Abstract
Rationale
Calcium/calmodulin-dependent protein kinase II (CaMKII) is activated in heart failure (HF) and can contribute to arrhythmias induced by β-adrenergic receptor-mediated sarcoplasmic reticulum calcium leak.
Objective
To evaluate the effect of CaMKII inhibition on ventricular tachycardia (VT) induction in conscious HF and naïve rabbits.
Methods and Results
Nonischemic HF was induced by aortic insufficiency and constriction. Electrocardiograms were recorded in rabbits pretreated with vehicle (saline) or the CaMKII inhibitor KN-93 (300 μg/kg); VT was induced by infusion of increasing doses of norepinephrine (NE, 1.56-25 μg/kg/min) in naïve (n = 8) and HF (n = 7) rabbits. With saline, median VT dose threshold in HF was 6.25 versus 12.5 μg/kg/min NE in naïve rabbits (p = 0.06). Pretreatment with KN-93 significantly increased VT threshold in HF and naïve rabbits (median = 25 μg/kg/min, p < 0.05 versus saline for both groups). Mean cycle length of VT initiation was shorter in HF (221 ± 20 ms) than naïve (296 ± 23 ms, p < 0.05) rabbits with saline; this difference was not significant after treatment with KN-93.
Conclusions
KN-93 significantly reduced arrhythmia inducibility and slowed initiation of VT, suggesting that CaMKII inhibition may have antiarrhythmic effects in the failing human heart.
Keywords: calcium/calmodulin-dependent protein kinase II, norepinephrine, arrhythmia, antiarrhythmic drugs, heart failure
Introduction
Recently, calcium/calmodulin-dependent protein kinase II (CaMKII) has emerged as a promising target to address arrhythmogenesis in heart failure (HF). CaMKII, which is activated in the failing heart,[1] is a critical regulator of multiple ion channels and calcium-handling proteins implicated in arrhythmogenesis. Despite abundant in vitro data linking CaMKII to arrhythmias in hypertrophied or failing hearts (see Swaninathan et al. for review[1]), few studies have directly tested the antiarrhythmic effect of CaMKII inhibition in HF in vivo,[2–5] with those that have largely limited to studies using transgenic mouse models. It is well established that mice exhibit profound differences in action potential (AP) shape, ionic currents, calcium handling and contractile protein isoforms compared to either rabbit or human hearts.[6, 7] Thus, in vivo tests of CaMKII inhibition are lacking in clinically relevant animal models of HF. We have extensively characterized molecular mechanisms underlying CaMKII activation in our well-characterized arrhythmogenic model of nonischemic HF, including showing that enhanced CaMKII activation and CaMKII-dependent phosphorylation of the cardiac ryanodine receptor (RyR2) are involved in enhanced diastolic sarcoplasmic reticulum (SR) calcium leak in HF.[8] These HF rabbits have many hallmarks of human HF, including contractile dysfunction, spontaneous ventricular arrhythmias, a 10% incidence of sudden death,[9] spontaneous SR calcium release,[10, 11] and delayed afterdepolarizations (DADs).[8, 10] This model is ideally suited to study the antiarrhythmic effects of CaMKII inhibition. The goal of these studies was to determine whether inhibition of CaMKII is effective in decreasing arrhythmias in the failing heart.
Materials and Methods
Animals
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Animal Care and Use Committee of the University of Alabama at Birmingham.
Naïve (n = 8) and HF (n = 7) adult New Zealand White rabbits of either sex (approximately 50% of each sex in each group, no statistically significant differences in the proportions for sex) were used in this study. Nonischemic HF was induced by combined aortic insufficiency and subsequent (at least 2 weeks after the initial surgery) abdominal aortic constriction, as has been previously described.[9, 10, 12] Prior to the induction of HF, each rabbit underwent echocardiographic examination to assess baseline contractile function. Measurements of left ventricular end-diastolic (LVEDD) and left ventricular end-systolic (LVESD) dimensions (cm) were obtained from M-mode echocardiograms. Fractional shortening (FS) was computed as: FS (%) = (LVEDD – LVESD)/LVEDD. Echoes were repeated after the induction of HF to confirm the presence of contractile dysfunction in the HF rabbits.[9, 12] Rabbits were studied 6.6 ± 1.3 months after aortic constriction. In this model, greater than 10% of the HF rabbits die suddenly, with 24-hour Holter monitoring demonstrating frequent nonreentrant premature ventricular complexes and spontaneous runs of nonsustained ventricular tachycardia (VT) in 90% of these animals.[9, 12]
Chemicals and reagents
Norepinephrine bitartrate (NE, 1 mg/mL) was purchased from Hospira (Levophed®; Lake Forest, IL). For in vivo infusion studies, NE was diluted with sterile saline on the day of the experiment. A low concentration (12.5 μg/mL) and a high concentration (100 μg/mL) NE working solution were prepared to allow for a wide range of NE doses (1.56, 3.13, 6.25, 12.5, and 25.0 μg/kg/min) to be infused by a syringe pump. The CaMKII-inhibitor KN-93 was purchased from Calbiochem (San Diego, CA). KN-93 was dissolved in sterile water (1 mg/mL) fresh daily, further diluted with sterile saline based on animal weight, and then filter sterilized (0.22 μm pore size) to yield a final volume of 3 mL at a dose of 300 μg/kg, which has previously been shown to inhibit CaMKII in rabbits in vivo.[13]
NE infusion protocol
Given that residual β-adrenergic responsiveness contributes to enhanced arrhythmogenesis in HF,[10] we designed an infusion protocol using the adrenergic agonist NE to induce VT in vivo. By using increasing doses of NE, we were able to determine a threshold for arrhythmia inducibility for each individual rabbit (naïve and HF). Conscious rabbits were placed in a restrainer in a quiet room. An arterial line was placed in the median ear artery and connected to a blood pressure transducer to allow monitoring of blood pressure during the course of the study. In some instances we were unable to obtain consistent blood pressure measurements throughout the entirety of the study, particularly in HF rabbits where paired blood pressure recordings were only obtained in 3 of the 7 HF rabbits. For Table 3, blood pressure data was only compared for studies in which paired data were available. A 22-gauge catheter was placed in the lateral ear vein for intravenous infusion. Needle-electrodes were placed subcutaneously at the proximal portion of each limb and a 6-lead electrocardiogram (ECG) was recorded using a Bard Electrophysiology system (C.R. Bard Inc; Lowell, MA) to monitor heart rate and rhythm. ECG signals were recorded at 4 kHz sampling with a low frequency cutoff at 0.1 Hz, high frequency cutoff at 100 Hz, and a notch filter at 60 Hz.
Table 3.
Arterial blood pressure after pretreatment with saline (vehicle) or KN-93.
| SBP (mmHg) | DBP (mmHg) | MAP (mmHg) | ||
|---|---|---|---|---|
| Naïve | vehicle | 106.7 ± 3.9 | 80.5 ± 2.3 | 89.3 ± 2.4 |
| KN-93 | 104.1 ± 2.4 | 78.6 ± 1.3 | 87.1 ± 1.6 | |
| HF | vehicle | 74.3 ± 4.8* | 55.2 ± 7.5* | 61.6 ± 6.1* |
| KN-93 | 78.3 ± 3.1* | 54.3 ± 2.6* | 62.3 ± 2.8* |
SBP: systolic blood pressure, DBP: diastolic blood pressure, MAP: mean arterial pressure. Naïve, n = 7; HF, n = 3; mean ± SE;
p < 0.05 vs naïve.
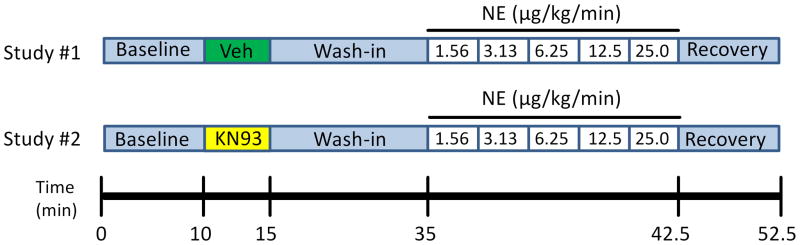
To test the effect of the CaMKII-inhibitor KN-93 on arrhythmia inducibility, increasing doses of NE were infused after pretreatment with either KN-93 (300 μg/kg) or vehicle (saline) in paired studies conducted at least 1 week apart. A diagram of the NE infusion protocol is depicted in Figure 1. After a 10 minute baseline period, the conscious rabbits were pretreated with an intravenous bolus (3 mL delivered over 5 minutes) of either KN-93 (300 μg/kg dissolved in 3 mL of saline) or vehicle (3 mL saline). This was followed by a 20 minute wash-in period to allow the drug to take effect. At the end of the wash-in period, a syringe pump (Medex Inc.; Duluth, GA) was used to infuse increasing doses of NE: 1.56 – 25.0 μg/kg/min. Each dose was delivered for 90 seconds. Once VT was induced, the infusion was stopped and the animal was allowed to recover (the ECG was recorded for 10 minutes after the cessation of the NE infusion). For the purpose of this study, VT was defined as 5 consecutive wide complex ventricular beats with a mean and/or minimum cycle length (CL) less than the baseline CL during normal sinus rhythm.
Figure 1.
Diagram of the NE infusion protocol. In paired studies, 6-lead ECGs were recorded in conscious naïve (n = 8) and HF (n = 7) rabbits. A 10 minute baseline period was followed by pretreatment with saline (vehicle, Veh) or the CaMKII inhibitor KN-93. After a 20 minute wash-in period, increasing doses of NE (1.56 – 25 μg/kg/min) were infused intravenously for 90 seconds at each dose. Once VT occurred, the infusion was stopped and the rabbit was monitored for a 10 minute recovery period to verify the return of sinus rhythm.
Data Analysis
All electrophysiologic measurements were made using the Lab System Pro v.2.6a software package on the Bard Electrophysiology system. The dose threshold was defined as the dose of NE at which VT was induced, and reported as the median dose for summary data. ECG parameters measured during each stage of the protocol included the PR interval, QRS duration, RR interval, and the QT interval. The corrected QT interval (QTc) was calculated using a regression algorithm specifically created for use in conscious rabbits: QTc = QT/(RR)0.72.[14] Measurements from 5 consecutive beats during sinus rhythm were taken at the end of each stage of the protocol; results are reported as mean ± standard error of the mean (SE). The VT threshold events were characterized by duration (number of beats) and rate. In addition to the overall mean VT CL, the initial (first 5 CLs), terminal (last 5 CLs), and minimum (shortest CL of the VT event) CLs were reported (mean ± SE) for the threshold VT event. The coupling interval between the last sinus beat and the first beat of the VT (S-VT) was also measured, as well as the mean CL of the 5 consecutive sinus beats preceding the VT (Pre-VT mean). Systolic and diastolic blood pressure (SBP and DBP, respectively) were continuously monitored, and mean arterial pressure (MAP) was calculated as MAP (mmHg) = DBP + (SBP − DBP)/3.
Statistical analyses were performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC). Descriptive statistics were computed for all study variables; thresholds were reported as median values. The exact Wilcoxon signed-rank was used to compare the vehicle-pretreated dose thresholds to the KN93-pretreated dose thresholds (paired data). Comparisons of the dose thresholds between the groups (HF and naïve) were performed using the exact Wilcoxon rank-sum test. Fisher’s exact test was used to compare proportions (for sex) between the groups. Since no significant sex differences were detected, the male and female datasets were combined to increase sample size. For all other data, the unpaired t-test was used to assess differences between means for HF versus naïve, and the paired t-test was used to assess changes in means for vehicle versus KN-93. Nonparametric statistical analyses (including the exact Wilcoxon rank-sum test and Wilcoxon signed-rank test) corresponding to the prior analyses were performed, and these analyses yielded results that are similar to those obtained by the prior analyses. All statistical tests were two-sided and were performed using a significance level of 5% (i.e. α = 0.05).
Results
Echocardiographic assessment of in vivo contractile function
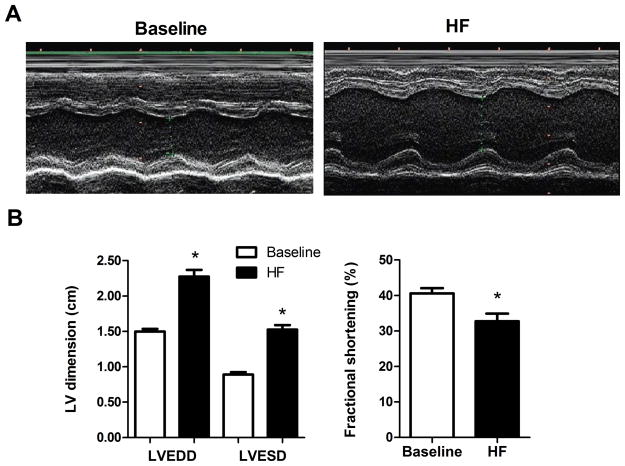
Induction of nonischemic HF by aortic insufficiency and aortic constriction led to significant cardiac enlargement and contractile dysfunction (Figure 2). M-mode echocardiograms showed that in the failing hearts (HF), LVEDD was increased 52.0 ± 5.2% and LVESD was increased 72.6 ± 9.7% compared to their baseline (pre-HF induction) values (p < 0.001). This led to a significant decrease in FS from 40.6 ± 1.5% at baseline to 32.7 ± 2.2% after HF induction (Figure 2, p < 0.05).
Figure 2.
Echocardiographic assessment of failing hearts. A) Paired representative m-mode echocardiograms pre- (Baseline) and post- induction of HF showing changes in the left ventricular end-diastolic (LVEDD) and left ventricular end-systolic (LVESD) dimensions (cm). B) Summary data for changes in LVEDD, LVESD, and fractional shortening (FS) associated with the induction of HF. *p < 0.05 HF vs Baseline.
CaMKII inhibition increases the threshold for VT
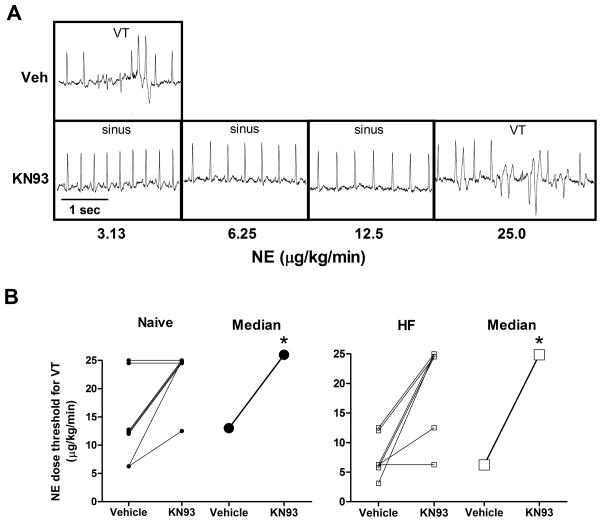
With vehicle pretreatment (saline), subsequent NE infusion induced VT in all animals, and all but one (naïve) animal when pretreated with KN-93. In all cases, VT self-terminated upon cessation of the NE infusion. Shown in Figure 3A are ECG traces from paired studies in a HF rabbit. When pretreated with saline, this HF rabbit developed VT at 3.13 μg/kg/min NE. After pretreatment with KN-93, the maximal dose of NE (25 μg/kg/min) was required to induce VT in this rabbit. Figure 3B shows the paired and median VT thresholds for all naïve (left) and HF (right) rabbits. During the vehicle studies, there was a strong trend (p = 0.06) for the VT threshold to be lower in HF than the naïve group (median = 6.25 versus 12.5 μg/kg/min, respectively). Pretreatment with KN-93 significantly increased the VT threshold in both naive and HF rabbits (median = 25.0 μg/kg/min, p < 0.05 versus vehicle for both groups).
Figure 3.
Pretreatment with KN-93 increases the arrhythmia threshold during NE infusion. A) ECG traces from paired studies in a HF rabbit during pretreatment with saline (Veh) or KN-93. With Veh, VT was induced during infusion of NE at 3.13 μg/kg/min. With KN-93, VT was not induced until the maximal dose, 25 μg/kg/min. B) Paired observations of individual (small symbols) and median (large symbols) values of the NE dose threshold for the induction of VT with pretreatment of saline (Vehicle) or KN-93 in naïve (n = 8, left) and HF (n = 7, right) rabbits. *p < 0.05 KN-93 vs Vehicle
CaMKII inhibition slows VT initiation in HF rabbits
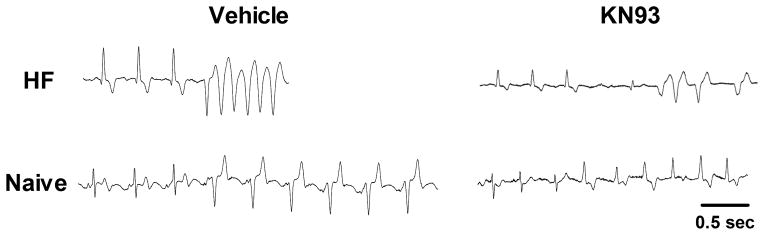
In addition to establishing the VT threshold, we also examined the characteristics of the VT events in HF versus naïve rabbits, as well as the effects of CaMKII inhibition. With vehicle, the initial VT CL (mean of the first 5 VT CLs) was significantly shorter (i.e. faster rate) in HF (CL = 221 ± 20 ms) than naïve rabbits (CL = 296 ± 23 ms, p < 0.05; Figure 4 and Table 1). Pretreatment with KN-93 selectively increased the initial VT CL (slower rate) in HF rabbits such that the difference with naïve rabbits was no longer significant (naïve: CL = 266 ± 27 ms, HF: CL = 267 ± 20 ms). Thus, once the threshold for VT was reached, in HF (but not naïve) rabbits the VT started at a slower rate when treated with KN-93. KN-93 did not affect the sinus rate preceding VT initiation (pre-VT), sinus to VT coupling interval (S-VT), nor any of the other VT CLs (Table 1) in either naïve or HF rabbits. Similarly, KN-93 did not change the duration (number of beats) of the threshold VT events (Table 1), or the number of ventricular beats (VT or ectopic) observed in the first 30 seconds after the initiation of the sentinel run of VT (saline: 67.5 ± 26.4 versus KN-93: 64.8 ± 22.0 beats, p = 0.87).
Figure 4.
Initiation of VT is faster in HF than naïve rabbits with saline pretreatment. Representative ECG traces at the time of VT initiation. Paired ECGs for HF (top) and naïve (bottom) rabbits are shown during pretreatment with saline (Vehicle, left) and KN-93 (right). Shown in each trace are the last 3 sinus beats preceding VT initiation. Note that during treatment with vehicle, the VT initiation (mean of first 5 CLs of VT) is faster in HF than naïve; this difference was diminished during pretreatment with KN-93. Mean cycle lengths of VT initiation shown for HF vehicle and KN93, and naïve vehicle and KN93 were 193, 254, 402 and 304 ms, respectively.
Table 1.
Ventricular tachycardia (VT) characteristics during the threshold event
| Sinus cycle length (ms)
|
# VT beats | VT cycle length (ms)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Pre-VT mean | S-VT | Initial | Mean | Min | Termination | |||
| Naïve | vehicle | 355.7 ± 38.9 | 339.8 ± 35.4 | 45.8 ± 26.9 | 295.9 ± 23.4 | 287.3 ± 18.6 | 183.0 ± 24.1 | 299.3 ± 19.6 |
| KN-93 | 369.0 ± 38.2 | 320.3 ± 36.0 | 146.6 ± 53.2 | 265.7 ± 26.6 | 241.5 ± 16.2 | 155.3 ± 16.7 | 245.5 ± 23.5 | |
| HF | vehicle | 349.3 ± 36.6 | 306.9 ± 35.0 | 75.1 ± 45.6 | 220.9 ± 19.6* | 237.5 ± 14.0 | 156.0 ± 16.3 | 274.8 ± 22.1 |
| KN-93 | 366.7 ± 53.2 | 299.3 ± 42.7 | 47.9 ± 40.9 | 267.0 ± 19.8 | 266.3 ± 20.7 | 191.9 ± 17.3 | 286.7 ± 17.3 | |
Naïve, n = 8; HF, n = 7; mean ± SE;
p < 0.05 vs naïve
Lack of electrophysiologic effects during sinus rhythm with CaMKII inhibition
We assessed the effects of KN-93 on several ECG parameters during sinus rhythm at the end of the wash-in period (see diagram of protocol in Figure 1). As shown in Table 2, the PR interval and QRS duration were significantly longer in HF than naïve rabbits, as is commonly found clinically in HF patients.[15–17] There were no significant differences in HF and naïve rabbits for the QTc or RR intervals. Pretreatment with KN-93 did not significantly change the PR, QTc, or RR intervals, nor the QRS duration in HF or naïve rabbits (p = NS versus vehicle for all). While MAP was significantly higher in naïve than HF rabbits, pretreatment with KN-93 had no effect on MAP (Table 3).
Table 2.
Electrophysiologic properties during sinus rhythm after pretreatment with saline (vehicle) or KN-93.
| PR (ms) | QRS (ms) | RR (ms) | QTc (ms) | ||
|---|---|---|---|---|---|
| Naïve | vehicle | 68.1 ± 1.6 | 38.0 ± 1.4 | 263.7 ± 8.8 | 389.9 ± 7.2 |
| KN-93 | 68.6 ± 1.6 | 37.0 ± 0.9 | 273.8 ± 11.4 | 376.7 ± 8.2 | |
| HF | vehicle | 77.6 ± 2.0* | 44.3 ± 2.3* | 264.4 ± 14.7 | 383.8 ± 6.1 |
| KN-93 | 75.8 ± 1.4* | 45.3 ± 2.5* | 272.0 ± 10.1 | 396.9 ± 14.0 |
Naïve, n = 8; HF, n = 7; mean ± SE;
p < 0.05 vs naïve
Discussion
It is widely accepted that in HF CaMKII signaling modulates key ion channels and SR calcium handling proteins leading to proarrhythmic effects such as QT prolongation,[18] early afterdepolarizations (EADs),[2, 3] spontaneous SR calcium leak,[8, 19] spontaneous calcium waves,[11] and DADs.[3] However, direct evidence of the effects of CaMKII inhibition on arrhythmogenesis from in vivo studies in clinically relevant models of HF is lacking. We sought to examine the antiarrhythmic effects of CaMKII inhibition in a HF model that more closely mimics human HF, and in a species whose AP and calcium transient characteristics are more similar to human.[7]
Previous characterization of our nonischemic HF rabbit model has shown that both spontaneous and induced arrhythmias are due to a nonreentrant mechanism.[9, 10, 12] Isolated myocytes from these HF rabbits exhibited spontaneous SR calcium release leading to DAD-mediated triggered activity.[10] Subsequent mechanistic studies extensively assessing the expression and phosphorylation status of key calcium handling proteins provided further insight into the nature of the observed spontaneous calcium release. We found that in our HF rabbits, there was a 50% decrease in RyR2 mRNA (versus controls) and 30% decrease in RyR2 protein expression.[8] Furthermore, the expression and activation of CaMKII in the RyR2 macromolecular complex was increased by 96% and 105%, respectively, while the expression of calmodulin, FK-506 binding protein 12.6, and phosphatases 1 and 2a were reduced by 30–45%, which led to enhanced RyR2 phosphorylation.[8] Phospholamban phosphorylation was increased at the CaMKII site, and CaMKII inhibition with KN-93 increased SR calcium content.[8] These changes were associated with an increase in diastolic SR calcium leak that was reduced by inhibiting CaMKII (but not protein kinase A), thereby implicating CaMKII-dependent phosphorylation of RyR2 as a mechanism for spontaneous calcium release in HF.[8] Indeed, decreasing SR calcium leak in these HF myocytes by inhibiting CaMKII with KN-93 resulted in fewer spontaneous calcium waves during treatment with the adrenergic agonist isoproterenol.[11, 19] However, the question remained whether inhibiting CaMKII (with KN-93) could prevent arrhythmias in vivo, particularly in a clinically relevant HF model.
In CaMKIIδC overexpressing mice with HF, the frequency of induced arrhythmias decreased after pretreatment with KN-93.[3] In a knock-in mouse model expressing RyR2 with a CaMKII phospho-mimetic mutation, transaortic constriction led to increased mortality.[4] In two (out of 10) cases, these deaths were attributed to spontaneous VT and genetic ablation of the CaMKII phosphorylation site significantly reduced the incidence of pacing-induced VT.[4] In contrast to the studies in mice, CaMKII inhibition by KN-93 in ischemic HF rabbits with drug-induced torsades de pointes (TdP) failed to significantly reduce TdP incidence or time of onset.[5] This difference may be due to the HF etiology or the mechanism of TdP induction (IKr inhibition resulting in AP duration (APD) prolongation and EADs), although KN-93 has been shown to suppress EADs induced by oxidative stress in rabbit ventricular myocytes[20] and EAD-mediated arrhythmias in transgenic mice overexpressing a constitutively active form of CaMKIV.[2] In the current study, in vivo pretreatment with KN-93 increased the arrhythmia threshold without altering heart rate (i.e. RR interval) or the QTc interval (Table 2), which suggests ventricular repolarization was unaffected, thereby arguing against a reentrant mechanism or even an APD-dependent triggered mechanism (i.e. EADs). Altogether, this evidence suggests KN-93 increased the threshold for VT by suppressing CaMKII-dependent DAD-mediated nonreentrant arrhythmias.
Further analysis of the threshold VT events revealed that the antiarrhythmic effect of KN-93 in HF rabbits was not simply the result of shifting the threshold, but it actually slowed VT. Thus, when treated with KN-93, despite occurring at a higher dose of NE, the threshold VT events started at a slower rate (i.e. longer CL). Consistent with this finding, CaMKII inhibition with KN-93 has been shown to decrease calcium spark frequency[3, 4, 21] and the frequency of nonstimulated contractile events (i.e. spontaneous calcium waves).[3, 21] Similarly, Curran et al.[11] found that treatment with KN-93 delayed the initiation time (longer coupling interval) for pacing-induced spontaneous calcium waves.
A potential limitation of previous studies testing CaMKII inhibition in vivo[3, 4] was the use of anesthetized animals for arrhythmia testing, as anesthetic agents have been shown to modulate the inducibility of ventricular arrhythmias in vivo.[22] A key feature of our protocol was that arrhythmia testing was conducted in unanesthetized animals. The infusion of NE, the endogenous catecholamine that has been shown to induce DADs in failing rabbit and human hearts,[23] provides a novel approach for testing antiarrhythmic treatments in a conscious animal model of HF. Our results show that pretreatment with KN-93 increases the NE threshold for arrhythmia inducibility in conscious rabbits, independent of effects on heart rate or QT interval, and slowed the initiation of VT. These findings indicate CaMKII inhibition as a viable target for future antiarrhythmic therapy in HF.
Acknowledgments
Funding: This work was supported by an American Heart Association Predoctoral Fellowship (GSH) and grants from the NIH (SMP and DMB, P01-HL80101; RO, UL1TR001417) and Fondation LeDucq (SMP and DMB, 08CVD01).
We would like to thank Frank Vance, Shannon Salter, Dennis Rollins, Yujie Zhu, and Sharon Melnick for their technical assistance during the infusion studies.
References
- 1.Swaminathan PD, et al. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110(12):1661–77. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Y, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106(10):1288–93. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 3.Sag CM, et al. Calcium/calmodulin-dependent protein kinase II contributes to cardiac arrhythmogenesis in heart failure. Circ Heart Fail. 2009;2(6):664–75. doi: 10.1161/CIRCHEARTFAILURE.109.865279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Oort RJ, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122(25):2669–79. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kijtawornrat A, et al. Effects of sarcolemmal Ca(2+) entry, ryanodine function, and kinase inhibitors on a rabbit model of heart failure. Int Heart J. 2010;51(4):285–90. doi: 10.1536/ihj.51.285. [DOI] [PubMed] [Google Scholar]
- 6.Wagner S, et al. Ca/calmodulin kinase II differentially modulates potassium currents. Circ Arrhythm Electrophysiol. 2009;2(3):285–94. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pogwizd SM, Bers DM. Rabbit models of heart disease. Drug Discovery Today: Disease Models. 2008;5(3):185–193. doi: 10.1016/j.ddmod.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ai X, et al. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97(12):1314–22. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 9.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation. 1995;92(4):1034–48. doi: 10.1161/01.cir.92.4.1034. [DOI] [PubMed] [Google Scholar]
- 10.Pogwizd SM, et al. Arrhythmogenesis and contractile dysfunction in heart failure: Roles of sodium-calcium exchange, inward rectifier potassium current, and residual beta-adrenergic responsiveness. Circ Res. 2001;88(11):1159–67. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- 11.Curran J, et al. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca(2+)-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010;49(1):25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pogwizd SM, et al. Upregulation of Na(+)/Ca(2+) exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res. 1999;85(11):1009–19. doi: 10.1161/01.res.85.11.1009. [DOI] [PubMed] [Google Scholar]
- 13.Lange M, et al. Desflurane-induced postconditioning is mediated by beta-adrenergic signaling: role of beta 1- and beta 2-adrenergic receptors, protein kinase A, and calcium/calmodulin-dependent protein kinase II. Anesthesiology. 2009;110(3):516–28. doi: 10.1097/ALN.0b013e318197ff62. [DOI] [PubMed] [Google Scholar]
- 14.Kijtawornrat A, et al. Assessment of drug-induced QT interval prolongation in conscious rabbits. J Pharmacol Toxicol Methods. 2006;53(2):168–73. doi: 10.1016/j.vascn.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Wessler BS, et al. Clinical Implications of the PR Interval in Patients Hospitalized for Worsening Heart Failure and Reduced Ejection Fraction: Analysis of The EVEREST Study. Journal of Cardiac Failure. 20(8):S103–S104. [Google Scholar]
- 16.Kutyifa V, et al. PR interval identifies clinical response in patients with non-left bundle branch block: a Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy substudy. Circ Arrhythm Electrophysiol. 2014;7(4):645–51. doi: 10.1161/CIRCEP.113.001299. [DOI] [PubMed] [Google Scholar]
- 17.Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46(12):2183–92. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 18.Wagner S, et al. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116(12):3127–38. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res. 2003;93(7):592–4. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 20.Xie LH, et al. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104(1):79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonano LA, et al. Calcium-calmodulin kinase II mediates digitalis-induced arrhythmias. Circ Arrhythm Electrophysiol. 2011;4(6):947–57. doi: 10.1161/CIRCEP.111.964908. [DOI] [PubMed] [Google Scholar]
- 22.Hunt GB, Ross DL. Comparison of effects of three anesthetic agents on induction of ventricular tachycardia in a canine model of myocardial infarction. Circulation. 1988;78(1):221–6. doi: 10.1161/01.cir.78.1.221. [DOI] [PubMed] [Google Scholar]
- 23.Vermeulen JT, et al. Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovasc Res. 1994;28(10):1547–54. doi: 10.1093/cvr/28.10.1547. [DOI] [PubMed] [Google Scholar]