Abstract
Trimethyltin (TMT) toxicity causes histopathological damage in the hippocampus and induces seizure behaviors in mice. The lesions and symptoms recover spontaneously over time; however, little is known about the precise mechanisms underlying this recovery from TMT toxicity. We investigated changes in the brain-derived neurotrophic factor/extracellular signal-regulated kinases (BDNF/ERK) signaling pathways in the mouse hippocampus following TMT toxicity. Mice (7 weeks old, C57BL/6) administered TMT (2.6 mg/kg intraperitoneally) showed acute and severe neurodegeneration with increased TUNEL-positive cells in the dentate gyrus (DG) of the hippocampus. The mRNA and protein levels of BDNF in the hippocampus were elevated by TMT treatment. Immunohistochemical analysis showed that TMT treatment markedly increased phosphorylated ERK1/2 expression in the mouse hippocampus 1-4 days after TMT treatment, although the intensity of ERK immunoreactivity in mossy fiber decreased at 1-8 days post-treatment. In addition, ERK-immunopositive cells were localized predominantly in doublecortin-positive immature progenitor neurons in the DG. In primary cultured immature hippocampal neurons (4 days in vitro), BDNF treatment alleviated TMT-induced neurotoxicity, via activation of the ERK signaling pathway. Thus, we suggest that BDNF/ERK signaling pathways may be associated with cell differentiation and survival of immature progenitor neurons, and will eventually lead to spontaneous recovery in TMT-induced hippocampal neurodegeneration.
Keywords: Trimethyltin, Hippocampus, BDNF, ERK, Spontaneous recovery
1. Introduction
The organotin compound trimethyltin (TMT) induces selective neurodegeneration in the mammalian central nervous system, particularly the hippocampus (Geloso et al., 2011). TMT-treated animals show neuronal death and glial activation in the hippocampus (Fiedorowicz et al., 2001; Kim et al., 2014b). They also exhibit cognitive deficits and behavioral changes, including memory loss, learning impairment, hyperactivity, aggression, and seizures (Besser et al., 1987; Fabrizi et al., 2015; Kim et al., 2013).
Previous studies in experimental animals have reported spontaneous recoveries from various brain injuries, including traumatic brain injury, stroke, seizure, and chemical insults (Blaiss et al., 2011; Lee et al., 2011; Park et al., 2014; Wachter et al., 2010). Mice also recover spontaneously from TMT-induced hippocampal lesions and seizure behaviors (Kim et al., 2014a; Kim et al., 2015; Yang et al., 2012). One hypothesis to explain this recovery emphasizes that neural stem cells (NSCs) and neural progenitor cells (NPCs) in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG) and the subventricular zone of the anterior lateral ventricle, where adult neurogenesis takes place, replace the damaged neurons and glial cells after TMT toxicity (Ogita et al., 2005). However, the precise intracellular mechanisms of this endogenous and spontaneous recovery remain unclear.
Extracellular signal-regulated kinases (ERK) 1 and 2, members of the mitogen-activated protein kinase (MAPK) superfamily, are activated when extracellular stimuli, such as neurotransmitters, neurotrophic factors, and growth factors, bind to the upstream receptors of ERK1/2 under physiological conditions (Grewal et al., 1999; Segal and Greenberg, 1996). Translocation of the activated ERK1/2 to the nucleus then leads to diverse cellular responses, including gene transcription, protein synthesis, ion channel modulation, dendritic spine stabilization, cell cycle progression, and cell survival, through cell-specific combinations of downstream substrates (Garcia et al., 2002; Khokhlatchev et al., 1998). ERK1/2 activation is not only found in normal states, but is also found in pathological conditions, such as viral infection, DNA injury, oxidative stress, epilepsy, and ischemic stroke (Adderley and Fitzgerald, 1999; Berkeley et al., 2002; Lannuzel et al., 1997; Slevin et al., 2000). Likewise, the roles of ERK1/2 activation following brain insults seem to be complex and implicated in both neurodegeneration and neuroprotection. In certain brain injuries, including damage elicited by H2O2, amyloid beta, and 6-hydroxydopammine, ERK activation may lead to cell death (Bhat and Zhang, 1999; Kulich and Chu, 2001; Rapoport and Ferreira, 2000). Alessandrini et al. (1999) reported that increased ERK phosphorylation was seen in infarct areas of the brain cortex in mice following occlusion of the middle cerebral artery, and pre-treatment with the MAPK kinase (MEK)/ERK inhibitor PD98059 reduced infarct size and relieved the neurological deficits. In contrast, there is also accumulating evidence showing that the ERK1/2 pathway is required for neuroprotection (Sanchez et al., 2012). Hossain et al. (2013) reported that plasmalogens rescued nutrient deprivation-induced cell death by reducing apoptotic action via activation of Akt and ERK1/2 pathways. In addition, Pignataro et al. (2013) demonstrated that ERK1/2 phosphorylation was involved in the neuroprotection produced by remote ischemic post-conditioning. However, the role(s) of ERK1/2 in neurodegenerative models remain(s) unclear.
Several studies have shown that increased endogenous neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), can activate various intracellular signaling pathways including ERK signalings (Choi et al., 2008; Wuhanqimuge et al., 2013). BDNF may interact with other signaling pathways and co-regulate cellular functions including survival, differentiation, and metabolism (Mendoza et al., 2011; Yun et al., 2005). Activation of BDNF by brain insults may play an important role in neuroprotection, and the neuroprotective effect can be achieved through activating several downstream kinases and transcription factors (Schinelli et al., 2001; Vanhoutte et al., 1999), resulting in the transcription of survival-associated genes (Svaren et al., 1996; Veyrac et al., 2013). However, little is known about the precise roles of BDNF/cellular signaling pathways interaction in chemical-induced hippocampal neurodegeneration.
In the present study, we examined changes in BDNF/ERK1/2 signaling in the hippocampus of mice after TMT treatment, and whether BDNF/ERK signaling may be involved in the spontaneous recovery against TMT-induced hippocampal neurotoxicity.
2. Materials and methods
2.1. Animals and drug treatments
Male C57BL/6J mice (7 weeks old) were obtained from a specific pathogen-free colony maintained by Daehan Biolink Co. (Chungbuk, South Korea). The care and handling of animals conformed to all of the current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85–23, 1985, revised 1996). The Institutional Animal Care and Use Committee of Chonnam National University approved all of the protocols used in this study (approval no. CNU IACUC-YB-2012-18). All of the experiments were conducted in a manner that minimized the numbers of animals used and the suffering caused.
TMT (trimethyltin hydroxide; Wako, Osaka, Japan) was dissolved in sterile 0.9% (w/v) saline. Time-dependent effects of TMT on the adult mouse hippocampus were observed after intraperitoneal administration of the material (2.6 mg/kg). The vehicle control group was injected with 0.9% (w/v) saline. Seizure tests were performed in a brightly lit area (40 × 40 cm; 250 lux; n = 10 mice/group). Behavioral changes were scored as follows: (1) aggression; (2) weak tremor; (3) systemic tremor; (4) tremor and spasmodic gait; and (5) death (Kim et al., 2014b; Yoneyama et al., 2008).
2.2. Antibodies
Monoclonal rabbit anti-phospho-ERK1/2 (Thr202/Tyr204), monoclonal rabbit anti-ERK1/2, and monoclonal mouse anti-glial fibrillary acidic protein (GFAP) were purchased from Cell Signaling Technology (Beverly, MA, USA). Monoclonal mouse anti-nestin and polyclonal rabbit anti-BDNF were from Millipore (Temecula, CA, USA). Monoclonal mouse anti-neuronal nuclei (NeuN), monoclonal rat anti-CD68, and polyclonal goat anti-doublecortin (DCX) were obtained from Abcam (Cambridge, MA, USA), Serotec (Oxford, UK), and Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. Monoclonal mouse anti-β-actin, and for immunofluorescent staining, fluorescein isothiocyanate (FITC) and tetramethyl rhodamine isothiocyanate (TRITC)-conjugated secondary antibodies, were purchased from Sigma-Aldrich (St. Louis, MO, USA). For immunoblot analysis, horseradish peroxidase (HRP)-conjugated anti-rabbit IgG and anti-mouse IgG were from Thermo Fisher Scientific (Waltham, MA, USA).
2.3. Preparation of free-floating sections
Mice were sacrificed 1, 2, 4 and 8 days after injection of TMT for histological examination of brain tissue. Mice in the vehicle-treated control group were sacrificed 4 days after injection. The animals were anesthetized and perfused with 4% (w/v) paraformaldehyde (PFA) in phosphate-buffered saline (PBS, pH 7.4). After perfusion, the brains were removed immediately and post-fixed in 4% (w/v) PFA in PBS for 2 days at 4°C. The brains were suspended in 30% (w/v) sucrose for 4 days, and embedded in tissue-embedding medium (Miles Inc., Elkhart, IN, USA). The hemispheres were sectioned at the brain region approximately 1.44–1.56 mm from the median border, commencing at the beginning of the ventral hippocampus and extending towards the medial border of the hippocampus, using a sliding microtome (SM2010R; Leica Microsystems, Wetzlar, Germany). Free-floating serial sagittal sections (30 μm thick) were collected in 12 wells filled with PBS.
2.4. TUNEL
DNA fragmentation was detected by in situ nick end labeling (terminal deoxynucleotidyl transferase [TdT]-mediated dUTP nick end-labeling, TUNEL) performed using an ApopTag® in situ apoptosis detection kit (Intergen, Purchase, NY, USA), according to the manufacturer's protocol.
2.5. RNA extraction, cDNA synthesis, and quantitative real-time reverse transcription PCR
To measure mRNA levels, mice were sacrificed and hippocampi were dissected 1, 2, 4, and 8 days (n = 7 mice/group) after injection of TMT. Mice in the vehicle-treated control group were sacrificed 4 days after injection. Total RNA was isolated using RNAeasy® Lipid Tissue Mini Kits (Qiagen, Hilden, Germany), according to the manufacturer's instructions. RNA concentrations were determined by measuring the optical density of solutions with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific). First-strand complementary DNA (cDNA) was prepared using random primers (Takara Bio, Tokyo, Japan) and Superscript™ II reverse transcriptase (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. cDNA solutions were diluted to 8 ng/μL with RNase-free water and stored at −70°C. Quantitative real-time reverse transcription PCR (qRT-PCR) amplification was performed using TOPreal qPCR 2× PreMIX solution (Enzynomics, Daejeon, South Korea) on a Stratagene MX3000P platform (Agilent Technologies, Santa Clara, CA, USA), according to the manufacturer's instructions. The primers used for qRT-PCR are shown in Table 1. The thermal profile featured pre-incubation at 94°C for 10 min, followed by 45 cycles of denaturation (94°C, 15 s), annealing (55°C, 30 s), and elongation (72°C, 20 s). A melting curve was constructed to verify that only a single product was amplified. Amplification curves were generated with the aid of the built-in software, and threshold cycle values were determined. All of the readings were normalized to those of the reference gene, β-actin. Results are expressed as mean-percentage changes compared to vehicle-treated controls using the 2-ΔΔCT method (Livak and Schmittgen, 2001).
Table 1.
Primer sequences for qRT-PCR analysis
| Gene | Accession No. | Primer sequence | Product size (bp) |
|---|---|---|---|
| BDNF | NM_001048142.1 | FWD 5’-TGGCTGACACTTTTGAGCAC-3’ | 188 |
| RVS 5’-GTTTGCGGCATCCAGGTAAT-3’ | |||
| TrkB | NM_001025074.1 | FWD 5’-TGTGGCTCAAGACTCTCCAG-3’ | 170 |
| RVS 5’-AGACTTTCCTTCCTCCACGG-3’ | |||
| β-actin | NM_007393.3 | FWD 5’-GTGCTGTCCCTGTATGCCTC-3’ | 175 |
| RVS 5’-CACGCTCGGTCAGGATCTTC-3’ |
Abbreviations: BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase B; FWD, forward; RVS, reverse.
2.6. Western blot analysis
Mice were sacrificed and the hippocampi dissected 1, 2, 4 and 8 days (n = 3 mice/group) after injection of TMT. Mice in the vehicle-treated control group were sacrificed 4 days after injection. Mouse hippocampi were immediately individually immersed in buffer H (50 mM β-glycerophosphate, 1.5 mM ethylene glycol tetraacetic acid, 0.1 mM Na3VO4, 1 mM dithiothreitol, 10 μg/mL aprotinin, 2 μg/mL pepstatin, 10 μg/mL leupeptin, and 1 mM phenylmethanesulfonyl fluoride, pH 7.4), and sonicated for 8 s. Sodium dodecyl sulfate (SDS) sample buffer (4×) was added to each homogenized sample, and the samples were heated at 100°C for 10 min.
In an in vitro study, medium was completely removed from the hippocampal cell culture by aspiration, and buffer H was added to each culture (n = 3 cultures/condition). Cells from each culture were scraped and sonicated for 4 s. Then, SDS sample buffer (2×) was added, and the samples were heated at 100°C for 10 min.
Immunoblotting was performed as previously described (Son et al., 2015). Briefly, the resolved proteins were separated by 10% SDS-PAGE (Bio-Rad, Hercules, CA, USA) and transferred onto membranes, which were then incubated with primary antibodies (rabbit anti-BDNF and rabbit anti-phospho-ERK1/2 [Thr202/Tyr204]) in PBS containing 0.1% Tween 20 (Sigma-Aldrich), 1% normal goat serum (NGS; Vector Laboratories, Burlingame, CA, USA) and 0.5% bovine serum albumin (BSA; BioShop, Burlington, Canada) overnight at 4°C. Antibodies for phospho-ERK1/2 and BDNF were used at 1:1,000 and 1:500 dilution, respectively. After extensive washing and incubation with HRP-conjugated anti-rabbit IgG in PBS containing 0.1% Tween 20, 1% NGS and 0.5% BSA for 2 h at room temperature (RT), signals were developed with a chemiluminescence kit (SuperSignal® West Pico; Thermo Fisher Scientific) and read on a C-DiGit® Blot Scanner (LI-COR, Lincoln, NE, USA). To quantify the phosphorylation of ERK1/2 and BDNF, the membranes were reprobed with an antibody to total ERK1/2 (1:1,000 dilution) and β-actin (1:10,000 dilution), respectively. Several exposure times were used to obtain signals in the linear range. The bands were quantified using the Scion Image software (Scion, Frederick, MD, USA).
2.7. Immunohistochemistry on free-floating sections
First, endogenous peroxidase activity in the free-floating sections was blocked with 0.3% (v/v) hydrogen peroxide in distilled water for 20 min, and the sections were then blocked with 5% (v/v) NGS in 0.3% (v/v) Triton X-100 for 1 h at RT. Next, sections were incubated with rabbit anti-phospho-ERK1/2 [Thr202/Tyr204] (1:400 dilution) primary antibody in antibody dilution buffer (Invitrogen) for 1 day at 4°C. After washing, sections were reacted with biotinylated goat anti-rabbit IgG (Vector ABC Elite Kit; Vector Laboratories) for 1 h at RT, washed, and incubated for 1 h at RT with an avidin-biotin peroxidase complex (Vector ABC Elite Kit), prepared according to the manufacturer's instructions. After washing, the peroxidase reaction was initiated using a diaminobenzidine substrate (contained in the DAB kit; Vector Labs.), prepared according to the manufacturer's instructions. As a control, the primary antibody was omitted. Immunohistochemically stained specimens were examined using a BX-40 instrument (Olympus, Tokyo, Japan) fitted with an eXcope X3 digital camera (DIXI Optics, Daejeon, South Korea).
The intensity of hippocampal phospho-ERK1/2 expression was measured with the aid of the ImageJ software (NIH, Bethesda, MD, USA). Two non-overlapping sections (approximately 240 μm apart) were chosen from each animal and the intensities in subregions (e.g., granular cell layer [GCL]/SGZ, molecular layer [ML], mossy fiber [MF] and cornu ammonis [CA] 1) were measured. The photomicrographs were changed to 8-bit and the threshold was adjusted on every tissue for background subtraction. The intensities were digitized into levels 0-255. All measurements were performed by the same individual who was blinded to the experimental conditions. The mean intensity of the two-three sections of each mouse was assigned the value of 1, and the levels of intensity were calculated as means ± standard errors (SEs) (n = 5 mice/group).
2.8. Double immunofluorescence staining
Co-localization of phospho-ERK with cellular markers (i.e., DCX, nestin, GFAP, NeuN, and CD68) was examined using double immunofluorescence labeling on free-floating sections 2 days after TMT treatment. Briefly, sections were deactivated by the action of endogenous peroxidase for 20 min and blocked with 5% (v/v) NGS (Vector ABC Elite Kit) in 0.3% (v/v) Triton X-100 for 1 h. Next, sections were incubated with primary antibodies (rabbit anti-phospho-ERK1/2 [Thr202/Tyr204] [1:100 dilution], goat anti-DCX [1:15,000 dilution], mouse anti-nestin [1:400 dilution], mouse anti-GFAP [1:800 dilution], mouse anti-NeuN [1:200 dilution], and rat anti-CD68 [1:400 dilution]) overnight at 4°C. After washing, bound primary antibodies were tagged with the appropriate secondary antibodies (FITC-labeled anti-rabbit IgG, mouse IgG or goat IgG, and TRITC-labeled anti-rabbit IgG, mouse IgG, or rat IgG) for 1.5 h at RT. After washing, the sections were counterstained with 4’,6-diamidino-2-phenylindole·2HCl (DAPI; Thermo Fisher Scientific) and mounted. The double immunofluorescence-stained specimens were observed using a LSM 5 Pascal laser scanning confocal microscopy (Carl Zeiss, Oberkochen, Germany).
2.9. Primary hippocampal cell culture and drug treatment
The primary hippocampal cell culture method has been previously described (Kim et al., 2013; Yang et al., 2011). Briefly, hippocampi were dissected from C57BL/6J mice pups at postnatal day 1, and prepared for culturing. After dissection, tissues were chopped and digested with 10 units/mL papain (Worthington Biochemical, Lakewood, NJ, USA) and 100 units/mL DNase I (Roche, Basel, Switzerland) in dissociation buffer at 37°C for 30 min. The digestion was triturated with Neurobasal A medium (Invitrogen). The cells were seeded at a density of 0.4×106 cells/well on poly-D-lysine hydrobromide (150 μg/mL; Sigma-Aldrich)-coated 24-well plates (Nunc; Thermo Fisher Scientific). Neurobasal A was replaced 1 h after plating with growth Neurobasal A medium, including 1× B27 supplement (Invitrogen), 100 units/mL penicillin, 0.1 mg/mL streptomycin, and 0.5 mM glutamine (Invitrogen). All of the cultures were maintained at 37°C and 5% CO2. To examine the inhibitory effect of the ERK signaling pathway, U0126 (Cell Signaling Technology; 10 μM) or PD98059 (Sigma-Aldrich; 20 μM) dissolved in dimethyl sulfoxide (DMSO) was added 1 h before TMT treatment (10 μM) at 4 days in vitro (DIV). The final concentration of DMSO did not exceed 0.2%. To evaluate the effects of BDNF on TMT-treated hippocampal neurons (4 DIV), BDNF (Sigma-Aldrich; 10 ng/mL) was added 30 min before TMT treatment. BDNF and TMT were dissolved in sterile 0.9% (w/v) saline. All of the cultures were assayed or collected 24 h after TMT treatment, and cells were observed under a phase-contrast microscope (DM IRB; Leica Microsystems).
2.10. Cytotoxicity evaluation
Cytotoxicity in hippocampal-cultured neurons was evaluated using a lactate dehydrogenase (LDH) release assay (n = 4–6 cultures/condition). A commercially available LDH cytotoxicity assay kit from Biovision (Mountain View, CA, USA) was used as recommended by the manufacturer. The optical densities at 450 nm were determined using a microplate reader (Emax, Molecular Devices, Crawley, UK).
2.11. Statistical analysis
All of the data are reported as means ± SEs. After TMT injection, clinical scores relative to those of vehicle-treated controls and a peak stage of seizure score were analyzed by one-way repeated measures analysis of variance (Ivanova and Beyer) followed by pair-wise comparisons. For in vivo biochemical analyses, statistically significant differences between the vehicle-treated and TMT-treated groups were determined using two-tailed Student's t-tests. For the in vitro study, the data were analyzed by one-way ANOVA followed by the Student-Newman-Keuls post hoc test for multiple comparisons. In all of the analyses, p < 0.05 was considered to be statistically significant.
3. Results
3.1. Changes in seizure behaviors and histological findings in the hippocampi of TMT-treated mice
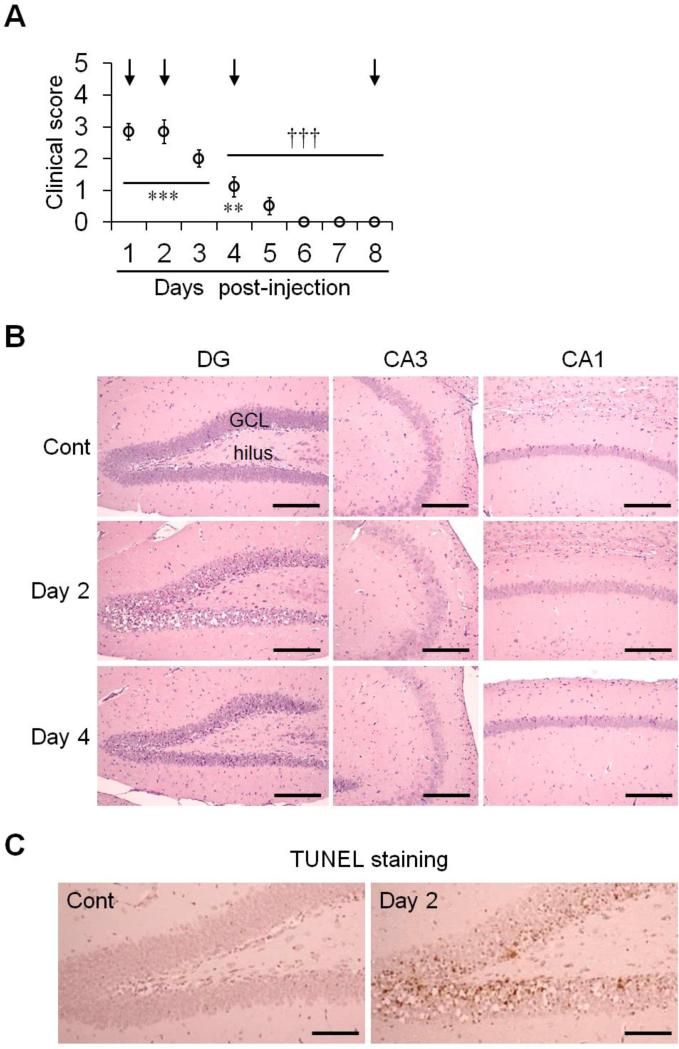
We identified and recorded the clinical symptoms, and followed histopathological changes daily for 8 days after TMT injection in mice. Seizures developed rapidly on day 1 after TMT treatment, peaked on day 2, and then decreased (Fig. 1A, n = 10 mice/group), consistent with data from our previous studies on TMT treatment in mice (Kim et al., 2013; Kim et al., 2014a; Kim et al., 2014b; Lee et al., 2014). Clinical symptoms in the recovery stage (days 4–8 post-injection) were significantly decreased compared to the peak stage (day 2 post-injection; Fig. 1A). Histopathological analysis was performed using hematoxylin and eosin (H&E) staining on days 1, 2, 4, and 8 after TMT treatment. Under low magnification, no significant change in general hippocampal structure was detected in vehicle-treated or test animals (data not shown). Under high magnification, the CA1 and CA3 did not show prominent histopathological changes. However, eosinophilic cytoplasm, nuclear pyknosis, and nuclear karyolysis were found in the GCL of the DG following TMT treatment. Thus, TMT treatment induced selective neuronal cell death/apoptosis in the mouse hippocampal DG. These pathological changes were conspicuous, and marked cell loss was observed on day 2. However, the lesions were attenuated by day 4, although changes were still identifiable (Fig. 1B), and histologically recovered by day 8 (data not shown). Furthermore, we used TUNEL staining to confirm TMT-induced apoptotic neuronal cell death. Significant TUNEL staining was observed in the GCL of the DG on day 2 following TMT treatment (Fig. 1C). These results demonstrate a correlation between spontaneous recovery of seizure behaviors and the restoration of the histopathological lesions in the hippocampus.
Fig. 1.
Clinical seizure scores and histological findings in the hippocampus of mice treated with TMT. (A) Clinical symptoms were scored (0–5) for 8 days after TMT injection. Mice were sacrificed at the times indicated by the arrows. The data are reported as the means ± SEs (n = 10 per group). ** p < 0.01, *** p < 0.001, vs. vehicle-treated controls; ††† p < 0.001, vs. peak stage of seizure scores (day 2 post-injection). (B) Histopathological analysis was performed using H&E staining at 2 and 4 days post-treatment. (C) Apoptotic neuronal cell death was determined by TUNEL staining in the hippocampus 2 days post-treatment. DG, dentate gyrus; CA, cornu ammonis; GCL, granular cell layer; Cont, controls. Scale bars represent 180 μm (B) and 130 μm (C).
3.2. Increased expression of BDNF and TrkB in the hippocampus of TMT-treated mice
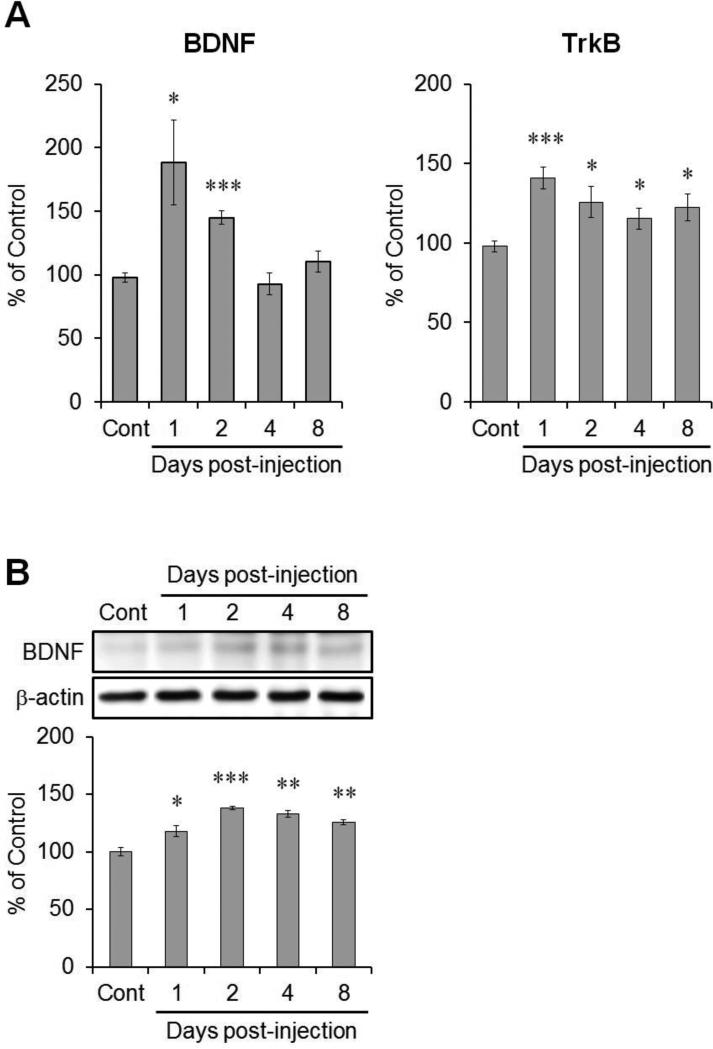
To determine changes in BDNF/TrkB signaling in the mouse hippocampus following TMT injection, we investigated the levels of mRNA encoding BDNF and TrkB in the hippocampus. BDNF mRNA levels increased significantly 1 and 2 days post-treatment (188% and 145% versus the control value, respectively), and then returned to control levels (Fig. 2A, left, n = 7 mice/group). Levels of TrkB mRNA increased significantly 1–8 days post-treatment (141%, 126%, 115%, and 122% versus the control value, respectively; Fig. 2A, right, n = 7 mice/group).
Fig. 2.
Changes in BDNF and TrkB expression in the hippocampus following TMT injection. (A) The bar graphs show levels of mRNA encoding BDNF and its receptor, TrkB (n = 7 per group). (B) Representative immunoblot images of BDNF in the hippocampus post-treatment. Bar graphs show a significant increase in BDNF expression in the hippocampus 1–8 days post-treatment (n = 3 per group). β-actin was used for normalization. The data are reported as the means ± SEs. * p < 0.05, ** p < 0.01, *** p < 0.001, vs. vehicle-treated controls. Cont, controls.
Changes in BDNF protein levels were examined in the hippocampus following TMT injection by Western blot analysis (n = 3 mice/group). Levels were increased significantly at days 1–8 post-injection (118%, 138%, 133%, and 125% versus the control value, respectively; Fig. 2B).
3.3. Spatiotemporal expression of phosphorylated ERK1/2 after TMT treatment
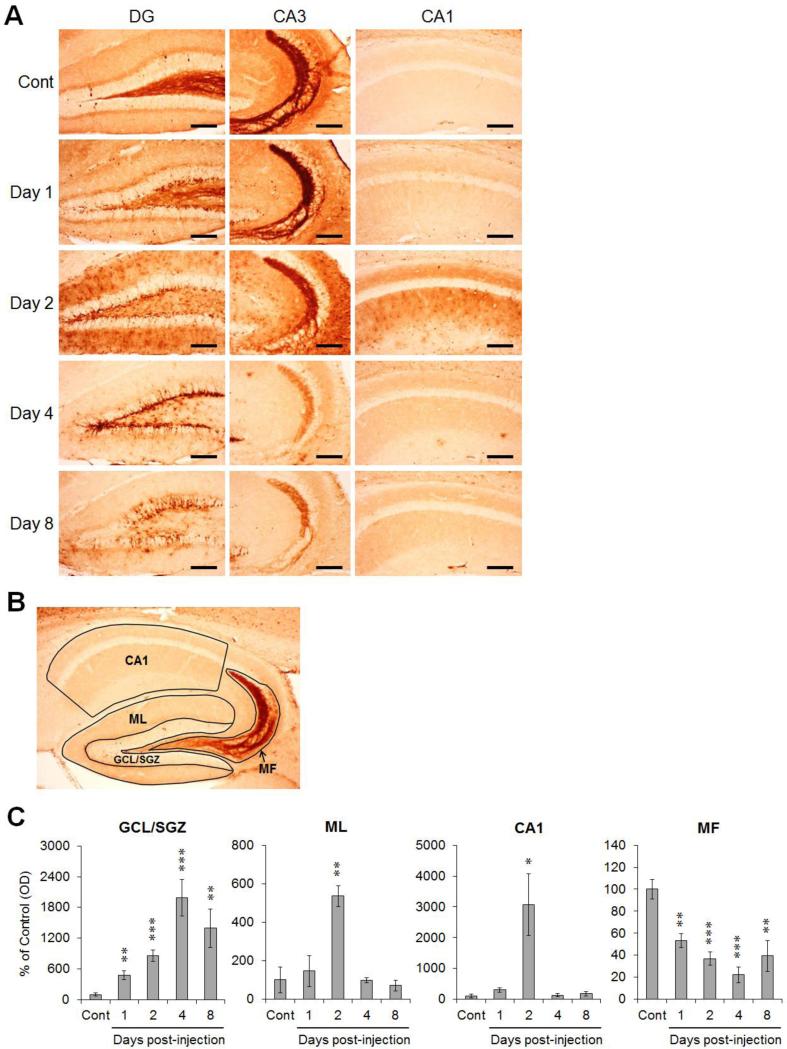
We confirmed spatiotemporal changes in the phosphorylated form of ERK1/2 in TMT-treated hippocampal subregions by immunohistochemistry. Figure 3A shows representative photomicrographs for the expression of phospho-ERK1/2 in TMT-treated mouse hippocampus. In vehicle-treated controls, we observed a few immunoreactivities in both dendrites and cell bodies along the GCL of the DG, and immunostained suprapyramidal and intra/infrapyramidal MF in the CA3. In TMT-treated mice, the markedly enhanced staining for phospho-ERK1/2 in the GCL/SGZ was confirmed on day 1-8, and the immunoreactivity in the ML of DG and the stratum radiatum of the CA1 was conspicuously increased on day 2. Whereas the upregulation in the DG and CA1, we observed that the immunoreactivities of phospho-ERK1/2 in the MF of the hilus and CA3 faded at days 1-8 post-injection. However, we identified that the disappeared immunoreactivity in intra/infra-pyramidal MF on day 4 was slightly turned up on day 8 after TMT treatment.
Fig. 3.
Spatiotemporal pattern of ERK1/2 activation in the hippocampus following TMT injection. (A) Representative photomicrographs of phospho-ERK1/2 immunoreactivity show regionally variable expression of phospho-ERK1/2 in the hippocampus. (B) Schematic diagram showing the hippocampal subdivision for intensity measurement (GCL/SGZ, ML, MF and CA1). (C) Intensities of phospho-ERK1/2 expression in the hippocampal subregions following TMT treatment. Bar graphs show a significant increase of ERK1/2 activation in the GCL/SGZ, ML and CA1, and a substantial reduction of the expression in the MF after TMT treatment. The data are reported as the means ± SEs (n = 5 per group). * p < 0.05, ** p < 0.01, *** p < 0.001, vs. vehicle-treated controls. DG, dentate gyrus; CA, cornu ammonis; Cont, controls; GCL, granular cell layer; SGZ, subgranular zone; ML, molecular layer; MF, mossy fiber. Scale bars represent 120 μm (A).
Then, we assessed the intensity of phospho-ERK1/2 in each hippocampal subregions (GCL/SGZ, ML, CA1 and MF) according to the division as shown in Fig. 3B (Fig. 3C, n = 5 mice/group). The intensity in GCL/SGZ significantly increased at 1-8 days and peaked at 4 days post-treatment (approximately 477%, 857%, 1988%, and 1393% versus the control value, respectively). The intensities in ML and CA1 reached their peaks at 2 days post-treatment (approximately 535% and 3072% versus the control value, respectively). The upregulation of phospho-ERK1/2 in the GCL/SGZ, ML and CA1 subregions was distinctly observed, whereas the intensity for MF significantly decreased at 1-8 days post-treatment (approximately 53%, 37%, 22%, and 39% versus the control value, respectively).
3.4. TMT-induced phospho-ERK1/2 elevation was mostly restricted to immature neurons and NSCs/NPCs rather than mature neurons and glia
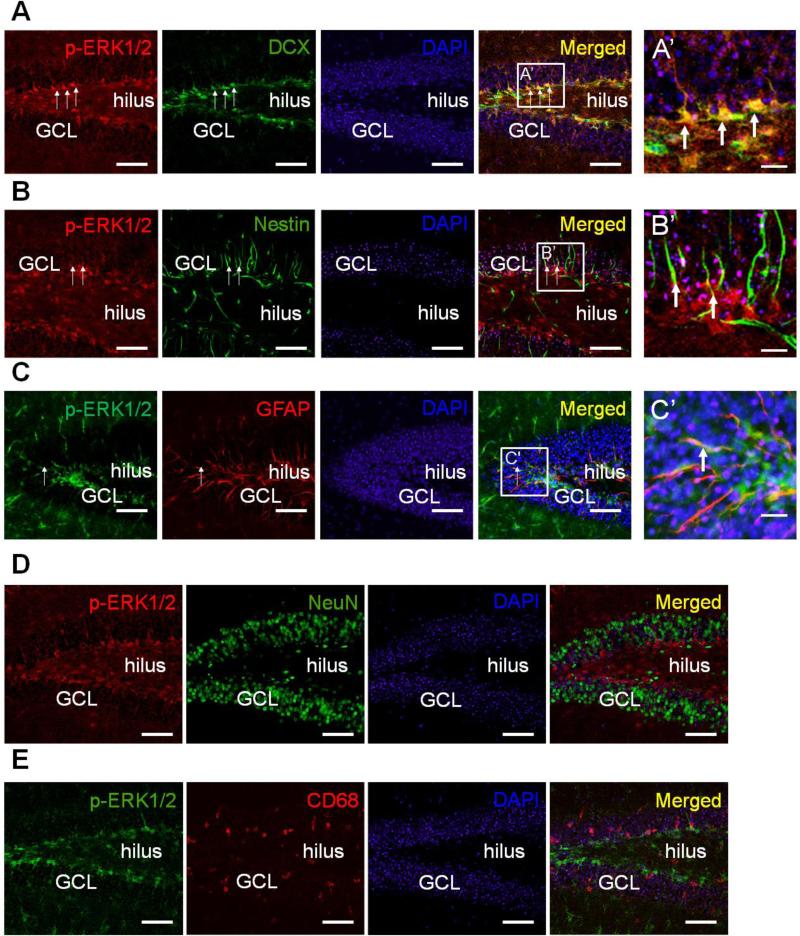
To confirm the location of phospho-ERK1/2 in the hippocampi of TMT-treated mice, we performed double immunofluorescence staining for phospho-ERK1/2 and DCX (marker of immature progenitor neurons), nestin (marker of NSCs), GFAP (marker of NPCs)(Liu et al., 2010), NeuN (marker of mature neurons), or CD68 (marker of activated microglia) 2 days post-treatment, when the neuropathological lesions were severe in GCL/SGZ (Fig. 4). Figure 4A–C show that phospho-ERK1/2-expressing cells in the GCL/SGZ of the DG co-expressed DCX, nestin and GFAP (arrows in right panels). Most phospho-ERK1/2-positive cells co-expressed DCX as cell somae in the SGZ (Fig. 4A). A few phospho-ERK1/2-positive cells localized in nestin- or GFAP-positive cells, and the form of the double-positive cells was detected as processes in the GCL (Fig. 4B and C). However, phospho-ERK1/2 was not expressed in NeuN-positive or CD68-positive cells in the DG (Fig. 4D and E).
Fig. 4.
Representative photomicrographs of double immunofluorescent staining for phospho-ERK1/2 and either DCX, nestin, GFAP, NeuN, or CD68 in the hippocampi of TMT-treated mice on day 2. (A) Double immunostaining for phospho-ERK1/2 (red) and DCX (green) in the DG. (B) Double immunostaining for phospho-ERK1/2 (red) and nestin (green) in the DG. (C) Double immunostaining for phospho-ERK1/2 (green) and GFAP (red) in the DG. The squares indicate magnified regions in the merged photos, and co-expressing cells with phospho-ERK1/2 and each cellular marker are indicated by arrows under low and high magnifications (A’-C’). (D) Double immunostaining for phospho-ERK1/2 (red) and NeuN (green) in the DG. (E) Double immunostaining for phospho-ERK1/2 (green) and CD68 (red) in the DG. Cell nuclei were counterstained with DAPI (blue). GCL, granular cell layer. Scale bars represent 90 μm and 20 μm under low and high magnification, respectively.
3.5. BDNF treatment reduces TMT-induced toxicity in cultured immature hippocampal neurons
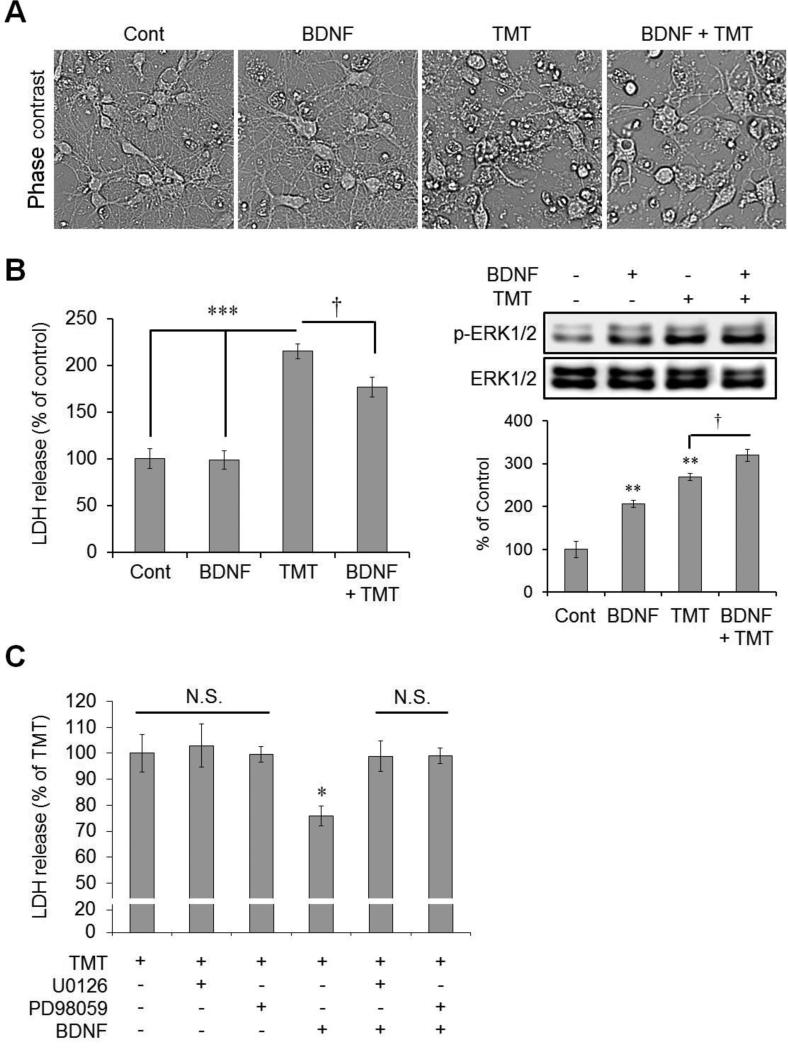
After confirming that endogenous BDNF was increased and expressed similarly to the temporal pattern of ERK in TMT-treated mice, we assessed the effect of BDNF treatment in TMT-treated hippocampal-cultured immature neurons. In phase-contrast images, we confirmed that BDNF treatment reduced TMT-induced cell death (Fig. 5A). Consistent with the phase-contrast images, data from the LDH release assay revealed that TMT treatment significantly increased LDH release 24 h post-treatment (215% versus the control value and 218% versus the BDNF-treated value), and BDNF treatment protected against TMT-induced cell death (LDH release decreased to 177% versus the control value) (Fig. 5B, left, n = 6 cultures/condition). Next, we confirmed changes in the expression of phospho-ERK1/2 by Western blotting 24 h after drug treatment (Fig. 5B, right). Each BDNF and TMT treatment markedly increased the expression of phospho-ERK1/2 compared to the controls (BDNF alone and TMT alone: 206% and 269% versus the control value, respectively). Treatment with both significantly increased expression compared to the TMT alone-treated group (319% versus the control value; Fig. 5B, right, n = 3 cultures/condition). Figure 5C shows that the pre-treatment with MEK1/2 inhibitors, U0126 and PD98059, significantly reduced the protective effect of BDNF in TMT-treated neurons (LDH release values 24 h after TMT treatment were as follows: 76% in the group treated with TMT and BDNF; 99% in the group treated with TMT, U0126 and BDNF; 99% that of the TMT-treated condition value in the group treated with TMT, PD98059, and BDNF) (n = 4 cultures/condition).
Fig. 5.
Cytoprotective effects of BDNF via ERK activation in hippocampal neurons against TMT-induced neurotoxicity. (A) Representative phase-contrast photomicrographs in immature hippocampal neurons 24 h after TMT treatment. BDNF (10 ng/mL) was pre-treated 30 min before TMT (10 μM) treatment at 4 DIV. (B) Left bar graphs show analysis of relative LDH levels following either BDNF (10 ng/mL) or TMT (10 μM) treatment at 4 DIV (n = 6 per group). LDH release assay was performed 24 h after TMT treatment. † p < 0.05, *** p < 0.001, vs. groups indicated by the bars. Right panels show the immunoblotting results for p-ERK1/2 and ERK1/2 in immature hippocampal neurons after BDNF or TMT treatment (n = 3 per group). The cells were collected for Western blot analysis 24 h after TMT treatment. ** p < 0.01, vs. vehicle-treated controls; † p < 0.05, vs. groups indicated by the bar. (C) Bar graphs show that MEK1/2 inhibitors (U0126 and PD98059) diminished the cytoprotective effect of BDNF against TMT-induced neurotoxicity significantly (n = 4 per group). Either U0126 (10 μM) or PD98059 (20 μM) was pre-treated 1 h before and BDNF (10 ng/mL) was pre-treated 30 min before TMT (10 μM) treatment at 4 DIV. LDH release assay was performed 24 h after TMT treatment. N.S., not significant; * p < 0.05 vs. the five other groups. The data are reported as the means ± SEs. Cont, controls.
4. Discussion
TMT is a neurotoxic reagent that damages the mammalian limbic system. Neurodegeneration from TMT toxicity results from complex events, including neuroinflammation, glutamate excitotoxicity, intracellular calcium overload, oxidative stress, mitochondrial dysfunction, and impaired neurotransmission (Corvino et al., 2013; Doctor et al., 1982; Geloso et al., 2011; Kim da and Kim, 2015; Naalsund et al., 1985; Piacentini et al., 2008). Mice exposed to TMT exhibit seizure behaviors, but these symptoms resolve spontaneously (Kim et al., 2013). Consistently, TMT induces neuronal degeneration, but the lesion subsequently recovers, and it has been shown that newborn neurons replace the damaged ones in the SGZ (Harry et al., 2004; Ogita et al., 2005). After TMT injection, the course of clinical symptoms in mice is consistent with the histopathological changes in the mouse hippocampus (Lee et al., 2014). This study further showed that the TMT-induced injury was most severe on day 2 in the hippocampal DGs, but the lesions were alleviated by 4 days post-injection; the severity of cell loss and the number of TUNEL-positive cells remarkably decreased in the DG at 4 days post-injection, consistent with the clinical symptoms.
Biological processes regulated by ERK1/2 include cellular transcription and translation, cell cycle progression, proliferation and differentiation (Garcia et al., 2002; Zhou et al., 2009). In some brain injuries, ERK activation leads to cell death (Alessandrini et al., 1999; Bhat and Zhang, 1999; Kulich and Chu, 2001; Rapoport and Ferreira, 2000). Conversely, several studies have shown that the ERK1/2 pathway is required for neuroprotection (Hossain et al., 2013; Sanchez et al., 2012). In a model of TMT-induced neurodegeneration, Casalbore et al. (2010) showed that phosphatidylinositol 3-kinase (PI3K)/Akt and ERK1/2 activation, triggered by BDNF overexpression, attenuated the TMT-induced neurotoxicity in NSCs. In contrast, a recent study by Qing et al. (2013) reported that pre-treatment with the MEK-ERK1/2 inhibitor U0126 reduced TMT-induced apoptosis in human neuroblastoma SY5Y cells via upregulation of survival factors, Bcl-2 and XIAP, suggesting that ERK signaling may play a role in neuronal cell death. In the present study, we observed that ERK1/2 was markedly activated in the mouse hippocampus following TMT treatment, although the temporal expression tendency was differently observed within each subregions of the hippocampus. Here, we suggest that the ERK pathway may play an important role in TMT-induced neurodegeneration.
Although various extracellular cues can activate the ERK pathway, we focused on the BDNF/TrkB interaction, as a potential survival signaling activating ERK in TMT-induced toxicity. BDNF is a member of the neurotrophin family and plays a role in the proliferation and differentiation of NSCs/NPCs (Islam et al., 2009) and the survival of immature neurons through its receptor, TrkB or p75 (Jones et al., 1994). BDNF levels are very low in the normal adult brain (Matsumoto et al., 2008), but, the levels increase in some brain injuries (Choi et al., 2008; Sathanoori et al., 2004). The main cell sources for increasing BDNF following injuries may be glial cells (Dougherty et al., 2000), and BDNF may act in an autocrine-paracrine fashion (Schwartz et al., 1997). Increasing the BDNF/TrkB interaction could stimulate downstream signaling events, such as the PI3K/Akt and ERK pathways (Nakazawa et al., 2002). The present study showed that the levels of mRNA encoding BDNF and TrkB and protein levels of BDNF increased significantly in the TMT-treated hippocampus. The temporal changes in BDNF and TrkB were similar to the expression pattern of phospho-ERK1/2. Furthermore, our in vitro study showed that treatment with exogenous BDNF reduced TMT-induced neuronal damages in immature hippocampal neurons. BDNF-mediated neuronal protection depended on ERK1/2 activity, because treatment with the MEK1/2 inhibitors U0126 and PD98059 significantly reduced the rescuing effects of BDNF on TMT-induced hippocampal neuronal death. Thus, we suggest that ERK activation could have a protective effect in TMT-induced hippocampal neurodegeneration. Then, the ERK pathway, activated by increased endogenous BDNF in brain injuries, could stimulate downstream kinases and transcription factors for neuroprotection.
ERK1/2 activation can be differently produced in the same cell as well as in different cell types, depending on the duration of the signal, the subcellular localization of the components of the ERK cascade, signals provided by other pathways, and the cellular energetic state (Hetman and Gozdz, 2004; Marshall, 1995). Mebratu and Tesfaigzi (2009) reported that nuclear ERK1/2 promoted cellular survival via activation of multiple transcription factors. In the present study, TMT toxicity activated ERK, and the increased ERK1/2 expression was localized in the DCX-positive surviving immature progenitor neurons in the SGZ and a few dendritic branches of nestin and GFAP-positive NSCs/NPCs in the GCL of hippocampal DGs. GFAP is also thought as an astrocyte marker, however, in the current study, the morphology of GFAP in ERK-expressing cells is a radial glia in the GCL. In an epilepsy model, most phospho-ERK labeling was expressed in nestin and GFAP-positive NSCs/NPCs, and some was expressed in NeuroD-positive cells (later-stage NPCs, indicating neuronal identity (Pleasure et al., 2000)), but not in DCX or NeuN-positive cells, at the onset of pilocarpine-induced seizures (Li et al., 2010). However, Houser et al. (2008) showed that phospho-ERK was expressed in numerous NeuN-positive mature neurons at slightly longer intervals after pilocarpine-induced seizures. In addition, Matsumoto et al. (2006) suggested that sodium orthovanadate may promote the proliferation of progenitor cells after focal cerebral ischemia, showing ERK1/2 was activated in DCX-positive immature progenitor neurons. Thus, ERK expression at different stages of neurogenesis among the studies may be due to variation in the characteristics of each neurodegenerative model and the time intervals for sacrifice after drug treatment. Consequently, we suggest that the ERK pathway may play important roles in differentiating immature neurons as well as NSCs/NPCs against chemical-induced hippocampal neurotoxicity, and may further act in the mechanism of spontaneous recovery from TMT toxicity.
The present study also showed that phospho-ERK1/2 immunoreactivity in the MF faded 1-8 days post-treatment. The intensity value on day 8 was increased compared to that on day 4 in the MF, and we confirmed that the immunoreactivity in intra/infrapyramidal MF was slightly recovered on day 8 after TMT toxicity. MF connecting the DG with the CA3 subregion is able to rapidly alter their neurochemical content in diverse ways (Jaffe and Gutierrez, 2007). In MF axon terminals, ERK activation contributes to regulating the distribution and recycling of synaptic vesicles in neurons, thereby supporting a role in synapse rearrangement and synaptic plasticity (Schenk et al., 2005; Vara et al., 2009). Here, the change in ERK activation in the MF of the DG following TMT exposure suggests two possibilities. First, TMT-induced zinc depletion could reduce ERK activation in the MF. Under normal states, MF terminals show high levels of zinc and ERK activation, and synaptic vesicular zinc modulates presynaptic ERK activation (Sindreu et al., 2011); however, the depletion of zinc in the MF is caused by TMT toxicity (Chang, 1987). Thus, depletion of zinc in MF following TMT exposure may result in the decrease in ERK activation, and the spontaneous recovery process could restore the zinc concentration, followed by ERK activation. Second, MF sprouting is a process of synaptic rearrangement and a common neuropathological finding in limbic seizure-related disorders (e.g., epilepsy), and it occurs for weeks after recovery from seizures (Berkeley et al., 2002; Dudek and Shao, 2004). During TMT toxicity, MF connections between the DG and CA3 might be lost during the stage of TMT-induced DG granular cell injury. However, in recovery from TMT-induced seizures and neuronal injury, MF outgrowth from newly born granule cells would occur, and the connection from granule cells of DG to CA3 would rebuild and be rearranged. However, additional work is required to examine the precise morphological changes in the MF and the related mechanisms in the DG following TMT exposure.
5. Conclusion
In the present study, we investigated the mechanisms underlying the spontaneous recovery after TMT toxicity. The temporal profile of ERK1/2 activation was similar to the expression patterns of BDNF/TrkB, and activated ERK1/2 was expressed in NSCs/NPCs and immature progenitor neurons in TMT-treated hippocampal DGs. In hippocampal-cultured neurons, exogenous BDNF alleviated TMT-induced toxicity through activation of ERK signaling. These evidences suggest that BDNF/ERK signaling in NSCs/NPCs and immature progenitor neurons might play important roles in the spontaneous recovery from TMT-induced hippocampal neurodegeneration, by promoting cell differentiation and survival, and an understanding of the cellular signalings may clinically provide a therapeutic approach for neurodegeneration including TMT-induced seizure.
Highlights.
Trimethyltin (TMT) increased ERK1/2 activation in the mouse hippocampus.
BDNF/TrkB expression was similar to that of ERK1/2 activation after TMT treatment.
ERK1/2 was mainly activated in immature progenitor neurons in TMT-treated hippocampal DGs.
Exogenous BDNF alleviated TMT-induced in vitro toxicity via the ERK activation.
BDNF/ERK signaling may play roles in spontaneous recovery from TMT-induced toxicity.
Acknowledgments
The animal experiment in this study was supported by the Animal Medical Institute of Chonnam National University. This work was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (NRF-2012R1A1B4001262 to CM) and NIH grant, U.S.A. (R01MH093445 to HW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J. Biol. Chem. 1999;274:5038–5046. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- Alessandrini A, Namura S, Moskowitz MA, Bonventre JV. MEK1 protein kinase inhibition protects against damage resulting from focal cerebral ischemia. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12866–12869. doi: 10.1073/pnas.96.22.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J. Neurochem. 2002;82:192–201. doi: 10.1046/j.1471-4159.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- Besser R, Kramer G, Thumler R, Bohl J, Gutmann L, Hopf HC. Acute trimethyltin limbic-cerebellar syndrome. Neurology. 1987;37:945–950. doi: 10.1212/wnl.37.6.945. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P. Hydrogen peroxide activation of multiple mitogen-activated protein kinases in an oligodendrocyte cell line: role of extracellular signal-regulated kinase in hydrogen peroxide-induced cell death. J. Neurochem. 1999;72:112–119. doi: 10.1046/j.1471-4159.1999.0720112.x. [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Yu TS, Zhang G, Chen J, Dimchev G, Parada LF, Powell CM, Kernie SG. Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J. Neurosci. 2011;31:4906–4916. doi: 10.1523/JNEUROSCI.5265-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casalbore P, Barone I, Felsani A, D'Agnano I, Michetti F, Maira G, Cenciarelli C. Neural stem cells modified to express BDNF antagonize trimethyltin-induced neurotoxicity through PI3K/Akt and MAP kinase pathways. J. Cell Physiol. 2010;224:710–721. doi: 10.1002/jcp.22170. [DOI] [PubMed] [Google Scholar]
- Chang LW. Possible pathogenic mechanisms on trimethyltin-induced lesions in the hippocampus of adult and neonatal rats : An overview. Biol. Trace Elem. Res. 1987;13:77–88. doi: 10.1007/BF02796623. [DOI] [PubMed] [Google Scholar]
- Choi YS, Cho HY, Hoyt KR, Naegele JR, Obrietan K. IGF-1 receptor-mediated ERK/MAPK signaling couples status epilepticus to progenitor cell proliferation in the subgranular layer of the dentate gyrus. Glia. 2008;56:791–800. doi: 10.1002/glia.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvino V, Marchese E, Michetti F, Geloso MC. Neuroprotective strategies in hippocampal neurodegeneration induced by the neurotoxicant trimethyltin. Neurochem. Res. 2013;38:240–253. doi: 10.1007/s11064-012-0932-9. [DOI] [PubMed] [Google Scholar]
- Doctor SV, Costa LG, Kendall DA, Murphy SD. Trimethyltin inhibits uptake of neurotransmitters into mouse forebrain synaptosomes. Toxicology. 1982;25:213–221. doi: 10.1016/0300-483x(82)90031-2. [DOI] [PubMed] [Google Scholar]
- Dougherty KD, Dreyfus CF, Black IB. Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol. Dis. 2000;7:574–585. doi: 10.1006/nbdi.2000.0318. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Shao LR. Mossy fiber sprouting and recurrent excitation: direct electrophysiologic evidence and potential implications. Epilepsy Curr. 2004;4:184–187. doi: 10.1111/j.1535-7597.2004.04507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizi C, Pompili E, De Vito S, Somma F, Catizone A, Ricci G, Lenzi P, Fornai F, Fumagalli L. Impairment of the autophagic flux in astrocytes intoxicated by trimethyltin. Neurotoxicology. 2015;52:12–22. doi: 10.1016/j.neuro.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz A, Figiel I, Kaminska B, Zaremba M, Wilk S, Oderfeld-Nowak B. Dentate granule neuron apoptosis and glia activation in murine hippocampus induced by trimethyltin exposure. Brain Res. 2001;912:116–127. doi: 10.1016/s0006-8993(01)02675-0. [DOI] [PubMed] [Google Scholar]
- Garcia J, Ye Y, Arranz V, Letourneux C, Pezeron G, Porteu F. IEX-1: a new ERK substrate involved in both ERK survival activity and ERK activation. EMBO J. 2002;21:5151–5163. doi: 10.1093/emboj/cdf488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geloso MC, Corvino V, Michetti F. Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem. Int. 2011;58:729–738. doi: 10.1016/j.neuint.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr. Opin. Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- Harry GJ, McPherson CA, Wine RN, Atkinson K, Lefebvre d'Hellencourt C. Trimethyltin-induced neurogenesis in the murine hippocampus. Neurotox. Res. 2004;5:623–627. doi: 10.1007/BF03033182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetman M, Gozdz A. Role of extracellular signal regulated kinases 1 and 2 in neuronal survival. Eur. J. Biochem. 2004;271:2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- Hossain MS, Ifuku M, Take S, Kawamura J, Miake K, Katafuchi T. Plasmalogens rescue neuronal cell death through an activation of AKT and ERK survival signaling. PLoS One. 2013;8:e83508. doi: 10.1371/journal.pone.0083508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Huang CS, Peng Z. Dynamic seizure-related changes in extracellular signal-regulated kinase activation in a mouse model of temporal lobe epilepsy. Neuroscience. 2008;156:222–237. doi: 10.1016/j.neuroscience.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam O, Loo TX, Heese K. Brain-derived neurotrophic factor (BDNF) has proliferative effects on neural stem cells through the truncated TRK-B receptor, MAP kinase, AKT, and STAT-3 signaling pathways. Curr. Neurovasc. Res. 2009;6:42–53. doi: 10.2174/156720209787466028. [DOI] [PubMed] [Google Scholar]
- Ivanova T, Beyer C. Pre- and postnatal expression of brain-derived neurotrophic factor mRNA/protein and tyrosine protein kinase receptor B mRNA in the mouse hippocampus. Neurosci Lett. 2001;307:21–24. doi: 10.1016/s0304-3940(01)01905-x. [DOI] [PubMed] [Google Scholar]
- Jaffe DB, Gutierrez R. Mossy fiber synaptic transmission: communication from the dentate gyrus to area CA3. Prog. Brain Res. 2007;163:109–132. doi: 10.1016/S0079-6123(07)63006-4. [DOI] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokhlatchev AV, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb MH. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- Kim da J, Kim YS. Trimethyltin-Induced Microglial Activation via NADPH Oxidase and MAPKs Pathway in BV-2 Microglial Cells. Mediators Inflamm. 2015;2015:729509. doi: 10.1155/2015/729509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yang M, Kim SH, Kim JC, Wang H, Shin T, Moon C. Possible role of the glycogen synthase kinase-3 signaling pathway in trimethyltin-induced hippocampal neurodegeneration in mice. PLoS One. 2013;8:e70356. doi: 10.1371/journal.pone.0070356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Yang M, Kim J, Song L, Lee S, Son Y, Kang S, Bae CS, Kim JC, Kim SH, Shin T, Wang H, Moon C. Developmental and degenerative modulation of brain-derived neurotrophic factor transcript variants in the mouse hippocampus. Int. J. Dev. Neurosci. 2014a;38C:68–73. doi: 10.1016/j.ijdevneu.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Kim J, Yang M, Son Y, Jang H, Kim D, Kim JC, Kim SH, Kang MJ, Im HI, Shin T, Moon C. Glial activation with concurrent up-regulation of inflammatory mediators in trimethyltin-induced neurotoxicity in mice. Acta Histochem. 2014b;116:1490–1500. doi: 10.1016/j.acthis.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Kim J, Son Y, Kim J, Lee S, Kang S, Park K, Kim SH, Kim JC, Kim J, Takayama C, Im HI, Yang M, Shin T, Moon C. Developmental and degenerative modulation of GABAergic transmission in the mouse hippocampus. Int. J. Dev. Neurosci. 2015 doi: 10.1016/j.ijdevneu.2015.08.009. 10.1016/j.ijdevneu.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: implications for Parkinson's disease. J. Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannuzel A, Barnier JV, Hery C, Huynh VT, Guibert B, Gray F, Vincent JD, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann. Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- Lee S, Ueno M, Yamashita T. Axonal remodeling for motor recovery after traumatic brain injury requires downregulation of gamma-aminobutyric acid signaling. Cell Death Dis. 2011;2:e133. doi: 10.1038/cddis.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Yang M, Kim J, Kim J, Son Y, Kwon S, Kim SH, Kim JC, Kang SS, Wang H, Shin T, Moon C. Nestin expression and glial response in the hippocampus of mice after trimethyltin treatment. Acta Histochem. 2014;116:1276–1288. doi: 10.1016/j.acthis.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Li Y, Peng Z, Xiao B, Houser CR. Activation of ERK by spontaneous seizures in neural progenitors of the dentate gyrus in a mouse model of epilepsy. Exp. Neurol. 2010;224:133–145. doi: 10.1016/j.expneurol.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Namba T, Liu J, Suzuki R, Shioda S, Seki T. Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus. Neuroscience. 2010;166:241–251. doi: 10.1016/j.neuroscience.2009.12.026. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto J, Morioka M, Hasegawa Y, Kawano T, Yoshinaga Y, Maeda T, Yano S, Kai Y, Fukunaga K, Kuratsu J. Sodium orthovanadate enhances proliferation of progenitor cells in the adult rat subventricular zone after focal cerebral ischemia. J. Pharmacol. Exp. Ther. 2006;318:982–991. doi: 10.1124/jpet.106.104562. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Rauskolb S, Polack M, Klose J, Kolbeck R, Korte M, Barde YA. Biosynthesis and processing of endogenous BDNF: CNS neurons store and secrete BDNF, not pro-BDNF. Nat. Neurosci. 2008;11:131–133. doi: 10.1038/nn2038. [DOI] [PubMed] [Google Scholar]
- Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: Is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem. Sci. 2011;36:320–328. doi: 10.1016/j.tibs.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naalsund LU, Allen CN, Fonnum F. Changes in neurobiological parameters in the hippocampus after exposure to trimethyltin. Neurotoxicology. 1985;6:145–158. [PubMed] [Google Scholar]
- Nakazawa T, Tamai M, Mori N. Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest. Ophthalmol. Vis. Sci. 2002;43:3319–3326. [PubMed] [Google Scholar]
- Ogita K, Nishiyama N, Sugiyama C, Higuchi K, Yoneyama M, Yoneda Y. Regeneration of granule neurons after lesioning of hippocampal dentate gyrus: evaluation using adult mice treated with trimethyltin chloride as a model. J. Neurosci. Res. 2005;82:609–621. doi: 10.1002/jnr.20678. [DOI] [PubMed] [Google Scholar]
- Park SY, Marasini S, Kim GH, Ku T, Choi C, Park MY, Kim EH, Lee YD, Suh-Kim H, Kim SS. A method for generate a mouse model of stroke: evaluation of parameters for blood flow, behavior, and survival. Exp. Neurobiol. 2014;23:104–114. doi: 10.5607/en.2014.23.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini R, Gangitano C, Ceccariglia S, Del Fa A, Azzena GB, Michetti F, Grassi C. Dysregulation of intracellular calcium homeostasis is responsible for neuronal death in an experimental model of selective hippocampal degeneration induced by trimethyltin. J. Neurochem. 2008;105:2109–2121. doi: 10.1111/j.1471-4159.2008.05297.x. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Esposito E, Sirabella R, Vinciguerra A, Cuomo O, Di Renzo G, Annunziato L. nNOS and p-ERK involvement in the neuroprotection exerted by remote postconditioning in rats subjected to transient middle cerebral artery occlusion. Neurobiol. Dis. 2013;54:105–114. doi: 10.1016/j.nbd.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Collins AE, Lowenstein DH. Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J. Neurosci. 2000;20:6095–6105. doi: 10.1523/JNEUROSCI.20-16-06095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y, Liang Y, Du Q, Fan P, Xu H, Xu Y, Shi N. Apoptosis induced by trimethyltin chloride in human neuroblastoma cells SY5Y is regulated by a balance and cross-talk between NF-kappaB and MAPKs signaling pathways. Arch. Toxicol. 2013;87:1273–1285. doi: 10.1007/s00204-013-1021-9. [DOI] [PubMed] [Google Scholar]
- Rapoport M, Ferreira A. PD98059 prevents neurite degeneration induced by fibrillar beta-amyloid in mature hippocampal neurons. J. Neurochem. 2000;74:125–133. doi: 10.1046/j.1471-4159.2000.0740125.x. [DOI] [PubMed] [Google Scholar]
- Sanchez A, Tripathy D, Yin X, Luo J, Martinez J, Grammas P. Pigment epithelium-derived factor (PEDF) protects cortical neurons in vitro from oxidant injury by activation of extracellular signal-regulated kinase (ERK) 1/2 and induction of Bcl-2. Neurosci. Res. 2012;72:1–8. doi: 10.1016/j.neures.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathanoori M, Dias BG, Nair AR, Banerjee SB, Tole S, Vaidya VA. Differential regulation of multiple brain-derived neurotrophic factor transcripts in the postnatal and adult rat hippocampus during development, and in response to kainate administration. Brain Res. Mol. Brain Res. 2004;130:170–177. doi: 10.1016/j.molbrainres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Schenk U, Menna E, Kim T, Passafaro M, Chang S, De Camilli P, Matteoli M. A novel pathway for presynaptic mitogen-activated kinase activation via AMPA receptors. J. Neurosci. 2005;25:1654–1663. doi: 10.1523/JNEUROSCI.3074-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinelli S, Zanassi P, Paolillo M, Wang H, Feliciello A, Gallo V. Stimulation of endothelin B receptors in astrocytes induces cAMP response element-binding protein phosphorylation and c-fos expression via multiple mitogen-activated protein kinase signaling pathways. J. Neurosci. 2001;21:8842–8853. doi: 10.1523/JNEUROSCI.21-22-08842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA. Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu. Rev. Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Sindreu C, Palmiter RD, Storm DR. Zinc transporter ZnT-3 regulates presynaptic Erk1/2 signaling and hippocampus-dependent memory. Proc. Natl. Acad. Sci. U. S. A. 2011;108:3366–3370. doi: 10.1073/pnas.1019166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Slowik A, Rubio F, Szczudlik A, Gaffney J. Activation of MAP kinase (ERK-1/ERK-2), tyrosine kinase and VEGF in the human brain following acute ischaemic stroke. Neuroreport. 2000;11:2759–2764. doi: 10.1097/00001756-200008210-00030. [DOI] [PubMed] [Google Scholar]
- Son Y, Yang M, Kang S, Lee S, Kim J, Kim J, Park S, Kim JS, Jo SK, Jung U, Shin T, Kim SH, Wang H, Moon C. Cranial irradiation regulates CREB-BDNF signaling and variant BDNF transcript levels in the mouse hippocampus. Neurobiol Learn Mem. 2015;121:12–19. doi: 10.1016/j.nlm.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Svaren J, Sevetson BR, Apel ED, Zimonjic DB, Popescu NC, Milbrandt J. NAB2, a corepressor of NGFI-A (Egr-1) and Krox20, is induced by proliferative and differentiative stimuli. Mol. Cell. Biol. 1996;16:3545–3553. doi: 10.1128/mcb.16.7.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol. Cell. Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara H, Onofri F, Benfenati F, Sassoe-Pognetto M, Giustetto M. ERK activation in axonal varicosities modulates presynaptic plasticity in the CA3 region of the hippocampus through synapsin I. Proc. Natl. Acad. Sci. U. S. A. 2009;106:9872–9877. doi: 10.1073/pnas.0900077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veyrac A, Gros A, Bruel-Jungerman E, Rochefort C, Kleine Borgmann FB, Jessberger S, Laroche S. Zif268/egr1 gene controls the selection, maturation and functional integration of adult hippocampal newborn neurons by learning. Proc. Natl. Acad. Sci. U. S. A. 2013;110:7062–7067. doi: 10.1073/pnas.1220558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachter B, Schurger S, Rolinger J, von Ameln-Mayerhofer A, Berg D, Wagner HJ, Kueppers E. Effect of 6-hydroxydopamine (6-OHDA) on proliferation of glial cells in the rat cortex and striatum: evidence for de-differentiation of resident astrocytes. Cell Tissue Res. 2010;342:147–160. doi: 10.1007/s00441-010-1061-x. [DOI] [PubMed] [Google Scholar]
- Wuhanqimuge, Itakura A, Matsuki Y, Tanaka M, Arioka M. Lysophosphatidylcholine enhances NGF-induced MAPK and Akt signals through the extracellular domain of TrkA in PC12 cells. FEBS Open Bio. 2013;3:243–251. doi: 10.1016/j.fob.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Kim JS, Kim J, Kim SH, Kim JC, Kim J, Wang H, Shin T, Moon C. Neurotoxicity of methotrexate to hippocampal cells in vivo and in vitro. Biochem. Pharmacol. 2011;82:72–80. doi: 10.1016/j.bcp.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Yang M, Kim J, Kim T, Kim SH, Kim JC, Kim J, Takayama C, Hayashi A, Joo HG, Shin T, Moon C. Possible involvement of galectin-3 in microglial activation in the hippocampus with trimethyltin treatment. Neurochem. Int. 2012;61:955–962. doi: 10.1016/j.neuint.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Nishiyama N, Shuto M, Sugiyama C, Kawada K, Seko K, Nagashima R, Ogita K. In vivo depletion of endogenous glutathione facilitates trimethyltin-induced neuronal damage in the dentate gyrus of mice by enhancing oxidative stress. Neurochem. Int. 2008;52:761–769. doi: 10.1016/j.neuint.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY. Both ERK and Wnt/beta-catenin pathways are involved in Wnt3a-induced proliferation. J. Cell. Sci. 2005;118:313–322. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]
- Zhou X, Moon C, Zheng F, Luo Y, Soellner D, Nunez JL, Wang H. N-methyl-D-aspartate-stimulated ERK1/2 signaling and the transcriptional up-regulation of plasticity-related genes are developmentally regulated following in vitro neuronal maturation. J. Neurosci. Res. 2009;87:2632–2644. doi: 10.1002/jnr.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]