Abstract
Methamphetamine (MA) is a widely abused, highly addictive, psychostimulant that elicits pronounced deficits in neurocognitive function related to hypo-functioning of the prefrontal cortex (PFC). Our understanding of how repeated methamphetamine impacts excitatory glutamatergic transmission within the PFC is limited, as is information about the relation between PFC glutamate and addiction vulnerability/resiliency. In vivo microdialysis and immunoblotting studies characterized the effects of methamphetamine (10 injections of 2 mg/kg, IP) upon extracellular glutamate in C57BL/6J mice and upon glutamate receptor and transporter expression, within the medial PFC. Glutamatergic correlates of both genetic and idiopathic variance in MA preference/intake were determined through studies of high versus low MA-drinking selectively bred mouse lines (MAHDR versus MALDR, respectively) and inbred C57BL/6J mice exhibiting spontaneously divergent place-conditioning phenotypes. Repeated methamphetamine sensitized drug-induced glutamate release and lowered indices of NMDA receptor expression in C57BL/6J mice, but did not alter basal extracellular glutamate content or total protein expression of Homer proteins, or metabotropic or AMPA glutamate receptors. Elevated basal glutamate, blunted methamphetamine-induced glutamate release and ERK activation, as well as reduced protein expression of mGlu2/3 and Homer2a/b were all correlated biochemical traits of selection for high versus low methamphetamine drinking, and Homer2a/b levels were inversely correlated with the motivational valence of methamphetamine in C57BL/6J mice. These data provide novel evidence that repeated, low-dose, methamphetamine is sufficient to perturb pre- and post-synaptic aspects of glutamate transmission within the medial PFC and that glutamate anomalies within this region may contribute to both genetic and idiopathic variance in methamphetamine addiction vulnerability/resiliency.
Keywords: prefrontal cortex, Homer proteins, metabotropic glutamate receptor, NMDA receptor, addiction vulnerability, methamphetamine, sensitization
INTRODUCTION
Methamphetamine (MA) abuse poses major health and socioeconomic problems, with the United Nations Office on Drugs and Crime (2015) reporting MA as one of the most commonly abused illicit drugs worldwide. Clinically, MA abuse is associated with gross perturbations in executive function and other aspects of cognition governed by the activity of the prefrontal cortex (PFC) (e.g., Barr et al., 2006; Goldstein & Volkow, 2011; Ornstein et al., 2000; Rusyniak, 2011). These deficits correlate with PFC metabolic hypoactivity (e.g., Baicy & London, 2007; Berman et al., 2008; Goldstein & Volkow, 2011; Kim et al., 2005; Nestor et al., 2011; Salo et al., 2009). Although a significant body of evidence supports a role for PFC dopamine signaling anomalies in drug-related deficits (Chang et al., 2007; McCann et al., 2008), hypofrontality in MA addiction may also relate to perturbations in PFC excitatory glutamate transmission, as evidenced by enduring reductions in glutamate/glutamine content in MA-abstinent individuals (Ernst & Chang, 2008). However, it is unclear if glutamatergic abnormalities or neurocognitive/PFC dysfunction in MA-abusing/addicted humans is a precedent or an antecedent of addiction, as clinical studies do not allow disentanglement in a systematic, experimentally-controlled, manner.
MA-associated neurocognitive deficits can be recapitulated in animal models (e.g., Groman et al., 2013; Henry et al., 2010; Parsegian et al., 2011; Scofield et al., 2015), enabling direct examination of cause-effect relations between drug experience and addiction-related neuropsychological outcomes. Furthermore, genetic animal models can be used to identify mechanisms involved in addiction vulnerability/resiliency. As with clinical studies, the majority of basic science research concerning the biobehavioral correlates of MA addiction-related traits has focused on dopamine dysregulation, notably dopamine neurotoxicity within the dorsal striatum (e.g., Groman et al., 2013; Schwendt et al., 2009), and studies employing high-dose, binge-like, MA regimens indicate a critical role for drug-elicited glutamate hyperactivity in neurotoxicity (e.g., Stephans & Yamamoto, 1994,1995). However, acute, subtoxic, MA injection is sufficient to impact extracellular glutamate within both the cell body and terminal regions of excitatory corticostriatal projections (Han et al., 2012; Shoblock et al., 2003) that are highly implicated in addiction neurocircuitry (Koob & Volkow 2010; London et al., 2014). Similarly, a history of intravenous MA self-administration in rats augmented burst firing within PFC glutamate neurons (Parsegian et al., 2011) and elicited enduring changes in extracellular glutamate within both the PFC and ventral striatum (Lominac et al., 2012; Parsegian & See 2014). Thus, enduring PFC glutamate plasticity occurs in both human MA addicts and animal models of MA abuse/addiction warranting deeper consideration as a potential substrate in MA addiction etiology.
These studies aimed to test the hypothesis that low-dose MA exposure is sufficient to elicit enduring changes in glutamate-relevant biochemistry within the medial PFC (mPFC). As hypo-frontality may be a precedent or antecedent of MA addiction, we also tested the hypothesis that individual variance in MA-taking and –preference relates to mPFC glutamatergic anomalies by studying both non-genetic individual variation and using a genetic model of MA addiction vulnerability/resiliency. Herein, subchronic MA dosing elicited enduring glutamate sensitization within the mPFC and indices of reduced MA-induced glutamate transmission are implicated as biochemical correlates of both idiopathic and genetic MA addiction vulnerability.
MATERIALS & METHODS
SUBJECTS AND MA TREATMENT
The majority of the studies used adult (8 weeks old), male, C57BL/6J (B6) mice obtained from The Jackson Laboratory (Sacramento, CA). As male mice were not available at the time of study, adult (6–8 weeks old) female mice from the MA High Drinking (MAHDR) and MA Low Drinking (MALDR) lines, generated at the Portland VA Medical Center (see Wheeler et al., 2009), were shipped to the University of California Santa Barbara and quarantined for six weeks prior to study. Prior investigation indicates that males and females of these lines exhibit comparable differences in MA drinking and MA-related reward and aversion phenotypes (Wheeler et al., 2009; Shabani et al., 2011; Shabani et al., 2012b). Mice were housed in groups of 4 in standard mouse polycarbonate cages under a 12 h light-dark cycle (lights on: 07:00) and food and water were available ad libitum. MA (Sigma-Aldrich, St. Louis, MO) was administered by intraperitoneal (IP) injection at a volume of 10 ml/kg and a dose of 2 mg/kg; an equivalent volume of 0.9% saline was administered IP for control injections. All experimental protocols and animal care, including room temperature and air exchange conditions, were consistent with the guidelines provided by the National Institute of Health Guide for Care and Use of Laboratory Animals (revised 2014) and were approved by the Institutional Animal Care and Use Committees (IACUC) of the University of California Santa Barbara and Oregon Health and Science University.
STEREOTAXIC SURGERY
For microdialysis, mice underwent stereotaxic surgery to implant stainless steel guide cannulae (7 mm, 20 gauge; Eagle Stainless; Warminster, PA) above the mPFC using procedures identical to those described in our recent work (e.g., Ary et al., 2013; Lominac et al., 2014). All surgeries were performed under isoflurane anesthesia (1.5–2%), using oxygen as the carrier gas. Once anesthetized, a mouse was placed in a Kopf stereotaxic device and its head was stabilized with tooth and ear bars. The skull was then exposed and leveled. Holes were drilled based on coordinates from Bregma (AP: +1.8 mm, ML: ±0.5 mm; DV −1.0mm), according to the Paxinos and Franklin (2007) mouse brain atlas. The guide cannulae were then lowered to 2 mm above the mPFC and were fixed in place with light-cured dental resin. Surgical incisions were closed, using tissue adhesive as necessary. Dummy cannulae (24 gauge; length equivalent to guide cannulae) were inserted into the guide cannulae to reduce externalization. Animals were administered the non-steroidal, anti-inflammatory, banamine (2 mg/kg, SC), once during the surgical procedure and then twice a day for the first 48 h post-operation. Animal health was monitored daily following surgery and all mice were allowed at least 5 days recovery prior to injection or microdialysis procedures. Prior to any statistical analyses of the data, probe placements within the mPFC were verified using microscopic analysis of Nissl-stained coronal sections.
MA-INDUCED GLUTAMATE SENSITIZATION
The first experiment examined the shorter- and longer-term effects of repeated, non-contingent, injections of MA upon basal extracellular glutamate content and sensitization of glutamate release within the mPFC of B6 mice. To ensure equivalent MA-dosing across subjects, mice were injected with 2 mg/kg MA, once a day, for 10 days. This dosing was reported by our group to elicit MA-induced sensitization of dopamine release within the PFC of B6 mice (Lominac et al., 2014) and is similar to regimens reported to elicit behavioral sensitization in rodents (e.g., Broom & Yamamoto, 2005; Szumlinski et al., 2000). Control animals received daily injections of SAL. At either 1 or 21 days of withdrawal, mice underwent microdialysis procedures or were rapidly decapitated to obtain mPFC tissue (see below).
IN VIVO MICRODIALYSIS PROCEDURES
The in vivo microdialysis procedures for estimating basal extracellular glutamate content using no net-flux methods, and for examining for the changes in extracellular glutamate elicited by an IP injection of 1 mg/kg MA injection using conventional microdialysis methods, were similar to those described in recent studies (e.g., Ary et al., 2013; Lominac et al., 2014). Counterbalancing hemispheres across subjects, mice were lightly restrained to remove the dummy cannula and insert a microdialysis probe (24-gauge, 10 mm in length with ∼1.7 mm of active membrane) unilaterally into the mPFC. The probe was then connected to a liquid swivel (Instech, Plymouth Meeting, PA), and fitted with tubing connected to an automated syringe pump (KD Scientific, Thermo-Fisher) that perfused microdialysis buffer (146 nM NaCl, 1.0 mM MgCl2, 1.7 mM KCl, 1.2 mM CaCl2, pH = 7.4) at a rate of 2 µl/min. After 3 h of probe equilibration, dialysate collection began and occurred at 20-min intervals for 3–4 h, depending upon the study. The dialysate was collected in vials containing 10 µl of preservative (0.075 µM NaH2PO4, 25 µM EDTA, 0.0017 µM 1-octansulfonic acid, 10% acetonitrile (v/v), pH = 3.0), as described previously (Lominac et al., 2014), and was then stored at −80°C until assay. Once the session was complete, animals were lightly restrained to remove the probe and reinsert a sterile dummy cannula. Depending on guide cannula patency, animals underwent a second microdialysis session in which the probe was inserted into the opposite hemisphere. For the study of MA-induced glutamate sensitization in B6 mice, the second microdialysis session was conducted at 21 days following the end of repeated MA/SAL injection to index long-term effects of repeated MA treatment, and separate groups of B6 mice were employed for the no net-flux versus conventional microdialysis procedures. As the number of MAHDR/MALDR mice available for study was limited, the first microdialysis session employed no net-flux procedures to estimate genotypic differences in basal glutamate content and the second microdialysis session, conducted 4–7 days later in the opposite hemisphere, employed conventional microdialysis procedures to study genotypic differences in the glutamate response to a single acute 2 mg/kg MA injection.
For all microdialysis sessions, baseline glutamate levels were established over a 1-h sampling period. Mice examined under no net-flux procedures were then perfused with increasing glutamate concentrations (2.5, 5 and 10 µM) for 1 h per concentration. To determine the point of no net flux (y=0; an estimate of extracellular content) and the extraction fraction (Ed; an index of neurotransmitter clearance/release), linear regression analyses were performed as conducted in previous work (Ary et al., 2013; Haider et al., 2015; Szumlinski et al., 2004, 2005, 2008b). Following the 1-h baseline sampling period, mice tested for the sensitization of MA-induced glutamate release were injected with either 1 mg/kg MA (B6 study) or 2 mg/kg MA (MAH/LDR study) and dialysate was collected, at 20-min intervals, for another 3 h, as in our previous study (Lominac et al., 2014). The data obtained under conventional procedures was normalized to the average baseline levels of glutamate to better illustrate group differences in glutamate responsiveness.
HPLC DETECTION OF GLUTAMATE
High pressure liquid chromatography (HPLC) methods for detecting glutamate in dialysate and chromatography procedures were identical to those in previous work (Ary et al., 2013; Ben-Shahar et al., 2012; Goulding et al., 2011; Haider et al., 2015; Lominac et al., 2012). The HPLC system consisted of a Coularray detector, a Model 542 autosampler and a Model 582 solvent delivery systems (ESA Inc., Bedford, MA, USA), with a detection limit of 0.01 fg/sample (20 µl/sample onto column). The mobile phase consisted of 100 mM NaH2PO4, 22% methanol (v/v), 3.5% acetonitrile (v/v) pH = 6.75 and glutamate was separated using a CAPCELL PAK C18 MG column (50 × 3.2 mm; Shiseido Company Ltd., Tokyo, Japan), eluting at 1.8 min. An ESA 5011A analytical cell with two electrodes (E1, +150 mV; E2, +550 mV) detected glutamate, following precolumn derivatization with o-phthalaldehyde (2.7 mg/ml) using the autosampler. The glutamate content in each sample was analyzed by peak height and was compared with an external standard curve for quantification using ESA Coularray for Windows software.
MA-INDUCED PLACE-CONDITIONING
While MAHDR/MALDR mice serve well for the study of the biochemical correlates of genetic vulnerability/resiliency to MA addiction-related behavioral traits (e.g., Eastwood et al., 2012; Lominac et al., 2014; Shabani et al., 2011, 2012a, 2012b; Wheeler et al., 2009), the limited number of MAHDR/MALDR mice available for study precluded any further study of the relation between addiction vulnerability/resiliency and mPFC glutamate. However, a serendipitous observation in our laboratory revealed marked behavioral heterogeneity, with respect to the motivational/affective valence of MA among commercially available, adult, male B6 mice (unpublished results). This offered us the opportunity to determine whether or not individual differences in MA-conditioned place-preference/aversion (CPP/CPA) related to protein indices of glutamate function within the mPFC, in an isogenic population of mice. Groups of B6 mice were subjected to a MA place-conditioning procedure identical to that employed previously by our laboratory (Lominac et al., 2014). Place-conditioning consisted of 3 phases: habituation (day 1, Pre-test), MA/SAL conditioning (days 2–9) and post-conditioning test (day 10, Post-test). The apparatus consisted of two distinct compartments – one with black and white marble-patterned walls and a textured floor, and the other with a smooth Plexiglas floor and wood-patterned walls. During the habituation and Post-test sessions, mice were allowed free access to both compartments of the apparatus for 15 minutes. During conditioning, mice were injected with 2 mg/kg MA, immediately prior to confinement in one of the compartments and, on alternating days, were injected with SAL and confined to the other compartment. Each conditioning session was 15 min in duration and mice received 4 conditioning sessions for each unconditioned stimulus. As overall, mice tend not to exhibit a strong preference for one compartment vs. the other during the Habituation session (e.g., Lominac et al., 2014), the time spent on SAL-paired side during the Post-test was subtracted from the time spent on the MA-paired side to get a CPP Score, which served to index the direction and magnitude of MA-conditioned reward. Mice exhibiting CPP scores >+100 sec were operationally defined/phenotyped as exhibiting a CPP, while mice exhibiting CPP Scores <-100 sec were operationally defined/phenotyped as exhibiting a CPA; mice with intermediate CPP Scores were operationally defined/phenotyped as ambivalent or Neutral. To control for effects of apparatus exposure, animal handling and injections on protein expression, a separate group of B6 mice underwent the place-conditioning procedures but received SAL injections in both compartments of the apparatus. Upon completion of the Post-test, the CPP Scores were calculated and subsets of CPP, CPA, Neutral and SAL mice (n=12–14/phenotype) were immediately decapitated to obtain tissue for immunoblotting (see below).
INTRACRANIAL DRUG INFUSION
Neuropharmacological approaches were employed to determine the functional relevance of endogenous glutamate tone within the mPFC for the manifestation of a MA-induced CPP in B6 mice. For this study, mice were fitted with bilateral guide cannulae, allowed a minimum of 5 days recovery and were then subjected to our MA-induced place-conditioning procedures, all as described above. Following the initial Post-test, the mice were subdivided into 3 groups slated to receive bilateral intra-mPFC infusion of water vehicle (VEH; volume = 0.25 µl/side), 50 µM of the mGlu2/3 agonist APDC [(2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate; Tocris Biosciences, Minneapolis, MN] or 300 µM of the non-selective EAAT reuptake inhibitor TBOA (DL-threo-β-Benzyloxyaspartic acid; Tocris Biosciences], immediately prior to a second Post-test. These drugs and doses were selected based on the results of a prior study demonstrating their efficacy to raise and lower alcohol intake in B6 mice (Kapasova & Szumlinski, 2008). There were no significant differences in the CPP Scores or total distance traveled between the 3 experimental groups prior to microinjection. The procedures for infusing these glutamatergic drugs into the mPFC were identical to those employed in our recent dopamine study (Lominac et al., 2014). In brief, dummy cannulae were removed, 33-gauge microinjectors (9 mm in length) were lowered into the guide cannulae and drugs infused at a rate of 0.25 µl/min for a period of 1 min. The microinjectors were left in place for an additional 1 minute and then removed. The dummy cannulae were replaced and then mice were placed into the place-conditioning apparatus with the open divider and their behavioral response was recorded for 15 min. A comparison of the CPP Scores from the initial Post-test and the microinjection Post-test by ANOVA indicated whether or not intra-mPFC microinjection influenced the magnitude or direction of the conditioned response.
IMMUNOBLOTTING
In all, 3 immunoblotting experiments were conducted. To determine the shorter- and longer-term effects of a sensitizing regimen of MA (10 injections of 2 mg/kg) upon protein expression, the mPFC was excised from B6 mice at 1 or 21 days of withdrawal from repeated treatment. An illustration of the dissection is provided in Fig. 2B. To determine the protein correlates of selection for high versus low MA drinking, a subset of MAHDR/MALDR mice was injected acutely with 2 mg/kg MA, while another subset was injected acutely with SAL and the mPFC dissected out 3 h later, in a manner consistent with earlier mRNA studies of these lines (Wheeler et al., 2009). In all 3 studies, we examined the expression of the metabotropic glutamate receptor (mGluR) subtypes mGlu1, mGlu5, and mGlu2/3, the NMDA and AMPA glutamate receptor subunits, Homer proteins, and excitatory amino acid transporters, as these proteins have been implicated in the neurobiology of stimulant addiction (e.g., Ary et al., 2013; Ben-Shahar et al., 2009, 2013; Ghasemazadeh et al., 2003; Kalivas et al., 2005; Melendez et al., 2005; Swanson et al., 2001; Szumlinski et al., 2004). General immunoblotting procedures were performed as described previously by our group (Ary et al., 2013; Goulding et al., 2011; Quadir et al., 2015). The mPFC was dissected out from a 1-mm coronal section and kept frozen at −80°C until assay. Anti-Homer2a/b (Cosmo Bio USA Inc., Carlsbad, CA, USA), anti-Homer1b/c (GeneTex Inc., Irvine, CA, USA), anti-mGlu5 (Millipore, Billerica, MA, USA), anti-NR2a and anti-NR2b (Calbiochem, San Diego, CA, USA), anti-GluA1 (Upstate Cell Signaling Solutions, Lake Placid, NY, USA), anti-mGlu2/3 (Upstate Cell Signaling Solutions, Lake Placid, NY, USA), anti-EAAT 1 (Cell Signaling Biotechnology, Beverly, MA), anti-EAAT 2 (Cell Signaling Biotechnology, Beverly, MA), and anti-EAAT3 (Santa Cruz Biotechnology, Santa Cruz, CA) rabbit polyclonal antibodies were used. In addition, mouse monoclonal anti-GluN1 (Upstate Cell Signaling Solutions, Lake Placid, NY, USA), and polyclonal anti-mGlu1α antibody (BD Transduction Laboratories) were also used. Unless otherwise specified, all antibodies were applied at a 1:300 – 1:2000 dilution. After primary antibody incubation, membranes were washed, prior to being incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Millipore; 1:40,000–1:80,000 dilution) or anti-mouse secondary antibody (Millipore; 1:40,0000–1:80,0000) for 90 minutes. Membranes were then washed again and immunoreactive bands were detected by enhanced chemiluminescence using either ECL Plus (GE Healthcare) or Pierce SuperSignal West Femto (Fisher Scientific). Rabbit anti-calnexin polycolonal primary antibody (Enzo Life Sciences, Farmingdale, NY) was used to standardize protein loading and membrane transfer. ImageJ was used to quantify immunoreactivity of each protein. Protein/calnexin ratios were used to normalize immunoreactivity of each protein with its respective calnexin value. For group comparisons, values of experimental animals were expressed as a percentage of control animals on each gel. The data were analyzed using t-tests (MAHDR versus MALDR) or ANOVAs, followed by post-hoc comparisons as appropriate. α=0.05 for all analyses.
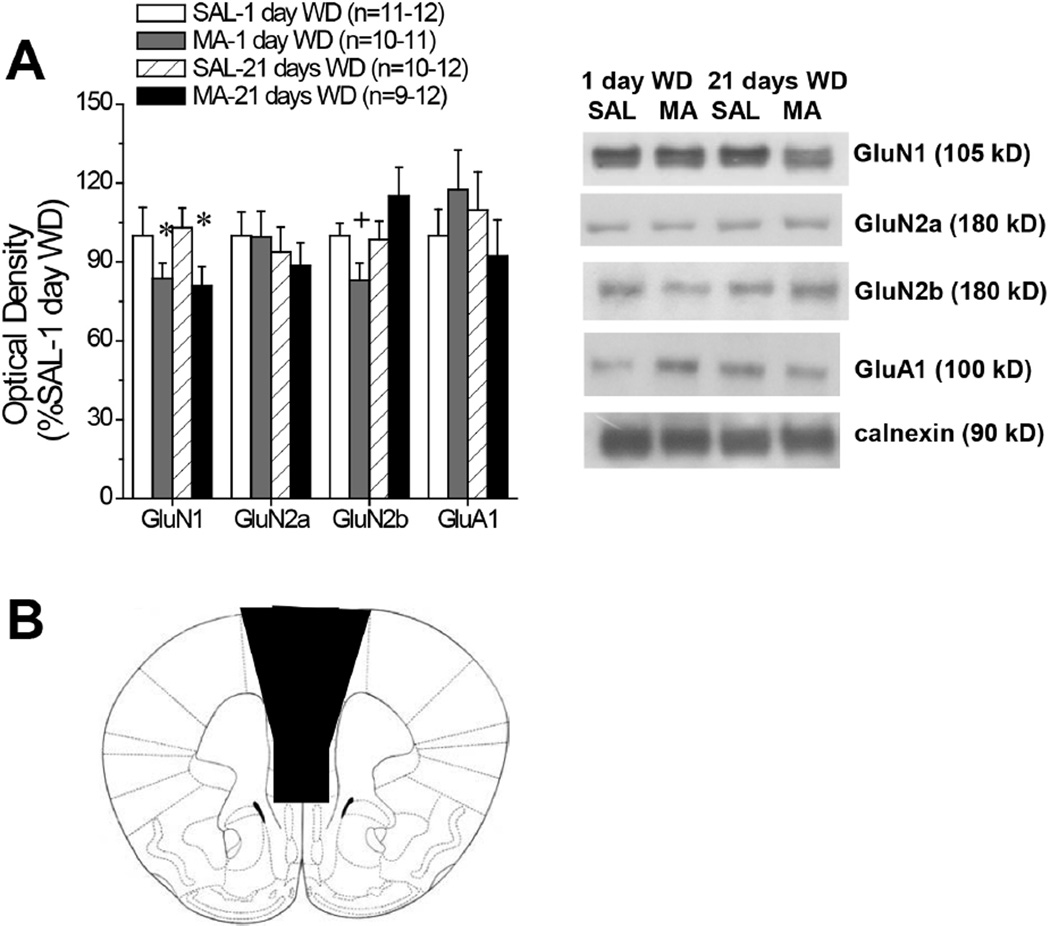
Figure 2. Summary of the effects of withdrawal from repeated MA upon indices of glutamate NMDA receptor subunit levels within the mPFC of B6 mice.
(A) Summary of SAL-MA differences in the protein expression of ionotropic glutamate receptor subunits within the mPFC of B6 mice, determined at 1 versus 21 days withdrawal. The immunoblotting data are expressed as a percent of the average SAL-1 day WD controls. Samples sizes are indicated in parentheses. Representative immunoblots are also provided. *denotes main Pretreatment effect (p<0.05); +denotes different from SAL-1 day WD (test for simple main effects). (B) Depiction of the gross dissection of the mPFC employed to obtain tissue for immunoblotting.
RESULTS
Repeated MA elicits enduring glutamate sensitization within mPFC of B6 mice
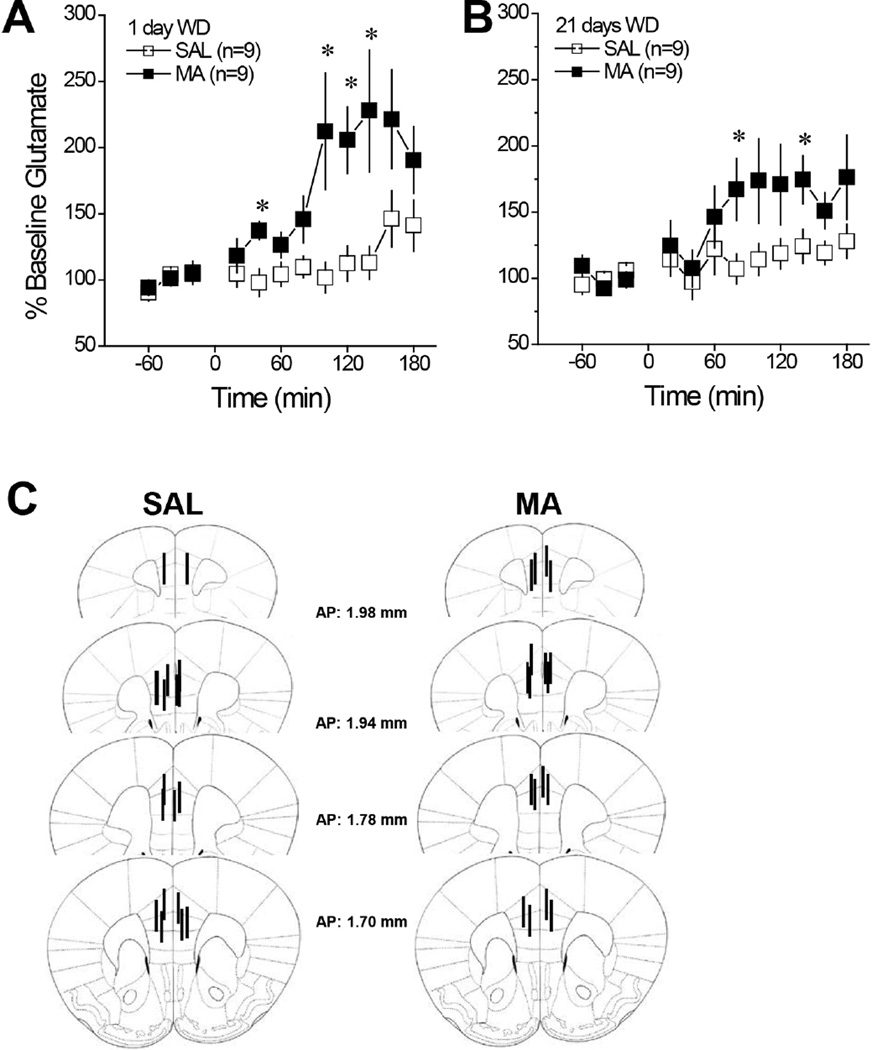
As probe patency was maintained in all B6 mice tested for MA-induced glutamate sensitization, the data were analyzed using a Treatment (MA vs. SAL) X Withdrawal (1 vs. 21 days) X Time (12, 20-min bins) ANOVA, with repeated measures on both the Withdrawal and Time factors. As depicted in Fig. 1A & 1B, repeated MA (10 injections of 2 mg/kg) sensitized the capacity of a 1 mg/kg MA challenge injection to elevate mPFC levels of extracellular glutamate; in MA-treated mice, the onset of MA-induced glutamate release was advanced in time and the magnitude of the rise was approximately double that of SAL controls, irrespective of the withdrawal period [Treatment: F(1,32)=10.39, p=0.003; Time: F(11,352)=11.60, p<0.0001; Treatment X Time: F(11,352)=4.80, p<0.0001; Withdrawal effect and interactions, all p’s>0.10]. Thus, repeated, non-contingent injections of MA induce glutamate sensitization within the mPFC that manifests very early in withdrawal and endures for at least 3 weeks following the last MA administration. A summary of the location of the microdialysis probes within the mPFC is provided in Fig. 1C.
Figure 1. Summary of the effects of withdrawal from repeated MA upon indices of glutamate transmission within the mPFC of B6 mice.
When administered at either 1 day withdrawal (WD) (A) or 21 days WD (B), a 1 mg/kg MA injection elevated extracellular glutamate in mice treated repeatedly with saline (SAL); however, the rise was more robust in mice with a history of repeated methamphetamine (MA) treatment (10 injections of 2 mg/kg), irrespective of withdrawal time-point. For panels A and B, *p<0.05 vs. SAL (tests for simple effects). (C) Cartoon illustrating the location of the microdialysis probe membranes within the mPFC of the B6 mice employed in the conventional in vivo microdialysis study. All mice that underwent in vivo microdialysis procedures exhibited placements comparable to those illustrated, with the majority of the probes localized to the prelimbic cortex.
Repeated MA does not alter basal extracelluar levels of glutamate within the mPFC of B6 mice
An analysis of the average basal extracellular glutamate levels determined during the hour prior to MA challenge did not indicate any significant effect of prior drug history upon baseline glutamate (Table 1; Treatment X Withdrawal ANOVA, all p’s>0.25). As the results of conventional microdialysis procedures can be influenced by differences in probe recovery, we conducted no net-flux microdialysis procedures to replicate the lack of group differences in basal glutamate levels. As summarized in Table 1, SAL-MA differences were not apparent for either y=0 (estimate of basal extracellular glutamate content) or for the extraction fraction (Ed), which serves to index glutamate reuptake/release (for both variables: Treatment X Withdrawal ANOVA, all p’s>0.20). These data indicate that a 10-day history of non-contingent MA does not alter basal extracellular glutamate context within the mPFC of B6 mice.
Table 1.
Summary of the results obtained from in vivo microdialysis studies of the mPFC of B6 mice treated repeatedly with either saline (SAL) or methamphetamine (MA) (10 × 2 mg/kg). MA-SAL differences were not noted for baseline glutamate levels derived from the experiment employing conventional microdialysis procedures nor were group differences observed for either y=0 (estimate of basal extracellular glutamate content) or the extraction fraction (Ed; index of clearance/release) in the experiment employing no net-flux microdialysis procedures.
| Repeated Treatment: | SAL | MA | ||
|---|---|---|---|---|
| Withdrawal: | 1 day | 21 days | 1 day | 21 days |
| Baseline glutamate (pg/20 µl) | 10.21 ± 3.40 | 9.03 ± 1.31 | 11.02 ± 3.84 | 6.11 ± 1.50 |
| Y=0 (µM) | 5.58 ± 0.27 | 5.31 ± 0.08 | 5.42 ± 0.17 | 5.53 ± 0.16 |
| Ed | 0.86 ± 0.04 | 0.90 ± 0.04 | 0.87 ± 0.07 | 0.89 ± 0.04 |
Repeated MA produces few changes in the expression of glutamate receptor-related proteins within the mPFC of B6 mice
To our surprise, B6 mice treated repeatedly with MA (10 injections of 2 mg/kg) exhibited very few protein changes within the mPFC, relative to their SAL controls. In fact, of all the glutamate-related proteins examined, the only two changes observed were a withdrawal-independent reduction in GluN1 (Fig. 2A) [Treatment effect: F(1,40)=5.57, p=0.02; other p’s>0.70] and a Treatment X Withdrawal interaction for GluN2b (Fig. 2A) [Treatment X Withdrawal: F(1,36)=5.07, p=0.03]. Simple main effects analyses indicated that this latter interaction reflected a MA-induced reduction in GluN2b expression during early withdrawal (p<0.05), that was no longer apparent at the later withdrawal time-point (p>0.05). As summarized in Table 2, we observed no differences in the expression of mGluRs, Homer proteins or EEATs that might account for the sensitization of MA-induced glutamate release described above. Although there was a general tendency for reduced kinase phosphorylation within the mPFC of MA-experienced mice during later withdrawal, Treatment X Withdrawal ANOVAs failed to detect any group differences with respect to the activational state (i.e. ratio of phosphorylated to total protein levels) of PI3K, Akt, ERK or PKCε (Table 2).
Table 2.
Summary of the effects of repeated methamphetamine (MA; 10 × 2 mg/kg, IP) or saline (SAL) injections upon the total levels of glutamate-related proteins within the mPFC of B6 mice, assayed by immunoblotting at either 1 or 21 days withdrawal (WD). Treatment X Withdrawal ANOVAs failed to indicate any significant group differences (p’s>0.05). Samples sizes are indicated in parentheses.
| 1 Day WD | 21 DAYS WD | |||
|---|---|---|---|---|
| Protein | SAL | MA | SAL | MA |
| mGlu1 | 100 ± 6.19 (8) |
97.67 ± 8.03 (11) |
111.13 ± 7.06 (11) |
94.51 ± 8.53 (12) |
| mGlu5 | 100 ± 8.39 (9) |
88.31 ± 6.27 (11) |
110.37 ± 8.55 (12) |
99.56 ± 9.16 (12) |
| mGlu2/3 | 100 ± 4.62 (11) |
95.68 ± 7.67 (11) |
94.99 ± 6.54 (12) |
106.67 ± 9.91 (12) |
| Homer1b/c | 100 ± 8.34 (12) |
104.28 ± 10.66 (11) |
106.79 ± 11.6 (11) |
113.86 ± 8.56 (12) |
| Homer2 | 100 ± 5.76 (12) |
101.35 ± 8.65 (11) |
91.1 ± 9.61 (11) |
98.14 ± 8.39 (11) |
| EAAT2 | 100 ± 4.23 (9) |
100.11 ± 5.16 (11) |
103.73 ± 5.85 (12) |
98.38 ± 6.02 (12) |
| EAAT3 | 100 ± 7.98 (10) |
93.04 ± 70.76 (10) |
100.28 ± 7.25 (11) |
96.14 ± 9.0 (12) |
| PI3K | 100 ± 7.62 (12) |
110.17 ± 10.78 (11) |
119.01 ± 12.19 (12) |
105.33 ± 12.24 (12) |
| p(Tyr)p85α PI3K binding motif | 100 ± 7.6 (11) |
87.08 ± 9.16 (11) |
93.81 ± 10.12 (12) |
93.58 ± 11.41 (11) |
| ERK | 100 ± 6.67 (12) |
110.69 ± 10.55 (11) |
112.54 ± 8.66 (12) |
123.18 ± 8.04 (12) |
| pERK | 100 ± 6.65 (12) |
94.69 ± 13.27 (11) |
89.96 ± 10.7 (12) |
78 ± 5.08 (12) |
| pERK: ERK | 100 ± 9.81 (9) |
105.55 ± 16.27 (11) |
95.69 ± 13.64 (12) |
73.77 ± 3.81 (12) |
| AKT | 100 ± 5.38 (11) |
105.42 ± 10.43 (11) |
103.35 ± 10.48 (12) |
104.63 ± 8.56 (12) |
| pAKT | 100 ± 11.24 (9) |
114.66 ± 10.92 (11) |
107.38 ± 9.03 (11) |
96.36 ± 8.73 (12) |
| pAKT: AKT | 100 ± 13.98 (12) | 95 ± 12.63 (11) |
90.61 ± 9.51 (11) |
80.15 ± 10.17 (12) |
| PKCε | 100 ± 5.55 (11) |
97.55 ± 7.55 (11) |
96.72 ± 5.26 (12) |
100.53 ± 6.85 (12) |
| pPKCε | 100 ± 7.03 (12) |
102.24 ± 11.21 (11) |
102.29 ± 11.45 (11) |
94.66 ± 12.28 (11) |
| pPKCε: PKCε | 100 ± 10.53 (12) | 101.43 ± 13.33 (11) |
100.26 ± 13.24 (11) |
85.88 ± 12.99 (12) |
mPFC glutamate correlates of selection for a high versus low MA drinking phenotype
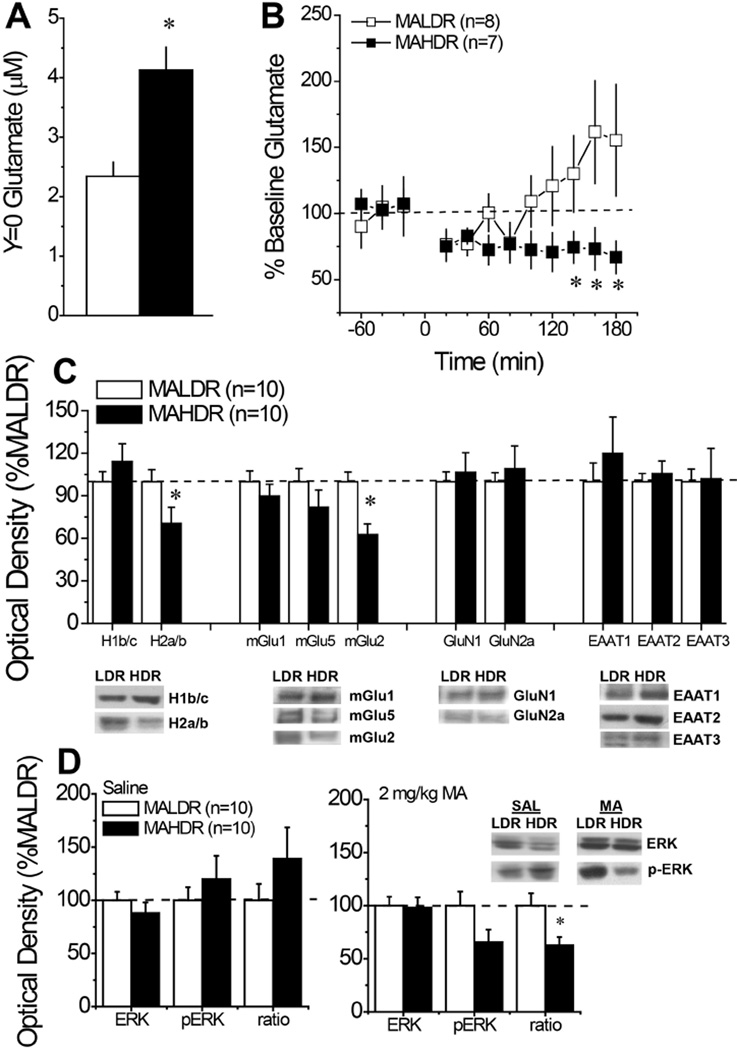
A marked line difference was apparent with respect to the basal extracellular glutamate content within the mPFC between MAHDR and MALDR mice when determined using no net-flux microdialysis procedures. As illustrated in Fig. 3A, MAHDR animals exhibited glutamate levels that were double those of MALDR mice [t(16)=4.20, p=0.001]. Despite this marked difference in glutamate content, there were no line differences in the Ed (MAHDR: 0.99 ± 0.08 vs. MALDR: 0.87 ± 0.06; p=0.25). Interestingly, a marked line difference was also noted for the glutamate response to an acute injection of 2 mg/kg MA (Fig. 3B) [Line X Time: F(11,143)=2.46, p=0.008]. As observed for B6 mice injected acutely with MA (see SAL in Fig. 2A), acute MA tended to elevate glutamate in MALDR mice, but the rise was not significantly above baseline levels (Time effect, p=0.06). In stark contrast, the same MA injection produced a statistically significant drop in extracellular glutamate, below baseline, in MAHDR mice [Time effect: F(11,66)=3.11, p=0.002]. Simple main effects analyses indicated a significant line difference in the glutamate response to MA during the last 40 min of testing (p<0.05).
Figure 3. Summary of the mPFC glutamate correlates of selection for high versus low MA drinking.
(A) No net-flux in vivo microdialysis procedures demonstrated higher basal extracellular glutamate content within the mPFC of mice selectively bred for high MA drinking (MAHDR), relative to those selectively bred for low MA drinking (MALDR). *p<0.05 vs. MALDR (t-test). (B) When injected acutely with 2 mg/kg MA, MAHDR mice exhibited a reduction in extracellular glutamate that was not apparent in MALDR mice, and the lines differed in glutamate content at later time periods. *p<0.05 vs. MALDR at the indicated time points (tests for simple main effects). (C) An immunoblotting analysis of line differences in protein expression revealed lower Homer2a/b (H2a/b) and lower mGlu2/3 expression within the mPFC of MAHDR versus MALDR mice, but no differences in Homer1b/c (H1b/c) expression or the expression of other glutamate-related proteins. (D) MA-naïve/saline-injected MAHDR mice tended to exhibit higher relative p-ERK expression within mPFC; however, when injected with 2 mg/kg MA, MAHDR mice exhibited a reduction in relative p-ERK expression. For Panels C and D, *p<0.05 vs. MALDR (t-tests).
When the expression of glutamate-related proteins was examined, SAL-injected MAHDR mice exhibited reduced expression of both Homer2a/b [t(18)=2.08, p=0.05] and mGlu2/3 [t(18)=3.66, p=0.002], but there were no line differences in Homer1b/c, mGlu1/5, GluN1/2a or in EAAT1/2/3. Unfortunately, due to technical difficulties, there was insufficient tissue sample from SAL-injected mice to examine for line differences in GluN2b expression or the activational state of many of the kinases examined in MA-sensitized B6 mice (Table 3). One exception was ERK and as illustrated in Fig. 3D, a line difference was not apparent for total ERK expression within mPFC, irrespective of acute treatment. However, SAL-injected MAHDR mice tended towards elevated total and relative p-ERK expression, although neither of these measures was statistically significant (Fig. 3D left panel; t-tests, p’s>0.20). More interestingly, line differences were observed in mice injected acutely with 2 mg/kg MA, with MAHDR exhibiting lower relative p-ERK expression [Fig. 3D right panel; for p-ERK: p=0.07; for ratio: t(18)=2.69, p=0.02]. These latter results for ERK activity corroborate the results of the in vivo microdialysis experiment and support the notion that high genetic vulnerability to MA addiction is associated with blunted MA-responsiveness within the mPFC.
Table 3.
Comparison of the major findings from the present study of glutamate-related correlates of: (1) MA-induced sensitization in B6 mice (MA-injected) versus repeated saline controls (SAL-injected); 2) genetic vulnerability/resiliency to MA addiction as determined in MAHDR and MALDR selected lines of mice; and 3) idiopathic vulnerability/resilience to MA addiction determined in B6 mice demonstrating a conditioned place-preference (CPP) or a conditioned place-aversion (CPA) when tested under our MA-induced place-conditioning procedures.
| MA- vs SAL-injected | MAHDR vs MALDR | CPP vs CPA | |
|---|---|---|---|
| Basal extracellular glutamate content | – | ↑ | n.d. |
| MA-elicited glutamate release | ↑ (1 & 21 days WD) | ↓ | n.d. |
| GluN1 levels | ↓ (1 & 21 days WD) | – | – |
| GluN2b levels | ↓ (1 day WD) | n.d. | ↓ |
| mGlu2/3 | – | ↓ | n.d. |
| mGlu5 | – | – | ↓ |
| Homer2a/b | – | ↓ | ↓ |
| pERK:ERK ratio | – | ↓ (2 mg/kg MA) | ↑1 |
↑ denotes relative increase, ↓ denotes relative decrease, – denotes no change, n.d. denotes not determined.
data presented in Campbell et al. (under review).
Increasing endogenous glutamate within mPFC promotes MA-preference
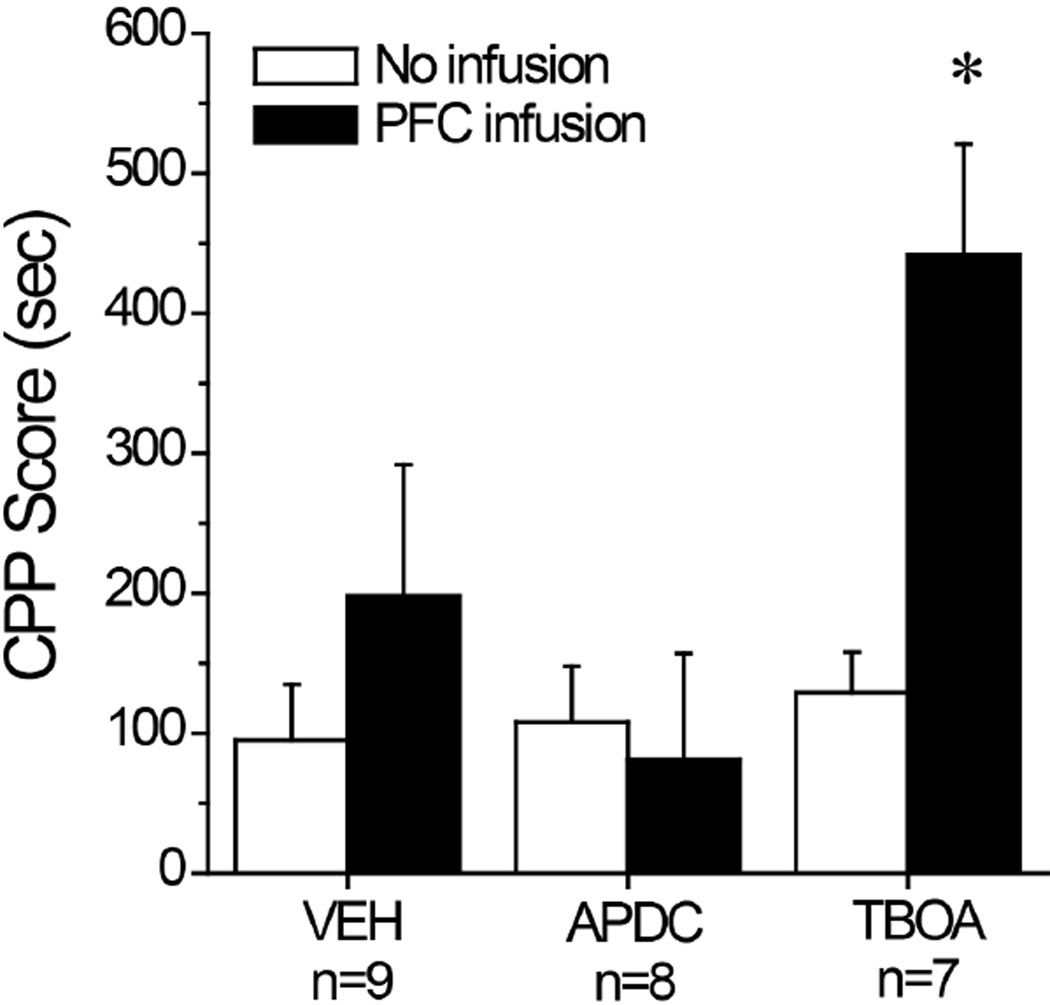
As only a limited number of MAH/LDR mice were available for study, we employed a neuropharmacological approach to study the relevance of basal extracellular glutamate for the manifestation of a MA-induced CPP. A comparison of CPP Scores before and after intra-mPFC microinjection of the EAAT reuptake inhibitor TBOA, the mGlu2/3 autoreceptor agonist APDC or vehicle control indicated an active role for mPFC glutamate in the magnitude of a place-preference (Fig. 4) [Drug effect: F(2,21)=4.40, p=0.03; Test effect: F(1,21)=5.35, p=0.03; Drug X Test interaction: F(2,21)=2.93, p=0.08]. Relative to their initial Post-test, the magnitude of the MA-conditioned response was increased in TBOA-infused mice, as indicated by the results of tests for simple main effects analyses [for TBOA, p<0.05; for vehicle and APDC, p’s>0.05]. In contrast, mPFC manipulations of endogenous glutamate tone did not alter the locomotor activity of the mice during the test for MA-conditioning, relative to that exhibited during the baseline Post-test (data not shown; Drug X Test ANOVA, all p’s>0.30). These data indicate that increasing and decreasing endogenous glutamate tone within the mPFC is sufficient to augment and reduce the magnitude of a MA-induced CPP in B6 mice in a manner unrelated to changes in locomotor activity.
Figure 4. Summary of the effects of raising and lower endogenous glutamate within the mPFC upon the expression of a MA-conditioned place-preference.
Summary of the average CPP Score (time on MA-paired compartment minus time on SAL-paired compartment) exhibited by B6 mice for a Post-conditioning test conducted prior to neuropharmacological procedures (No Infusion) or following infusion with vehicle (VEH), 50 µM of the mGlu2/3 autoreceptor agonist APDC or 300 µM of the non-selective EAAT inhibitor TBOA. Sample sizes are indicated in their respective datasets. *p<0.05 vs. VEH and APDC (LSD post-hoc tests).
mPFC glutamate correlates of individual differences in the motivational valence of MA
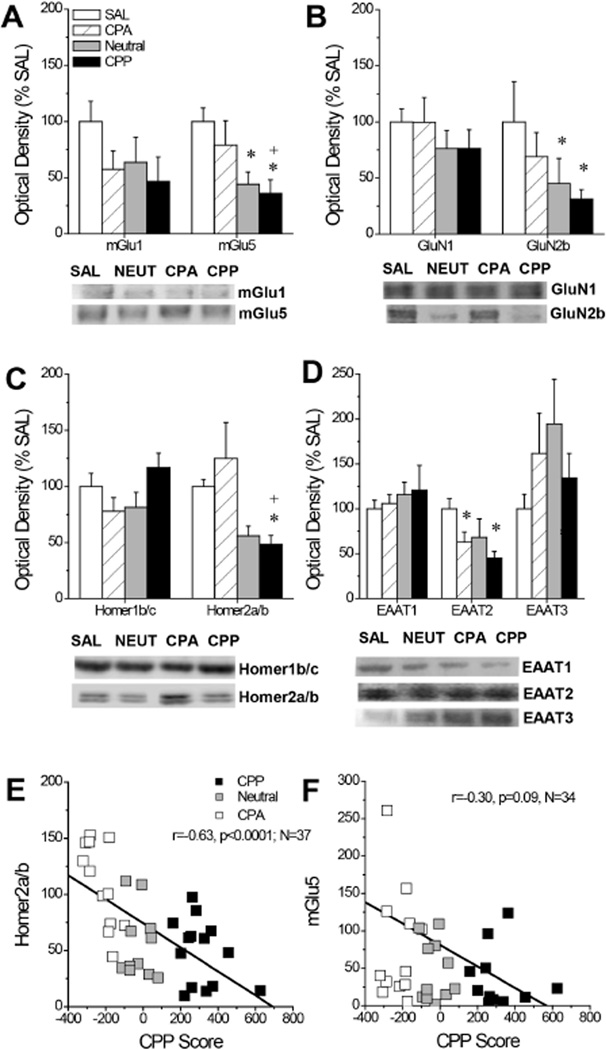
The final series of experiments employed immunoblotting in B6 mice expressing divergent MA-induced place-conditioning phenotypes to examine for associations between idiopathic differences in MA preference/aversion and the expression of glutamate-related proteins within the mPFC. Despite the marked differences in the direction and/or magnitude of their MA-conditioned responses, mPFC levels of mGlu1 did not differ between SAL-conditioned B6 mice and B6 mice phenotyped as CPP, Neutral or CPA following MA place-conditioning (Fig. 5A; p=0.28). However, phenotypic differences were observed for mGlu5 levels (Fig. 5A) [F(3,45)=3.17, p=0.03], and LSD post-hoc tests indicated that CPP mice exhibited lower mGlu5 expression relative to both CPA (p=0.05) and SAL controls (p=0.01). There were no phenotypic differences in GluN1 (p=0.62), although GluN2b levels were significantly lower in both Neutral and CPP mice, relative to SAL controls (Fig. 5B) [F(3,38)=2.57, p=0.04; LSD post-hoc tests: SAL vs. Neutral, p=0.01; SAL vs. CPP, p=0.04]. While Homer1b/c levels did not vary with phenotype (one-way ANOVA, p=0.11), Homer2a/b expression was lower in CPP mice relative to both CPA and SAL controls (Fig. 5C) [F(3,43)=4.45, p=0.008; LSD post-hoc tests: CPP vs. CPA, p=0.002; CPP vs. SAL, p=0.05]. We observed no group differences in EAAT1 expression (p=0.56), however, repeated MA treatment lowered the levels of EAAT2 [F(2,45)=3.61, p=0.02], with both CPA and CPP mice exhibiting statistically significant reductions in EAAT2 expression, relative to SAL controls (Fig. 5D; LSD post-hoc tests, p’s<0.04). In contrast, repeated MA elevated EAAT3 expression, irrespective of phenotype; however, group differences were not statistically reliable (Fig. 5D; one-way ANOVA, p>0.25). Given the step-wise pattern of group differences in mGlu5, GluN2b, and Homer2a/b, we conducted correlational analyses between the individual CPP Scores for the mice employed in the immunoblotting study and their protein expression. These results revealed a significant inverse relation between CPP Score and mPFC levels of Homer2a/b (Fig. 5E; r=-0.63, p<0.0001; N=38) and a near-significant trend for an inverse relation between CPP Score and mPFC levels of mGlu5 (Fig. 5E; r=-0.30, p=0.08; N=38). No other correlations approached statistical significance (p’s>0.25). These data extend the results from MAHDR/MALDR above by indicating that lower Homer2a/b is a biochemical correlate of the positive motivational/affective valence of MA in inbred B6 mice.
Figure 5. Summary of the mPFC glutamate correlates of individual differences in the motivational valence of MA.
Average optical densities and representative immunoblots of our proteins of interest for mice conditioned to saline only (SAL), as well as Neutral mice (NEUT), and mice exhibiting a CPA or CPP following MA-induced place-conditioning procedures. (A) While mGlu1 levels tended to be lowered by MA-conditioning, mGlu5 expression appeared to be inversely related to CPP Score. (B) GluN1 levels were unchanged by MA-conditioning, while GluN2a levels appeared to be inversely related to CPP Score. (C) Homer1b/c levels were not changed by MA-conditioning, while Homer2a/b levels varied inversely with CPP Score. (D) EAAT1 levels were unchanged by MA-conditioning, while EAAT2 and EAAT3 levels were, respectively, lower and higher in MA-conditioned mice but did not vary systematically with CPP Score. (E) Correlational analyses indicated a significant inverse correlation between CPP Score and mPFC Homer2a/b expression. (F) Correlational analyses revealed a non-significant trend toward an inverse relation between CPP Score mPFC mGlu5 expression. Sample sizes ranged from 11–14/phenotype. *p<0.05 vs. SAL; +p<0.05 vs. CPA (LSD post-hoc tests).
DISCUSSION
Herein we show that repeated, non-contingent, injections of subtoxic MA doses elicit enduring sensitization of MA-induced mPFC glutamate release in B6 mice, accompanied by reduced GluN1 subunit expression. In a genetic model of MA addiction vulnerability/resiliency, elevated basal extracellular glutamate content, but blunted MA-stimulated release and mPFC cellular activity (as determined by ERK phosphorylation) were present in the high MA intake line, as were reduced mPFC levels of Homer2a/b and mGlu2/3 in drug-naïve animals. Neuropharmacological study of B6 mice demonstrated a cause-effect relation between endogenous glutamate tone within the mPFC and the magnitude of a MA-induced CPP. Finally, Homer2a/b, mGlu5 and GluN2b levels within the mPFC were inversely correlated with MA-induced CPP in B6 mice. The major findings of this study are summarized in Table 3 and their implications for the etiology of MA addiction are discussed below.
Repeated MA exposure sensitizes MA-induced glutamate release within mPFC
Human MA addicts exhibit enduring anomalies in glutamine/glutamate within PFC (Ernst & Chang, 2008) that are consistent with the pronounced hypofrontality observed in addicted individuals theorized to underpin their deficits in executive processing and cognition (see Introduction). Implicating drug-taking history as causative to anomalous excitatory PFC neurotransmission, withdrawal from MA self-administration results in a number of glutamate-related anomalies within PFC of rodents (Parsegian et al., 2011; Parsegian & See, 2014; Schwendt et al., 2012; but see Herrold et al., 2013a). While possessing high face validity for the human condition, a draw-back of voluntary drug self-administration procedures relates to individual differences in drug intake that can confound data interpretation (Sanchis-Segura & Spanagel, 2006) and certain glutamate-related effects of MA self-administration vary within PFC as a function of post-MA extinction training (Schwedt et al., 2012). As such, we examined the glutamatergic consequences of mere withdrawal from a repeated, non-contingent, MA injection regimen that elicits a withdrawal-dependent increase in extracellular dopamine content within mPFC and sensitizes MA-induced dopamine release in this region (Lominac et al., 2014).However, In contrast to a recent MA self-administration study in rats (Parsegian & See, 2014), we failed to detect any effect of MA exposure or withdrawal upon basal extracellular glutamate content within the mPFC in B6 mice (Table 1). As microdialysis probe localization was comparable between the two studies, the discrepancy in results likely reflects factors associated with the route or temporal pattern of MA administration, control over intake, exposure to extinction training, or species differences. Indeed, control over intravenous MA intake influences the magnitude and time-course of intravenous MA’s effects upon basal extracellular dopamine and glutamate content, respectively, within the nucleus accumbens (Lominac et al., 2012) and the effects of MA self-administration upon both extracellular glutamate content within nucleus accumbens, as well glutamate autoreceptor expression within mPFC can vary with extinction training vs. withdrawal (Lominac et al., 2012 vs. Parsegian & See, 2014; Schwendt et al., 2012). This apparent discrepancy between the effects of self-administered vs injected MA argues that non-pharmacological factors associated with drug-taking may be key to regulating MA-induced changes in basal extracellular glutamate within mPFC. Indeed, re-exposure to MA-associated cues exerts strong influences upon extracellular glutamate in rats with a history of MA self-administration (Parsegian & See, 2014) and our prior immunoblotting results from cocaine self-administering rats points to important interactions between stimulant abstinence and re-exposure to drug-associated stimuli in regulating both the manifestation and direction of biochemical changes within PFC subregions (see Ben-Shahar et al., 2013; Gould et al., 2015).
Expression of the obligatory GluN1 subunit of the NMDA receptor was reduced in MA-sensitized mice at both withdrawal time-points and these animals also exhibited reduced total GluN2b expression during short-term withdrawal (Fig. 2A). It is interesting to note that impaired NMDA receptor function within PFC relates to higher rates of burst firing of PFC neurons (e.g., Homayoun & Moghaddam, 2006), deficits in executive processing/cognition (c.f., Castner & Williams, 2007; MacDonald & Chafee, 2006), greater spontaneous and MA-induced locomotor hyperactivity (e.g., Del Arco & Mora, 2008; Han et al., 2012), as well as greater dopamine release within both the cell body and terminal regions of corticoaccumbens projections (e.g., Homayoun et al., 2004; Lorrain et al., 2003; Takahata & Moghaddam, 1998) in a manner consistent with observed in MA-experienced animals (e.g., Lominac et al., 2012, 2014; Parsegian et al., 2011; Parsegian & See, 2014; Reichel et al., 2011; Szumlinski et al., 2000). Notwithstanding recent evidence that repeated MA alters the expression of GluA2 AMPA receptor subunits with mPFC (Herrold et al., 2013a), the present data supports the notion that a history of repeated MA exposure produces an enduring deficit in NMDA receptor function within PFC that contributes to the neurocognitive pathologies characteristic of MA abuse and addiction (e.g., Baicy & London, 2007; Berman et al., 2008; Goldstein & Volkow, 2011; Kim et al., 2005; Nestor et al., 2011; Salo et al., 2009). In contrast, we did not detect any MA-induced changes in the total protein expression of mGluRs or EAATs (Table 2) that regulate extracellular levels of glutamate within the mPFC (see Melendez et al., 2005), which is consistent with a similar recent immunoblotting study of MA-injected rodents (Herrold et al. 2013a). Currently, it is difficult to reconcile the discrepancies in our extant knowledge of how NMDA receptors regulate basal extracellular glutamate within mPFC (e.g., Gruss et al., 1999; Han et al., 2012; Lorrain et al., 2003; Moghaddam et al., 1997; Zuo et al., 2006) to make any firm predictions regarding how low NMDA receptor expression within PFC might contribute to or be impacted by MA-induced glutamate sensitization within this region.
PFC glutamate and genetic vulnerability to high oral MA intake
MAH/LDR mice were selectively bred for high versus low oral MA intake under long (18-h) access, 2-bottle-choice procedures to provide a genetic model of MA addiction vulnerability/resiliency (Wheeler et al., 2009; Shabani et al., 2011), and have since been demonstrated to diverge with respect to a number of MA addiction-related variables in a manner consistent with their selected phenotype (Eastwood et al. 2012; Shabani et al. 2011, 2012a, 2012b; Wheeler et al. 2009). In prior in vivo microdialysis studies, MAHDR mice exhibited reduced basal extracellular dopamine content within mPFC and heightened dopamine responsiveness to an acute challenge of MA, relative to their MALDR counterparts (Lominac et al., 2014). An opposite extracellular glutamate profile was observed herein within the mPFC, with MAHDR animals exhibiting higher basal extracellular glutamate content and a markedly blunted glutamate response to an acute MA injection, relative to MALDR mice (Fig. 3A, B). An inverse relation between extracellular dopamine and glutamate within the mPFC has been observed in studies of the neurochemical consequences of excessive cocaine-taking (Ben-Shahar et al., 2012; Shin et al., 2015) and is consistent with reports demonstrating antagonistic dopamine-glutamate interactions within the PFC of stimulant-naïve animals (e.g., Abekawa et al., 2000; Homayoun et al., 2004; Lorrain et al., 2003). At the present time, we cannot discern whether or not the elevated basal glutamate content observed within MAHDR mice (Fig. 3A) directly reflects their low dopamine levels or vice versa. Nevertheless, the results of the present neuropharmacological study indicate clearly that increased endogenous mPFC glutamate is sufficient to augment the expression of a MA-induced CPP in B6 mice (Fig. 4), supporting an active, but perhaps not necessary, role for mPFC extracellular glutamate in regulating the motivational/affective valence of MA that is consistent with the MA intake and CPP phenotypes of MAH/LDR mice (Eastwood et al., 2012; Shabani et al., 2011, 2012a, 2012b; Wheeler et al., 2009).
Line differences were observed with respect to total mGlu2/3 receptor expression within the mPFC of MAH/LDR mice, with MAHDR mice exhibiting lower mGlu2/3 expression (Fig. 3C). As mGlu2/3 receptors are presynaptically localized on glutamatergic neurons within mPFC and inhibit glutamate release (c.f., Conn & Pin, 1997], reduced mGlu2/3-mediated autoinhibition could account, either in whole or in part, for the elevated basal glutamate content observed in MAHDR mice (Fig. 3A). Alternatively, mGlu2/3 receptors located on GABAergic interneurons may also play a role in the heterosynaptic suppression of GABA release within mPFC (c.f., Schoepp, 2001), which would also be predicted to increase the basal hyperactivity of glutamate terminals within mPFC of MAHDR animals. That reduced mPFC expression of mGlu2/3 is a correlate of selection for high genetic vulnerability to MA addiction-related behaviors is particularly interesting in light of evidence for an enduring down-regulation in both total and cell surface mGlu2/3 expression within the mPFC in rats exhibiting escalated MA intake, the latter of which is resistant to extinction (Schwedt et al., 2012). Thus, it would appear that reduced mGlu2/3 expression within the mPFC may be both a precedent and an antecedent of MA addiction that might elevate basal glutamate tone within the mPFC (Fig. 3A), increase the basal level of burst firing of mPFC neurons and reduce the signal-to-noise ratio that is required for normal neurocognitive function (Parsegian et al., 2011). Supporting blunted PFC activation in MAHDR mice, an acute injection of MA lowered both extracellular glutamate levels (Fig. 3B) and indices of ERK activation (Fig. 3D) within their mPFC. In contrast, acute MA injection elicited a modest elevation in mPFC glutamate levels in MALDR mice that was in line with that observed in MA-naïve B6 animals (Fig. 2 vs. Fig. 3B), which is fitting with their comparable levels of MA intake reported recently (Eastwood et al., 2012; Harkness et al., 2015), and resistance of MALDR mice to MA-induced CPP (Wheeler et al., 2009; Shabani et al., 2011). MAHDR mice also exhibit impaired retention of spatial memory (Olsen et al., 2013), providing some evidence for neurocognitive dysfunction in this genetic model of MA addiction vulnerability. Although the molecular bases of MAH/LDR differences in addiction vulnerability and neurocognitive processing require further study, they may relate to line differences in mPFC Homer2a/b levels (Fig. 3C), as this glutamate receptor scaffolding protein can regulate basal extracellular glutamate content with mPFC to impact cocaine reward (Ary et al., 2013). Although earlier studies of MAH/LDR mice indicate that different heritable factors contribute to increased risk for MA versus cocaine abuse (Gubner et al., 2013), the important role for corticoaccumbens Homer2a/b expression in the regulation of both cocaine and alcohol intake (c.f., Szumlinski et al., 2008a) warrant further investigation into the functional relevance of mPFC Homer2a/b expression in MA addiction-related behavioral anomalies.
PFC glutamate and idiopathic risk for MA addiction
Supporting the potential importance of low mPFC Homer2a/b expression and MA reward are the results of the immunoblotting study of tissues from B6 mice that exhibited divergent MA place-conditioning responses. This study is viewed as complementary to that involving MAH/LDR mice and it was hypothesized at the outset of this study that the identification of common biochemical correlates of high versus low risk for MA addiction across these models would be of particularly high significance for the human condition. It is interesting that the only protein correlate of high MA reward in common between the studies of MAH/LDR and CPP/CPA mice was reduced total expression of Homer2a/b (see Table 3). In fact, mPFC Homer2a/b expression was the only biochemical measure that correlated with CPP Score in our B6 study and did so inversely (Fig 4G). Such a result lends credence to the possibility that Homer2a/b expression within the mPFC may be a negative regulator of risk for MA abuse and an on-going study in the laboratory applies virus-mediated transgenic approaches to test this hypothesis.
Despite equivalent MA-conditioning, both MA-ambivalent Neutral and MA-preferring CPP, but not MA-avoiding CPA, mice differed from SAL-conditioned controls with respect to mPFC expression of mGlu5, GluN2b or Homer2a/b (Fig. 4C–E) indicating a potential antecedent or differential neuroplastic changes associated with the subjective valence of MA exposure. Similarly, reduced cell surface expression of mGlu5 has been reported previously within mPFC of rats expressing a MA-induced CPP (Herrold et al., 2011). The coincident reduction in mGlu5, Homer2a/b and GluN2b is consistent with the important role played by Homer proteins in scaffolding Group1 mGluRs and NMDA receptors within the postsynaptic density (c.f., Shiraishi-Yamaguichi & Furichi, 2007) and prior evidence that Homer2 expression regulates the levels of both mGlu5 and GluN2b within the mPFC of B6 mice (Ary et al., 2013). Together, the present results suggest that idiopathic hyper-sensitivity to MA-induced perturbations in Homer2-dependent scaffolding of glutamate receptors within the mPFC may increase risk for MA abuse.
The reduced expression of mGlu5/Homer2 in MA-conditioned CPP/Neutral mice is also interesting in light of our failure to detect significant effects of 10 daily MA injections, administered in the home cage, upon the expression of either protein in B6 mice (Fig. 2A, Table 2). Further, irrespective of their CPP Scores, the MA-conditioned B6 mice exhibited reduced expression of mGlu1 and EAATs within mPFC (Fig. 4C–F), while there was absolutely no indication of any MA effect upon these proteins in drug-sensitized animals (Table 1). As the B6 mice tested for MA-induced place-conditioning were re-exposed to the MA-paired context in the absence of any IP injection (and thus, any injection-related interoceptive or exteroceptive cues), the arguably more robust MA effects observed in MA-conditioned animals may reflect the relative contribution of MA-associated contextual factors to glutamate plasticity within mPFC, as indicated by studies of MA self-administering rats (Parsegian & See, 2014; Schwendt et al., 2012). Moreover, akin to the present results, distinct glutamate receptor-related changes, including reduced mGlu5 expression, are observed within the mPFC of rats subjected to MA-induced place-conditioning procedures, but not in animals that are MA-injected in the home cage (Herrold et al., 2011, 2013b). Thus, the apparent resiliency of CPA mice to MA-induced changes in certain glutamate receptor proteins may very well reflect less time spent in the MA-associated context. Indeed, re-exposure to the drug-paired context/cues can elicit very rapid (within 2 h) reductions in the expression of glutamate receptors and Homer scaffolding proteins within mPFC that are either not apparent in, or opposite to that of, drug-experienced animals not re-exposed to the drug-context (Ben-Shahar et al., 2013; Gould et al., 2015). As drug-associated cues/contexts exert powerful and enduring control over cognition and behavior in addicted individuals, greater research efforts should focus on understanding how individual differences in drug-environment associative learning might contribute to both genetic and idiopathic risk for MA abuse and addiction, as well as on improving our understanding of how MA-environment interactions impact the neuropharmacology of the motivational circuitry of relevance to the treatment of MA abuse.
Acknowledgments
This work was funded, in part, by NIH grant DA024038 to Karen K. Szumlinski, NIH grant DA027525 to Tod E. Kippin, as well as funding from the Department of Veterans Affairs and NIDA grant P50 DA018165 to Tamara J. Phillips.
LIST OF ABBREVIATIONS
- B6
C57BL/6J
- CPA
conditioned place-aversion
- CPP
conditioned place-preference
- EAAT
excitatory amino acid transporter
- GluA
AMPA glutamate receptor subunit
- GluN
NMDA glutamate receptor subunit
- MA
methamphetamine
- MAHDR
methamphetamine high drinking
- MALDR
methamphetamine low drinking
- mGluR
metabotropic glutamate receptor
- mPFC
medial prefrontal cortex
- PFC
prefrontal cortex
- SAL
saline
Footnotes
None of the authors of this report have any financial conflicts of interest to declare related to this work.
REFERENCES
- Abekawa T, Ohmori T, Ito K, Koyama T. D1 dopamine receptor activation reduces extracellular glutamate and GABA concentrations in the medial prefrontal cortex. Brain Res. 2000;867(1–2):250–254. doi: 10.1016/s0006-8993(00)02298-8. [DOI] [PubMed] [Google Scholar]
- Ary AW, Lominac KD, Wroten MG, Williams AR, Campbell RR, Ben-Shahar O, von Jonquieres G, Klugmann M, Szumlinski KK. Imbalances in prefrontal cortex CC-Homer1 versus -Homer2 expression promote cocaine-seeking behavior. J. Neurosci. 2013;33:8101–8113. doi: 10.1523/JNEUROSCI.1727-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baicy K, London ED. Corticolimbic dysregulation and chronic methamphetamine abuse. Addiction. 2007;102(Suppl 1):5–15. doi: 10.1111/j.1360-0443.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Barr AM, Panenka WJ, MacEwan GW, Thornton AE, Lang DJ, Honer WG, Lecomte T. The need for speed: an update on methamphetamine addiction. J Psychiatry Neurosci. 2006;31:301–313. [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Obara I, Ary AW, Ma N, Mangiardi MA, Medina RL, Szumlinski KK. Extended daily access to cocaine results in distinct alterations in Homer 1b/c and NMDA receptor subunit expression within the medial prefrontal cortex. Synapse. 2009;63(7):598–609. doi: 10.1002/syn.20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Szumlinski KK, Lominac KD, Cohen A, Gordon E, Ploense KL, DeMartini J, Bernstein N, Rudy NM, Nabhan AN, Sacramento A, Pagano K, Carosso GA, Woodward N. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addict Biol. 2012;17:746–757. doi: 10.1111/j.1369-1600.2011.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar O, Sacramento AD, Miller BW, Webb SM, Wroten MG, Silva HE, Caruana AL, Gordon EJ, Ploense KL, Ditzhazy J, Kippin TE, Szumlinski KK. Deficits in ventromedial prefrontal cortex group 1 metabotropic glutamate receptor function mediate resistance to extinction during protracted withdrawal from an extensive history of cocaine self-administration. J Neurosci. 2013;33(2):495–506a. doi: 10.1523/JNEUROSCI.3710-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman S, O’Neill J, Fears S, Bartzokis G, London ED. Abuse of amphetamines and structural abnormalities in the brain. Ann N Y Acad Sci. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom SL, Yamamoto BK. Effects of subchronic methamphetamine exposure on basal dopamine and stress-induced dopamine release in the nucleus accumbens shell of rats. Psychopharmacology. 2005;181:467–476. doi: 10.1007/s00213-005-0007-6. [DOI] [PubMed] [Google Scholar]
- Castner SA, Williams GV. Tuning the engine of cognition: a focus on NMDA/D1 receptor interactions in prefrontal cortex. Brain Cogn. 2007;63(2):94–122. doi: 10.1016/j.bandc.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex. Pharmacol Biochem Behav. 2008;90(2):226–235. doi: 10.1016/j.pbb.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Eastwood EC, Barkley-Levenson AM, Phillips TJ. Methamphetamine drinking microstructure in mice bred to drink high or low amounts of methamphetamine. Behav Brain Res. 2014;272:111–120. doi: 10.1016/j.bbr.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol. 2008;3:165–172. doi: 10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Permenter LK, Lake RW, Kalivas PW. Nucleus accumbens Homer proteins regulate behavioral sensitization to cocaine. Ann N Y Acad Sci. 2003;1003:395–397. doi: 10.1196/annals.1300.034. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould AT, Sacramento AD, Wroten MG, Miller BW, von Jonquieres G, Klugmann M, Ben-Shahar O, Szumlinski KK. Cocaine-elicited imbalances in ventromedial prefrontal cortex Homer1 versus Homer2 expression: implications for relapse. Addict Biol. 2015;20(1):148–157. doi: 10.1111/adb.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, Szumlinski KK. Accumbens Homer2-mediated signaling: a factor contributing to mouse strain differences in alcohol drinking? Genes Brain Behav. 2011;10:111–126. doi: 10.1111/j.1601-183X.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA, London ED, Jentsch JD. Dysregulation of D□-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci. 2013;32(17):5843–852. doi: 10.1523/JNEUROSCI.0029-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruss M, Bredenkötter M, Braun K. N-methyl-D-aspartate receptor-mediated modulation of monoaminergic metabolites and amino acids in the chick forebrain: an in vivo microdialysis and electrophysiology study. J Neurobiol. 1999;40(1):116–135. doi: 10.1002/(sici)1097-4695(199907)40:1<116::aid-neu10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Gubner NR, Reed C, McKinnon CS, Phillips TJ. Unique genetic factors influence sensitivity to the rewarding and aversive effects of methamphetamine versus cocaine. Behav Brain Res. 2013;256:420–427. doi: 10.1016/j.bbr.2013.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider A, Woodward NC, Lominac KD, Sacramento AD, Klugmann M, Bell RL, Szumlinski KK. Homer2 within the nucleus accumbens core bidirectionally regulates alcohol intake by both P and Wistar rats. Alcohol. 2015;S0741–8329(15):20281–289. doi: 10.1016/j.alcohol.2015.03.009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Wang F, Qi J, Wang F, Zhang L, Zhao S, Song M, Wu C, Yang J. NMDA receptors in the medial prefrontal cortex and the dorsal hippocampus regulate methamphetamine-induced hyperactivity and extracellular amino acid release in mice. Behav Brain Res. 2012;232(1):44–52. doi: 10.1016/j.bbr.2012.03.038. [DOI] [PubMed] [Google Scholar]
- Harkness JH, Shi X, Janowsky A, Phillips TJ. Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits. Neuropsychopharmacology. 2015;40:2175–84. doi: 10.1038/npp.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry PK, Murnane KS, Votaw JR, Howell LL. Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav. 2010;4:212–219. doi: 10.1007/s11682-010-9100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrold AA, Voigt RM, Napier TC. Brain region-selective cellular redistribution of mGlu5 but not GABA(B) receptors following methamphetamine-induced associative learning. Synapse. 2011;65(12):1333–343. doi: 10.1002/syn.20968. [DOI] [PubMed] [Google Scholar]
- Herrold AA, Persons AL, Napier TC. Cellular distribution of AMPA receptor subunits and mGlu5 following acute and repeated administration of morphine or methamphetamine. J Neurochem. 2013a;126(4):503–517. doi: 10.1111/jnc.12323. [DOI] [PubMed] [Google Scholar]
- Herrold AA, Voigt RM, Napier TC. mGluR5 is necessary for maintenance of methamphetamine-induced associative learning. Eur Neuropsychopharmacol. 2013b;23(7):691–696. doi: 10.1016/j.euroneuro.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Stefani MR, Adams BW, Tamagan GD, Moghaddam B. Functional Interaction Between NMDA and mGlu5 Receptors: Effects on Working Memory, Instrumental Learning, Motor Behaviors, and Dopamine Release. Neuropsychopharmacology. 2004;29(7):1259–269. doi: 10.1038/sj.npp.1300417. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26(31):8025–39. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45(5):647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32(4):617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Sung YH, Lee HY, Lee DS, Jeong DU, Renshaw PF. Frontal glucose hypometabolism in abstinent methamphetamine users. Neuropsychopharmacology. 2005;30(7):1383–391. doi: 10.1038/sj.npp.1300699. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Oleson EB, Pava M, Klugmann M, Schwarz MK, Seeburg PH, During MJ, Worley PF, Kalivas PW, Szumlinski KK. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25(50):11586–11594. doi: 10.1523/JNEUROSCI.3764-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE. Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology. 2012;37(3):707–722. doi: 10.1038/npp.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Phillips TJ, Szumlinski KK. Mesocorticolimbic monoamine correlates of methamphetamine sensitization and motivation. Front Systems Neurosci. 2014;8:70. doi: 10.3389/fnsys.2014.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London ED, Kohno M, Morales AM, Ballard ME. Chronic methamphetamine abuse and corticostriatal deficits revealed by neuroimaging. Brain Res. 2014 doi: 10.1016/j.brainres.2014.10.044. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117(3):697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Chafee MV. Translational and developmental perspective on N-methyl-D-aspartate synaptic deficits in schizophrenia. Dev Psychopathol. 2006;18(3):853–876. [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62(2):91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Vuthiganon J, Kalivas PW. Regulation of extracellular glutamate in the prefrontal cortex: focus on the cystine glutamate exchanger and group I metabotropic glutamate receptors. J Pharmacol Exp Ther. 2005;314:139–147. doi: 10.1124/jpet.104.081521. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17(8):2921–927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194(3):287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RH, Allen CN, Derkach VA, Phillips TJ, Belknap JK, Raber J. Impaired memory and reduced sensitivity to the circadian period lengthening effects of methamphetamine in mice selected for high methamphetamine consumption. Behav Brain Res. 2013;256:197–204. doi: 10.1016/j.bbr.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, Robbins TW. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology. 2000;23(2):113–126. doi: 10.1016/S0893-133X(00)00097-X. [DOI] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Jr, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69(3):253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, See RE. Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology. 2014;39(4):811–822. doi: 10.1038/npp.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Maryland Heights, MO: Academic Press; 2007. [Google Scholar]
- Quadir SG, Santos JR, Campbell RR, Wroten MG, Singh N, Holloway JJ, Bal SK, Camarini R, Szumlinski KK. Homer2 regulates alcohol and stress cross-sensitization. Addict Biol. 2015 Apr 27; doi: 10.1111/adb.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36(4):782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyniak DE. Neurologic manifestations of chronic methamphetamine abuse. Neurol Clin. 2011;29:641–655. doi: 10.1016/j.ncl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: a functional magnetic resonance imaging study. Biol Psychiatry. 2009;65(8):706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict Biol. 2006;11(1):2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See RE, Pacchioni AM, McGinty JF, Kalivas PW. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharmacol Exp Ther. 2009;331(2):555–562. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Trantham-Davidson H, Schwendt M, Leong KC, Peters J, See RE, Reichel CM. Failure to Recognize Novelty after Extended Methamphetamine Self-Administration Results from Loss of Long-Term Depression in the Perirhinal Cortex. Neuropsychopharmacology. 2015 Apr 13; doi: 10.1038/npp.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, McKinnon CS, Reed CR, Cunningham CL, Phillips TJ. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav. 2011;10:625–636. doi: 10.1111/j.1601-183X.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, Dobbs LK, Ford MM, Mark GP, Finn DA, Phillips TJ. A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology. 2012a;62:2169–2177. doi: 10.1016/j.neuropharm.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabani S, Mckinnon CS, Cunningham CL, Phillips TJ. Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology. 2012b;62:1134–1141. doi: 10.1016/j.neuropharm.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CB, Serchia MM, Shahin JR, Ruppert-Majer MA, Kippin TE, Szumlinski KK. Incubation of cocaine-craving related to glutamate overflow within ventromedial prefrontal cortex. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirashi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8(2):206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Sullivan EB, Maisonneuve IM, Glick SD. Neurochemical and behavioral differences between d-methamphetamine and d-amphetamine in rats. Psychopharmacology (Berl) 2003;165(4):359–369. doi: 10.1007/s00213-002-1288-7. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: Roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Effect of repeated methamphetamine administration on dopamine and glutamate efflux in rat prefrontal cortex. Brain Res. 1995;700:99–106. doi: 10.1016/0006-8993(95)00938-m. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD. Homers regulate drug-induced neuroplasticity: implications for addiction. Biochem Pharmacol. 2008a;75(1):112–133. doi: 10.1016/j.bcp.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008b;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Balogun MY, Maisonneuve IM, Glick SD. Interactions between iboga agents and methamphetamine sensitization: studies of locomotion and stereotypy in rats. Psychopharmacology (Berl) 2000;151(2–3):234–241. doi: 10.1007/s002130000478. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Rohrer J, Klugmann M, Griffin W, 3rd, Toda S, Champtiaux NP, Berry T, Tu JC, Shealy SE, During MJ, Middaugh LD, Worley PF, Kalivas PW. Homer proteins regulate vulnerability to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25(30):7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Glutamatergic regulation of basal and stimulus-activated dopamine release in the prefrontal cortex. J Neurochem. 1998;71(4):1443–449. doi: 10.1046/j.1471-4159.1998.71041443.x. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2015. 2015 (United Nations publication, Sales No. E.15.XI.6) [Google Scholar]
- Wheeler JM, Reed C, Burkhard-Kasch S, Li, Cunningham CL, Janowsky A, Franken FH, Wiren KM, Hashimoto JG, Scibelli AC, Phillips TJ. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav. 2009;8:758–771. doi: 10.1111/j.1601-183X.2009.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo DY, Zhang YH, Cao Y, Wu CF, Tanaka M, Wu YL. Effect of acute and chronic MK-801 administration on extracellular glutamate and ascorbic acid release in the prefrontal cortex of freely moving mice on line with open-field behavior. Life Sci. 2006;78(19):2172–178. doi: 10.1016/j.lfs.2005.09.022. [DOI] [PubMed] [Google Scholar]