et al. 2001; Resnick et al. 2003; Brickman et al. 2006; Xuan et al. 2013). White matter damage can be measured by examining white matter hyperintensities (WMH) on fluid attenuated inversion recovery (FLAIR) images from magnetic resonance imaging (MRI). More recent studies have employed diffusion tensor imaging (DTI) to assess white matter integrity, which can be sensitive to microstructural changes in the absence of fully developed lesions (Filippi et al. 2001). Fractional anisotropy (FA) is a commonly reported scalar metric derived from the diffusion tensor that provides a measure of the directionality of water diffusion. Research has shown that while FA and WMH are related, they likely reflect distinct structural manifestations of white matter (Vernooij et al. 2009; Meier et al. 2012; Maillard et al. 2013; Aine et al. 2014), and thus may both be valuable for assessing the extent of white matter disturbances associated with HIV.
While studies show that people with HIV experience premature cognitive decline and white matter damage, there is lack of consensus in the literature as to whether the effects of age and HIV are additive, contributing independently to cognitive and neurological decline, or synergistic, having a greater than additive effect, potentially due to exacerbation of common mechanisms of neurological damage (Cohen et al. 2015). Some studies report interactive effects of age and HIV on neurological and cognitive functioning (Sacktor et al. 2010; Chang et al. 2013; Seider et al. 2014), such that older people with HIV (e.g. over age 55) experience greater deficits compared to younger HIV infected people and younger and older seronegative controls. Others suggest the effects are additive (Ances et al. 2012; Becker et al. 2012; Thomas et al. 2013; Nir et al. 2014; Pfefferbaum et al. 2014), such that people with HIV have greater levels of neurological dysfunction, but decline with age at the same rate as people without HIV.
To address these issues, the current study investigated whether aging, in the context of HIV infection, was associated with greater white matter damage as measured by WMH and FA. The relationship between the two white matter measures was also examined. The use of a quantified measure of whole-brain WMH as well as FA measures of white matter integrity in key regions of interest (ROIs) provides a more thorough evaluation of the association between age, HIV, and cerebral white matter than prior research, which may use qualitative WMH measurements or only one measure of white matter damage. Our investigation of FA was focused on ROIs that are particularly relevant to HIV, including frontal white matter, pathways to and from the basal ganglia, and the corpus callosum. We also examined other large white matter ROIs, though they were not expected to show strong HIV associated effects. We predict that overall WMH and ROIs vulnerable to HIV will show the greatest evidence of interactive effects of age and HIV.
Methods
Participants
Participants were recruited from the outpatient Immunology Center of the Miriam Hospital and the Brown University Center for AIDS Research as part of an NIH-sponsored study of HIV associated brain dysfunction. HIV seronegative (HIV-) controls were either recruited because they were family or friends of the seropositive (HIV+) participants, or they responded to fliers posted in the community. The study was approved by the IRB and informed consent was obtained from all participants. Prospective participants were excluded if they had any of the following: history of head injury (loss of consciousness > 10 min); neurologic condition such as dementia, seizure disorder, stroke, or opportunistic brain infection; major psychiatric illness that might affect brain function such as schizophrenia, untreated bipolar disorder, or any other psychotic or thought disorder; or recent history of substance use as defined by substance dependence in the past six months or a positive urine toxicology screen for cocaine, opiates, or illicit stimulants or sedatives. HIV infection was documented by enzyme-linked immunosorbent assay (ELISA) and confirmed by Western blot. Participants were evaluated for active hepatitis C virus (HCV) infection, which was defined as detectable serum HCV by polymerase chain reaction.
One hundred and seventy-three participants received MRIs. Of these, 36 participants were excluded due to diffusion MRI data quality, and 12 excluded due to FLAIR data quality involving the presence of significant motion or other imaging artifacts. Thus, 137 participants (88 HIV+ and 49 HIV-) were included in analyses of DTI data and 161 participants (101 HIV+, 60 HIV-) were used for WMH analyses. Thirty-two participants were uniquely included in WMH analyses (had good FLAIR quality but poor DTI quality) while 8 participants were uniquely included in FA analyses (had good DTI quality but poor FLAIR quality); therefore, 129 participants were included in all analyses conducted, and any analyses involving both WMH and FA data pertain only to these 129 participants. There were no significant differences between the 161 participants used for WMH analyses and the 137 used for FA analyses in terms of clinical or demographic characteristics.
Demographic characteristics of the 137 participants with good DTI quality are presented in Table 1. These 137 were chosen because there were several FA ROIs and only one WMH outcome variable, thus the majority of analyses conducted pertain to FA data and these 137 participants. Participant ages ranged from 23 – 79. The HIV- group had more years of education and a smaller proportion with active HCV infection. Among the HIV+ participants, the average time since diagnosis was 12.5 years, 83% were on cART, 33% had active HCV infection, 58% had a history of AIDS (CD4 nadir < 200), 68% had undetectable plasma HIV RNA (< 75 copies/ml), and the average current CD4 cell count was 451. These indicate a low burden of infection in the majority of participants at the time of the study.
Table 1. Sample characteristics.
| HIV+ (n = 88) | HIV- (n = 49) | |||
|---|---|---|---|---|
| Demographic characteristics | Mean (SD) | Range | Mean (SD) | Range |
|
| ||||
| Age (years) | 45.2 (9.9) | 23 – 65 | 44.1 (13.0) | 25 – 79 |
| Education (years)* | 12.6 (2.2) | 6 – 18 | 14.0 (3.2) | 8 – 20 |
| % male | 65.9 | 57.1 | ||
| % Caucasian | 55.7 | 71.4 | ||
|
| ||||
| Clinical characteristics | ||||
|
| ||||
| % with active HCV infection* | 33.3 | 10.2 | ||
| HIV duration (years) | 12.5 (7.0) | 0 – 26 | ||
| % undetectable HIV RNA | 67.9 | |||
| Current CD4 | 451 (241) | 56 – 1320 | ||
| % with history of AIDSa | 58.1 | |||
| % on cART | 82.8 | |||
One-way ANOVA or Pearson's x2, HIV+ vs. HIV-, p < .05
History of AIDS defined as CD4 nadir < 200
MRI Data Acquisition
All MRI data were acquired at the Brown University MRI Research Facility using a Siemens Trio 3T scanner. High-resolution structural MRI of the whole brain was acquired in the sagittal plane using a T1-weighted MPRAGE pulse sequence (TE/TR = 3.06/2,250 ms, flip angle = 9°, slice thickness = 0.86 mm) and in the axial plane using a T2-weighted FLAIR TSE sequence (TE/TR 149/9,000 ms, flip angle = 120°, slice thickness = 3 mm no gap, interleaved). Diffusion-weighted images (DWI) covering the whole brain were acquired using a double-spin-echo echo-planar pulse sequence in the axial plane plane with 64 diffusion gradient directions, b-value = 1000 ×/mm2, TE = 103 ms, TR = 10060 ms, in-plane resolution = 1.77×1.77 mm, and slice thickness = 1.8 mm. Ten images with no diffusion encoding were acquired as baseline for diffusion tensor fitting. Due to an MRI imager upgrade during the study, 27 of the subjects were scanned with identical acquisition parameters, except for TR = 10,100 ms. Accordingly, protocol type was accounted for in statistical analysis as a covariate.
White Matter Hyperintensity Analysis
WMH were quantified using a semi-automated approach. T1 images were skull-stripped and segmented using the automated FreeSurfer procedures (Fischl 2012). All segmentations were visually inspected and judged to be of adequate quality. Each T1 image was then rigid-body registered to the corresponding FLAIR images using FMRIB Software Library (FSL) FMRIB's Linear Image Registration Tool (FLIRT) (Jenkinson & Smith 2001; Jenkinson et al. 2002; Greve & Fischl 2009), and the resulting transformations were applied to the FreeSurfer segmentation file using a nearest-neighbor interpolation to preserve the integer volume labels. A white matter mask for each participant was then created based on the segmentation and eroded by one voxel in all directions to eliminate residual skull and cerebrospinal fluid voxels. The FLAIR images were skull-stripped using the T1 skull-strip images and corresponding transformations, then normalized for field inhomogeneity using FSL FAST (Zhang et al. 2001). White matter voxels from the FLAIR images were then extracted using the eroded mask created above.
A threshold level of 1.25 SD above the median intensity of white matter voxels was created through an iterative process with visual inspection as an optimal point to identify white matter hyperintensity for each brain. A WMH mask of all white matter voxels that exceeded the threshold intensity was then created. To minimize false positives from voxels included due to minor movement artifact or change in tissue density, all clusters smaller than 5 voxels were removed. WMH masks were then manually edited according to strict rules designed to identify and remove false WMH inclusions that occurred from transitioning between grey and white matter in a consistent fashion across brains. A final volumetric measure of WMH was then derived from the final WMH mask. To account for individual differences in head size, volumetric measures of whole-brain WMH were adjusted based on intracranial volume (ICV) by creating a percentage (WMH/ICV*100). Thus, in all analyses, WMH refers to the percentage of ICV that is WMH.
To determine measurement reliability of this WMH quantification process, 14 FLAIR images with varying degrees of WMH burden were independently rated using a well-validated visual rating scale (Appel et al. 2009). Correlation between methods was 0.83, indicating high inter-method reliability.
Diffusion Tensor Analysis
Diffusion tensor analysis was performed using FSL (Smith et al. 2004; Woolrich et al. 2009; Jenkinson et al. 2012). Non-DWI images were co-registered to correct for movement using FSL FLIRT rigid-body registrations. The images were then averaged to create a baseline for subsequent tensor fitting. To account for movement and eddy current distortions, the DWIs were registered to the non-DWI baseline image using 12-parameter affine registrations. The diffusion gradient vectors for each individual DWI were adjusted according to the corresponding affine transformations to account for the spatial transformations (Alexander et al. 2001). In order to avoid negative eigenvalues, diffusion tensor estimations were performed using a nonlinear iterative method (Cox 1996). The three principal eigenvectors were then computed, as were associated eigenvalues of the tensor characterizing the diffusion ellipsoid. Fractional anisotropy (FA) was derived from the eigenvalues using standard formulas (Basser & Jones 2002). The FA data were then fed into standard TBSS skeletonization to derive a common skeleton in MNI space onto which each person's FA data were projected. The Johns Hopkins ICBM-DTI-81 atlas was then used to segment the TBSS skeleton, and average FA values computed for each ROI bilaterally.
Six ROIs were chosen based on relevance to HIV disease: the anterior corona radiata (Pomara et al. 2001; Xuan et al. 2013), genu of the corpus callosum (Thurnher et al. 2005; Wu et al. 2006; Xuan et al. 2013), all areas of the internal capsules (Aylward et al. 1993; Ernst et al. 2000; Nath et al. 2000; Pomara et al. 2001), and the cerebral peduncles, which are an extension of the posterior limbs of the internal capsules. The 9 other white matter ROIs, which were not expected to be influenced by HIV, were examined as a control comparison condition to evaluate the specificity of early HIV associated effects.
Statistical Analysis
All statistical analyses were performed using IBM SPSS Statistics version 21. Alpha was set at .05, two-tailed. Differences in demographic and clinical variables between HIV+ and HIV- participants were examined using analysis of variance (ANOVA) for continuous variables and Pearson's chi-square tests for categorical variables.
To assess the interactive effects of age and HIV status, an age by HIV interaction term was created by multiplying the age and HIV variables, then orthogonalizing the product to avoid multicollinearity among predictors. This was achieved by regressing the effects of age and HIV out of the product term in a process called residual centering (Little et al. 2006; Marsh et al. 2007). To explain, a linear regression was conducted with age and HIV as independent variables and the age by HIV product term as the dependent variable. The unstandardized residuals from the regression were compiled as a new continuous variable, which represents the variance unexplained by the model, or unique interaction between age and HIV that is not related to age or HIV individually.
A linear regression was performed to examine how age, HIV status, and their interaction relate to WMH. The dependent variable in the regression analysis was WMH and the independent variables were age, HIV status, and the orthogonalized age by HIV product term. To examine how age, HIV status, and their interaction affect white matter integrity, separate linear regression analyses were conducted for each of the white matter ROIs. In these regression analyses, FA for each ROI was the dependent variable, independent variables of interest were age, HIV status, and the orthogonalized age by HIV interaction term, and DTI protocol was included as a control variable. A backwards selection linear regression analysis was then conducted to assess which white matter measure of interest (WMH and the six FA measures of interest) was most closely associated with the age by HIV interaction.
Since years of education and HCV differed between HIV groups, correlational analyses examined the relationship between these two variables and the white matter measures of interest. Hierarchical regressions entering either HCV or education in the first model and then adding age, HIV, and the age by HIV interaction in the second model were conducted to examine the impact of HCV or education on the outcomes of interest.
Partial correlational analyses were used to assess the relationship between WMH and FA for each of the 15 white matter ROIs, controlling for DTI protocol. Finally, to examine the influence of clinical variables related to HIV on white matter damage independent of age, stepwise regressions using backward elimination were conducted for WMH and each of the 6 ROIs expected to show HIV associated effects. The following predictors were used: active HCV infection (yes/no), HIV duration (years), detectable HIV RNA (yes/no), current CD4 cell count, history of AIDS (yes/no, defined as CD4 nadir < 200), and current cART use (yes/no). This statistical method begins with a full regression model, using all predictors, and removes the variable that will most improve the model once removed, continuing until the model is optimal.
Results
White Matter as a Function of Age and HIV
Age, HIV status, and the age by HIV interaction explained significant variability in WMH (R2 = .069, F [3, 156] = 3.83, p = .011). There was a significant main effect of age (β = .179, p = .023), such that older age was associated with greater WMH. The main effect of HIV was not significant (β = .054, p = .486). However, the age by HIV interaction effect was significant (β = .203, p = .010). To deconstruct the interaction term, follow-up regression analyses were conducted for HIV+ and HIV- participants separately with age as the independent variable and WMH as the outcome variable. For the HIV- group, age was not associated with WMH (β = -.058, p = .661), but for the HIV+ group, older age was associated with greater WMH (β = .269, p = .007). Figure 1 depicts WMH as a function of age for HIV+ and HIV- groups. WMH are presented as a percentage of total intracranial volume, and best-fit lines are displayed with 95% confidence bands.
Fig. 1.
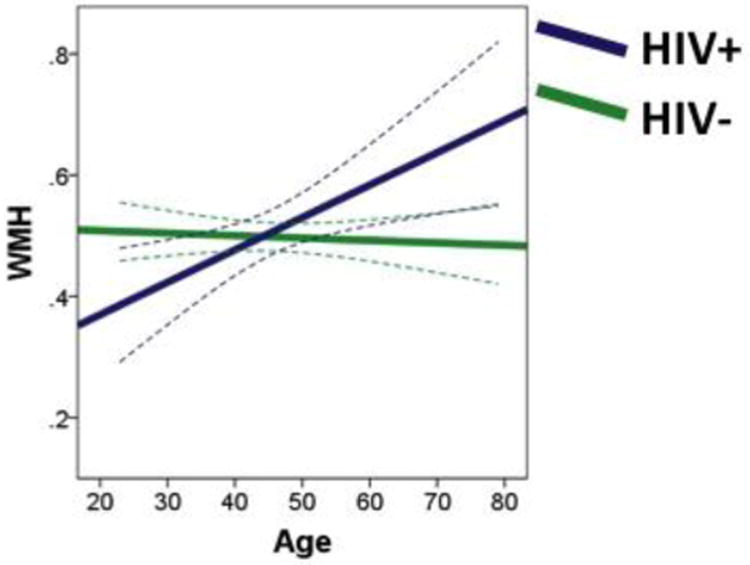
The HIV+ group shows greater increase in a whole-brain volumetric measure of white matter hyperintensities (WMH) with age vs. HIV- participants, depicting how age exacerbates HIV associated white matter damage. Results are displayed as best-fit lines with 95% confidence bands. Data are extrapolated for HIV+ individuals over age 65.
Table 2 displays the regression coefficients for each of the 15 FA analyses, with the 6 ROIs expected to show HIV and age interactive effects listed first. Regression equations were significant for all white matter ROIs. A significant main effect for HIV was not found for any white matter ROI. A significant main effect of age was found in most of the ROIs examined, where greater age was associated with lower FA. Older age was associated with lower FA in the anterior, posterior, and superior corona radiata, the inferior and superior longitudinal fasciculi, the posterior thalamic radiations, the body and genu of the corpus callosum, the anterior and posterior limbs of the internal capsules, the cerebral peduncles, and the external capsules.
Table 2. Regression coefficients for FA analyses.
| Age | HIV | Age by HIV | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| β | p | β | p | β | p | |
| Anterior Corona Radiata | -.479 | <.001* | -.015 | .835 | -.142 | .042* |
| Anterior Limb of the Internal Capsule | -.290 | <.001* | .003 | .973 | -.145 | .065 |
| Retrolenticular of the Internal Capsule | -.063 | .422 | -.064 | .431 | -.075 | .340 |
| Posterior Limb of the Internal Capsule | -.285 | .001* | .078 | .366 | -.198 | .018* |
| Cerebral Peduncle | -.317 | <.001* | -.016 | .841 | -.184 | .016* |
| Genu of the Corpus Callosum | -.264 | <.001* | <.001 | 1.0 | -.024 | .706 |
| Body of the Corpus Callosum | -.171 | .017* | -.044 | .555 | -.070 | .324 |
| Splenium of the Corpus Callosum | -.053 | .462 | .026 | .728 | -.039 | .589 |
| External Capsule | -.355 | <.001* | -.080 | .347 | -.087 | .289 |
| Inferior Longitudinal Fasciculus | -.143 | .040* | -.004 | .955 | -.031 | .653 |
| Middle Cerebellar Peduncle | -.031 | .684 | .003 | .969 | -.044 | .569 |
| Posterior Corona Radiata | -.321 | <.001* | .054 | .502 | -.097 | .213 |
| Posterior Thalamic Radiations | -.322 | <.001* | .012 | .859 | -.086 | .196 |
| Superior Corona Radiata | -.420 | <.001* | -.049 | .552 | -.134 | .093 |
| Superior Longitudinal Fasciculus | -.316 | <.001* | .007 | .936 | -.079 | .341 |
p < .05
There was a significant interaction effect of age and HIV for FA in the anterior corona radiata, posterior limbs of the internal capsules, and the cerebral peduncles. These regions are displayed in Figure 2. To deconstruct the interaction term, follow-up regression analyses were conducted for HIV+ and HIV- participants separately with age as the independent variable and FA as the outcome variable, controlling for DTI protocol. While there was a significant relationship between increased age and decreased FA of the anterior corona radiata in the HIV- group (β = -.388, p = .003), the relationship was significantly stronger in the HIV+ group (β = -.551, p < .001) (Fig. 3a). For FA of the posterior limbs of the internal capsules (Fig. 3b), greater age was associated with lower FA in the HIV+ group (β = -.398, p < .001), whereas there was no relationship between age and FA in the HIV- group (β = -.107, p = .468). A similar pattern was observed for FA in the cerebral peduncles (Fig. 3c), for which greater age was associated with lower FA among people with HIV (β = -.409, p < .001), whereas there was no relationship between age and FA among seronegative participants (β = -.177, p = .219).
Fig. 2.
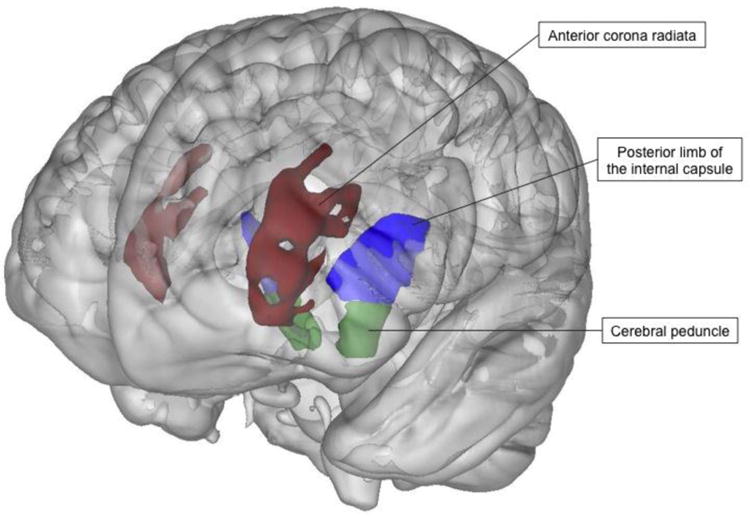
White matter areas for which the age by HIV interaction was significant (p < .05). HIV+ participants showed stronger associations between older age and reduced FA compared to HIV- participants in the anterior corona radiata (β = -.142), posterior limbs of the internal capsules (β = -.198), and cerebral peduncles (β = -.184), showing that older age exacerbates HIV associated decreases of white matter integrity in these regions.
Fig. 3.
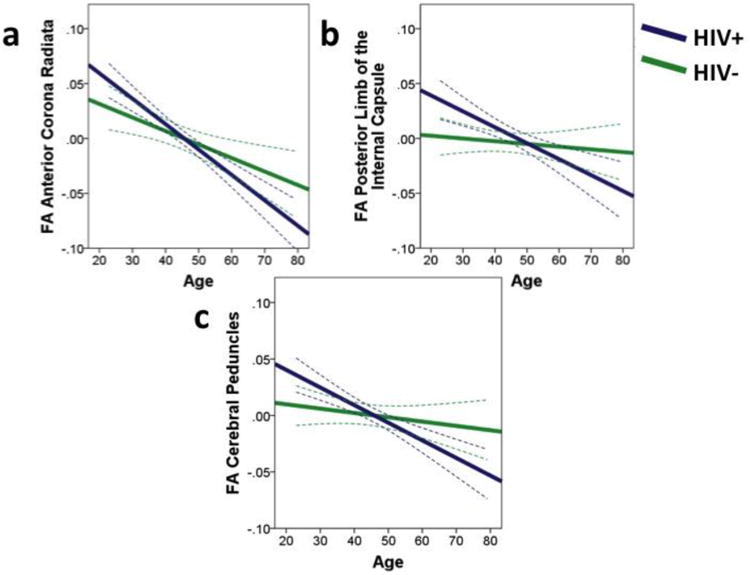
Fractional anisotropy (FA) of white matter regions of interest as a function of age for HIV- and HIV+ groups. The HIV+ group shows greater FA decline with age in a the anterior corona radiata, b the posterior limbs of the internal capsules, and c the cerebral peduncles. Results are displayed as best-fit lines with 95% confidence bands. Data are extrapolated for HIV+ individuals over age 65.
In an effort to determine whether the interactive effects of age and HIV were evident among participants who had undetectable HIV and HCV viral loads, secondary analyses were conducted on this subgroup. The subgroup for WMH analyses included 43 HIV+ and 53 HIV- participants (mean age = 46.5, standard deviation = 11.8). Analyses revealed a significant main effect of HIV on WMH (β = -.198, p = .050) such that those with HIV had overall fewer WMH. The age by HIV interaction remained significant (β = .246, p = .029). When examining HIV groups individually, HIV+ participants had a significant association between greater age and greater WMH (β = .329, p = .034) whereas no association was found for the HIV- participants (β = -.073, p = .606). FA analyses included 42 HIV+ and 44 HIV- participants (mean age = 44.8, standard deviation = 11.9). Lower FA of the anterior corona radiata was associated with greater age (β = -.504, p < .001) and the age by HIV interaction (β = -.193, p = .036), lower FA of the genu of the corpus callosum was associated with greater age (β = -.240, p = .003), lower FA of the anterior limbs of the internal capsules was associated with greater age (β = -.336, p = .001) and the age by HIV interaction (β = -.209, p = .043), lower FA of the posterior limbs of the internal capsules was associated with older age (β = -.287, p = .012) and the age by HIV interaction (β = -.233, p = .041), and lower FA of the cerebral peduncles was associated with older age (β = -.353, p = .001) and the age by HIV interaction (β = -.216, p = .044). All interaction effects were as expected, with negative associations between age and FA for the HIV+ group and either weaker or absent associations between age and FA in the HIV- group. In sum, the interaction between age and HIV was even stronger among healthier participants who did not have detectable HIV or HCV coinfection.
The Influence of HCV and Education
Given that HIV+ and HIV- participants differed in terms of years of education and current HCV infection, correlational analyses were conducted to examine the relationship between these variables and white matter variables of interest. This was followed by hierarchical regressions to assess whether accounting for the effects of HCV and education altered the effects of interest. HCV correlated with WMH (r = .264, p = .001) and FA of the anterior corona radiata (r = -.190, p = .028), while education was correlated with FA of the posterior limbs of the internal capsules (r = -.191, p = .027). Controlling for the effects of HCV and education did not significantly alter any findings.
The Relationship Between WMH and FA
Correlational analyses revealed moderate negative correlations between WMH and FA for all 15 white matter ROIs studied. Table 3 lists correlation coefficients and corresponding p-values. Results are depicted in Figure 4.
Table 3.
Partial correlations between WMH and FA, controlling for DTI protocol.
| r | p | |
|---|---|---|
| Anterior Corona Radiata | -.367 | <.001 |
| Anterior Limb of the Internal Capsule | -.426 | <.001 |
| Retrolenticular of the Internal Capsule | -.263 | .003 |
| Posterior Limb of the Internal Capsule | -.204 | .022 |
| Cerebral Peduncle | -.381 | <.001 |
| Genu of the Corpus Callosum | -.380 | <.001 |
| Body of the Corpus Callosum | -.401 | <.001 |
| Splenium of the Corpus Callosum | -.388 | <.001 |
| External Capsule | -.389 | <.001 |
| Inferior Longitudinal Fasciculus | -.404 | <.001 |
| Middle Cerebellar Peduncle | -.268 | .002 |
| Posterior Corona Radiata | -.342 | <.001 |
| Posterior Thalamic Radiations | -.425 | <.001 |
| Superior Corona Radiata | -.338 | <.001 |
| Superior Longitudinal Fasciculus | -.470 | <.001 |
Fig. 4.
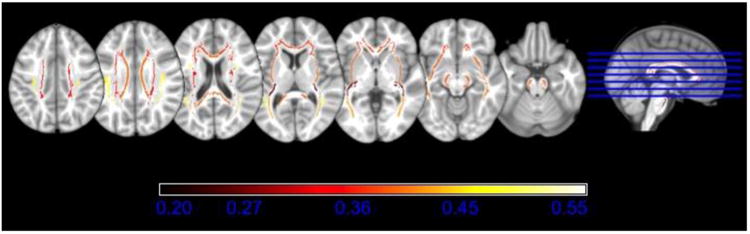
White matter regions of interest (ROIs) color-coded by the correlation with whole-brain white matter hyperintensities (WMH). Greater WMH were moderately correlated (-.204 ≤ r ≤ -.470) with lower fractional anisotropy in all ROIs and all relationships were significant (p < .05).
Which Measure is Most Sensitive to the Interaction of Age and HIV?
To assess which white matter measure of interest had the strongest association with the age by HIV interaction effect, a follow-up stepwise regression with backwards elimination was conducted with the white matter measures of interest, namely WMH and FA values for the anterior corona radiata, the anterior and posterior limbs and retrolenticular of the internal capsules, the genu of the corpus callosum, and the cerebral peduncles, as predictors and the orthogonalized age by HIV interaction term as the outcome variable. Results of this process revealed that all models were non-significant until the final model, in which WMH was the only predictor, explaining 3.2% of the variance in the interaction effect (R2 = .032, F [1, 124] = 4.06, p = .046).
The Impact of HIV Associated Clinical Factors
Stepwise regression analyses with backwards selection were used to create optimal models predicting white matter measures using HIV clinical variables. Of the 6 ROIs expected to show HIV associated effects, only the anterior corona radiata was related to HIV variables. The optimal model predicting FA of the anterior corona radiata explained 13.4% of the variability in FA (R2 = .134, F [3, 80] = 4.11, p = .009) using history of AIDS (β = -.26, p = .027), current CD4 (β = -.24, p = .037), and active HCV infection (β = -.22, p = .042). HIV variables explained 16.4% of the variance in WMH (R2 = .164, F [3, 93] = 6.08, p = .001) with a model that included active HCV infection (β = .319, p = .001), current CD4 (β = .238, p = .021), and history of AIDS (β = .207, p = .044).
Discussion
Our results suggest that age exacerbates HIV associated white matter damage such that with increased age, people with HIV have greater WMH and reduced FA compared to younger HIV+ individuals and older and younger seronegative controls. Measures of whole-brain WMH revealed a relationship between age and greater white matter damage in HIV+ participants only. Furthermore, HIV associated decreases in frontal and subcortical white matter integrity became more pronounced with age, as measured by steeper FA declines with age compared to seronegative controls in the anterior corona radiata, posterior limbs of the internal capsules, and cerebral peduncles. Interactive effects of age and HIV persisted when examining a subgroup free of HCV coinfection and detectable HIV RNA, suggesting that these effects exist even in infected individuals who are successfully medicated and free of HCV comorbidity.
The direction and magnitude of the combined impact of age and HIV were similar for both WMH and FA. Additionally, these measures were moderately correlated. Thus, both measures reflect similar patterns in this population and are both sensitive to white matter damage. However, when WMH and FA were simultaneously examined, WMH was retained as the variable most strongly associated with the age by HIV interaction. The variance accounted for by WMH was not substantially improved by DTI metrics. This implies that, at the very least, FA measures were comparable to WMH in their sensitivity to this interaction in this cohort. This also suggests that WMH should not be discarded as a potentially useful clinical measure in an aging HIV+ population. Furthermore, WMH may be easier to radiologically obtain, quantify, and qualitatively assess, using FLAIR images from MRI, whereas DTI measures require complex analysis to obtain findings. WMH have been a long-standing and clinically useful biomarker of white matter damage for a variety of pathological conditions, and results from the present study indicate that it continues to be clinically relevant.
Despite this evidence for the value of WMH when assessing age effects among people with HIV, it is important to note that reduced FA was associated with increased age regardless of HIV status. Age related FA reductions were widespread, consistent with previous research showing detrimental effects of age on white matter integrity (Pfefferbaum et al. 2000; Good et al. 2001; Abe et al. 2002; Resnick et al. 2003; Head et al. 2004; Brickman et al. 2006; Grieve et al. 2007; Meier et al. 2012). Given that the mean age of HIV- participants was 44 and the maximum was 79, it can be concluded that FA is sensitive to age related white matter changes in people who are not yet very advanced in age.
On the other hand, WMH did not vary with age in the seronegative controls. This is perhaps not surprising, as prior studies of people at advanced ages show that WMH are common in people beyond age 80, but are minimal prior to age 60 (Bradley et al. 1984; Garde et al. 2000; Hopkins et al. 2006). The current HIV- cohort was, on average, below age 60, so significant white matter abnormalities on FLAIR were not expected. Accordingly, whole-brain WMH measurement among seronegative adults may be less informative than DTI measures. Additional research is warranted to better understand whether there are regional cerebral WMH differences that give more information with respect to age associated white matter abnormalities in those with and without HIV. It will also be important to investigate the evolution of WMH relative to changes in FA as they occur in people aging with HIV.
The finding that HIV associated FA reductions were strongest in frontal and subcortical regions is consistent with our hypotheses, developed based on studies showing initial changes in frontal (Pomara et al. 2001; Xuan et al. 2013) and subcortical (Pomara et al. 2001) white matter of people with HIV as well as the historical associations between HIV and subcortical brain changes (Aylward et al. 1993; Ernst et al. 2000; Nath et al. 2000). The majority of HIV+ participants in the current study were taking cART and had well-controlled viral loads, so they would not be expected to show devastating neurological changes, but rather evidence of the earliest signs of damage. Still, HIV+ participants had levels of WMH greater than would be expected for healthy people of a similar age, and greater frontal and subcortical damage compared to controls. Furthermore, the mean age of the participants with HIV was 45 and the oldest was only 65. Thus, white matter damage is occurring in HIV+ individuals before late life, in middle age. Combined with data showing that frontal regions are typically the first affected by normal aging (Head et al. 2004; Ardekani et al. 2007; Grieve et al. 2007), these results indicate that effects associated with age and HIV are compounding in HIV+ individuals aged 50 and older.
Both WMH and FA abnormalities were greater among older HIV infected people, supporting the study hypothesis that HIV associated white matter damage worsens with age. Yet, whether HIV and age act as additive or interactive risk factors for neurological damage is controversial. Age and HIV have been shown to have interactive neurological and cognitive effects (Sacktor et al. 2010; Chang et al. 2013; Seider et al. 2014), though some research suggests that the effects are additive rather than synergistic (Ances et al. 2012; Becker et al. 2012; Nir et al. 2014). Evidence for the interactive influence of HIV and age comes from the finding that neurocognitive impairments among older people with HIV are more prevalent than would be expected from HIV or age related risks alone (Bhatia et al. 2012). Furthermore, prior studies show interactive age and HIV effects in association with greater functional activation abnormalities in left frontal brain regions (Chang et al. 2013), greater neuroinflammation and fewer markers of neuronal viability in a magnetic resonance spectroscopy (MRS) study (Ernst & Chang 2004), and greater declines in memory (Seider et al. 2014), psychomotor speed (Sacktor et al. 2010), and daily functioning (Morgan et al. 2012) in older participants with HIV. For a discussion of mechanisms of neurological damage that are common to both HIV and advanced age, see (Cohen et al. 2015).
Analysis of the relationship between clinical factors and white matter measures among the HIV infected people revealed significant associations between HCV coinfection, history of AIDS, and current CD4 levels. A positive history of AIDS was associated with greater white matter damage, which corroborates previous findings (Zhu et al. 2013). Thus, prior immune compromise may produce lasting neurological effects, even after immune reconstitution. HCV coinfection was also associated with greater white matter damage. HCV affects liver function, leading to inflammatory responses in the body and the brain, and has been associated with greater cognitive dysfunction in people with HIV (Devlin et al. 2012). Surprisingly, greater CD4 levels were related to greater white matter damage. Although larger CD4 cell counts have been associated with white matter abnormalities and reduced volume in prior research (Jernigan et al. 2011; Fennema-Notestine et al. 2013), the mechanism behind this association remains unclear.
Given that age and duration of infection are closely related, it is possible that age effects might have been explained by duration of infection rather than aging per se. However, HIV duration as measured by time since diagnosis was not a significant predictor of white matter damage in the current cohort. Therefore, it can be concluded that the observed age effects were independent of HIV duration and truly reflected the effects of aging.
The present study is a cross-sectional design and thus has several inherent limitations. We cannot determine that the white matter structure in these study participants changed from a premorbid state, nor can we conclude that it reflected damaged caused directly by HIV. Additionally, inferences were made about the effects of age on white matter, but only in a longitudinal design would we be able to directly measure age related changes and assure absence of cohort effect. Future longitudinal studies would allow for a more direct observation of the effects of age, HIV, and comorbid conditions on white matter structure and integrity.
In conclusion, aging and HIV are associated with white matter damage and interact as risk factors for WMH increase and FA decline. FLAIR imaging may still be a clinically relevant tool to measure white matter abnormalities in middle-aged and older people with HIV, whereas DTI may be more useful for assessing age related white matter changes in middle-aged seronegative adults. Given that the current study population was relatively healthy and functionally intact, findings reflect early changes in white matter, which occur in frontal and subcortical areas in the context of HIV. Discovering early signs of decline gives an opportunity to aggressively treat modifiable risk factors for white matter damage, such as HCV, before the development of significant functional impairments. As the population with HIV ages, it will continue to be important to investigate the effects of the disease, comorbid conditions, and age on brain structure and function.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (R01 MH074368), the Lifespan/Tufts/Brown Center for AIDS Research (P30 AI042853), the National Institute of Alcohol Abuse and Alcoholism (P01 AA019072), the National Institute of Neurological Disorders and Stroke (R01 NS080655), the Enigma Center for Worldwide Medicine (U54 EB020403), and the Southern HIV Alcohol Research Consortium (U24 AA022002). Support was also provided by the McKnight Brain Research Foundation and the University of Florida Center for Cognitive Aging and Memory.
Abbreviations
- BBB
blood brain barrier
- cART
combination antiretroviral therapy
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FLAIR
fluid attenuated inversion recovery
- HCV
Hepatitis C virus
- HIV
Human Immunodeficiency Virus-1
- HIV+
HIV positive
- HIV-
HIV negative
- ICV
intracranial volume
- MRI
magnetic resonance imaging
- ROI
region of interest
- TBSS
tract-based spatial statistics
- WMH
white matter hyperintensities
Footnotes
The authors declare that they have no conflict of interest.
References
- Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K. Normal aging in the central nervous system: Quantitative mr diffusion-tensor analysis. Neurobiol Aging. 2002;23(3):433–441. doi: 10.1016/S0197-4580(01)00318-9. [DOI] [PubMed] [Google Scholar]
- Aine CJ, Sanfratello L, Adair JC, Knoefel JE, Qualls C, Lundy SL, Caprihan A, Stone D, Stephen JM. Characterization of a normal control group: Are they healthy? Neuroimage. 2014;84:796–809. doi: 10.1016/j.neuroimage.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance images. IEEE Trans Med Imaging. 2001;20(11):1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of hiv, aging, and haart on brain volumetric measures. J Acquir Immune Defic Syndr. 2012;59(5):469–477. doi: 10.1097/QAI.0b013e318249db17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel J, Potter E, Bhatia N, Shen Q, Zhao W, Greig MT, Raj A, Barker WW, Potter H, Schofield E, Wu Y, Loewenstein DA, Duara R. Association of white matter hyperintensity measurements on brain mr imaging with cognitive status, medial temporal atrophy, and cardiovascular risk factors. AJNR Am J Neuroradiol. 2009;30(10):1870–1876. doi: 10.3174/ajnr.A1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magn Reson Imaging. 2007;25(2):154–167. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Henderer B, McCarthur Jea. Reduced basal ganglia volume in hiv-1 associated dementia: Results from quantitative neuroimaging. Neurology. 1993;43:2099–2104. doi: 10.1212/WNL.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor mri: Theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15(7-8):456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Becker JT, Lopez OL, Dew MA, Aizenstein HJ. Prevalence of cognitive disorders differs as a function of age in hiv virus infection. Aids. 2004;18(1):S11–18. [PubMed] [Google Scholar]
- Becker JT, Maruca V, Kingsley LA, Sanders JM, Alger JR, Barker PB, Goodkin K, Martin E, Miller EN, Ragin A, Sacktor N, Selnes O, Multicenter ACS. Factors affecting brain structure in men with hiv disease in the post-haart era. Neuroradiology. 2012;54(2):113–121. doi: 10.1007/s00234-011-0854-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, Rowley HA, Lazar M, Alexander AL, Johnson SC. White matter in aging and cognition: A cross-sectional study of microstructure in adults aged eighteen to eighty-three. Dev Neuropsychol. 2010;35(3):257–277. doi: 10.1080/87565641003696775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia R, Ryscavage P, Taiwo B. Accelerated aging and human immunodeficiency virus infection: Emerging challenges of growing older in the era of successful antiretroviral therapy. J Neurovirol. 2012;18(4):247–255. doi: 10.1007/s13365-011-0073-y. [DOI] [PubMed] [Google Scholar]
- Bradley W, Waluch V, Brant-Zawadzki M, Vadley R, Wycoff R. Patchy periventricular white matter lesions in the elderly: A common observation during nmr imaging. Noninvasive Med Imaging. 1984;1:35–41. [Google Scholar]
- Brickman AM, Zimmerman ME, Paul RH, Grieve SM, Tate DF, Cohen RA, Williams LM, Clark CR, Gordon E. Regional white matter and neuropsychological functioning across the adult lifespan. Biol Psychiatry. 2006;60(5):444–453. doi: 10.1016/j.biopsych.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chang L, Holt JL, Yakupov R, Jiang CS, Ernst T. Lower cognitive reserve in the aging human immunodeficiency virus-infected brain. Neurobiol Aging. 2013;34(4):1240–1253. doi: 10.1016/j.neurobiolaging.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Boland R, Paul R, Tashima KT, Schoenbaum EE, Celentano DD, Schuman P, Smith DK, Carpenter CC. Neurocognitive performance enhanced by highly active antiretroviral therapy in hiv-infected women. Aids. 2001;15(3):341–345. doi: 10.1097/00002030-200102160-00007. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Seider TR, Navia B. Hiv effects on age-associated neurocognitive dysfunction: Premature cognitive aging or neurodegenerative disease? Alzheimers Res Ther. 2015;7(1):37. doi: 10.1186/s13195-015-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. Afni: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Detels R, Munoz A, McFarlane G, Kingsley LA, Margolick JB, Giorgi J, Schrager LK, Phair JP. Effectiveness of potent antiretroviral therapy on time to aids and death in men with known hiv infection duration. Multicenter aids cohort study investigators. JAMA. 1998;280(17):1497–1503. doi: 10.1001/jama.280.17.1497. [DOI] [PubMed] [Google Scholar]
- Devlin KN, Gongvatana A, Clark US, Chasman JD, Westbrook ML, Tashima KT, Navia B, Cohen RA. Neurocognitive effects of hiv, hepatitis c, and substance use history. J Int Neuropsychol Soc. 2012;18(1):68–78. doi: 10.1017/S1355617711001408. doi:S1355617711001408[pii]10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE, Janoff EN, Justice AC, Kuritzkes D, Nayfield SG, Plaeger SF, Schmader KE, Ashworth JR, Campanelli C, Clayton CP, Rada B, Woolard NF, High KP. Aging and infectious diseases: Workshop on hiv infection and aging: What is known and future research directions. Clin Infect Dis. 2008;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Effect of aging on brain metabolism in antiretroviral-naive hiv patients. AIDS. 2004;18(1):S61–67. [PubMed] [Google Scholar]
- Ernst T, Itti E, Itti L, Chang L. Changes in cerebral metabolism are detected prior to perfusion changes in early hiv-cmc: A coregistered (1)h mrs and spect study. J Magn Reson Imaging. 2000;12(6):859–865. doi: 10.1002/1522-2586(200012)12:6<859::aid-jmri8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Ellis RJ, Archibald SL, Jernigan TL, Letendre SL, Notestine RJ, Taylor MJ, Theilmann RJ, Julaton MD, Croteau DJ, Wolfson T, Heaton RK, Gamst AC, Franklin DR, Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I, Group C. Increases in brain white matter abnormalities and subcortical gray matter are linked to cd4 recovery in hiv infection. J Neurovirol. 2013;19(4):393–401. doi: 10.1007/s13365-013-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi C, Ulug A, Ryan E, Ferrando S, Van Gorp W. Diffusion tensor imaging of patients with hiv and normal-appearing white matter on mr images of the brain. AJNR Am J Neuroradiol. 2001;22(2):277–283. [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for hiv-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Fischl B. Freesurfer. Neuroimage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: A longitudinal study. Lancet. 2000;356(9230):628–634. doi: 10.1016/S0140-6736(00)02604-0. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009;48(1):63–72. doi: 10.1016/j.neuroimage.2009.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: A diffusion tensor mr imaging study. AJNR Am J Neuroradiol. 2007;28(2):226–235. [PMC free article] [PubMed] [Google Scholar]
- Grueter BE, Schulz UG. Age-related cerebral white matter disease (leukoaraiosis): A review. Postgrad Med J. 2012;88(1036):79–87. doi: 10.1136/postgradmedj-2011-130307. [DOI] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex. 2004;14(4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. J Neuroimaging. 2006;16(3):243–251. doi: 10.1111/j.1552-6569.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Taylor MJ, Theilmann RJ, Julaton MD, Notestine RJ, Wolfson T, Letendre SL, Ellis RJ, Heaton RK, Gamst AC, Franklin DR, Jr, Clifford DB, Collier AC, Gelman BB, Marra C, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Grant I, Group C. Clinical factors related to brain structure in hiv: The charter study. J Neurovirol. 2011;17(3):248–257. doi: 10.1007/s13365-011-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TD, Bovaird JA, Widaman KF. On the merits of orthogonalizing powered and product terms: Implications for modeling interactions among latent variables. Structural Equation Modeling. 2006;13(4):497–519. doi: 10.1207/s15328007sem1304_1. [DOI] [Google Scholar]
- Mack K, Ory M. Aids and older americans at the end of the twentieth century. Journal of Acquired Immune Deficiency Syndrome. 2003;33(2):568–572. doi: 10.1097/00126334-200306012-00003. [DOI] [PubMed] [Google Scholar]
- Maillard P, Carmichael O, Harvey D, Fletcher E, Reed B, Mungas D, DeCarli C. Flair and diffusion mri signals are independent predictors of white matter hyperintensities. AJNR Am J Neuroradiol. 2013;34(1):54–61. doi: 10.3174/ajnr.A3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredi R, Nanetti A, Valentini R, Calza L, Chiodo F. Frequency, epidemiology, risk factors, clinical and bacteriological features of enterococcal disease in patients with hiv infection in a decade survey. New Microbiol. 2002;25(2):179–186. [PubMed] [Google Scholar]
- Marsh HW, Wen Z, Hau KT, Little TD, Bovaird JA, Widaman KF. Unconstrained structural equation models of latent interactions: Contrasting residual-and mean-centered approaches. Structural Equation Modeling. 2007;14(4):570–580. doi: 10.1080/10705510701303921. [DOI] [Google Scholar]
- Meier IB, Manly JJ, Provenzano FA, Louie KS, Wasserman BT, Griffith EY, Hector JT, Allocco E, Brickman AM. White matter predictors of cognitive functioning in older adults. J Int Neuropsychol Soc. 2012;18(3):414–427. doi: 10.1017/S1355617712000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan EE, Iudicello JE, Weber E, Duarte NA, Riggs PK, Delano-Wood L, Ellis R, Grant I, Woods SP Group HIVNRP. Synergistic effects of hiv infection and older age on daily functioning. J Acquir Immune Defic Syndr. 2012;61(3):341–348. doi: 10.1097/QAI.0b013e31826bfc53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with aids dementia. J Psychopharmacol. 2000;14(3):222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nir TM, Jahanshad N, Busovaca E, Wendelken L, Nicolas K, Thompson PM, Valcour VG. Mapping white matter integrity in elderly people with hiv. Hum Brain Mapp. 2014;35(3):975–992. doi: 10.1002/hbm.22228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rogosa DA, Rosenbloom MJ, Chu W, Sassoon SA, Kemper CA, Deresinski S, Rohlfing T, Zahr NM, Sullivan EV. Accelerated aging of selective brain structures in human immunodeficiency virus infection: A controlled, longitudinal magnetic resonance imaging study. Neurobiol Aging. 2014;35(7):1755–1768. doi: 10.1016/j.neurobiolaging.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44(2):259–268. doi: 10.1002/1522-2594(200008)44:2<259∷AID-MRM13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Pomara N, Crandall DT, Choi SJ, Johnson G, Lim KO. White matter abnormalities in hiv-1 infection: A diffusion tensor imaging study. Psychiatry Res. 2001;106(1):15–24. doi: 10.1016/S0925-4927(00)00082-2. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, Skolasky RL, Cox C, Selnes O, Becker JT, Cohen B, Martin E, Miller EN, Multicenter ACS. Longitudinal psychomotor speed performance in human immunodeficiency virus-seropositive individuals: Impact of age and serostatus. J Neurovirol. 2010;16(5):335–341. doi: 10.3109/13550284.2010.504249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider TR, Luo X, Gongvatana A, Devlin K, de la Monte S, Chasman JD, Peisi Y, Tashima K, Navia B, Cohen RA. Verbal memory declines more rapidly with age in hiv infected versus uninfected adults. Submitted for publication. Journal of Clinical and Experimental Neuropsychology. 2014;36(4):356–367. doi: 10.1080/13803395.2014.892061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in hiv patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural mr image analysis and implementation as fsl. Neuroimage. 2004;23(1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thomas JB, Brier MR, Snyder AZ, Vaida FF, Ances BM. Pathways to neurodegeneration: Effects of hiv and aging on resting-state functional connectivity. Neurology. 2013;80(13):1186–1193. doi: 10.1212/WNL.0b013e318288792b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnher MM, Castillo M, Stadler A, Rieger A, Schmid B, Sundgren PC. Diffusion-tensor mr imaging of the brain in human immunodeficiency virus-positive patients. AJNR Am J Neuroradiol. 2005;26(9):2275–2281. [PMC free article] [PubMed] [Google Scholar]
- van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F study Anoc. Life expectancy of recently diagnosed asymptomatic hiv-infected patients approaches that of uninfected individuals. AIDS. 2010;24(10):1527–1535. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Vrooman HA, Wielopolski PA, Krestin GP, Hofman A, Niessen WJ, Van der Lugt A, Breteler MM. White matter microstructural integrity and cognitive function in a general elderly population. Arch Gen Psychiatry. 2009;66(5):545–553. doi: 10.1001/archgenpsychiatry.2009.5. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in fsl. Neuroimage. 2009;45(1 Suppl):S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Wu Y, Storey P, Cohen BA, Epstein LG, Edelman RR, Ragin AB. Diffusion alterations in corpus callosum of patients with hiv. AJNR Am J Neuroradiol. 2006;27(3):656–660. [PMC free article] [PubMed] [Google Scholar]
- Xuan A, Wang GB, Shi DP, Xu JL, Li YL. Initial study of magnetic resonance diffusion tensor imaging in brain white matter of early aids patients. Chin Med J (Engl) 2013;126(14):2720–2724. [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain mr images through a hidden markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhu T, Zhong J, Hu R, Tivarus M, Ekholm S, Harezlak J, Ombao H, Navia B, Cohen R, Schifitto G. Patterns of white matter injury in hiv infection after partial immune reconstitution: A dti tract-based spatial statistics study. J Neurovirol. 2013;19(1):10–23. doi: 10.1007/s13365-012-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


