Abstract
Ultraviolet blood irradiation (UBI) was extensively used in the 1940s and 1950s to treat many diseases including septicemia, pneumonia, tuberculosis, arthritis, asthma and even poliomyelitis. The early studies were carried out by several physicians in USA and published in the American Journal of Surgery. However with the development of antibiotics, the use of UBI declined and it has now been called “the cure that time forgot”. Later studies were mostly performed by Russian workers and in other Eastern countries, and the modern view in Western countries is that UBI remains highly controversial. This review discusses the potential of UBI as an alternative approach to current methods used to treat infections, as an immune-modulating therapy and as a method for normalizing blood parameters. Low and mild doses of UV kill microorganisms by damaging the DNA, while any DNA damage in host cells can be rapidly repaired by DNA repair enzymes. However the use of UBI to treat septicemia cannot be solely due to UV-mediated killing of bacteria in the bloodstream, as only 5–7% of blood volume needs to be treated with UV to produce the optimum benefit, and higher doses can be damaging. There may be some similarities to extracorporeal photopheresis (ECP) using psoralens and UVA irradiation. However there are differences between UBI and ECP in that UBI tends to stimulate the immune system, while ECP tends to be immunosuppressive. With the recent emergence of bacteria that are resistant to all known antibiotics, UBI should be more investigated as an alternative approach to infections, and as an immune-modulating therapy.
Keywords: ultraviolet irradiation of blood, systemic infections, DNA repair, blood cells, phagocytes, lymphocytes, extracorporeal photopheresis, bone marrow, cytokines
1 Historical Introduction
Ultraviolet (UV) radiation is part of the electromagnetic spectrum with a wavelength range (100–400 nm) shorter than that of visible light (400–700 nm), but longer than x-rays (<100 nm). UV radiation is divided into four distinct spectral areas including vacuum UV (100–200 nm), UVC (200–280 nm), UVB (280–315 nm) and UVA (315–400 nm).
In 1801 Johann Wilhelm Ritter, a Polish physicist working at the University of Jena in Germany discovered a form of light beyond the violet end of the spectrum that he called “Chemical Rays” and which later became known as “Ultraviolet” light [1]. In 1845, Bonnet [2] first reported that sunlight could be used to treat tuberculosis arthritis (a bacterial infection of the joints).
In the second half of the 19th century, the therapeutic application of sunlight (known as heliotherapy) gradually became popular. In 1855, Rikli from Switzerland opened a thermal station in Veldes in Slovenia for the provision of heliotherapy [3]. In 1877, Downes and Blunt discovered [4] by chance that sunlight could kill bacteria. They noted that sugar water placed on a window-sill turned cloudy in the shade but remained clear while kept in the sun. Upon microscopic examination of the two solutions, they realized that bacteria were growing in the shaded solution but not in the one exposed to sunlight.
In 1904, the Danish physician Niels Finsen was awarded the Nobel Prize in Physiology or Medicine for his work on UV treatment of various skin conditions. He had a success rate of 98% in thousands of cases, mostly the form of cutaneous tuberculosis known as lupus vulgaris [5]. Walter H Ude reported a series of 100 cases of erysipelas (a cutaneous infection caused by Streptococcus pyogenes) in the 1920s, that were treated with high cure rates using UV skin irradiation [6].
Emmett K Knott (Figure 1) in Seattle, WA reasoned that the beneficial effect of UV irradiation to the skin might (at least partly) be explained by the irradiation of blood circulating in the superficial capillaries of the skin. With his collaborator Edblom, an irradiation chamber was constructed to allow direct exposure of the blood to UV light. The irradiation chamber was circular and contained a labyrinthine passage connecting the inlet and outlet ports underneath the quartz window that formed the top of the chamber. The irradiation chamber was so designed as to provide maximum turbulence in order: (a) to prevent the formation of a film of blood on the chamber window that would absorb and filter out much of the UV; (b) to insure that all the blood passing through the chamber was equally exposed to UV [7].
Figure 1.

Emmett K Knott of Seattle, WA.
Knott and co-workers then carried out a series of experiments using UV irradiation of blood extracted from dogs that had been intravenously infected with Staphlyococcus aureus and hemolytic Streptococcus, and then the treated blood was reinfused. They found that it was unnecessary to deliver a sufficient exposure to the blood to kill all the bacteria directly. It was also found unnecessary to expose the total blood volume in the dogs. The optimum amount of blood to be irradiated was determined to be only 5–7% of the estimated blood volume or approximately 3.5 mL per kg of body weight. Exceeding these limits led to loss of the benefits of the therapy. All the treated dogs recovered from an overwhelming infection (while many dogs in the control group died), and none showed any ill effects after four months of observation [7].
The first treatment on a human took place in 1928 when a patient was determined to be in a moribund state after a septic abortion complicated by hemolytic streptococcus septicemia. UBI therapy was commenced as a last resort, and the patient responded to treatment and made a full recovery [7]. She proceeded to give birth to two children.
Hancock and Knott [8] had similar success in another patient with advanced hemolytic streptococcal septicemia. These workers noted that in the majority of cases, a marked cyanosis was present at the time of initiation of UBI. It was noted that during (or immediately following) the treatment a rapid relief of the cyanosis occurred with improvement in respiration accompanied by a noticeable flushing of the skin with a distinct loss of pallor.
These observations led to application of UBI in patients suffering from pneumonia. In a series of 75 cases in which the diagnoses of pneumonia were confirmed by X-rays, all patients responded well to UBI with a rapid fall in temperature, disappearance of cyanosis (often within 3–5 minutes), cessation of delirium if present, a marked reduction in pulse rate and a rapid resolution of pulmonary consolidation. A shortening of the time of hospitalization and convalescence occurred regularly.
The knowledge gained in these successful studies led to the redesign of the irradiation chamber to give a more thoroughly uniform exposure and led to the “Knott Technic of Ultraviolet Blood Irradiation.” A number of redesigned irradiation units (Figure 2) were manufactured and placed in the hands of physicians interested in the procedure, so that more clinical data could be accumulated [7]. The technique involved removing approximately 3.5 mL/kg venous blood, citrating it for anticoagulation, and passing it through a radiation chamber and reinfusing it. Exposure time per given unit amount was approximately 10 seconds, at a peak wavelength of 253.7 nm (ultraviolet C) provided by a mercury quartz burner and immediately re-perfused [7].
Figure 2.

The Knott Hemo-Irradiator.
George P Miley at the Hahnemann Hospital, Philadelphia, PA published a series of articles on the use of the procedure in the treatment of thrombophlebitis, staphylococcal septicemia, peritonitis, botulism, poliomyelitis, non-healing wounds, and asthma [9–22].
Henry A Barrett at the Willard Parker Hospital in New York City, in 1940 reported on 110 cases including a number of infections. Twenty-nine different conditions were described as responding including the following: infectious arthritis, septic abortion, osteoarthritis, tuberculosis glands, chronic blepharitis, mastoiditis, uveitis, furunculosis, chronic paranasal sinusitis, acne vulgaris, and secondary anemia [23, 24].
EV Rebbeck at the Shadyside Hospital in Pittsburgh, PA, reported the use of UBI in Escherichia coli septicemia, post-abortion sepsis, puerperal sepsis, peritonitis, and typhoid fever [25–29].
Robert C Olney at the Providence Hospital, Lincoln, NE, treated biliary disease, pelvic cellulitis and viral hepatitis with UBI [30–32].
UV irradiation of blood was hailed as a miracle therapy for treating serious infections in the 1940s and 1950s. However in an ironic quirk of fate, this time period coincided with the widespread introduction of penicillin antibiotics, which were rapidly found to be an even bigger miracle therapy. Moreover another major success of UBI, which was becoming used to treat polio, was also eclipsed by the introduction of the Salk vaccine. Starting in the 1960s UBI fell into disuse in the West and has now been called “the cure that time forgot” [33].
In this review, we will discuss the mechanisms and the potential of UBI as an alternative approach to infections and as a new method to modulate the immune system. Our goal is to remind people to continue to do more research and explore more clinical uses. The topics include the efficacy of UBI for infections (both bacterial and viral), to treat autoimmune disease, disease, the possible mechanisms of action, and a comparison with extracorporeal photopheresis.
2 Mechanisms of action of UBI
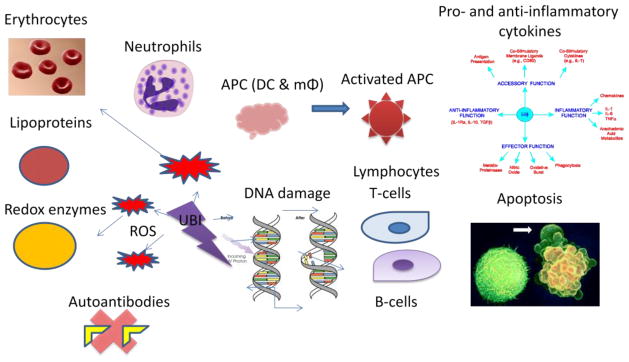
The use of UBI has been described to affect many different components of the blood. UBI can alter the function of leukocytes as proven in many in vitro studies. UV can increase stimulator cells in mixed leukocyte cultures, modulate helper cells in mitogen-stimulated cultures, UV can also reverse cytokine production and block cytokine release. UV can disturb cell membrane mobilization (Figure 3)
Figure 3.
Some mechanisms of action of UBI.
2.1 Effect on red cells
Anaerobic conditions were reported to strongly restrict the process by which long wave ultraviolet light could induce loss of K+ ions by red blood cells. Kabat showed that UV-irradiation could have an effect on the osmotic properties of red blood cells, altering their submicroscopic structure and affecting the metabolism of adenine nucleotides. Irradiation times (60, 120, 180, 240 and 300 minutes) were used. ATP decreased while content while ADP, AMP and adenine compounds increased. It was also found that hypotonic Na+ and K+ ion exchange and hematocrit values increased. [34]
UV light irradiation on Rh-positive blood significantly increased the immunosorption activity. Vasil’eva et al [35] studied varying irradiation levels of UV on both red blood cells and leucocyte-thrombocyte suspensions. The immunosorption activity increased immediately after irradiation in the whole blood and red blood cells, however, the immunosorption capacity in leucocytic – thrombocytic suspensions was lost after two days later.
A two-phase polymer system including polydextran was used to study a one-hour UV exposure of blood for autotransfusion. They found that the cell surface properties of circulating erythrocytes were altered, which contributed to the prolongation and more effective therapeutic benefit of autotransfusion [36]. Snopov et al [37] suggested that some structural disturbances in the state of the erythrocyte glycocalyx were related to UV-irradiation when it was used as a clinical treatment. Cytochemical and isoserological methods were used to show that blood autotransfusions were improved after UV irradiation.
Ichiki et al [38] showed that the erythrocyte cellular volume and the membrane potential were changed by UV irradiation. Lower doses (< 0.1 J/cm2) increased polymorphonuclear leukocyte production of peroxides (H2O2) which was the most pronounced among different blood cells, However an increased dose decreased the production, while the peroxide production in platelets was lowest at the lower dose, but it increased abruptly at doses above 0.4 J/cm2.
2.2 Effects on Neutrophils
The pro-oxidative effects of UBI on neutrophils could be inhibited by arachidonate or lysophosphatidylcholine (LPC), as well as the complex-forming agent alpha-tocopherol. These compounds inhibited the interaction of UVR with phagocytes [39]. In chronic inflammatory disease, the concentration of large IC-IgG, IgM, and small IC-IgM immunocomplexes showed a linear and inverted correlation when UBI was carried out on autotransfused blood [40]. The function of UV-B irradiated mononuclear cells derived from human peripheral blood could be enhanced by deoxyribonucleoside supplementation, and also T-lymphocyte survival was enhanced after UV-B or UV-C exposure [41]
Artiukhov suggested that nitric oxide (NO) generation by photomodified neutrophils was due to the activation of iNOS synthesis that was de novo upregulated by UV-irradiation, which also had an effect on TNF-alpha production. Irradiation with a lower dose (75.5J/m2) improved the maintenance of physiological homeostasis through an effect relative to the native level of NO. While higher doses (755 and 2265 J/m2) were delivered to neutrophils this led to different effects by increasing the concentration of NO metabolites. Cells treated with UV-irradiation in the presence of cycloheximide (a transcriptional inhibitor of protein synthesis) could prevent the activation of iNOS synthesis. High dose UV-irradiation (755 J/m2) of blood cells showed a positive correlation between NO and TNF-alpha concentrations [42].
Zor’kina carried out a series of thirty-day rabbit experiments, suggesting that alleviation of chronic stress with hypodynamia after UBI, was caused by neutrophilic mobilization and lowered coagulation. These effects contributed to improvement of body function under long-term hypodynamia and lessening of chronic stress. UBI enhanced an adaptive process to reduce stress through activated neutrophils, lowering of disseminated intravascular coagulation, and changed atherogenic metabolism[43].
2.3 Effects on lymphocytes
Although UBI has several disadvantages including a lack of depth penetration and limited absorption by targeted cells, it can be useful in organ transplantation and in blood transfusion particularly in the UVB range, since immunological function and immunogenicity could be suppressed in a dose-dependent manner. Although UBI can decrease lymphocyte viability, UVC irradiation appears to be the most effective among the three spectral regions. UVB and UVC irradiation can abolish proliferative and stimulatory ability as well as the accessory/antigen-presenting ability of leukocytes in vitro. Cell-surface properties, calcium mobilization, cytokine production and release, and other sub cellular processes could be changed by UV irradiation [44]. Areltt et al [45] used the “Comet“ assay for strand breakage (single cell gel electrophoresis) as an indicator of nucleotide-excision repair to prove that circulating human T–lymphocytes were exquisitely hypersensitive to the DNA-damaging and lethal effects of UV-B radiation, raising the possibility that UV-B may make a contribution to immunosuppression via a direct effect on extracapilliary T-lymphocytes.
Schieven et al observed that after surface immunoglobulin cross-linking, UV-induced tyrosine phosphorylation in B cells was very similar to that seen after Ca2+ signaling in T cells. This means that the UV irradiation effect on lymphocyte function could induce both tyrosine phosphorylation and Ca2+ signals. Ca2+ channels in lymphocyte membranes are sensitive to UV irradiation, and moreover UV radiation can cause damage DNA through activation of cellular signal-transduction processes. UV radiation depending on dose and wavelength can not only induce tyrosine phosphorylation in lymphocytes, but also induce Ca2+ signals in Jurkat T cells and associated proteins synthesis. Furthermore, the pattern of surface immunoglobulin cross-linking was very similar to the UV-irradiated B cells and Ca2+-treated T-cells. In this research it was found that CD4+ and CD8+ normal human T-lymphocyte cells gave strong reactions during UV-irradiation induced producing Ca2+ responses [46].
In another similar study, Spielberg et al [47] found that UV-induced inhibition of lymphocytes accompanied by a disruption of Ca2+ homeostasis, and compared the UV effect with gamma irradiation, which have different effects on lymphocyte membranes. They found the presence of Ca2+ channels in lymphocyte membranes that were sensitive to UV irradiation. Indo-1 and cytofluorometry, was used to measure [Ca2+]i kinetics was in UVC- or UVB-exposed human peripheral blood leukocytes (PBL) and Jurkat cells in parallel with functional assays. The UV-induced [Ca2+]i rise was predominantly due to influx of extracellular calcium, and it was more pronounced in T than in non-T cells. It was observed that [Ca2+]i increased within 2–3 h of irradiation; these increases were UV-dose dependent and reached maxima of 240% and 180% above baseline level (130 nM) for UVB and UVC. The UV-induced more [Ca2+]i rise in T cells than in non-T cells, due to the influx of extracellular calcium. UV-induced calcium shifts and UV irradiation on the plasma membrane decreased the sensitivity of response to phyto hemagglutinin (PHA) and its ability to stimulate a mixed leukocyte culture, because UV produces [Ca2+]i shifts.
A series of studies confirmed that UVR irradiated lymphocytes were not able to induce allogeneic cells in a mixed lymphocyte culture (MLC) as first reported by Lindahl-Kiessling [48–50]. Clusters formed by specialized accessory cells such as dendritic cells (DC), after mitogenic or allogenic stimulation, were necessary for lymphocyte activation to occur. Aprile found that UV irradiation of DC before culture completely abrogated the accessory activity and was able to block both cluster formation and proliferation [51].
UV-induced differentiation of human lymphocytes could accelerate the repair of UV-irradiation damage in these cells [52]. Exposure to UV irradiation was more effective than combination of UV-irradiation with methyl methanesulfonate (MMS) in the unscheduled DNA synthesis value, especially when MMS was given prior to the UV-irradiation (either at 2 hour or 26 hours incubation) because the MMS has an effect on the DNA repair polymerase by alkylating DNA [53]. Photo modification of HLA-D/DR antigens could be a trigger mechanism for activation of immunocopetent cells by UV-irradiation. Lymphocytes were isolated from a mixture of non-irradiated and UBI irradiated blood at different ratios (1:10, 1:40, 1:160) [54].
Pamphilon reported that platelet concentrates (PC) could become non-immunogenic after being irradiated with ultraviolet light (UVL) and stored for 5 d in DuPont Stericell containers. Lactate levels, beta-thromboglobulin and platelet factor were increased, while glucose levels were decreased with an irradiation dose of 3000 J/m2 at a mean wavelength of 310 nm in DuPont Stericell bags [55]. Ultraviolet B (UVB) irradiation of platelet concentrate (PCs) accelerated downregulation of CD14 and nonspecifically increased the loss of monocytes by inhibiting the upregulation of ICAM-1 and HLA-DR [56]. However, UV radiation of platelet concentrates reduced the induced immunological response in a cell suspension [57–59].
Deeg et al studied a model where administering blood transfusions to littermate dogs led to rejection of bone marrow grafts even though the grafts were DLA-identical, while untransfused dogs uniformly achieved sustained engraftment. UBI of the blood before transfusion prevented bone marrow graft rejection in vivo. 9.2 Gy of total body irradiation (TBI) was also used and 2.8±2.1×108/Kg donor marrow cells were infused, and whole blood was exposed for 30 minutes to UV light for 1.35 J/cm2, then injected into the recipient dogs. The control group transfused with sham-exposed blood rejected grafts, while no rejection appeared in the treatment group, which received UV-exposed blood before transplanted marrow. UV irradiation of blood lessened activation of DC by eliminating a critical DC-dependent signal; therefore subsequent DLA-identical marrow graft was successfully engrafted [60].
Oluwole et al [61] suggested that transfusion of UV-irradiated blood into recipients could be used prior to heart transplantation to inhibit immune response and reduce lymphocyte reaction. Three strains of rats (ACI, Lewis, W/F) were used for heart transplantation in his research. When ACI rats received a Lewis rat heart, giving 1 mL transfusion of donor-type blood with or without UV-irradiation transfusion at 1,2, and 3 weeks prior to the transplantation, the mixed lymphocyte reaction with ACI lymphocytes showed a weaker response to Lewis lymphocytes than without UBI and the similar results were obtained with the other two strains of heart transplantation. UV irradiation of donor rhesus-positive blood can be used for increase in therapeutic effect of blood exchange transfusion in children with rhesus-conflict hemolytic disease [62].
Kovacs et al [63] found that DNA repair synthesis was dependent on the dose of UV-C light between 2 and 16 J/cm2. This was evaluated in irradiated and unirradiated lymphocytes in 51 healthy blood donors. Irradiation (253.7 nm) of 2,4,8 and 16 J/m2 was used, then DNA synthesis was measured by [3H] thymidine incorporation in the presence of hydroxyurea (2mM/2 ×106 cells) added 30 min before irradiation to inhibit the DNA-replicative synthesis. No significant age-related difference was seen between 17 and 74 years.
Teunissen et al [64] suggested that UVB radiation neither selectively affects Th1 or Th2 nor CD4 or CD8 T cell subsets. Compared with different dose of UVB irradiation, although the phototoxic effect was not immediately apparent, low doses of UVB (LD50: 0.5–1 mJ/cm2) irradiation were sufficient to kill most of T cells after 48–72 hours. There was a dose dependent reduction of all cytokines (IL-2, IL-4, IL-5, IFN-γ, TNF-a) 72h after irradiation. This fall in cytokine production was correlated with loss of viability so the reduction of cytokine production may be caused directly by cell death. However, the ratio of CD4+ or CD8+ T cell subsets, and the expression of CD4 and CD8 compared with the un-irradiated control, was not altered by UVB, suggesting that neither of the two T cell subsets was selectively affected.
2.4 Effects on phagocytic cells
Phagocytic activity (PhA) was one of the first mechanisms to be proposed to explain the immunocorrection by UBI therapy, In Samoı̆lova’s research, non-irradiated blood mixed with 1:10 volumes of irradiated blood were used to test PhA of monocytes and granulocytes. An increase of 1.4–1.7 times in PhA compared with non-irradiated blood, was seen when UV-irradiated blood was transfused into healthy adults. The enhancement of PhA depended on its initial level and may occur simultaneously with structural changes of the cell surface components [65].
Simon et al [66] showed that UVB could convert Langerhans cells (LC) or splenic adherent cells (SAC) from an immunogenic to a tolerogenic type of APC (LC or SAC). In his research, single dose of irradiation (200J/m2) was used on LC and SAC. The Th1 loss of response after preincubation with keyhole limpet hemocyanin (KLH) was studied with UVB-LC or UVB-SAC. Furthermore, the loss of responsiveness was not related to the release of soluble suppressor factors but was Ag-specific, MHC-restricted, and did not last for a long time. Functional of allogeneic LC or SAC delivery a costimulatory signal(s) was interferes by UVB, because unresponsiveness by UVB-LC or UVB-SAC could not induce by unirradiated allogeneic SAC.
UV-irradiation increased phagocytic activity of human monocytes and granulocytes; the improvement in phagocytic index was related to the irradiation dose, and the initial level. A lower initial level would increase proportionately more than a higher initial level after UV-irradiation. It was found that UV irradiation enhanced the phagocytic activity directly [67].
2.5 Effects on low-density lipoprotein (LDL)
Roshchupkin et al [68] found that UV irradiation played a core role in lipid peroxidation in the membrane of blood cells. UV irradiation on blood stimulated arachidonic acid to be produced by a cyclooxygenase catalyzed reaction. UV induced a process of dark lipid autoperoxidation that continued for some time afterwards producing free radicals. It contributed to lipid photoperoxidation producing lipid hydroperoxides.
An UV irradiated lipid emulsion greatly enhanced reactive oxygen species (ROS) production by monocytes. Highly atherogenic oxidized LDL could be generated in the circulation. UV irradiation of the lipid emulsion called “Lipofundin” (largely consisting of linoleic acid oxidized either by lipoxygenase, Fe3+ or ultraviolet irradiation) was injected into rabbits. Blood samples were taken from the ear vein with EDTA before and 6 hours after lipofundin treatment. Though UV-oxidized lipofundin induce less chemiluminescence from monocytes compared with Fe3+ oxidation, it lasted 2.3 times longer. UV–oxidized lipofundin could more effectively stimulate H2O2 production by cells, than LDL altered by monocytes, even with the same concentration of thiobarbituric acid reactive substance (TBARS). Six hours after injection of oxidized lipofundin, the lipid peroxide content was significantly increased; however neutral lipids of LDL separated from rabbit plasma showed no significantly difference to the monocyte-oxidized human LDL [69].
Salmon [70] found that UVB (280–315 nm) irradiation could easily damage LDL and high density lipoprotein (HDL) tryptophan (Trp) residues. The TBARS assay was used to measure the photooxidation of tryptophan residues which was accompanied by the peroxidation of low and high density lipoprotein unsaturated fatty acids. Vitamin E and carotenoids naturally carried by low and high density lipoproteins, were also rapidly destroyed by UVB. However UVA radiation did not destroy tryptophan residue and lipid photoperoxidation.
UV radiation (wavelength range 290–385 nm) easily oxidized lipoproteins contained in the suction blister fluid of healthy volunteers, which is a good representative of the interstitial fluid feeding the epidermal cells. Apolipoprotein B of LDL and apolipoprotein A-I and II were all changed in the same way under UV irradiation. The single tryptophan residue of albumin was highly susceptible to photo-oxidation during irradiation. UVA irradiation of undiluted suction blister fluid induced apo-A-I aggregation; however, purified lipoproteins were not degraded. During UV irradiation of suction blister fluid, antigenic apolipoprotein B is fragmented and polymerized. Activated oxygen radicals in the suction blister fluid during UV irradiation were derived from lipid peroxidation in HDL. Furthermore, they suggested that lipid peroxidation of was caused by a radical chain reaction and could transfer the initial photodamage. UV-light irradiation could play an important role in triggering inflammation and the degeneration caused by induced lipoprotein photo-oxidation with systemic effects. [71]
2.6 Effects on redox status
Artyukhov et al [72] found that dose-dependent UV-irradiation could activate the myeloperoxidase (MPO) and the NADPH-oxidase systems and lipid peroxide (LPO) concentration in donor blood. Two doses of UV-light were used (75.5 and 151.0 J/m2 ) in UV-induced priming of neutrophils (NP). A higher dose activated more free radicals and H2O2 from NP than a lower dose. Two groups were divided by the type of relationship between MPO activity and UV light dose (from 75.5 to 1510J/m2). A low enzyme activity (group 1) increased under the effect of UV exposure in doses of 75.5 and 151.0 J/m2, while in group 2 this parameter decreased. MPO activity showed the same result in dose-dependent UV-irradiation; however increasing the dose to 1510J/m2 did not increase the activity of MPO. In the next series of experiments, LPO concentration was evaluated after UV exposure of the blood. Two groups of donors were distinguished by the relationship between blood content of LPO and UV exposure dose. UV irradiation at low doses (75.5–151.0 J/m2) decreased initially high LPO and increased initially low LPO levels. In phagocytes, NADPH-oxidase plays one of the most important role of photoacceptors for UV light. Which cause the superoxide concentration to increase after UV-irradiation by activating the enzyme complex. UV irradiation decreases intracellular pH that is raised by activation of NADPH-oxidase complex.
UBI can reduce the free radical damage and elevate the activity of antioxidant enzymes after spinal cord injury in rabbits. 186 rabbits were divided into 4 groups randomly, (control, blood transfusion, injured and UBI). UV irradiation (wavelength 253.7nm, 5.68×10−3 J/cm2) were used in the treatment group at 47, 60 and 72 hours after surgery. Free radical signals (FR), malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione peroxidase (GSH-PX) were measured. In the treatment group, SOD and GSH-PX were highly increased and showed significant differences compared with other groups; while FR and MDA decreased significantly in the UBI groups compared to the other groups. UV-irradiated blood decreased MDA and FR content in the spinal cord tissue. They also suggested that two factors contributed to increased SOD and GSH-PX activity: one was that UV irradiation induced the (lowered) SOD, GSH-PX return to normal levels, the other was that a decrease in the formation of FR, led to SOD and GSH-PX increases, especially at 48 and 72 hours after injury [73].
3 Extracorporeal photopheresis (ECP) overview
As UBI has certain factors in common with the medical procedure known as extracorporeal photopheresis (ECP) we believe it is useful to compare and contrast the two techniques. ECP is an apheresis-based immunomodulatory therapy which involves ultraviolet A (UVA) irradiation of autologous peripheral blood mononuclear cells (PBMCs) exposed to the photosensitizing drug 8-methoxypsoralen (8-MOP). ECP has been widely used as an immunotherapy for cutaneous T cell lymphoma (CTCL) since it received US Food and Drug Administration (FDA) approval in 1988. There are a numbers of features of ECP that distinguish it from other immunologic therapies, such as its action as a cancer immune-stimulator and an immune-modulator in the transplant setting; induction of antigen presenting cells (APC); and its ability to modify processed leukocytes [74]. ECP has been studied for treatment of other autoimmune-mediated disorders and for prevention of organ allograft rejection. It is especially beneficial for CTCL and graft-versus host disease (GVHD).
3.1 ECP therapy treatment
The standard schedule of ECP treatment involves 2 successive days at 4 week intervals. Tens of thousands of patients afflicted with CTCL, organ transplant rejection, GVHD, Crohn’s disease and type 1 diabetes [75–80] have been benefited by ECP since the first report of the systemic efficacy of ECP by Edelson [81] in 1987. In his studies, treatment of skin manifestations in patients with cutaneous T-cell lymphoma (CTCL) achieved a response rate of greater than 70% compared with other forms of treatment. Wollnia [82] combined alpha-interferon and ECP treatment for fourteen patients (all male) aged 38 to 72 years with CTCL of the mycosis fungoides type, stage IIa/IIb, achieving a total response rate of 56%.
3.2 Mechanism of ECP
UVA activated 8-MOP causes formation of cross-links between the pyrimidine bases of DNA of sister strands, causing apoptosis of the extracorporeally targeted lymphocytes [83]. ECP can reduce erythrodermic CTCL caused by intact CD8 T cells and prolongs survival with minimal toxicity [84]. Two immune effects of ECP have been confirmed: one is immunostimulatory effects against neoplastic cells in CTCL, the other is immunosuppressive effects against T-cell-mediated disorders such as GVHD [85].
3.3 Comparison between UBI and ECP
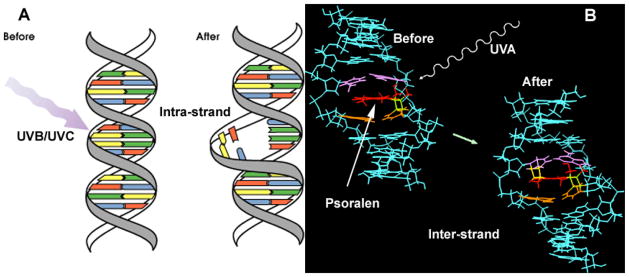
As far as we can tell ECP has never been tested against the systemic bacterial infections that were treated so successfully by UBI between 1930 and 1950. Both UBI and ECP can have immunostimulatory and immunosuppressive effects depending on the dose employed and the disease that is being treated. The type of DNA damage is different between UBI and ECP. UBI causes formation of thymine dimers and 6:4 photoproducts, which are intra-strand crosslinks, while ECP causes formation of inter-strand cross-links when the photoactivated psoralen reacts with nucleic acid base residues in both strands [86].
4. Conclusion
UBI had originally been an American discovery, but then transitioned to being more studied in Russia and other eastern countries, which had long concentrated on physical therapies for many diseases, which were more usually treated with drugs in the West. Over the years its acceptance by the broad medical community has been hindered by uncertainties about its mechanism of action. Confusion has been caused by the widely held idea that since UV is used for sterilization of water and instruments; therefore its use against infection must also rely on UV-mediated direct destruction of pathogens. Another highly confusing aspect is the wide assortment of diseases that have been claimed to be successfully treated by UBI. It is often held that something that appears to be “too good to be true” usually is.
It is clear that the effectiveness UBI is critically dependent on the dose of UV employed. In fact the dose-response is governed by the concept of hormesis [87], where a small dose is beneficial, but when the dose is increased the benefit is lost, and if the dose is further increased then damaging effects can be produced In fact Knott’s original studies using dogs found that only 5–7% of the total blood volume should be treated to have the optimum benefit [7]. UV radiation is well known to produce DNA damage, and cells with DNA damage that is unable to be repaired will undergo apoptosis. It is uncertain to what extent the cell death caused by UV irradiation is necessary for the beneficial effects. It should not be forgotten that the original Knott technic used UVC irradiation from a low-pressure mercury lamp (253.7 nm). Many of the laboratory studies reported above have used UVB light (280–315 nm). It is possible that there are major differences between these two wavelengths of UV light. Interest in UVB has to a great extent been driven by the field of photodermatology, that seeks to understand the damaging effects of UV exposure to the skin in sunlight [88]. This has led to accumulation of a large body of knowledge on the immunosuppressive effects of UVB, in addition to its carcinogenic effects. Since the UVC wavelengths in sunlight are absorbed by the ozone layer, and do not reach the earth’s surface, the biological effects of UVC have been somewhat neglected.
It is still uncertain which of the many plausible mechanisms covered above really contribute to the success of UBI. Is it the production of reactive oxygen species caused by UV irradiation? Is it the activation of phagocytes such as neutrophils, monocytes and macrophages? Is it an alteration in lymphocyte subsets leading to differences in Th1 and Th2 profiles. Is it due to alteration in the secretion of cytokines? What factor is responsible for the marked increase in oxygen-carrying capacity of the blood that was noted by the early pioneers? There are many questions still to be answered.
In the last decade the problem of multi-antibiotic resistant bacteria has grown relentlessly. Multidrug-resistant (MDR) and pandrug-resistant (PDR) bacterial strains and their related infections are emerging threats to public health throughout the world [89]. These are associated with approximately two-fold higher mortality rates and considerably prolonged hospital admissions [90]. The infections caused by antibiotic resistant strains are often exceptionally hard to treat due to the limited range of therapeutic options [91]. Recently in Feb 2015, the Review on Antimicrobial Resistance stated “Drug-resistant infections could kill an extra 10 million people across the world every year by 2050 if they are not tackled. By this date they could also cost the world around $100 trillion in lost output: more than the size of the current world economy, and roughly equivalent to the world losing the output of the UK economy every year, for 35 years.” [92]
Sepsis is an uncontrolled response to infection involving massive cytokine release, widespread inflammation, which leads to blood clots and leaky vessels. Multi-organ failure can follow. Every year, severe sepsis strikes more than a million Americans. It is estimated that between 28–50% percent of these people die. Patients with sepsis are usually treated in hospital intensive care units with broad-spectrum antibiotics, oxygen and intravenous fluids to maintain normal blood oxygen levels and blood pressure. Despite decades of research, no drugs that specifically target the aggressive immune response that characterizes sepsis have been developed [93].
We would like to propose that UBI be reconsidered and re-investigated as a treatment for systemic infections caused by multi-drug resistant Gram-positive and Gram-negative bacteria in patients who are running out of (or who have already run out) of options. Patients at risk of death from sepsis could also be considered as candidates for UBI. Further research is required into the mechanisms of action of UBI. The present confusion about exactly what is happening during and after the treatment is playing a large role in the controversy about whether UBI could ever be a mainstream medical therapy, or must remain sidelined in the “alternative and complementary” category where it has been allowed to be forgotten for the last 50 years, and sometimes referred to as “photoluminescence therapy”.
Figure 4.
Comparison of DNA damage produced by (A) UVB or UVC (intra-strand cross-links); and (B) DNA damage produced by psoralens and UVA (ECP, interstrand cross-links)
Acknowledgments
Research in the Hamblin laboratory is supported by US NIH grant R01AI050875.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frercksa J, Weberb H, Wiesenfeldt G. Reception and discovery: the nature of Johann Wilhelm Ritter’s invisible rays. Studies in History and Philosophy of Science Part A. 2009;40:143–156. [Google Scholar]
- 2.Bonnet A. Traite des Maladies des Articulations. Bailliere; Paris: 1845. [Google Scholar]
- 3.Barth J, Kohler U. Photodermatologie in Dresden-ein historischer Abriss. Festschrift anlasslich des 75. Geburtstages von Prof. Dr. Dr. Dr. h.c. H.-E. Kleine-Natrop (1917–1985) Dresden. 1992 [Google Scholar]
- 4.Downes A, Blunt TP. Researches on the effect of light upon bacteria and other organisms. Proc Royal Soc London. 1877;26:488–500. [Google Scholar]
- 5.Finsen NR. Phototherapy. Edward Arnold; London: 1901. [Google Scholar]
- 6.Ude WH. Ultraviolet Radiation Therapy in Erysipelas. Radiology. 1929;13:504. [Google Scholar]
- 7.Knott EK. Development of ultraviolet blood irradiation. Am J Surg. 1948;76:165–171. doi: 10.1016/0002-9610(48)90068-3. [DOI] [PubMed] [Google Scholar]
- 8.Hancock VKK, EK Irradiated blood transfusion in the treatment of infections. Northwest Med. 1934:200. [Google Scholar]
- 9.Miley G, Christensen JA. Ultraviolet blood irradiation further studies in acute infections. Am J Surg. 1947;LxxIII:486–493. doi: 10.1016/0002-9610(47)90330-9. [DOI] [PubMed] [Google Scholar]
- 10.Miley G. Uv irradiation non healing wounds. Am J Surg. 1944;LXV:368–372. [Google Scholar]
- 11.Miley GP. Recovery from botulism coma following ultraviolet blood irradiation. The Review of gastroenterology. 1946;13:17–19. [PubMed] [Google Scholar]
- 12.Miley GP, Seidel RE, Christensen JA. Ultraviolet blood irradiation therapy of apparently intractable bronchial asthma. Archives of physical medicine and rehabilitation. 1946;27:24–29. [PubMed] [Google Scholar]
- 13.Miley G. The control of acute thrombophlebitis with ultraviolet blood irradiation therapy. Am J Surg. 1943:354–360. [Google Scholar]
- 14.Miley G. Efficacy of ultraviolet blood irraidation therapy in the control of staphylococcemias. Am J Surg. 1944:313–322. [Google Scholar]
- 15.Miley G. Ultraviolet blood irraidation therapy in acute poliomyelitis. Arch Phys Therapy. 1944:651–656. [Google Scholar]
- 16.Miley G. Disapperance of hemolytic staphylococcus aureus septicemia following ultraviolet blood irradiation therapy. Am J Surg. 1943:241–245. [Google Scholar]
- 17.Miley G. The knott technic of ultraviolet blood irradiation in acute pyogenic infections. New York state Med. 1942:38–46. [Google Scholar]
- 18.Miley G. Present status of ultraviolet blood irradiation (Knott technic) Arch Phys Therapy. 1944:368–372. [Google Scholar]
- 19.Miley G. Ultravilet blood irradiation. Arch Phys Therapy. 1942:536. [Google Scholar]
- 20.Miley G. Ultraviolet blood irradiation therapy (knott technic) in acute pyogenic infections. Am J Surg. 1942:493. doi: 10.1016/0002-9610(49)90239-1. [DOI] [PubMed] [Google Scholar]
- 21.Miley G. The knott technic of ultraviolet blood irradiation as a control of infection in peritonitis. The Review of gastroenterology. 1943:1. [Google Scholar]
- 22.Miley GP, Seidel RE, Christensen JA. Preliminary report of results observed in eighty cases of intractable bronchial asthma. Arch Phys Therapy. 1943:533. [Google Scholar]
- 23.Barrett HA. The irradiation of autotransfused blood by ultraviolet spectral energy. Result of therapy in 110 cases. Med clin North America. 1940 721.1040. [Google Scholar]
- 24.Barrett HA. Five years’ experience with hemo-irradiation according to the Knott technic. Am J Surg. 1943;61:42–53. [Google Scholar]
- 25.Rebbeck EW. Double septicemia following prostatectomy treated by the knott technic of ultraviolet blood irradiation. Am J Surg. 1942;57:536–538. [Google Scholar]
- 26.Rebbeck EW. Preoperative hemo-irradiations. Am J Surg. 1943;61:259–265. [Google Scholar]
- 27.Rebbeck EW. Ultraviolet irradiation of autotransfused blood in the treatment of puerperal sepsis. Am J Surg. 1941;54:691–700. [Google Scholar]
- 28.Rebbeck EW. Ultraviolet irradiation of autotransfused blood in the treatment of postabortional sepsis. Am J Surg. 1942;55:476–486. [Google Scholar]
- 29.Rebbeck EW. Ultraviolet Irradiation of Blood in the Treatment Of Escherichia coli Septicemia. Arch Phys Therap. 1943:158–167. [Google Scholar]
- 30.Olney RC. Ultraviolet blood irradiation in biliary disease; Knott method. Am J Surg. 1946;72:235–237. doi: 10.1016/0002-9610(46)90417-5. [DOI] [PubMed] [Google Scholar]
- 31.Olney RC. Ultraviolet blood irradiation treatment of pelvic cellulitis; Knott method. Am J Surg. 1947;74:440–443. doi: 10.1016/0002-9610(47)90136-0. [DOI] [PubMed] [Google Scholar]
- 32.Olney RC. Treatment of viral hepatitis with the Knott technic of blood irradiation. Am J Surg. 1955;90:402–409. doi: 10.1016/0002-9610(55)90777-7. [DOI] [PubMed] [Google Scholar]
- 33.Rowen RJ. Ultraviolet Blood Irradiation Therapy (Photo-Oxidation): The Cure That Time Forgot. Int J Biosocial Med Research. 14:115–132. [Google Scholar]
- 34.Kabat IA, Sysa J, Zakrzewska I, Leyko W. Effect of UV-irradiation of shifts of energy-rich phosphate compounds: ADP, ATP and AXP in human red blood cells represented by a trigonometrical polynomial. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene Erste Abteilung Originale Reihe B: Hygiene, praventive Medizin. 1976;162:393–401. [PubMed] [Google Scholar]
- 35.Vasil’eva ZF, Samoilova KA, Shtil’bans VI, Obolenskaia KD, Vitiuk NG. Changes of immunosorption properties in the blood and its components at various times after UV-irradiation. Gematologiia i transfuziologiia. 1991;36:26–27. [PubMed] [Google Scholar]
- 36.Vasil’eva ZF, Samoilova KA, Shtil’bans VI, Obolenskaia KD, Vitiuk NG. Changes of immunosorption properties in the blood and its components at various times after UV-irradiation. Gematologiia i transfuziologiia. 1991;36:26–27. [PubMed] [Google Scholar]
- 37.Snopov SA, Aritsishevskaia RA, Samoilova KA, Marchenko AV, Dutkevich IG. Functional and structural changes in the surface of human erythrocytes following irradiation with ultraviolet rays of various wave lengths. V. Modification of the glycocalyx in autotransfusions of UV-irradiated blood. Tsitologiia. 1989;31:696–705. [PubMed] [Google Scholar]
- 38.Ichiki H, Sakurada H, Kamo N, Takahashi TA, Sekiguchi S. Generation of active oxygens, cell deformation and membrane potential changes upon UV-B irradiation in human blood cells. Biological & pharmaceutical bulletin. 1994;17:1065–1069. doi: 10.1248/bpb.17.1065. [DOI] [PubMed] [Google Scholar]
- 39.Savage JE, Theron AJ, Anderson R. Activation of neutrophil membrane-associated oxidative metabolism by ultraviolet radiation. The Journal of investigative dermatology. 1993;101:532–536. doi: 10.1111/1523-1747.ep12365905. [DOI] [PubMed] [Google Scholar]
- 40.Ivanov EM, Kapshienko IN, Tril NM. Effect of the UV irradiation of autologous blood on the humoral link in the immune response of patients with chronic inflammatory processes. Voprosy kurortologii, fizioterapii, i lechebnoi fizicheskoi kultury. 1989:45–47. [PubMed] [Google Scholar]
- 41.Green MH, Waugh AP, Lowe JE, Harcourt SA, Cole J, Arlett CF. Effect of deoxyribonucleosides on the hypersensitivity of human peripheral blood lymphocytes to UV-B and UV-C irradiation. Mutation research. 1994;315:25–32. doi: 10.1016/0921-8777(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 42.Artiukhov VF, Gusinskaia VV, Mikhileva EA. Level of nitric oxide and tumor necrosis factor-alpha production by human blood neutrophils under UV-irradiation. Radiatsionnaia biologiia, radioecologiia/Rossiiskaia akademiia nauk. 2005;45:576–580. [PubMed] [Google Scholar]
- 43.Zor’kina AV, Inchina VI, Kostin Ia V. Effect of UV-irradiation of blood on the course of adaptation to conditions of hypodynamia. Patologicheskaia fiziologiia i eksperimental’naia terapiia. 1996:22–24. [PubMed] [Google Scholar]
- 44.Deeg HJ. Ultraviolet irradiation in transplantation biology. Manipulation of immunity and immunogenicity. Transplantation. 1988;45:845–851. doi: 10.1097/00007890-198805000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Arlett CF, Lowe JE, Harcourt SA, Waugh AP, Cole J, Roza L, Diffey BL, Mori T, Nikaido O, Green MH. Hypersensitivity of human lymphocytes to UV-B and solar irradiation. Cancer research. 1993;53:609–614. [PubMed] [Google Scholar]
- 46.Schieven GL, Ledbetter JA. Ultraviolet radiation induces differential calcium signals in human peripheral blood lymphocyte subsets. Journal of immunotherapy with emphasis on tumor immunology: official journal of the Society for Biological Therapy. 1993;14:221–225. doi: 10.1097/00002371-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 47.Spielberg H, June CH, Blair OC, Nystrom-Rosander C, Cereb N, Deeg HJ. UV irradiation of lymphocytes triggers an increase in intracellular Ca2+ and prevents lectin-stimulated Ca2+ mobilization: evidence for UV- and nifedipine-sensitive Ca2+ channels. Experimental hematology. 1991;19:742–748. [PubMed] [Google Scholar]
- 48.Pamphilon DH, Corbin SA, Saunders J, Tandy NP. Applications of ultraviolet light in the preparation of platelet concentrates. Transfusion. 1989;29:379–383. doi: 10.1046/j.1537-2995.1989.29589284134.x. [DOI] [PubMed] [Google Scholar]
- 49.Lindahl-Kiessling K, Safwenberg J. Inability of UV-irradiated lymphocytes to stimulate allogeneic cells in mixed lymphocyte culture. International archives of allergy and applied immunology. 1971;41:670–678. doi: 10.1159/000230559. [DOI] [PubMed] [Google Scholar]
- 50.Slater LM, Murray S, Liu J, Hudelson B. Dissimilar effects of ultraviolet light on HLA-D and HLA-DR antigens. Tissue antigens. 1980;15:431–435. doi: 10.1111/j.1399-0039.1980.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 51.Aprile J, Deeg HJ. Ultraviolet irradiation of canine dendritic cells prevents mitogen-induced cluster formation and lymphocyte proliferation. Transplantation. 1986;42:653–660. doi: 10.1097/00007890-198612000-00015. [DOI] [PubMed] [Google Scholar]
- 52.Genter EI, Zhestianikov VD, Mikhel’son VM, Prokof’eva VV. DNA repair in the UV irradiation of human peripheral blood lymphocytes (healthy donors and xeroderma pigmentosum patients) in relation to the dedifferentiation process in phytohemagglutinin exposure. Tsitologiia. 1984;26:599–604. [PubMed] [Google Scholar]
- 53.Genter EI, Mikhel’son VM, Zhestianikov VD. The modifying action of methylmethane sulfonate on unscheduled DNA synthesis in the UV irradiation of human peripheral blood lymphocytes. Radiobiologiia. 1989;29:562–564. [PubMed] [Google Scholar]
- 54.Volgareva EV, Volgarev AP, Samoilova KA. The effect of UV irradiation and of UV-irradiated autologous blood on the functional state of human peripheral blood lymphocytes. Tsitologiia. 1990;32:1217–1224. [PubMed] [Google Scholar]
- 55.Pamphilon DH, Potter M, Cutts M, Meenaghan M, Rogers W, Slade RR, Saunders J, Tandy NP, Fraser ID. Platelet concentrates irradiated with ultraviolet light retain satisfactory in vitro storage characteristics and in vivo survival. British journal of haematology. 1990;75:240–244. doi: 10.1111/j.1365-2141.1990.tb02656.x. [DOI] [PubMed] [Google Scholar]
- 56.Fiebig E, Lane TA. Effect of storage and ultraviolet B irradiation on CD14-bearing antigen-presenting cells (monocytes) in platelet concentrates. Transfusion. 1994;34:846–851. doi: 10.1046/j.1537-2995.1994.341095026968.x. [DOI] [PubMed] [Google Scholar]
- 57.Kahn RA, Duffy BF, Rodey GG. Ultraviolet irradiation of platelet concentrate abrogates lymphocyte activation without affecting platelet function in vitro. Transfusion. 1985;25:547–550. doi: 10.1046/j.1537-2995.1985.25686071428.x. [DOI] [PubMed] [Google Scholar]
- 58.Andreu G, Boccaccio C, Klaren J, Lecrubier C, Pirenne F, Garcia I, Baudard M, Devers L, Fournel JJ. The role of UV radiation in the prevention of human leukocyte antigen alloimmunization. Transfusion medicine reviews. 1992;6:212–224. doi: 10.1016/s0887-7963(92)70171-0. [DOI] [PubMed] [Google Scholar]
- 59.Tandy NP, Pamphilon DH. Platelet transfusions irradiated with ultraviolet-B light may have a role in reducing recipient alloimmunization. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 1991;2:383–388. doi: 10.1097/00001721-199104000-00025. [DOI] [PubMed] [Google Scholar]
- 60.Deeg HJ, Aprile J, Graham TC, Appelbaum FR, Storb R. Ultraviolet irradiation of blood prevents transfusion-induced sensitization and marrow graft rejection in dogs. Blood. 1986;67:537–539. [PubMed] [Google Scholar]
- 61.Oluwole SF, Iga C, Lau H, Hardy MA. Prolongation of rat heart allografts by donor-specific blood transfusion treated with ultraviolet irradiation. The Journal of heart transplantation. 1985;4:385–389. [PubMed] [Google Scholar]
- 62.Vasil’eva ZF, Shtil’bans VI, Samoilova KS, Obolenskaia KD. The activation of the immunosorptive properties of blood during its UV irradiation at therapeutic doses. Biulleten’ eksperimental’noi biologii i meditsiny. 1989;108:689–691. [PubMed] [Google Scholar]
- 63.Kovacs E, Weber W, Muller H. Age-related variation in the DNA-repair synthesis after UV-C irradiation in unstimulated lymphocytes of healthy blood donors. Mutation research. 1984;131:231–237. doi: 10.1016/0167-8817(84)90030-0. [DOI] [PubMed] [Google Scholar]
- 64.Teunissen MB, Sylva-Steenland RM, Bos JD. Effect of low-dose ultraviolet-B radiation on the function of human T lymphocytes in vitro. Clinical and experimental immunology. 1993;94:208–213. doi: 10.1111/j.1365-2249.1993.tb06002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Samoilova KA, Obolenskaia KD, Freidlin IS. Changes in the leukocyte phagocytic activity of donor blood after its UV irradiation. II. Simulation of the effect of the autotransfusion of UV-irradiated blood. Tsitologiia. 1987;29:1048–1055. [PubMed] [Google Scholar]
- 66.Simon JC, Tigelaar RE, Bergstresser PR, Edelbaum D, Cruz PD., Jr Ultraviolet B radiation converts Langerhans cells from immunogenic to tolerogenic antigen-presenting cells. Induction of specific clonal anergy in CD4+ T helper 1 cells. Journal of immunology. 1991;146:485–491. [PubMed] [Google Scholar]
- 67.Obolenskaia KD, Freidlin IS, Samoilova KA. Changes in the leukocyte phagocytic activity of donor blood after its UV irradiation. I. Its relation to the irradiation dose and initial level of phagocytic activity. Tsitologiia. 1987;29:948–954. [PubMed] [Google Scholar]
- 68.Roshchupkin DI, Murina MA. Free-radical and cyclooxygenase-catalyzed lipid peroxidation in membranes of blood cells under UV irradiation. Membrane & cell biology. 1998;12:279–286. [PubMed] [Google Scholar]
- 69.Gorog P. Activation of human blood monocytes by oxidized polyunsaturated fatty acids: a possible mechanism for the generation of lipid peroxides in the circulation. International journal of experimental pathology. 1991;72:227–237. [PMC free article] [PubMed] [Google Scholar]
- 70.Salmon S, Maziere JC, Santus R, Morliere P, Bouchemal N. UVB-induced photoperoxidation of lipids of human low and high density lipoproteins. A possible role of tryptophan residues. Photochemistry and photobiology. 1990;52:541–545. doi: 10.1111/j.1751-1097.1990.tb01797.x. [DOI] [PubMed] [Google Scholar]
- 71.Salmon S, Haigle J, Bazin M, Santus R, Maziere JC, Dubertret L. Alteration of lipoproteins of suction blister fluid by UV radiation. Journal of photochemistry and photobiology B, Biology. 1996;33:233–238. doi: 10.1016/1011-1344(95)07260-8. [DOI] [PubMed] [Google Scholar]
- 72.Artyukhov VG, Iskusnykh AY, Basharina OV, Konstantinova TS. Effect of UV irradiation on functional activity of donor blood neutrophils. Bulletin of experimental biology and medicine. 2005;139:313–315. doi: 10.1007/s10517-005-0280-8. [DOI] [PubMed] [Google Scholar]
- 73.Dong Y, Shou T, Zhou Y, Jiang S, Hua X. Ultraviolet blood irradiation and oxygenation affects free radicals and antioxidase after rabbit spinal cord injury. Chinese medical journal. 2000;113:991–995. [PubMed] [Google Scholar]
- 74.Edelson RL. Mechanistic insights into extracorporeal photochemotherapy: efficient induction of monocyte-to-dendritic cell maturation. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2014;50:322–329. doi: 10.1016/j.transci.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Child FJ, Ratnavel R, Watkins P, Samson D, Apperley J, Ball J, Taylor P, Russell-Jones R. Extracorporeal photopheresis (ECP) in the treatment of chronic graft-versus-host disease (GVHD) Bone marrow transplantation. 1999;23:881–887. doi: 10.1038/sj.bmt.1701733. [DOI] [PubMed] [Google Scholar]
- 76.Atta M, Papanicolaou N, Tsirigotis P. The role of extracorporeal photopheresis in the treatment of cutaneous T-cell lymphomas. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2012;46:195–202. doi: 10.1016/j.transci.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 77.de Waure C, Capri S, Veneziano MA, Specchia ML, Cadeddu C, Di Nardo F, Ferriero AM, Gennari F, Hamilton C, Mancuso A, Quaranta G, Raponi M, Valerio L, Gensini G, Ricciardi W. Extracorporeal Photopheresis for Second-Line Treatment of Chronic Graft-versus-Host Diseases: Results from a Health Technology Assessment in Italy. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2015;18:457–466. doi: 10.1016/j.jval.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Patel J, Klapper E, Shafi H, Kobashigawa JA. Extracorporeal photopheresis in heart transplant rejection. Transfusion and apheresis science: official journal of the World Apheresis Association: official journal of the European Society for Haemapheresis. 2015;52:167–170. doi: 10.1016/j.transci.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 79.Reinisch W, Knobler R, Rutgeerts PJ, Ochsenkuhn T, Anderson F, von Tirpitz C, Kaatz M, Janneke van der Woude C, Parenti D, Mannon PJ. Extracorporeal photopheresis (ECP) in patients with steroid-dependent Crohn’s disease: an open-label, multicenter, prospective trial. Inflammatory bowel diseases. 2013;19:293–300. doi: 10.1002/ibd.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ludvigsson J, Samuelsson U, Ernerudh J, Johansson C, Stenhammar L, Berlin G. Photopheresis at onset of type 1 diabetes: a randomised, double blind, placebo controlled trial. Archives of disease in childhood. 2001;85:149–154. doi: 10.1136/adc.85.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Edelson R, Berger C, Gasparro F, Jegasothy B, Heald P, Wintroub B, Vonderheid E, Knobler R, Wolff K, Plewig G, et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. The New England journal of medicine. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 82.Wollina U, Looks A, Meyer J, Knopf B, Koch HJ, Liebold K, Hipler UC. Treatment of stage II cutaneous T-cell lymphoma with interferon alfa-2a and extracorporeal photochemotherapy: a prospective controlled trial. Journal of the American Academy of Dermatology. 2001;44:253–260. doi: 10.1067/mjd.2001.110645. [DOI] [PubMed] [Google Scholar]
- 83.Santella RM, Dharmaraja N, Gasparro FP, Edelson RL. Monoclonal antibodies to DNA modified by 8-methoxypsoralen and ultraviolet A light. Nucleic acids research. 1985;13:2533–2544. doi: 10.1093/nar/13.7.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heald P, Rook A, Perez M, Wintroub B, Knobler R, Jegasothy B, Gasparro F, Berger C, Edelson R. Treatment of erythrodermic cutaneous T-cell lymphoma with extracorporeal photochemotherapy. Journal of the American Academy of Dermatology. 1992;27:427–433. doi: 10.1016/0190-9622(92)70212-x. [DOI] [PubMed] [Google Scholar]
- 85.Hart JW, Shiue LH, Shpall EJ, Alousi AM. Extracorporeal photopheresis in the treatment of graft-versus-host disease: evidence and opinion. Therapeutic advances in hematology. 2013;4:320–334. doi: 10.1177/2040620713490316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cole RS. Repair of interstrand cross-links in DNA induced by psoralen plus light. Yale J Biol Med. 1973;46:492. [PMC free article] [PubMed] [Google Scholar]
- 87.Calabrese EJ. Hormesis: from mainstream to therapy. J Cell Commun Signal. 2014;8:289–291. doi: 10.1007/s12079-014-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krutmann J, Morita A, Chung JH. Sun exposure: what molecular photodermatology tells us about its good and bad sides. The Journal of investigative dermatology. 2012;132:976–984. doi: 10.1038/jid.2011.394. [DOI] [PubMed] [Google Scholar]
- 89.Kraus CN. Low hanging fruit in infectious disease drug development. Current opinion in microbiology. 2008;11:434–438. doi: 10.1016/j.mib.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 90.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, Kumarasamy K, Livermore DM, Maya JJ, Nordmann P, Patel JB, Paterson DL, Pitout J, Villegas MV, Wang H, Woodford N, Quinn JP. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. The Lancet Infectious diseases. 2013;13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yoneyama H, Katsumata R. Antibiotic resistance in bacteria and its future for novel antibiotic development. Bioscience, biotechnology, and biochemistry. 2006;70:1060–1075. doi: 10.1271/bbb.70.1060. [DOI] [PubMed] [Google Scholar]
- 92.O’Neill J. Review on Antimicrobial Resistance: Tackling a Global Health Crisis. Initial Steps. 2015 [Google Scholar]
- 93.Fink MP, Warren HS. Strategies to improve drug development for sepsis. Nat Rev Drug Discov. 2014;13:741–758. doi: 10.1038/nrd4368. [DOI] [PubMed] [Google Scholar]