Abstract
Pancreas ductal adenocarcinoma (PDAC) has one of the worst five-year survival rates of all solid tumors, and thus new treatment strategies are urgently needed. Here we report that targeting Bruton’s Tyrosine Kinase (BTK), a key B cell and macrophage kinase, restores T cell-dependent anti-tumor immune responses, thereby inhibiting PDAC growth and improving responsiveness to standard-of-care chemotherapy (CTX). We report that PDAC tumor growth depends on crosstalk between B cells and FcRγ+ tumor-associated macrophages, resulting in TH2-type macrophage programming via BTK activation in a phosphatidylinositide 3-kinase (PI3K)γ-dependent manner. Treatment of PDAC-bearing mice with the BTK inhibitor PCI32765 (ibrutinib) or by PI3Kγ inhibition reprogrammed macrophages toward a TH1 phenotype that fostered CD8+ T cell cytotoxicity, and suppressed PDAC growth, indicating that BTK signaling mediates PDAC immunosuppression. These data indicate that pharmacological inhibition of BTK in PDAC can reactivate adaptive immune responses, presenting a new therapeutic modality for this devastating tumor type.
Keywords: chemotherapy, gemcitabine, B cells, macrophages, BTK, PI3Kγ, pancreatic ductal adenocarcinoma, desmoplasia
INTRODUCTION
The contributions of tissue resident and recruited leukocytes to the progression of solid tumors is now a widely accepted mechanism of pathogenesis (1, 2), and one that is gaining notable traction in the clinic (3). While many studies have dissected the numerous activities leukocytes exert towards regulating neoplastic progression, tractable immune-based targets for anti-cancer therapy are only just emerging.
Ductal adenocarcinoma of the pancreas (PDAC) is a devastating disease with one of the lowest five-year survival rates of all solid tumors (4). Currently, 7.2% of newly diagnosed pancreatic cancer patients will survive 5 years following diagnosis, due in part to the fact that PDAC is rarely detected at an early stage; instead, the majority of patients present with locally unresectable or metastatic disease (5). Since standard therapies have only a modest impact on survival (6, 7), novel therapeutic and diagnostic strategies are urgently needed.
A characteristic feature of PDAC tumors is the presence of abundant infiltrating leukocytes representing both lymphoid and myeloid lineages (8); thus, we sought to identify functionally significant immune-based programs regulating pancreatic carcinogenesis that would be tractable for therapeutic intervention. We report here that B cell-macrophage interactions promote PI3Kγ- and Bruton’s tyrosine kinase (BTK)-dependent macrophage TH2 polarization, leading to immune suppression and PDAC progression. Using mouse models of PDAC, targeted inhibition of BTK, a common signaling molecule in B cells and myeloid cells, resulted in slowed PDAC tumor growth, abated immune suppression, impeded late-stage tumor growth and improved responsiveness to standard-of-care chemotherapy (CTX), indicating that targeting BTK therapeutically could provide long-term antitumor control for this devastating malignancy.
RESULTS
B cells and FcRγ-positive cells in human PDAC
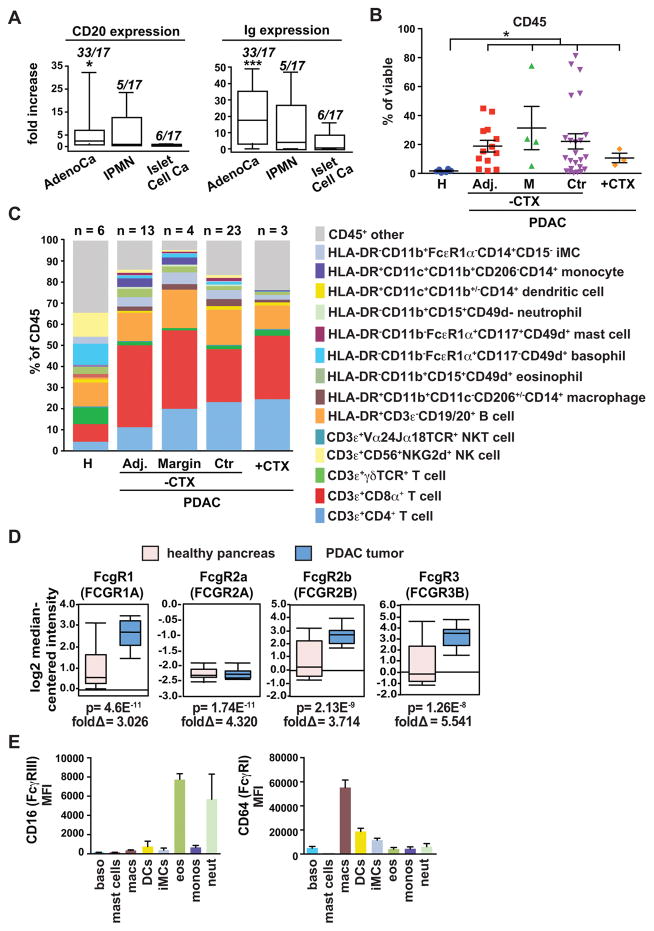
We previously identified a protumorigenic role for CD20+ B cells in solid tumors using murine models of squamous cell carcinogenesis, and demonstrated that therapeutic depletion of B cells in SCC-bearing mice resulted in an improved response to CTX by CD8+ T cell-dependent mechanisms (9). To identify which human solid tumors might be regulated by protumorigenic B cells, we examined cDNA microarrays of ~3,000 human tumors to assess levels of CD20 and immunoglobulin (Ig) mRNA expression relative to corresponding normal tissue (9). As expected, human SCCs of the vulva and head and neck exhibited high expression of both mRNAs (9). Human PDACs also exhibited increased CD20 and Ig expression relative to corresponding healthy pancreas tissue, whereas intrapapillary mucinous neoplasias (IPMN) and islet cell carcinomas did not (Fig. 1A). Using an independent data set, we confirmed increased expression of CD20, IgG3 and IgM mRNA in human PDACs (Supplementary Fig. S1A) and correlated with significantly increased plasma IgG in late-stage PDAC patients (Supplementary Fig. S1B). To quantitatively evaluate presence of specific leukocyte lineages in healthy pancreata versus regions of resected PDACs, we evaluated fresh single cell suspensions from surgically resected healthy pancreata and primary human PDAC tumors by polychromatic flow cytometry (FACS; Supplementary Fig. S1C). We found that CD45+ leukocyte infiltration of PDAC tumor was significantly increased as compared to healthy pancreas tissue (Fig. 1B), and in PDAC from either chemo-naïve or chemo-treated patients, tumors were dominated by B cells, CD4+ and CD8+ T cells (Fig. 1C; Supplementary Fig. S1D), similar to reports from other studies (8).
Figure 1. Leukocytes in human PDAC.
A. Relative CD20 and Ig mRNA expression in human pancreatic ductal adenocarcinoma (AdenoCa; n=33), intrapapillary mucinous neoplasias (IPMN; n=5), and islet cell carcinomas (Islet Cell Ca; n=6), as compared to healthy pancreas tissue (n=17) assessed by Affymatrix Human U133 Plus 2 microarrays. Data are represented as box-and-whisker plots depicting median fold change value compared to normal tissue, displaying the first and third quartiles at the end of each box, with the maximum and minimum at the ends of the whiskers. Statistical significance determined via Wilcoxon rank-sum test with *p< 0.05, ***p< 0.001. B. FACS-quantitation of CD45+ cells as a percentage of viable cells from pancreas tissues reflecting healthy (H) pancreata (n=6), pancreas tissue adjacent to PDAC (Adj; n=13), PDAC tumor margins (M; n=4) and tumor centers (Ctr; n=23) from patients who had not received prior chemotherapy (−CTX), and PDACs (n=3) from patients treated with standard-of-care CTX (+CTX). Each data point reflects an individual piece of tissue. C. Leukocyte complexity of healthy human pancreas (n=6), and pancreas tissue isolated from patients with PDAC evaluated by polychromatic flow cytometry of single cell suspensions and evaluated for expression of the lineage markers shown. Data represent mean leukocyte complexity for leukocyte lineages shown (right) in tumor tissue resected from healthy pancreata (H; n=6), pacreata tissue adjacent to PDAC (Adj; n=13), tissue resected from PDAC margins (n=4) or tumor centers (Ctr; n=23), and PDACs (n=3) from patients treated with standard-of-care CTX (+CTX). Data reflecting individual populations is provided in Fig. S1D. D. mRNA expression of FcγR isoforms from humans with healthy pancreas vs. patients with PDAC tumors. Data was compiled from gene expression available in Oncomine (https://www.oncomine.org). P values and fold change in gene expression are shown. E. FACS histogram showing relative frequency of CD64 (FcγR1) and CD16 (FcγRIII) expression on leukocytes infiltrating human PDAC. Data shown are reflective of 20 human PDAC. Lineage markers for cell types shown are as indicated in panel C and reflect basophils (baso), mast cells, macrophages (macs) dendritic cells (DCs), inflammatory monocytes (iMCs), eosinophils (eos), monocytes (monos) and neutrophils (neut). Adjacent (Adj.) normal indicates tissue isolated from pancreas tissue proximal to resected PDAC tumors.
Based on our previous data indicating that B cells regulate protumorigenic programing of Ig receptor gamma (FcγR)-positive myeloid cells (9), we next evaluated publicly available data sets for FcγR expression. We found that FCGR1, FCGR2B and FCGR3B mRNAs were increased in PDACs as compared to healthy pancreas (Fig. 1D). In addition, we evaluated the frequency of leukocytes expressing CD64 (FcγR1) and CD16 (FcγRIII), the activating forms of FcRγ, in human PDAC tumors, and found highest levels of CD64 on macrophages, dendritic cells, and immature monocytes, and highest levels of CD16 instead on eosinophils and neutrophils (Fig 1E). Based on these collective data, we hypothesized that, similar to murine SCCs, B cells co-operate with FcRγ-positive myeloid cells to foster PDAC tumorigenesis.
B cells and FcRγ-positive myeloid cells foster PDAC tumorigenesis
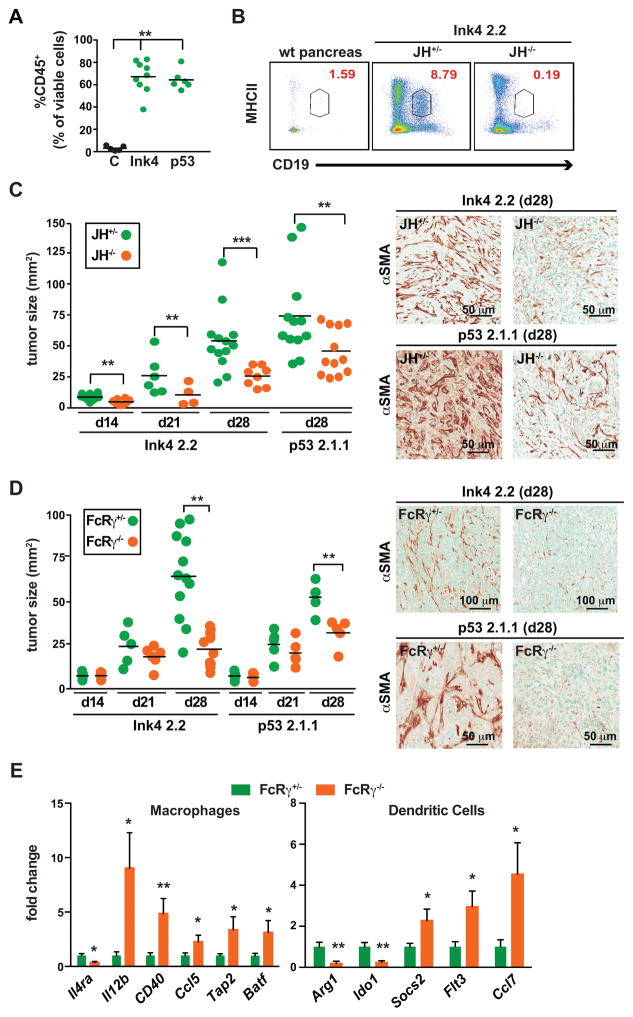
To test the hypothesis that B cells collaborate with myeloid cells to promote PDAC tumorigenesis, we investigated tumor growth of two syngeneic murine PDAC cell lines derived from primary pancreatic carcinomas of transgenic Pdx-Cre; LSL-KrasG12D mice (10–12) harboring null mutations in p16Ink4a (Ink4 2.2) or p53 (p53 2.1) (13–16). Both cell lines generated PDACs that were histologically similar to PDACs from transgenic mice from which they were derived (Supplementary Fig. S2A–C). PDAC tumors derived from both cell lines also exhibited similar infiltration by CD45+ leukocytes as a percentage of viable cells in tumors (Fig. 2A; Supplementary Fig. S2D), as well as significant B cell infiltration as compared to wildtype pancreas tissue as revealed by FACS analysis, predominated by IgMhiCD23+ transitional 2 cells, IgMhiCD23−CD5+ B1a cells, IgMhiCD23−CD5−CD1dlo B1b cells, IgMloCD23+CD5− follicular B cells, IgMloCD23− memory B cells, and to lesser extents, B cells reflecting marginal zone, regulatory, plasma blast and plasma cells (Fig 2B; Supplementary Fig. S2E), and/or immunohistochemical analysis (Supplementary Fig. S2A).
Figure 2. Orthotopic PDAC growth is regulated by B cells and FcRγ-positive myeloid cells.
A. Murine PDAC cell lines, derived from Pdx-Cre LSL-KrasG12D mice harboring an Ink4a/Arfflox/+ allele (Ink4 2.2) or a p53flox/+ allele (p53 2.1.1), were injected orthotopically into the pancreas, and subsequent tumors evaluated by FACS for CD45-positive cellular infiltrates. Graph shows percentage of CD45+ cells as a percentage of viable cells. B. Representative CD19+MHCII+ FACS plot gated on viable CD45+ cells from tumor naïve pancreas and Ink4 2.2-implanted PDAC tumors in syngeneic JH+/− and JH−/− mice evaluated 28 days after implantation. C. Ink4 2.2 and p53 2.1.1-derived PDACs were quantitatively evaluated in syngeneic JH+/− and JH−/− mice for tumor area in serial H&E-stained tissue sections reflecting tumors isolated on day 14, 21, and 28 post-implantation. On right, representative photomicrographs reflecting immuno-detection of αSMA in end-stage Ink4 2.2 and p53 2.1.1-derived orthotopic PDAC tumors. D. Ink4 2.2 and p53 2.1.1-derived PDACs were quantitatively evaluated in syngeneic FcRγ+/− and FcRγ−/− mice for tumor area in serial H&E-stained tissue sections reflecting tumors isolated on day 14, 21, and 28 post-implantation. On right, representative photomicrographs reflecting immuno-detection of αSMA in end-stage Ink4 2.2 and p53 2.1.1-derived orthotopic PDAC tumors. E. qRT-PCR analysis from cDNA reflecting FACS-purified macrophages (MØ = CD45+CD11b+MHCII+F4/80+Ly6C−) or dendritic cells (DC = CD45+CD11b+MHCII+F4/80−CD11c+) from Ink4 2.2 PDAC-derived tumors harvested from d21 FcRγ+/− (n=10) or FcRγ−/− (n=8) mice. Displayed are fold changes in genes that reached statistically significant differences. Data compiled from two independent experiments. For graphs in panels A, C–E, statistical means are shown, with p values determined by either student’s T test or one-way Anova when analyzing more than two groups, with *p < 0.05, **p < 0. 0.01, ***p < 0.001. For graphs in C and D, each data point reflects mean tumor size from one mouse based on quantitative morphometry of 5 FFPE sections, resulting from 3 independent experiments.
To determine if B cells or FcRγ-positive myeloid cells imparted a growth advantage to orthotopic PDACs, cell lines were implanted into syngeneic B cell-proficient (JH+/+ or +/−) or B cell-deficient JH−/− mice (Fig. 2B and C). JH−/− mice possess a deletion in the J segment of the Ig heavy chain locus and thus do not express IgM or IgG, and thus have no mature B cells in bone marrow or periphery due to blocked B cell differentiation at the large, CD43+ precursor stage (17). Tumor cells were also implanted into Ig receptor FcRγ-proficient (FcRγ+/− or +/+) and FcRγ-deficient (−/−) mice (18), and tumor growth kinetics and characteristics evaluated longitudinally (Fig. 2C and D; Supplementary Fig. S2B and C). PDACs grown in either B cell-deficient or FcRγ-deficient mice were significantly smaller than littermate controls (Fig. 2C and D), and exhibited significantly reduced desmoplasia, as determined by alpha smooth muscle actin (αSMA) immunoreactivity (Fig. 2C and D) and Gomori trichrome staining (Supplementary Fig. S2B and C).
Similar to human PDAC expression of FCGR1, FCGR2B and FCGR3B mRNAs (Fig. 1D), we evaluated expression of CD64 FcγRI and CD16/CD32 FcγRII/III by FACS (Supplementary Fig. S2F) and found high level expression on tumor infiltrating macrophages and DCs, and to a lesser degree monocytes and neutrophils (Supplementary Fig. S2F). Macrophages, DCs, monocytes and neutrophils infiltrating Ink4 2-2-derived PDAC tumors grown in FcRγ-deficient (FcRγ−/−) mice exhibited no expression of either FcγRI or FcγRII/III molecules (Supplementary Fig. S2G). Since we previously reported that FcRγ−/− macrophages in SCC tumors have a shifted gene expression profile favoring expression of mRNAs characterizing TH1 phenotypes (9, 19), we isolated CD45+CD11b+MHCII+F4/80+Ly6C− macrophages and CD45+CD11b+MHCII+F4/80−CD11c+ DCs from Ink4 2.2-derived PDAC tumors harvested from FcRγ-proficient versus –deficient mice, and evaluated a panel of characteristic TH1 (IL12b, Cd40, Ccl5, Tap2, Batf, Flt3, Ccl7, Socs2) and TH2 (IL4ra, Arg1, Ido1) mRNAs, and found significant skewing of both cell types towards a TH1 phenotype in FcRγ-deficient mice (Fig. 2E). This data, together with diminished PDAC growth observed in B cell null JH−/− mice, and Ig receptor null FcRγ−/− mice thus supported the hypothesis that B cells, as well as subsets of myeloid cells influence PDAC tumor growth.
B cell and myeloid cell signaling pathways in PDAC
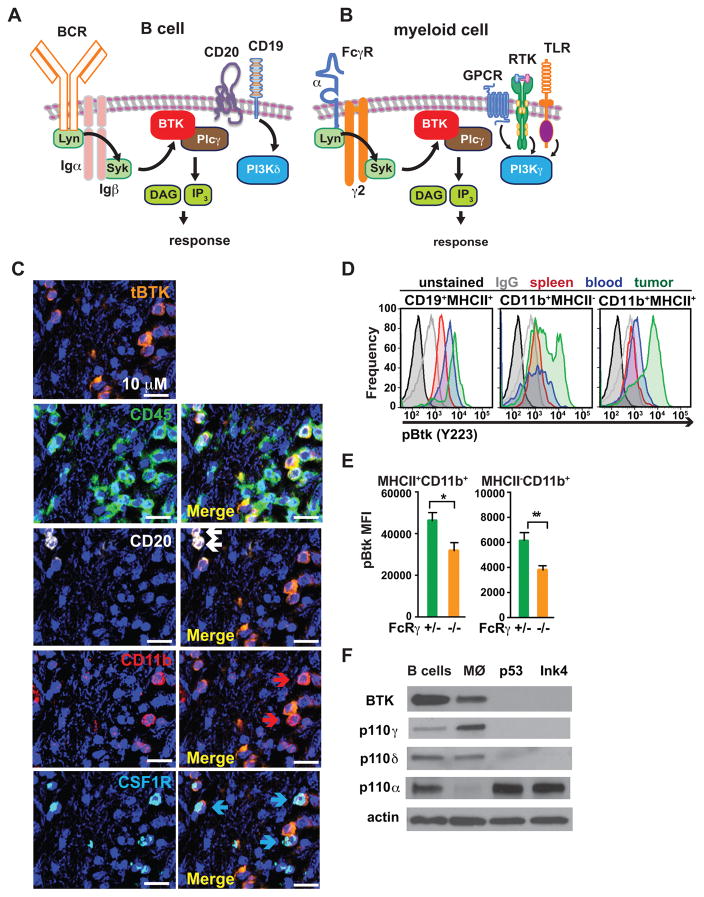
Based on these findings and our previous data indicating that B cells and tumor-associated macrophages impede cytotoxic T cell (CTL) activity to regulate solid tumor development (19, 20), we hypothesized that inhibitors of common signaling pathways active in both B cells and infiltrating macrophages, such as BTK or PI3K signaling, might be efficacious against PDAC tumors (Fig. 3A and B). BTK is a member of the Tec family of cytoplasmic protein tyrosine kinases expressed by multiple hematopoietic-lineage cells; in B lymphocytes, BTK is activated by the B cell receptor (BCR) pathway, while in macrophages, BTK is activated downstream of FcRγ by spleen tyrosine kinase (Syk) (21, 22). Thus, we examined human PDAC specimens for presence of BTK+ cells in tissue sections and found BTK immunoreactivity in CD45+ leukocytes, CD20+ B cells, CD11b+ and colony stimulating factor 1 receptor (CSF1R)-positive myeloid cells, (Fig. 3C, Supplementary Fig. S3A). In murine PDAC tumors, we identified activated BTK (pBTK) cells in single cell suspensions most prominently in tumor resident CD19+MHCII+ B cells and CD11b+ myeloid cells (Fig. 3D). Relative to unstimulated cells, the mean fluorescent intensity (MFI) of pBTK significantly increased in B cells and myeloid cells following BCR and FcγRII/III-stimulation, respectively (Supplementary Fig. S3B). Importantly, BTK activation was reduced in FcRγ-deficient myeloid cells infiltrating orthotopic PDAC tumors (Fig. 3E). As macrophage depletion via administration of a depleting colony stimulating factor (CSF1) antibody (αCSF1 mAb) (9, 20, 23) (Supplementary Fig. S3C), and B cell depletion through loss of the JH locus (Fig. 2C) significantly reduced PDAC tumor growth in vivo, we postulated that BTK+ B cells and macrophages were promoting PDAC growth.
Figure 3. BTK and PI3K in PDAC-infiltrating B cells and myeloid cells.
A–B. Cartoons depicting BCR- and FcRγ-activated BTK signaling in B cells and myeloid cells. C. Representative photomicrograph showing immunodetection of BTK, CD45, CD20, CD11b and CSF1R in a human PDAC FFPE section. Arrows in ‘merged’ images indicate double-positive cells. Magnification is shown. 6 different human PDAC tumors were evaluated, and data shown is reflective of all. D. Intracellular FACS detection of activated phosphoBTK (pBTK) (Y223) in single cells harvested from peripheral blood, spleen, and tumor tissue of Ink4 2.2-implanted syngeneic mice, gated on CD19+MHCII+ B cells, CD11b+MHCII− and CD11b+MHCII+ myeloid cells. Also shown are unstained and IgG1 isotype control stained cells. Representative data from one experiment (n=7 mice) reflective of 2 independent experiments is shown. E. Intracellular FACS detection of pBTK (Y223) in MHCII+CD11b+ and MHCII−CD11b+ cells isolated from end-stage Ink4 2.2 PDAC tumors from syngeneic FcRγ+/− or FcRγ−/− mice. Data from one representative experiment (n=8 mice per experimental group) is shown and is reflective of 2 independent experiments. F. Cropped western blot images showing expression of BTK, PI3Kγ (p110γ), PI3Kδ (p110δ), PI3Kα (p110α) and actin, in murine PDAC-derived B cells, primary murine macrophages (MØ), and cultured PDAC clones p53 2.1.1 (p53) and Ink4a 2.2 (Ink4).
PI3Kγ activates macrophage BTK to promote PDAC growth
As PI3Kinases can activate BTK to promote phospholipase C (PLC)γ-dependent signaling in hematopoietic cells (21, 24), we explored the roles of BTK and PI3Kinases in B cell-macrophage interactions during PDAC tumorigenesis. Four unique isoforms of Class I PI3Ks (p110α, β, δ and γ) regulate PI3K signaling in cells (2). We found that primary murine macrophages express high levels of BTK and PI3Kγ (p110γ), but lower levels of PI3Kδ (p110δ), while B cells express high levels of BTK, PI3Kα (p110α) and PI3Kδ, but low levels of PI3Kγ; neoplastic PDAC tumor cells express only PI3Kα and PI3Kβ (p110β) (Fig. 3F; Supplementary Fig. S3D). As PI3Kγ and BTK are similarly expressed in myeloid cells, and the PI3K isoform p110γ selectively promotes PLCγ-dependent integrin α4β1 activation leading to myeloid cell recruitment to tumors (25), we speculated that PI3Kγ might activate BTK to promote myeloid cell recruitment during PDAC progression.
To characterize the interactions between PI3Kγ and BTK in myeloid cells, we first compared their roles in mediating integrin α4β1 activation and cell adhesion, processes that are required for myeloid cell trafficking into tumors (25, 26). To achieve this, we used pharmacological inhibitors and siRNA-mediated knockdown of Btk, as well as pharmacological and genetic disruption of p110γ (PI3Kγ). To inhibit BTK, we utilized an FDA-approved BTK inhibitor PCI-32765 (ibrutinib) (27). PCI-32765 has an IC50 of 0.5 nM for BTK and exhibits cross-reactivity to BLK and BMX (IC50=0.5 nM), ITK (IC50 = 11 nM), and TEC (IC50 = 78 nM) (22). To inhibit p110γ, we utilized the investigational PI3Kγ/δ inhibitor TG100-115, which has an IC50 of 83 nM for PI3Kγ, 238 nM for PI3Kδ and >1000nM for PI3Kα and β (25, 28).
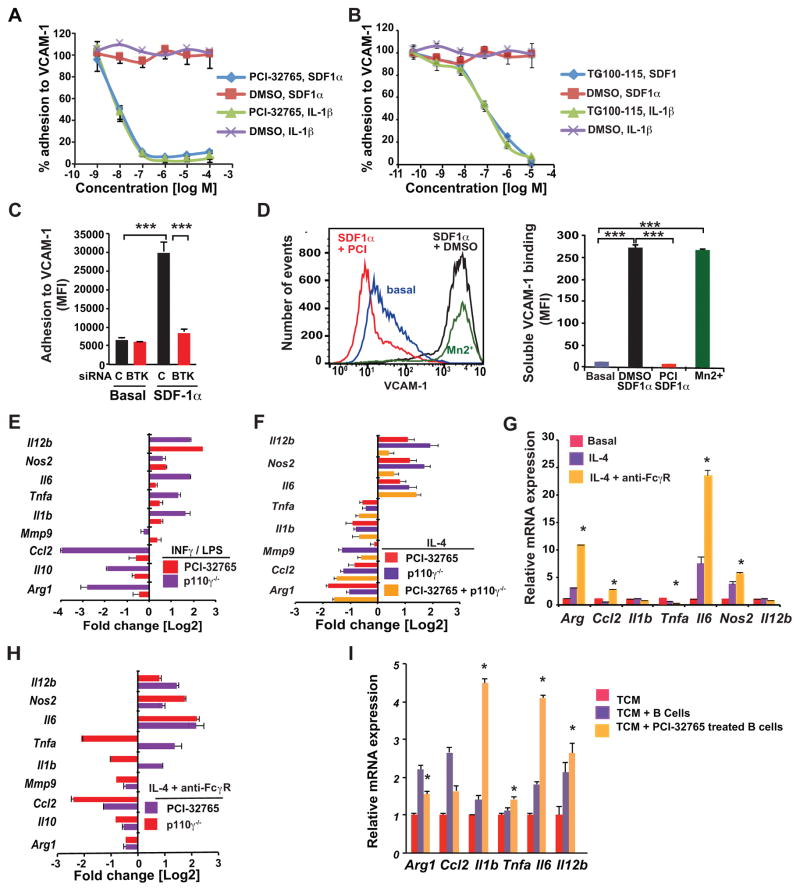
We previously found that stromal cell-derived factor 1 (SDF-1)α and interleukin (IL)-1β induce adhesion of primary macrophages to vascular cell adhesion protein (VCAM)-1 coated surfaces (21–23); both PCI-32765 and TG100-115 significantly suppressed SDF-1 and IL-1β mediated cell adhesion, with IC50’s of 10 nM and 100 nM, respectively (Fig. 4A–B). In support of these findings, Btk knockdown (Supplementary Fig. S4A), similar to p110γ deletion and knockdown (21–23), suppressed adhesion of primary myeloid cells to VCAM-1 (Fig. 4C). PCI-32765 and p110γ inhibition also suppressed CD11b+ myeloid cell adhesion to vascular endothelial cell (EC) monolayers (Supplementary Fig. S4B), and PCI-32765 also suppressed B cell adhesion to EC (Supplementary Fig. S4C). In addition, siRNA-mediated knockdown of PLCγ2, a key BTK and PI3Kγ signaling intermediate, (21, 25) (see schematic in Fig. 3A–B), suppressed myeloid cell adhesion (Supplementary Fig. S4D and E). Together, these findings support the hypothesis that PI3Kγ, BTK and PLCγ2 regulate similar signaling pathways in myeloid cells that contribute to PDAC progression.
Figure 4. FcRγ-signaling regulates BTK activation and mediates PDAC growth.
A–B. Effect of the BTK inhibitor PCI-32765 (A), and the p110γ inhibitor TG100-115 (B) versus vehicle control (DMSO) on IL-1β– and SDF-1-stimulated adhesion of primary myeloid cells to VCAM-1 (n=3). C. Effect of siRNA-mediated BTK knockdown on SDF-1α-stimulated myeloid cell adhesion to VCAM-1 (n=3). D. Effect of PCI-32765 (PCI) on SDF-1α-induced integrin α4β1 activation, as detected by binding of fluorescent VCAM-1 to myeloid cells (n=3); left, FACS profiles and right, quantification of mean fluorescence intensity. Positive control: extracellular Mn2+ treatment. E–F. Log2-fold change of gene expression in (E) LPS/IFNγ-in vitro polarized macrophages treated with PCI-32765 or p110γ−/− macrophages, (F) p110γ−/−, PCI-3276-treated, and p110γ−/− PCI-32765-treated IL-4-in vitro polarized primary murine macrophages (n=3). G. Relative mRNA expression of macrophages polarized with IL-4 or IL-4+FcRγ crosslinking (n=3). H. Log2-fold change of gene expression in IL-4-stimulated FcRγ-cross-linked p110γ−/− or PCI-3276-treated primary murine macrophages (n=3). I. Relative mRNA expression of macrophages co-cultured in p53 2.1.1 tumor cell conditioned medium (TCM) with tumor derived B cells (n=3) or PCI-32765 treated B cells. Data shown are mean +/− SEM of biological replicates and were validated in 3 or more separate experiments. Significance testing was performed by one way Anova with Tukey’s posthoc testing for multiple pairwise testing or by Student’s t test, where p>0.05 unless other specified, * P < 0.05, ** P < 0.01, and *** P < 0.001. For mRNA expression studies, p<0.01 unless otherwise indicated.
Based on these findings, we surmised that PI3Kγ might activate a BTK-signaling pathway to promote integrin α4β1 activation on macrophages prior to cell adhesion. In support of this, BTK inhibition, similar to PI3Kγ inhibition (25), suppressed integrin activation, as measured by cytokine-induced fluorescent VCAM-1 binding to myeloid cells (Fig. 4D). In this assay, whereas VCAM-1 binding to macrophages is rapidly stimulated by SDF-1α or positive control Mn2+, VCAM-1 binding was completely inhibited in the presence of PCI-32765 (Fig. 4D). Because BTK activation, as measured by autophosphorylation on Y223, was suppressed in macrophages lacking PI3Kγ (p110γ−/−) (Supplementary Fig. S4F), we concluded that PI3Kγ activates a BTK-PLCγ2 pathway to promote integrin activation, myeloid cell adhesion and, potentially, myeloid cell recruitment to tumors in vivo.
Since we previously reported PI3Kγ regulates pro-tumorigenic properties of macrophages in vivo (25), we asked whether PI3Kγ activated BTK to promote pro-tumor macrophage polarization. Interferon gamma (IFNγ/lipopolysaccharide (LPS) signaling induces macrophage expression of TH1 cytokines including IL-12, tumor necrosis factor (TNF)α, IL-6, IL-1β, and nitric oxide synthase (Nos)2, and inhibits expression of TH2 immune-suppressive cytokines, including Arginase (Arg)1 and transforming growth factor (TGF)β in vitro, whereas IL-4 signaling instead inhibits expression of TH1 cytokines and stimulates expression of TH2 factors in vitro (29, 30). We undertook an analysis of relative mRNA levels in IFNγ- or IL-4-stimulated control and p110γ−/− or PCI-32765-treated macrophages by RT-PCR. Analysis of the change in mRNA levels between control and p110γ−/− or PCI-32765-treated macrophages indicated that genetic or pharmacological inhibition of either PI3Kγ or BTK induced TH1 skewing of IFNγ/LPS-stimulated macrophages, as expression of Il-12, Il-6, Tnfa, Il-1b and Nos2 was enhanced, and expression of Arg1, Il-10, and Ccl2 was reduced (Fig. 4E; Supplementary Fig. S4G and H). Inhibition of either PI3Kγ or BTK by pharmacological or genetic approaches similarly induced TH1 skewing of IL-4-stimulated macrophages while also inhibiting Il-1b and Tnfa expression (Fig. 4F; Supplementary Fig. S4I). Combination of BTK inhibitors with PI3Kγ−/− macrophages had little additional effects on TH1 skewing of macrophage gene expression profiles (Fig. 4F), indicating that BTK and PI3Kγ regulate similar macrophage polarization pathways. Importantly, these observations also reveal that PI3Kγ and BTK similarly promote TH2 macrophage polarization and restrain TH1 polarization, indicating that these kinases may promote pro-tumor TH2 macrophage polarization in vivo.
Since macrophage FcγR signaling promotes PDAC growth (Fig. 2), we investigated the consequences of FcγR crosslinking on macrophage polarization by incubating macrophages with anti-FcγR antibodies followed by secondary antibodies. FcγR crosslinking of IL-4-polarized macrophages enhanced TH2 skewing, as macrophages displayed enhanced expression of key TH2 factors, including Arg1 and Ccl2 (Fig. 4G). Importantly, inhibition of either PI3Kγ or BTK suppressed FcγR-stimulated TH2 cytokine expression and promoted TH1 cytokine expression, with the exception that PI3Kγ inhibition suppressed, while BTK inhibition stimulated, Tnfa and Il-1b expression (Fig. 4H). These minor differences in FcγR-stimulated gene expression indicate that PI3Kγ regulates both BTK-dependent and -independent gene expression pathways. Taken together, these results support that hypothesis that PI3Kγ activates BTK to promote macrophage TH2 polarization.
We previously reported that B cell-derived Ig containing immune complexes stimulate FcγR crosslinking to promote macrophage TH2 cytokine expression (19). To determine if BTK regulated B cell-directed expression of TH2 cytokines in macrophages, we co-cultured PDAC-derived B cells with primary macrophages in co-culture chambers in vitro. Under these conditions, B cells and macrophages were separated by a filter allowing only passage of macromolecules between chambers. In the presence of PDAC cell conditioned medium, tumor-derived B cells enhanced macrophage TH2 skewing; however, pre-treatment of PDAC-derived B cells with PCI-32765 suppressed this and instead enhanced expression of TH1 cytokines (Fig. 4I). As B cell-derived immune complexes promote macrophage TH2 cytokine expression (8), these results indicate that B cells promote the immunosuppressive, pro-tumor properties of macrophages in a manner that depends on BTK in both B cells and macrophages.
To evaluate the relative contributions of BTK in B cells and macrophages during tumor progression in vivo, B cells isolated from spleens of PDAC (p53 2.1.1)-bearing animals or Gr1−CD11b+ myeloid cells (Supplementary Fig. S5A) isolated from tumors of PDAC (p53 2.1.1)-bearing animals were incubated with PCI-32765 or vehicle, mixed with tumor cells and implanted in animals. Untreated B cells and myeloid cells had no significant effect on tumor growth, while pre-treatment of B cells or myeloid cells with BTK inhibitor prior to implantation significantly suppressed tumor growth (Fig. 5A). These results support the hypothesis that BTK in both B cells and myeloid cells promote PDAC tumor growth.
Figure 5. PI3Kγ and BTK promote PDAC progression.
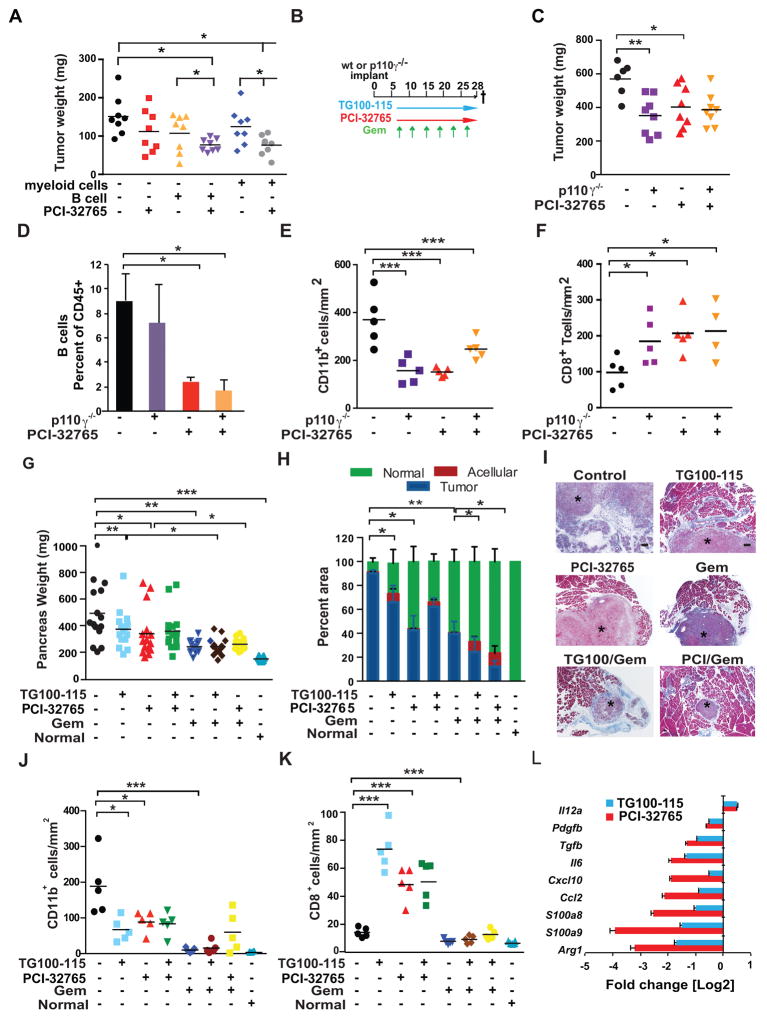
A. Tumor burden in mice implanted with admixed p53 2.1.1 cells together with PCI-32765- or vehicle-pretreated B cells or myeloid cells as compared to untreated or PCI-32765 post-implantation treatment alone. B. Schemas for early treatment schedule for PDAC-bearing mice with administration of PCI-32765 in drinking water (0.016% w/v) and/or the p110γ inhibitor TG100-115 (2.5 mg/kg BID given i.p.) beginning on day 7 post-implantation, and gemcitabine (Gem; 15 mg/kg, i.v.) in p53 2.1.1 derived PDAC-bearing mice. C. End-stage p53 2.2 PDAC tumor weights from syngeneic PI3Kγ-deficient (p110γ−/−) or wildtype mice treated with or without PCI-32765 (n=10–15/mice per experimental group). D. Tumor-derived B cells as a percentage of CD45+ cells in treated tumors from ‘C’, as determined by FAC analysis. E. Number of CD11b+ cells/mm2 in treated tumors from ‘C’. F. Number of CD8+ T cells/mm2 in treated tumors from ‘C’. G. Effect of TG100-115, PCI-32765, gemcitabine (Gem) and combinations of these agents on growth to end-stage of orthotopic p53 2.2 PDAC tumors (n=10–15/mice per experimental group) depicted as total pancreas weight after 3 weeks of treatment as compared to pancreas weight of tumor-naïve mice. H. Percent residual tumor, normal acinar tissue and acellular fibrotic tissue in pancreata of tumors depicted in panel ‘G’. I. Representative photomicrographs showing Masson’s trichrome stained images of PDAC tumors (*) treated as shown reflecting quantitation in panels G and H. J. Number of CD11b+ cells/mm2 in treated tumors depicted in panel ‘C’, compared to normal pancreata. K. CD8+ T cells/mm2 in treated tumors depicted in panel ‘C’. L. Log base 2 fold change in gene expression in tumors between control and TG100-115 or control and PCI-32765 treatments (n=15). mRNAs for which TG100-115-treated tumors showed statistically significant differences from control tumors are shown. Data shown are mean +/− SEM of biological replicates and were validated in 3 or more separate experiments. Significance testing was performed by one way Anova with Tukey’s posthoc testing for multiple pairwise testing or by Student’s t test, where p>0.05 unless other specified, * P < 0.05, ** P < 0.01, and *** P < 0.001. ns indicates not statistically different. For panels A, C, E–G, J–K each data point reflects an individual tumor with statistical means shown. For mRNA expression studies, p<0.01 unless otherwise indicated.
As neither BTK nor PI3Kγ inhibitors directly affected the viability of macrophages (Supplementary Fig. S5B) or PDAC tumor cells (Supplementary Fig. S5C and D), we evaluated whether PI3Kγ regulated BTK activity during PDAC growth in vivo by systemically treating wildtype (WT) and p110γ−/− mice bearing p53 2.1.1 PDAC tumors with or without PCI-32765 (Fig. 5B). Analogous to clinical scenarios observed in patients (31), mice receiving PCI-32765 exhibited transient lymphocytosis in peripheral blood of tumor bearing mice that resolved by the study termination (Supplementary Fig. S5E). PDAC tumor growth was similarly suppressed in p110γ−/− animals and in WT mice treated with PCI-32765, while PCI-32765 treatment of p110γ−/− mice had no additive effect on tumor growth (Fig. 5C). Importantly, p110γ deletion and PCI- 32765 inhibitor treatment suppressed B cell and CD11b+ myeloid cell infiltration (Fig. 5D–E; Supplementary Fig. S5F) as predicted by results from the in vitro cell adhesion studies (Fig. 4A–B). p110γ deletion and PCI-32765 treatment also increased CD8+ T cell presence in tumors (Fig. 5F).
To evaluate the effect of combined treatment of PDAC tumors with PI3Kγ and BTK pharmacological inhibitors, we treated mice bearing orthotopic p53 2.1.1 PDAC tumors with PI3Kγ and BTK inhibitors alone, together, or each in combination with gemcitabine (Gem; Fig. 5G). TG100-115 and PCI-32765 both suppressed PDAC tumor growth, as reflected in weights of pancreata 28 days post-inoculation, but the combination of the two inhibitors had no additive effect (Fig. 5G). Gem monotherapy suppressed tumor growth as measured by pancreas weight, whereas Gem plus TG100-115 or Gem plus PCI-32765 had no additional impact on overall pancreas weight (Fig. 5G). However, histological examination of end-stage tumors revealed little residual live tumor in pancreata treated with Gem plus PCI-32765 or TG100-115, indicating additive effects of the two therapies with Gem (Fig. 5H–I). Importantly, PI3Kγ and BTK inhibitors suppressed CD11b+ myeloid cell infiltration (Fig. 5J; Supplementary Fig. S5G), and increased CD8+ T cell residency of tumors (Fig. 5J) similar to results observed in Fig. 5E–F. The combination of PI3Kγ and BTK inhibitors had no additional effects beyond those of each inhibitor alone, supporting the conclusion that PI3Kγ and BTK regulate the same pathway in vivo as well as in vitro (Fig. 4E–H). In addition, all Gem-treated pancreata exhibited fewer CD11b+ and CD8+ T cells than other treatments, as very little tumor tissue remained in these pancreata after 28 days (Fig. 5I–K). As PI3Kγ and BTK inhibition suppressed TH2 and stimulated TH1 cytokine expression in in vitro cultured macrophages (Fig. 4E–I), in the tumor microenvironment (Fig. 5L) and in tumor-derived myeloid cells (Supplementary Fig. S5H), these studies indicate that BTK and PI3Kγ regulate macrophage and T cell programming in vivo as well as in vitro. Together these data support the conclusion that PI3Kγ and BTK promote a pathway leading to TH2 polarization during PDAC progression. Furthermore, these data indicate that PI3Kγ and BTK inhibitors could be useful therapeutic approaches to treat early PDAC tumors.
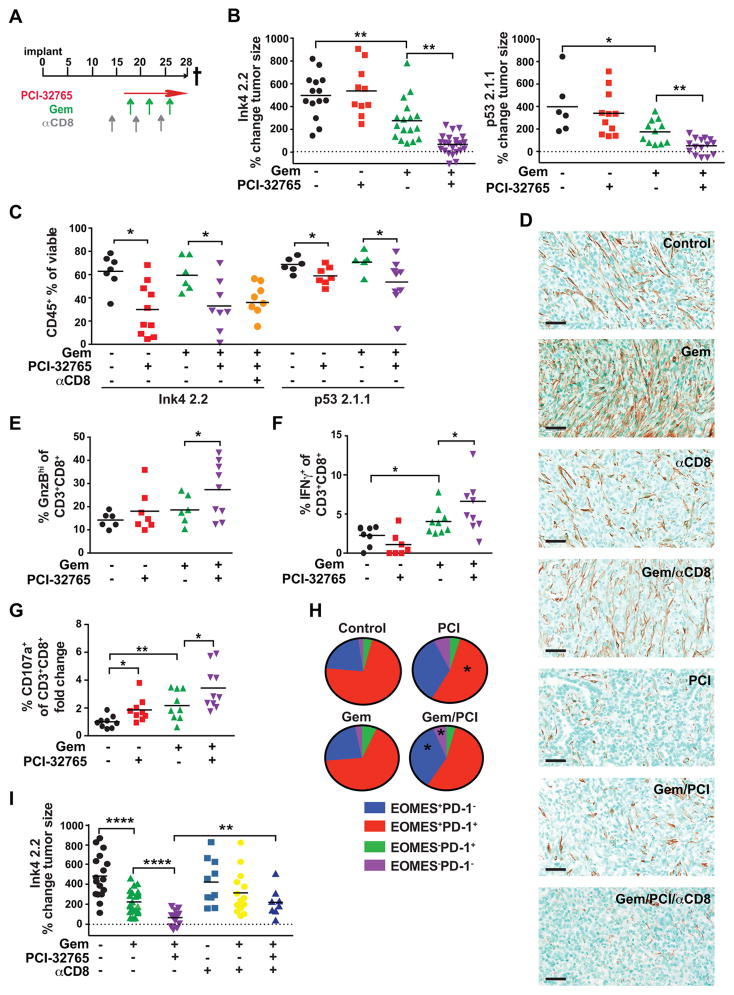
Clinically, patients with PDAC typically present at late-stage; thus, we sought to determine if therapeutic administration of PCI-32765 was efficacious in late-stage PDAC tumors as monotherapy, or in combination with Gem (Fig. 6A). PDAC growth in mice treated with combination PCI-32765/Gem administered at a late-stage resulted in significantly reduced tumor size at end stage (Fig. 6B). Decreased tumor burden was associated with reduced presence of CD45+ leukocytes (Fig. 6C; Supplementary Fig. S6A), associated with dynamic changes in the B cell compartment whereby Gem treatment reduced presence of memory B cells, BTKi resulted in increased frequency of T2 and B1b cells and reduced frequency of follicular and marginal zone B cells (Supplementary Fig. S2D), and decreased desmoplasia (Fig. 6D; Supplementary Fig. S6A). Importantly, we also observed a significant increase in intratumoral frequency of granzyme BHI, IFNγ+, and extracellular CD107a+ CD8+ T cells indicative of their recent activation and degranulation (Fig 6E–G; Supplementary Fig. S6A). In agreement with these data, there was an enhanced presence of programmed death (PD)-1−EOMES+ late effector and PD-1−EOMES− short-term memory CD8+ T cell phenotypes (Fig 6H; Supplementary Fig. S6B), that correlated with TH1-skewing of BTKi-treated tumors (Fig. 5L). This increased presence of effector CD8+T cell phenotypes was functionally significant as CD8+ T cell-depletion reinstated tumor growth to levels similar to Gem-treated mice (Fig. 6I; Supplementary Fig. S6C), without reinstating CD45+ leukocyte infiltration (Fig. 6C) or desmoplasia (Fig. 6D) observed in untreated tumors. Taken together, these results indicate that myeloid cell PI3Kγ activates BTK, thereby promoting myeloid cell recruitment to PDACs and T cell-mediated immune suppression, and importantly, indicate that pharmacologic inhibition of PI3Kγ or BTK activates a TH1 immune response that suppresses tumor growth.
Figure 6. Btk–activated signaling regulates PDAC tumorigenesis.
A. Schemas for therapeutic administration of PCI-32765 (in drinking water [0.16% w/v] beginning on day 14 post-implantation and gemcitabine (Gem; 15 mg/kg, i.v., beginning on day 18) to late-stage PDAC tumor-bearing mice. 500 μg depleting αCD8 mAb antibody administered i.p. on day 15, 20, and 25. B. Percent change in Ink4 2.2 (left) or p53 2.1.1 (right) tumor size from day 14 to day 27 post-implantation measured by ultrasonography and assessed following treatment with vehicle (−), PCI-32765, or Gem, and combinations as indicated. For Ink4 2.2, data from 3 independent experiments are shown reflective of 5 independent experiments. For p53 2.1.1, data from one experiment is shown and is representative of 2 independent experiments. C. CD45+ leukocyte frequency from end-stage tumors as a percentage of total viable cells determined by FACS analysis of single cell suspensions of tumors from treatment groups indicated. Data from 2 independent experiments are shown and representative of 5 independent studies. D. Representative photomicrographs showing immune-detection of alpha-smooth muscle actin (αSMA) in mice bearing end-stage Ink4 2.2 PDAC tumors treated as shown. Scale bars = 100 μm. E. Percentage of granzyme B-positive (GnzB) cells of CD3+CD8+ cells as determined by FACS of end-stage Ink4 2.2 PDAC tumors treated as shown. Data from 2 independent experiments are shown and representative of 4 independent studies. F. Percentage of IFN-γ+CD8+ T cells of CD3+CD8+ cells as determined by intracellular FACS assessment of ex vivo stimulated Ink4 2.2 PDAC tumors harvested from mice at day 23 post-implant. Data from 2 independent experiments are shown. G. Fold change of percent of CD107a+CD8+ T cells of CD3+CD8+ cells analyzed by FACS of Ink4 2.2 PDAC tumors harvested from mice at day 23 post-implant. Data from 2 independent experiments are shown. H. FACS analysis of Ink4 2.2 tumors from day 23 time-points for percentages of PD-1+EOMES+ populations of CD3+CD8+ T cells. Data from 2 independent experiments is shown. Statistical significance was determined using the student’s T test or one-way Anova when analyzing more than two groups. I. Percent change in Ink4 2.2 tumor size from day 14 to day 27 post-implantation measured by ultrasonography and assessed following treatment with vehicle (−), PCI-32765, or Gem, and combinations in mice also administered depleting monoclonal antibodies for CD8+ (αCD8) T cells as indicated. Data from 2 independent experiments is shown. * p < 0.05, ** p < 0.01, *** p < 0.001. For panels B–C and E–G and I, each data point reflects an individual tumor with statistical means shown.
DISCUSSION
Pancreatic ductal adenocarcinoma is traditionally thought to be an immunologically silent malignancy, but recent therapeutic strategies have demonstrated that effective immune-mediated tumor cell death can be invoked to combat disease dissemination (32–34). The fundamental principle of these strategies is that CD8+ T cells can be mobilized to recognize and eliminate malignant cells if suppressive barriers to immunity are abated. Data presented herein reveal a critical role for B cells and FcRγ-activated myeloid cells, and in particular macrophages, in regulating functionality of CD8+ T cells in PDAC, and identify BTK and PI3Kγ as key regulators of tumor immune suppression. Our studies demonstrate that both human and murine PDACs exhibit increased BTK activation in tumor-resident CD20+, CD11b+ and FcγRI/III+ cells relative to leukocytes in the periphery. BTK or PI3Kγ inhibition as monotherapy in early stage PDAC, or in combination with Gem in late-stage PDAC slowed progression of orthotopic tumors in a manner dependent on T cells. Coincident with slowed tumor growth, the percentage of CD8+ T cells with enhanced effector molecule expression increased in PDAC tumors that were functionally significant as CD8+ T cell-depletion eliminated benefits of combined BTK inhibitor/Gem therapy. An increase in effector and memory CD8+ T cell phenotypes was also observed midway through therapy, and thus consistent with other reports regarding CD8+ T cell responses to various immunotherapies (35). Based on these data and reports from other groups (36, 37), we anticipate that targeted inhibition of BTK with therapies like ibrutinib may synergize with immune checkpoint inhibitors and/or select anti-cancer vaccines to further bolster T cell activation for long term durable anti-tumor immunity (36). Notably, this hypothesis is currently being tested in patients with PDAC and HNSCC in two clinical trials (ClinicalTrials.gov Identifier: NCT02436668 and NCT02454179).
While protumorigenic activities of B cells have not been widely investigated, we and other groups have reported direct and indirect protumorigenic roles for tumor-infiltrating, as well as peripheral B cells. In squamous carcinomas and melanomas, humoral immunity and IL-10 secreting B1 cells induce TH2-type programing of diverse myeloid cell types that foster tumor angiogenesis and CD8+ T cell suppression (9, 19, 38, 39). In B cell-infiltrated prostate cancers (40), immunosuppressive B cells and plasmocytes promote survival of androgen-independent prostatic epithelial cells via lymphotoxin-mediated NF-κB regulation (41), and inhibit efficacy of immunogenic CTX by IL-10 and immune checkpoint-mediated suppression of CTLs (42). CTL suppression in these contexts is consistent with in vitro data revealing that IFNγ release from CD8+ T cells and NK cells is increased when B cells are absent, whereas presence of B cells or B cell-derived IL-10 associates instead with reduced IFNγ (43). Results reported herein lend further support for the role B cells play in mediating tumorigenesis and response to cytotoxic therapy, and further identify BTK as a tractable target for anti-cancer therapy. Based on decreased presence of IgMloCD23+CD5− follicular and IgMloCD23− memory B cells correlating with improved outcome and increased CTL bioactivity in BTKi-treated PDAC-bearing mice (Supplementary Fig. S2E), we anticipate operative activities for these B cell subsets in either directly driving PDAC-associated T cell suppression or in regulating CTL-mediated responses to cytotoxic therapy. At earlier stages of PDAC progression however, IL-35-producing B cells promote Ras-driven neoplasia (44) and B1b cell recruitment, tethered to oxygen sensing programs, associate with early PDAC expansion (45), consistent with our results revealing dampened PDAC growth in B cell-deficient mice (Fig. 2). Together, these studies support both direct and indirect effects of B cells in regulating discrete aspects of tumorigenesis culminating in regulation of T cell responses to neoplasia and cytotoxic therapy.
While identifying BTK as a B cell-intrinsic target, studies herein further identify macrophage BTK, and its regulation by FcRγ- and PI3Kγ-mediated signaling, with subsequent effects on macrophage polarization, immune suppression and induction of desmoplastic stroma. Although one prior study linked the Class IA PI3Ks to BTK activation in B cells, recent studies have reported that these proteins regulate similar but distinct signaling pathways in B cells (33, 34). Our studies instead reveal the selective role of the Class IB PI3K isoform PI3Kγ in regulating BTK to control the macrophage response to the tumor microenvironment. PDACs from BTKi and PI3Kγ-inhibitor-treated mice exhibited a TH1-polarized phenotype with mRNAs encoding stimulators of CD8+ T cell cytotoxic activity. These observations are similar to anti-pathogen immunological states, and are necessary for immunogenic cell death of tumor cells (46) further supporting the hypothesis that BTK signaling regulates macrophage functionality and ultimately immunosuppression in PDAC tumors. Enhanced anti-tumor responses were observed when tumor-bearing mice were treated with BTKi therapy for an extended period of time commencing when PDAC tumors were small, as compared to later/larger stage tumors, supporting the general tenant that earlier detection and therapy in PDAC improves outcome.
Regarding BTK-regulated desmoplastic responses in PDACs, data presented herein are consistent with reported roles for macrophages in regulating muscle fibrosis and pancreatitis-associated fibrosis via pathways involving Arginine, TGFβ. MMP-9, IL-10, and other TH2-type proteins (47, 48). Soucek and colleagues also recently reported an important role for mast cells expressing BTK in regulating desmoplastic stroma of PDAC tumors (37). While murine PDAC tumors evaluated herein did exhibit significant presence of mast cells, BTK inhibition did not alter the presence of degranulating mast cells within tumor stroma (Supplementary Fig. S6D). Thus, if BTK regulates mast cell-induced desmoplasia, it likely does so via regulation of a protein secretion pathway, as opposed to a degranulation-mediated mechanism. Moreover, since CD8+ T cell depletion of PCI-32765/GEM-treated mice reversed the benefit of combination therapy (Fig 6I), without impacting decreased desmoplasia of treated tumors (Fig. 6D), these data indicate that decreased PDAC tumor growth due to BTK inhibition is dependent on B cell and/or myeloid cell reprogramming and bolstering of TH1-type immune responses, and independent of desmoplasia that is characteristic of PDAC tumors.
Macrophage activation and polarization state are subject to a multitude of signals distinct in various tumor microenvironments. In mammary carcinomas, macrophages are recruited to tumor parenchyma by CSF1 expressed by mammary epithelial cells (49), and then polarized to a TH2 effector state following stimulation by IL-4 produced by infiltrating CD4+ T cells (50, 51). TH2-skewed macrophages in turn indirectly regulate CD8+ T cell functionality via their high level expression of IL-10, thereby impairing dendritic cell maturation and expression of IL-12 (20). In contrast, macrophages in SCCs acquire a pro-tumoral TH2-type phenotype following Ig-containing immune complex activation of FcγR receptors (19), which in turn represses expression of macrophage-derived chemokines required for CD8+ T cell infiltration (9). Whereas some of this same biology is preserved between pancreas and cutaneous microenvironments, other facets may play a more significant role in the pancreas including mechanisms regulating B1b cell recruitment into areas of hypoxia (45), and/or downstream of oncogenic Ras and CXCL13 (44). Future studies will determine why these tissue-specific differences exist, the appropriate patient populations likely to respond to these immunotherapy strategies, molecular mechanisms underlying paracrine BTK regulation of CD8+ T cell functionality, and the degree to which mast cells and macrophages differentially play a role downstream of BTK in regulating desmoplasia associated with PDAC tumorigenesis. As the BTK inhibitor, e.g., ibrutinib is FDA-approved for treatment of leukemia (27), our studies indicate that BTK inhibitors could be rapidly evaluated as new therapeutic agent for pancreatic cancer.
METHODS
Freshly-isolated human PDAC samples and peripheral blood
All human samples for IHC, pathology, FACS and plasma analysis were obtained and studied under informed consent in accordance with the Declaration of Helsinki and acquired through the Oregon Pancreatic Tumor Registry and IRB protocol #3609.
Human PDA tissue microarray
Microarray data reflecting CD20 and Ig mRNA in human tumor samples were queried from a commercially available data set (BioExpress System, Gene Logic) originally generated on the Human Genome U133 Plus 2.0 Array (Affymetrix) and normalized by standard robust multichip average procedure. A single probe set of the highest variance among samples was chosen to represent CD20 (228592_at; MS4A1) and Ig (211430_s_at; IGHG1, IGHG2, IGHV4-31, IGHM), respectively. To ensure data consistency, results from additional probe sets were compared to a single probe set. Median value was used to calculate fold change of expression in tumor tissue compared to normal tissue. Statistical analyses were performed using Wilcoxon rank-sum test to compare mRNA expression levels to their corresponding normal tissue controls.
Immunohistochemistry and Immunofluorescence
Detailed procedures are provided in the Supplementary Methods
Cell lines
Ink4 2.2 and p53 2.1.1 cell lines were derived from primary PDAC tumors (FVB/N) of male transgenic Pdx-Cre; LSL-KrasG12D mice harboring null mutations in p53 and p16Ink4a (10–16). Passage 3 of the cell lines were obtained in 2011 directly from the Hanahan laboratory where they were derived and initially expanded. mCherry transfectants were expanded and frozen at low passage for use in the Coussens and Varner labs. Cells used in these studies were authenticated by gene expression in 2012 (RNASeq), whole exome analysis (in 2015) and BTK inhibitor sensitivity analyses (2013). All cell lines were tested for mycoplasma contamination and grown in DMEM/10% FBS/1.0% Pen-Strep on plastic coated with 50 μg/ml rat tail collagen I (BD Biosciences).
Animal Husbandry and in vivo studies
All animal experiments were performed with approval from the Institutional Animal Care and Use Committees of the University of California, San Diego or Oregon Health & Science University. Generation and characterization of B cell-deficient JH−/− (deletion in J segment of the Ig heavy chain locus and hence express no IgM or IgG and have no mature B cells), FcRγ−/− and p110γ−/− mice have been described previously (17, 18, 25). All mice used for orthotopic implantation of PDAC cells were male FVB/n, 7–12 weeks of age. Mice receiving monoclonal antibodies for cellular depletion and/or cytokine neutralization were administered i.p. αCD8 (2.43, BioXcell) was administered on day 15, day 20 and day 25 post-implantation at 500 μg/mouse; and αCSF-1 (clone, BioXcell) was administered at 1.0 mg/mouse on d21 and 500 μg/mouse on d26. PCI-32765 (BTKi) was delivered ad libitum in drinking water at 0.16% w/v in a 2.0% β-hydroxycyclodextrin (Sigma) solution beginning on day 18 and continued until the end of the study. Gemcitabine was administered i.v. at 15 mg/kg on days 18, 22 and 26 post-implantation. Alternatively, FVB/n mice inoculated orthotopically with p53 2.1.1 PDAC cells were treated from day 7 with PCI-32765 (0.016% w/v in 2.0% β-hydroxycyclodextrin ad libitum in drinking water) or TG100-115 (2.5 mg/kg i.p., BID) with or without Gemcitabine (15 mg/kg on d7, 10, 13, 16, 19, 22, and 25 post-implantation). Prior to surgery, mice were anesthetized with isofluorane and abdominal hair surrounding the surgical site removed. A left abdominal flank incision (1.5 cm) was made and spleen and adherent pancreas exteriorized. PDAC cells (1.0 × 103 cells), mixed with 50% matrigel and 50% serum free DMEM in a total volume of 30 μl were injected orthotopically into the tail of the pancreas of syngeneic 7–12 week-old male mice using a 30 gauge insulin needle, resulting in the appearance of a fluid bleb. Pancreas and spleen were then returned to their original position within the peritoneal cavity, followed by suture of the peritoneum and stapling of the skin. Staples were removed 7 days later and tumor growth was monitored in situ using a Vevo 2100 small animal ultrasound (Visual Sonics) at day 14 and day 27 following implantation. PDAC tumors were quantitated with ultrasound based on area (mm2) of the largest face of the tumor and percent change from day 14 to day 27 was graphed, by weight in grams, or morphometrically using serial H&E stained tissue sections by evaluating tumor area every 500 μm through the entire tissue depth and calculating the average tumor area/section. Tissues were formalin fixed or frozen in OCT for histological analysis. RNA was isolated from tissues flash frozen in liquid nitrogen using the methods described for macrophage polarization.
Peripheral blood analysis
Blood was collected via cardiac puncture at necropsy or by saphenous vein at specific time points during study and placed into EDTA-coated tubes to prevent clotting. CBC analysis was acquired on a Cell Dyn 3700 analyzer. Plasma was collected by spinning whole blood at 13,000 RPM for 5 minutes, then cell free supernatant collected and snap frozen for later analysis.
ELISA
Plasma from mice was thawed and diluted 1:10,000 for assay of Ig levels and specific isotype. Concentrations of Ig in mouse plasma were determined using a standard curve and ELISA reagents from Southern Biotec. Human IgG was analyzed via the Human IgG Subclass Profile kit from Novex-Life Technologies. Human samples were courtesy of the Oregon Pancreas Tumor Registry (OPTR). Data is displayed as the fold increase relative to tumor naïve mice.
Statistical considerations
For all in vitro studies, experiments were performed 3 or more times with n=3 or more per experiment group. In vivo experiments were performed at least twice, with n=10–15 randomly assigned mice/group for orthotopic tumor studies in wildtype and p110γ−/− mice, and in mice treated with vehicle, PCI-32765 and TG100-115. A sample size of 15 mice/group provided 80% power to detect mean difference of 1 standard deviation (SD) between two groups (based on a two-sample t-test with 2-sided 5% significance level). Prior to statistical analyses, data were examined for possible outliers using the Grubbs test. Data were normalized to the standard where applicable. Significance testing was performed by one-way Anova with Tukey’s posthoc testing for multiple pairwise testing or by parametric or nonparametric Student’s t test as appropriate. A two-sample t-test (two groups) and ANOVA (multiple groups) were used when data were normally distributed, and a Wilcoxon rank sum test (two groups) when data were not. All mouse studies were randomized for treatment by imaging all mice at d14 post-implantation and equally distributing mice into appropriate groups based on tumor size so that all groups began with a similar mean average (10–11 mm2) prior to treatment. Blinded assignment of mice into treatment groups, tumor measurement and tumor analysis was performed by coding mice with randomly assigned mouse numbers, with the key unknown to operators until experiments were completed. Treatment studies were further blinded to evaluate tumor growth in response to therapy by having one investigator implant the mice and treat them while another investigator performed imaging at the indicated time-points.
Additional detailed methods can be found in Supplementary Materials.
Supplementary Material
Statement of Significance.
We report that Bruton’s Tyrosine kinase (BTK) regulates B cell and macrophage-mediated T cell suppression in pancreas adenocarcinomas (PDAC). Inhibition of BTK with the FDA-approved inhibitor ibrutinib restores T cell-dependent anti-tumor immune responses to inhibit PDAC growth and improves responsiveness to chemotherapy, presenting a new therapeutic modality for pancreas cancer.
Acknowledgments
The authors thank Xiaodan Song, Justin Tibbitts, Teresa Beechwood, Jo Hill and Amy Li for regulatory and technical assistance; Jason Link for facilitating acquisition of human pancreas samples and HIPAA-compliant clinical data; Sushil Kumar and Terry Medler for helpful discussion; and support from the Knight Cancer Center Flow Cytometry, Bioinformatics, and Advanced Light Microscopy shared resources.
GRANT SUPPORT
The authors acknowledge support from T32AI078903-04 to AJG, T32HL098062 to MMK, and T32CA009523 and T32CA121938 to SG, R01CA167426-03S1 to AVN, support from the NCI/NIH (R01CA167426, R01CA126820 and R01CA083133) to JAV and support from the NCI/NIH (R01CA130980, R01CA140943, R01CA15531, U54CA163123), the Department of Defense Breast Cancer Research Program (W81XWH-11-1-0702), the Susan G Komen Foundation (KG110560 and KG111084), the Brenden-Colson Center for Pancreatic Health, and a Stand Up To Cancer – Lustgarten Foundation Pancreatic Cancer Convergence Dream Team Translational Research Grant (SU2C-AACR-DT14-14) to LMC. Stand Up To Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research.
Footnotes
Conflicts: Betty Y. Chang is an employee of Pharmacyclics, Inc. LMC and JAV received PCI-32765 free of charge from Pharmacyclics, Inc. who had no role in experimental design, data preparation or interpretation. LMC is on the PCYC-1137-CA Steering Committee.
Author contributions: NIA developed the orthotopic PDAC model with p53 and Ink cell lines; BR developed FACS panels for evaluating leukocytes in PDAC tumors for complexity and transcriptome analysis; TT developed and conducted the multiplex IHC analysis for evaluating leukocytes in human PDAC; AJG identified BTK+ cells in murine PDAC, conducted FACS studies on human pancreas and PDAC, and studies using αCD8 and αCD4 mAb, αCSF1 mAb, PCI-32764 and Gem, and evaluated resultant data with assistance from SML; MT, PO and DH generated and characterized p53 and Ink cell lines; GK, MT, and BS provided human pancreas tissues and tumors; BI evaluated human cDNA libraries for CD20 and Ig gene expression; BYC provided PCI-32765 (ibrutinib) and based on the PK/PD properties of the drug, developed oral formulations and dosing schedules used in animal models. Studies comparing BTK and PI3Kγ in cell proliferation, cell adhesion and integrin activation were performed by MMK and AVN. In vitro and in vivo macrophage gene expression analysis was performed by SG, AVN and MMK. In vivo analysis of PI3Kγ and BTK inhibition was performed by MMK. JAV directed studies comparing BTK and PI3Kγ in vitro and in early stage PDAC tumors. LMC directed studies evaluating B cells, FcRγ-positive cells, and mice treated at late-stage with PCI-32764 and Gem. JAV and LMC conceived the studies and co-wrote the manuscript with assistance from AJG and MMK.
References
- 1.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Schmid MC, Varner JA. Myeloid cells in tumor inflammation. Vascular cell. 2012;4:14. doi: 10.1186/2045-824X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–91. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.SEER.cancer.gov [Internet] Rockville: National Cancer Institute; c2015. Available from: http://seer.cancer.gov/statfacts/html/pancreas.html. [Google Scholar]
- 5.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 6.Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 7.Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:4548–54. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shibuya KC, Goel VK, Xiong W, Sham JG, Pollack SM, Leahy AM, et al. Pancreatic ductal adenocarcinoma contains an effector and regulatory immune cell infiltrate that is altered by multimodal neoadjuvant treatment. PLoS One. 2014;9:e96565. doi: 10.1371/journal.pone.0096565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Affara NI, Ruffell B, Medler TR, Gunderson AJ, Johansson M, Bornstein S, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–21. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes & development. 2001;15:3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 12.Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–87. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 13.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 14.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes & development. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- 15.Bardeesy N, Aguirre AJ, Chu GC, Cheng KH, Lopez LV, Hezel AF, et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc Natl Acad Sci U S A. 2006;103:5947–52. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–57. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Trounstine M, Alt FW, Young F, Kurahara C, Loring JF, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. International immunology. 1993;5:647–56. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 18.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–29. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 19.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–34. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–37. doi: 10.1016/j.ccell.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Gorter DJ, Beuling EA, Kersseboom R, Middendorp S, van Gils JM, Hendriks RW, et al. Bruton’s tyrosine kinase and phospholipase Cgamma2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26:93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14:219–32. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 23.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discovery. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmid MC, Franco I, Kang SW, Hirsch E, Quilliam LA, Varner JA. PI3-kinase gamma promotes Rap1a-mediated activation of myeloid cell integrin alpha4beta1, leading to tumor inflammation and growth. PLoS One. 2013;8:e60226. doi: 10.1371/journal.pone.0060226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3Kgamma, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19:715–27. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, et al. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res. 2011;71:6965–75. doi: 10.1158/0008-5472.CAN-11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dangi-Garimella S. FDA grants accelerated approval for ibrutinib for CLL. The American journal of managed care. 2014;20:SP145. [PubMed] [Google Scholar]
- 28.Palanki MS, Dneprovskaia E, Doukas J, Fine RM, Hood J, Kang X, et al. Discovery of 3,3′-(2,4-diaminopteridine-6,7-diyl)diphenol as an isozyme-selective inhibitor of PI3K for the treatment of ischemia reperfusion injury associated with myocardial infarction. J Med Chem. 2007;50:4279–94. doi: 10.1021/jm051056c. [DOI] [PubMed] [Google Scholar]
- 29.Bonecchi R, Sozzani S, Stine JT, Luini W, D’Amico G, Allavena P, et al. Divergent effects of interleukin-4 and interferon-gamma on macrophage-derived chemokine production: an amplification circuit of polarized T helper 2 responses. Blood. 1998;92:2668–71. [PubMed] [Google Scholar]
- 30.Biswas SK, Gangi L, Paul S, Schioppa T, Saccani A, Sironi M, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation) Blood. 2006;107:2112–22. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 31.Benson MJ, Rodriguez V, von Schack D, Keegan S, Cook TA, Edmonds J, et al. Modeling the clinical phenotype of BTK inhibition in the mature murine immune system. J Immunol. 2014;193:185–97. doi: 10.4049/jimmunol.1302570. [DOI] [PubMed] [Google Scholar]
- 32.Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-Induced GM-CSF Production Promotes the Development of Pancreatic Neoplasia. Cancer Cell. 2012;21:836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keenan BP, Saenger Y, Kafrouni MI, Leubner A, Lauer P, Maitra A, et al. A Listeria vaccine and depletion of T-regulatory cells activate immunity against early stage pancreatic intraepithelial neoplasms and prolong survival of mice. Gastroenterology. 2014;146:1784–94 e6. doi: 10.1053/j.gastro.2014.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sagiv-Barfi I, Kohrt HE, Czerwinski DK, Ng PP, Chang BY, Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masso-Valles D, Jauset T, Serrano E, Sodir NM, Pedersen K, Affara NI, et al. Ibrutinib exerts potent antifibrotic and antitumor activities in mouse models of pancreatic adenocarcinoma. Cancer Res. 2015;75:1675–81. doi: 10.1158/0008-5472.CAN-14-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schioppa T, Moore R, Thompson RG, Rosser EC, Kulbe H, Nedospasov S, et al. B regulatory cells and the tumor-promoting actions of TNF-alpha during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–7. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, et al. Macrophage polarization to a unique phenotype driven by B cells. European journal of immunology. 2010;40:2296–307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 40.Woo JR, Liss MA, Muldong MT, Palazzi K, Strasner A, Ammirante M, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12:30. doi: 10.1186/1479-5876-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ammirante M, Luo JL, Grivennikov S, Dedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–6. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shalapour S, Font-Burgada J, Di Caro G, Zhong Z, Sanchez-Lopez E, Dhar D, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–8. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 44.Pylayeva-Gupta Y, Das S, Handler JS, JHajdu CH, Coffre M, Koralov S, et al. IL-35 producing B cells promote the development of pancreatic neoplasia. Cancer Discovery. 2016 doi: 10.1158/2159-8290.CD-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee KE, Spata M, Bayne LJ, Biza EL, Durham AC, Allman D, et al. HIF1alpha deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer Discovery. 2016 doi: 10.1158/2159-8290.CD-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell-autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nat Med. 2014;20:1301–9. doi: 10.1038/nm.3708. [DOI] [PubMed] [Google Scholar]
- 47.Xue J, Sharma V, Hsieh MH, Chawla A, Murali R, Pandol SJ, et al. Alternatively activated macrophages promote pancreatic fibrosis in chronic pancreatitis. Nature communications. 2015;6:7158. doi: 10.1038/ncomms8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lemos DR, Babaeijandaghi F, Low M, Chang CK, Lee ST, Fiore D, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–94. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 49.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes & development. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.