Abstract
Background
Anaphylaxis is a potentially life-threatening allergic reaction. The risk of anaphylaxis after vaccination has not been well described in adults or with newer vaccines in children.
Objective
We sought to estimate the incidence of anaphylaxis after vaccines and describe the demographic and clinical characteristics of confirmed cases of anaphylaxis.
Methods
Using health care data from the Vaccine Safety Datalink, we determined rates of anaphylaxis after vaccination in children and adults. We first identified all patients with a vaccination record from January 2009 through December 2011 and used diagnostic and procedure codes to identify potential anaphylaxis cases. Medical records of potential cases were reviewed. Confirmed cases met the Brighton Collaboration definition for anaphylaxis and had to be determined to be vaccine triggered. We calculated the incidence of anaphylaxis after all vaccines combined and for selected individual vaccines.
Results
We identified 33 confirmed vaccine-triggered anaphylaxis cases that occurred after 25,173,965 vaccine doses. The rate of anaphylaxis was 1.31 (95% CI, 0.90-1.84) per million vaccine doses. The incidence did not vary significantly by age, and there was a nonsignificant female predominance. Vaccine-specific rates included 1.35 (95% CI, 0.65-2.47) per million doses for inactivated trivalent influenza vaccine (10 cases, 7,434,628 doses given alone) and 1.83 (95% CI, 0.22-6.63) per million doses for inactivated monovalent influenza vaccine (2 cases, 1,090,279 doses given alone). The onset of symptoms among cases was within 30 minutes (8 cases), 30 to less than 120 minutes (8 cases), 2 to less than 4 hours (10 cases), 4 to 8 hours (2 cases), the next day (1 case), and not documented (4 cases).
Conclusion
Anaphylaxis after vaccination is rare in all age groups. Despite its rarity, anaphylaxis is a potentially life-threatening medical emergency that vaccine providers need to be prepared to treat.
Keywords: Anaphylaxis, vaccine safety, immunization
Anaphylaxis is an acute, systemic, and potentially lethal hypersensitivity reaction with multiple organ system involvement.1-4 Anaphylaxis can occur after exposure to allergens from a variety of sources, including food, venom, drugs, and immunizations. Virtually all vaccines have the potential to trigger anaphylaxis.5,6 Recently, a committee of the Institute of Medicine (IOM) concluded that epidemiologic and mechanistic evidence convincingly supports a causal relationship between anaphylaxis and several childhood and adolescent vaccines (measles, mumps, rubella [MMR] vaccine; varicella vaccine; influenza vaccine; hepatitis B vaccine; diphtheria toxoid-, tetanus toxoid-, and acellular pertussis antigen-containing vaccines; and meningococcal vaccine), favors acceptance of a causal relationship for the human papillomavirus vaccine (HPV; mechanistic evidence only), and is inadequate for hepatitis A vaccine (HAV).7 Vaccine components that might be allergenic include the vaccine antigen, residual animal protein, antimicrobial agents, preservatives, stabilizers, or other vaccine components.8 Individual vaccine components that have been implicated in acute vaccine reactions include egg protein, gelatin, milk proteins, and potentially other additives. Natural rubber latex, which can be contained in the syringe plunger, the tips on prefilled syringes, and vial stoppers, is another potential cause of anaphylaxis.9
The life-threatening nature of anaphylaxis and the acceptance of a causal relationship with certain vaccines make estimation of the magnitude of risk after vaccination an important priority for research into vaccine safety. Current data are limited for quantifying the risk of anaphylaxis associated with vaccination in general or with specific vaccines. Given the infrequency with which anaphylaxis occurs, large populations are necessary to study this exposure-disease relationship. The Vaccine Safety Datalink (VSD), a collaboration between the Centers for Disease Control and Prevention’s Immunization Safety Office and several integrated health care systems across the United States, offers such a population.10 The only population-based data on the risk of anaphylaxis associated with vaccination are from a much-cited historical VSD study conducted by Bohlke et al11 covering the years 1991-1997. At the time of the study by Bohlke et al,11 the VSD comprised 4 sites with a population of approximately 6 million persons, and the focus was on the safety of childhood immunizations; there was limited use of influenza vaccine in the pediatric population. Bohlke et al11 used an ad hoc algorithm in their study to classify anaphylaxis cases. Based on 5 confirmed cases, the incidence rate of anaphylaxis in children and adolescents was estimated to be 0.65 (95% CI, 0.21-1.53) cases per million vaccine doses.11
Recently, since the report by Bohlke et al,11 there have been changes to the recommended childhood and adult immunization schedules, with broader age groups recommended to receive certain vaccines and the introduction of several new vaccines and vaccine combinations. Importantly, influenza vaccine has been generally recommended for the entire US population aged 6 months or greater, with more than 100 million doses of influenza vaccine administered annually. Because eggs are used in the production of most influenza vaccines, these contain small amounts of ovalbumin, and potential anaphylactic reactions among patients with egg allergy are an important safety concern for influenza vaccines. A recent advance in vaccine safety science has been the Brighton Collaboration’s development of standardized case definitions and guidelines for data collection, analysis, and presentation. In 2007, the Brighton Collaboration published a standardized case definition for anaphylaxis after vaccinations.12 Lastly, since the Bohlke et al report,11 VSD has expanded to a total of 9 sites with the ability to monitor vaccine safety in a larger and more diverse population of both children and adults.13
Therefore we conducted a study using recent VSD data and applied the Brighton criteria in our analysis. Our study objectives included (1) estimating the incidence of anaphylaxis after all vaccines combined and for individual vaccines (when dose numbers were sufficient to allow vaccine-specific analysis [eg, influenza vaccines]) and (2) describing the demographic and clinical characteristics of confirmed cases of anaphylaxis.
METHODS
Study population
Our study population included all children and adults enrolled in the health plans at 9 VSD sites who received 1 or more vaccines during the period from January 1, 2009 through December 31, 2011. Currently, the VSD has data on approximately 9.3 million subjects available annually, including 2.1 million children and 7.2 million adults.13 The participating VSD sites maintain automated databases of health care encounters, including immunization registries with detailed information on vaccines administered; have the capability to access written or electronic medical records and other data sources to provide detailed information on specific health care encounters; and provide integrated health care services to their members so that the full spectrum of health care from outpatient department (OPD) and emergency department (ED) visits to hospitalizations can be captured. The study protocol was approved by institutional review boards at the Centers for Disease Control and Prevention and each VSD site, and it was determined that informed consent was not required.
Case identification
We identified potential cases of anaphylaxis based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (Table I). Our primary search in automated data of the OPD, inpatient/hospital, and ED settings identified either (1) a specific anaphylaxis code (995.0, anaphylactic shock; 999.4, anaphylactic shock caused by serum) occurring 0 to 2 days after vaccination, with day 0 being the day of vaccination, or (2) an external cause of injury code (E948.0 through E948.9, any adverse reaction from bacterial vaccines; E949.0 through E949.9, any adverse reaction from other vaccines and biological substances) occurring on day 0. To restrict to only incident cases, we excluded any of the above visits if the same code had been used at another visit in the prior 2 days.
TABLE I.
Search algorithm for potential cases of anaphylaxis after vaccination
| Adverse event | ICD-9 codes | Postvaccination observation window (d)* |
Medical setting | First episode in what period? |
|
|---|---|---|---|---|---|
| Primary screen | Anaphylaxis | 995.0, 999.4 | 0-2 | Outpatient, inpatient, ED | First in 2 d |
|
| |||||
| E codes | E948.0-948.9 E949.0-949.9 | 0 | Outpatient, inpatient, ED | First in 2 d | |
|
| |||||
| Secondary screen | Allergic reactions | 995.1, 995.3, 708.0, 708.1, 708.9, 695.1, 995.20, 995.21, 995.27, 995.29 |
0 | Outpatient, inpatient, ED | First in 42 d |
E codes, External cause of injury codes.
Day 0 represents day of vaccination.
Previous studies have suggested that some anaphylaxis cases are coded as other allergic reactions, and therefore we conducted a 2-phase secondary search of the electronic data to identify all vaccine-exposed persons with nonspecific codes for selected allergic episodes (restricted to diagnoses occurring only on day 0 and without a prior diagnosis in the preceding 42 days). The ICD-9-CM codes included can be found in Table I. At each VSD site, a site-specific algorithm was used to screen these subjects’ automated data to include only those patients with allergic episodes who also received epinephrine within 24 hours of vaccination for further case validation.
Case validation
Medical chart review was conducted at VSD sites by trained personnel on all potential anaphylaxis cases identified in the automated search by using a standardized chart abstraction instrument (Fig 1). The purpose was to obtain clinical information to document a clinical diagnosis of anaphylaxis, including whether the diagnosis was recorded in the chart by the treating provider.
FIG 1.
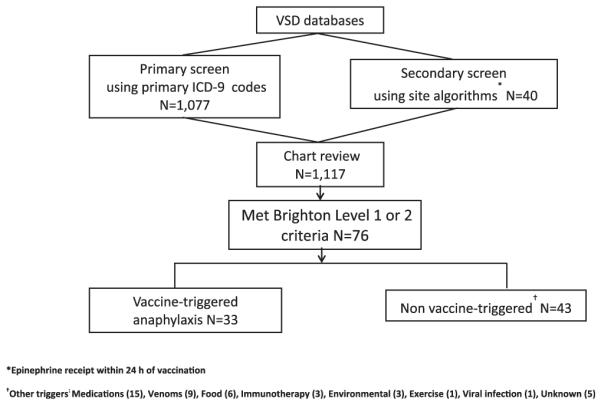
Search strategy for anaphylaxis cases, 2009-2011.
All completed chart abstraction forms that indicated anaphylaxis, possible anaphylaxis, and allergic episode were adjudicated by 2 medical officers (M.M.M. and L.S.) using the Brighton criteria for anaphylaxis (Tables II and III).12 Although the Brighton criteria specify a “sudden onset and rapid progression” of signs and symptoms of anaphylaxis, an exact timeframe is not specified.
TABLE II.
Brighton Collaboration case definition of anaphylaxis12
| For all levels of diagnostic certainty: |
| Anaphylaxis is a clinical syndrome characterized by |
| • sudden onset AND |
| • rapid progression of signs and symptoms AND |
| • multiple (≥2) organ systems, as follows: |
| Level 1 of diagnostic certainty |
| • ≥1 major dermatological AND |
| • ≥1 major cardiovascular AND/OR ≥1 major respiratory criterion |
| Level 2 of diagnostic certainty |
| • ≥ 1 major cardiovascular AND ≥1 major respiratory criterion |
| OR |
| • ≥1 major cardiovascular OR respiratory criterion AND |
| • ≥1 minor criterion involving ≥1 different system (other than cardiovascular or respiratory systems) |
| • (≥1 major dermatologic) AND (≥1 minor cardiovascular AND/OR minor respiratory criterion) |
| Level 3 of diagnostic certainty |
| • ≥1 minor cardiovascular OR respiratory criterion AND |
| • ≥1 minor criterion from each of ≥2 different systems/categories |
TABLE III.
Major and minor criteria used in the case definition of anaphylaxis: Brighton Collaboration Criteria12
| Major criteria | Minor criteria | |
|---|---|---|
| Dermatologic or mucosal |
|
|
|
| ||
| Cardiovascular |
|
|
|
| ||
| Respiratory |
|
|
|
| ||
| Gastrointestinal |
|
|
|
| ||
| Laboratory |
|
|
Not hereditary angioedema.
Vaccination and other variables
During the 3-year study period, vaccine exposure was determined by using automated data. In addition, medical chart abstraction of potential cases included vaccine, vaccine manufacturer, and lot number. Additional data obtained from the electronic records included sex, date of birth, VSD site, and health plan enrollment dates. Medical chart abstraction of potential cases also collected information on any prior history of and/or specific drug therapy for potentially atopic conditions, including anaphylaxis, asthma, atopic dermatitis, allergic bronchitis, rhinitis, bronchiolitis, and specific allergies (eg, to egg or medications), as well as clinical details on the anaphylactic episode of interest and follow-up management. Information about potential nonvaccine causes of anaphylaxis (eg, egg and medication allergy) was also collected. We classified anaphylaxis cases as vaccine triggered or not from information in the medical chart, including health care provider’s assessment and exposure to vaccines and other nonvaccine triggers in relation to timing of onset of the anaphylactic episode.
Analyses
Using the number of validated cases of anaphylaxis and the number of vaccine doses administered, we calculated the incidence per million doses administered and exact 95% confidence limits of anaphylaxis after vaccination for all vaccines combined and for select vaccines with adequate numbers to allow calculation.14 SAS 9.3 software (SAS Institute, Cary, NC) was used for analysis.
RESULTS
From January 1, 2009 through December 31, 2011, we identified a total of 17,606,500 vaccination visits at which a total 25,173,965 vaccine doses were administered. Among all visits, 1,117 potential anaphylaxis cases were identified by using electronic data. We identified 76 cases of chart-confirmed anaphylaxis (Brighton Levels 1 and 2); 33 anaphylaxis cases were associated with vaccination, and 43 were attributed to other causes.
Characteristics of the 33 cases of postvaccination anaphylaxis are summarized in Table IV. Five cases were identified by screening for epinephrine administration and were not materially different from other cases. There were no deaths, and only 1 (3%) patient was hospitalized. There was a female predominance (20 female vs 13 male patients), and the age range was 4 to 65 years (median, 17 years). Children (<18 years) were predominantly male, but adults were predominantly female. Information on race was available for 25 (76%) cases: among male patients, 8 were white and 2 were black, and among female patients, all 15 were white. The onset of symptoms among cases was within 30 minutes (8 cases), 30 to less than 120 minutes (8 cases), 2 to less than 4 hours (10 cases), 4 to 8 hours (2 cases), the next day (1 case), and not documented (4 cases). All cases received specific drug therapy, including epinephrine in 15 (45%), antihistamine in 28 (85%), corticosteroid in 17 (52%), H2-blocker in 7 (21%), nebulized bronchodilator in 13 (39%), oxygen in 5 (15%), and intravenous therapy in 5 (15%) cases. Three (9%) cases after evaluation were prescribed epinephrine autoinjectors, and 5 (15%) cases were referred to an allergist.
TABLE IV.
Vaccine-triggered anaphylaxis cases: demographic and clinical characteristics
| No. of cases (n = 33) | Percent | |
|---|---|---|
| Year | ||
|
| ||
| 2009 | 11 | 33 |
|
| ||
| 2010 | 8 | 24 |
|
| ||
| 2011 | 14 | 42 |
|
| ||
| Setting | ||
|
| ||
| ED | 13 | 39 |
|
| ||
| OPD | 20 | 61 |
|
| ||
| Age group (y) | ||
|
| ||
| 0-17 | 18 | 55 |
|
| ||
| 18-49 | 9 | 27 |
|
| ||
| ≥50 | 6 | 18 |
|
| ||
| Sex | ||
|
| ||
| Female | 20 | 61 |
|
| ||
| Race | ||
|
| ||
| White | 24 | 73 |
|
| ||
| Black | 2 | 6 |
|
| ||
| Unknown | 7 | 21 |
|
| ||
| Past history | ||
|
| ||
| Atopy | 28 | 85 |
|
| ||
| Anaphylaxis* | 3 | 9 |
|
| ||
| Time to onset | ||
|
| ||
| <30 min | 8 | 24 |
|
| ||
| 30 to <120 min | 8 | 24 |
|
| ||
| 2 to <4 h | 10 | 30 |
|
| ||
| 4 to 20 h | 3 | 10 |
|
| ||
| Not documented | 4 | 12 |
|
| ||
| Treatment of episode | ||
|
| ||
| Epinephrine | 15† | 45 |
|
| ||
| Antihistamine | 28 | 85 |
|
| ||
| Corticosteroid | 17 | 52 |
|
| ||
| H2-blocker | 7 | 21 |
|
| ||
| Bronchodilator | 13 | 39 |
|
| ||
| Oxygen | 5 | 15 |
|
| ||
| Intravenous therapy | 5 | 15 |
|
| ||
| Outcome | ||
|
| ||
| Recovered | 33 | 100 |
|
| ||
| Follow-up care | ||
|
| ||
| Inpatient | 1 | 3 |
|
| ||
| Epinephrine autoinjector prescribed | 3 | 9 |
|
| ||
| Referred to allergist | 5 | 15 |
Two patients after TIV and 1 patient after aspirin.
Brighton Level 1=6, 50%; Brighton Level 2 = 9, 43%.
Selected clinical details on the individual cases are presented in Table V. All cases were classified as Brighton Level 1 (12 [36%]) or Level 2 (21 [64%]). Twenty-eight (85%) cases included a history of atopy. Of the atopic cases, 3 included a prior history of anaphylaxis, and an additional 16 included either a prior diagnosis of asthma (n = 12) or receipt of specific therapy suggesting an asthma diagnosis (n = 4); 9 of the 10 nonasthmatic atopic cases included a history of prior allergies, predominantly to food and antibiotics (Table V). Predominant signs and symptoms involved dermatologic and respiratory systems.
TABLE V.
Selected clinical findings of 33 vaccine-triggered anaphylaxis cases, VSD, 2009-2011
| Case no. |
Age (y)/ race/sex |
History of atopy | Vaccines | Onset after vaccination | Setting of initial evaluation |
Diagnosing provider’s specialty/ attributed trigger |
Symptoms | Brighton level |
Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 5/B/M | Fish, peanuts, eggs | MMR, DTaP-IPV, varicella |
Onset <30 min | Clinic | Pediatrician/vaccine | General pruritus, wheeze, cough, vomiting | 2 | Epinephrine, oral antihistamine, antiemetic | Patient recovered |
|
| ||||||||||
| 2 | 26/W/F | Asthma | TIV, PPV23 | Onset not documented | ED | ED doctor/vaccine | Wheeze (bilateral), SOB, ↑HR, nasal congestion, nausea | 2 | Nebulizer | Patient recovered |
|
| ||||||||||
| 3 | 4/?/F | Asthma, atopic dermatitis | TIV | Onset 30 to <60 min | Clinic | FNP/vaccine | Generalized urticaria, wheeze, respiratory distress, vomiting | 1 | Epinephrine, oral antihistamine, O2, albuterol nebulizer | Patient recovered |
|
| ||||||||||
| 4 | 15/W/F | Asthma, other allergy (DTaP) | Tdap, HPV4 | Onset 2 h | Clinic | Pediatrician/vaccine | Angioedema, generalized urticaria, erythema and pruritus, SOB, abdominal pain |
2 | Oral antihistamine, bronchodilators | Patient recovered |
|
| ||||||||||
| 5 | 12/W/F | Allergy to penicillin | TIV | Onset 1 d after vaccine | Inpatient (overnight) |
ED doctor/vaccine | Generalized urticaria, pruritus and rash, ↑HR, syncope, BP 117/72 mm Hg, “lump in throat” |
2 | Epinephrine, parenteral antihistamine, parenteral H2-blocker, oral steroid, O2, other (antiemetic, lidocaine cream) |
Patient recovered, referred to allergist (patient’s skin test and RAST results both negative for latex), prescribed epinephrine autoinjector |
|
| ||||||||||
| 6 | 62/W/F | Hives after sulfa and cephalexin | Hepatitis, Tdap | Onset 30 to <60 min | Clinic | ED doctor/vaccine | Flushing, SOB, nausea, vomiting, abdominal pain | 2 | Parenteral antihistamine, parenteral H2-blocker, parenteral steroid, O2, other (ASA, nitroglycerin, MS) |
Patient recovered |
|
| ||||||||||
| 7 | 17/W/F | Other (latex gloves) | HPV4, MMR, MCV4 | Onset 2 h | ED | ED doctor/vaccine (latex in Sanofi stopper) |
Generalized “prickle” sensation, dizziness, ↑HR, wheeze, throat closure, dry cough |
2 | Epinephrine, oral antihistamine, oral steroid | Patient recovered, referred to allergist, prescribed epinephrine autoinjector |
|
| ||||||||||
| 8 | 65/W/M | Asthma, allergic rhinitis, other (allergy shots) | HZV and allergy shot (received before event) |
Onset <30 min | Clinic | LPN and INT doctor/ allergy shot |
Generalized pruritus, wheeze, SOB, cough | 2 | Epinephrine, parenteral antihistamine, albuterol nebulizer |
Patient recovered |
|
| ||||||||||
| 9 | 56/W/M | Other (amoxicillin) | TIV, Tdap | Onset 60 to <90 min | Clinic | FP doctor/vaccine | Angioedema, SOB, vomiting, fatigue | 2 | Oral antihistamine | Patient recovered |
|
| ||||||||||
| 10 | 36/W/F | Contact dermatitis, allergic rhinitis | TIV, Tdap | Onset 3 h | Clinic | Urgent care doctor/ vaccine |
Angioedema, “choking” cough, ↑HR, nausea | 2 | Oral antihistamine, oral steroid, other (cool compress) | Patient recovered |
|
| ||||||||||
| 11 | 14/W/F | Asthma, allergic conjunctivitis and rhinitis | TIV | Onset 2 h | Clinic | Pediatrician/vaccine | Wheeze, vomiting | 2 | Oral antihistamine, bronchodilators | Patient recovered |
|
| ||||||||||
| 12 | 5/W/M | Asthma, other (cashew, pistachio nuts) | TIV | Onset 3 h | ED | ED doctor/vaccine | Generalized urticaria, pruritus and rash, SOB | 2 | Oral antihistamine, oral steroid | Patient recovered |
|
| ||||||||||
| 13 | 29/W/F | None | TIV (administered 1 d before) | Onset 8 h | ED | ED doctor/vaccine | Angioedema, swelling (lips, uvula, mouth), generalized urticaria, pruritus and rash, SOB, cough |
1 | Oral antihistamine, oral H2-blocker, oral steroid | Patient recovered, referred to PCP |
|
| ||||||||||
| 14 | 55/W/F | None | TIV | Onset 6 h | ED | ED doctor/vaccine | Wheeze, SOB, red and itchy eyes | 2 | Oral antihistamine, oral steroid, bronchodilators, other (fluids and rest) |
Patient recovered, referred to PCP |
|
| ||||||||||
| 15 | 7/?/M | Anaphylaxis, atopic dermatitis, other (egg, gelatin, TIV and other vaccines) |
Hepatitis A, varicella | Onset 30 to <60 min | Clinic | ED doctor/vaccine | Angioedema, swelling (lips), throat closure, red itchy eyes, cough, erythema and rash, vomiting |
1 | Epinephrine, oral antihistamine, oral H2-blocker, parenteral steroid, IVT, bronchodilator |
Patient recovered, referred to allergist |
|
| ||||||||||
| 16 | 64/?/F | Asthma, other (multiple medications) | PPV23 | Onset not documented | Clinic | INT doctor/vaccine | Wheeze (bilateral), dizziness, nausea | 2 | Bronchodilators | Patient recovered |
|
| ||||||||||
| 17 | 9/?/F | None | TIV | Onset <30 min | Clinic | ED doctor and pediatrician/vaccine |
Angioedema (eyelid), cough | 2 | Epinephrine, oral antihistamine, oral steroid, bronchodilators, other (icepack) |
Patient recovered, referred to pediatrician |
|
| ||||||||||
| 18 | 44/W/F | Anaphylaxis, asthma, allergic rhinitis, other (ASA) |
TIV | Onset <30 min | ED | ED doctor/vaccine | Generalized pruritus and rash, wheeze, SOB, throat tightening, hoarse voice, cough, vomiting and diarrhea |
2 | Parenteral antihistamine, oral steroid, O2, bronchodilators | Patient recovered |
|
| ||||||||||
| 19 | 12/W/M | Asthma, atopic dermatitis, allergic rhinitis, other (egg) | MCV4, MMRV, Tdap | Onset <3 h | Clinic | Pediatrician/vaccine | Generalized urticaria, pruritus and rash, cough | 2 | Oral antihistamine, other (cool compress) | Patient recovered |
|
| ||||||||||
| 20 | 8/W/M | Contact dermatitis, allergic rhinitis | MIV | Onset <30 min | ED | ED doctor/vaccine | Angioedema (periorbital), wheeze, SOB | 1 | Oral antihistamine, oral steroid, bronchodilators | Patient recovered |
|
| ||||||||||
| 21 | 6/W/F | Anaphylaxis, allergic rhinitis, reactive airway disease, other (TIV) |
TIV | Onset 60 to <90 min afte 5 test doses vaccine |
Clinic | Allergist/vaccine | Erythema (throat), generalized pruritus and rash, cough | 2 | Epinephrine, oral antihistamine | Patient recovered |
|
| ||||||||||
| 22 | 4/?/M | None | DTaP, IPV, MMR | Onset <60 min | Clinic | ED doctor and pediatrician/vaccine |
Angioedema, generalized urticaria, erythema, rash, red itchy eyes, wheeze, ↑RR, cough |
1 | Oral antihistamine, parenteral steroids | Patient recovered, referred to allergist and pediatrician, prescribed epinephrine autoinjector |
|
| ||||||||||
| 23 | 62/?/F | Other (penicillin class medications) | HZV (administered at work same day) |
Onset not documented | Clinic | Physician’s assistant/ vaccine |
Generalized pruritus, erythema, tongue swelling, chest tightness | 1 | Parenteral antihistamine, oral H2-blocker | Patient recovered |
|
| ||||||||||
| 24 | 4/W/M | Asthma, allergic conjunctivitis and rhinitis | DTaP, MMR, IPV, varicella | Onset <30 min | Clinic | Allergist/vaccine | Facial swelling, erythema and pruritus, wheeze (bilateral), swelling (posterior oropharynx), rhinorrhea, cough, vomiting |
1 | Epinephrine, bronchodilators | Patient recovered, referred to allergist |
|
| ||||||||||
| 25 | 12/W/M | None | Rabies IM (history of cat bite in Mexico) |
Onset 30 to <60 min | ED | ED doctor/vaccine | Angioedema (eyelids, lips), generalized rash, facial erythema and urticaria, wheeze, swelling (lips, throat) |
1 | Epinephrine, parenteral antihistamine, parenteral H2-blocker, parenteral steroid, O2, other (continued antibiotic) |
Patient recovered, referred to pediatrician |
|
| ||||||||||
| 26 | 11/?/M | None | MCV4, Tdap, varicella, hepatitis A |
Onset not documented | Clinic | Pediatrician/vaccine | Injection site urticaria & erythema, measured ↓BP | 2 | Oral antihistamine | Patient recovered |
|
| ||||||||||
| 27 | 44/B/M | Allergic rhinitis, other (penicillin class medications) | Hepatitis A | Onset 2 h | ED | ED/vaccine | Generalized erythema and rash, red itchy eyes, ↑HR, palpitations, swelling (throat), “chest heaviness” |
1 | Oral antihistamine, acetaminophen | Patient recovered, referred to PCP |
|
| ||||||||||
| 28 | 27/W/F | None | MIV, TIV | Onset 2 h | ED | ED doctor/vaccine | Generalized urticaria, pruritus and rash, angioedema, swelling (tonsils) |
1 | Epinephrine, parenteral steroid | Patient recovered |
|
| ||||||||||
| 29* | 11/W/M | Asthma, other (fluconazole, penicillins) | Varicella, Tdap, MCV4 | Onset immediate | Clinic | FPP/vaccine | Angioedema (lips), swelling (tongue) | 2 | Epinephrine, oral antihistamine, oral steroid | Patient recovered |
|
| ||||||||||
| 30* | 15/W/F | Allergic rhinitis, other (shellfish, methylprednisolone) | Tdap, varicella | Onset 30 to <60 min | Clinic | FPP/vaccine | Angioedema (lips), swelling (tongue) | 2 | Epinephrine, oral antihistamine, oral steroid | Patient recovered |
|
| ||||||||||
| 31* | 37/W/F | Asthma, other (codeine and cephalexin) | MIV | Onset 2 h | ED | ED doctor/vaccine | Generalized “prickle” sensation, SOB, swelling (throat), “tingling” lips |
2 | Epinephrine, parenteral antihistamine, other (lorazepam) | Patient recovered, referred to PCP |
|
| ||||||||||
| 32* | 35/W/F | Other (shellfish, latex, amoxicillin, ciprofloxacin, gentamicin) | TIV | Onset <30 min | Clinic | FP doctor/ vaccine |
Generalized pruritus, swelling (tongue), SOB, throat closure | 2 | Epinephrine, parenteral antihistamine, IVT, bronchodilator (albuterol) |
Patient recovered |
|
| ||||||||||
| 33* | 44/W/F | Asthma, allergic rhinitis, other (eggs, sulfonamides) | Tdap | Onset 2 h | ED | ED doctor and INT/ vaccine |
Generalized urticaria, erythema and rash, measured ↓BP, LOC, nausea, diaphoretic |
1 | Epinephrine, oral antihistamine, parenteral H2-blocker, parenteral steroid, IVT, bronchodilators |
Patient recovered, referred to PCP |
ASA, Aspirin; B, black; BP, blood pressure; F, female; FNP, family nurse practitioner; FPP, family practice provider; HR, heart rate; IM, intramuscular; INT, internist; IPV, inactivated polio vaccine; IVT, intravenous therapy; LOC, loss of consciousness; LPN, licensed practical nurse; M, male; MCV4, meningococcal conjugate vaccine; MMRV, measles, mumps, rubella, and varicella vaccine; MS, morphine sulfate controlled-release; NSAID, nonsteroidal anti-inflammatory drug; PCP, primary care physician; RR, respiratory rate; SOB, shortness of breath; W, white.
Identified by means of screening for epinephrine administration.
Specific vaccine exposures are shown in Table VI; 18 (55%) cases received a single vaccine, and 15 (45%) received 2 or more vaccines concomitantly. The most frequent vaccines identified included inactivated influenza and tetanus diphtheria acellular pertussis vaccines (Tdap/DTaP).
TABLE VI.
Vaccination-triggered anaphylaxis cases by vaccine exposures (n = 33)
| Vaccine | Alone | With other vaccines |
Other concomitant vaccine(s)* |
|---|---|---|---|
| TIV | 10 | 4 | MIV, Tdap (2), PPSV23 |
|
| |||
| MIV | 2 | 1 | TIV |
|
| |||
| Tdap | 1 | 8 | TIV (2), HPV4, HAV, VAR, MCV4+MMRV, MCV4+VAR, MCV4+VA R +HAV |
|
| |||
| PPSV23 | 1 | 1 | TIV |
|
| |||
| HAV | 1 | 3 | Tdap, Tdap+MCV4+VAR, VAR |
|
| |||
| HZV | 2 | 0 | — |
|
| |||
| Rabies | 1 | 0 | — |
|
| |||
| HPV4 | — | 2 | Tdap, MMR+MCV4 |
|
| |||
| MMR | — | 3 | DTaP-IPV+AR, DTaP+IPV+VAR, HPV4+ MCV4 |
|
| |||
| MMRV | — | 2 | Tdap+MCV4, DTaP+IPV |
|
| |||
| MCV4 | — | 4 | Tdap+MMRV, Tdap+VAR, Tdap+VA R + HAV, HPV4+MMR |
|
| |||
| VAR | — | 5 | Tdap, Tdap+MCV4+HAV, DTaP-IPV+ MMR, HAV, Tdap+MCV4 |
|
| |||
| DTaP-IPV | — | 1 | MMR+VAR |
|
| |||
| DTaP | — | 2 | MMRV+IPV, MMR+IPV+VAR |
|
| |||
| IPV | — | 2 | DTaP+MMRV, DTaP+MMR+VAR |
IPV, Inactivated polio vaccine; MCV4, meningococcal conjugate vaccine; MMRV, measles, mumps, rubella, and varicella vaccine; PPSV23, pneumococcal polysaccharide vaccine (23-valent); VAR, varicella vaccine.
A total of 15 (45%) cases received 2 or more concomitant vaccines.
The rate of postvaccination anaphylaxis for all vaccines was 33 cases per 25,173,965 doses or 1.31 (95% CI, 0.90-1.84) per million vaccine doses (Table VII). Rates were similar by age, although the rate was lower in the oldest age group. The rate in female subjects was slightly higher than in male subjects. Rates after specific vaccines were difficult to quantify because most were administered concomitantly with other vaccines. The cases that followed administration of a single vaccine involved predominantly trivalent influenza vaccine (TIV), for which the rate was estimated to be 1.35 per million vaccine doses (Table VIII). The highest rates followed herpes zoster vaccine (HZV) and rabies vaccine, but these were based on only 2 and 1 case(s), respectively, and had wide CIs (Table VIII). The rate for postvaccination anaphylaxis for cases that did not involve TIV was 19 cases per 14,394,021 doses or 1.32 (95% CI, 0.79-2.06) per million vaccine doses.
TABLE VII.
Incidence of vaccination-triggered anaphylaxis by age, sex, year, and vaccine type
| No. of cases | Doses administered | Rate (/106 doses) | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|
| Age group (y) | |||||
|
| |||||
| 0-17 | 18 | 12,403,201 | 1.45 | 0.86 | 2.29 |
|
| |||||
| 18-49 | 9 | 5,063,802 | 1.78 | 0.81 | 3.37 |
|
| |||||
| ≥50 | 6 | 7,706,962 | 0.78 | 0.29 | 1.69 |
|
| |||||
| Sex | |||||
|
| |||||
| Female | 20 | 13,770,592 | 1.45 | 0.89 | 2.24 |
|
| |||||
| Male | 13 | 11,403,373 | 1.14 | 0.61 | 1.95 |
|
| |||||
| Year | |||||
|
| |||||
| 2009 | 11 | 8,535,631 | 1.29 | 0.64 | 2.31 |
|
| |||||
| 2010 | 8 | 8,207,595 | 0.98 | 0.42 | 1.92 |
|
| |||||
| 2011 | 14 | 8,430,739 | 1.66 | 0.91 | 2.79 |
|
| |||||
| Vaccine* | |||||
|
| |||||
| Any hepatitis B | 0 | 1,287,074 | 0 | 0.00 | 2.87 |
|
| |||||
| RV1 | 0 | 57,517 | 0 | 0.00 | 64.13 |
|
| |||||
| RV5 | 0 | 636,756 | 0 | 0.00 | 5.79 |
|
| |||||
| Any DTaP | 3 | 1,449,370 | 2.07 | 0.43 | 6.05 |
|
| |||||
| Any HIB | 0 | 1,143,025 | 0 | 0.00 | 3.23 |
|
| |||||
| PCV7 | 0 | 558,201 | 0 | 0.00 | 6.61 |
|
| |||||
| PCV13 | 0 | 742,467 | 0 | 0.00 | 4.97 |
|
| |||||
| PPSV23 | 2 | 698,482 | 2.86 | 0.35 | 10.34 |
|
| |||||
| Any IPV | 2 | 1,215,163 | 1.65 | 0.20 | 5.95 |
|
| |||||
| TIV | 14 | 8,830,935 | 1.59 | 0.87 | 2.66 |
|
| |||||
| LAIV | 0 | 530,737 | 0 | 0.00 | 6.95 |
|
| |||||
| MIV | 3 | 1,422,921 | 2.11 | 0.43 | 6.16 |
|
| |||||
| LAMV | 0 | 298,721 | 0 | 0.00 | 12.35 |
|
| |||||
| Other influenza | 0 | 36,338 | 0 | 0.00 | 101.51 |
|
| |||||
| Any influenza | 17 | 11,119,652 | 1.53 | 0.89 | 2.45 |
|
| |||||
| MMR | 3 | 584,103 | 5.14 | 1.06 | 15.01 |
|
| |||||
| MMRV | 2 | 100,897 | 19.8 | 2.40 | 71.60 |
|
| |||||
| VAR | 6 | 866,129 | 6.93 | 2.54 | 15.08 |
|
| |||||
| HAV | 4 | 1,197,047 | 3.34 | 0.91 | 8.56 |
|
| |||||
| Tdap | 9 | 3,116,161 | 2.89 | 1.32 | 5.48 |
|
| |||||
| Td | 0 | 203,970 | 0 | 0.00 | 18.09 |
|
| |||||
| HPV4 | 1 | 775,833 | 1.29 | 0.03 | 7.18 |
|
| |||||
| MCV4 | 4 | 649,199 | 6.16 | 1.68 | 15.78 |
|
| |||||
| HZV | 2 | 304,001 | 6.58 | 0.80 | 23.77 |
|
| |||||
| Rabies | 1 | 18,041 | 55.43 | 1.40 | 308.79 |
|
| |||||
| Typhoid | 0 | 164,483 | 0 | 0.00 | 22.43 |
|
| |||||
| YFV | 0 | 34,176 | 0 | 0.00 | 107.93 |
|
| |||||
| JEV | 0 | 4,448 | 0 | 0.00 | 828.99 |
|
| |||||
| Anthrax | 0 | 81 | 0 | 0.00 | 44520.26 |
|
| |||||
| Smallpox | 0 | 31 | 0 | 0.00 | 112188.75 |
|
| |||||
| All vaccines | 33 | 25,173,965 | 1.31 | 0.90 | 1.84 |
HIB, Haemophilus influenzae type B vaccine; HPV4, quadrivalent human papillomavirus vaccine; IPV, inactivated polio vaccine; JEV, Japanese encephalitis vaccine; LAIV, live attenuated influenza vaccine; LAMV, live attenuated monovalent influenza vaccine; MCV4, meningococcal conjugate vaccine; MMRV, measles, mumps, rubella, and varicella vaccine; RV, rotavirus vaccine; PCV, pneumococcal conjugate vaccine; PPSV23, pneumococcal polysaccharide vaccine (23-valent); VAR, varicella vaccine; YFV, yellow fever vaccine.
Total count greater than 33 for vaccines received because some cases received more than 1 vaccine. This includes doses administered alone and coadministered with other vaccines.
TABLE VIII.
Vaccine-specific incidence of anaphylaxis
| Vaccine (administered alone) | No. of cases (n = 18) | Doses administered alone* | Rate (/106 doses) | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
| TIV | 10 | 7,434,628 | 1.35 | 0.65 | 2.47 |
|
| |||||
| MIV | 2 | 1,090,279 | 1.83 | 0.22 | 6.63 |
|
| |||||
| Tdap | 1 | 1,951,153 | 0.51 | 0.01 | 2.86 |
|
| |||||
| PPSV23 | 1 | 403,803 | 2.48 | 0.06 | 13.80 |
|
| |||||
| HAV | 1 | 296,271 | 3.38 | 0.09 | 18.81 |
|
| |||||
| HZV | 2 | 208,407 | 9.60 | 1.16 | 34.67 |
|
| |||||
| Rabies | 1 | 11,619 | 86.1 | 2.18 | 479.43 |
PPSV23, Pneumococcal polysaccharide vaccine (23-valent).
Doses of specified vaccine administered without any other concomitant vaccines.
When analyzed based on vaccination visits rather than vaccination doses, the rate of postvaccination anaphylaxis for all vaccines was 33 cases per 17,606,500 vaccination visits or 1.87 (95% CI 1.29-2.63) per million vaccination visits. The rate based on visits was higher than the per-dose rate because more than 1 vaccine was often administered at a vaccination visit. The median number of vaccines received at each visit was 2 (range, 1-14) among 0- to 17-year-olds, 1 (range, 1-12) among 18- to 49-year-olds, and 1 (range, 1-12) among those 50 years and older. The rate for postvaccination anaphylaxis for cases that did not involve TIV was 19 cases per 8,857,787 vaccination visits or 2.15 (95% CI, 1.29-3.35) per million vaccination visits.
DISCUSSION
In a large population-based study with extensive case finding, we identified 33 confirmed cases of anaphylaxis after administration of 25,173,965 vaccine doses or 1.31 (95% CI, 0.90-1.84) cases per million vaccine doses. With the largest number of doses and the largest number of cases, inactivated TIV was the major contributor to the number of vaccine-triggered anaphylaxis cases in the population, although the rate (1.35 [95% CI, 0.65-2.47] cases per million vaccine doses of TIV given alone) was similar to the rate for all vaccines. Our overall rate of postvaccination anaphylaxis was slightly higher than the previous study conducted in the VSD by Bohlke et al,11 which identified 5 cases of vaccine-associated anaphylaxis after administration of 7,644,049 vaccine doses or 0.65 (95% CI, 0.21-1.53) cases per million doses. However, that study was limited to children and adolescents and lacked outpatient data on a majority of subjects.11 The changes to the recommended childhood and adult immunization schedules and the introduction of new vaccines/vaccine combinations since the study by Bohlke et al11 stimulated our systematic re-evaluation of postvaccination anaphylaxis cases in the VSD. We used the same ICD-9-CM codes for anaphylaxis but supplemented our search strategy with allergy codes in conjunction with epinephrine-dispensing codes. In addition, we used the Brighton criteria, which facilitates comparison of our results with those of other studies of anaphylaxis.12 Our study population was considerably larger than the previous VSD study, and data on adults and the OPD setting at all sites were included. In recent years, TIV has become by far the most commonly administered vaccine in the United States, and we were able to provide the first estimates of anaphylaxis risk after TIV administration.
Reviews by IOM committees have found that anaphylaxis can be caused by several vaccines.7 However, the IOM review concentrated on childhood and adolescent vaccines, as did the study by Bohlke et al.11 We identified anaphylaxis cases for all the vaccines that IOM found to be causally related to anaphylaxis with the exception of hepatitis B, which we did not find administered in association with any anaphylaxis cases. Additionally, primarily because we included both adults and children, we identified anaphylaxis after several vaccines not included in the earlier IOM reports (monovalent influenza vaccine [MIV], Tdap, pneumococcal polysaccharide vaccine [23-valent], HAV, HZV, and rabies vaccine). To the extent possible and using available information (including from Sanofi Pasteur, Paris, France), we determined the ovalbumin content of the influenza vaccines administered in our cases was less than 1 μg/mL for TIV and less than 5 μg/mL for MIV, respectively.15
Our study is subject to certain potential limitations. Our ability to estimate rates for individual vaccines, with the exception of TIV, was limited because of small numbers of anaphylaxis cases and/or vaccine doses administered or concomitant administration with other vaccines. We used a broad search strategy that supplemented specific anaphylaxis codes with allergy codes in conjunction with epinephrine-dispensing codes. Nonetheless, we might have missed some cases of anaphylaxis because only about half of the confirmed cases of anaphylaxis had been treated with epinephrine, which could have resulted in missing some anaphylaxis cases that received an allergy code but were not treated with epinephrine. The strengths of this study included the large population, the use of Brighton Collaboration case definition, the broad algorithm used to identify potential cases, and the use of chart review to confirm cases. We considered it important to use the published Brighton Collaboration case definition for postvaccination anaphylaxis for our study.12 In vaccine safety Brighton Collaboration definitions are generally accepted as the gold standard surveillance case definitions for postvaccination adverse events, including anaphylaxis. The Brighton Collaboration case definition of anaphylaxis is also used in human vaccine clinical trials. Other clinical algorithms to identify anaphylaxis regardless of cause have been proposed.16,17 On review, one third (11/33) of our cases do not meet the more specific 2nd National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium clinical criteria,17 and 7 (64%) of these patients did not receive epinephrine.
We identified 3 recent reports of postvaccination anaphylaxis that used the Brighton criteria, all from spontaneous reporting systems. In a 2012 United Kingdom report, investigators analyzed data during September 2008 through October 2009 for reports in subjects less than 16 years old through the “orange card” pediatrician surveillance system and found 7 Brighton-validated cases with patients who recovered without sequelae; no cases followed an estimated 5.5 million doses of infant and preschool primary schedule immunizations, including MMR and influenza vaccines.18 Although denominators were unavailable for all vaccines, and thus the overall incidence was not calculated, 2 estimated incidence rates were reported (12.0 per 100,000 doses of single component measles vaccine and 1.4 cases per million doses of the bivalent HPV). In an Australian HPV4 school-based vaccination program in 12- to 18-year-olds, a rate of 2.6 per 100,000 doses was calculated (7 cases and all recovered among 269,680 doses administered).19 Lastly, in a 2009 H1N1 vaccination campaign in Quebec, Canada, a reporting rate of anaphylaxis of 8 per million distributed doses was reported after 2009 AS03-adjuvanted pandemic H1N1 vaccine (Arepanrix; GlaxoSmithKline, Mississauga, Ontario, Canada).20 A follow-up study conducted by the vaccine manufacturer of reports after Pandemrix (GlaxoSmithKline Biologicals S.A., Rixensart, Belgium) or Arepanrix to the worldwide safety database (62/72 reports after Arepanrix were from Canada) found no evidence the reporting rate was increased compared with rates for other vaccines.21 Data from the Vaccine Adverse Event Reporting System in the United States, where only unadjuvanated 2009 H1N1 pandemic influenza vaccines were used, found a reporting rate of 1.4 per million doses.22
Our finding of no deaths among our cases is consistent with the above reports.18-20 (The death from the Canadian report above did not meet the Brighton case definition for anaphylaxis.20) In a review of reports after TIV in adults to the US Vaccine Adverse Event Reporting System from 1990-2005, of 371 deaths, 4 were reported as anaphylaxis; during this period, an estimated 747.1 million doses of TIV were administered.23 In addition, a recent review of fatal anaphylaxis cases during 1999-2010, which used the National Center for Health Statistics Multiple Cause of Death Database, found 1446 (58%) 2458 anaphylaxis-related deaths were attributable to medications.24 However, most (74%) drug-induced anaphylaxis-related deaths had no identified culprit drug. Among the 368 in which the implicated medication was specified, in only 10 (3%) was this categorized as “serum”; postvaccination anaphylaxis deaths were not identified separately.24
Contrary to Bohlke et al,11 who found patients in 4 of 5 cases were aged less than 2 years, we identified no cases in children less than 4 years old, and the median age of the patients in our cases was 17 years (range, 4-65 years). Of the total 1117 charts reviewed, 380 were identified provisionally as anaphylaxis, possible anaphylaxis, or allergy, and 135 (36%) of these cases were among children 5 years or younger. Only 2 were validated anaphylaxis cases (both Brighton Level 2), and neither was attributed to vaccine. A 1-year-old healthy female subject 48 hours after receipt of DTaP and HAV vaccines had anaphylaxis 3 hours after receiving amoxicillin for an ear infection and recovered after parenteral antihistamine. A 1-year-old male subject with a history of chronic urticaria had anaphylaxis 24 hours after receipt of DTaP, TIV, MMR, and varicella vaccines and recovered after epinephrine, antihistamine, and oral and topical corticosteroids; however, anaphylaxis was attributed to milk allergy and on follow-up by an allergist, he was found to have positive skin test results for eggs, peanuts, and almonds. Our finding of no validated postvaccination anaphylaxis cases among children less than 2 years old is consistent with the recent United Kingdom experience, which reported no anaphylaxis events after routine infant and preschool immunizations.18 Factors potentially contributing to the lack of infants and toddlers among our cases might include difficulty making a diagnosis of anaphylaxis and/or applying Brighton criteria in these age groups. Sargant et al25 suggested the tendency for anaphylaxis in children to present most frequently with respiratory features has raised concern about the potential for diagnostic confusion with acute asthma, and consideration of the diagnosis is recommended in all children presenting with severe asthma.
Our study identified a female predominance among adults. The Bohlke et al11 and Erlewyn-Lajeunesse et al18 studies had small case numbers and did not report cases’ sex, and the Brotherton et al19 report was an HPV4 vaccination campaign that targeted female subjects only. However, the Rouleau et al20 safety study of 2009 pandemic H1N1 influenza vaccine found after adjusting for doses administered that female subjects were represented 3.9-fold more often than male subjects among their anaphylaxis reports, and among reports included in Tavares et al,21 the proportion of anaphylaxis cases, which met Brighton Levels 1 to 3 was higher in female compared with male subjects (84% vs 16%). In these 2 reports the rates were highest in women of childbearing age, and although the effect of a greater female tendency to report could not be ruled out in these passive surveillance studies, a biologic basis for the difference was hypothesized.20,21 Importantly, in our population-based study female preponderance was seen only in adults and not in children. Fourteen (70%) of 20 female subjects were of childbearing age (ie, aged 10-50 years). In general, anaphylaxis and immediate hypersensitivity, particularly drug allergy, occur more frequently in women of childbearing age.26-32 Sex-specific differences in innate, humoral, and cell-mediated immune responses to vaccination have also been reported.33-35 In addition, sex differences in adverse events (fever, pain, and inflammation) after immunization have been noted for several vaccines, including influenza36,37 and MMR38,39 vaccines. Although precise biological mechanisms underlying sex-specific responses to vaccines are unknown, genetic and hormonal factors are considered important.33 Sex hormones have been shown to modulate immune responses,40,41 and Hox et al42 recently found that sex-specific differences in a mouse model of anaphylaxis were due to the female steroid estradiol.
The finding that 28 (85%) cases had pre-existing atopic disease (3 with prior anaphylaxis, 16 with asthma, and 9 with specific prior allergies) is consistent with earlier reports emphasizing coexisting atopic disease, particularly asthma, as being clinical risk factors for anaphylaxis.29,30,43 Although epinephrine is recommended as the drug of choice for anaphylaxis,3,44 this therapy, as documented in the medical records, was administered in only 15 (45%) of our cases compared with antihistamines in 28 (85%) cases and corticosteroids in 17 (52%) cases. Only 3 (9%) cases were documented to have been prescribed epinephrine autoinjectors, and only 5 (15%) were known to have been referred to an allergist/allergy clinic for follow-up. The 2010 Joint American Academy of Allergy, Asthma & Immunology/ American College of Allergy, Asthma & Immunology/Joint Council of Allergy, Asthma & Immunology Task Force on Practice Parameters for the management of anaphylaxis clearly state that if initial assessment supports potential anaphylaxis, the immediate intervention is to assess the patient’s airway, breathing, circulation, and mentation; inject epinephrine; and re-evaluate for repeat injection, if necessary.3 After resolution, the patient should be provided with autoinjectable epinephrine, and in circumstances in which an allergist/immunologist is not already involved, it is strongly recommended that such consultation should be obtained.3 Lack of use of epinephrine and these other recommendations is not specific to our study and has been commented on by other investigators.3,45-48
Our study, which is based on a very large population receiving currently used vaccines, confirms the rarity of postvaccination anaphylaxis overall and after specific vaccines. Although anaphylaxis after immunization is rare, its immediate onset (usually within minutes) and life-threatening nature require that all personnel and facilities providing vaccinations have procedures in place for anaphylaxis management. Additional provider education concerning current recommendations for treatment and follow-up appears to be warranted.
Clinical implications.
Anaphylaxis after vaccination is rare in all age groups. Despite its rarity, anaphylaxis is a potentially life-threatening medical emergency that vaccine providers need to be prepared to treat.
Acknowledgments
The findings and conclusions of this report are those of the authors and do not necessarily represent the official policy or position of the Centers for Disease Control and Prevention (CDC). Use of trade names and commercial sources is for identification only and does not imply endorsement by the CDC. The Vaccine Safety Datalink Project is funded by the CDC. This study was supported by the CDC, and no external funding was secured.
Abbreviations
- DT
Diphtheria and tetanus toxoids
- DTaP and Tdap
Diphtheria, tetanus, and acellular pertussis vaccines
- ED
Emergency department
- HAV
Hepatitis A vaccine
- HPV
Human papillomavirus vaccine
- HZV
Herpes zoster vaccine
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- IOM
Institute of Medicine
- MIV
Monovalent influenza vaccine
- MMR
Measles, mumps, rubella vaccine
- OPD
Outpatient department
- TIV
Trivalent influenza vaccine
- VSD
Vaccine Safety Datalink
Footnotes
Disclosure of potential conflict of interest: L. Sukumaran has received research support from the National Institutes of Health (NIH). N. P. Klein has received research and travel support, as well as payment for writing/reviewing the manuscript, from the Centers for Disease Control and Prevention (CDC) and has received research support from Sanofi Pasteur, GlaxoSmithKline, Novartis, MedImmune, Protein Science, Merck, Pfizer, and Nuron Biotech. S. J. Hambidge has received research support from the CDC Vaccine Safety Datalink. G. M. Lee and L. A. Jackson have received research support from the CDC. S. A. Irving has received research and travel support from the CDC. J. P. King has received research support from the CDC. The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Austen KF. Disease of immediate type hypersensitivity. In: Isselbacher KJ, Braunwald E, Wilson JD, Martin JB, Fauci AS, Kasper DL, editors. Harrison’s principles of internal medicine. 13th McGraw-Hill; New York: 1994. pp. 1630–8. [Google Scholar]
- 2.Simmons FE. Anaphylaxis. J Allergy Immunol. 2010;125(suppl):S161–81. doi: 10.1016/j.jaci.2009.12.981. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman P, Nicklas RA, Oppenheimer J, Kemp SF, Lang DM, Bernstein DI, et al. The diagnosis and management of anaphylaxis practice parameter: 2010 update. J Allergy Clin Immunol. 2010;126:477–80. doi: 10.1016/j.jaci.2010.06.022. e1-42. [DOI] [PubMed] [Google Scholar]
- 4.Simons FE, Ardusso LR, Bilo B, El-Gamal YM, Ledford DK, Ring J, et al. World Allergy Organization guidelines for the assessment and management of anaphylaxis. J Allergy Clin Immunol. 2011;127(593):e1–22. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention General recommendations on immunization—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–64. [PubMed] [Google Scholar]
- 6.Kelso JM, Li JT, Nicklas RA, Blessing-Moore J, Cox L, Lang DM, et al. Adverse reactions to vaccines. Ann Allergy Asthma Immunol. 2009;103(4 Suppl 2):S1–14. doi: 10.1016/s1081-1206(10)60350-x. [DOI] [PubMed] [Google Scholar]
- 7.Stratton K, Ford A, Rusch E, Clayton EW. Institute of Medicine. National Academies Press; Washington (DC): 2011. Adverse effects of vaccines: evidence and causality. [PubMed] [Google Scholar]
- 8.Grabenstein JD. Clinical management of hypersensitivities to vaccine components. Hospital Pharmacy. 1997;32:77–87. [Google Scholar]
- 9.Lear JT, English JS. Anaphylaxis after hepatitis B vaccination. Lancet. 1995;345:1249. doi: 10.1016/s0140-6736(95)92039-0. [DOI] [PubMed] [Google Scholar]
- 10.Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring vaccine safety. Pediatrics. 2011;127(suppl):S45–53. doi: 10.1542/peds.2010-1722H. [DOI] [PubMed] [Google Scholar]
- 11.Bohlke K, Davis RL, Marcy SM, Braun MM, DeStefano F, Black SB, et al. Risk of anaphylaxis after vaccination of children and adolescents. Pediatrics. 2003;112:815–20. doi: 10.1542/peds.112.4.815. [DOI] [PubMed] [Google Scholar]
- 12.Ruggeberg JU, Gold MS, Bayas JM, Blum MD, Bonhoeffer J, Friedlander S, et al. Anaphylaxis: case definition and guidelines for data collection, analysis and presentation of immunization safety data. Vaccine. 2007;25:5675–84. doi: 10.1016/j.vaccine.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 13.McNeil MM, Gee J, Weintraub ES, Belongia EA, Lee GM, Glanz JM, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine. 2014;32:5390–8. doi: 10.1016/j.vaccine.2014.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothman KJ, Boice JD., Jr . Epidemiologic analysis with a programmable calculator. National Institutes of Health; Bethesda (MD): 1979. pp. 31–2. NIH publication no. 79-1649. [Google Scholar]
- 15.Grohskopf LA, Olsen SJ, Sokolow LZ, Bresee JS, Cox NJ, Broder KR, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2014-15 influenza season. MMWR Morb Mortal Wkly Rep. 2014;63:691–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson HA, Muñoz-Furlong A, Bock SA, Schmitt C, Bass R, Chowdhury BA, et al. Symposium on the definition and management of anaphylaxis: summary report. J Allergy Clin Immunol. 2005;115:584–92. doi: 10.1016/j.jaci.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Sampson HA, Muñoz-Furlong A, Campbell RL, Adkinson NF, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report—second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network Symposium. J Allergy Clin Immunol. 2006;117:391–7. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 18.Erlewyn-Lajeunesse M, Hunt LP, Heath PT, Finn A. Anaphylaxis as an adverse event following immunization in the UK and Ireland. Arch Dis Child. 2012;97:487–90. doi: 10.1136/archdischild-2011-301163. [DOI] [PubMed] [Google Scholar]
- 19.Brotherton JM, Gold MS, Kemp AS, McIntyre PB, Burgess MA, Campbell-Lloyd S, et al. Anaphylaxis following quadrivalent human papillomavirus vaccination. CMAJ. 2008;179:525–33. doi: 10.1503/cmaj.080916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rouleau I, De Serres G, Drolet JP, Skowronski DM, Ouakki M, Toth A, et al. Increased risk of anaphylaxis following administration of 2009 AS03-adjuvanted monovalent pandemic A/H1N1 (H1N1pdm09) vaccine. Vaccine. 2013;31:5989–96. doi: 10.1016/j.vaccine.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Tavares F, Delaigle A, Slavin D, Bauchau V, Fries L, Siefert H. Anaphylaxis following H1N1 pandemic vaccines: safety data in perspective. Vaccine. 2011;29:6402–7. doi: 10.1016/j.vaccine.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, et al. Adverse events following influenza A (H1N1) monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009-January 31, 2010. Vaccine. 2010;28:7248–55. doi: 10.1016/j.vaccine.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 23.Vellozzi C, Burwen DR, Dobardzic A, Ball R, Haber P. Safety of trivalent inactivated influenza vaccines in adults: background for pandemic influenza vaccine safety monitoring. Vaccine. 2009;27:2114–20. doi: 10.1016/j.vaccine.2009.01.125. [DOI] [PubMed] [Google Scholar]
- 24.Jershow E, Lin RY, Scaperotti MM, McGinn AP. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic association. J Allergy Clin Immunol. 2014;134:1318–28. doi: 10.1016/j.jaci.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sargant N, Erlewyn-Lajeunesse M, Benger J. Does anaphylaxis masquerade as asthma in children? Emerg Med J. 2015;32:83–4. doi: 10.1136/emermed-2014-203603. [DOI] [PubMed] [Google Scholar]
- 26.Erlwyn-Lajeunesse M, Dymond S, Slade I, Mansfield HL, Fish R, Jones O, et al. Diagnostic utility of two case definitions for anaphylaxis: a comparison using retrospective case notes analysis in the UK. Drug Saf. 2010;33:57–64. doi: 10.2165/11318970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman PL. Anaphylaxis. In: Adkinson N, Bochner B, Busse W, Holgate S, Lemanske R, Simons F, editors. Middleton’s allergy: principles and practice. 7th Mosby; Philadelphia: 2008. pp. 1027–50. [Google Scholar]
- 28.Sheikh A, Hippisley-Cox J, Newton J, Fenty J. Trends in national incidence, lifetime prevalence and adrenaline prescribing for anaphylaxis in England. J R Soc Med. 2008;101:139–43. doi: 10.1258/jrsm.2008.070306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonz'alez-Per'ez A, Aponte Z, Vidaurre CF, Rodriguez LA. Anaphylaxis epidemiology in patients with and patients without asthma: a United Kingdom database review. J Allergy Clin Immunol. 2010;125:1098–104. doi: 10.1016/j.jaci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Clark S, Wei W, Rudders SA, Camargo CA., Jr Risk factors for severe anaphylaxis in patients receiving anaphylaxis treatment in US emergency departments and hospitals. J Allergy Clin Immunol. 2014;134:1125–30. doi: 10.1016/j.jaci.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Chen W, Mempel M, Schober W, Behrendt H, Ring J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy. 2008;63:1418–27. doi: 10.1111/j.1398-9995.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 32.Decker WW, Campbell RL, Manivannan V, Luke A, Sauver JL, Weaver A, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122:1161–5. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook IF. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–5. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 34.Cook IF. Sex differences in injection site reactions with human vaccines. Hum Vaccin. 2009;5:441–9. doi: 10.4161/hv.8476. [DOI] [PubMed] [Google Scholar]
- 35.Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010;10:338–49. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Haang CY, Cox NJ, et al. Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Arch Intern Med. 2008;156:1546–50. doi: 10.1001/archinternmed.2008.513. [DOI] [PubMed] [Google Scholar]
- 37.Nichol KL, Margolis KL, Lind A, Murdoch M, McFadden R, Hauge M, et al. Side effects associated with influenza vaccination in healthy working adults: a randomized placebo-controlled trial. Arch Intern Med. 1996;156:1546–50. [PubMed] [Google Scholar]
- 38.Khalil MK, Al-Mazrou YY, Al-Ghamdi YS, Tumsah S, Al-Jeffri M, Meshkhas A. Effect of gender on reporting of MMR adverse events in Saudi Arabia. East Mediter Health J. 2003;9:152–8. [PubMed] [Google Scholar]
- 39.Benjamin CM, Chew GC, Silman AJ. Joint and limb symptoms in children after immunization with measles, mumps, and rubella vaccine. BMJ. 1992;304:1075–8. doi: 10.1136/bmj.304.6834.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pennell LM, Galligan CL, Fish EN. Sex affects immunity. J Autoimmun. 2012;38:1075–8. doi: 10.1016/j.jaut.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 41.Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol. 2001;1:983–93. doi: 10.1016/s1567-5769(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 42.Hox V, Desai A, Bandara G, Gilfillan AM, Metcalfe DD, Olivera A. Estrogen increases the severity of anaphylaxis in female mice through enhanced endothelial nitric acid oxide synthetase expression and nitric oxide production. J Allergy Clin Immunol. 2015;135:729–36. doi: 10.1016/j.jaci.2014.11.003. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iribarren C, Tolstykh IV, Miller MK, Eisner MD. Asthma and the prospective risk of anaphylactic shock and other allergy diagnoses in a large integrated health care delivery system. Ann Allergy Asthma Immunol. 2010;104:371–7. doi: 10.1016/j.anai.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Simons FER. Pharmacologic treatment of anaphylaxis: can the evidence base be strengthened? Curr Opin Allergy Clin Immunol. 2010;10:384–93. doi: 10.1097/ACI.0b013e32833c2038. [DOI] [PubMed] [Google Scholar]
- 45.Campbell RL, Luke A, Weaver AL, Sauver JL, St, Bergstralh EJ, Li JT, et al. Prescriptions for self-injectable epinephrine and follow-up referral in emergency department patients presenting with anaphylaxis. Ann Allergy Asthma Immunol. 2008;101:631–6. doi: 10.1016/S1081-1206(10)60227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell RL, Park MA, Kieber MA, Sangil L, Hagan JB. Outcomes of allergy/immunology follow-up after an emergency department evaluation for anaphylaxis. J Allergy Clin Immunol Pract. 2015;3:88–93. doi: 10.1016/j.jaip.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Rudders SA, Clark S, Wei W, Camargo CA. Longitudinal study of 954 patients with stinging insect anaphylaxis. Ann Allergy Asthma Immunol. 2013;111:199–204. doi: 10.1016/j.anai.2013.06.020. e1. [DOI] [PubMed] [Google Scholar]
- 48.Dudley LS, Mansour MI, Merlin MA. Epinephrine for anaphylaxis: underutilized and unavailable. West J Emerg Med. 2015;16:385–7. doi: 10.5811/westjem.2015.3.25337. [DOI] [PMC free article] [PubMed] [Google Scholar]


