Abstract
Considerable strides have been made over the past 20 years in our understanding of the ligands, receptor subtypes, signal transduction mechanisms and biological actions comprising the endocannabinoid system. From the ever-expanding number of studies that have been conducted during this time, it has become increasingly clear that sex differences are the cornerstone of cannabinoid-regulated biology. Available evidence has demonstrated that these sex differences endure in the absence of gonadal steroids, and are modulated by the acute, activational effects of these hormones. This review focuses on select aspects of sexually differentiated, cannabinoid-regulated biology, with a particular emphasis on the control of energy balance. It is anticipated that it will lend impactful insight into the pervasive and diverse disparities in how males and females respond to cannabinoids – from the organismal level down to the molecular level. Additionally, it will furnish a newfound appreciation for the need to recalibrate our thinking in terms of how cannabinoids are used as therapeutic adjuvants for a broad range of clinical disorders and associated comorbidities, including body wasting and obesity.
Keywords: cannabinoid, sex difference, estradiol, testosterone, AMP-activated protein kinase, glutamate, retrograde signaling, energy balance, cachexia, obesity
1. Introduction
The marijuana plant (i.e., cannabis sativa, cannabis indica) has played a diversified role in many cultures and societies over the millennia. It has been used for purposes ranging from the textile to the religious to the medicinal (Hamarneh, 1972; Mikuriya, 1969; Touw, 1981). The medicinal properties of cannabis have been leveraged through the ages by the Indians, Chinese, Greeks, Assyrians, Persians, Egyptians, Algerians, Moroccans, Hindus, Buddhists, Jews and Muslims as an analgesic, anesthetic, appetite stimulant and euphoriant (Brunner, 1973; Hamarneh, 1972; Hindmarch, 1972; Mikuriya, 1969; Touw, 1981; Winek, 1977). Cannabis was introduced to Western medicine in the 19th century (Mikuriya, 1969), and various formulations could be found in the Materia Medica of the U.S. Pharmacopoeia up until 1941; removed only after Congress passed the Marihuana Tax Act in 1937 (Brunner, 1973; Mikuriya, 1969; Winek, 1977).
Of the 60 or so cannabinoid compounds found in cannabis, the primary psychotropic constituent is Δ9-tetrahydrocannabinol (THC) (Mechoulam and Gaoni, 1965). THC binds two Gi/o-coupled receptors, each with seven transmembrane-spanning domains arranged in a serpentine fashion – namely, the cannabinoid CB1 and CB2 receptors (Gérard et al., 1991; Matsuda et al., 1990; Munro et al., 1993). More recently, it has been shown that THC activates a third G protein-coupled receptor, GPR55, that is apparently involved in the regulation of gastrointestinal motility, insulin secretion and adiposity (Lauckner et al., 2008; Lin et al., 2011; Moreno-Navarrete et al., 2012; Romero-Zerbo et al., 2011). Another major cannabinoid, cannabidiol (CBD), is found in relative abundance in cannabis sativa (Hillig and Mahlberg, 2004). It binds to CB1 and CB2 receptors with comparatively lower affinity than does THC, and is an antagonist at the GPR55 receptor (Matsuda et al., 1990; Munro et al., 1993; Ross, 2009). This pharmacological profile may help explain the ability of CBD to ameliorate encephalitis, enteritis and pancreatitis (Li et al., 2013; Lin et al., 2011). The two principle endogenous cannabinoids, anandamide and 2-arachidonoyl glycerol (2-AG), are derived from arachidonic acid and act as agonists at CB1 receptors (Devane et al., 1992; Ishac et al., 1996; Yoshida et al., 2006) and, in the case of the latter, CB2 receptors (Van Sickle et al., 2005). There is also evidence that L-α-lysophosphatidylinositol acts as an endogenous agonist at the GPR55 receptor (Moreno-Navarrete et al., 2012).
As can be inferred from the above two paragraphs, the array of biological processes regulated by the endocannbinoid system is quite diverse. It ranges from the regulation of pain processing to energy balance to inflammation, although this is by no means exhaustive. A striking feature about cannabinoid-regulated biology is the degree of sexual disparity between males and females. This review will cover recent advances in our understanding of sex differences in: 1) the metabolic disposition of cannabinoids, 2) cannabinoid abuse, 3) cannabinoid-induced antinociception, and 4) cannabinoid-induced changed in energy homeostasis, with a particular emphasis on the latter. It is anticipated that this review will impart new insights into the diversity of sex differences in, and gonadal steroid hormonal influences on, cannabinoid-regulated biology. Moreover, it should provide a newfound appreciation for how gender and endocrine status can guide decisions on whether or when to use cannabinoid ligands as therapeutic adjuncts for the treatment of conditions ranging from HIV/AIDS- or cancer-related cachexia to obesity.
2. Sex Differences in Cannabinoid Metabolism
Exogenous cannabinoids like THC are metabolized primarily by the liver. In male rats, THC is transformed in vivo either by hydroxylation at, in order of prevalence, the 11-, 8- and 3-carbon positions of the dibenzopyran backbone. However, 11-OH-THC is the predominant metabolite produced in female rats, followed by lesser amounts of THC-11-oic acid and 8α, 11-diOH-THC (Narimatsu et al., 1991). Liver microsomes prepared from female rats also reveal the existence of a metabolite with an oxidized methyl group at the 9-carbon position, known as 9α, 10α-epoxyhexahydrocannabinol. This compound is second only to 11-OH-THC in abundance, but represents only a minor component of the metabolic profile for THC in males (Narimatsu et al., 1991). In addition, the ability of microsomal aldehyde oxygenase to convert substrates like 11-oxo-THC and 9-anthraldehyde to their corresponding carboxylic acids is sexually differentiated. Slightly lower activity is observed for the metabolism of 11-oxo-THC (per total amount of cytochrome P450), and more robust activity is seen for the metabolism of 9-anthraldehyde, in female mice than in their male counterparts (Watanabe et al., 1992). Moreover, the activity of alcohol oxygenase that converts 7-OH-THC to 7-oxo-THC is higher in female guinea pigs than in males (Matsunaga et al., 1997). The converse is true for rats, in which the activity of the microsomal alcohol oxygenase is ~3× higher in males than in females (Matsunaga et al., 2000). Finally, CBD is converted by guinea pig liver microsomes to 7-OH, 6-OH and 4-OH variants (Yamamoto et al., 1991). CBD also undergoes hepatic conversion to cannabielsoin in several rodent species, and the male rat microsomal production of this metabolite is over twice the rate as that observed in females (Yamamoto et al., 1991). A listing of the cannabinoid agonists, antagonists and metabolites discussed throughout this review can be found in Table 1.
Table 1.
A list of the cannabinoid agonists, antagonists and metabolites described in this review.
| Compound | Abbreviation/Nickname | Function/Purpose |
|---|---|---|
| 2-arachidonoyl glycerol | 2-AG | Endogenous CB1/2 receptor agonist |
| Δ9-tetrahydrocannabinol | THC | Cannabinoid CB1/2 receptor agonist |
| 7-hydroxy-THC | 7-OH-THC | THC metabolite |
| 7-oxo-THC | N/A | THC metabolite |
| 8α, 11-dihydroxy-THC | 8α, 11-diOH-THC | THC metabolite |
| 9α, 10α-epoxyhexahydrocannabinol | N/A | THC metabolite |
| 11-hydroxy-THC | 11-OH-THC | Primary THC metabolite |
| Cannabidiol | CBD | Cannabinoid receptor ligand |
| 4-hydroxy-CBD | 4-OH-CBD | CBD metabolite |
| 6-hydroxy-CBD | 6-OH-CBD | CBD metabolite |
| 7-hydroxy-CBD | 7-OH-CBD | CBD metabolite |
| Cannabielsoin | N/A | CBD metabolite |
| CP 55,940 | N/A | CB1/2 receptor agonist |
| L-α-lysophosphatidylinositol | N/A | GPR55 agonist |
| Marinol (dronabinol) | N/A | Oral THC formulation |
| N-arachidonoylethanolamine | Anandamide | Endogenous CB1 receptor agonist |
| Rimonabant | N/A | CB1 receptor antagonist |
| THC-11-oic acid | N/A | THC metabolite |
With regard to endogenous cannabinoids, adolescent female rats exhibit higher levels of fatty acid amide hydrolase (FAAH; responsible for anandamide degradation) in the frontal cortex of the brain, and lower levels of monoglycerol lipase (MAGL; responsible for 2-AG degradation) in the ventral striatum and amygdala, than do their male counterparts (Marco et al., 2014). The levels of these enzymes are influenced by disruptive early life events in a sexually differentiated fashion. Indeed, maternal deprivation increased their expression in the frontal cortex of adolescent male rats, whereas it increased their expression in the hippocampus of adolescent female rats (Marco et al., 2014). The studies described above utilized gonadally intact animals, and so at this point the organizational and activational roles of gonadal steroid hormones in sexually disparate cannabinoid metabolism remain undefined.
3. Sex Differences in Cannabinoid Abuse
Data from early clinical studies in humans dating back to the 1970s indicated that men consume cannabis at a faster rate than women. Perez-Reyes and coworkers reported that they take more puffs per unit time, and exhibit a shorter interval between puffs (Perez-Reyes et al., 1981). This pattern of consumption resulted in higher THC levels in men than in women. Interestingly, the subjective, psychological effects reported by the male and female subjects were nearly identical, which the authors attributed to the men having a larger volume of distribution than the women (Perez-Reyes et al., 1981). On the other hand, Penetar and colleagues demonstrated that men were more sensitive to the cardiovascular effects of cannabis inhalation (i.e., increased heart rate), which was mirrored by subjective behavioral ratings of cannabis intoxication as assessed through visual analog scales and the Addiction Research Center Inventory (Penetar et al., 2005). These effects were further enhanced by nicotine pre-treatment (Penetar et al., 2005). In more contemporary studies in Europe, Swedish men were much more likely to be apprehended for driving under the influence of cannabis than women, and had higher circulating levels of THC in samples taken at the time of arrest as well (Jones et al., 2008). Likewise, in France, more men presented to the emergency department over a two-year period ranging from 2009–2011 for mental and behavioral disturbances related to cannabinoid intoxication than did women (Le Querrec et al., 2015). Moreover, in the United States, males are more likely to use the emergently popular, synthetic cannabinoid formulation K2 (a.k.a., spice) than women (Hu et al., 2011). By contrast, no sex differences were reported for cannabis-induced impairment in simulated driving performance (Anderson et al., 2010). In addition, women appear to be more sensitive to cannabinoidinduced exacerbation of the postural syncope associated with the transition from the reclined to the standing position (Mathew et al., 2003). This is in keeping with recent trends observed with other drugs of abuse. Indeed, women currently exhibit a more rapid progression from first cannabis use to cannabinoid use disorder, and provide heightened, subjective behavioral ratings in response to cannabis that are associated with abuse liability (Cooper and Haney, 2014; Hernandez-Avila et al., 2004). The findings of Lundahl and Greenwald demonstrated that men self-report as feeling “down” when exposed to cannabis-associated cues, whereas women exhibit reduced compulsivity as assessed by the Marijuana Craving Questionaire following oral THC ingestion (Lundahl and Greenwald, 2015). Moreover, while men exhibit greater stability in the duration of the dependence episodes across their adult lifespan (Duncan et al., 2015), women are at greater risk for commencing cannabis use at younger ages and engaging in high-frequency alcohol and marijuana use that ultimately leads to a subsequent diagnosis of alcohol use disorder (Buu et al., 2014). These more recent findings are consistent with animal studies showing that female rats self-administer higher amounts of the cannabinoid receptor agonist WIN 55,212-2, and more rapidly acquire stable intake than do males (Fattore et al., 2007). Unfortunately, the clinical studies were not designed to assess the endocrine status of the participants, and thus there is no information on the potential organizational and/or activational effects of gonadal steroids in the sex differences described for cannabinoid abuse. However, estradiol reduces CB1 receptor binding in the prefrontal cortex and amygdala of ovariectomized rats; areas that are involved in the pathophysiologic sequelae of addiction (Castelli et al., 2014). It also increases locomotor activity and reduces prepulse inhibition of the startle reflex; behavioral effects suggestive of a greater vulnerability to cannabis addiction than that seen in males (Castelli et al., 2014).
4. Sex Differences in Cannabinoid-Induced Antinociception
Cannabinoid receptor agonists produce antinociception by altering neurotransmission within spinal and supraspinal pain-processing circuits (Hama and Sagen, 2011; Stein et al., 1996; Vaughan et al., 2000; Wallace et al., 2003). In addition, cannabinoid receptor agonists are effective and well tolerated when used to treat pain arising from a number of different etiologies (Lynch et al., 2006; Lynch and Ware, 2015; Toth et al., 2012; Woolridge et al., 2005). Interestingly, female rats are more sensitive to the cannabinoid receptor agonist-induced increase in the latency to withdraw the tail or paw in response to thermal or mechanical stimuli, respectively (Tseng and Craft, 2001). This sex difference involves the heightened conversion of THC in females to bioactive metabolites like the 11-OH-THC discussed earlier (Narimatsu et al., 1991; Tseng et al., 2004). Other contributing factors to these sex differences include the activation of both CB1 and CB2 receptors in females, whereas in males only the CB1 receptors contribute (Craft et al., 2012). The enhanced responsiveness observed in females extends to the development of antinociceptive tolerance upon chronic treatment with THC (Wakley et al., 2014b). Similarly, females are more sensitive, via both CB1 and CB2 receptors, to the ability of THC to counter the allodynia and hyperalgesia observed after a week-long administration of Freund’s adjuvant to the right hind paw than males are via activation of only CB1 receptors (Craft et al., 2013). On the other hand, Niu and coworkers showed using an orofacial myositis pain model that female rats required an ~30× higher dose of the CB1 receptor agonist arachidonylcyclopropylamide to produce the same reduction in mechanical hypersensitivity of the masseter muscle as seen in the male (Niu et al., 2012). Moreover, the antinociceptive efficacy of cannabinoids and other analgesic drugs is markedly attenuated in G protein-gated, inwardly-rectifying K+ channel (GIRK)2-null mice, and this diminution is more prominent in males than in females (Blednov et al., 2003). The activation of GIRK channels produces a slow inhibitory postsynaptic potential that hyperpolarizes the postsynaptic cell membrane to cause a cessation in neuronal firing (Hille, 1992). CB1 receptors couple to GIRK channels in AtT20 cells, Xenopus oocytes, and in hypothalamic neurons of male but not female guinea pigs (Farhang et al., 2009; Henry and Chavkin, 1995; Mackie et al., 1995). Thus, the more prevalent coupling of CB1 receptors to GIRK channels in neurons within the pain-processing circuitry of males may help explain the disparities in the decline of antinociceptive efficacy observed between male and female GIRK2-null mice.
The sex difference in cannabinoid-induced antinociception persists after gonadectomy (Wakley et al., 2015), but is also susceptible to the activational effects of gonadal steroids. For example, estradiol potentiates the THC-induced increase in the latency to react to noxious mechanical stimuli, an effect mirrored when comparing the responses seen in diestrus vs. estrus (Craft and Leitl, 2008; Wakley et al., 2014a). Likewise, levels of pain-regulating endocannabinoids in the female rat have been shown to fluctuate over the course of the estrous cycle in several brain regions (e.g., hypothalamus, hippocampus) and the pituitary gland; with the biggest changes occurring around the time of ovulation and behavioral estrus (Bradshaw et al., 2006). On the other hand, data from the orofacial myositis pain model indicate that testosterone promotes cytokine-induced upregulation of CB1 receptor expression in the trigeminal ganglia of male rats (Niu et al., 2012). By contrast, ovariectomy enhances cannabinoid-induced antinociception in mice, which is completely reversed by estradiol replacement (Anaraki et al., 2008). This discrepancy is indicative of a species difference in the cannabinoid regulation of pain processing, which has implications as to whether or how these data pertain to the human condition. It is also reflective of an issue that will arise again when discussing cannabinoid effects on energy balance. Lastly, the development of antinociceptive tolerance is not dependent on the activational effects of gonadal steroids, as the blunted THC response seen in chronically treated, gonadally intact rats is indistinguishable from that seen in gonadectomized or gonadectomized, steroid-treated animals (Wakley et al., 2015).
5. Sex Differences in the Cannabinoid Regulation of Energy Homeostasis
It is well accepted that the cannabinoid regulation of energy balance involves coordinated interactions between the gut, liver, pancreas, adipose tissue and the brain (Borgquist and Wagner, 2013). Cannabinoids regulate gastrointestinal motility and secretion (Borgquist and Wagner, 2013), and in humans dronabinol reduces gastric emptying to a greater extent in women than it does in men (Esfandyari et al., 2006). Within the hypothalamus of the brain, there are a number of neuroanatomical substrates through which cannabinoids can influence the feeding circuitry. The hypothalamic feeding circuitry comprises orexigenic and anorexigenic components spread amongst several different hypothalamic nuclei (for review see (Viveros et al., 2011a). Orexigenic components can be found in the form of orexin- and melanin-concentrating hormone-containing somata in the lateral hypothalamic area, as well as neuropeptide Y (NPY)-/agouti-related peptide (AgRP)- and ghrelin-containing somata in the hypothalamic arcuate nucleus (ARC). Anorexigenic elements of the hypothalamic feeding circuitry include the ventromedial nucleus that, when lesioned, results in rampant hyperphagia and obesity (Stricker, 1978). The steroidogenic factor (SF)-1-containing neurons in the VMN provide a principal source of anorexigenic output from this region (Dhillon et al., 2006). The ARC contains another critical appetite-suppressing cell population known as the proopiomelanocortin (POMC) neurons. The POMC precursor peptide is posttranslationally modified to yield two neuropeptides involved in the regulation of energy balance – namely, α-melanocyte-stimulating hormone and β-endorphin. The vast majority of POMC neurons co-express another anorexigenic neuropeptide called cocaine- and amphetamine-regulated transcript. All of these afferent elements of the hypothalamic feeding circuitry project to, and make synaptic contact with, parvocellular corticotropin-releasing hormone (CRH) neurons in the hypothalamic paraventricular nucleus. This indicates that these CRH neurons represent a point of convergence and integration that is important for the generation of an efferent response (for review see (Viveros et al., 2011a). The focal administration of CB1 receptor agonists into hypothalamic nuclei such as the PVN and VMN increases energy intake (Jamshidi and Taylor, 2001; Verty et al., 2005). Along similar lines, the levels of hypothalamic endocannabinoids are influenced by peripheral appetite-regulating hormones. For example, anorexigenic leptin decreases the amounts of anandamide and 2-AG (Di Marzo et al., 2001), whereas orexigenic ghrelin increases them (Kola et al., 2008).
The first demonstration of sex differences in the cannabinoid regulation of energy intake came from Miller and coworkers, where they showed that fourth ventricular administration of the cannabinoid receptor agonist CP 55,940 stimulates the consumption of sweetened condensed milk in male rats at a 10× lower dose than that observed in females (Miller et al., 2004). This was corroborated several years later using our guinea pig animal model, when we showed the cannabinoid receptor agonist WIN 55,212-2 stimulated cumulative energy intake to a greater extent in orchidectomized males than it did in ovariectomized females (Diaz et al., 2009). This was associated with sexually disparate changes in meal pattern; with males exhibiting cannabinoid-induced increases in meal size, meal frequency and meal duration, and females exhibiting an increase in meal frequency only (Diaz et al., 2009). In addition, males are more sensitive to the hypothermic effects of cannabinoid receptor agonists; exhibiting an ~0.5 °C greater reduction in core body temperature in response to WIN 55,212-2 than did females (Diaz et al., 2009). We have resolved these sex differences in the cannabinoid regulation of energy homeostasis down to the level of the hypothalamic feeding circuitry, where the pleiotropic actions of cannabinoids inhibit the excitability of anorexigenic POMC neurons in a sexually discrepant manner. In POMC neurons from orchidectomized male guinea pigs, CB1 receptor agonists activate postsynaptic GIRK channels that cause an outward current and hyperpolarization that, in turn, cause a cessation in neuronal firing (Ho et al., 2007). In POMC neurons from ovariectomized female guinea pigs, CB1 receptors couple to postsynaptic Kv4.2 channels underlying A-type K+ currents (IA). This coupling augments the IA by causing a rightward shift in the inactivation curve, which lessens the magnitude of the hyperpolarization necessary to transition the Kv4.2 channels from the inactivated to the closed state, and increases the number of channels available to oppose a subsequent depolarizing stimulus (Tang et al., 2005). This, in turn, slows the rate of rise of the ramp-like depolarization toward threshold, increases the interspike interval, and reduces firing rate.
Cannabinoids also exert presynaptic effects on excitatory and inhibitory inputs impinging on POMC neurons. CB1 receptor agonists are equipotent in their ability to presynaptically inhibit glutamatergic input onto POMC neurons from gonadectomized male and female guinea pigs (Diaz et al., 2009). However, they presynaptically inhibit GABAergic input with a ~6× lower potency in POMC neurons from orchidectomized male guinea pigs than they do in those from ovariectomized female guinea pigs (Diaz et al., 2009). It therefore stands to reason that cannabinoids acutely enhance inhibitory tone onto POMC neurons to a greater extent in males than they do in females. This would be consistent with the findings of Corchero and co-workers, who demonstrated that chronically administered THC increased POMC gene expression more robustly in male rats than in female rats (Corchero et al., 2001).
Sex differences in the cannabinoid regulation of energy balance persist in the absence of gonadal steroids, and are forged by organizational effects of these hormones. However, we have compelling evidence that activational effects also play an important role in this process. Both estradiol in the female and testosterone in the male are ideally suited to influence various neuroanatomical substrates comprising the hypothalamic feeding circuitry. For example, estradiol increases POMC gene expression and β-endorphin secretion in rodents and primates (Cheung and Hammer, 1995; Ferin et al., 1984; Roepke et al., 2008), and decreases the gene expression and secretion of NPY from N-38, mHypoE-42 and mHypoA-2/12 cell lines (Dhillon and Belsham, 2011; Titolo et al., 2008). Estradiol replacement in ovariectomized females reduces energy intake per se, and rapidly and markedly attenuates the increase in energy intake caused by WIN 55,212-2, and also reduces the magnitude and duration of the agonistinduced hypothermia (Kellert et al., 2009). Again, this can be drilled down to the level of the hypothalamic feeding circuitry, where estradiol rapidly diminishes the endocannabinoid-mediated, retrograde inhibition of glutamatergic input onto POMC neurons as assessed through depolarization-induced suppression of excitation (DSE), as well as the enhancement of the IA in these cells (Borgquist et al., 2015a; Kellert et al., 2009).
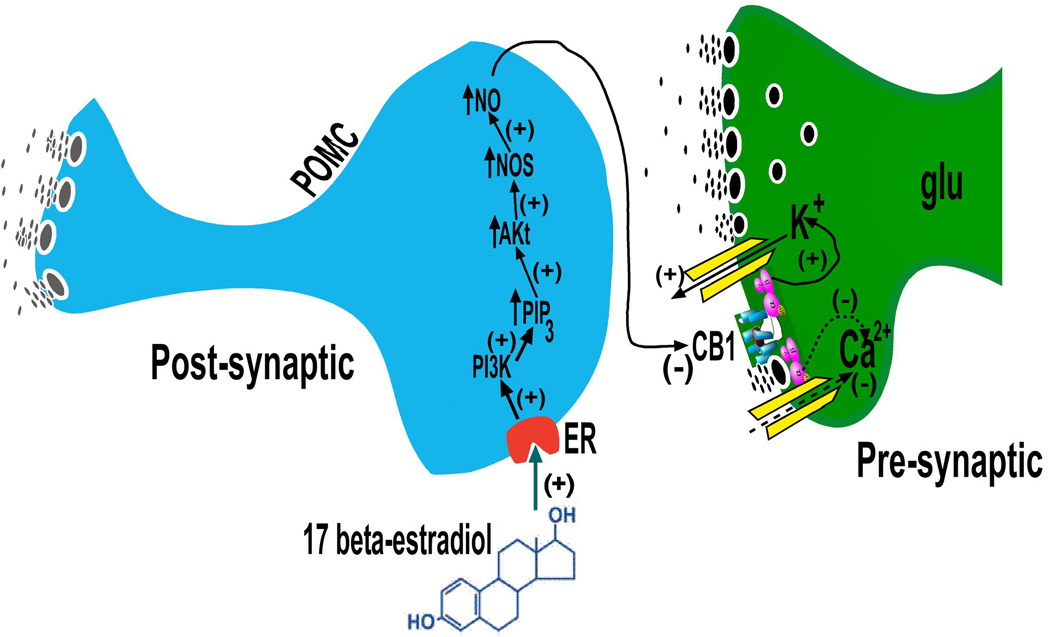
This estrogenic disruption in endocannabinoid signaling is due to activation of estrogen receptor (ER)α and the putative Gq-coupled membrane ER (Gq-mER) (Washburn et al., 2013). Activation of these receptors by estradiol elicits a signal transduction cascade involving phosphatidylinositol-3-kinase (PI3K), protein kinase C (PKC), protein kinase A (PKA) and neuronal nitric oxide synthase (nNOS) to unravel the coupling of the presynaptic CB1 receptors to their effector systems (Borgquist et al., 2015a; Mela et al., 2015; Washburn et al., 2013) (Figure 1). PI3K is a signaling molecule that is activated upon both ERα and ERβ stimulation (Gingerich and Krukoff, 2008; Smith et al., 2013), as well as by other anorexigenic hormones such as leptin and insulin (Hill et al., 2008; Mela et al., 2015; Qiu et al., 2014), within various elements of the hypothalamic feeding circuitry. PI3K signaling in murine POMC neurons plays an important role in the ERα-mediated regulation of appetite and insulin sensitivity (Zhu et al., 2015). It, along with PKC and PKA, are also involved in the Gq-mER-mediated uncoupling of other metabotropic, Gi/o-linked receptors like the μ-opioid and GABAB receptors from their GIRK channels in POMC neurons (Kelly et al., 2002; Malyala et al., 2008; Qiu et al., 2006). Similarly, nNOS is a signaling molecule that is located downstream of PI3K (Gingerich and Krukoff, 2008; Haynes et al., 2003), decreased by fasting, and activated by leptin to help carry out, at least in part, the effects of the adipostat on energy homeostasis (Leshan et al., 2012; Otukonyong et al., 2000). Collectively, these findings are largely consistent with the fact that estradiol downregulates CB1 receptors in the rat hypothalamus (Riebe et al., 2010).
Figure 1.
Schematic illustration of how estradiol impairs CB1 receptor-mediated signaling at glutamatergic inputs onto POMC neurons. Estrogens rapidly uncouple cannabinoid CB1 receptors from their effector systems in the glutamatergic nerve terminal by activating nNOS. This is achieved through an increase in PI3K, which enhances phosphatidylinositol triphosphate (PIP3), in turn up-regulating Akt leading to augmented nNOS. The increased enzyme activity may reduce endocannabinoid tone either by inhibiting synthesis, enhancing breakdown, or promoting reuptake and removal from the synaptic cleft. Alternatively, the nitric oxide produced may act retrogradely to uncouple the CB1 receptor from its effector system(s) in the glutamatergic nerve terminal. Modified from (Mela et al., 2015).
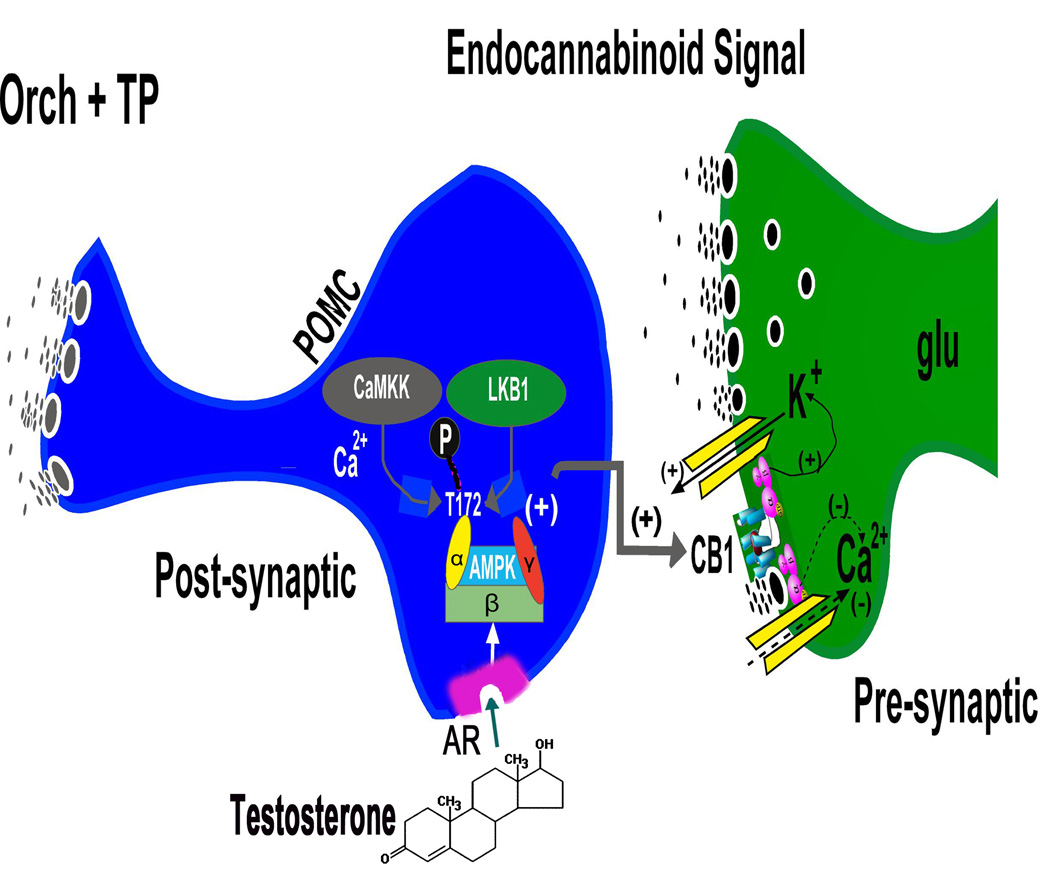
Conversely, testosterone replacement in orchidectomized males increases energy intake per se, an effect that is blocked by the CB1 receptor antagonist AM251 (Borgquist et al., 2015b). This effect can be attributed to a testosterone-induced increase in inhibitory GABAergic input onto POMC neurons, as well as a potentiation of endocannabinoid-mediated retrograde inhibition of glutamatergic input via DSE (Borgquist et al., 2015b). Testosterone increases the phosphorylation of AMP-activated kinase (AMPK) in the ARC (Borgquist et al., 2015b). Moreover, the testosterone-induced potentiation of DSE is blocked by the AMPK inhibitor compound C, and mimicked by AMPK activator metformin during recordings in slices from vehicle-treated males (Borgquist et al., 2015b), which indicates that AMPK is a signaling molecule integral to the androgenic regulation of endocannabinoid-mediated retrograde inhibition and thus energy homeostasis (Figure 2). AMPK is a cellular energy sensor, whose activity is increased by phosphorylation of the threonine residue at the 172nd position of the catalytic α subunit via liver kinase β1 or Ca2+/calmodulin-activated protein kinase kinase β (Hardie et al., 2012). This occurs in response to elevated AMP:ATP and ADP:ATP ratios brought on by metabolic stresses and a negative energy balance (Hardie et al., 2012). This triggers biochemical and behavioral processes that ultimately lead to increased production of ATP. In addition, the activity of AMPK within the hypothalamus is stimulated by orexigenic hormones like ghrelin (Andersson et al., 2004; Kola et al., 2008) and inhibited by anorexigenic hormones like leptin (Andersson et al., 2004; Minokoshi et al., 2004). Thus, testosterone, by virtue of activating AMPK in the ARC within the hypothalamic feeding circuitry, compels the organism to increase its consumption of energetic substrates.
Figure 2.
A schematic illustrating the testosterone-induced enhancement of CB1 receptor-mediated signaling at glutamatergic inputs impinging upon POMC neurons. Testosterone produces hyperphagia and heightens cannabinoid tone at the CB1 receptors in the glutamatergic nerve terminals by activating cellular energy sensor, AMPK. The activation of this heterotrimeric AMPK complex is due to upstream kinases like Ca2+/calmodulin-dependent protein kinase kinase (CaMKK) and liver kinase B1 (LKB1) via phosphorylation of threonine residue 172 of the α subunit that occurs in response to increases in intracellular calcium and the AMP/ATP ratio. This, in turn, leads to increased endocannabinoid tone either by enhancing synthesis, inhibiting breakdown, or attenuating reuptake and removal from the synaptic cleft. The endocannabinoids could then act retrogradely at the CB1 receptor to augment the cannabinoid-induced presynaptic inhibition of glutamate release. Modified from (Borgquist et al., 2015b).
As mentioned above, there are species differences concerning the sexually disparate cannabinoid regulation of pain processing. The same is likely to hold true for the cannabinoid regulation of energy balance. Estradiol negatively modulates metabotropic, Gi/o-coupled receptor function in guinea pigs, rats and mice (Mela et al., 2015; Qiu et al., 2006). However, there is striking variability in how (or if) cannabinoids influence POMC neuronal excitability to affect changes in energy intake in mice. For example, in enhanced green fluorescent protein-POMC mice, exogenously administered cannabinoid receptor agonists presynaptically inhibit glutamatergic input onto POMC neurons, but no retrograde inhibition of this input by endocannabinoids was observed (Hentges et al., 2005). In Ay mice that overexpress AgRP, changes in energy intake caused by CB1 receptor activation or blockade do not appear to involve POMC neurons, but rather mesolimbic dopamine neurons emanating in the ventral tegmental area (Sinnayah et al., 2008). On the other hand, in POMC-cre mice, CB1 receptor activation directly excites POMC neurons, and stimulates the expression of proteases that increase the production of β-endorphin during posttranslational processing (Koch et al., 2015). Thus, not only are there species differences in the cannabinoid regulation of energy balance, but there are also clear strain differences among the different lines of transgenic mice. Given these disparities, it is therefore not surprising that no one has attempted to investigate sex differences in the cannabinoid regulation of energy homeostasis in mice.
The litany of anecdotal and experimental evidence that cannabinoid receptor agonists augment energy intake has effectively translated into the use of cannabis and oral formulations of THC like marinol or dronabinol as therapeutic adjuncts in the treatment of cancer- and HIV/AIDS-related cachexia. In all of the available literature extolling the efficacy of these compounds to ameliorate the lack of appetite and body wasting, whether it comes in the form of case reports, epidemiological studies or randomized, double blind, placebo controlled investigations, the overwhelming majority of the study participants were male (Beal et al., 1995; Haney et al., 2005; Nelson et al., 1994; Sacks et al., 1990; Woolridge et al., 2005). However, in the one study in which the gender ratio was more evenly distributed, neither cannabis extract nor THC produced significant increases in appetite or quality of life relative to placebo-treated controls (Strasser et al., 2006). These observations provide some credence to the idea that sex differences in the cannabinoid regulation of appetite extend to humans. In addition, the discrepant activational effects of gonadal steroids on the cannabinoid regulation of energy balance that we have found in our guinea pig animal model indicate that androgens could be used in conjunction with either cannabis or oral THC to combat cachexia.
The synthetic androgen nandrolone has proven effective in increasing lean body mass, fat-free mass, and other body composition measures such as body cell mass and intracellular water in HIV-infected men experiencing low to moderate weight loss (Storer et al., 2005). It has also been shown to produce a modest yet statistically insignificant increase in energy intake and appetite score (Batterham and Garsia, 2001). This suggests that the effectiveness of nandrolone in improving cachexic symptoms is due primarily to its anabolic and metabolic actions (Bardin, 1996; Bhasin et al., 1997), and is consistent with our findings that testosterone per se increases O2 consumption, CO2 and metabolic heat production, as well as core body temperature (Borgquist et al., 2015b). On the other hand, dronabinol significantly increases appetite in patients with HIV/AIDS-related cachexia, but is comparatively less effective in increasing body weight (Batterham and Garsia, 2001; Beal et al., 1995). However, to date there are no published accounts describing how nandrolone and dronabinol directly compare with one another, or how combined therapy might maximize gains in appetite and weight gain. Bear in mind that during the female ovarian cycle, energy intake is at its lowest during the periovulatory phase, when estradiol is the preeminent constituent of the gonadal steroid hormonal milieu, and at its highest during the progesterone-dominated luteal phase (Johnson et al., 1994). Therefore, it should not be surprising that the synthetic progesterone derivative, megestrol acetate, can effectively counter the cachexia associated with HIV/AIDS. Indeed, it is comparatively more efficacious than nandrolone in increasing appetite, energy intake, weight gain and adiposity (Batterham and Garsia, 2001). In addition, it increases appetite and weight gain to a significantly higher degree than does dronabinol (Jatoi et al., 2002). Interestingly, a study investigating the effects of combined megestrol acetate and nandrolone decanoate reported significant elevations in weight gain and fat-free mass but not adiposity (Cuerda et al., 2005).
The negative modulatory effects of estradiol on the cannabinoid regulation of energy homeostasis that we demonstrated in the female guinea pig would suggest that cannabinoid sensitivity fluctuates over the course of the reproductive cycle. Accordingly, one might predict that cannabinoid responsiveness would be at its lowest during the estradiol-dominated follicular and periovulatory phases of the reproductive cycle. Likewise, it follows that under hypoestrogenic states brought on by primary amenorrhea (e.g., anorexia nervosa) or secondary amenorrhea (e.g., menopause), CB1 receptor-mediated signaling would proceed to the fullest extent possible. Indeed, dronabinol significantly increased weight gain in women with anorexia nervosa of at least five years duration (Andries et al., 2014), which is consistent with findings that THC diminished activity-based anorexia in female rats as assessed by THC-induced increases in energy intake, decreases in running wheel activity, reductions in weight loss, decreases in thermogenesis in brown adipose tissue and decrements in lipolysis in white adipose tissue (Verty et al., 2011). While the hypoestrogenemia that is associated with anorexia certainly appears to confer responsiveness to the appetite-stimulating properties of cannabinoid receptor agonists, different allelic forms of the CB1 receptor (CNR1) gene are reported to be preferentially transmitted in the binging/purging and restricting types of anorexia nervosa (Siegfried et al., 2004). In addition, a point mutation in the gene encoding for GPR55 that substitutes a valine for a glycine residue at the 195th position diminishes agonist-stimulated phosphorylation of extracellular signal-regulated kinase, and is more abundant in Japanese women diagnosed with anorexia nervosa (Ishiguro et al., 2011).
On the other hand, the RIO-North American clinical trial that examined the anti-obesity effects of Rimonabant comprised ~80% women, over half of whom were 45 years or older (Pi-Sunyer et al., 2006). Rimonibant effectively reduced body weight, waist circumference, circulating triglycerides and fasting insulin levels, and elevated HDL cholesterol levels (Pi-Sunyer et al., 2006). Were it not for the increased incidence of a few adverse side effects (i.e., nausea, anxiety, depressed mood) experienced by a small percentage of the study participants, it is hard to imagine this drug not being approved by the Food and Drug Administration or remaining on the market in Europe. Given that ≥40% were either peri- or post-menopausal women, the subsiding of the estradiol-induced attenuation of CB1 receptor-mediated signaling could constitute a major reason behind the enhanced endocannabinoid tone that is blocked by Rimonabant. However, adult female rats in which obesity was induced by the antipsychotic olanzapine exhibited reduced CB1 receptor binding in the ARC (Weston-Green et al., 2012).
A major source of ARC CB1 receptors comes from SF-1-containing VMN neurons. The selective knockout of the CB1 receptor in these neurons leads to a lean phenotype associated with increased sympathetic tone, lipolysis and leptin sensitivity in normal chow-fed mice, but an obese phenotype concomitant with leptin resistance in animals fed a high-fat diet (Cardinal et al., 2014). Thus, these SF-1-containing, CB1 receptor-bearing VMN neurons, the preponderance of which also express the vesicular glutamate transporter (Cardinal et al., 2014), may serve as a molecular switch that confers metabolic flexibility in the face of an ever-changing diet. The axon terminals of these cells synapse with POMC neurons in the ARC (Lindberg et al., 2013). As such, they likely represent a critical target for endocannabinoid-mediated retrograde inhibition of excitatory input impinging on POMC neurons.
It has long been known that energy balance is inexorably linked to reproductive status (Sinchak and Wagner, 2012). It should therefore not be surprising that there are sex differences in the manner in which cannabinoids affect reproductive behavior. For example, women are more likely to report an increase in sexual desire following the consumption of low to moderate amounts of cannabis, whereas men are more apt to experience decrements in sexual performance due to the suppression of the reproductive axis and erectile dysfunction (Gorzalka et al., 2010). This parallels findings from animal studies, in which THC facilitates sexual receptivity in ovariectomized, estradiol-primed and progesterone-treated female rats (Mani et al., 2001), whereas it decreases copulatory behavior, increases mount latency, and blocks the associated catecholaminergic discharge in the mediobasal hypothalamus of male rats (Murphy et al., 1994).
6. Summary and Concluding Remarks
From the research carried out over the past several decades, it is clear that sex differences in cannabinoid-regulated biology are pervasive and far-reaching. While this review focused on the most recent advances into our understanding of sex differences in the metabolic fate, abuse liability, as well as the antinociceptive and appetite-stimulating properties of cannabinoids, it should not be misconstrued that this list is by any means comprehensive. Indeed, there are sex differences reported for the endocannabinoid-mediated neonatal development of the amygdala (Krebs-Kraft et al., 2010), the THC-induced impairment of spatial learning during adolescence and adulthood (Cha et al., 2007), as well as a number of behavioral and endocrine disturbances that develop as a result of chronic or subchronic exposure to cannabinoid receptor agonists during adolescence (Viveros et al., 2011b).
Although the disparities spotlighted in this review have been more thoroughly investigated, there is still much more work that needs to be done in order to complete our understanding. For example, we still do not know whether gonadal steroids exert activational effects on the metabolic disposition of cannabinoids, how the sequelae varies over the course of the estrous cycle, or whether this phenomenon is found in humans and non-human primates. High doses of progesterone and testosterone stimulate the conversion of 7β-OH-Δ8-THC to 7-oxo-Δ8-THC in microsomes from male monkey liver (Funahashi et al., 2005), but it is not known if there are underlying sex differences with regard to cannabinoid metabolism in the primate. Sex differences in cannabinoid abuse have been documented in both humans and animal models, although information pertaining to the activational effects of gonadal steroids, and to the potential fluctuations that may be observed over the reproductive cycle, is non-existent. We have a more complete picture regarding how gonadal steroids modulate cannabinoid-induced antinociception, but further investigation into the gonadal steroid receptor-mediated transduction mechanisms that modulate cannabinoid receptor signaling within the spinal and supraspinal pain-processing sites is warranted, as is a determination of which animal model best reflects the human condition. We have also gleaned some considerable mechanistic insights into how gonadal steroids influence the cannabinoid regulation of the hypothalamic feeding circuitry and how this impacts cannabinoid-induced changes in energy balance. However, we have yet to see how effectively this translates to the human condition, and how (or if) we can parlay this information into appropriately tailored theraupeutic strategies in men and women to help combat HIV/AIDS- and cancer-related cachexia as well as obesity.
What is more, most of the discussion concerning the activational effects of gonadal steroids centers around estradiol and testosterone; comparatively little work has been done on potential modulatory effects of progesterone on sexually discrepant, cannabinoid-regulated biology. Furthermore, CB2 receptors are expressed in CNS structures such as the brainstem and hypothalamus (Gong et al., 2006; Van Sickle et al., 2005), and evidence suggests that these receptors contribute to sexually differentiated, cannabinoid-induced antinociception (Craft et al., 2012). Moreover, loss-of-function mutations at the GPR55 receptor confer vulnerability to the development of anorexia nervosa (Ishiguro et al., 2011). It would therefore be worthwhile to explore the role of the CB2 and GPR55 receptors in the regulation of energy balance, whether this regulation is sexually differentiated, and if so, whether this regulation is subject to activational effects of gonadal steroids. The culmination of research findings spanning over two decades has made it abundantly clear that sexually distinct cannabinoid-regulated biology is the norm, rather than the exception. At the same time, they provide an indelible reminder that there is much more to be accomplished in elucidating the molecular underpinnings of these inherent differences between males and females, and how these can be leveraged to better serve humanity.
Highlights.
Sex differences are fundamental to cannabinoid-regulated biology
Males are more sensitive to the appetite-stimulating properties of cannabinoids
Sex steroid hormones have divergent roles in determining cannabinoid sensitivity
Acknowledgments
Support provided by PHS Grants DA024314 and HD058638, as well as an intramural research grant from Western University of Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anaraki DK, Sianati S, Sadeghi M, Ghasemi M, Javadi P, Mehr SE, Dehpour AR. Modulation by female sex hormones of the cannabinoid-induced catalepsy and analgesia in ovariectomized mice. Eur. J. Pharmacol. 2008;586:189–196. doi: 10.1016/j.ejphar.2008.02.055. [DOI] [PubMed] [Google Scholar]
- 2.Anderson BM, Rizzo M, Block RI, Pearlson GD, O'Leary DS. Sex differences in the effects of marijuana on simulated driving performance. J. Psychoactive Drugs. 2010;42:19–30. doi: 10.1080/02791072.2010.10399782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J. Biol. Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- 4.Andries A, Frystyk J, Flyvbjerg A, Støving RK. Dronabinol in severe, enduring anorexia nervosa: a randomized controlled trial. Int. J. Eat. Disord. 2014;47:18–23. doi: 10.1002/eat.22173. [DOI] [PubMed] [Google Scholar]
- 5.Bardin CW. The anabolic action of testosterone. New England J. Med. 1996;335:52–53. doi: 10.1056/NEJM199607043350111. [DOI] [PubMed] [Google Scholar]
- 6.Batterham MJ, Garsia R. A comparison of megestrol acetate, nandrolone decanoate and dietary counselling for HIV associated weight loss. Int. J. Androl. 2001;24:232–240. doi: 10.1046/j.1365-2605.2001.00291.x. [DOI] [PubMed] [Google Scholar]
- 7.Beal JE, Olson R, Laubenstein L, Morales JO, Bellman P, Yangco B, Lefkowitz L, Plasse TF, Shepard KV. Dronabinol as a treatment for anorexia associated with weight loss in patients with AIDS. J. Pain Symptom Manage. 1995;10:89–97. doi: 10.1016/0885-3924(94)00117-4. [DOI] [PubMed] [Google Scholar]
- 8.Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J. Clin. Endocrinol. Metab. 1997;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- 9.Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: activation of GIRK2 channels. Proc. Natl. Acad. Sci. 2003;100:277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borgquist A, Meza C, Wagner EJ. Role of neuronal nitric oxide synthase in the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. J. Neurophysiol. 2015a;113:904–914. doi: 10.1152/jn.00615.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgquist A, Meza C, Wagner EJ. The role of AMP-activated protein kinase in the androgenic potentiation of cannabinoid-induced changes in energy homeostasis. Am. J. Physiol. Endocrinol. Metab. 2015b;308:E482–E495. doi: 10.1152/ajpendo.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borgquist A, Wagner EJ. On the cannabinoid regulation of energy homeostasis: past, present and future. In: Murillo-Rodriguez E, Onaivi ES, Darmani NA, Wagner EJ, editors. Endocannabinoids: Molecular, Pharmacological, Behavioral and Clinical Features. Oak Park, IL, USA: Bentham Science; 2013. pp. 60–91. [Google Scholar]
- 13.Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R349–R358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- 14.Brunner TF. Marijuana in ancient Greece and Rome? The literary evidence. Bull. Hist. Med. 1973;47:344–355. [PubMed] [Google Scholar]
- 15.Buu A, Dabrowska A, Mygrants M, Puttler LI, Jester JM, Zucker RA. Gender differences in the developmental risk of onset of alcohol, nicotine, and marijuana use and the effects of nicotine and marijuana use on alcohol outcomes. J. Stud. Alcohol Drugs. 2014;75:850–858. doi: 10.15288/jsad.2014.75.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardinal P, André C, Quarta C, Bellocchio L, Clark S, Elie M, Leste-Lasserre T, Maitre M, Gonzales D, Cannich A, Pagotto U, Marsicano G, Cota D. CB1 cannabinoid receptor in SF1-expressing neurons of the ventromedial hypothalamus determines metabolic responses to diet and leptin. Mol. Metab. 2014;3:705–716. doi: 10.1016/j.molmet.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castelli MP, Fadda P, Casu A, Spano MS, Casti A, Fratta W, Fattore L. Male and female rats differ in brain cannabinoid CB1 receptor density and function and in behavioural traits predisposing to drug addiction: effect of ovarian hormones. Curr. Pharm. Des. 2014;20:2100–2113. doi: 10.2174/13816128113199990430. [DOI] [PubMed] [Google Scholar]
- 18.Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav. Pharmacol. 2007;18:563–569. doi: 10.1097/FBP.0b013e3282ee7b7e. [DOI] [PubMed] [Google Scholar]
- 19.Cheung S, Hammer RP. Gonadal steroid hormone regulation of proopiomelanocortin gene expression in arcuate neurons that innervate the medial preoptic area of the rat. Neuroendocrinology. 1995;62:283–292. doi: 10.1159/000127015. [DOI] [PubMed] [Google Scholar]
- 20.Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 2014;136:85–91. doi: 10.1016/j.drugalcdep.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corchero J, Manzanares J, Fuentes JA. Role of gonadal steroids in the corticotropinreleasing hormone and proopiomelanocortin gene expression response to Δ9-tetrahydrocannabinol in the hypothalamus of the rat. Neuroendocrinology. 2001;74:185–192. doi: 10.1159/000054685. [DOI] [PubMed] [Google Scholar]
- 22.Craft RM, Kandasamy R, Davis SM. Sex differences in anti-allodynic, antihyperanalgesic and anti-edema effects of Δ9-tetrahydrocannabinol in the rat. Pain. 2013;154:1709–1717. doi: 10.1016/j.pain.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of Δ9-tetrahydrocannabinol in male and female rats. Eur. J. Pharmacol. 2008;578:37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Craft RM, Wakley AA, Tsutsui KT, Laggart JD. Sex differences in cannabinoid 1 vs. cannabinoid 2 receptor-selective antagonism of antinociception produced by Δ9-tetrahydrocannabinol and CP55,940 in the rat. J. Pharmacol. Exp. Ther. 2012;340:787–8000. doi: 10.1124/jpet.111.188540. [DOI] [PubMed] [Google Scholar]
- 25.Cuerda C, Zugasti A, Bretón I, Camblor M, Miralles P, García P. Treatment with nandrolone decanoate and megestrol acetate in HIV-infected men. Nutr. Clin. Pract. 2005;20:93–97. doi: 10.1177/011542650502000193. [DOI] [PubMed] [Google Scholar]
- 26.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 27.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Dhillon SS, Belsham DD. Estrogen inhibits NPY secretion through membrane-associated estrogen receptor (ER)-α in clonal, immortalized hypothalamic neurons. Int. J. Obesity. 2011;35:198–207. doi: 10.1038/ijo.2010.124. [DOI] [PubMed] [Google Scholar]
- 29.Di Marzo V, Goparahu SK, Wang L, Liu J, Bátkai S, Járai Z, Fezza F, Miura GI, Palmiter RD, Sugiura T, Kunos G. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature. 2001;410:822–825. doi: 10.1038/35071088. [DOI] [PubMed] [Google Scholar]
- 30.Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duncan SC, Gau JM, Farmer RF, Seeley JR, Kosty DB. Comorbidity and temporal relations of alcohol and cannabis use disorders from youth through adulthood. Drug Alcohol Depend. 2015;149:80–86. doi: 10.1016/j.drugalcdep.2015.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esfandyari T, Camilleri M, Ferber I, Burton D, Baxter K, Zinsmeister AR. Effect of a cannabinoid agonist on gastrointestinal transit and postprandial satiation in healthy human subjects: a randomized, placebo-controlled study. Neurogastroenterol. Motil. 2006;18:831–838. doi: 10.1111/j.1365-2982.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 33.Farhang B, Diaz S, Tang SL, Wagner EJ. Sex differences in the cannabinoid regulation of energy homeostasis. Psychoneuroendocrinology. 2009;34S:S237–S246. doi: 10.1016/j.psyneuen.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br. J. Pharmacol. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferin M, Van Vugt D, Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent Prog. Horm. Res. 1984;40:441–485. doi: 10.1016/b978-0-12-571140-1.50015-3. [DOI] [PubMed] [Google Scholar]
- 36.Funahashi T, Tanaka Y, Yamaori S, Kimura T, Matsunaga T, Ohmori S, Kageyama T, Yamamoto I, Watanabe K. Stimulatory effects of testosterone and progesterone on the NADH- and NADPH-dependent oxidation of 7β-hydroxy-Δ8-tetrahydrocannabinol to 7-oxo-Δ8-tetrahydrocannabinol in monkey liver microsomes. Drug Metab. Pharmacokinet. 2005;20:358–367. doi: 10.2133/dmpk.20.358. [DOI] [PubMed] [Google Scholar]
- 37.Gérard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem. J. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gingerich S, Krukoff TL. Activation of ERβ increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology. 2008;55:878–885. doi: 10.1016/j.neuropharm.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 39.Gong J-P, Onaivi ES, Ishiguro H, Liu Q-R, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: Immunohistochemical localization in the rat brain. Brain Res. 2006;1071:10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 40.Gorzalka BB, Hill MN, Chang SCH. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm. Behav. 2010;58:91–99. doi: 10.1016/j.yhbeh.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Hama A, Sagen J. Activation of spinal and supraspinal cannabinoid-1 receptors leads to antinociception in a rat model of neuropathic spinal cord injury pain. Brain Res. 2011;1412:44–54. doi: 10.1016/j.brainres.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamarneh S. Pharmacy in medievil Islam and the history of drug addiction. Med. Hist. 1972;16:226–237. doi: 10.1017/s0025727300017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haney M, Rabkin J, Gunderson E, Foltin RW. Dronabinol and marijuana in HIV+ marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology (Berl) 2005;181:170–178. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]
- 44.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, Collinge M, Sessa WC, Bender JR. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J. Biol. Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 46.Henry DJ, Chavkin C. Activation of inwardly rectifying potassium channels (GIRK1) by coexpressed rat brain cannabinoid receptors in Xenopus oocytes. Neurosci. Lett. 1995;186:91–94. doi: 10.1016/0304-3940(95)11289-9. [DOI] [PubMed] [Google Scholar]
- 47.Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J. Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J. Clin. Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hille B. Potassium Channels and Chloride Channels. In: Hille B, editor. Ion Channels of Excitable Membranes. Sunderland, MA, USA: Sinauer Associates, Inc; 1992. pp. 115–139. [Google Scholar]
- 51.Hillig KW, Mahlberg PG. A chemotaxonomic analysis of cannabinoid variation in Cannabis (Cannabaceae) Am. J. Botany. 2004;91:966–975. doi: 10.3732/ajb.91.6.966. [DOI] [PubMed] [Google Scholar]
- 52.Hindmarch I. A social history of the use of Cannabis Sativa. Contemp. Rev. 1972;220:252–257. [PubMed] [Google Scholar]
- 53.Ho J, Cox JM, Wagner EJ. Cannabinoid-induced hyperphagia: Correlation with inhibition of proopiomelanocortin neurons? Physiol. Behav. 2007;92:507–519. doi: 10.1016/j.physbeh.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu X, Primack BA, Barnett TE, Cook RL. College students and use of K2: an emerging drug of abuse in young persons. Subst. Abuse Treat. Prev. Policy. 2011;6:16-. doi: 10.1186/1747-597X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishac EJN, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishiguro H, Onaivi ES, Horiuchi Y, Imai K, Komaki G, Ishikawa T, Suzuki M, Watanabe Y, Ando T, Higuchi S, Arinami T. Functional polymorphism in the GPR55 gene is associated with anorexia nervosa. Synapse. 2011;65:103–108. doi: 10.1002/syn.20821. [DOI] [PubMed] [Google Scholar]
- 57.Jamshidi N, Taylor DA. Anandamide administration into the ventromedial hypothalamus stimulates appetite in rats. Br. J. Pharmacol. 2001;134:1151–1154. doi: 10.1038/sj.bjp.0704379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jatoi A, Windschitl HE, Loprinzi CL, Sloan JA, Dakhil SR, Mailliard JA, Pundaleeka S, Kardinal CG, Fitch TR, Krook JE, Novotny PJ, Christensen B. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a north central cancer treatment group study. J. Clin. Oncol. 2002;20:567–573. doi: 10.1200/JCO.2002.20.2.567. [DOI] [PubMed] [Google Scholar]
- 59.Johnson WG, Corrigan SA, Lemmon CR, Bergeron KB, Crusco AH. Energy regulation over the menstrual cycle. Physiol. Behav. 1994;56:523–527. doi: 10.1016/0031-9384(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 60.Jones AW, Holmgren A, Kugelberg FC. Driving under the influence of cannabis: a 10-year study of age and gender differences in the concentrations of tetrahydrocannabinol in blood. Addiction. 2008;103:452–461. doi: 10.1111/j.1360-0443.2007.02091.x. [DOI] [PubMed] [Google Scholar]
- 61.Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur. J. Pharmacol. 2009;622:15–24. doi: 10.1016/j.ejphar.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelly MJ, Rønnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ. Estrogen modulation of K+ channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids. 2002;67:447–456. doi: 10.1016/s0039-128x(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 63.Koch M, Varela L, Kim JG, Kim JD, Hernández-Nuño F, Simonds SE, Castorena CM, Vianna CR, Elmquist JK, Morozov YM, Rakic P, Bechmann I, Cowley MA, Szigeti-Buck K, Dietrich MO, Gao X-B, Diano S, Horvath TL. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, Amin F, Harvey-White J, Liposits Z, Kunos G, Grossman AB, Fekete C, Korbonits M. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS ONE. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krebs-Kraft DL, Hill MN, Hillard CJ, McCarthy MM. Sex difference in cell proliferation in developing rat amygdala mediated by endocannabinoids has implications for social behavior. Proc. Natl. Acad. Sci. 2010;107:20535–20540. doi: 10.1073/pnas.1005003107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lauckner JE, Jensen JB, Chen H-Y, Lu H-C, Hille B, Mackie K. GPR55 is an cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc. Natl. Acad. Sci. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Le Querrec F, Bounes V, Mestre ML, Azema O, Longeaux N, Gallart J-C. Sex and age differences in ED patients with mental and behavioral disorders due to psychoactive substance use. Am. J. Emerg. Med. 2015 doi: 10.1016/j.ajem.2015.06.068. [DOI] [PubMed] [Google Scholar]
- 68.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG. Leptin action via hypothalamic nitric oxide synthase-1 neurons controls energy balance. Nature Medicine. 2012;18:820–823. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li K, Feng J-Y, Li Y-Y, Yuece B, Lin X-H, Yu L-Y, Li Y-N, Feng Y-J, Storr M. Anti-inflammatory role of cannabidiol and O-1602 in cerulin-induced acute pancreatitis in mice. Pancreas. 2013;42:123–129. doi: 10.1097/MPA.0b013e318259f6f0. [DOI] [PubMed] [Google Scholar]
- 70.Lin X-H, Yuece B, Li Y-Y, Feng Y-J, Feng J-Y, Yu L-Y, Li K, Li Y-N, Storr M. A novel CB receptor GPR55 and its ligands are involved in regulation of gut movement in rodents. Neurogastroenterol. Motil. 2011;23:862–e342. doi: 10.1111/j.1365-2982.2011.01742.x. [DOI] [PubMed] [Google Scholar]
- 71.Lindberg D, Chen P, Li C. Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to the autonomic centers of the hypothalamus and hindbrain. J. Comp. Neurol. 2013;521:3167–3190. doi: 10.1002/cne.23338. [DOI] [PubMed] [Google Scholar]
- 72.Lundahl LH, Greenwald MK. Effect of oral THC pretreatment on marijuana cue-induced responses in cannabis dependent volunteers. Drug Alcohol Depend. 2015;149:187–193. doi: 10.1016/j.drugalcdep.2015.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J. Neuroimmune Pharmacol. 2015;10:293–301. doi: 10.1007/s11481-015-9600-6. [DOI] [PubMed] [Google Scholar]
- 74.Lynch ME, Young J, Clark AJ. A case series of patients using medical marihuana for management of chronic pain under the Canadian Marihuana Medical Access Regulations. J. Pain Symptom Manage. 2006;32:497–501. doi: 10.1016/j.jpainsymman.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 75.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J. Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Malyala A, Zhang C, Bryant DN, Kelly MJ, Rønnekleiv OK. PI3K signaling effects in hypothalamic neurons mediated by estrogen. J. Comp. Neurol. 2008;506:895–911. doi: 10.1002/cne.21584. [DOI] [PubMed] [Google Scholar]
- 77.Mani SK, Mitchell A, O'Malley BW. Progesterone receptor and dopamine receptors are required in Δ9-tetrahydrocannabinol modulation of sexual receptivity in female rats. Proc. Natl. Acad. Sci. 2001;98:1249–1254. doi: 10.1073/pnas.031563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marco EM, Echeverry-Alzate V, López-Moreno JA, Giné E, Peñasco S, Viveros MP. Consequences of early life stress on the expression of endocannabinoid-related genes in the rat brain. Behav. Pharmacol. 2014;25:547–5566. doi: 10.1097/FBP.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 79.Mathew RJ, Wilson WH, Davis R. Postural syncope after marijuana: a transcranial Doppler study of the hemodynamics. Pharmacol. Biochem. Behav. 2003;75:309–318. doi: 10.1016/s0091-3057(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 80.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 81.Matsunaga T, Shibayama K, Higuchi S, Tanaka H, Watanabe K, Yamamoto I. Characterization of microsomal alcohol oxygenase catalyzing the oxidation of 7-hydroxy-Δ8-tetrahydrocannabinol to 7-oxo-Δ8-tetrahydrocannabinol in rat liver. Biol. Pharm. Bull. 2000;23:43–46. doi: 10.1248/bpb.23.43. [DOI] [PubMed] [Google Scholar]
- 82.Matsunaga T, Tanaka H, Komura A, Watanabe K, Yamamoto I, Yoshimura H. Microsomal alcohol oxygenase: purification and characterization of a cytochrome P450 responsible for oxidation of 7-hydroxy-Δ8-tetrahydrocannabinol and 7-oxo-Δ8-tetrahydrocannabinol in guinea pig liver. Arch. Biochem. Biophysics. 1997;348:56–64. doi: 10.1006/abbi.1997.0390. [DOI] [PubMed] [Google Scholar]
- 83.Mechoulam R, Gaoni Y. A total synthesis of dl-Δ1-tetrahydrocannabinol, the active constituent of hashish. J. Am. Chem. Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- 84.Mela V, Vargas A, Meza C, Kachani M, Wagner EJ. Modulatory influences of estradiol and other anorexigenic hormones on metabotropic, Gi/o-coupled receptor function in the hypothalamic control of energy homeostasis. J. Steroid Biochem. Mol. Biol. 2015 doi: 10.1016/j.jsbmb.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mikuriya TH. Marijuana in medicine: past, present and future. Calif. Med. 1969;110:34–40. [PMC free article] [PubMed] [Google Scholar]
- 86.Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol. Behav. 2004;80:611–616. doi: 10.1016/j.physbeh.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 87.Minokoshi Y, Alquier T, Furukawa N, Kim Y-B, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–574. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 88.Moreno-Navarrete JM, Catalán V, Whyte L, Díaz-Arteaga A, Vázquez-Martínez R, Rotellar R, Guzmán R, Gómez-Ambrosi J, Pulido MR, Russell WR, Imbernon M, Ross RA, Malagón MM, Dieguez C, Fernández-Real JM, Frühbeck G, Nogueiras R. The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity. Diabetes. 2012;61:281–291. doi: 10.2337/db11-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 90.Murphy LL, Gher J, Steger RW, Bartke A. Effects of delta9-tetrahydrocannabinol on copulatory behavior and neuroendocrine responses of male rats to female conspecifics. Pharmacol. Biochem. Behav. 1994;48:1011–1017. doi: 10.1016/0091-3057(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 91.Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of Δ9-tetrahydrocannabinol in the rat. Biochem. Pharmacol. 1991;41:1187–1194. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- 92.Nelson K, Walsh D, Deeter P, Sheehan F. A phase II study of delta-9-tetrahydrocannabinol for appetite stimulation in cancer-associated anorexia. J. Palliat. Care. 1994;10:14–18. [PubMed] [Google Scholar]
- 93.Niu KY, Zhang Y, Ro JY. Effects of gonadal hormones on the peripheral cannabinoid receptor 1 (CB1R) system under a myositis condition in rats. Pain. 2012;153:2283–2291. doi: 10.1016/j.pain.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Otukonyong EE, Okutani F, Takahashi S, Murata T, Morioka N, Kaba H, Higuchi T. Effect of food deprivation and leptin repletion on the plasma levels of estrogen (E2) and NADPH-d reactivity in the ventromedial and arcuate nuclei of the hypothalamus in the female rats. Brain Res. 2000;887:70–79. doi: 10.1016/s0006-8993(00)02969-3. [DOI] [PubMed] [Google Scholar]
- 95.Penetar DM, Kouri EM, Gross MM, McCarthy EM, Rhee CK, Peters EN, Lukas SE. Transdermal nicotine alters some of marijuana's effects in male and female volunteers. Drug Alcohol Depend. 2005;79:211–223. doi: 10.1016/j.drugalcdep.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 96.Perez-Reyes M, Owens SM, Di Guiseppi S. The clinical pharmacology and dynamics of marihuana cigarette smoking. J. Clin. Pharmacol. 1981;21:201S-207S. doi: 10.1002/j.1552-4604.1981.tb02596.x. [DOI] [PubMed] [Google Scholar]
- 97.Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J. Effect of Rimonabant, a Cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients. JAMA. 2006;295:761–775. doi: 10.1001/jama.295.7.761. [DOI] [PubMed] [Google Scholar]
- 98.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Rønnekleiv OK, Kelly MJ. A g-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J. Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Rønnekleiv OK, Kelly MJ. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metabolism. 2014;19:682–693. doi: 10.1016/j.cmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Riebe CJN, Hill MN, Lee TTY, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–1269. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149:6113–6124. doi: 10.1210/en.2008-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romero-Zerbo SY, Rafacho A, Díaz-Arteaga A, Suárez J, Quesada I, Imbernon M, Ross RA, Dieguez C, Rodríguez de Fonseca F, Nogueiras R, Nadal A, Bermúdez-Silva, F- J. A role for putative cannabinoid receptor GPR55 in the islets of Langerhans. J. Endocrinol. 2011;211:177–185. doi: 10.1530/JOE-11-0166. [DOI] [PubMed] [Google Scholar]
- 103.Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol. Sci. 2009;30:156–163. doi: 10.1016/j.tips.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 104.Sacks N, Hutcheson JR, Watts JM, Webb RE. Case report: the effect of tetrahydrocannabinol on food intake during chemotherapy. J. Am. Coll. Nutr. 1990;9:630–632. doi: 10.1080/07315724.1990.10720419. [DOI] [PubMed] [Google Scholar]
- 105.Siegfried Z, Kanyas K, Latzer Y, Karni O, Bloch M, Lerer B, Berry EM. Association study of cannabinoid receptor gene (CNR1) alleles and anorexia nervosa: differences between restricting and bingeing/purging subtypes. Am. J. Med. Genet. 2004;125B:126–130. doi: 10.1002/ajmg.b.20089. [DOI] [PubMed] [Google Scholar]
- 106.Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front. Neuroendocrinol. 2012;33:342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sinnayah P, Jobst EE, Rathner JA, Caldera-Siu AD, Tonelli-Lemos L, Eusterbrock AJ, Enriori PJ, Pothos EN, Grove KL, Cowley MA. Feeding induced by cannabinoids is mediated independently of the melanocortin system. PLoS ONE. 2008;3:e2202-. doi: 10.1371/journal.pone.0002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol. Am. J. Physiol. Endocrinol. Metab. 2013;305:E362–E640. doi: 10.1152/ajpendo.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stein EA, Fuller SA, Edgemond WS, Campbell WB. Physiological and behavioural effects of the endogenous cannabinoid, arachidonylethanolamide (anandamide), in the rat. Br. J. Pharmacol. 1996;119:107–114. doi: 10.1111/j.1476-5381.1996.tb15683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Storer TW, Woodhouse LJ, Sattler F, Singh AB, Schroeder ET, Beck K, Padero M, Mac P, Yarasheski KE, Geurts P, Willemsen A, Harms MK, Bhasin S. A randomized, placebo-controlled trial of nandrolone decanoate in human immunodeficiency virus-infected men with mild to moderate weight loss with recombinant human growth hormone as active reference treatment. J. Clin. Endocrinol. Metab. 2005;90:4474–4482. doi: 10.1210/jc.2005-0275. [DOI] [PubMed] [Google Scholar]
- 111.Strasser F, Luftner D, Possinger K, Ernst G, Ruhstaller T, Meissner W, Ko Y-D, Schnelle M, Reif M, Cerny T. Comparison of orally administered cannabis extract and delta-9-tetrahydrocannabinol in treating patients with cancer-related anorexia-cachexia syndrome: A multicenter, phase III, randomized, double-blind, placebo-controlled clinical trial from the cannabis-study-group. J. Clin. Oncol. 2006;24:3394–3400. doi: 10.1200/JCO.2005.05.1847. [DOI] [PubMed] [Google Scholar]
- 112.Stricker EM. Hyperphagia. New England J. Med. 1978;298:1010–1013. doi: 10.1056/NEJM197805042981809. [DOI] [PubMed] [Google Scholar]
- 113.Tang SL, Tran V, Wagner EJ. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. J. Neurophysiol. 2005;94:2983–2986. doi: 10.1152/jn.01187.2004. [DOI] [PubMed] [Google Scholar]
- 114.Titolo D, Mayer CM, Dhillon SS, Cai F, Belsham DD. Estrogen facilitates both phosphadtidylinositol 3-kinase and ERK1/2 mitogen-activated protein kinase membrane signaling required for long-term neuropeptide Y transcriptional regulation in clonal, immortalized neurons. J. Neurosci. 2008;28:6473–6482. doi: 10.1523/JNEUROSCI.0514-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Toth C, Mawani S, Brady S, Chan C, Liu C, Mehina E, Garven A, Bestard J, Korngut L. An enriched enrollment, randomized withdrawal, flexible-dose, double-blind, placebo-controlled, parallel assessment efficacy study of nabilone as adjuvant in the treatment of diabetic peripheral neuropathic pain. Pain. 2012;153:2073–2082. doi: 10.1016/j.pain.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 116.Touw M. The religious and medical uses of Cannabis in China, India and Tibet. J. Psychoactive Drugs. 1981;13:23–34. doi: 10.1080/02791072.1981.10471447. [DOI] [PubMed] [Google Scholar]
- 117.Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur. J. Pharmacol. 2001;430:41–47. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- 118.Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Δ9-tetrahydrocannabinol-induced behavioral effects in rats. Behav. Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 119.Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 120.Vaughan CW, Connor M, Bagley EE, Christie MJ. Actions of cannabinoids on membrane properties and synaptic transmission in rat periaqueductal gray neurons in vitro. Mol. Pharmacol. 2000;57:288–295. [PubMed] [Google Scholar]
- 121.Verty ANA, Evetts MJ, Crouch GJ, McGregor IS, Stefanidis A, Oldfield BJ. The cannabinoid receptor agonist THC attenuates weight loss in a rodent model of activity-based anorexia. Neuropsychopharmacology. 2011;36:1349–1358. doi: 10.1038/npp.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verty ANA, McGregor IS, Mallet PE. Paraventricular hypothalamic CB1 cannabinoid receptors are involved in the feeding stimulatory effects of Δ9-tetrahydrocannabinol. Neuropharmacology. 2005;49:1101–1109. doi: 10.1016/j.neuropharm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 123.Viveros M-P, Bermúdez-Silva F-J, Lopez-Rodriguez A-B, Wagner EJ. The endocannabinoid system as a pharmacological target derived from its CNS role in energy homeostasis and reward: applications in eating disorders and addiction. Pharmaceuticals. 2011a;4:1101–1136. doi: 10.3390/ph4081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Viveros M-P, Marco-López EM, López-Gallardo M, Garcia-Segura LM, Wagner EJ. Framework for sex differences in adolescent biology: a focus on cannabinoids. Neurosci. Biobehav. Rev. 2011b;35:1740–1751. doi: 10.1016/j.neubiorev.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wakley AA, McBride AA, Vaughn LK, Craft RM. Cyclic ovarian hormone modulation of supraspinal Δ9-tetrahydrocannabinol-induced antinociception and cannabinoid receptor binding in the female rat. Pharmacol. Biochem. Behav. 2014a;124:269–277. doi: 10.1016/j.pbb.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 126.Wakley AA, Wiley JL, Craft RM. Sex differences in antinociceptive tolerance to delta-9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2014b;143:22–28. doi: 10.1016/j.drugalcdep.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wakley AA, Wiley JL, Craft RM. Gonadal hormones do not alter the development of antinociceptive tolerance to delta-9-tetrahydrocannabinol in adult rats. Pharmacol. Biochem. Behav. 2015;133:111–121. doi: 10.1016/j.pbb.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wallace VCJ, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J. Neurosci. 2003;23:3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Washburn N, Borgquist A, Wang K, Jeffery GS, Kelly MJ, Wagner EJ. Receptor subtypes and signal transduction mechanisms contributing to the estrogenic attenuation of cannabinoid-induced changes in energy homeostasis. Neuroendocrinology. 2013;97:160–175. doi: 10.1159/000338669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Watanabe K, Matsunaga T, Narimatsu S, Yamamoto I, Yoshimura H. Sex difference in hepatic microsomal aldehyde oxygenase activity in different strains of mice. Res. Comm. Chem. Pathol. Pharmacol. 1992;78:373–376. [PubMed] [Google Scholar]
- 131.Weston-Green K, Huang X-F, Deng C. Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain. PLoS ONE. 2012;7:e33548. doi: 10.1371/journal.pone.0033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Winek CL. Some historical aspects of marijuana. Clin. Toxicol. 1977;10:242–253. doi: 10.3109/15563657708987969. [DOI] [PubMed] [Google Scholar]
- 133.Woolridge E, Barton S, Samuel J, Osorio J, Dougherty A, Holdcroft A. Cannabis use in HIV for pain and other medical symptoms. J. Pain Symptom Manage. 2005;29:358–367. doi: 10.1016/j.jpainsymman.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 134.Yamamoto I, Gohda H, Narimatsu S, Watanabe K, Yoshimura H. Cannabielsoin as a new metabolite of cannabidiol in mammals. Pharmacol. Biochem. Behav. 1991;40:541–546. doi: 10.1016/0091-3057(91)90360-e. [DOI] [PubMed] [Google Scholar]
- 135.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-α around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonyl glycerol, and presynaptic cannabinoid CB1 receptor. J. Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu L, Xu P, Cao X, Yang Y, Hinton AO, Xia Y, Saito K, Yan X, Zou F, Ding H, Wang C, Yan C, Saha P, Khan SA, Zhao J, Fukuda M, Tong Q, Clegg DJ, Chan L, Xu Y. The ERα-PI3K cascade in proopiomelanocortin progenitor neurons regulates feeding and glucose balance in female mice. Endocrinology. 2015;156:4474–4491. doi: 10.1210/en.2015-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]