Abstract
Orexin (ORX, also known as hypocretin, HRCT) neurons are located exclusively in the posterior hypothalamus and are involved in a wide range of behaviors, including motivation for drugs of abuse such as alcohol. Hypothalamic subregions contain functionally distinct populations of ORX neurons that may play different roles in regulating drug- and alcohol-motivated behaviors. To investigate the role of ORX neurons in alcohol seeking, we measured Fos activation of ORX neurons in rats following three different measures of alcohol seeking and preference: 1) context-induced reinstatement, or ABA renewal, 2) cue-induced reinstatement of extinguished responding for alcohol, and 3) a home cage task in which preference for alcohol (vs. water) was measured in the absence of either reinforcer. We found significant activation of ORX neurons in multiple subregions across all three behavioral tests. Notably, ORX neuron activation in lateral hypothalamus (LH) correlated with the degree of seeking in context reinstatement and degree of preference in home cage preference testing. In addition, Fos activation in ORX neurons in dorsomedial (DMH) and perifornical (PF) areas was correlated with context- and home cage seeking/preference, respectively. Surprisingly, we found no relationship between the degree of cue-induced reinstatement and ORX neuron activation in any region despite robust activation overall during reinstatement. These results demonstrate a strong relationship between ORX neuron activation and alcohol seeking/preference, but one that is differentially expressed across ORX field subregions depending on reinstatement modality.
Keywords: alcoholism, reward, lateral hypothalamus, Fos, reinstatement
Introduction
The orexin/hypocretin (ORX/HCRT) system consists of a population of neurons located exclusively in the hypothalamus that produces the peptides ORX-A (HCRT-1) and ORX-B (HCRT-2), from the prepro-orexin/hypocretin peptide (de Lecea et al., 1998; Sakurai et al., 1998). Since the first publications related to this system in 1998, it has been widely appreciated that the ORX system participates in a number of behaviors critical for the survival of an organism (de Lecea & Sutcliffe, 1999; Sakurai, 2007; Tsujino & Sakurai, 2013; Gao & Horvath, 2014; Mahler et al., 2014). The ORX system plays an important role in regulating sleep/wake activity, and dysregulation of the ORX system is associated with narcolepsy with cataplexy (Sakurai, 2007; de Lecea & Huerta, 2014). In addition, we and others have demonstrated a strong association between the ORX system and multiple types of motivated behavior (Harris et al., 2005; Aston-Jones et al., 2009; Borgland et al., 2009; Thompson & Borgland, 2011; Tsujino & Sakurai, 2013; Mahler et al., 2014; Merlo Pich & Melotto, 2014; Yeoh et al., 2014). The ORX system is involved in feeding behaviors (Sakurai et al., 1998), and also has a profound influence on motivation for drugs of abuse (Mahler et al., 2012), including alcohol (Lawrence, 2010; Mahler et al., 2012; Brown & Lawrence, 2013). Lawrence and colleagues first reported an association between the ORX system and alcohol seeking, demonstrating that ethanol (EtOH) consumption increased ORX mRNA, and that pharmacological blockade of the ORX-1 receptor (OX1R) decreased EtOH seeking behaviors (Lawrence et al., 2006). The effect of OX1R antagonism on alcohol seeking and drinking has been demonstrated by a number of groups (Lawrence, 2010; Mahler et al., 2012; Khoo & Brown, 2014). We and others showed that blocking OX1R activity decreases alcohol seeking preferentially in highly-motivated animals (Moorman & Aston-Jones, 2009; Anderson et al., 2014; Olney et al., 2015). This relationship between motivation for reward and ORX signaling led us to propose that the ORX system plays a major role in motivational activation, in which ORX neuron activation scales with intensity of motivational drive (Mahler et al., 2014).
Further work is needed to refine our understanding of the relationship between ORX neuron activation and motivational state, particularly with respect to alcohol seeking. Although several reports have documented that ORX receptor antagonism decreases motivation for alcohol (Richards et al., 2008; Moorman & Aston-Jones, 2009; Jupp et al., 2011; Anderson et al., 2014; Olney et al., 2015), few studies have shown an effect of blocking OX1Rs or OX2Rs on alcohol seeking (Dhaher et al., 2010) or alcohol preference (Moorman & Aston-Jones, 2009; Voorhees & Cunningham, 2011; Brown et al., 2013; Anderson et al., 2014; Barson et al., 2015). Moreover, this evidence is limited by findings that certain ORX receptor antagonists may exhibit reduced selectivity in certain conditions (Hollander et al., 2012; McElhinny et al., 2012; Merlo Pich & Melotto, 2014; Winrow & Renger, 2014).
The above findings indicate the need to measure activity of ORX neurons to better understand their roles in alcohol-related behaviors. An important and additional factor that supports this conclusion is that ORX neuron populations play multiple, diverse roles in motivated behaviors (Tsujino & Sakurai, 2013; Mahler et al., 2014). In particular, ORX neurons located in the lateral region of the orexin cell field function in motivation and reward-seeking whereas more medial ORX neurons (e.g., DMH and PF) are more involved in arousal and stress (Estabrooke et al., 2001; Harris & Aston-Jones, 2006). This topographic dichotomy in ORX neuron function has been demonstrated for multiple drugs of abuse and natural rewards (Harris et al., 2005; Harris et al., 2007), as well as for alcoholic beer seeking (Hamlin et al., 2007). However, the specific association between regional ORX neuron activation and alcohol seeking has not been completely described.
A focus on activities of ORX neurons is also warranted by the fact that ORX neurons co-release multiple neurotransmitters and peptides including ORX, dynorphin and glutamate, among others (Chou et al., 2001; Schone & Burdakov, 2012; Muschamp et al., 2014). Behaviors that activate ORX neurons such as alcohol seeking (Hamlin et al., 2007; Dayas et al., 2008; Kallupi et al., 2010; Millan et al., 2010), likely simultaneously release these co-transmitters indicating that a focus on ORX release itself (via e.g., pharmacology alone) may only reveal part of the picture. Despite this need, there have been a limited number of studies of ORX neuron activity during alcohol seeking, and these have focused on one behavioral paradigm (context-induced reinstatement or renewal) (Hamlin et al., 2007; Dayas et al., 2008).
We used Fos immunohistochemistry to measure activities of ORX neurons across a range of alcohol seeking behaviors, including during context-induced (ABA renewal) or cue-induced reinstatement of extinguished responding for alcohol, and also during a test of alcohol-free home cage alcohol seeking. We found that ORX neurons were activated in all three tests, and that the relationship between ORX neuron activation and alcohol seeking differed based on hypothalamic subregion and type of test. These results reveal that ORX neural activity varies substantially with different reinstatement paradigms, implying subtle topographically specific roles for ORX neurons in different aspects of alcohol seeking.
Materials and Methods
Subjects
Male Sprague-Dawley rats (n = 48; initial weight approximately 300–350 g; Charles River, Wilmington, MA) were single-housed under a reversed 12-h light/dark cycle (lights off at 6 a.m.) and had ad libitum access to food and water. Animals were housed in a temperature- and humidity-controlled animal facility at MUSC (AAALAC-accredited). All procedures were approved by the Medical University of South Carolina’s Institutional Animal Care and Use Committee and conducted according to specifications of the NIH as outlined in the Guide for the Care and Use of Laboratory Animals.
Procedures
Animals were trained to drink 20% EtOH (95% EtOH (AAPER, Shelbyville, KY) and filtered water) using the intermittent access (IA) paradigm (Wise, 1975; Simms et al., 2008; Moorman & Aston-Jones, 2009). In this procedure, rats received either 20% EtOH or water for 24 h on alternating days for 2 weeks in home cages with ad lib access to food and water. After IA access to develop alcohol drinking, animals were tested in one of the three following paradigms (Experiments 1–3).
Context-induced reinstatement
In Experiment 1, 16 rats were initially trained to self-administer 20% EtOH on an FR1 schedule. Training and testing occurred in sound-attenuated operant boxes with two levers and a reward well (Med-Associates). Active lever presses resulted in delivery of 0.1 ml of 20% EtOH to a reward well, which was paired with a tone and light stimulus above the active lever. Presses on the inactive lever produced no outcome but were recorded. Head entries into the alcohol-rewarded well were detected using an infrared beam break and recorded. Entries were rewarded (immediately following lever press) or non-rewarded (> 1 sec after lever press or during the intertrial interval). Intertrial intervals were 20 sec, during which time houselights were turned on and lever pressing produced no response but was recorded.
For ABA renewal/context-reinstatement, animals were separated into two groups and trained in separate rooms. Testing rooms were located in different parts of the building, had different spatial configurations, and were shared with different cohorts of rats from other tests. Furthermore, rats were trained in the same operant chamber for the duration of the self-administration period, ensuring a strong familiarity with the “A” context for comparison with the “B” context. After 3 weeks of training, animals were transitioned to FR2 and then FR3 self-administration for an additional 10 days. Rats were then switched to new operant chambers in the opposite rooms for extinction sessions. Lever pressing was extinguished for 10 days: lever presses produced no alcohol, lights or tones but were recorded. At the end of extinction, all animals emitted less than 20 presses per session. The day following extinction, animals were returned to the room and operant chamber in which they originally received alcohol (“A” context) and were tested in a reinstatement session. Lever presses and well-entries were recorded but no stimuli or alcohol was delivered. Rats were perfused 1.5 hours after the start of the reinstatement session and brains were processed for Fos and ORX immunohistochemistry.
Cue-induced reinstatement
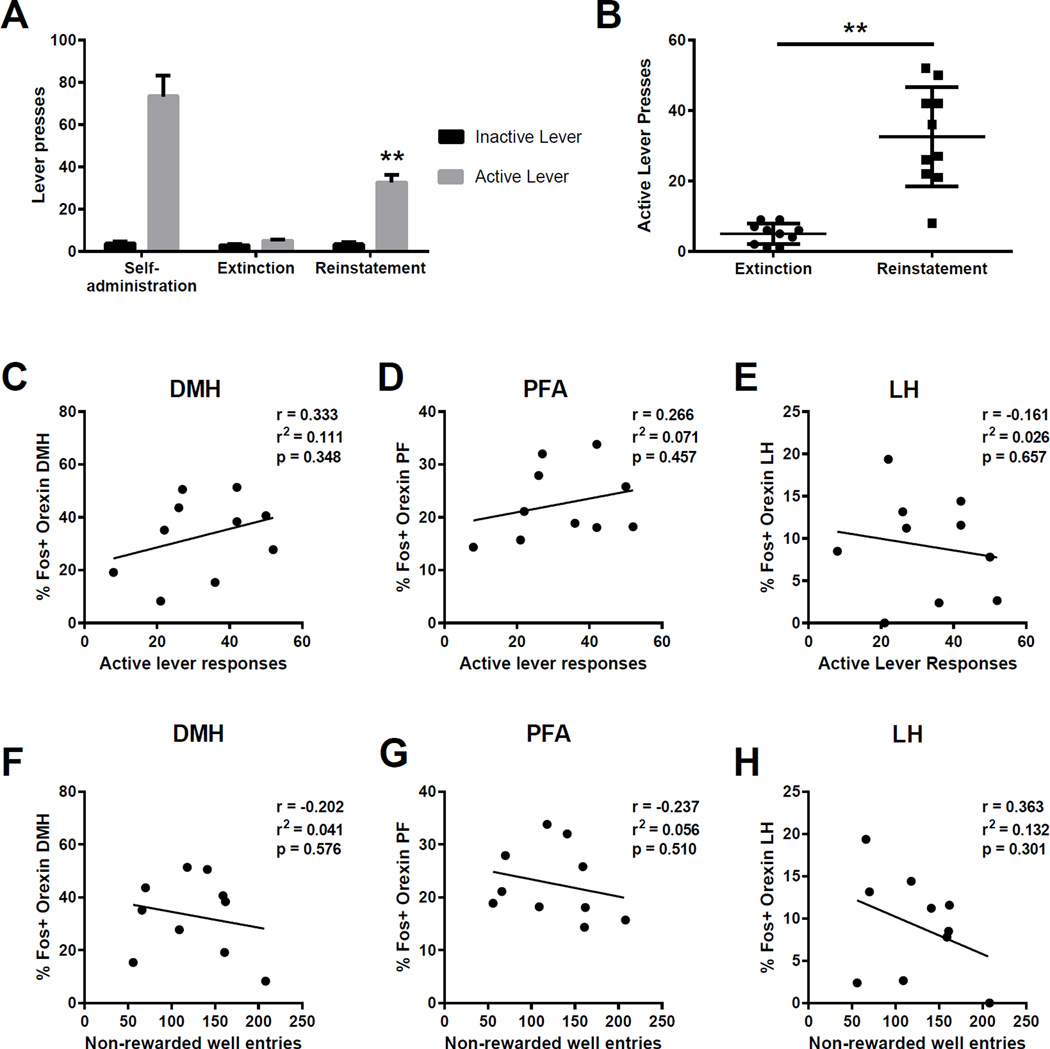
In Experiment 2, 16 rats were initially trained to self-administer 20% EtOH on an FR1 schedule, as in Experiment 1. FR1 training lasted for 13 days at which point animals were trained on FR2 (3 days), and finally FR3 (15 days). Rats were then extinguished for 10 days (in the same environment). At the end of extinction, all animals except one emitted less than 20 presses per session. This animal was excluded from further analysis. Rats then received a series of 3 cue-induced reinstatement tests, in which active lever presses produced light-tone cues previously associated with alcohol, but no alcohol. Each reinstatement test was separated by at least 4 days of extinction training. Rats were perfused 1.5 hours after the start of the final reinstatement session and brains processed to visualize Fos and ORX neurons. On days 10–12 of self-administration and on the first 2 days of cue-induced reinstatement, animals received treatment with SB-334867. The results from these tests are reported elsewhere (Moorman et al., submitted). SB self-administration test days were followed by three additional self-administration or extinction sessions to minimize the impact of SB administration on subsequent behavioral tests, and no effect of previous treatment with SB was observed. Cued-reinstatement was significant and robust on the final test session as shown in Figure 3.
Figure 3.
Cue-induced reinstatement of ethanol seeking. (A) Rats were trained to respond for EtOH paired with light-tone cues. Lever pressing was extinguished in sessions where both EtOH and cues were withheld. On test day, lever responses resulted in delivery of light-tone cues but no EtOH, and resulted in significant reinstatement of active lever responding. (B) As with context-induced reinstatement, there was significant variability in active lever responding during cued reinstatement test sessions. (C–E) Responding on the active lever during cue-induced reinstatement was not correlated with the percentage of Fos-positive orexin neurons in any of the subregions examined. (F–H) Similarly, there was no relationship between the activation of orexin neurons and the number of non-rewarded well entries the animals made throughout the reinstatement session. ** p<0.01.
Home cage conditioned response testing
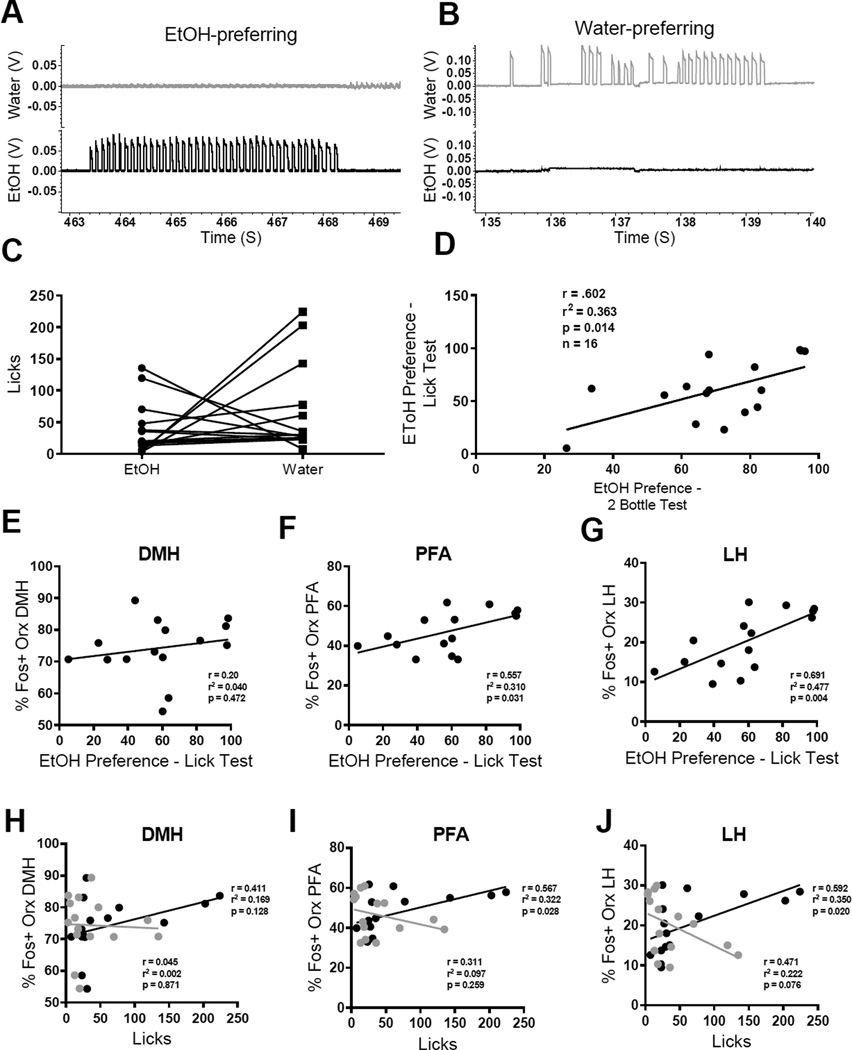
In Experiment 3, 16 rats received one week of 2-bottle choice testing (20% EtOH or water) for 2 hours per day to characterize EtOH preference and acclimatize them to conditioned responding. Animals received a conditioned response test on the last day of testing. For this, empty bottles were present on both sides of the home cage, similar to 2-bottle testing. Sipper tubes of the empty bottles were filled with either EtOH- or water-scented cotton to serve as odor cues. The amount of time spent licking each bottle was measured for 15 minutes using previously described techniques (Hayar et al., 2006). Briefly, wires were connected to the metal sipper tubes and grid floors at the bottom of the home cages. These wires were led to a CED recording system (Cambridge Electronic Design, Cambridge, UK; Spike2 software) and licks were detected as junction potentials when rats made contact between tube and ground (see Figure 4). Rats were perfused for Fos immunohistochemistry 1.5 hours after the start of the conditioned response test.
Figure 4.
Orexin neurons are activated during expression of ethanol preference. (A,B) Preference for EtOH was measured in the home cage using a junction potential detection method, as per previous studies (Hayar et al., 2006). This method allowed us to quantify the number of licks each animal made on the EtOH versus water bottles. (C) Rats sampled both EtOH and water tubes. Sampling of EtOH vs. water was variable across rats. (D) Preference for EtOH on test day was positively, and significantly, correlated with animals’ preference for EtOH on the 2-bottle choice test during the week prior to testing. (E– G) Animals’ preference for EtOH was positively, and significantly, correlated with percentage of Fos-positive orexin neurons in PF (F) and LH (G), but not DMH (E), subdivisions. (H–J) Activation of orexin neurons in PF (I) and LH (J) was also positively, and significantly, correlated with the number of licks animals’ made on the EtOH (black), but not water (gray), bottles.
Perfusion and immunohistochemistry
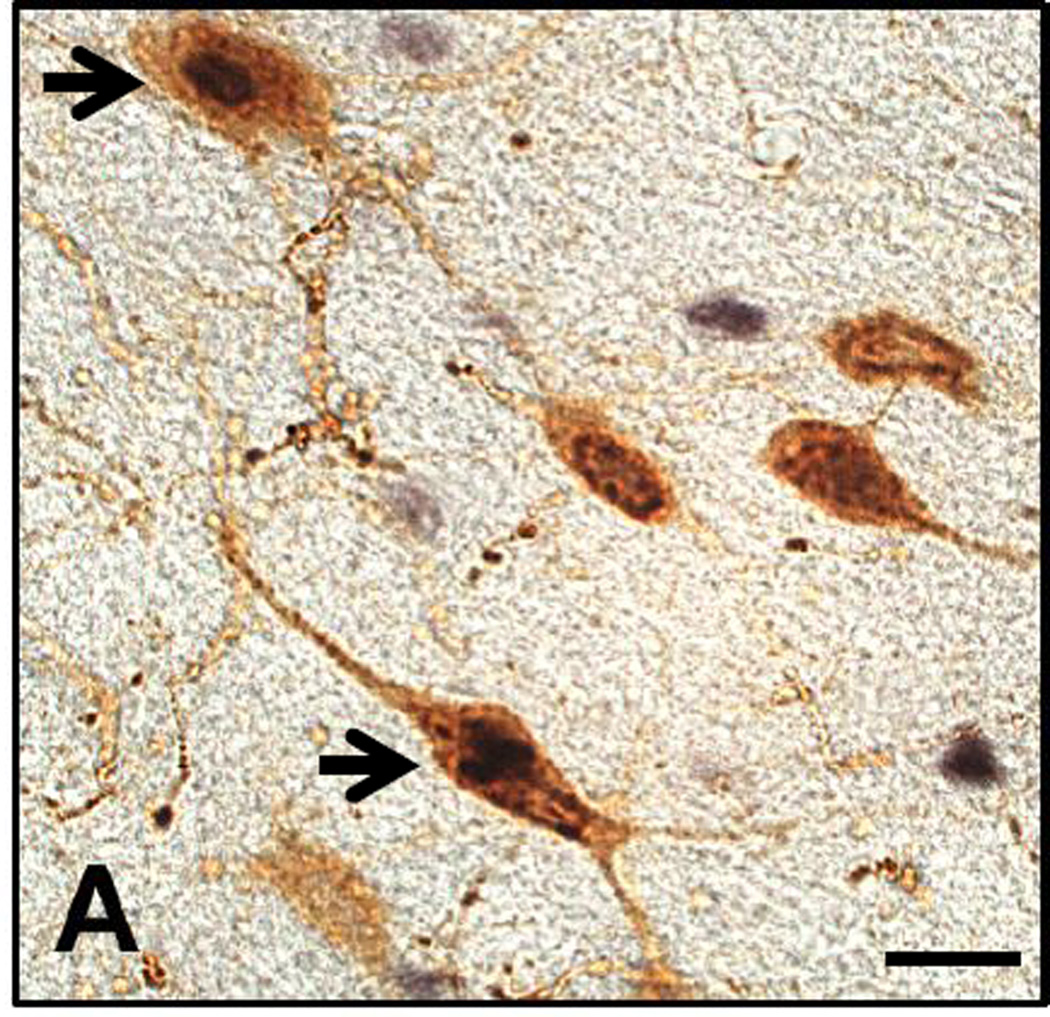
After tests, animals were deeply anesthetized and perfused with 0.9% saline followed by 4% paraformaldehyde. Brains were postfixed overnight in 4% paraformaldehyde and cryoprotected in 20% sucrose. Brains were rapidly frozen using dry ice, and 40 µm-thick sections through the extent of the lateral hypothalamic area were cut on a cryostat and stored in phosphate-buffered saline (PBS). Sections were stained for Fos and ORX as previously described (Mahler & Aston-Jones, 2012; Richardson & Aston-Jones, 2012; Sartor & Aston-Jones, 2012b; Cason & Aston-Jones, 2013). Briefly, sections were washed in 0.1% H2O2 in PBS for 15 minutes, incubated with 2% normal donkey serum (NDS, Jackson Labs) and PBS + 0.3% Triton-X (PBST) for 2 hours, and then incubated overnight in rabbit anti-Fos primary antibody (1 : 5000; Santa Cruz) in NDS and PBST. Sections were then incubated for 2 hours in biotynylated secondary (donkey anti-rabbit, 1:500, Jackson Labs) followed by 1.5 hours in avidin-biotin complex (1:500, Vector). Fos was labeled using 3,3’ diaminobenzidine (DAB) + nickel ammonium sulfate to yield a blue-black nuclear reaction product. Sections were then transferred to an overnight incubation in goat anti-orexin-A primary antibody (1:500, Santa Cruz), followed by a 2-hour incubation in biotinylated secondary (donkey anti-goat, 1:500, Jackson Labs) and then 1.5 hours in avidin-biotin complex (1:500, Vector). Orexin-A was visualized using DAB (no nickel) to produce a brown stain of ORX neuron cytoplasm (Figure 1). Tissue was rinsed three times with either PBS or PBST between each stage. Sections were then mounted and coverslipped for visualization.
Figure 1.
High-power photomicrograph of coronal section through the lateral hypothalamus immunolabelled for Fos-protein (black nuclear stain) and orexin-A (brown cytoplasmic stain). Arrows indicate co-labeled neurons. Other orexin neurons without Fos staining and Fos staining in non-orexin neurons are shown. Scale bar = 20 µm.
Measurement of Fos-activated orexin neurons
Hypothalamic sections were imaged with a 10× objective on a Leica DMRXA microscope using a QImagning camera and were quantified using Openlab image processing software (Improvision, Ltd), ImageJ (NIH) or Stereo Investigator software (MBF Bioscience). We determined the percentage of ORX neurons that also expressed Fos, as a measurement of ORX neuron activation. Fos-activated ORX neurons were counted in three sections taken from the ORX-expressing region of the hypothalamus in each animal. Two sections per animal were counted in 3 animals in Experiment 2 (cueinduced reinstatement). ORX-positive and Fos/ORX-positive neurons were counted bilaterally in each section. Three subregions of the orexin cell field were analyzed independently.
Data analysis
All statistical analyses were conducted using GraphPad Prism (Version 5.1). In Experiments 1 and 2, behavioral measures were numbers of active and inactive lever presses in extinction vs. reinstatement/renewal and were compared using student t-tests. For Experiments 1 and 2, animals that did not reinstate above the group extinction average were excluded from Fos correlation analyses, as was one animal in Experiment 2 that did not extinguish. In Experiment 3, preference measures (EtOH/(EtOH+water)) were numbers of licks on each sipper tube measured on test day (see Figure 4 A–C), or ml of fluid consumed (see Figure 4D) compared using student t-tests. One animal was removed from analysis due to corrupted lickometer detection. In all three experiments, the percentages of ORX+ neurons that were also Fos+ (double-labeled neurons) were correlated with number of behavioral responses (active lever presses in Experiments 1 and 2; EtOH-lickometer preference in Experiment 3). Correlations were calculated separately for double-labeled neurons in lateral, perifornical, and medial LHA.
Results
Experiment 1: Context-induced reinstatement of ethanol seeking
Animals exhibited robust context-induced reinstatement of alcohol seeking
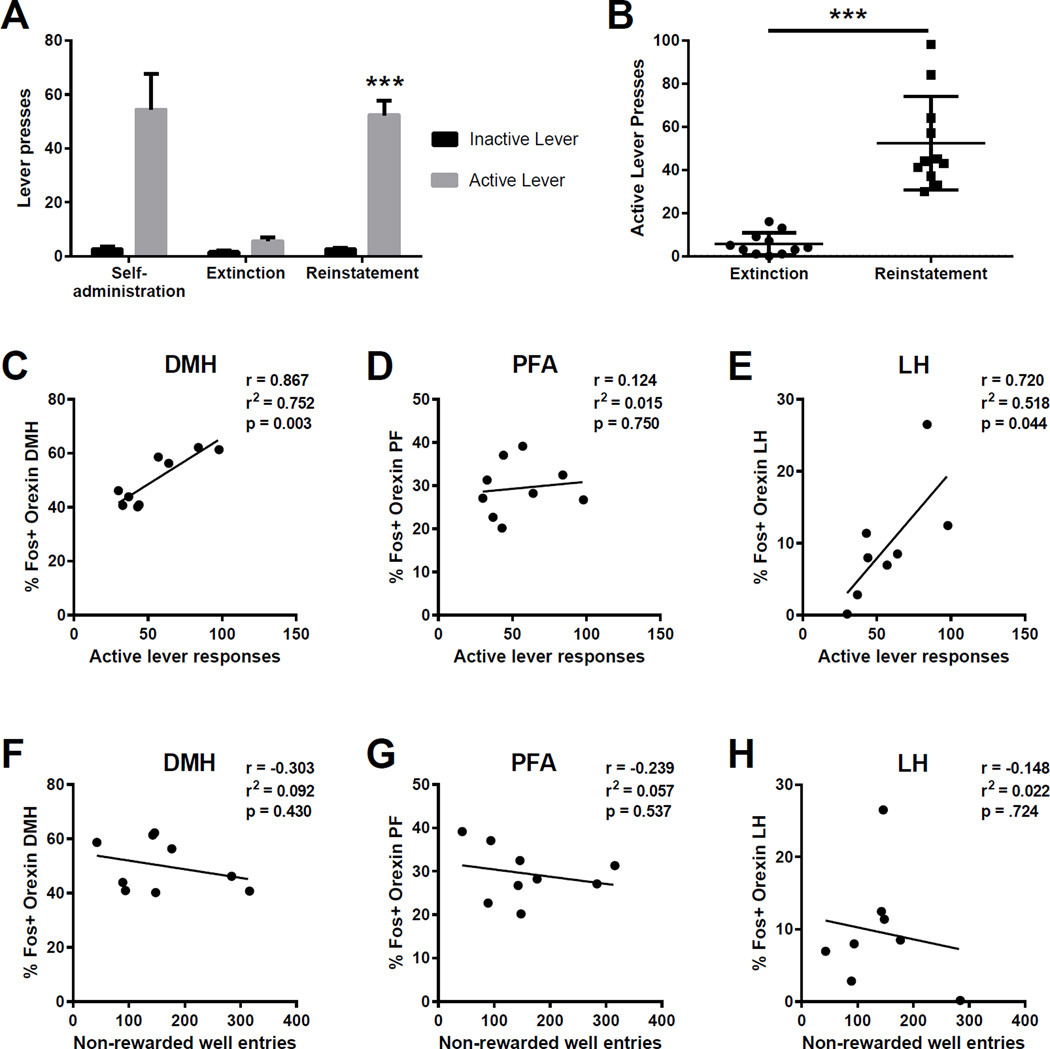
Two groups of animals were trained to respond for EtOH in operant chambers located in two separate rooms. Responding on the active and inactive levers, as well as the total number of infusions earned, were statistically similar for both training groups across FR1, FR2 and FR3 training (p>0.05 for all t-tests, data not shown). On the final three days of self-administration training, rats exhibited an average of 74.2 (±12.7 SEM) active lever responses, 3.0 (±1.2 SEM) inactive lever responses and earned 19.3 (±2.8 SEM) EtOH rewards (Figure 2A). Following self-administration training, rats were switched to the opposite room for extinction training over a period of 10 days. Both training groups extinguished at similar rates, and on the final day of extinction, exhibited an average of 5.6 (±1.3 SEM) active lever responses and 1.6 (±0.7 SEM) inactive lever responses (Figure 2A). Finally, rats were returned to the room where they originally received alcohol and tested for renewal of EtOH seeking. Animals exhibited robust reinstatement behavior, as evidenced by a significant increase in active lever responding as compared to the final day of extinction (t(20)=6.959, p<0.001; Figure 2A,B). Responding on the inactive lever did not differ between test day and the final day of extinction (p>0.05; Figure 2A), indicating a specific reinstatement of EtOH seeking. Reinstatement behavior was identical across the two training groups (p>0.05, data not shown).
Figure 2.
Context-induced reinstatement of ethanol seeking. Rats were trained to respond for EtOH in two separate groups in similar operant chambers located in two distinct lab rooms, to provide different contexts. There were no behavioral differences between groups in terms of self-administration, extinction or reinstatement behavior. (A) Animals exhibited a strong preference for responding on the active lever (versus the inactive lever) during self-administration (left). Lever responding was then extinguished in the opposite room (middle). On test day, animals were returned to the room in which they originally learned to respond for EtOH, resulting in a significant reinstatement of responding on the active lever only (right). (B) There was significant variability in reinstatement behavior (active lever responding) as a result of returning to the original self-administration context. (C–D) Responding on the active lever during context-induced reinstatement was significantly, and positively, correlated with the percentage of Fos-positive orexin neurons in DMH (C) and LH (E), but not the PF (D). (F–H) Activation of orexin neurons was not correlated with the number of non-rewarded well entries animals made throughout the reinstatement session, a measure of non-goal-directed locomotor activity. *** p<0.001.
Context-induced reinstatement of EtOH seeking is positively correlated with activation of ORX neurons in DMH and LH areas
Next, we assessed whether there was a relationship between the magnitudes of context-induced reinstatement behavior and activation of ORX neurons in DMH, PF and LH areas. We observed a significant, positive correlation between active lever responses during reinstatement testing and the percentage of Fos+ ORX neurons in the DMH (r=0.867, p<0.01; Figure 2C) and LH (r=0.720, p<0.05; Figure 2E) subregions. No such relationship was observed in the PF area (r=0.012, p>0.05; Figure 2D).
We also examined the relationship between the percentage of Fos+ ORX neurons and the number of non-rewarded head entries the animals made into the reward well during the reinstatement test as a measure of general locomotor activity. Importantly, we did not observe a significant relationship between Fos activation and non-rewarded well-entries in any of the hypothalamic subregions (p>0.05; Figure 2 F–H), indicating that Fos induction in DMH and LH ORX neurons is not related to non-specific locomotor behavior.
Experiment 2: Cue-induced reinstatement of ethanol seeking
Animals exhibited robust cue-induced reinstatement of alcohol seeking
Animals were trained to respond for EtOH, the delivery of which was paired with light-tone cues. Over the final three days of FR3 self-administration training, animals exhibited an average of 91.0 (±8.1 SEM) active lever responses and 3.0 (±0.8 SEM) inactive lever responses, and earned 28 (±2.4 SEM) EtOH rewards (Figure 3A). Lever pressing was extinguished in the same context, but in the absence of light-tone cues and EtOH rewards. On the extinction day prior to reinstatement testing, animals exhibited an average of 5.0 (±0.8 SEM) active lever presses and 2.9 (±0.7 SEM) inactive lever responses. On test day, active lever presses resulted in the delivery of light-tone cues previously paired with EtOH, but no delivery of EtOH. Animals exhibited significant cue-induced reinstatement of EtOH seeking on test day, as evidenced by a significant increase in active lever responding (32.6±3.5 SEM) compared to extinction levels (t(18)=6.048, p<0.001; Figure 3A, B). We did not observe a significant increase in inactive lever responding on test day (p>0.05; Figure 3A).
Cue-induced reinstatement of EtOH seeking is not correlated with activation of ORX neurons
As in Experiment 1, we assessed whether there was a relationship between cueinduced reinstatement behavior and activation of ORX neurons. Interestingly, there were no significant correlations between active lever responding on test day and the percentage of Fos+ ORX neurons in all hypothalamic subregions (Figure 3 C–E). There also was no correlation between ORX neuron activation and general locomotor activity, as assessed by non-rewarded entries the animals made into the reward well during testing (p>.05).
Experiment 3: Home cage conditioned response testing
Animals exhibited significant variability in EtOH preference on 2-bottle choice and test day
Animals received 1 week of 2-bottle choice testing to characterize preference for EtOH. During this period, animals on average consumed 4.6 ml (±0.3 SEM) of EtOH (1.8±0.11 g/kg) and 2.1 ml (±0.3 SEM) of water in each 2-hour session. On the final day of testing, animals received a conditioned response test, whereby the number of licks on each bottle was measured using a junction potential detection method (see Methods and Hayar et al., 2006). Examples of junction potential recordings of EtOH-preferring versus water-preferring rats are shown in Figure 4 A,B. Preference for the EtOH vs. water bottle varied greatly between rats (Figure 4C) and was significantly positively correlated with individual preference on the 2-bottle task (r=0.602, p<0.05; Figure 4D).
EtOH preference was positively correlated with activation of ORX-positive neurons in PF and LH areas
Next we examined the relationship between each animal’s EtOH preference score (EtOH /(EtOH+water)) on test day and the level of activation of ORX neurons in DMH, PF and LH areas (Figure 4E–G). There was no relationship between EtOH preference and the number of Fos-positive ORX neurons in the DMH (r=0.20, p>0.05). In contrast, there was a significant, positive correlation between each animal’s EtOH preference score and the number of Fos+ ORX neurons in both the PF (r=.557, p<0.05) and LH (r=0.691, p<0.005) areas.
To confirm that activation of ORX neurons was specifically linked to alcohol seeking, we also examined whether there was a relationship between the number of licks made on the EtOH versus water bottles and Fos-positive ORX neurons. Interestingly, there was no relationship between licks on the water bottle and the percentage of Fos+ ORX neurons in any of the regions examined (p>.05; Figure 4 H–J; gray symbols). In contrast, there was a significant, positive correlation between the number of licks each animal made on the EtOH bottles and the percentage of Fos+ ORX neurons in the PF (r=0.567, p<0.05) and LH (r=0.592, p<0.05) areas.
Discussion
The present results demonstrate three main findings related to the activation of ORX neurons during EtOH seeking. In Experiment 1, we observed strong activation of ORX neurons during context-induced reinstatement, or renewal, of EtOH seeking, in line with previous observations (Hamlin et al., 2007; Dayas et al., 2008). Furthermore, we found that the activation of ORX neurons in the lateral and medial, but not perifornical, hypothalamus were significantly and positively correlated with context-induced EtOH seeking. In Experiment 2, there was no correlation between Fos activation of ORX neurons and cue-induced EtOH seeking in any of the three regions. This effect was somewhat surprising given the significant relationships seen between ORX Fos and context-induced reinstatement, but is in line with previous studies (Mahler & Aston-Jones, 2012), as discussed further below. Finally, in Experiment 3 we measured home cage EtOH seeking and preference in the absence of EtOH/water and observed a strong correlation between ORX neuron activation and EtOH preference in lateral ORX neurons, a weaker but still significant correlation in perifornical ORX neurons, and no correlation for medial ORX neurons. This relationship was driven by a significant correlation between ORX neuron activation and EtOH seeking, but not water seeking. Thus, EtOH seeking was associated with ORX neuron activation in two behavioral paradigms (context-induced and home cage) but not a third (cue-induced). In sum, these results show a strong association between ORX neuron activation and EtOH seeking, but reveal subregional and behavioral differences underlying this relationship.
The relationship between ORX neuron activation and context-induced reinstatement, or renewal, of EtOH seeking was not surprising. Dayas and colleagues showed that exposure to environmental stimuli previously associated with EtOH induced both reinstatement of EtOH seeking and increased Fos activation of ORX neurons in both lateral/perifornical (analyzed together) and dorsomedial LHA as compared to a null stimulus which produced little responding and less Fos activation (Dayas et al., 2008). These results are in line with ours in that both lateral and medial ORX neurons were engaged during reinstatement, though our results extend these findings to show direct correlations between intensity of seeking and activation of ORX neurons. Hamlin and colleagues found a similar increase in ORX neuron Fos activation in rats undergoing ABA renewal testing for alcoholic beer (Hamlin et al., 2007). Interestingly, these authors also found a correlation between ORX neuron activation and beer seeking in the ABA context, in line with our findings. The authors did not report tests for correlations in medial or perifornical ORX neurons, possibly due to their observation of no differences in the relatively high ORX-Fos expression in those regions during ABA renewal.
Other studies reveal correlated activity of ORX neurons with seeking for other rewards as well. Hamlin et al (Hamlin et al., 2008) found medial and perifornical, but not lateral, ORX neuron activation for ABA renewal of cocaine seeking but did not report correlations with seeking. Also, work from our group and others has shown that Fos activation of lateral ORX neurons is significantly correlated with the strength of conditioned place preference (another type of association between context and reward) for cocaine, morphine, or food, (Harris et al., 2005; Harris et al., 2007; Richardson & Aston-Jones, 2012; Sartor & Aston-Jones, 2012a; Sartor & Aston-Jones, 2012b; Lasheras et al., 2015). Thus, results from multiple studies, including the present one, demonstrate that ORX neuron activation, particularly that of lateral ORX neurons, is upregulated when the context produces reward seeking or preference.
It is unclear why lateral and medial, but not perifornical, ORX neurons were activated during context-induced ETOH seeking. One hypothesis is that context-induced reinstatement produced motivation to acquire reward along with frustration in not receiving reward. Under the assumption that lateral and medial ORX neurons encode positively- and negatively-valenced motivators respectively (Harris & Aston-Jones, 2006), one might expect to see activation of both populations in this test. Another hypothesis put forward by McGregor and colleagues is that operant behavior for reward activates both lateral and medial ORX neurons due, in part, to the enhanced motor activity involved in operant behavior (McGregor et al., 2011). Of note, perifornical ORX neurons were overall relatively strongly activated in these tests. Perifornical neurons may contribute an arousal-related signal (Harris & Aston-Jones, 2006), which may be binary in nature and less related to degree of positive or negative motivation. These proposals are somewhat speculative and need to be tested in behavioral paradigms that extract out each subcomponent or reinstatement to determine the specific relationships to ORX neuron activity.
It was interesting that we did not observe an association between ORX-Fos activation and strength of cue-induced reinstatement, given the fact that ORX receptor antagonism decreases this form of reinstatement for alcohol and other drugs of abuse (Mahler et al., 2012; Brown & Lawrence, 2013). However, we noted that few studies have investigated the influence of cue-induced reinstatement on ORX Fos activation. One study of cue-induced cocaine reinstatement, also from our group, reported no correlation between degree of reinstatement and ORX neuron activation (Mahler & Aston-Jones, 2012). A second study found increased activation of lateral and perifornical ORX neurons in cue-induced reinstatement for nicotine seeking in mice, although the authors did not report potential correlations with degree of reinstatement (Plaza-Zabala et al., 2013). These results are in contrast with the relatively reliable influence of context-induced reinstatement of seeking and place conditioning on Fos in ORX neurons, as described above. We hypothesize that persistent environmental or contextual cues drive ORX neurons for sufficient time to induce strong Fos expression, whereas transient discrete cues, that may transiently activate ORX neurons and drive reinstatement, may not elicit sufficiently prolonged ORX neuron activation to produce Fos. Along these lines, Millan and colleagues reported Fos activation of lateral and perifornical ORX neurons in reinstatement of beer seeking driven by NAc shell inactivation, which presumably tonically disinhibited ORX neurons (Millan et al., 2010) throughout the course of the reinstatement session. Similarly, Kallupi and colleagues found increased Fos activation of lateral and perifornical ORX neurons during neuropeptide-S-induced reinstatement of cocaine seeking (Kallupi et al., 2010), a manipulation that also induces reinstatement of alcohol seeking. This hypothesis needs to be explored in additional behavioral paradigms to identify the specific nature of stimuli that produce Fos activation of ORX neurons.
Finally, we observed strongly correlated Fos activation of lateral and perifornical ORX neurons in home cage 2-bottle lick tests in the absence of either EtOH or water. The rationale for using this test was to minimize additional factors present in operant tasks such as elevated locomotor activity or complex cognitive processes (e.g., decision-making) associated with an operant procedure, and attempt to identify specific ORX neuron correlates of alcohol preference in the absence of alcohol ingestion. Importantly, preference scores on the test day were highly correlated with 2-bottle test scores when EtOH and water were available (Figure 4 D), and ORX neuron Fos activation was correlated with alcohol bottle licks in addition to preference (Figure 4 E–J). These combined findings indicate that the lateral ORX neuron activity observed was strongly related to alcohol preference, in line with measures of preference for other drugs and natural rewards using tasks such as CPP (Harris et al., 2005; Harris et al., 2007; Richardson & Aston-Jones, 2012; Sartor & Aston-Jones, 2012a; Sartor & Aston-Jones, 2012b; Lasheras et al., 2015), though see (Voorhees & Cunningham, 2011). The absence of medial ORX neuron activation also supports this region’s potential role in aversive processes and associated negative reinforcing contributions to motivated behavior. Perifornical neuronal Fos also was correlated (though to a lesser degree) with alcohol preference. Notably, some studies have reported perifornical ORX neuron activation in reinstatement and other aspects of reward seeking (Dayas et al., 2008; Kallupi et al., 2010; Millan et al., 2010; Cason & Aston-Jones, 2013; Plaza-Zabala et al., 2013; Cole et al., 2015), although others have not (Baldo et al., 2004; Harris et al., 2005; Harris et al., 2007; Richardson & Aston-Jones, 2012; Sartor & Aston-Jones, 2012a; Sartor & Aston-Jones, 2012b; Lasheras et al., 2015). This underscores the need to further investigate the subpopulations of ORX neurons and their relationships to different aspects of both reward-seeking as well as other behaviors (arousal, stress, etc.).
These differences in activation of orexin neuron subpopulations across studies may result from at least two factors. First, categorization of ORX neurons (e.g., medial vs lateral boundaries) may differ across studies. One option might be to focus more on multidimensional profiling of ORX neurons considering, for example, projection target, in addition to medial/lateral location (Richardson & Aston-Jones, 2012). Second, behavioral paradigms differ across studies, making comparison of activation of ORX neuron populations difficult. In general, data from both pharmacological and immunohistochemical measures of activation demonstrate that ORX neurons, particularly those in the lateral orexin cell regions, are directly involved in regulating motivation for both natural and drug reward (Mahler et al., 2014). In particular, a clear and consistent relationship of this nature has been demonstrated for alcohol seeking (Lawrence, 2010). Future work is needed to understand which ORX neurons are involved in specific aspects of alcohol (and other drug) seeking so that certain elements of addiction such as compulsive craving can be targeted through the ORX system while leaving natural reward processes and other functions of the ORX system (e.g., regulation of arousal) intact.
Acknowledgments
Supported by PHS grants R21-DA032005, R37/R01-DA006214, P50-AA010761, UL1-RR029882, NHMRC CJ Martin Fellowship 1072706.
Footnotes
The authors declare no conflicts of interest regarding this work.
References
- Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM. Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Frontiers in neuroscience. 2014;8:33. doi: 10.3389/fnins.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, Richardson KA. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 2009;56(Suppl 1):112–121. doi: 10.1016/j.neuropharm.2008.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- Barson JR, Ho HT, Leibowitz SF. Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addiction biology. 2015;20:469–481. doi: 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Khoo SY, Lawrence AJ. Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2013;16:2067–2079. doi: 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- Brown RM, Lawrence AJ. Ascending orexinergic pathways and alcohol-seeking. Current opinion in neurobiology. 2013;23:467–472. doi: 10.1016/j.conb.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Cason AM, Aston-Jones G. Role of orexin/hypocretin in conditioned sucrose-seeking in rats. Psychopharmacology (Berl) 2013;226:155–165. doi: 10.1007/s00213-012-2902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, Willie JT, Beuckmann CT, Chemelli RM, Sakurai T, Yanagisawa M, Saper CB, Scammell TE. Orexin (hypocretin) neurons contain dynorphin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Hobin MP, Petrovich GD. Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience. 2015;286:187–202. doi: 10.1016/j.neuroscience.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biological psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Frontiers in pharmacology. 2014;5:16. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Sutcliffe JG. The hypocretins/orexins: novel hypothalamic neuropeptides involved in different physiological systems. Cell Mol Life Sci. 1999;56:473–480. doi: 10.1007/s000180050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA. The Orexin-1 Receptor Antagonist SB-334867 Reduces Alcohol Relapse Drinking, but not Alcohol-Seeking, in Alcohol-Preferring (P) Rats. Journal of addiction medicine. 2010;4:153–159. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XB, Horvath T. Function and dysfunction of hypocretin/orexin: an energetics point of view. Annual review of neuroscience. 2014;37:101–116. doi: 10.1146/annurev-neuro-071013-013855. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Clemens KJ, McNally GP. Renewal of extinguished cocaine-seeking. Neuroscience. 2008;151:659–670. doi: 10.1016/j.neuroscience.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends in neurosciences. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behavioural brain research. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Bryant JL, Boughter JD, Heck DH. A low-cost solution to measure mouse licking in an electrophysiological setup with a standard analog-to-digital converter. J Neurosci Methods. 2006;153:203–207. doi: 10.1016/j.jneumeth.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Frontiers in behavioral neuroscience. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krivdic B, Krstew E, Lawrence AJ. The orexin(1) receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain research. 2011;1391:54–59. doi: 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, de Lecea L, Ciccocioppo R. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo SY, Brown RM. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS drugs. 2014;28:713–730. doi: 10.1007/s40263-014-0179-x. [DOI] [PubMed] [Google Scholar]
- Lasheras MC, Laorden ML, Milanes MV, Nunez C. Corticotropin-releasing factor 1 receptor mediates the activity of the reward system evoked by morphine-induced conditioned place preference. Neuropharmacology. 2015;95:168–180. doi: 10.1016/j.neuropharm.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ. Regulation of alcohol-seeking by orexin (hypocretin) neurons. Brain research. 2010;1314:124–129. doi: 10.1016/j.brainres.2009.07.072. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. British journal of pharmacology. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:13309–13326. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nature neuroscience. 2014;17:1298–1303. doi: 10.1038/nn.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Progress in brain research. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinny CJ, Jr, Lewin AH, Mascarella SW, Runyon S, Brieaddy L, Carroll FI. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorg Med Chem Lett. 2012;22:6661–6664. doi: 10.1016/j.bmcl.2012.08.109. [DOI] [PubMed] [Google Scholar]
- McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo Pich E, Melotto S. Orexin 1 receptor antagonists in compulsive behavior and anxiety: possible therapeutic use. Frontiers in neuroscience. 2014;8:26. doi: 10.3389/fnins.2014.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin-1 receptor antagonism decreases ethanol consumption and preference selectively in high-ethanol--preferring Sprague--Dawley rats. Alcohol. 2009;43:379–386. doi: 10.1016/j.alcohol.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA., Jr Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE. Binge-like consumption of ethanol and other salient reinforcers is blocked by orexin-1 receptor inhibition and leads to a reduction of hypothalamic orexin immunoreactivity. Alcohol Clin Exp Res. 2015;39:21–29. doi: 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Flores A, Martin-Garcia E, Saravia R, Maldonado R, Berrendero F. A role for hypocretin/orexin receptor-1 in cue-induced reinstatement of nicotine-seeking behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38:1724–1736. doi: 10.1038/npp.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, Bartlett SE. Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 2008;199:109–117. doi: 10.1007/s00213-008-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KA, Aston-Jones G. Lateral hypothalamic orexin/hypocretin neurons that project to ventral tegmental area are differentially activated with morphine preference. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3809–3817. doi: 10.1523/JNEUROSCI.3917-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nature reviews. Neuroscience. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur J Neurosci. 2012a;36:3549–3558. doi: 10.1111/j.1460-9568.2012.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012b;32:4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schone C, Burdakov D. Glutamate and GABA as rapid effectors of hypothalamic "peptidergic" neurons. Frontiers in behavioral neuroscience. 2012;6:81. doi: 10.3389/fnbeh.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, Bartlett SE. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JL, Borgland SL. A role for hypocretin/orexin in motivation. Behavioural brain research. 2011;217:446–453. doi: 10.1016/j.bbr.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Frontiers in behavioral neuroscience. 2013;7:28. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees CM, Cunningham CL. Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacology (Berl) 2011;214:805–818. doi: 10.1007/s00213-010-2082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winrow CJ, Renger JJ. Discovery and development of orexin receptor antagonists as therapeutics for insomnia. British journal of pharmacology. 2014;171:283–293. doi: 10.1111/bph.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Maximization of ethanol intake in the rat. Adv Exp Med Biol. 1975;59:279–294. doi: 10.1007/978-1-4757-0632-1_19. [DOI] [PubMed] [Google Scholar]
- Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Frontiers in neuroscience. 2014;8:36. doi: 10.3389/fnins.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]