Abstract
Ovarian cancer is the deadliest gynecologic cancer, due in large part to the diagnosis of advanced stage disease, the development of platinum resistance, and inadequate treatment alternatives. Recent studies by our group and others have shown that T-type Ca2+ channels play a reinforcing role in cancer cell proliferation, cell cycle progression and apoptosis evasion. Therefore, we investigated whether T-type Ca2+ channels affect ovarian tumor growth and response to platinum agents. Inhibition of T-type Ca2+ channels with mibefradil or by silencing expression resulted in growth suppression in ovarian cancer cells with a simultaneous increase in apoptosis, which was accompanied by decreased expression of the anti-apoptotic gene survivin (BIRC5). Analysis of intracellular signaling revealed mibefradil reduced AKT phosphorylation, increased the levels and nuclear retention of FOXO transcription factors that repress BIRC5 expression, and decreased expression of FoxM1, which promotes BIRC5 expression. Combining carboplatin with mibefradil synergistically increased apoptosis in vitro. Importantly, mibefradil rendered platinum-resistant ovarian tumors sensitive to carboplatin in a mouse model of peritoneal metastasis. Together, the data provide rationale for future use of T-type channel antagonists together with platinum agents for the treatment of ovarian cancer.
Keywords: mibefradil, carboplatin, T-type calcium channels, apoptosis, survivin, drug synergy
Introduction
Ovarian cancer is the deadliest gynecologic malignancy and the fifth leading cause of all cancer-related death in women. According to SEER estimates, approximately 22,000 women in the United States will be diagnosed with ovarian cancer, and more than 14,000 patients will die of this disease (1). Greater than 75% of the incident cases are detected after metastatic spread (Stages III and IV), where the survival rate at five years is only 10-20%. Standard treatment consists of surgical cytoreduction and cytotoxic chemotherapy. While the majority of advanced stage patients achieve a complete response to standard platinum/taxane based chemotherapy, as many as 80% recur within 2 years and will eventually succumb to platinum-resistant disease. Despite advances in cytotoxic therapy, resistance to platinum agents remains a significant hurdle to overcome.
Calcium (Ca2+) is a crucial second messenger in all eukaryotic cells (2). The relationship between Ca2+ signaling and cardiovascular or neurological diseases has been studied for decades. Indeed, classes of drugs targeting particular Ca2+ channels are used to treat hypertension, angina and other cardiovascular conditions. More recently, the contribution of over-expression and/or aberrant activation of Ca2+ specific channels and Ca2+ regulated intracellular pathways to cancer progression has been reported (3). Voltage-activated Ca2+ channels provide a pathway for rapid influx of Ca2+ into cells. Among them, the low voltage-activated Ca2+ channel family (Cav3, commonly called T-type Ca2+ channels) is functionally linked to many physiological processes (4) and are aberrantly expressed in cancer, including ovarian tumors and cell lines (5,6). Recent data demonstrate that inhibition of T-type Ca2+ channels in ovarian cancer cells disturbs cell cycle progression, decreases proliferation and enhances cell death (5,6). However, the detailed molecular mechanisms of this inhibition are still unknown. We previously reported that T-type Ca2+ channel blockers, specifically mibefradil, induce apoptosis in glioblastoma and colon cancer cells by suppression of the PI3K/AKT pathway (7) and activation of p53 and the p38/MAPK pathway (8), respectively. Upon T-type Ca2+ channel inhibition, apoptosis is manifested as changes in expression of several pro- and anti-apoptotic proteins (7,8), including the anti-apoptotic protein survivin.
Survivin (encoded by BIRC5) is one of the anti-apoptotic proteins belonging to the inhibitor of apoptosis (IAP) family (9). It is important for inhibition of apoptosis and essential in the regulation of mitosis (10), autophagy (11) and DNA damage repair (12). Survivin is overexpressed in many cancers, including ovarian (13–17), and as such could serve as both molecular target and biomarker for response to therapy and patient survival (18,19). Several mechanisms regulating survivin gene expression and activity in cancer cells have been reported, including transcriptional regulation (20), post-translational modification such as phosphorylation, acetylation and ubiquitination (21), and shuttling between the nucleus and cytoplasm (22). However, non-malignant cells also use those pathways to control normal physiological functions of survivin. Therefore, we postulate that inhibiting survivin expression by blocking upstream pathways that are de-regulated in cancer would allow for more specific targeting of survivin in cancer versus normal tissues. One such pathway involves PI3K/AKT regulation of forkhead box transcription factors acting either as activators (FoxM1 (23,24)) or repressors (FoxO1/FoxO3a (25,26)) of BIRC5 expression.
Abnormal expression of anti-apoptotic proteins is commonly observed in ovarian tumors and associated with their aggressiveness and resistance to cytotoxic therapy (16,27). Therefore, intervention in cell death pathways is considered an effective approach to increase cancer response to therapy (18,28). Here, we investigated the contribution of T-type Ca2+ channels to ovarian cancer cell growth and tumor progression. We report that loss of expression or inhibition of T-type Ca2+ channels with mibefradil induced apoptosis in cultured ovarian cancer cell lines. Apoptosis was accompanied by decreased AKT phosphorylation and alterations in FoxO and FoxM1 expression culminating in reduced survivin expression. Importantly, pretreatment of platinum-resistant ovarian cancer cells with mibefradil rendered them sensitive to carboplatin in vitro and significantly hindered tumor growth in vivo. Together, our data support the rationale for using T-type Ca2+ channel blockers in the treatment of platinum-resistant ovarian cancer.
Materials and Methods
Cell culture and drug treatment
Human ovarian cancer cell lines were purchased from ATCC (Manassas, VA) or Sigma-Aldrich (St. Louis, MO); SKOV3.ip1 were generously provided by Dr. Anil Sood (MD Anderson). They were maintained in a 37°C/ 5% CO2 humidified chamber in RPMI-1640 supplemented with 10% FBS (A2780 and A2780Cis) or with sodium bicarbonate, sodium pyruvate and 10% FBS (IGROV-1) or McCoy's supplemented with 15% FBS (SKOV3.ip1). A2780Cis cells were cultured in 1 μmol/L cisplatin (cisPt) every third passage. None of the lines have been tested or authenticated. All cell culture materials and supplies were from Life Technologies GIBCO (Grand Island, NY). Mibefradil (Mib), a generous gift from Cavion LLC (Charlottesville, VA), was suspended in DMSO; cisPt (Sigma-Aldrich) was suspended in sterile water. Carboplatin (carboPt) was obtained from the University of Virginia pharmacy as a 10 mg/ml stock solution in phosphate-buffered saline (PBS). Cells were treated with the indicated concentrations of antagonists or platinum agents at 37°C for times indicated as single agents (see Figure Legends). Drug combination studies were performed by treating cells for 24 hours with the indicated concentrations of Mib and then replacing the Mib-containing media with media containing the indicated concentrations of carboplatin or vehicle for another 24-72 hours.
Small-interfering RNA (siRNA) transfection
Cells were transfected with 25 nmol/L of siRNA targeted against CACNA1G or CACNA1H T-type channels subunits (Supplementary Table 1), or non-targeted scrambled control siRNA, using Lipofectamine RNAiMax (Life Technologies) as described previously (8). After 72 hours cells were harvested and processed for total RNA isolation or subjected to proliferation, cell death or Western blot assays.
Reverse transcriptase quantitative PCR (RT-qPCR) for gene expression
Total RNA was isolated using Mini Plus RNeasy Kit (Qiagen, Valencia, CA), and 1 μg was used for cDNA synthesis using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Each quantitative PCR (qPCR) reaction was done in triplicate using SsoFast EvaGreen Supermix (Bio-Rad), including 50 ng of cDNA as a template and 0.5 μmol/L of specific primers (Supplementary Table 2 and (8,29)). Conditions for amplification were as follows: initial denaturation 98°C for 30 sec, then 40 cycles of denaturation for 5 sec at 98°C and annealing with extension for 5 sec at 62°C. Relative gene expression of specific genes of T-type Ca2+ channels or BIRC5 was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH), β-glucouronidase (GUS) or β-actin expression and calculated by the formula 2−ΔΔCt by subtracting the Ct value of GAPDH, GUS or β-actin and then the Ct value of untreated control (30).
Cell viability, metabolic activity and proliferation
The viability of treated cells was assessed using trypan blue exclusion. Following treatment cells were collected by trypsinization, stained with trypan blue (0.04%) for 10 min and the total cells and percentage of non-viable cells were counted using automated Cell Counter (Bio-Rad). Proliferation/viability was determined by Alamar Blue (Life Technologies) after 72 hours drug treatment. The proliferation rate was assessed by staining cells treated with indicated drugs or vehicle control for 72 hours with sulforhodamine B (SRB, Sigma-Aldrich) or using the CyQuant assay following the manufacturer’s instructions (Life Technologies). For drug combination studies, cells were treated first with Mib for 24 hours, Mib-containing media was then replaced with either fresh media or media containing carboplatin, and the cells were incubated continuously at 37°C for additional 24 hours. For A2780Cis and IGROV-1 cells the results for Mib induced drug synergy with carboPt from CyQuant proliferation assay were confirmed with longer time incubation with carboPt (72 hours) in SRB assay.
Cell cycle distribution
Cells were allowed to attach/recover overnight and treated with studied agents (or sham-treated) for 0-24 hours. Bromodeoxyuridine (BrdU, BD Pharmnigen, San Jose, CA) was added for the last hour of drug incubation to a final concentration of 10 μmol/L. Samples were collected (including floating cells) and processed using BD Pharmingen BrdU Flow Kit according to the instruction manual. Two-dimensional (BrdU-FITC vs. 7-AAD) flow cytometry analyses were performed on a FACS Calibur instrument, quantified using CellQuest software and analyzed using FlowJo or ModFit Software (Flow Cytometry Core, University of Virginia).
Apoptosis
Mechanism of cell death induced by Mib, carboPt or the combination of both was evaluated by annexin V-FITC/propidium iodide (PI) staining (BD Bioscience). Briefly, the cells were plated for 24 hours, treated with Mib (6 μmol/L) alone and/or in combination with increasing concentrations of carboPt (1-10 μg/mL), collected, washed with PBS and stained with Annexin V-FITC and PI for 15 min at room temperature according to the manufacturer suggestions (BD Pharmingen). Live cells were analyzed within one hour by 2D flow cytometry (Flow Cytometry Core, University of Virginia).
Western blotting
Following different incubation times with the drug, the cells were collected, washed with ice-cold PBS and lysed in modified RIPA buffer (Tris-HCl 50 mmol/L, NaCl 150 mmol/L, glycerol 10%, EDTA 5 mmol/L, EGTA 5 mmol/L, Triton X-100 0.5%, deoxycholate 0.5%, CHAPS 0.5%, protease/phosphatase inhibitors). To assess intracellular distribution of proteins, the cells were processed with NE-PER reagents (Pierce/Thermo Scientific, Pittsburgh, PA) to separate nuclear and cytosolic proteins. Equal amounts of protein were resolved on 4-20% gradient gels (TGX Criterion, Bio-Rad), transferred to nitrocellulose and probed with specific antibodies against: Rb phospho-Ser780 (Cell Signaling Tech., CST #9307), Rb (CST #9309), cyclin D1 (CST #2926), survivin (CST #2808), PARP (Santa Cruz Biotech., SCB sc-8007), cleaved caspase 9 (CST #9505), cleaved caspase 7 (CST #8438), AKT phospho-Ser473 (CST #4051), AKT phospho-Thr308 (CST #2965), AKT (CST #9272), FoxM1 (CST #5436), FoxO1 (CST #2880), FoxO3a (CST #2497), lamin A/C (SCB sc-7292), β-actin (Sigma A5316), α-tubulin (Sigma T6074). Quantification of signal intensity was performed using a two-color Li-COR Odyssey Imager and software (Li-COR Biosciences, Lincoln, NE).
Chromatin immunoprecipitation (ChIP)
Chromatin immunoprecipitation was performed according to the published protocol (31). Briefly, cells were treated with 10 μmol/L Mib (or 0.1% DMSO) for 24 hours. Protein-DNA macromolecules were cross-linked with 1% formaldehyde for 10 min at room temperature. Cells were washed and scraped in PBS, collected by centrifugation at 1,000 g for 10 min at 4°C, re-suspended in lysis buffer containing 1% SDS, protease inhibitors (Pierce), and sonicated using Branson Sonifier 400 W Cell Disruptor to 200-1,000 bp DNA size fractions. Extracts were cleared by centrifugation and diluted with 9 volumes of Low Salt Buffer (Upstate Biotechnology, Lake Placid, NY) plus protease inhibitors and the following specific rabbit polyclonal antibodies were added for overnight incubation: anti-FoxM1 (Santa Cruz Biotechnology, K-19, sc-500 X), anti-FoxO1 (anti-FKHR, Santa Cruz Biotechnology, H-128, sc-11350 X), anti-FoxO3a (anti-FKHRL1, Santa Cruz Biotechnology, sc-11351 X) or IgG (2 μg). Protein-DNA complexes were collected with Dynabeads Protein G (Life Technologies) and washed according to the protocol (Upstate). Complexes were dissociated from beads using 1% SDS buffer containing 0.1 mol/L NaHCO3, DNA crosslinks were reversed with heat (65°C for 4 hours), and digested with proteinase K for one hour at 37°C. DNA was phenol-chloroform extracted and precipitated with 0.7 volume of 100% isopropanol. Mib-induced enrichment of FoxO1/FoxO3a binding or loss of FoxM1 binding to specific sites within BIRC5 promoter was determined based on the absolute quantification in qPCR reaction normalized to the Input. Specific primers were designed using Primer3 software (32) and were in proximity to a specific binding site for FoxM1 binding according to previously published information (33) (Supplementary Table 3, Supplementary Fig S4E). qPCR was performed using IQ Syber Green Supermix (Bio-Rad) as follows: one cycle, 3 min at 95°C, 40 cycles of 95°C for 15 sec, 62°C for 30 sec and 72°C for 30 sec.
In vivo human ovarian cancer xenografts
All animal experiments were performed following approval from the Institutional Animal Care and Use Committee at the University of Virginia. Six to 8 week-old female athymic nude mice (Harlan Laboratories) were injected intraperitoneally (IP) with 200 μL PBS containing 106 A2780Cis cells stably expressing luciferase following lentiviral transduction, which did not affect cellular response to Mib. Tumor-bearing mice were treated in one of 4 arms as follows: 1) 40 mg/kg Mib in water via gavage every 6 hours for 5 days (2 cycles); 2) 25 mg/kg carboplatin in PBS via IP injection; 3) Mib and carboplatin as described; or 4) vehicle control. The dosing schedule was as follows: d1 – inject cells, d4-8 and d13-17 – mibfradil, d9 and d18 – carboplatin. The experiment was performed twice with 4-5 mice per treatment arm per experiment; the total number of mice analyzed (n) is indicated in the figure legend. Mice were observed 2-3 times per week by laboratory personnel and monitored for signs of distress (i.e., changes in appearance, respiration, activity, etc.) and weighed; mice showing signs of distress or losing greater than 15% body weight were euthanized and examined for tumor. Tumor burden was assessed in one experiment (n=4-5 per treatment arm) on days 4, 8, 13, 18 and 24 after tumor cell injection by measuring light emission following IP luciferin administration as an indication of luciferase activity using an IVIS imaging system (Molecular Imaging Core, University of Virginia). Total flux (photons/sec) was determined for the entire abdominal cavity per mouse and normalized to the mean total flux of control-treated mice imaged on day 4. Upon experimental termination (d28), mice were euthanized and tumor burden evaluated upon necropsy by counting the number of tumor nodules and weighing the omentum (primary site of tumor implantation) and any additional tumor nodules. Formalin-fixed, paraffin-embedded tissues were sectioned and H&E stained (University of Virginia Research Histology Core) to evaluate microscopic tumor burden.
Data analysis
All values were expressed as the means of at least three independent experiments ± SEM. Results were compared using one-way ANOVA followed by Tukey’s multiple comparisons test. A P-value of less than 0.05 indicated statistically significant differences between observed effects. Drug induced synergy between mibefradil and carboplatin was determined by Median Dose Effect analysis (34) using CompuSyn software (ComboSyn). Combination Index (CI) values <1.0 denote synergistic interactions. Tumor incidence from the mouse experiments was evaluated using Fisher’s Exact Test. All other data from mouse experiments were analyzed using 2-way ANOVA followed by Tukey’s multiple comparisons test.
Results
T-type Ca2+ channel expression and sensitivity to inhibition or downregulation in ovarian cancer cells
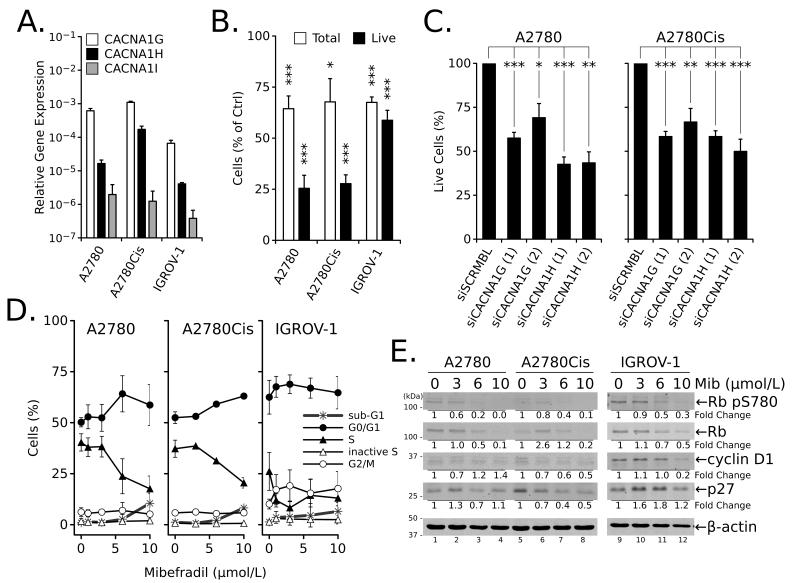
The contribution of T-type channels to ovarian cancer cell growth was evaluated using a relatively selective antagonist, mibefradil (Mib) (35), or siRNA knockdown of individual channels. Similar to a previous report (5), RT-qPCR confirmed the expression of the T-type Ca2+ channel genes, CACNA1G (Cav3.1), CACNA1H (Cav3.2) and CACNA1I (Cav3.3) in three ovarian cancer cell lines including A2780, A2780Cis, and IGROV-1, although to different extents (Fig. 1A). Cells were subjected to increasing concentrations of Mib for 72 hours, and viability was determined with the Alamar Blue assay. The results showed that Mib decreased cell viability and generated EC50 values ranging from 6 to 13 μmol/L (Table 1).
Figure 1.
Ovarian cancer cell lines express T-type Ca2+ channels and are sensitive to inhibition. (A) Expression of mRNA for Cav3.1 (CACNA1G), Cav3.2 (CACNA1H) and Cav3.3 (CACNA1I) in ovarian cancer cell lines normalized to GAPDH expression. (B) Percentage of total and viable (live, trypan blue negative) A2780, A2780Cis, and IGROV-1 cells relative to control (100%) after treatment with 10 μmol/L Mib for 24 hours. (C) Percentage of live (trypan blue negative) A2780 or A2780Cis cells after transfection with siRNA against T-type channel isoforms (two different siRNAs per gene) for 72 hours. (D) Effects of increasing concentrations of Mib on cell cycle in ovarian cancer cells treated for 24 hours. (E) Western blot analysis of cell cycle-related proteins in total cell extracts isolated from ovarian cancer cells treated with increasing concentrations of Mib for 24 hours. All values are the mean ± SEM from ≥ three independent experiments; *, P < 0.05; **, P < 0.01; ***, P < 0.001; NS, P > 0.05 relative to untreated or siSCRMBL controls.
Table 1.
Viability of ovarian cancer cells treated with mibefradil or carboplatin.
| Cell line | Mibefradil EC50 ± SEM (μmol/L) |
Carboplatin EC50 ± SEM (μg/mL) |
|---|---|---|
| A2780 | 6.6 ± 0.7 | 5.9 ± 0.83 |
| A2780Cis | 7.7 ± 0.8 | 19 ± 2.3 |
| IGROV-1 | 9.6 ± 0.8 | 14 ± 2.1 |
| SKOV3.ip1 | 13 ± 2.2 | 18 ± 3.3 |
Cells were treated with mibefradil continuously for 72 hours, and viability was determined by Alamar Blue assay as described (see Material and Methods). Values represent the average of ≥ three independent experiments ± SEM. EC50 – drug concentration that decreased viability 50%.
To determine whether Mib inhibited proliferation or induced cell death, cells were treated for 24 hours with 10 μmol/L Mib and evaluated via trypan blue exclusion. The data showed a 25-40% reduction in the total number of cells (Fig. 1B) relative to control mock-treated cells (100%, not shown) for all cell lines. Moreover, the number of viable/live A2780 and A2780Cis cells was 25% of the total, indicating increased cell death. The effects of Mib were confirmed using siRNA to individual T-type channels (Supplementary Table 1), where a 35-50% decrease in the percentage of live cells was observed in both A2780 and A2780Cis cells (Fig. 1C, Supplementary Fig. 1).
The effects of Mib on cell cycle progression in ovarian cancer cells was examined by treating cells with increasing concentrations of Mib for 24 hours. All cell lines showed a dose-dependent decrease in the number of cells in active S phase (BrdU-incorporating) and an increase in the number of cells in G1 (all cell lines) and/or G2 (IGROV-1) phases (Fig. 1D). The inhibition of cell cycle progression after treatment with Mib is supported by dose-dependent decreases in the expression of cyclin D1, p27 and Rb as well as decreased Rb phosphorylation, consistent with reduced entry into S phase (Fig. 1E). These results concur with previously reported induction of G1 arrest and inhibition of proliferation in A2780 cells treated with T-type channel antagonists (5,6) or transfected with specific siRNA (5).
Blocking T-type Ca2+ channels induced apoptosis in ovarian cancer cells through decreased expression of survivin
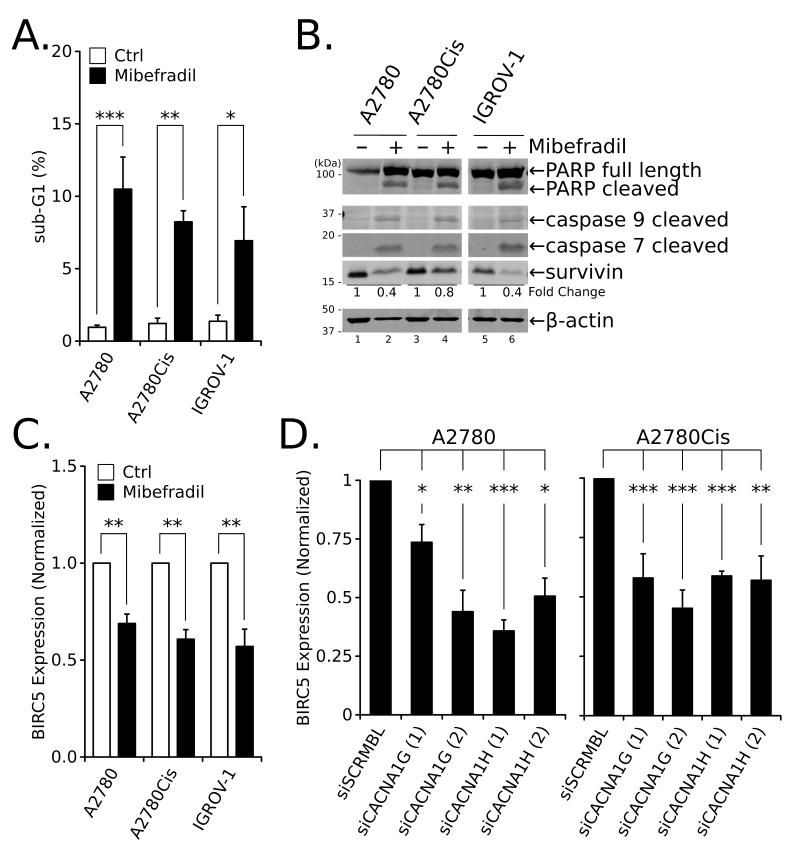
In addition to changes in cell cycle progression, Mib treatment promoted a dose-dependent increase in the sub-G1 population (Figs. 1D and 2A). Treatment with 10 μmol/L Mib for 24 hours also increased cleaved PARP, caspase 9, and caspase 7 (Fig. 2B), well-established markers of apoptosis, and reduced expression of survivin, an anti-apoptotic factor. Indeed, survivin protein (Fig. 2B) and mRNA (BIRC5; Fig. 2C) levels decreased 20-60% and 30-50%, respectively, following 24 hours of treatment with 10 μmol/L Mib relative to untreated cells. Furthermore, reduced CACNA1G or CACNA1H expression following transfection with two different siRNA oligomers directed against each gene diminished survivin (Supplementary Fig. 2) and BIRC5 expression (Fig. 2D) approximately 30-50% relative to control cells (siSCRMBL).
Figure 2.
Inhibition of T-type Ca2+ channels induces apoptosis and lowers survivin expression in ovarian cancer cells. (A) The sub-G1 population in cells treated with 10 μmol/L Mib for 24 hours was determined by flow cytometry. (B) Western blot analysis of markers of apoptotic cell death in total cell extracts from ovarian cancer cells treated with 10 μmol/L Mib for 24 hours. (C) Expression of BIRC5 mRNA determined by quantitative RT-PCR in ovarian cancer cells treated with Mib for 24 hours at 6 μmol/L (A2780) or 10 μmol/L (A2780Cis and IGROV-1). (D) Expression of BIRC5 mRNA determined by quantitative RT-PCR in A2780 and A2780Cis cells 72 hours after transfection with siRNA against T-type channels isoforms. All values are the mean ± SEM from ≥ two independent experiments; **, P < 0.01; ***, P < 0.001.
Inhibition of T-type Ca2+ channels targeted the PI3K/AKT pathway and forkhead box transcription factors
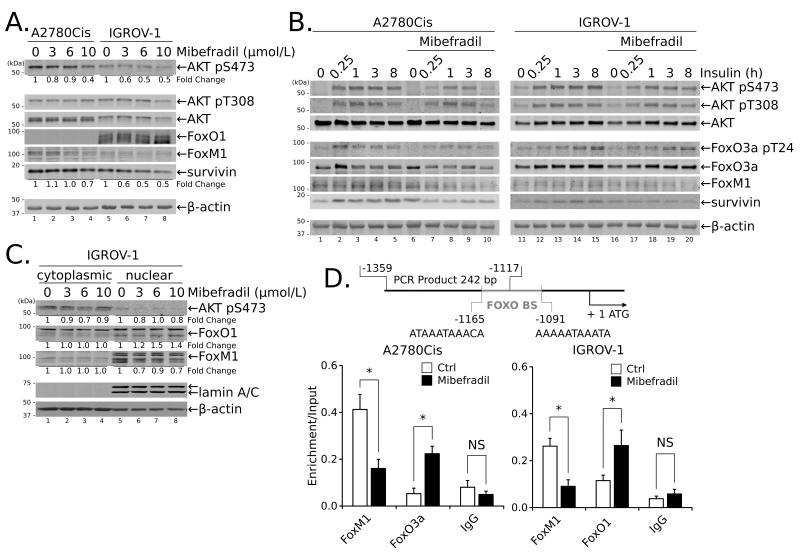
One mechanism that regulates survivin gene expression depends on forkhead box transcription factors: FoxM1 acting as an activator (36), and FOXO proteins (FoxO3a and/or FoxO1) acting as repressors (33). FOXO proteins in turn are regulated by PI3K/AKT pathway (37). To determine if T-type Ca2+ channels regulated survivin gene expression through this pathway, ovarian cancer cells were treated with increasing concentrations of Mib and examined for AKT phosphorylation and changes in expression of FoxM1 and FOXO proteins. As demonstrated in Fig. 3A, Mib modestly reduced basal AKT phosphorylation and FoxM1 protein expression in A2780Cis and IGROV-1 cells in a concentration dependent manner. The Mib-dependent reduction of FoxM1 expression in IGROV-1 cells was more obvious in the nuclear fraction (Fig. 3C). Mib also increased basal levels and nuclear retention of FoxO1 protein in IGROV-1 cells (Figs. 3A and 3C) and FoxO3a protein in A2780Cis cells (not shown). Mib had a more pronounced effect on stimulated expression. Stimulation of serum-starved A2780Cis and IGROV-1 cells with insulin (10 μg/mL) for increasing periods of time produced maximal phosphorylation of AKT in both cell lines within 15 minutes, which was maintained for 8 hours (Fig. 3B). Pretreatment with Mib (10 μmol/L for 60 minutes) delayed peak AKT phosphorylation to 1 hour and decreased the time during which it was maintained (Fig. 3B). Mib treatment inhibited insulin-stimulated AKT activity as evidenced by reduced phosphorylation of GSK-3β and PRAS40 (Supplementary Fig. 3A), direct substrates of AKT. Similarly, FoxO3a phosphorylation (A2780Cis), FoxM1 and survivin expression were reduced with Mib pretreatment of insulin-stimulated cells (Fig. 3B). Conversely, treatment with the Ser/Thr phosphatase inhibitor Calyculin A prevented the loss of AKT phosphorylation and preserved FoxM1 and survivin expression following Mib treatment (Supplementary Fig. 3B). Together, the data demonstrate that Mib suppresses activation of the PI3K/AKT pathway, resulting in increased protein expression and nuclear retention of FoxO1 and/or FoxO3a, and decreased FoxM1 protein expression, all of which have been implicated in the transcriptional suppression of BIRC5 expression.
Figure 3.
Treatment with Mib reduces survivin expression in ovarian cancer cells through disruption of PI3K/AKT and Forkhead box protein activities and sub-cellular localization. (A) A2780Cis and IGROV-1 cells were treated with the indicated concentrations of Mib for 24 hours prior to blotting for AKT phosphorylation and FoxO1 and FoxM1 protein expression. (B) Cells were treated with 10 μmol/L Mib for 1 hour followed by stimulation with 10 μg/ml insulin for up to 8 hours. (C) Subcellular localization of FoxO1 and FoxM1 was determined by blotting cytoplasmic or nuclear extracts of IGROV-1 cells treated with increasing concentrations of Mib for 24 hours. (D) A2780Cis cells or IGROV cells were treated with Mib (10 μmol/L) or DMSO for 24 hours, cross-linked and subjected to ChIP using antibodies specific for the indicated proteins; rabbit IgG served as a negative control. Enrichment in binding of specific proteins was determined based on the amplification of immunoprecipitated DNA by qPCR of the BIRC5 promoter using specific primers spanning the FOXO binding sites (FOXO BS; schematic on top). The data were normalized to the corresponding input DNA as described (see Materials and Methods) and are presented as the mean from two independent experiments ± SEM; *, P < 0.05, **, P < 0.01.
Mib inhibits BIRC5 transcription by regulating promoter occupancy
FOXO proteins regulate BIRC5 expression through direct binding to the promoter (36,38) (Fig. 3D). Suppression of BIRC5 expression is associated with loss of FoxM1, a transcriptional activator and recruitment of transcriptional repressors, including FoxO1 and FoxO3a (38), which in turn bind histone deacetylases (HDACs) to facilitate chromatin remodeling (reviewed in (20)). To investigate whether inhibition of BIRC5 expression occurs at the level of chromatin, we analyzed the binding of transcriptional regulators to the BIRC5 promoter using chromatin immunoprecipitation (ChIP) and specific antibodies for FoxM1, FoxO3a or FoxO1. Treatment of A2780Cis cells or IGROV-1 cells with Mib (10 μmol/L) resulted in a significant decrease in FoxM1 binding to the BIRC5 promoter as compared to DMSO-treated control (Fig. 3D). At the same time, Mib treatment increased specific binding of the transcriptional repressors FoxO3a (A2780Cis) or FoxO1 (IGROV-1) to the BIRC5 promoter (Fig. 3D). These data together with the previous observations that Mib treatment decreased survivin mRNA (Fig. 2C and Fig. 2D) and protein expression (Fig. 2B and Fig. 3A) support the notion that T-type Ca2+ channels regulate survivin gene expression by controlling AKT activity and binding of FoxM1/FoxO1 proteins within the BIRC5 promoter.
Mibefradil synergized with carboplatin to inhibit ovarian cancer cell growth in vitro
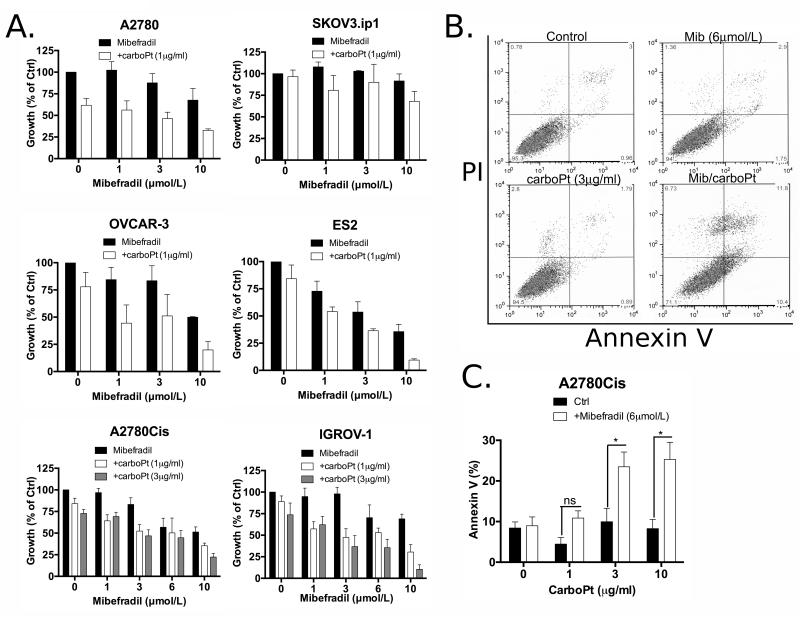
The drug-induced decrease in expression of the anti-apoptotic protein, survivin, as well as the suppression of AKT/FoxM1 and activation of FOXO signaling pathways are valid circuitry for anti-cancer drug intervention (39). For women with advanced-stage ovarian cancer, platinum resistance is one of the major obstacles impairing successful treatment. Therefore, we investigated the ability of Mib to act as sensitizing agent to carboplatin (carboPt) using selected ovarian cancer cells in vitro. Cells were treated with increasing concentrations of Mib (0-10 μmol/L) for 24h followed by incubation for another 24h in fresh media or media with the indicated concentrations of carboPt (Fig. 4A). With the exception of SKOV3.ip1, Mib alone inhibited growth of all cell lines to varying degrees (Fig. 4A). Pre-treatment of ovarian cancer cells with Mib increased the response to sub-lethal concentrations of carboPt (Table 1) in a dose-dependent manner in all cell lines with the exception of SKOV3.ip1 (Fig. 4A). Growth of A2780Cis cells was inhibited approximately 20% following treatment with either 3 μmol/L of Mib or 1-3 μg/mL of carboPt (EC50=19 ± 2.3 μg/ml, Table 1) alone; sequential treatment of Mib (3 μmol/L) followed by carboPt (1 or 3 μg/mL) resulted in 50% growth inhibition (Fig. 4A). Similarly IGROV-1 or OVCAR-3 cells treated with Mib (3 μmol/L) or carboPt (1 or 3 μg/mL) showed marginal growth inhibition (~10%), while the combination resulted in greater than 50% growth inhibition (Fig. 4A). However, A2780 cells were sensitive to carboPt (50% decrease with 1 μg/mL), which was modestly affected by pre-treatment with Mib, and ES2 cells showed a dose-dependent decrease in growth with Mib alone that was marginally affected by the addition of carboPt (Fig. 4A). To determine whether the growth inhibitory effects were synergistic or additive, the Chou-Talalay method was used to obtain combination indices (CIs, Supplementary Table 4); values lower than one indicate drug synergy, while those close to one indicate additive effects (34). The analysis indicated drug synergy for A2780Cis, IGROV-1 and OVCAR-3 cells, while the effects of Mib and carboPt were additive in SKOV-3.ip1, A2780 and ES2 cells (Supplementary Table 4). The synergistic growth inhibition induced by Mib and carboPt was likely due to an increase in apoptosis. A2780Cis cells treated with Mib and carboPt for 24h, induced a significantly high level of apoptosis (~25%) compared to the control, as measured by Annexin V-FITC/7-AAD staining (Figs. 4B and 4C) and PARP cleavage (Supplementary Figs. 4A and 4C); neither Mib (6 μmol/L) nor carboPt (0.1-30 μg/mL) alone stimulated apoptosis above control (Figs. 4B and 4C, 4E; Supplementary Figs. 4A and 4C). Mib treatment or reduced expression of CACNA1G or CACNA1H but not carboPt decreased survivin expression and no further decrease was observed when combined; however, the combination increased γH2AX (Supplementary Fig. 4), indicating an increase in DNA double strand breaks that was not observed with Mib or carboPt alone.
Figure 4.
T-type Ca2+ channel inhibitor mibefradil sensitizes ovarian cancer cells to carboplatin. (A) A2780, SKOV3.ip1, OVCAR3, ES2, A2780Cis or IGROV-1 cells were treated with the indicated concentrations of Mib for 24 hours followed by 24 hours of treatment with the indicated concentrations of carboPt, and growth was determined by CyQuant assay (for A2780, SKOV3.ip1, OVCAR-3, ES2 cells) and SRB assay (for A2780Cis, IGROV-1). Graphs represent data from ≥ two independent experiments ± SEM. (B) Representative histograms of A2780Cis cells treated for 24 hours with Mib (6 μmol/L), carboPt (3 μg/mL) or the combination of both and stained for Annexin V and PI. (C) Quantification of apoptosis induction in A2780Cis cells by the combined treatment of Mib (6 μmol/L) and carboPt (1, 3 or 10 μg/mL) as measured by Annexin V-FITC/PI staining. The percentage of apoptotic cells includes a sum of the early apoptosis (bottom right quadrant) and late apoptotic cells (top right quadrant). Graph represents data from > three independent experiments ± SEM; *, P < 0.05.
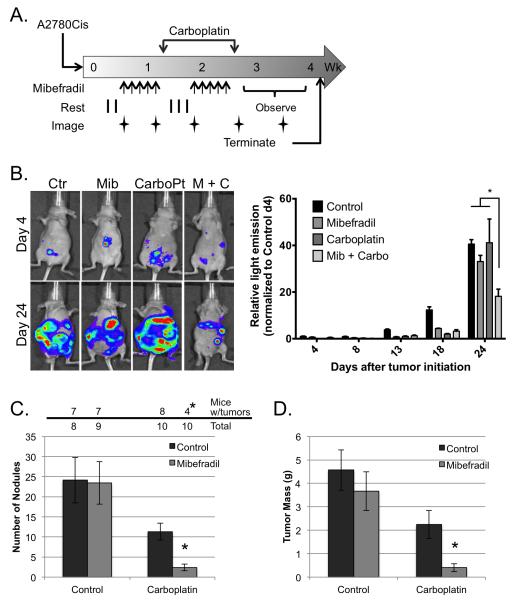
Mibefradil improved the response of peritoneal ovarian cancer growth to carboplatin in a mouse model
Intraperitoneal (IP) injection of human ovarian cancer cells into nude mice mimics many aspects of Stage III ovarian cancer including extensive peritoneal dissemination and the development of ascites. This model was employed to evaluate whether Mib sensitizes ovarian cancer cells to carboPt in vivo. Following IP injection of comparatively platinum-resistant, luciferase-expressing A2780Cis cells, mice were divided into 4 treatment arms: Mib alone, carboPt alone, Mib followed by carboPt or vehicle control. Three days after tumor initiation, mice received daily injections of Mib or vehicle for 5 days followed by a single dose of carboPt or vehicle; this schedule was repeated 3 days after the carboPt dosing (see Materials and Methods, Fig. 5A). Bioluminescent imaging was performed prior to treatment on the indicated days (Figs. 5A and 5B) and revealed an increase in tumor growth over time for all groups (Fig. 5B). However, mice that received the combination treatment showed a significant reduction in light emission compared to control or either single treatment arm (Fig. 5B). Upon necropsy, visible tumor nodules were observed throughout the peritoneal cavity and most prominently on the omentum and mesenteric fat in greater than 75% of the mice in the control or single treatment arms compared to only 40% of the mice treated with both Mib and carboPt (Fig. 5C). Mice receiving Mib plus carboPt had an average of 3 visible tumor nodules within the peritoneum compared to 24 for control or Mib alone and 11 for carboPt alone (Fig. 5C). While the number of tumor nodules in the carboPt treated mice was lower than control, the difference was not statistically significant. As an additional measure of tumor burden, the omentum, mesentery and visible tumor nodules were dissected from surrounding tissue and weighed. The tumor weight in mice receiving the dual treatment was 0.41 ± 0.17 g, which was significantly lower than control (4.56 ± 0.86) or either single treatment (Mib – 3.67 ± 0.82 and carboPt – 2.25 ± 0.59) (Fig. 5D). Again, the difference between carboPt treatment alone and control was not significant. Together, these observations demonstrate that Mib enhanced the response of relatively resistant cells to carboPt in vivo thus supporting the sensitization data observed in vitro and indicating that combination therapy might be effective in treating women with ovarian cancer.
Figure 5.
Mibefradil sensitizes human ovarian cancer cells to carboplatin in a mouse model of peritoneal metastasis. (A) Schematic representation of treatment protocol. Mice were injected with 106 A2780Cis cells (day 0), treated daily with mibefradil days 4-8 and 13-17 (arrows), and twice with carboplatin one day following the last dose of mibefradil (days 9 and 18). Whole body images of light emission were obtained on days 4, 8, 13, 18 and 24. Imaging was performed prior to administration of treatment. (B) Left panel depicts representative luminescent images from individual mice in each of the 4 treatment groups taken on days 4 and 24. Total light emission was obtained from each of 4 mice per treatment arm on the indicated days after tumor initiation; values were normalized to images of control treated mice taken on day 4 (right panel). Data represent mean ± SEM; *, P < 0.05 determined by 2-way ANOVA followed by Tukey’s multiple comparisons test. (C) Tumor incidence (top panel). The number of mice with visible tumor nodules at necropsy and the total number of mice per treatment arm are indicated. *, P < 0.05 determined with Fisher’s Exact Test. Tumor nodules (bottom panel). The number of macroscopic tumor nodules was counted from each mouse upon necropsy. Data represent the mean nodule count ± SEM; *, P < 0,05, determined by 2-way ANOVA followed by Tukey’s multiple comparisons test. (D) Tumor weight. The omentum, mesentary and any visible tumor nodules were removed from each mouse and weighed. Data represent the mean tumor weight ± SEM; *, P < 0,05, determined by 2-way ANOVA followed by Tukey’s multiple comparisons test.
Discussion
The lack of effective therapeutics for the treatment of ovarian cancer has hampered the ability to improve prognosis for women diagnosed with this disease. Cytotoxic platinum-based agents, such as cisplatin and carboplatin, are an important part of first-line therapy in patients suffering from epithelial ovarian cancer. Unfortunately, the anticancer activity of platinum agents is impaired by tumor chemoresistance, either intrinsic or acquired. In effect, over 90% of patients with advanced recurrent ovarian cancer will die because of chemotherapeutic resistance. In the current study, we found that blocking T-type Ca2+ channels with a chemical antagonist or decreasing expression with siRNA reduced proliferation, induced pro-apoptotic responses, and sensitized ovarian cancer cells to carboplatin in vitro. Most importantly, the T-type channel antagonist mibefradil enhanced antitumor activity of carboplatin in vivo.
Blocking T-type channels in ovarian cancer cells produced 1) a cytostatic, anti-proliferative effect blocking cell growth in the G1 and/or G2 phases of the cell cycle, and 2) a cytotoxic, lethal effect associated with features of apoptosis (increase in sub-G1 population and cleavage of PARP, caspase 9 and caspase 7). These effects were independent of histopathological subtype (40) or p53 status (40–42) of the cells. Growth inhibition and G1 arrest following treatment with an antagonist or transfection with siRNA targeting T-type channels have been reported (5), and induction of apoptosis has been shown in ovarian cancer cells treated with KYS05090, a structurally dissimilar T-type channel antagonist (6). However, the detailed mechanism for induction of those effects has not been elucidated. The data presented here demonstrate that blocking T-type channels induces caspase-dependent apoptosis (Fig. 2B) associated with decreased survivin mRNA and protein expression (Figs. 2B and 2C).
Survivin is thought to be one of the most important anti-apoptotic factors in cancer cells. It is often overexpressed in ovarian cancers (in more than 70% of cases (15)), and correlates with poor prognostic parameters, such as high grade, histopathological type, p53 mutation, increased proliferation and chemoresistance (13,15,16,18). Kaplan-Meier survival analysis demonstrated that the patients with tumors overexpressing survivin have a short overall survival (16). As a result, inhibitors of survivin expression have been developed and tested in clinical trials, although with limited success (43–46).
Survivin is a direct downstream target of the PI3K/AKT pathway through FoxO transcription factors. Activation of the PI3K/AKT pathway results in inactivation of FoxO-containing transcription repressor complexes and increased expression of survivin (33,47). Indeed, mibefradil treatment decreased AKT phosphorylation and increased FoxO protein levels in ovarian cancer cells (Fig. 3). Furthermore, blocking T-type Ca2+ channels decreased FoxM1 expression. FoxM1 is often overexpressed in ovarian tumors (48), and its transcriptional levels increase with tumor grade, suggesting a role in the progression of ovarian cancer (49). While blocking T-type Ca2+ channels inhibits PI3K/AKT signaling to FoxO and reduces survivin expression, the direct molecular connection between T-type channels and PI3K/AKT/FoxO pathway is still unknown and likely involves Ca2+-dependent pathways.
Inhibition of survivin expression enhances the ability of platinum agents to induce apoptosis and increase DNA double strand breaks in ovarian cancer and non-small cell lung cancer cells (43,50). The ability of mibefradil to decrease survivin expression is consistent with the notion that it primes cells for apoptosis induced by chemotherapeutic agents, such as carboplatin. Indeed, treatment of ovarian cancer cells with both carboplatin and mibefradil increased cytotoxicity and drug synergy (Fig. 4 and Supplementary Table 4). Interestingly, mibefradil and carboplatin synergistically inhibited the growth of platinum-resistant A2780Cis and IGROV-1 cells while the inhibition of platinum-sensitive A2780 cells was mildly synergistic (Fig. 4), perhaps because each agent alone is capable of inhibiting growth at low drug concentrations. While the combination had no additional effect on survivin expression, treatment with mibefradil and carboplatin increased DNA double strand breaks (Supplementary Fig. 4C). Importantly, adding mibefradil pretreatment to carboplatin-based therapy in vivo resulted in a significant reduction in xenograft tumor burden in mice harboring platinum-resistant tumors (Fig. 5). This has important implications for the treatment of ovarian cancer patients, who frequently succumb from platinum-resistant disease. Thus, the data presented here support the use of T-type Ca2+ channel blockers, either alone or together with platinum agents in the treatment of ovarian cancer patients.
Supplementary Material
Acknowledgements
This work used IVIS bioluminescence scanner in the Molecular Imaging Core, which was purchased with support from NIH grant 1S10RR025694-01, and resources from the Flow Cytometry Core Facility and Research Histology Core. All cores are supported by the University of Virginia School of Medicine and the Cancer Center.
Financial Support: This study was supported in part by the University of Virginia Women’s Oncology Research Fund, Commonwealth Foundation for Cancer Research and NCI R01 CA142783 (J.K. Slack-Davis and E.V. Casarez), George Amorino Pilot Grant from the UVA Department of Radiation Oncology (B. Dziegielewska), and Cavion LLC research grant (J. Dziegielewski).
Footnotes
Preliminary account of these studies has been presented at AACR Annual Meetings 2013 and 2015.
Disclosure of Potential Conflict of Interest: LSG is a co-founder and a consultant for Cavion LLC. JD has received a research grant and is a consultant to Cavion LLC.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287:31666–73. doi: 10.1074/jbc.R112.343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev. 2003;83:117–61. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- 5.Li W, Zhang S-L, Wang N, Zhang B-B, Li M. Blockade of T-type Ca(2+) channels inhibits human ovarian cancer cell proliferation. Cancer Invest. 2011;29:339–46. doi: 10.3109/07357907.2011.568565. [DOI] [PubMed] [Google Scholar]
- 6.Jang SJ, Choi HW, Choi DL, Cho S, Rim H-K, Choi H-E, et al. In vitro cytotoxicity on human ovarian cancer cells by T-type calcium channel blockers. Bioorg Med Chem Lett. 2013;23:6656–62. doi: 10.1016/j.bmcl.2013.10.049. [DOI] [PubMed] [Google Scholar]
- 7.Valerie NCK, Dziegielewska B, Hosing AS, Augustin E, Gray LS, Brautigan DL, et al. Inhibition of T-type calcium channels disrupts Akt signaling and promotes apoptosis in glioblastoma cells. Biochem Pharmacol. 2013;85:888–97. doi: 10.1016/j.bcp.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Dziegielewska B, Brautigan DL, Larner JM, Dziegielewski J. T-type Ca2+ channel inhibition induces p53 dependent cell growth arrest and apoptosis through activation of p38-MAPK in colon cancer cells. Mol Cancer Res. 2014;12:348–58. doi: 10.1158/1541-7786.MCR-13-0485. [DOI] [PubMed] [Google Scholar]
- 9.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–9. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 10.Altieri DC. Survivin - The inconvenient IAP. Semin Cell Dev Biol. 2015 doi: 10.1016/j.semcdb.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Q, Chen Z, Diao X, Huang S. Induction of autophagy-dependent apoptosis by the survivin suppressant YM155 in prostate cancer cells. Cancer Lett. 2011;302:29–36. doi: 10.1016/j.canlet.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Capalbo G, Dittmann K, Weiss C, Reichert S, Hausmann E, Rödel C, et al. Radiation-induced survivin nuclear accumulation is linked to DNA damage repair. Int J Radiat Oncol Biol Phys. 2010;77:226–34. doi: 10.1016/j.ijrobp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Aune G, Stunes AK, Tingulstad S, Salvesen O, Syversen U, Torp SH. The proliferation markers Ki-67/MIB-1, phosphohistone H3, and survivin may contribute in the identification of aggressive ovarian carcinomas. Int J Clin Exp Pathol. 2011;4:444–53. [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Liang L, Yan X, Liu N, Gong L, Pan S, et al. Survivin status affects prognosis and chemosensitivity in epithelial ovarian cancer. Int J Gynecol Cancer. 2013;23:256–63. doi: 10.1097/IGC.0b013e31827ad2b8. [DOI] [PubMed] [Google Scholar]
- 15.Cohen C, Lohmann CM, Cotsonis G, Lawson D, Santoianni R. Survivin expression in ovarian carcinoma: correlation with apoptotic markers and prognosis. Mod Pathol. 2003;16:574–83. doi: 10.1097/01.MP.0000073868.31297.B0. [DOI] [PubMed] [Google Scholar]
- 16.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int J Oncol. 2002;21:315–20. [PubMed] [Google Scholar]
- 17.Zaffaroni N, Pennati M, Colella G, Perego P, Supino R, Gatti L, et al. Expression of the anti-apoptotic gene survivin correlates with taxol resistance in human ovarian cancer. Cell Mol Life Sci. 2002;59:1406–12. doi: 10.1007/s00018-002-8518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rödel F, Sprenger T, Kaina B, Liersch T, Rödel C, Fulda S, et al. Survivin as a prognostic/predictive marker and molecular target in cancer therapy. Curr Med Chem. 2012;19:3679–88. doi: 10.2174/092986712801661040. [DOI] [PubMed] [Google Scholar]
- 19.Altieri DC. Targeting survivin in cancer. Cancer Lett. 2013;332:225–8. doi: 10.1016/j.canlet.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boidot R, Végran F, Lizard-Nacol S. Transcriptional regulation of the survivin gene. Mol Biol Rep. 2014;41:233–40. doi: 10.1007/s11033-013-2856-0. [DOI] [PubMed] [Google Scholar]
- 21.Nogueira-Ferreira R, Vitorino R, Ferreira-Pinto MJ, Ferreira R, Henriques-Coelho T. Exploring the role of post-translational modifications on protein-protein interactions with survivin. Arch Biochem Biophys. 2013;538:64–70. doi: 10.1016/j.abb.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 22.Stauber RH, Mann W, Knauer SK. Nuclear and cytoplasmic survivin: molecular mechanism, prognostic, and therapeutic potential. Cancer Res. 2007;67:5999–6002. doi: 10.1158/0008-5472.CAN-07-0494. [DOI] [PubMed] [Google Scholar]
- 23.Ning Y, Li Q, Xiang H, Liu F, Cao J. Apoptosis induced by 7-difluoromethoxyl-5,4′-di-n-octyl genistein via the inactivation of FoxM1 in ovarian cancer cells. Oncol Rep. 2012;27:1857–64. doi: 10.3892/or.2012.1739. [DOI] [PubMed] [Google Scholar]
- 24.Jiang L, Cao X-C, Cao J-G, Liu F, Quan M-F, Sheng X-F, et al. Casticin induces ovarian cancer cell apoptosis by repressing FoxM1 through the activation of FOXO3a. Oncol Lett. 2013;5:1605–10. doi: 10.3892/ol.2013.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obexer P, Hagenbuchner J, Unterkircher T, Sachsenmaier N, Seifarth C, Böck G, et al. Repression of BIRC5/survivin by FOXO3/FKHRL1 sensitizes human neuroblastoma cells to DNA damage-induced apoptosis. Mol Biol Cell. 2009;20:2041–8. doi: 10.1091/mbc.E08-07-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakrabarty A, Bhola NE, Sutton C, Ghosh R, Kuba MG, Dave B, et al. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 2013;73:1190–200. doi: 10.1158/0008-5472.CAN-12-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Zheng F, Xing H, Gao Q, Wei W, Lu Y, et al. Resistance to chemotherapy-induced apoptosis via decreased caspase-3 activity and overexpression of antiapoptotic proteins in ovarian cancer. J Cancer Res Clin Oncol. 2004;130:423–8. doi: 10.1007/s00432-004-0556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Church DN, Talbot DC. Survivin in solid tumors: rationale for development of inhibitors. Curr Oncol Rep. 2012;14:120–8. doi: 10.1007/s11912-012-0215-2. [DOI] [PubMed] [Google Scholar]
- 29.Mariot P, Vanoverberghe K, Lalevee N, Rossier MF, Prevarskaya N. Overexpression of an alpha 1H (Cav3.2) T-type calcium channel during neuroendocrine differentiation of human prostate cancer cells. J Biol Chem. 2002;277:10824–33. doi: 10.1074/jbc.M108754200. [DOI] [PubMed] [Google Scholar]
- 30.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–22. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 31.Zhu N, Gu L, Findley HW, Chen C, Dong J-T, Yang L, et al. KLF5 Interacts with p53 in regulating survivin expression in acute lymphoblastic leukemia. J Biol Chem. 2006;281:14711–8. doi: 10.1074/jbc.M513810200. [DOI] [PubMed] [Google Scholar]
- 32.Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guha M, Plescia J, Leav I, Li J, Languino LR, Altieri DC. Endogenous tumor suppression mediated by PTEN involves survivin gene silencing. Cancer Res. 2009;69:4954–8. doi: 10.1158/0008-5472.CAN-09-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 35.Mishra SK, Hermsmeyer K. Selective inhibition of T-type Ca2+ channels by Ro 40-5967. Circ Res. 1994;75:144–8. doi: 10.1161/01.res.75.1.144. [DOI] [PubMed] [Google Scholar]
- 36.Wang I-C, Chen Y-J, Hughes D, Petrovic V, Major ML, Park HJ, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25:10875–94. doi: 10.1128/MCB.25.24.10875-10894.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 38.Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle. 2009;8:2708–10. doi: 10.4161/cc.8.17.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Wang Y, Wang Y, Yin X, He Y, Chen L, et al. FOXM1 modulates cisplatin sensitivity by regulating EXO1 in ovarian cancer. PLoS ONE. 2014;9:e96989. doi: 10.1371/journal.pone.0096989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126. doi: 10.1038/ncomms3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muscolini M, Montagni E, Caristi S, Nomura T, Kamada R, Di Agostino S, et al. Characterization of a new cancer-associated mutant of p53 with a missense mutation (K351N) in the tetramerization domain. Cell Cycle. 2009;8:3396–405. doi: 10.4161/cc.8.20.9910. [DOI] [PubMed] [Google Scholar]
- 42.Yaginuma Y, Westphal H. Abnormal structure and expression of the p53 gene in human ovarian carcinoma cell lines. Cancer Res. 1992;52:4196–9. [PubMed] [Google Scholar]
- 43.Mir R, Stanzani E, Martinez-Soler F, Villanueva A, Vidal A, Condom E, et al. YM155 sensitizes ovarian cancer cells to cisplatin inducing apoptosis and tumor regression. Gynecol Oncol. 2014;132:211–20. doi: 10.1016/j.ygyno.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Lewis KD, Samlowski W, Ward J, Catlett J, Cranmer L, Kirkwood J, et al. A multi-center phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Invest New Drugs. 2011;29:161–6. doi: 10.1007/s10637-009-9333-6. [DOI] [PubMed] [Google Scholar]
- 45.Kelly RJ, Thomas A, Rajan A, Chun G, Lopez-Chavez A, Szabo E, et al. A phase I/II study of sepantronium bromide (YM155, survivin suppressor) with paclitaxel and carboplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2013;24:2601–6. doi: 10.1093/annonc/mdt249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiechno P, Somer BG, Mellado B, Chłosta PL, Cervera Grau JM, Castellano D, et al. A randomised phase 2 study combining LY2181308 sodium (survivin antisense oligonucleotide) with first-line docetaxel/prednisone in patients with castration-resistant prostate cancer. Eur Urol. 2014;65:516–20. doi: 10.1016/j.eururo.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 47.Dansen TB, Burgering BMT. Unravelling the tumor-suppressive functions of FOXO proteins. Trends Cell Biol. 2008;18:421–9. doi: 10.1016/j.tcb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 48.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Llauradó M, Majem B, Castellví J, Cabrera S, Gil-Moreno A, Reventós J, et al. Analysis of gene expression regulated by the ETV5 transcription factor in OV90 ovarian cancer cells identifies FOXM1 overexpression in ovarian cancer. Mol Cancer Res. 2012;10:914–24. doi: 10.1158/1541-7786.MCR-11-0449. [DOI] [PubMed] [Google Scholar]
- 50.Iwasa T, Okamoto I, Takezawa K, Yamanaka K, Nakahara T, Kita A, et al. Marked anti-tumour activity of the combination of YM155, a novel survivin suppressant, and platinum-based drugs. Br J Cancer. 2010;103:36–42. doi: 10.1038/sj.bjc.6605713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.