Abstract
Variation in maternal care can lead to divergent developmental trajectories in offspring with implications for neuroendocrine function and behavioral phenotypes. Study of the long-term outcomes associated with mother-infant interactions suggests complex mechanisms linking the experience of variation in maternal care and these neurobiological consequences. Through integration of genetic, molecular, cellular, neuroanatomical, and neuroendocrine approaches, significant advances in our understanding of these complex pathways have been achieved. In this review, we will consider the impact of maternal care on male and female offspring development with a particular focus on the issues of timing and mechanism. Identifying the period of sensitivity to maternal care and the temporal dynamics of the molecular and neuroendocrine changes that are a consequence of maternal care represents a critical step in the study of mechanism.
Keywords: maternal, offspring, development, sensitive period, epigenetic, timing
1. INTRODUCTION
Parent-offspring interactions are a critical developmental cue to environmental quality and have the capacity to impact growth, survival, physiology, and behavior. In mammals, biparental care is a relatively rare occurrence and these interactions are primarily through the mother. The capacity of offspring to shift in development in response to the quality of mother-infant interactions may represent an important adaptive pathway that prepares offspring for the conditions of life [1]. Our understanding of the adaptive process and mechanisms underlying the effects of maternal care has been advanced by human longitudinal and laboratory animal studies. Overall, these studies have highlighted the impact of mother-infant interactions on multiple neuroendocrine systems, including the hypothalamic-pituitary-adrenal axis (HPA), the hypothalamic-pituitary-gonadal axis (HPG), and the mesolimbic dopamine (DA) system [2; 3; 4]. Within these systems, there is evidence for long-term transcriptional activation and repression in association with postnatal maternal care, prompting analyses of the impact of mother-infant interactions on epigenetic processes [5]. Epigenetic mechanisms, such as DNA methylation, post-translational histone modifications, and microRNAs have been implicated in studies of the impact of environmental experiences including nutrition [6; 7], toxins [8; 9], stress [10; 11], and social experiences [12; 13]. Variation in [5] or deprivation of [13; 14] maternal care has been demonstrated to induce long-term epigenetic alterations, with implications for the development of neural circuits and the function of these circuits in adulthood.
Plasticity of the brain in response to the quality of mother-infant interactions during the postnatal period suggests the presence of a sensitive period for the development of these systems and their associated physiological and behavioral outcomes. The notion of critical or sensitive periods has a strong foundation within research on sensory systems [15] and social imprinting [16] and suggests that there are windows of time during development in which experiences may be maximally effective in inducing neurobiological and behavioral change. However, in the case of the influence of maternal care, much of the evidence for a particular window of sensitivity is correlative and cross-fostering studies have primarily focused on dissociating the impact of genetic or prenatal vs. postnatal maternal care influences than on identifying postnatal sensitive periods. However, emerging evidence for these periods [17; 18], highlights the need to integrate the study of the temporal dynamics of developmental change when considering the influence of maternal care. Though the long-term effects of maternal care have been relatively well described, the process of change and the intermediary molecular and neurobiological effects that may shape the developing brain have not been systematically explored.
In this review, we will highlight research approaches that have been used to study the impact of maternal care on the developing brain in male and female offspring. We will discuss three specific approaches used primarily in laboratory rodents: 1) the impact of naturally occurring variations in maternal care, 2) communal rearing, and 3) the impact of home-cage disruption. Though there are many other approaches that have been implemented (e.g. neonatal handling, maternal separation), the methodologies we will focus on in this review possess similarities in their effects on both the quantitative and qualitative aspects of maternal care and are currently incorporating epigenetic analyses. We will describe the literature implicating epigenetic mechanisms in the long-term impact of maternal care within these paradigms, with a particular emphasis on the timing of epigenetic changes. Finally, we will explore the notion of critical or sensitive periods in the effects of maternal care and how current and future research approaches can further our understanding of the fundamental questions of the timing and reversibility of epigenetic and neurobiological impact of maternal care.
2. NEUROBIOLOGICAL AND BEHAVIORAL IMPACT OF VARIATION IN MATERNAL CARE
Decades of research has explored the impact of maternal care on the development of offspring using a variety of observational and experimental approaches to quantify or manipulate the quality of mother-infant interactions. Historically, there has been a particular focus on the impact of disruptions to these interactions leading to the establishment of maternal separation or deprivation approaches in non-human primates [19; 20] and rodents [21; 22]. However, longitudinal studies in humans have implicated maternal sensitivity to offspring cues and parental warmth to early- and later-life behavioral and neurobiological outcomes [23; 24]. Thus, variation in care rather than deprivation of care may be an appropriate strategy for studying long-term neurodevelopmental programming. Here, we will consider three approaches in which the impact of this variation can be examined in a laboratory setting: 1) naturally occurring variations in maternal care, 2) communal rearing, and 3) home-cage disruption.
2.1 Natural Variations in Maternal Care
Across species, there are naturally occurring variations in maternal care that predict long-term neurobiological and behavioral phenotypes in offspring. In humans, maternal sensitivity to infant cues is a normally distributed behavior, and infants that have experienced low vs. high maternal sensitivity exhibit increased indices of fearfulness, reduced positive joint attention, increased negative affect, increased aggression, social inhibition and greater right frontal electroencephalographic asymmetry [23; 25]. In non-human primates, high levels of postnatal over-protectiveness (high levels of approach, contact and restraint) in Chlorocebus pygerythrus is associated with reduced exploratory behavior in juvenile offspring [26] and the experience of higher rates of rejection (from mothers, fathers, and siblings) in Callithrix geoffroyi predicts elevated stress-induced urinary cortisol levels [27]. Individual differences in maternal behavior in rodents emerge even within the controlled conditions of the laboratory and form the basis of variation in offspring brain and behavior. In laboratory rats (Rattus norvegicus), observations of home-cage maternal behavior indicate that the experience of low vs. high licking/grooming (LG) from mothers during the postnatal period results in prolonged elevations in plasma corticosterone following stress exposure [28], reduced exploration of novel or anxiogenic environments [29], increased fearfulness [30], and impairments in learning and memory [31] in adult male Long-Evans rat offspring. Adult female offspring of low- compared to high-LG rat dams display increased sexual behavior [32] and reduced maternal behavior [33]. It should be noted that this methodological approach does not typically assess the LG received by individual pups but rather the overall LG “style” of the dam. There is significant stability in LG behavior by dams across subsequent litters and following cross-fostering [34], suggesting that pup characteristics likely do not account for LG status. However, there is considerable within-litter variation in the receipt of LG by pups, such that some pups receive more LG and some pups receive less LG regardless of the LG status of the dam [35; 36]. For example, sex differences in the receipt of LG have been observed in Long-Evans rats, such that males receive higher levels of LG than females [37]. This variation likely contributes to within-litter variation and sex differences in phenotype and the paradoxical findings regarding the effects of between-litter vs. within-litter variation in LG [35; 38].
The behavioral and physiological impact of maternal LG is mediated by alterations in the function of several neural/neuroendocrine systems. In male offspring, the focus of analyses has been on gene/protein targets implicated in stress reactivity, fear responses, and cognition. The increased stress reactivity of adult male offspring of low- vs. high-LG Long-Evans rat dams has been attributed to changes in gene expression and protein levels with hypothalamic and hippocampal regions associated with HPA function (see Table 1). Adult male offspring reared by low-LG dams, have elevated corticotrophin releasing factor (CRF) mRNA in the paraventricular nucleus of the hypothalamus [28] and decreased protein and mRNA levels of glucocorticoid receptors (GR) within the hippocampus which may account for the increased plasma adrenocorticotrophin (ACTH) and corticosterone levels in low-LG offspring following stress exposure [28; 33]. Enhanced fear responses in the offspring of low-LG dams may involve altered expression of subunits within the gamma-aminobutyric acid A receptor (GABAAR) in the amygdala and locus coeruleus, decreased hippocampal glutamate decarboxylase 1 (GAD1) mRNA [39], and increased CRH protein levels within the nucleus tractus solitarus [29; 40].
Table 1.
Impact of low vs. high maternal LG on neuroendocrine outcomes in adult male Long-Evans rats
| Brain region | Effect of low vs. high LG | Reference |
|---|---|---|
| mPFC | ↓ α1, ↓ α4 GABAAR subunit mRNA | Caldji et al. (2003) |
| ↑ stress-induced dopamine release; ↓ COMT protein | Zhang et al. (2005) | |
| ↑ dendritic complexity; ↓ reelin protein levels | Smit-Rigter et al. (2009) | |
| PVN | ↑ CRH mRNA | Liu et al. (1997) |
| hippocampus | ↓ GR mRNA and protein | Liu et al. (1997); Francis et al. (1999) |
| ↓ MR protein | Champagne et al. (2008) | |
| ↑ Nr3c1 DNA methylation; ↓ H3K9Ac and ↓ NGFI-A binding to Nr3c1 promoter |
Weaver et al (2004); Weaver et al. (2005) |
|
| ↑ ↓ H3K9Ac and DNA methylation Chr18; ↑ protocadherin mRNA |
McGowan et al. (2011) | |
| ↓ α1, ↓ β3 GABAAR subunit mRNA | Caldji et al. (2003) | |
| ↓ GAD1 mRNA; ↑ DNA methylation of Gad1 promoter; ↓ H3K9Ac and ↓ NGFI-A binding to Gad1 promoter |
Zhang et al. (2010) | |
| ↓ reelin mRNA; ↓ ATRX mRNA | Weaver et al. (2005) | |
| ↓ acetylcholine release; ↓ choline acetyltransferase activity; ↓ synaptophysin, ↓ NCAM protein |
Liu et al. (2000) | |
| ↑ BAX protein; ↑ apoptosis | Weaver et al. (2002) | |
| ↓ neuronal survival | Bredy et al. (2003) | |
| ↓ NR1, ↓ NR2A, ↓ NR2B, ↓ GluR1, ↓ GluR3 NMDAR subunit mRNA |
Liu et al. (2000) Bredy et al. (2004) |
|
| ↑ GluN2A, ↑ GluN2B, ↑ GluN1 NMDAR subunit mRNA | Bagot et al. (2012) | |
| ↓ EPSPs; ↓ population spike amplitudes; ↓ NMDAR binding; ↑ AMPAR binding |
Bredy et al. (2003) | |
| ↓ reduced dendritic spine density, complexity, and length |
Bagot et al. (2009); Champagne et al. (2008) |
|
| ↓ BDNF mRNA (exon IX); ↑ immature neurons (DCX) | van Hasselt et al. (2012) | |
| ↑ SCN2A mRNA (dorsal hippocampus only) | Nguyen et al. (2015) | |
| ↓ mGluR1 mRNA and protein; ↑ Grm1 promoter DNA methylation; ↓ H3K9Ac and H3K4me3 at Grm1 promoter |
Bagot et al. (2012) | |
| amygdala | ↓ benzodiazepine receptor binding | Caldji et al. (1998); Francis et al. (1999) |
| ↓ α1, ↑ α3, ↑ α4, ↓ α5, ↓ β2, ↓ β 3, ↓ γ1, ↓ γ2 GABAAR subunit mRNA; ↓ α1, ↓ α2, γ2 GABAAR subunit protein | Caldji et al. (2003) | |
| ↓ vasopressin V1a receptor binding (central nucleus) | Francis et al. (2002) | |
| locus coeruleus | ↓ benzodiazepine receptor binding; ↓α2 adrenoreceptor binding; ↑ CRH receptor binding |
Caldji et al. (1998) |
| ↓ α1, α2, ↓ β2, ↓ β3, ↓ γ2 GABAAR subunit mRNA | Caldji et al. (2003) | |
| nucleus tractus solitarius | ↑ CRH receptor binding | Caldji et al. (1998) |
Deficits in learning/memory in the offspring of low-LG dams may be a consequence of several cellular and molecular changes in the medial prefrontal cortex (mPFC) and hippocampus, including decreased protein levels of reelin, synaptophysin, brain-derived neurotrophic factor (BDNF), and neural cell adhesion molecule (NCAM) [31; 36; 41], altered hippocampal expression of subunits within N-methyl-D-aspartate receptor (NMDAR) [31; 42; 43], decreased hippocampal metabotropic glutamate receptor 1 (mGluR1) mRNA [44], decreased hippocampal dendritic complexity [45; 46], and decreased excitatory post-synaptic potentials (EPSPs) indicating impaired long-term potentiation (LTP) [45; 47]. The impact of maternal LG on these outcomes within the hippocampus appears to vary significantly between the dorsal and ventral regions, indicating the regional-specificity of these experience-induced effects [48]. Though impairments in cognition have been observed in low- compared to high-LG offspring, under conditions of HPA activation (elevated corticosterone), low-LG males show enhancements in LTP and cognitive performance [46]. These findings suggest that the function of these systems may be highly context-dependent, with “optimal” performance for low-LG offspring occurring under conditions of heightened stress and deficits in cognition occurring in these offspring under conditions of minimal stress. The context-dependency of these early life influences fits within a framework of predictive adaptive responses [49], in which developmental plasticity in response to environmental cues (i.e. neuroendocrine changes associated with reduced maternal care) prepares individuals for the environmental conditions of later life (i.e. a high stress context). Within this framework, the enhanced cognition observed in low-LG offspring under conditions of stress reflects the better match between the early and later life environments.
In contrast to adult male offspring, where outcomes related to stress physiology and cognition have been a primary focus, the study of female offspring of low- vs. high-LG rat dams has typically focused on reproductive behavior (see Table 2). Adult females of low-LG dams do exhibit reduced hippocampal LTP [36] and stress-induced enhancements in cognitive performance [50], however the neural basis of these phenotypes has not been explored in depth. This bias within the literature is typical of neuroendocrine studies of stress reactivity and cognition in general, where these systems are only explored in females when a specific hypothesis related to the influence of reproductive state (i.e. pregnancy, lactation) is being explored. In female offspring, the study of the influence of LG on reproduction (e.g. sexual and maternal behavior) has focused on hypothalamic regions sensitive to gonadal hormones and targets within the mesolimbic dopamine pathways that have been implicated in the motivation to engage in maternal behavior. The enhanced sexual behavior observed amongst low-LG females is associated with increased estrogen receptor alpha (ERα) mRNA in the anteroventral paraventricular nucleus of the hypothalamus (AVPN) and increased estrogen sensitivity of these steroid receptors in the AVPN and ventral medial hypothalamus (VMH) [2; 51]. However, these females exhibit reduced estrogen sensitivity within hypothalamic structures that play a functional role in maternal behavior. Female offspring of low- compared to high-LG dams have reduced estrogen-stimulated increases in neuronal activation within the medial preoptic area (MPOA), likely attributable to the reduced levels of ERα in this region [52; 53]. Oxytocin receptor protein levels are reduced in the female offspring of low-LG dams within the MPOA, lateral septum, bed nucleus of the stria terminalis (BNST), PVN, and central nucleus of the amygdala [54; 55]. The mesolimbic dopamine system is also shaped by maternal LG. Adult female offspring of low-LG rat dams have reduced dopaminergic projections from the ventral tegmental area (VTA) and reduced expression of LIM homeobox transcription factor 1 beta (Lmx1b) and BDNF in this region [4], which suggests a maternal care influence on factors that contribute to dopaminergic cell differentiation and survival in the ventral midbrain.
Table 2.
Impact of low vs. high maternal LG on neuroendocrine outcomes in adult female Long-Evans rats
| Brain Region | Effect of low vs. high LG | Reference |
|---|---|---|
| lateral septum | ↓ oxytocin receptor binding | Champagne et al. (2007) |
| ↓ estrogen-induced oxytocin receptor binding | Champagne et al. (2001) | |
| BNST | ↓ oxytocin receptor binding | Francis et al. (2002) |
| MPOA | ↓ oxytocin receptor binding | Champagne et al. (2007) |
| ↓ estrogen-induced oxytocin receptor binding | Champagne et al. (2001) | |
| ↓ ERα mRNA and protein; ↓ estrogen-induced cFos |
Champagne et al. (2003) | |
| ↑ Esr1 promoter DNA methylation ↓ Stat5b binding to the Esr1 promoter |
Champagne et al. (2006) | |
| ↓ OTR mRNA; ↓ ERα-IR; ↑ Esr1 promoter DNA methylation; ↓ H3K4me3 at Esr1 promoter |
Pẽna et al. (2013) | |
| ↑ estrogen-induced GnRH-IR | Cameron et al. (2008) | |
| anteroventral paraventricular nucleus of the hypothalamus | ↑ ERα mRNA; ↑ estrogen-induced pERα |
Cameron et al. (2008) |
| ventromedial hypothalamus (VMH) | ↑ pERα-IR (proestrus); ↓c-Fos-IR (proestrus) |
Cameron et al. (2011) |
| ↑ Stat5b binding to the Esr1 promoter | Cameron et al. (2008) | |
| hippocampus | ↓ EPSPs | van Hasselt et al. (2012) |
| PVN | ↓ oxytocin receptor binding | Champagne et al. (2007) |
| amygdala (CN) | ↓ oxytocin receptor binding | Francis et al. (2002) |
| VTA | ↓ TH-IR ↓ Lmx1b mRNA; ↓ BDNF mRNA |
Pẽna et al. (2014) |
Exploration of the impact of maternal care on the brain and behavior has typically focused on adult outcomes and illustrate the long-term impact of variation in mother-infant interactions. However, it is increasingly evident that phenotypic consequences of maternal LG emerge during development (see Table 3). In male rat offspring, reductions in hippocampal GR, BDNF, GAD1 and NMDAR subunit expression are apparent by approximately postnatal (PND) 6. At the time of weaning (PND 21), male offspring that have experienced low-LG have continued reductions in hippocampal BDNF and NMDAR subunit expression [31; 36] as well as reduced synaptophysin and NCAM protein levels leading to reduced neuronal survival [31; 56]. Amongst juvenile (PND 40) male offspring of low-LG rat dams, altered serotonin (5-HT) turnover rates are apparent in the PFC, ventral striatum, and hippocampus [57; 58]. In female offspring, variation in protein and mRNA levels within the MPOA and VTA associated with maternal LG can be observed as early as PND 6. Female pups that have experienced low-LG, have reduced ERα mRNA and protein levels within the MPOA and decreased dopaminergic projections from the VTA [4; 17; 59]. At this developmental time point, the expression of cyclin-dependent kinase inhibitor 1C (cdkn1c) is reduced, which may alter cell proliferation within the ventral midbrain of these offspring [4]. At PND21, female offspring of low-LG rat dams are observed to have reductions in ERα and ERβ mRNA within the MPOA, reduced Lmx1b mRNA within the VTA, and reduced dopamine receptor expression in the nucleus accumbens [4; 17]. Amongst, juvenile females, the reduction in MPOA ERα and reduced dopaminergic projections from the VTA associated with low-LG persists [4; 60]. The early emergence of these neurobiological effects of maternal care may account for the behavioral phenotypes that also emerge prior to adulthood. Juvenile male offspring of low-LG dams exhibit increased indices of anxiety-like behavior [57] and low-LG female offspring display decreased maternal sensitivity to donor pups [17]. Though these developmental studies do provide some insight into the timing of neurobiological change as a consequence of variation in maternal care, a more systematic use of this approach, in which brain-region specific changes in gene expression/protein are assessed at multiple timepoints during development, will be needed to understand the process by which maternal care induces long-term effects. Moreover, our current understanding of what gene targets/systems are affected by maternal LG, developmentally or in adulthood, is based on a priori selection of targets/systems rather than on genome-wide analyses, thus prohibiting conclusions regarding the cascade of changes that occur and the relationship between affected and un-affected target genes. In addition to implementation of un-biased approaches to gene target selection, future studies should also compare and contrast the developmental impact of LG in males and females on the same neuroendocrine/gene targets so as to identify sex differences in response to this critical environmental experience.
Table 3.
Developmental impact of low vs. high maternal LG on neuroendocrine outcomes in Long-Evans rats
| Brain region | Sex | Age | Effect of low vs. high LG | Reference |
|---|---|---|---|---|
| PFC | male | PND 40 | ↑5-HT turnover (ratio of 5H1AA/5-HT) | Masís-Calvo et al. (2013) |
| nucleus accumbens | female | PND 21 | ↓ DRD1, ↓ DRD2, ↓ DRD3 mRNA | Pẽna et al. (2014) |
| male | PND 40 | ↓5-HT turnover, ↓ TrkB mRNA | Sequeira-Cordero et al. (2013) | |
| ventral striatum | male | PND 40 | ↑ DOPAC | Masís-Calvo et al. (2013) |
| MPOA | female | PND 6 | ↓ ERα mRNA; ↓ Stat5b protein |
Champagne et al. (2006) |
| ↓ ERα-IR | Pẽna et al. (2013) | |||
| female | PND 21 | ↓ ERα, ↓ ERβ mRNA; ↑ Esr1 promoter DNA methylation; ↓ H3K4me3 at Esr1 promoter; ↑ H3K9me3 at Esr1 promoter |
Pẽna et al. (2013) | |
| female | PND 40 | ↓ ERα-IR | Pẽna et al. (2014) | |
| hippocampus | male | PND 4 | ↓ GAD1 mRNA | Zhang et al. (2010) |
| ↓ NGFI-A, CBP, NAB1, NAB2 and Sp1 protein | Hellstrom et al. (2012) | |||
| male | PND 6 | ↑ Nr3c1 DNA methylation | Weaver et al. (2004) | |
| ↓ GR mRNA and protein; ↓CBP, H3K9Ac and NGFI-A binding to the Nr3c1 promoter |
Weaver et al. (2007) | |||
| ↓ MBD2 mRNA | Weaver et al. (2014) | |||
| male | PND 8 | ↓ BDNF mRNA; ↓ NR2A, ↓ NR2B NMDAR subunit mRNA |
Liu et al. (2000) | |
| male | PND 21 | ↓ neuronal survival; ↑ apoptosis; ↓ bFGF |
Bredy et al. (2003) | |
| ↑ Nr3c1 DNA methylation | Weaver et al (2004) | |||
| ↓ BDNF mRNA (exon IX) | van Hasselt et al. (2012) | |||
| male | PND 18 | ↓ synaptophysin, ↓NCAM protein; ↓ NR2A, ↓ NR2B NMDAR subunit mRNA | Liu et al. (2000) | |
| male | PND 40 | ↓5-HT turnover | Sequeira-Cordero et al. (2013) | |
| VTA | female | PND 6 | ↓ TH-IR ↓ cdkn1c mRNA |
Pẽna et al. (2014) |
| female | PND 21 | ↓ Lmx1b mRNA | Pẽna et al. (2014) | |
| female | PND 40 | ↓ TH-IR | Pẽna et al. (2014) | |
2.2 Communal Rearing
In biparental species, it is evident that offspring development is altered in response to the absence of the father [61; 62]. In humans, longitudinal studies indicate that early-life transition from a two-biological-parent to single-parent family structure is associated with increased behavioral problems in children [63]. However, even in non-biparental species, the benefit of multiple caregivers can be observed. Studies of communal rearing in rodents (primarily mice; Mus musculus) indicate that lactating females that are co-housed can form a communal nest, whereby multiple lactating females engage in care toward a pooled group of offspring. This rearing experience has been demonstrated to increase maternal behavior (LG and nursing) of individual dams and to enhance growth rates of offspring relative to standard laboratory rearing (a single mouse dam rearing a single litter) [64; 65; 66]. Though the adaptive benefit of this rearing strategy for mothers likely depends on the degree of relatedness of the females participating in the communal care of the litters [67], from the perspective of the offspring, communal rearing can be viewed as an effective approach for enhancing both mother-infant and peer interactions [68]. However, similar to the case of natural variations in LG, the developmental impact of being reared in a communal nest may be modulated by the amount of care received by the individual pup. This within-nest phenomenon is particularly evident when nests are composed of pups of varying ages, where the youngest and oldest pups in the nest have been observed to receive relatively higher levels of maternal care compared to other age groups [69].
Communal rearing in mice has been found to be associated with reductions in anxiety-like and depressive-like behavior as well as increases in social interactions and increased social competence in adult male mouse offspring [66; 70; 71]. Communal rearing also alters responsiveness to antidepressants [72] and may buffer adult male offspring from the effects of social stress [73]. Within the brain, communal rearing is associated with increased BDNF protein levels within the hippocampus, hypothalamus, and striatum, increased nerve growth factor (NGF) protein levels in the hippocampus and hypothalamus [66] and decreased hippocampal 5-HT levels [74]. These increased neurotrophin levels are similar to the developmental effects of high-LG and may account for the increased cell survival observed within the hippocampus [70]. Oxytocin receptor levels are elevated within the anterior cortical nucleus of the amygdala (ACo), central amygdala (CeA), and dorsal posterior medial amygdala (dpMA) of communal vs. standard reared males, though this effect may be attributable to increased peer social interactions, rather than increased maternal care [68]. Though studies of the neurobiological and behavioral impact of communal rearing in mice have focused primarily on male offspring, comparison of the behavioral impact of communal vs. standard rearing on males and females suggests that this early social experience may influence depressive-like behavior more significantly in females and that males and females do show a differential response to postnatal rearing in a communal nest [75].
Variation in LG and the experience of communal rearing has been observed to induce multigenerational effects via alterations in the maternal behavior of female offspring. In Long-Evans rats, offspring of low-LG dams display increased LG in adulthood and this phenotype has also been observed in the grand-offspring generation [33; 34]. Similarly, female mouse offspring (Balb/c) that have been reared in a communal nest exhibit elevated maternal care toward their own offspring, with increases in both nursing and LG observed in these females when rearing their offspring under non-communal conditions [64]. These females also have reductions in anxiety-like behavior. Within the next generation (i.e. daughters of communally reared female mice), there are also indices of enhanced maternal behavior and enhanced growth of offspring [64]. Multigenerational effects on maternal behavior as a consequence of variation in LG in rats have been associated with variation in hypothalamic neuropeptide receptor levels [54], and these systems may also be involved in the transmission of the effects of communal rearing in mice. At PND 28 (weaning), female Balb/c mice that have experienced communal vs. standard rearing during their postnatal development have elevated oxytocin receptor protein levels in the lateral septum, endopiriform nucleus, agranular insular cortex, and BNST and reduced vasopressin 1a receptor (V1a) protein levels in the lateral septum. Among the daughters of these females (who have not been exposed directly to communal rearing), the increased levels of oxytocin receptors and decreased V1a receptors in the lateral septum persists and is observed in adulthood [64]. It is presumed that this maternal transmission of the impact of communal rearing is mediated by the variation in maternal care induced by this manipulation (though this has yet to be established via cross-fostering/cross-rearing manipulations), suggesting strong parallels between these two approaches in the study of the neurobiological impact of maternal care.
2.3 Home-cage Disruption
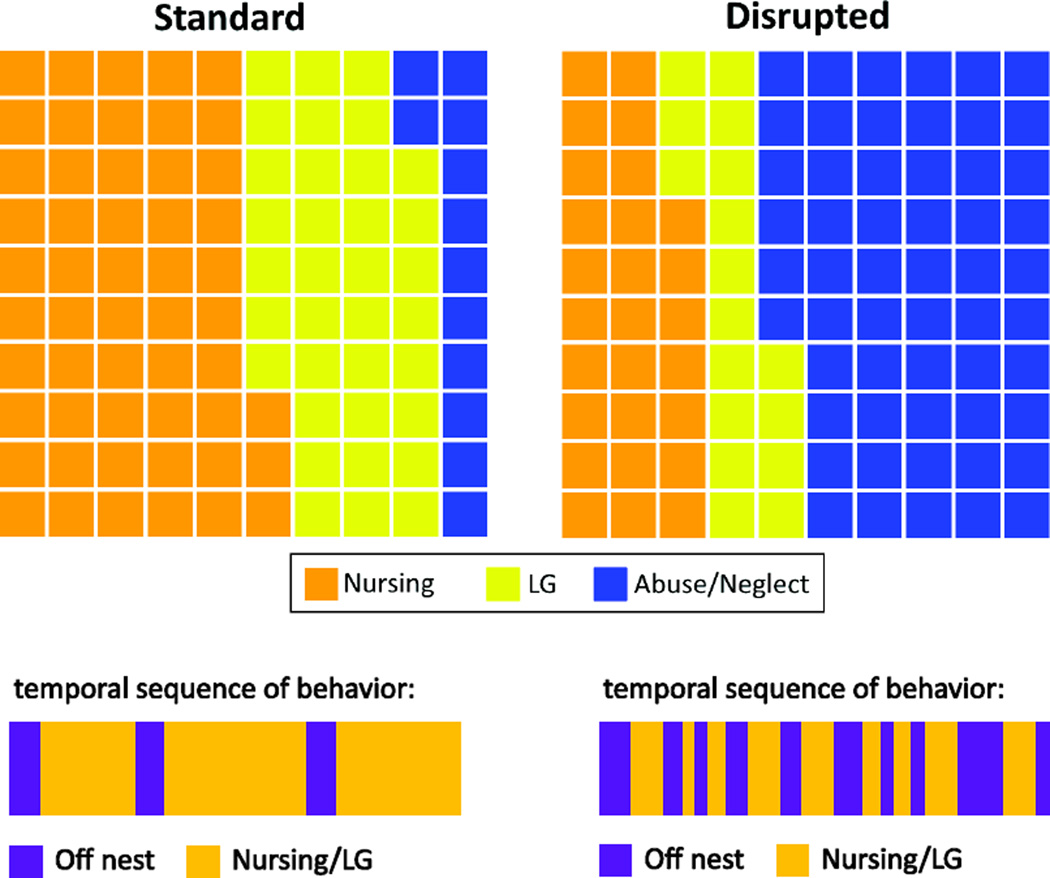
Though approaches to the study of the impact of maternal care on offspring development that involve variation in LG or communal rearing have typically focused on the quantity of maternal care as a critical feature, the quality of those interactions may also vary in these models and serve as a significant predictor of developmental outcomes. For example, Long-Evans rat dams that engage in high-LG display long continuous bouts of maternal care whereas low-LG dams engage in short bursts of maternal care that are juxtaposed with time off the nest [17]. This fragmentation of care can also be induced in laboratory rodents through disruption to the availability of nesting material in the home-cage (see Figure 1). Limiting the quantity of bedding material available to lactating rat dams results in reduced levels of nursing and LG and increases the amount of time pups are out of the nest and not in contact with dams [76; 77]. The shorter duration of bouts of maternal care observed results in an overall impact on the sequence of behavior – with the limited bedding condition inducing a more fragmented behavioral pattern [77]. Following a week of housing under limited bedding conditions, rat dams exhibit increased adrenal weights, increased basal plasma corticosterone levels, and decreased CRH mRNA within the PVN, suggesting that this manipulation serves as a chronic stressor [77]. However, if dams are returned to a standard rearing environment with appropriate levels of bedding after a week of limited-bedding exposure, maternal behavior toward pups is normalized. Thus, this approach can determine the impact of altered maternal care during a specific window during development. This manipulation can also increase the frequency of abusive maternal behavior (stepping on or roughly handling pups; see Figure 1) [78] which may also contribute to the developmental outcomes observed in offspring. This methodological approach may model the parental stress and disrupted parent-offspring interactions that are observed in humans and non-human primates under conditions of low or variable resource availability (i.e. low socioeconomic status; variable foraging demand) which have been associated with elevated CRF levels and increased behavioral problems in childhood [79; 80].
Figure 1.
Comparison of maternal care in rats under standard rearing conditions or following disruption. Removal of nesting materials from the home-cage is disruptive to maternal behavior at the level of frequency and pattern of care. Waffle chart indicating percentage of time spent in each behavior (one block = 1%). Frequency of abusive mother-infant interactions is increased following disruption and frequency of nurturing care (nursing and LG) is decreased following disruption. In addition, the pattern of behavior is altered. Home-cage disruptions lead to more fragmented bouts of maternal care due to frequent time not in contact with pups.
Immediately following a week of exposure (PND 2 to PND 9) to fragmented care within the limited nesting materials rearing approach, there are neuroendocrine changes induced suggestive that pups have experienced chronic stress. At PND 9 (immediately following disrupted maternal care), rat pups have increased adrenal weights, decreased body weights, and increased basal plasma levels of corticosterone [81; 82]. The mRNA levels of several target genes that regulate stress reactivity are altered by the experience of fragmented maternal care, including reduced CRF mRNA within the PVN, reduced hippocampal CRF1 receptor mRNA, and reduced GR mRNA within the PVN and frontal cortex [81]. Structural and molecular changes within the hippocampus suggest that fragmented maternal care also induces reduced synaptic plasticity. In mouse pups (C57BL/6) reared under conditions of reduced nesting material have reduced dendritic length and arborization in CA3 pyramidal neurons. In addition, these pups have reduced hippocampal protein levels of synaptophysin, post-synaptic protein PSD-95, nectin-3, and NMDA receptor subunit expression (NR1, NR2A) [83]. Exposure to this disruption in care also alters the response of rat pups to the mother and increases amygdala activation in response to maternal odors [78]. These altered behavioral and neural responses are suggestive of impairments in social attachment to the mother which may persist even when maternal behavior is normalized after PND 9.
The long-term effects of home cage disruption that persist to weaning and into adulthood, suggest that neural systems regulating response to stress and neural plasticity are particularly sensitive to the impact of this postnatal manipulation (see [84] for review). In mice, increased anxiety-like behavior in response to fragmented maternal care is evident in adulthood as are deficits in learning/memory [85; 86]. Within the adult brain, altered CRH mRNA within the PVN in mice [87] and reduced BDNF mRNA within the prefrontal cortex in rats [88] are associated with the postnatal experience of disrupted maternal care. However, temporal analyses indicate that the structural changes in the brain that may account for the behavioral phenotypes observed in this model may vary in expression from middle age to old age. For example, in middle age rats (4–5 months), reduced hippocampal dendritic complexity and length have been observed in offspring that have experienced disrupted maternal care [76]. However, indices of LTP have been observed to be impaired in old age (12 months) but not middle age offspring [76]. These findings suggest dynamic variation in the brain that is triggered by postnatal events but may express itself differently at a cellular and molecular level dependent on age.
Similar to approaches examining variation in frequency of LG and communal rearing, disruption to the home-cage environment has not typically examined the differential impact of this rearing environment on males vs. females. Many studies examining the immediate impact of this rearing experience on PND 9 pups have included both males and females but have not examined outcomes in these groups separately. In cases where this analysis has been conducted, it is evident that though there is significant overlap in the impact of disrupted maternal care on brain gene expression in males and females, there are also synaptic structural differences between males and females in response to this postnatal experience [83]. In adulthood, most outcomes have been studied exclusively in males. However, similar to the LG and communal rearing approaches, adult females do manifest changes in reproductive behavior. Lactating females rats that have experienced increased fragmented care during their postnatal development engage in a higher frequency of abusive care toward their own pups [88]. Consequently, the effects of disrupted maternal care may persist across generations via the transmission of abusive maternal care, though the mechanism of this transmission needs to be more thoroughly investigated, and may also involve prenatal factors [88].
3. MECHANISTIC PATHWAYS LINKING MATERNAL CARE TO OFFSPRING OUTCOMES
Variation in maternal care, whether it is occurring naturally or achieved through manipulation of the rearing environment, acts as a sensory signal to offspring with immediate consequences that shape developing neural systems. Both olfactory and tactile signals from the mother impact the developing brain. Neural activation is stimulated by odors associated with the early rearing environment, which may facilitate social learning [89]. This neural response to maternal odors is enhanced when combined with licking-like tactile stimulation [90], suggestive that both the presence of the mother and the care provided by the mother induce alterations in brain function. The somatosensory stimulation provided by licking has been demonstrated to increase serum lactate in the brain of newborn but not week-old rat pups [91]. Tactile stimulation in maternally separated PND 8–10 rat pups leads to increased brain levels of ornithine decarboxylase and growth hormone and decreased serum corticosterone, indicating that this stimulation can attenuate the effects of maternal separation [92]. These effects can also be observed at the level of gene expression, with licking-like tactile stimulation preventing maternal separation induced decreases in hippocampal GR mRNA and CRF mRNA in the PVN [93] and attenuating the effects of complete maternal deprivation [94]. In humans, preterm infants also show enhanced growth and neurodevelopment in response to postnatal tactile stimulation [95] and physical touch from mothers can attenuate infant stress responses [96]. Overall, there is significant support for the hypothesis that tactile simulation received by offspring during mother-infant interactions can influence the neural and physiological systems. However, given the diverse effects of maternal care on both short- and long-term developmental outcomes, it will be important to further explore how general signals (e.g. increased growth factors, energy availability, glucocorticoids) integrate with multiple neural systems to achieve specific cellular and molecular outcomes.
A mechanistic question that has been increasingly explored in the context of the impact of maternal care on brain development has focused on the changes in gene expression that are evident even in adulthood (see Table 1 and 2). The regulation of gene expression is a dynamic process, involving coordinated signals from hormones, cellular signaling pathways, and transcription factors. In the case of the long-term effects of variation in maternal care on gene expression, it is evident that a “cellular memory” of the events of postnatal development is predicting levels of mRNA within the brain. The stability of these effects suggests the role of epigenetic mechanisms. DNA methylation, post-translational histone modifications, and small non-coding RNAs are epigenetic molecular processes that alter gene expression without alteration to DNA sequence (see [97; 98; 99] for review). DNA methylation is generally considered the most stable of these processes, due to the strong covalent bond that chemically links the methy-group to cytosines within the DNA [98]. Though it had been assumed that epigenetic alterations had limited plasticity beyond the early stages of embryonic development, there is increasing evidence that these processes are highly dynamic throughout the lifespan in response to a variety of environmental signals. In particular, variation in the quality and/or quantity of maternal care is associated with epigenetic variation in the brain of offspring.
3.1 Epigenetic influence of maternal LG on the Nr3c1 gene
Hippocampal levels of GR serve a critical negative-feedback role within the HPA response to stress [100] and reduced levels of hippocampal GR among the male offspring of low-LG rat dams has been hypothesized to account for the heighted plasma corticosterone response to stress in these offspring [28]. Levels of hippocampal GR protein and mRNA are decreased in PND 6 male offspring that have experienced low levels of postnatal LG and this effect persists into adulthood [5; 28; 101]. Analysis of DNA methylation within the promoter region of the Nr3c1 gene, which encodes for GR, indicates that by PND 6 there is increased DNA methylation of Nr3c1 in offspring of low- compared to high-LG rat dams [5]. Group differences in DNA methylation are not observed prior to PND 6 and then remain constant at PND 21 and in adulthood (PND 90). This differential DNA methylation is particularly evident at the NGFI-A binding site proximal to the Nr3c1 transcription start site. NGFI-A [nerve growth factor-induced protein A; also known as EGR-1 (early growth response protein 1) or Zif268 (zinc finger protein 268)] is a transcription factor [102]. Low levels of maternal care are associated with decreased NGFI-A protein levels at PND 4 and reduced binding of NGFI-A to the Nr3c1 gene promoter at PND 6 [5; 101; 103]. These protein-DNA interactions may be downstream of thyroid hormone signaling that is induced acutely following LG. Plasma levels of triiodothyronine (T3) are increased immediately following mother-infant interactions in high-LG litters and the T3 precursor, thyroxine (T4), is elevated in the plasma of low-LG offspring [103]. This thyroid signaling is necessary for NGFI-A binding to the Nr3c1 gene promoter and is facilitated by 5-HT receptor activation. Provision of licking-like tactile stimulation can trigger these pathways resulting in dynamic epigenetic changes [103]. Levels of methyl-CpG binding domain proteins (MBDs), particularly MBD2, may facilitate these epigenetic effects as MBD2 mRNA levels are increased in PND 6 offspring of high-LG dams in the CA1 and dentate gyrus and levels of MBD2 expression are correlated with levels of GR expression [104].
Though epigenetic regulation of Nr3c1 in the hippocampus in response to maternal LG in rats has been studied in depth, it is important to note that other gene targets within the hippocampus are also altered in expression in response to low- vs. high-LG. In the adult male hippocampus, variation in maternal LG is associated with differential expression of over 900 genes [105]. Analyses of DNA methylation and histone modifications within chromosome 18 (which contains the Nr3c1 gene) indicates multiple loci at which there are increases or decreases in DNA methylation and histone acetylation (H3K9Ac) [106]. Target gene analyses indicate elevated DNA methylation and reduced histone acetylation within the Gad1 (encoding glutamate decarboxylase 1) and Grm1 (encoding metabotropic glutamate receptor 1) gene promoters [39; 44]. The broad epigenetic changes in the hippocampus in response to maternal LG may be due to alterations in genes that contribute generally to epigenetic regulation, such as MBDs and DNA methyltransferases, which have been shown to be altered in expression when comparing offspring of low- vs. high-LG dams [39; 104]. Though these changes emerge during the first week postnatal, it is evident that epigenetic plasticity is present in the adult brain, and pharmacological targeting of methylated CpGs or histones in adulthood can result in the reversibility of the epigenetic effects of postnatal LG [5; 107].
3.2 Epigenetic influence of maternal LG on the Esr1 gene
Sensitivity to hormones is a key determinant of postpartum maternal behavior in rodents, particularly the elevated estrogen levels that coincide with late pregnancy (see [108] for review). The genomic effects of estrogen are mediated through interactions with nuclear estrogen receptors, primarily estrogen receptor alpha (ERα; encoded by the Esr1 gene) and estrogen receptor beta (ERβ; encoded by the Esr2 gene) [109]. Thus, the reduced levels of hypothalamic ERα observed in the female offspring of low-LG rat dams acts to reduce estrogen sensitivity and may contribute to the reduced levels of maternal behavior observed in these offspring. Importantly, the reduced levels of ERα mRNA and protein are present in the developing brain, emerging at PND 6 [17; 59], prior to the hormonal activation of reproductive systems. Analyses of the levels of the transcription factor Stat5b (signal transducer and activator of transcription 5B) indicate that female offspring of high-LG dams have elevated hypothalamic Stat5b protein at PND 6 [59]. These elevations in Stat5b may promote increased transcriptional activity and reduce the likelihood of epigenetic gene silencing of Esr1. By PND 21, female offspring reared by high-LG dams have decreased DNA methylation within the promoter region of the Esr1 gene in comparison to offspring of low-LG dams [17]. Histone marks at the Esr1 gene promoter are also altered in association with LG, with histone tri-methylation at lysine 4 (H3K4me3) increased and histone tri-methylation at lysine 9 (H3K4me3) decreased in the hypothalamus of offspring reared by high-LG rat dams [17]. Collectively, these epigenetic marks contribute to a more accessible/active chromatin state in offspring of high-LG dams and transcriptional repression of Esr1 in the MPOA of offspring of low-LG dams.
Epigenetic regulation of Esr1 within the brain has been observed in response to prenatal exposure to endocrine disruptors [9] and may be modulated by hormonal exposure during development to generate sexual dimorphism in hypothalamic ERα levels [110]. Adult female rats express higher levels of ERα within the MPOA compared to male rats and this sex differences is associated with increased Esr1 gene promoter DNA methylation in males [111]. If females are provided with high levels licking-like tactile stimulation from PND 5–7, sex differences in Esr1 gene promoter DNA methylation are ablated due to increased Esr1 gene promoter DNA methylation in females. Thus, tactile stimulation comparable to that received via mother-infant interactions can alter the epigenetic state of Esr1. This effect of tactile stimulation on Esr1 is not specific to the MPOA and can also be observed in the developing amygdala [112]. Though Esr1 has been a primary focus of epigenetic studies of the impact of maternal care in females, it is unlikely that maternal influences are specific to this gene target. At PND 21, female offspring of high-LG dams also display increased ERβ mRNA levels within the MPOA [17] and increased expression of dopamine receptors within the nucleus accumbens [4], suggestive of broader epigenetic consequences of maternal care.
3.1.3 Epigenetic influence of communal rearing on the Bdnf gene
Neural plasticity is a critical feature of brain development and function, and underlies the ability to adapt to novel environments and experiences. The neurotrophin BDNF has been implicated in the process of neural plasticity (see [113] for review) and it is also evident that variation in maternal care can alter levels of BDNF in the brain. Among adult male offspring that have experienced communal rearing, there are increases in hippocampal BDNF [66]. Genetic and epigenetic analyses of the BDNF promoter reveal the complexity of this gene, which contains multiple promoter regions which are responsive to promoter-specific transcription factors and which generate tissue-specific transcripts [114]. These gene promoters also differ in their transcriptional response to epigenetic variation, indicated by pharmacological studies which induce decreased DNA methylation or increased histone acetylation [114]. Within the hippocampus of adult male mice that have experienced communal rearing, increased histone acetylation is associated with several Bdnf promoters, including promoter I, IV, and VII, suggesting a more transcriptionally active state [115]. This epigenetic variation may account for the increased plasticity of BDNF levels in communally reared offspring in response to novelty. Though DNA methylation has not been explored in this paradigm, it seems likely that promoter specific variation in this epigenetic mark may also be associated with communal rearing and contribute to resulting changes in BDNF mRNA and protein levels in adulthood.
3.1.4 Epigenetic influence of fragmented and abusive care on the Bdnf gene
Neural plasticity may also be impacted by disruption to the home-cage environment during the postnatal period and epigenetic analyses of the Bdnf gene suggest that while communal care promotes a more accessible chromatin state within the Bdnf gene, the converse is true in response to fragmented/abusive care. Exposure to fragmented/abusive care induced through limited nesting material is associated with increased DNA methylation within Bdnf promoter IX in the prefrontal cortex in rats at PND 8 and PND 30 [88]. In adulthood, this increased DNA methylation is observed in Bdnf promoters IV and IX in the prefrontal cortex and administration of zebularine, a drug that inhibits DNA methylation, can alter this epigenetic effect [88]. These epigenetic effects vary as a function of both sex, age, and the brain region being analyzed. At PND 30, abuse-exposed male rats have elevated Bdnf promoter IV DNA methylation in the prefrontal cortex whereas in females there is decreased DNA methylation in this genomic region. In adulthood, both males and females are observed to have increased DNA methylation in Bdnf promoter I in the prefrontal cortex, but at promoter IV only abuse-exposed females are observed to have increased DNA methylation [116]. At PND 8, abuse-exposed females have increased DNA methylation of Bdnf promoter IV in the ventral hippocampus [117]. Within the amygdala, abuse-exposure is associated with decreased BDNF promoter I DNA methylation in females and decreased Bdnf promoter IV DNA methylation in males. However, in adulthood the direction of effect of abusive care on Bdnf DNA methylation in the hippocampus and amygdala suggests dynamic epigenetic changes occurring across the lifespan [117]. This phenomenon is consistent with observed biphasic responses in studies of early life adversity and expression of the Bdnf gene [118].
Though BDNF has been the focus of much of the epigenetic analyses of the impact of abusive/fragmented care, the molecular changes within the Bdnf gene are likely part of broader epigenetic variation induced by this early life experience. Within the dorsal hippocampus, PND 30 male rat offspring that have experienced abusive care have increased global levels of DNA methylation whereas abuse-exposed female offspring have reduced global levels of DNA methylation within the ventral hippocampus [119]. Genes associated with epigenetic remodeling are also altered in expression in response to abusive care. At PND 30, there is decreased expression of the methyl-binding protein MeCp2 within the prefrontal cortex of abuse-exposed males [120]. In adulthood, expression of the DNA methyltransferases DNMT1 (in males) and DNMT3a (in males and females) in the prefrontal cortex are decreased by abuse-exposure. The decreased expression of MeCp2 in males is maintained in adulthood and decreased expression of Gadd45b (growth arrest and DNA-damage-inducible beta), a gene involved in activity-dependent reductions in DNA methylation, is observed in the prefrontal cortex of both males and females [120]. Reduced expression of the histone deacetylase 1 gene (Hdac1) is also observed in the prefrontal cortex of abuse-exposed adult male offspring, suggesting that post-translational histone modification may also account for the long-term impact of disruptions to mother-infant interactions.
4. TIMING AND SENSITIVE PERIODS
Though the postnatal period of development can be thought of generally as a sensitive period, a critical question within the context of studies of mother-infant interactions is regarding the temporal dynamics of the sensitivity to maternal care. Studies of variation in LG focus on the impact of high- vs. low-LG during the first week of life. This timing is when these dams are maximally divergent in their maternal care as LG and nursing decline in general frequency progressively throughout the postnatal period [121]. The importance of this time period are also highlighted by findings that neonatal handling in rats, a manipulation that enhances LG, is only effective in altering developmental outcomes if conducted during the first two weeks postnatal [122]. The timing of the effects of communal rearing are more difficult to discern as this rearing environment encompasses the entire postnatal period. In the case of the disruption to the home-cage environment, this manipulation is typically conducted during the first week postnatal, ending on PND 8–9. This manipulation, similar to maternal separation, offers a paradigm that allows for varying timing and duration of exposure – though the analyses of these variables have yet to be explored. Here we will explore how timing/sensitive periods has been explored within the context of social interactions occurring during development and how these studies contribute to our understanding of the temporal dynamics and mechanisms of response to maternal care.
4.1 Sensitive Periods for Behavioral Imprinting
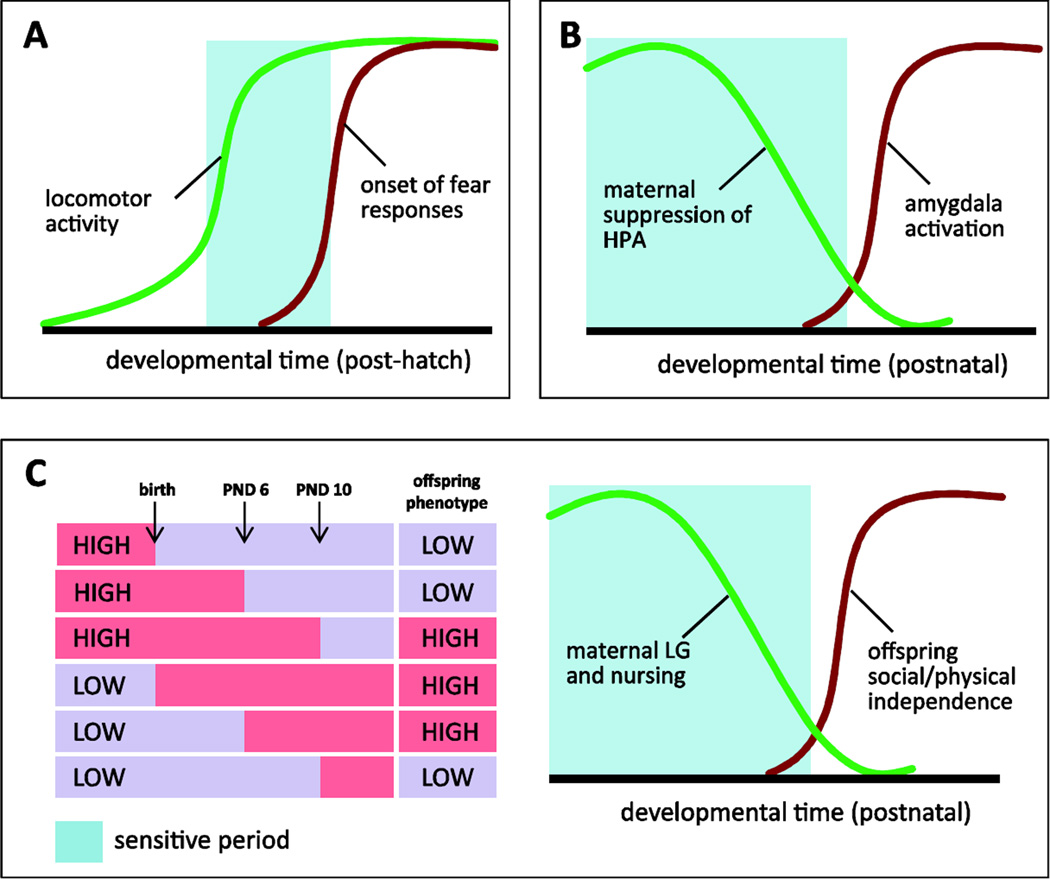
Behavioral imprinting, a phenomenon where newborn chicks form a long-term “attachment” to their parents following hatching, has been a classic model for exploring sensitive/critical periods for social learning (see Figure 2A). These studies have clearly identified a developmental window, during which time, exposure to the imprinting stimulus (typically the parent but can include any visual/auditory stimuli) is necessary to ensure behavioral imprinting. Within the lab, studies of behavioral imprinting involve post-hatching exposure to a stimulus (e.g. replica duck, geometic shape, color) followed by assessment of the amount of ambulatory behavior the chick will engage in to follow the stimulus upon re-exposure. In ducklings, exposure to the imprinting stimulus 16–17 hours post-hatch is maximally effective in generating a positive response (i.e. increased ambulatory behavior toward that stimulus), whereas exposure after 48 hours post-hatch generates minimal levels of imprinting [123]. It has been speculated, that the factors contributing to this temporally specific window of plasticity include the ability to engage in locomotor activity and the onset of fear responses [124]. Speed of locomotor activity in chicks reaches a peak 16–17 hours post-hatch and then plateaus. Thus, the ability to demonstrate imprinting will be limited until maximal ambulatory behavior can be achieved. Prior to post-hatch day 12, chicks do not engage in fear responses to the imprinting stimulus. However, after post-hatch day 17, an increasing percentage of chicks emit distress vocalizations when presented with the imprinting stimulus [124], thus promoting avoidance rather than approach behaviors. Interestingly, the quality of the social environment can alter the temporal dynamics of imprinting, with increased social contact prolonging the time period when imprinting is possible [125].
Figure 2.
Sensitive periods in the context of mother-infant interactions. (A) Behavioral imprinting studies have identified a sensitive period (blue shaded area) for imprinting during post-hatch development. This sensitive period is generated through increasing locomotor behavior of chicks which peaks at the start of the sensitive period. The end of the sensitive period is driven by the development of fear responses to the imprinting stimuli. B) Shock-odor conditioning in rat pups has identified a sensitive period for odor conditioning that starts to decline at PND 10. During this sensitive period, HPA responses and amygdala activation are attenuated. C) Cross-fostering between low and high LG dams indicates a sensitive period for maternal LG. Cross-fostering at birth or PND 6 is effective at shifting offspring phenotype toward that predicted by the foster dam. However, cross-fostering at PND 10 does not shift offspring phenotype. This sensitive period is likely mediated by the high levels of maternal care occurring during the first week postpartum, the heighted group differences in maternal LG during this time, and the increasing physical and social independence from the dam that occurs after this time.
The neural mechanisms involved in shaping the sensitive period for behavioral imprinting likely involve cellular/molecular changes that contribute to synaptic plasticity. Imprinting stimulates NMDA receptors [126] and pharmacological antagonism of these receptors can block the formation of imprinting [127]. Expression of the NR2B subunit of the NMDA receptor may create the windows of plasticity to imprinting. Expression levels of the NR2B subunit is elevated in the hyperpallium densocellulare post-hatch but then replaced by NR2A subunits at later developmental time points [128] and may account for reduced sensitivity to social learning. However, when NMDA receptors are pharmacologically blocked within the sensitive period and chicks are dark-reared (preventing imprinting opportunities), the sensitive period for imprinting can be extended to 8 days post-hatch [128]. Extension of the sensitive period can also be achieved through manipulation of the thyroid hormone system. Thyroid hormones peak at the time of hatching and inhibiting thyroid signaling can prevent imprinting [129]. Moreover, increasing T3 levels during the sensitive period can extend the sensitive period and increasing T3 levels beyond the sensitive period can re-open a period of sensitivity for behavioral imprinting [129]. Thyroid hormone levels can impact NMDA receptor function and subunit expression and so these hormonal signals likely interact with NMDA-mediated synaptic plasticity to shape the sensitive period to this early life experience [130]. Collectively, these neural and behavioral changes promote approach behaviors toward parents in developing offspring in the early phases of development.
4.2 Timing of Infant Attachment
Though behavioral imprinting can be achieved through use of abstract stimuli, this phenomenon is thought to serve primarily as a mechanism to achieve parent-infant attachment. In laboratory rodents, this phenomenon can also be established by examining olfactory conditioning in neonatal rat pups (see Figure 2B). Pairing a shock exposure with an odor can promote approach responses to the odor from birth to until PND 10 [131]. This approach learning is facilitated by an immature fear response system and low shock-induced plasma corticosterone levels characteristic of the stress hyporesponsive period [132]. From PND 10 onward, shock-odor pairings promote odor avoidance behaviors. Thus, there is a sensitive period for approach learning which may contribute to the formation of an attachment relationship. During the sensitive period, amygdala activation is suppressed during conditioning whereas avoidance responses occur in later development when amygdala activation is heightened during conditioning [131]. Levels of plasma corticosterone are also a critical modulator of this sensitive period. During the first two weeks postnatal, rat pups have an attenuated HPA response to stress [132]. This hyporesponsivity is associated with a period of high-levels of mother-infant interactions and absence of the mother during this period, through maternal separation, results in a robust elevation in plasma corticosterone levels in response to stress [133]. Within the postnatal period, elevations in corticosterone at PND 6 (but not PND5) can switch odor-shock conditioning to avoidance responses [18]. However, a premature switch occurring during the sensitive period does not appear to permanently close the sensitive period, indicated by the ability to induce approach behavior when a new odor is presented during subsequent conditioning trials [18]. Inhibition of corticosterone, either pharmacologically or through presence of the mother can extend the sensitive period for approach learning at PND 15 [18]. However, this appears to be the furthest time point in which approach rather than avoidance responses can be achieved through odor-shock pairings. The presence of the mother during post-sensitive period odor-shock conditioning inhibits amygdala activation and this effect is mediated through maternal suppression of pup corticosterone release [134]. These neuroendocrine characteristics of the neonatal rat pup may account for the approach behaviors observed in offspring exposed to abusive caregiving. Within the home-cage disruption paradigm, it has been noted that pups exposed to an abusive dam will continue to seek mother-infant interactions with the dam, despite the adversity associated with those interactions [135].
4.4 Impact of Cross-fostering
The impact of postnatal maternal care on development can be most clearly illustrated in cross-fostering or adoption studies. In humans, the impact of postnatal social deprivation in the form of institutional rearing has been found to more severely impact cognitive ability if individuals are adopted into families after six months of institutionalization [136] – suggesting that the duration of maternal absence is predictive of long-term outcomes. Intervention studies, in which institutional reared infants are fostered into a caregiving family, indicate that the HPA response to stress can be altered by altering the quality of the caregiving environment. Moreover, there appears to be a sensitive period for these effects, with intervention effects only evident if fostering is conducted prior to two years of age [137]. Cross-fostering studies in non-human primates indicate that the transmission of abusive caregiving behavior is related to the abusive phenotype of the rearing rather than the biological mother [138]. In rodents, cross-fostering at birth between phenotypically divergent individuals can result in a shift in phenotype in the direction of the foster/rearing mother, suggesting the impact of maternal care. For example, among rats selectively bred for emotionality (response to novelty), which generates high responders (HR; e.g. highly exploratory and impulsive) vs. low responders (LH; e.g. heightened anxiety- and depressive-like behavior), cross-fostering at birth between HR and LR results in reduced anxiety-like behavior and altered gene expression in the amygdala of LR offspring reared by HR dams [139]. In mice selectively bred for alcohol preference [high alcohol preference (HAP) vs. low alcohol preference (LAP)], cross-fostering at birth indicates that HAP pups reared by LAP dams have reduced levels of alcohol preference. However, an effect of cross-fostering on alcohol preference is not observed in LAP pups reared by HAP dams, indicating some constraints on this maternal influence [140]. Balb/c and C57BL/6 (B6) mice differ on multiple neurobiological and behavioral measures, including anxiety-like behavior [141], which is elevated in Balb/c mice, and maternal behavior, with Balb/c lactating females exhibiting comparatively less maternal LG toward pups [142]. Use of both prenatal (embryo transfer) and postnatal cross-fostering indicates that the phenotype of a B6 mouse can be shifted toward the phenotype of a Balb/c mouse on anxiety-like measures if the B6 embryo and developing pup are exposed to the Balb/c maternal environment [143]. In this case, postnatal cross-fostering alone was not sufficient to shift phenotype, indicating the influence of the prenatal period.
Exploration of the impact of natural variations in maternal behavior has used postnatal cross-fostering to illustrate the link between the experience of LG and the long-term consequences of LG observed in adulthood (see Tables 1 & 2). The LG status (low vs. high) of a dam is highly stable across subsequent litters allowing for a characterization of this phenotype prior to initiating cross-fostering [34]. Cross-fostering pups on the day of birth between low- and high-LG dams reveals that it is the rearing mother rather than the biological mother LG status that is predictive of exploratory behavior, GR expression, and Nr3c1 DNA methylation in adult male rat offspring [5; 33]. Thus, offspring born to a low-LG dam and cross-fostered at birth to a high-LG dam display phenotypes associated with high-LG. The converse is evident in offspring born to a high-LG dam and cross-fostered at birth to a low-LG dam. In the case of female offspring, levels of ERα mRNA in the MPOA and maternal LG displayed in adulthood are also predicted by the status of the rearing mother when cross-fostering is conducted on the day of birth [59]. However, this approach does not address the question of the constraints of the sensitive period during which LG can alter development. This question can be addressed by implementing the cross-fostering at later time points within the postnatal period. Recent studies have explored the impact of cross-fostering offspring between low- and high-LG rat dams on PND 6 and PND 10 to determine the period of sensitivity to postnatal LG [17] (see Figure 2C). Cross-fostering at PND 6 was found to shift levels of ERα mRNA in the MPOA and maternal sensitivity of juvenile offspring toward the phenotype of the foster dam. Thus, offspring that had experienced low levels of LG up until PND 6 who were then cross-fostered to a high-LG dam had elevated levels of ERα in the MPOA and increased maternal sensitivity (compared to non-fostered siblings). The converse was observed in offspring initially reared by a high-LG dam; cross-fostering at PND 6 resulted in reduced levels of ERα mRNA in the MPOA and decreased maternal sensitivity [17]. This alteration in ERα and maternal sensitivity is also observed through targeted manipulation of Esr1 expression in the developing hypothalamus at PND 4. Over-expression of Esr1 in the hypothalamus during this sensitive period results in increased ERα mRNA and protein in the MPOA and increased maternal sensitivity in offspring reared by low-LG dams [60]. However, sensitivity to LG appears to diminish by PND 10. Offspring reared initially by a low- or high-LG dam who were then cross-fostered to a high- or low-LG dam at PND 10 did not exhibit any change in ERα mRNA or maternal sensitivity [17]. Similar to studies of olfactory conditioning, these developmental outcomes have limited plasticity in response to the maternal environment beyond PND 10, suggesting temporal constraints on the sensitive period for the effects of maternal care.
One of the caveats of the cross-fostering approach is that the utility of this method in determining the causal impact of maternal care on development is dependent on the assumption that maternal phenotype is not altered by the phenotype/genotype of pups. However, as has been demonstrated repeatedly in rearing and cross-fostering studies, offspring can exert considerable influence on the quality of care they receive. For example, spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats display divergent levels of maternal care, with SHR dams engaging in elevated levels of LG and nursing compared to WKY dams [144]. However, when pups are cross-fostered on the day of birth between SHR and WKY dams, the maternal behavior of these dams shifts toward that of the pup strain. SHR dams caring for WKY pups display reduced levels of LG and nursing, whereas these behaviors are increased in WKY dams rearing SHR pups [145]. In mice, though B6 dams typically show reduced levels of nursing behavior when compared to dams of the 129S strain, rearing fostered B6 pups abolishes these strain differences in maternal behavior [146]. In the case of variation in LG, during the first week postnatal, group differences in maternal LG are maintained even when dams are rearing fostered pups [34]. However, the behavioral differences between low- vs. high-LG dams diminish across the postnatal period, due to overall decreases in both LG and pup nursing [121]. Pup age is a significant predictor of the amount of maternal care received [68] and the incentive value of the pups to the dam is significantly higher prior to PND 10 compared to later postpartum time points [147]. These changes in mother-infant interactions across time reflect the changing developmental needs of rodent pups and so it may be these naturally occurring shifts toward social and nutritional independence that shape the sensitive period to variation in maternal care.
5. CONCLUSIONS & FUTURE DIRECTIONS
Advances in our understanding of the mechanisms through which maternal care influences the developing brain have come through integration of both experimental and epidemiological studies and through analyses of behavioral, neuroendocrine, cellular, and molecular levels at which these influences manifest. Epigenetic changes have been demonstrated to be associated with the quantity and quality of maternal care experienced during development using a variety of experimental approaches that model both increases in and disruption to mother-infant interactions [5; 59; 88; 115]. It is also clear, that there are sensitive periods for the impact of maternal care that are shaped by neurobiological and behavioral changes that accompany the transition to independence [17; 18; 124; 148; 149]. However, these sensitive periods can be shifted or even re-opened through manipulation of neural systems involved in plasticity and it is certainly the case that epigenetic plasticity may continue throughout the lifespan [5; 107]. It is also likely, that social experiences that characterize each developmental stage may have the capability to alter neurobehavioral development. Though mother-infant interactions may have a sensitive period that ends pre-weaning, characteristics of the weaning process, interactions with peers, and experiences during reproduction should also be considered as developmentally meaningful signals that can alter brain function and behavior.
Though our knowledge of the mechanisms through which maternal care shapes offspring development has certainly expanded, there are many areas within this field of study that hold significant promise for elucidating the process and temporal dynamics of this maternal influence. (1) Timing of developmental and epigenetic changes. Though there have been increasing efforts to include time course analysis of the effects of maternal care, the systematic use of this approach is needed across paradigms. Molecular changes that transduce the influence of maternal care may not necessarily overlap with those mechanisms that maintain the changes over the lifespan. Thus, without these temporal insights, conclusions regarding process will be difficult to make. Cross-fostering between dams that vary in maternal phenotype or limiting exposure to communal rearing or home-cage disruption to specific time-points may also be revealing regarding the timing and sensitivity to developmental change. (2) Sex differences matter. Converging evidence indicates the differential impact of early life experiences on long-term developmental outcomes [9; 10; 75]. Despite this knowledge, the assessment of both males and females in basic neuroscience and pre-clinical studies is relatively rare. Though both male and female offspring have been assessed in the experimental approaches used to study the influence of maternal care, comparison of the impact of mothers on males vs. females is not systematically employed. Epigenetic analyses suggest that males and females have a differential molecular response to disruptions in maternal care and yet there is limited understanding of the factors that contribute to this sex difference. Policy changes may encourage the comparison of male and female offspring [150], and perhaps allow for better integration of HPG development with epigenetic and neural systems influenced by maternal care. (3) A complex maternal environment. Though variation in maternal care, particularly within the low- vs. high-LG model, implicates the tactile components of mothering as a critical mediating variable, mother-infant interactions are complex and likely involve multiple pathways of influence. In addition to tactile stimulation and the formation of odor preferences, mothers can shape development via hormones transmitted during nursing [151], thermoregulation during nursing [152] and through influence on the microbiome [153]. These maternal factors likely work collectively to shape development, interact with characteristics of the offspring, and create multiple possible developmental trajectories. (4) Developmental age. The quality of the early maternal/social environment may alter the pace of developmental change, thus adding an additional layer of complexity to the study of the timing of the effects of maternal care. The experience of low levels of maternal care is associated with early weaning and puberty onset [2; 149] and with accelerated maturation of fear systems [154], which may reduce the duration of the sensitive period to maternal care. Thus, it may be necessary to focus not on the changes that emerge at a given chronological age across groups but rather to examine whether those changes emerge earlier or later as a function of the quantity or quality of mother-infant interactions – highlighting the importance of timing to better understand mechanism.
HIGHLIGHTS.
Variation in maternal care induces both short- and long-term neurobiological effects.
Maternal care predicts brain area-specific gene-specific and global epigenetic effects.
There are sensitive periods for experiences related to mother-infant interactions.
Behavioral and neuroendocrine events define sensitive periods for maternal influences.
It is critical to understand timing in the context of maternal influences on development.
Acknowledgements
This research was funded by NIMH 1P50MH090964-01A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Cameron NM, Champagne FA, Parent C, Fish EW, Ozaki-Kuroda K, Meaney MJ. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci Biobehav Rev. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS One. 2008;3:e2210. doi: 10.1371/journal.pone.0002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 4.Pena CJ, Neugut YD, Calarco CA, Champagne FA. Effects of maternal care on the development of midbrain dopamine pathways and reward-directed behavior in female offspring. Eur J Neurosci. 2014;39:946–956. doi: 10.1111/ejn.12479. [DOI] [PubMed] [Google Scholar]
- 5.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 6.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 7.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roth TL, Zoladz PR, Sweatt JD, Diamond DM. Epigenetic modification of hippocampal Bdnf DNA in adult rats in an animal model of post-traumatic stress disorder. J Psychiatr Res. 2011 doi: 10.1016/j.jpsychires.2011.01.013. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollis F, Wang H, Dietz D, Gunjan A, Kabbaj M. The effects of repeated social defeat on long-term depressive-like behavior and short-term histone modifications in the hippocampus in male Sprague-Dawley rats. Psychopharmacology (Berl) 2010;211:69–77. doi: 10.1007/s00213-010-1869-9. [DOI] [PubMed] [Google Scholar]
- 13.Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OF, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12:1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- 14.Franklin TB, Russig H, Weiss IC, Graff J, Linder N, Michalon A, Vizi S, Mansuy IM. Epigenetic transmission of the impact of early stress across generations. Biol Psychiatry. 2010;68:408–415. doi: 10.1016/j.biopsych.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 15.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess EH. Imprinting, an effect of early experience, imprinting determines later social behavior in animals. Science. 1959;130:133–141. doi: 10.1126/science.130.3368.133. [DOI] [PubMed] [Google Scholar]
- 17.Pena CJ, Neugut YD, Champagne FA. Developmental timing of the effects of maternal care on gene expression and epigenetic regulation of hormone receptor levels in female rats. Endocrinology. 2013;154:4340–4351. doi: 10.1210/en.2013-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Dev Psychobiol. 2010;52:453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proc Natl Acad Sci U S A. 1965;54:90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suomi SJ, Collins ML, Harlow HF, Ruppenthal GC. Effects of maternal and peer separations on young monkeys. J Child Psychol Psychiatry. 1976;17:101–112. doi: 10.1111/j.1469-7610.1976.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 21.Hofer MA. Maternal separation affects infant rats' behavior. Behav Biol. 1973;9:629–633. doi: 10.1016/s0091-6773(73)80057-4. [DOI] [PubMed] [Google Scholar]
- 22.West JR. Use of pup in a cup model to study brain development. J Nutr. 1993;123:382–385. doi: 10.1093/jn/123.suppl_2.382. [DOI] [PubMed] [Google Scholar]
- 23.Hane AA, Henderson HA, Reeb-Sutherland BC, Fox NA. Ordinary variations in human maternal caregiving in infancy and biobehavioral development in early childhood: A follow-up study. Dev Psychobiol. 2010;52:558–567. doi: 10.1002/dev.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narita K, Takei Y, Suda M, Aoyama Y, Uehara T, Kosaka H, Amanuma M, Fukuda M, Mikuni M. Relationship of parental bonding styles with gray matter volume of dorsolateral prefrontal cortex in young adults. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Hane AA, Fox NA. Ordinary variations in maternal caregiving influence human infants' stress reactivity. Psychol Sci. 2006;17:550–556. doi: 10.1111/j.1467-9280.2006.01742.x. [DOI] [PubMed] [Google Scholar]
- 26.Fairbanks LA, McGuire MT. Long-term effects of early mothering behavior on responsiveness to the environment in vervet monkeys. Dev Psychobiol. 1988;21:711–724. doi: 10.1002/dev.420210708. [DOI] [PubMed] [Google Scholar]
- 27.Birnie AK, Taylor JH, Cavanaugh J, French JA. Quality of maternal and paternal care predicts later stress reactivity in the cooperatively-breeding marmoset (Callithrix geoffroyi) Psychoneuroendocrinology. 2013;38:3003–3014. doi: 10.1016/j.psyneuen.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 29.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menard JL, Champagne DL, Meaney MJ. Variations of maternal care differentially influence 'fear' reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience. 2004;129:297–308. doi: 10.1016/j.neuroscience.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 32.Cameron NM, Fish EW, Meaney MJ. Maternal influences on the sexual behavior and reproductive success of the female rat. Horm Behav. 2008;54:178–184. doi: 10.1016/j.yhbeh.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 34.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 35.Pan P, Fleming AS, Lawson D, Jenkins JM, McGowan PO. Within- and between-litter maternal care alter behavior and gene regulation in female offspring. Behav Neurosci. 2014;128:736–748. doi: 10.1037/bne0000014. [DOI] [PubMed] [Google Scholar]
- 36.van Hasselt FN, Cornelisse S, Zhang TY, Meaney MJ, Velzing EH, Krugers HJ, Joels M. Adult hippocampal glucocorticoid receptor expression and dentate synaptic plasticity correlate with maternal care received by individuals early in life. Hippocampus. 2012;22:255–266. doi: 10.1002/hipo.20892. [DOI] [PubMed] [Google Scholar]
- 37.Moore CL, Morelli GA. Mother rats interact differently with male and female offspring. J Comp Physiol Psychol. 1979;93:677–684. doi: 10.1037/h0077599. [DOI] [PubMed] [Google Scholar]
- 38.Ragan CM, Loken E, Stifter CA, Cavigelli SA. Within-litter variance in early rat pup-mother interactions and adult offspring responses to novelty. Dev Psychobiol. 2012;54:199–206. doi: 10.1002/dev.20581. [DOI] [PubMed] [Google Scholar]
- 39.Zhang TY, Hellstrom IC, Bagot RC, Wen X, Diorio J, Meaney MJ. Maternal care and DNA methylation of a glutamic acid decarboxylase 1 promoter in rat hippocampus. J Neurosci. 2010;30:13130–13137. doi: 10.1523/JNEUROSCI.1039-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–1959. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- 41.Smit-Rigter LA, Champagne DL, van Hooft JA. Lifelong impact of variations in maternal care on dendritic structure and function of cortical layer 2/3 pyramidal neurons in rat offspring. PLoS One. 2009;4:e5167. doi: 10.1371/journal.pone.0005167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bagot RC, Tse YC, Nguyen HB, Wong AS, Meaney MJ, Wong TP. Maternal care influences hippocampal N-methyl-D-aspartate receptor function and dynamic regulation by corticosterone in adulthood. Biol Psychiatry. 2012;72:491–498. doi: 10.1016/j.biopsych.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Bredy TW, Zhang TY, Grant RJ, Diorio J, Meaney MJ. Peripubertal environmental enrichment reverses the effects of maternal care on hippocampal development and glutamate receptor subunit expression. Eur J Neurosci. 2004;20:1355–1362. doi: 10.1111/j.1460-9568.2004.03599.x. [DOI] [PubMed] [Google Scholar]
- 44.Bagot RC, Zhang TY, Wen X, Nguyen TT, Nguyen HB, Diorio J, Wong TP, Meaney MJ. Variations in postnatal maternal care and the epigenetic regulation of metabotropic glutamate receptor 1 expression and hippocampal function in the rat. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17200–17207. doi: 10.1073/pnas.1204599109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92:292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Joels M, Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28:6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ. Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience. 2003;118:571–576. doi: 10.1016/s0306-4522(02)00918-1. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen HB, Bagot RC, Diorio J, Wong TP, Meaney MJ. Maternal care differentially affects neuronal excitability and synaptic plasticity in the dorsal and ventral hippocampus. Neuropsychopharmacology. 2015;40:1590–1599. doi: 10.1038/npp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bateson P, Gluckman P, Hanson M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J Physiol. 2014;592:2357–2368. doi: 10.1113/jphysiol.2014.271460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barha CK, Pawluski JL, Galea LA. Maternal care affects male and female offspring working memory and stress reactivity. Physiol Behav. 2007;92:939–950. doi: 10.1016/j.physbeh.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Cameron NM, Soehngen E, Meaney MJ. Variation in maternal care influences ventromedial hypothalamus activation in the rat. J Neuroendocrinol. 2011;23:393–400. doi: 10.1111/j.1365-2826.2011.02124.x. [DOI] [PubMed] [Google Scholar]
- 52.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4724. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- 54.Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- 55.Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- 56.Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–2909. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- 57.Masis-Calvo M, Sequeira-Cordero A, Mora-Gallegos A, Fornaguera-Trias J. Behavioral and neurochemical characterization of maternal care effects on juvenile Sprague-Dawley rats. Physiol Behav. 2013;118:212–217. doi: 10.1016/j.physbeh.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 58.Sequeira-Cordero A, Masis-Calvo M, Mora-Gallegos A, Fornaguera-Trias J. Maternal behavior as an early modulator of neurobehavioral offspring responses by Sprague-Dawley rats. Behav Brain Res. 2013;237:63–70. doi: 10.1016/j.bbr.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 59.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 60.Pena CJ, Champagne FA. Neonatal over-expression of estrogen receptor-alpha alters midbrain dopamine neuron development and reverses the effects of low maternal care in female offspring. Dev Neurobiol. 2014 doi: 10.1002/dneu.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao Y, Wu R, Tai F, Zhang X, Yu P, An X, Qiao X, Hao P. Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen receptor alpha mRNA expression in the MeA and NAcc, and serum oxytocin in mandarin voles. Horm Behav. 2014;65:57–65. doi: 10.1016/j.yhbeh.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 63.Ryan RM, Claessens A, Markowitz AJ. Associations between family structure change and child behavior problems: the moderating effect of family income. Child Dev. 2015;86:112–127. doi: 10.1111/cdev.12283. [DOI] [PubMed] [Google Scholar]
- 64.Curley JP, Davidson S, Bateseon P, Champagne FA. Social enrichment during postnatal development induces transgenerational effects on emotional and reproductive behavior in mice. Front Beh Neurosci. 2009 doi: 10.3389/neuro.08.025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sayler A, Salmon M. Communal nursing in mice: influence of multiple mothers on the growth of the young. Science. 1969;164:1309–1310. doi: 10.1126/science.164.3885.1309. [DOI] [PubMed] [Google Scholar]
- 66.Branchi I, D'Andrea I, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol Psychiatry. 2006;60:690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 67.Konig B. Maternal investment of communally nursing female house mice (Mus musculus domesticus) Behav Processes. 1993;30:61–73. doi: 10.1016/0376-6357(93)90012-G. [DOI] [PubMed] [Google Scholar]
- 68.Branchi I, Curley JP, D'Andrea I, Cirulli F, Champagne FA, Alleva E. Early interactions with mother and peers independently build adult social skills and shape BDNF and oxytocin receptor brain levels. Psychoneuroendocrinology. 2013;38:522–532. doi: 10.1016/j.psyneuen.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Branchi I, D'Andrea I, Gracci F, Santucci D, Alleva E. Birth spacing in the mouse communal nest shapes adult emotional and social behavior. Physiol Behav. 2009;96:532–539. doi: 10.1016/j.physbeh.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 70.Branchi I, D'Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, Alleva E. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and "depression"-like behavior. J Neurosci Res. 2006;83:965–973. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- 71.D'Andrea I, Alleva E, Branchi I. Communal nesting, an early social enrichment, affects social competences but not learning and memory abilities at adulthood. Behav Brain Res. 2007;183:60–66. doi: 10.1016/j.bbr.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 72.Branchi I, D'Andrea I, Cirulli F, Lipp HP, Alleva E. Shaping brain development: Mouse communal nesting blunts adult neuroendocrine and behavioral response to social stress and modifies chronic antidepressant treatment outcome. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 73.Branchi I, Santarelli S, D'Andrea I, Alleva E. Not all stressors are equal: early social enrichment favors resilience to social but not physical stress in male mice. Horm Behav. 2013;63:503–509. doi: 10.1016/j.yhbeh.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 74.Connors EJ, Migliore MM, Pillsbury SL, Shaik AN, Kentner AC. Environmental enrichment models a naturalistic form of maternal separation and shapes the anxiety response patterns of offspring. Psychoneuroendocrinology. 2015;52:153–167. doi: 10.1016/j.psyneuen.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 75.D'Andrea I, Gracci F, Alleva E, Branchi I. Early social enrichment provided by communal nest increases resilience to depression-like behavior more in female than in male mice. Behav Brain Res. 2010;215:71–76. doi: 10.1016/j.bbr.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 76.Brunson KL, Kramár E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154:1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coplan JD, Altemus M, Mathew SJ, Smith EL, Sharf B, Coplan PM, Kral JG, Gorman JM, Owens MJ, Nemeroff CB, Rosenblum LA. Synchronized maternal-infant elevations of primate CSF CRF concentrations in response to variable foraging demand. CNS Spectr. 2005;10:530–536. doi: 10.1017/s109285290001018x. [DOI] [PubMed] [Google Scholar]
- 80.Rijlaarsdam J, Stevens GW, van der Ende J, Hofman A, Jaddoe VW, Mackenbach JP, Verhulst FC, Tiemeier H. Economic disadvantage and young children's emotional and behavioral problems: mechanisms of risk. J Abnorm Child Psychol. 2013;41:125–137. doi: 10.1007/s10802-012-9655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Avishai-Eliner S, Gilles EE, Eghbal-Ahmadi M, Bar-El Y, Baram TZ. Altered regulation of gene and protein expression of hypothalamic-pituitary-adrenal axis components in an immature rat model of chronic stress. J Neuroendocrinol. 2001;13:799–807. doi: 10.1046/j.1365-2826.2001.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol. 1996;15:114–119. doi: 10.1016/0887-8994(96)00153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liao XM, Yang XD, Jia J, Li JT, Xie XM, Su YA, Schmidt MV, Si TM, Wang XD. Blockade of corticotropin-releasing hormone receptor 1 attenuates early-life stress-induced synaptic abnormalities in the neonatal hippocampus. Hippocampus. 2014;24:528–540. doi: 10.1002/hipo.22254. [DOI] [PubMed] [Google Scholar]
- 84.Chen Y, Baram TZ. Towards understanding how early-life stress re-programs cognitive and emotional brain networks. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang XD, Labermaier C, Holsboer F, Wurst W, Deussing JM, Muller MB, Schmidt MV. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur J Neurosci. 2012;36:2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x. [DOI] [PubMed] [Google Scholar]
- 86.Wang XD, Rammes G, Kraev I, Wolf M, Liebl C, Scharf SH, Rice CJ, Wurst W, Holsboer F, Deussing JM, Baram TZ, Stewart MG, Muller MB, Schmidt MV. Forebrain CRF(1) modulates early-life stress-programmed cognitive deficits. J Neurosci. 2011;31:13625–13634. doi: 10.1523/JNEUROSCI.2259-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Res Dev Brain Res. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- 90.Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986;392:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- 91.Alasmi MM, Pickens WL, Hoath SB. Effect of tactile stimulation on serum lactate in the newborn rat. Pediatr Res. 1997;41:857–861. doi: 10.1203/00006450-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 92.Pauk J, Kuhn CM, Field TM, Schanberg SM. Positive effects of tactile versus kinesthetic or vestibular stimulation on neuroendocrine and ODC activity in maternally-deprived rat pups. Life Sci. 1986;39:2081–2087. doi: 10.1016/0024-3205(86)90359-0. [DOI] [PubMed] [Google Scholar]
- 93.van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant's neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998;18:10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task--reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- 95.Field TM, Schanberg SM, Scafidi F, Bauer CR, Vega-Lahr N, Garcia R, Nystrom J, Kuhn CM. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics. 1986;77:654–658. [PubMed] [Google Scholar]
- 96.Feldman R, Singer M, Zagoory O. Touch attenuates infants' physiological reactivity to stress. Dev Sci. 2010;13:271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 97.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 98.Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. Embo J. 1998;17:4905–4908. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sato F, Tsuchiya S, Meltzer SJ, Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278:1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- 100.Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169–173. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- 101.Weaver IC, D'Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–1768. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 103.Hellstrom IC, Dhir SK, Diorio JC, Meaney MJ. Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade. Philos Trans R Soc Lond B Biol Sci. 2012;367:2495–2510. doi: 10.1098/rstb.2012.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weaver IC, Hellstrom IC, Brown SE, Andrews SD, Dymov S, Diorio J, Zhang TY, Szyf M, Meaney MJ. The methylated-DNA binding protein MBD2 enhances NGFI-A (egr-1)-mediated transcriptional activation of the glucocorticoid receptor. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McGowan PO, Suderman M, Sasaki A, Huang TC, Hallett M, Meaney MJ, Szyf M. Broad epigenetic signature of maternal care in the brain of adult rats. PLoS One. 2011;6:e14739. doi: 10.1371/journal.pone.0014739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 109.McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 110.Nugent BM, Wright CL, Shetty AC, Hodes GE, Lenz KM, Mahurkar A, Russo SJ, Devine SE, McCarthy MM. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. doi: 10.1038/nn.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. doi: 10.1210/en.2009-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Edelmann MN, Auger AP. Epigenetic impact of simulated maternal grooming on estrogen receptor alpha within the developing amygdala. Brain Behav Immun. 2011;25:1299–1304. doi: 10.1016/j.bbi.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cowansage KK, LeDoux JE, Monfils MH. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol. 2010;3:12–29. doi: 10.2174/1874467211003010012. [DOI] [PubMed] [Google Scholar]
- 114.Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. J Neurosci Res. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Branchi I, Karpova NN, D'Andrea I, Castren E, Alleva E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci Lett. 2011;495:168–172. doi: 10.1016/j.neulet.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 116.Blaze J, Scheuing L, Roth TL. Differential methylation of genes in the medial prefrontal cortex of developing and adult rats following exposure to maltreatment or nurturing care during infancy. Dev Neurosci. 2013;35:306–316. doi: 10.1159/000350716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roth TL, Matt S, Chen K, Blaze J. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Dev Psychobiol. 2014;56:1755–1763. doi: 10.1002/dev.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suri D, Veenit V, Sarkar A, Thiagarajan D, Kumar A, Nestler EJ, Galande S, Vaidya VA. Early stress evokes age-dependent biphasic changes in hippocampal neurogenesis, BDNF expression, and cognition. Biol Psychiatry. 2013;73:658–666. doi: 10.1016/j.biopsych.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Doherty TS, Forster A, Roth TL. Global and gene-specific DNA methylation alterations in the adolescent amygdala and hippocampus in an animal model of caregiver maltreatment. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Blaze J, Roth TL. Exposure to caregiver maltreatment alters expression levels of epigenetic regulators in the medial prefrontal cortex. Int J Dev Neurosci. 2013;31:804–810. doi: 10.1016/j.ijdevneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jensen Pena C, Champagne FA. Implications of temporal variation in maternal care for the prediction of neurobiological and behavioral outcomes in offspring. Behav Neurosci. 2013;127:33–46. doi: 10.1037/a0031219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- 123.Hess EH. Imprinting in animals. Sci American. 1958;198:81–90. [Google Scholar]
- 124.Hess EH. Two conditions limiting critical age for imprinting. J Comp Physiol Psychol. 1959;52 doi: 10.1037/h0049164. [DOI] [PubMed] [Google Scholar]
- 125.Canon P. Socialisation and imprinting in brown leghorn chicks. Animal Behav. 1959;7:26–34. [Google Scholar]
- 126.McCabe BJ, Horn G. Learning and memory: regional changes in N-methyl-D-aspartate receptors in the chick brain after imprinting. Proc Natl Acad Sci U S A. 1988;85:2849–2853. doi: 10.1073/pnas.85.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McCabe BJ, Davey JE, Horn G. Impairment of learning by localized injection of an N-methyl-D-aspartate receptor antagonist into the hyperstriatum ventrale of the domestic chick. Behav Neurosci. 1992;106:947–953. doi: 10.1037//0735-7044.106.6.947. [DOI] [PubMed] [Google Scholar]
- 128.Nakamori T, Sato K, Atoji Y, Kanamatsu T, Tanaka K, Ohki-Hamazaki H. Demonstration of a neural circuit critical for imprinting behavior in chicks. J Neurosci. 2010;30:4467–4480. doi: 10.1523/JNEUROSCI.3532-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamaguchi S, Aoki N, Kitajima T, Iikubo E, Katagiri S, Matsushima T, Homma KJ. Thyroid hormone determines the start of the sensitive period of imprinting and primes later learning. Nat Commun. 2012;3:1081. doi: 10.1038/ncomms2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee PR, Brady D, Koenig JI. Thyroid hormone regulation of N-methyl-D-aspartic acid receptor subunit mRNA expression in adult brain. J Neuroendocrinol. 2003;15:87–92. doi: 10.1046/j.1365-2826.2003.00959.x. [DOI] [PubMed] [Google Scholar]
- 131.Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 133.Cirulli F, Santucci D, Laviola G, Alleva E, Levine S. Behavioral and hormonal responses to stress in the newborn mouse: effects of maternal deprivation and chlordiazepoxide. Dev Psychobiol. 1994;27:301–316. doi: 10.1002/dev.420270505. [DOI] [PubMed] [Google Scholar]
- 134.Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 136.Beckett C, Maughan B, Rutter M, Castle J, Colvert E, Groothues C, Kreppner J, Stevens S, O'Connor TG, Sonuga-Barke EJ. Do the effects of early severe deprivation on cognition persist into early adolescence? Findings from the English and Romanian adoptees study. Child Dev. 2006;77:696–711. doi: 10.1111/j.1467-8624.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- 137.McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA., 3rd Causal effects of the early caregiving environment on development of stress response systems in children. Proc Natl Acad Sci U S A. 2015;112:5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc Natl Acad Sci U S A. 2005;102:9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cohen JL, Glover ME, Pugh PC, Fant AD, Simmons RK, Akil H, Kerman IA, Clinton SM. Maternal style selectively shapes amygdalar development and social behavior in rats genetically prone to high anxiety. Dev Neurosci. 2015;37:203–214. doi: 10.1159/000374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barrenha GD, Chester JA. Effects of cross-fostering on alcohol preference and correlated responses to selection in high- and low-alcohol-preferring mice. Alcohol Clin Exp Res. 2012;36:2065–2073. doi: 10.1111/j.1530-0277.2012.01839.x. [DOI] [PubMed] [Google Scholar]
- 141.Thompson WR. The inheritance of behavior: Behavioral differences in fifteen mouse strains. Can J of Psychol. 1953;7:145–155. doi: 10.1037/h0083586. [DOI] [PubMed] [Google Scholar]
- 142.Champagne FA, Curley JP, Keverne EB, Bateson PP. Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav. 2007;91:325–334. doi: 10.1016/j.physbeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 143.Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat Neurosci. 2003;6:445–446. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- 144.Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Maternal behavior of SHR rats and its relationship to offspring blood pressures. Dev Psychobiol. 1989;22:29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- 145.Cierpial MA, Murphy CA, McCarty R. Maternal behavior of spontaneously hypertensive and Wistar-Kyoto normotensive rats: effects of reciprocal cross-fostering of litters. Behav Neural Biol. 1990;54:90–96. doi: 10.1016/0163-1047(90)91271-c. [DOI] [PubMed] [Google Scholar]
- 146.Curley JP, Rock V, Moynihan AM, Bateson P, Keverne EB, Champagne FA. Developmental shifts in the behavioral phenotypes of inbred mice: the role of postnatal and juvenile social experiences. Behav Genet. 2010;40:220–232. doi: 10.1007/s10519-010-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seip KM, Morrell JI. Increasing the incentive salience of cocaine challenges preference for pup- over cocaine-associated stimuli during early postpartum: place preference and locomotor analyses in the lactating female rat. Psychopharmacology (Berl) 2007;194:309–319. doi: 10.1007/s00213-007-0841-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA. The meaning of weaning: influence of the weaning period on behavioral development in mice. Dev Neurosci. 2009;31:318–331. doi: 10.1159/000216543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Franks B, Champagne FA, Curley JP. Postnatal maternal care predicts divergent weaning strategies and the development of social behavior. Dev Psychobiol. doi: 10.1002/dev.21326. in press. [DOI] [PubMed] [Google Scholar]
- 150.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hinde K, Skibiel AL, Foster AB, Del Rosso L, Mendoza SP, Capitanio JP. Cortisol in mother's milk across lactation reflects maternal life history and predicts infant temperament. Behav Ecol. 2015;26:269–281. doi: 10.1093/beheco/aru186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jans JE, Woodside BC. Nest temperature: effects on maternal behavior, pup development, and interactions with handling. Dev Psychobiol. 1990;23:519–534. doi: 10.1002/dev.420230607. [DOI] [PubMed] [Google Scholar]
- 153.Daft JG, Ptacek T, Kumar R, Morrow C, Lorenz RG. Cross-fostering immediately after birth induces a permanent microbiota shift that is shaped by the nursing mother. Microbiome. 2015;3:17. doi: 10.1186/s40168-015-0080-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Callaghan BL, Richardson R. Maternal separation results in early emergence of adult-like fear and extinction learning in infant rats. Behav Neurosci. 2011;125:20–28. doi: 10.1037/a0022008. [DOI] [PubMed] [Google Scholar]