Abstract
Chikungunya, “that which bends up” in the Makonde dialect, is an emerging global health threat, with increasing incidence of neurological complications. Until 2013, Chikungunya infection had been largely restricted to East Africa and the Indian Ocean, with cases within the US reported to be from foreign travel. However, in 2014, over 1 million suspected cases were reported in the Americas, and a recently infected human could serve as an unwitting reservoir for the virus resulting in an epidemic in the continental US. Chikungunya infection is increasingly being associated with neurological sequelae. In this study, we sought to understand the role of astrocytes in the neuropathogenesis of Chikungunya infection. Even after virus has been cleared form the circulation, astrocytes were activated with regards to TLR2 expression. In addition white matter astrocytes were hypertrophic, with increased arbor volume in gray matter astrocytes. Combined, these would alter the number and distribution of synapses that each astrocyte would be capable of forming. These results provide the first evidence that Chikungunya infection induces morphometric and innate immune activation of astrocytes in vivo. Perturbed glia-neuron signaling could be a major driving factor in the development of Chikungunya-associated neuropathology.
INTRODUCTION
Chikungunya, is a single stranded RNA virus of the togaviridae family, the same family as Eastern and Western Equine Encephalitis viruses. The term Chikungunya comes from the Makonde dialect, “that which bends up” referring to posture that many of those infected adopt due to the extreme pain during infection [36]. Previous outbreaks of the Chikungunya virus in Reunion and India infected over 1.5 million people [15,35].
Until December 2013, Chikungunya infection had been largely restricted to East Africa and the Indian Ocean. This was when the first case of Chikungunya in the Western hemisphere was reported on Sint Maarten. As the Aedes mosquitoes that are responsible for the spread of the disease are also found in South and North America, there is no biological basis for this debilitating disease from becoming endemic in the United States. By the first half of 2014, there were over 1,500 cases of Chikungunya reported in the Caribbean and Southern United States, with an accelerating incidence (www.cdc.gov/chikungunya). While most of the cases within the US were reported to be from foreign travel, a recently infected human could serve as an unwitting reservoir for the virus resulting in an epidemic in the continental US.
Following a bite from an infected Aedes mosquito (either albopictus or aegypti species), there is a brief incubation period of up to ten days. Symptoms commonly include: fever (up to 40 °C), headache, nausea, edema, petechiae, and joint pain affecting multiple joints [5]. The fever commonly lasts for two days, but other symptoms including headaches and the characteristic prostration (for which the Makonde named the disease) can last for five to seven days. Older patients have reported lingering joint pain for over two years after the initial symptoms [31]. The virus can be found in synovial macrophages for up to 16 months post initial infection [14]. As both synovia and brain are immune privileged, we wondered what long term effects Chikungunya infection would affect the brain of primates.
Chikungunya infection is associated with neurological sequelae [8], and new strains of the virus are apparently becoming increasingly neuropathogenic [12]. The Chikugunya virus can be detected in brain within 2 days of experimental infection [11]. This infection induces increased expression of numerous genes associated with viral infection including IL-6 and TLR3 [24]. Both of these genes can be expressed by astrocytes during viral neuroinflammation, and astrocyte-derived cells have recently been shown to be capable of infection by Chikungunya virus [2].
In this study, we sought to understand the neuropathogenesis of Chikungunya infection. To this end, we examined frontal cortical brain tissues of cynomolgus macaques following subcutaneous infection with Chikungunya. In addition to detection of viral particles within brain tissues, we examined the innate immune activation of astrocytes and how this impacted their morphology and ability to form synapses.
We noted that even after virus has been cleared form the circulation, astrocytes were activated with regards to TLR2 expression. In addition there was hypertrophy of white matter astroglial cell bodies, together with increased grey matter arbor volume and decreased branching of processes. Combined, these would alter the number and distribution of synapses that each astrocyte would be capable of forming, perhaps accounting for some of the pain felt during the infection and after the main infection is cleared.
MATERIALS AND METHODS
Infection of animals
As is routine for vaccine studies [27], Cynomolgus macaques (Macaca fascicularis) of similar age (>4yr) and weighing 3–6 kg, free of simian immunodeficiency virus (SIV), simian type D retrovirus (SRV), simian T-lymphotropic virus (STLV), and alphavirus antibodies against western (WEEV), Venezuelan (VEEV), eastern equine encephalitis (EEEV), Sindbis (SINV), Semliki Forest (SFV), and Chikungunya (assayed by hemagglutination inhibition), were used. The study was approved by the Institutional Animal Care and Use Committee at Tulane University, and all animals were handled in accordance with guidance from the American Association for Accreditation of Laboratory Animal Care.
For this study seven animals received Chikungunya virus with a single subcutaneous inoculation in the upper deltoid of WT Chikunguna-LaReunion (5.0 log10 PFU in a volume of 100μL), as recently described [27]. Animals were monitored by telemetry and serial blood draws for 35 days before necropsy was performed. Brain tissues were placed in 10% zinc formalin for histopathological analysis. Control animals (n=3) were found in the extensive animal records database at TNPRC. As cynomolgus macaques are often used for vaccine studies, control animals with no experimental manipulations are, unfortunately, rare in our archive.
Immunofluorescence
A standard technique was followed for immunohistochemistry to detect TLR2 and GFAP co-expression [17]. Formalin-fixed, paraffin-embedded tissues were sectioned at 6 μm and mounted onto positively charged glass slides. Sections were baked for overnight at 60°C, deparaffinized in xylene, and then rehydrated in graded concentrations of ethanol. Antigen retrieval was carried out for 20 min using a steamer and a citrate-based antigen unmasking solution (Vector Labs, Burlingame, CA). Tissues were blocked in blocking buffer (Dako) for one hour at room temperature before antibodies were applied. Tissues were incubated with TLR2 (ab24192, Abcam) and GFAP primary antibody (GA-5, Sigma) overnight at 4°C, washed three times with PBS with 0.2% bovine serum albumin (Santa Cruz) (PBS/BSA), and then incubated in the dark for 60 min at room temperature with secondary antibodies directly conjugated with Alexa 488 (green) or Alexa 568 (red) (Molecular Probes/Invitrogen, Carlsbad, CA). Sections were washed three times in PBS/BSA, cover-slipped with Prolong Gold with DAPI (Molecular Probes/Invitrogen), and imaged on a Nikon Eclipse TE2000-U microscope.
Quantification of Astrocyte Morphology
We performed an established protocol for astrocyte morphometric analyses [16,32,17,25,20,19]. All samples were coded and analyzed randomly by a researcher blinded to animal number and condition. Images of non-overlapping fields in frontal cortical sections were captured by fluorescence microscopy at 40X objective (Nikon Eclipse TE2000-U) and analyzed using Neurolucida software (MBF Bioscience). Protoplasmic grey matter astrocytes reside in layers 2–6 and are complex cells with numerous fine processes [22]. We randomly selected astrocytes from layers 3–5 for our analysis. Fibrous white matter astrocytes identified along white matter tracts are generally less complex and were chosen randomly in the area of the anterior cingulate cortex. The fibrous white matter astrocytes were located significantly away from the grey-white matter interface so as not to be mistaken for Layer VI protoplasmic astrocytes. An average of 10 astrocytes with clear cell bodies and processes in both gray and white matter were chosen for reconstruction. The cells chosen were fully intact and did not have processes that touched the edges of the field. The resulting files generated by 2D reconstruction were analyzed with Neurolucida Explorer (MBF Bioscience), generating data of morphological measurements such as cell area, branching points (nodes), arbor length and volume.
Particle Analysis in ImageJ
An unbiased, reproducible automated particle analysis method was employed to quantify the expression of TLR2 in frontal cortex of control macaques and macaques infected with Chikugunya virus using ImageJ (v1.48u4) software [33]. Five to ten images per section were taken at 20X objective. The following sequence of events were used to analyze acquired .tiff images: 1. Open image, 2. Convert to 16-bit image, 3. Set threshold, 4. Analyze particle (Size 0.5 – Infinity, Circularity 0.00-1.00), 5. Save image, 6. Save particle information (count, total area, average size, and area fraction) into an Excel spreadsheet. Units were defined as default ImageJ settings (pixels). Tests for normal distribution of the acquired data (D’Agostino & Pearson omnibus normality test) and subsequent statistical tests for significance of difference between TLR2 expression in controls and dengue fever infected macaques was conducted using Student’s unpaired T test or Mann Whitney test. Significance was set at p < 0.05.
Statistical Analyses
Statistical analyses were performed using GraphPad Prism (version 5, GraphPad software, La Jolla, CA). Normality was assessed by Kolmogorov-Smirnov test, and data that passed normality were analyzed by two-tailed unpaired T-test. Data that were not distributed normally were assessed by Mann-Whitney test to determine significance between groups. Results are expressed as mean ± SEM. For all analyses, significance was set at p < 0.05.
RESULTS
Subcutaneous infection with La Reunion strain of Chikungunya results in seroconversion and replication competent virus
To determine if cynomolgus macaques were productively infected following subcutaneous injection of La Reunion stain virus, we monitored virus, antibody responses (IgG and IgM) and temperature longitudinally [27]. Animals treated with the Chikugunya virus reached peak viremia between 4.0 and 6.0 log10PFU/ml by day 2 post-challenge. IgM specific to Chikungunya was apparent by three days post infection, peaking 13 days post infection. IgG responses were evident by day 6 post infection, continuing to increase throughout the course of this study (not shown). Body temperatures were followed by an implanted subcutaneous radio telemetry transmitters (T34G-8; Konigsberg Instruments, Inc., Pasadena, CA). All infected animals exhibited hypothermia beginning 3–4 days, which was sustained for the duration of the study in 4/7 of the animals. Therefore, all of the animals that received subcutaneous Chikungunya became productively infected (as previously reported [27]). Brain sections were stained for Chikungunya envelope glycoprotein (clone CHK48, BEI Resources, Manassas, VA). At time of necropsy (35 days post infection) there was no immune-reactivity in any of the animals (results not shown).
Chikungunya infection leads to innate immune activation of astrocytes
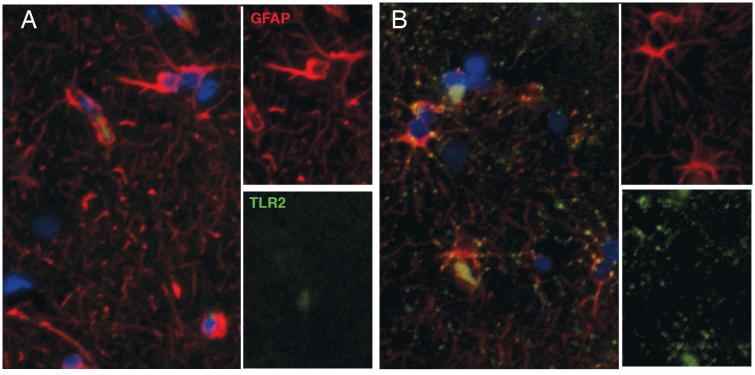
We have previously shown increased levels of TLR2 on astrocytes following activation in bacterial, lentiviral or behavioral diseases [17,20,19]. As we anticipated infection of the CNS with Chikungunya [24,11,2,4], we attempted to determine cell types activated. As shown in Figure 1, there were diffuse punta of TLR2 (green) staining throughout gray matter, in 2/3 controls (A) and all macaques infected with Chikungunya (B). It was noted that the double labeling was prominent perinuclearly and in primary processes. This was not nearly as bright as we have observed in SIV infection [17], but was present throughout gray matter. In the control animals, there was some double labeling, but this was present inconsistently. While much of the TLR2 was expressed on astrocyte processes, there was also TLR2 on areas that were GFAP immunonegative, suggesting other cell types expressed TLR2 also. Further studies would reveal precisely which cells.
Figure 1. Chikungunya infection induces qualitative changes in TLR2 expression.
Control cynomolgus macaques (A) had diffuse expression of TLR2 (green), mainly on astrocytes (red). Following subcutaneous infection with Chikungunya, it TLR2 expression became more pronounced, oftentimes in a perinuclear location (B).
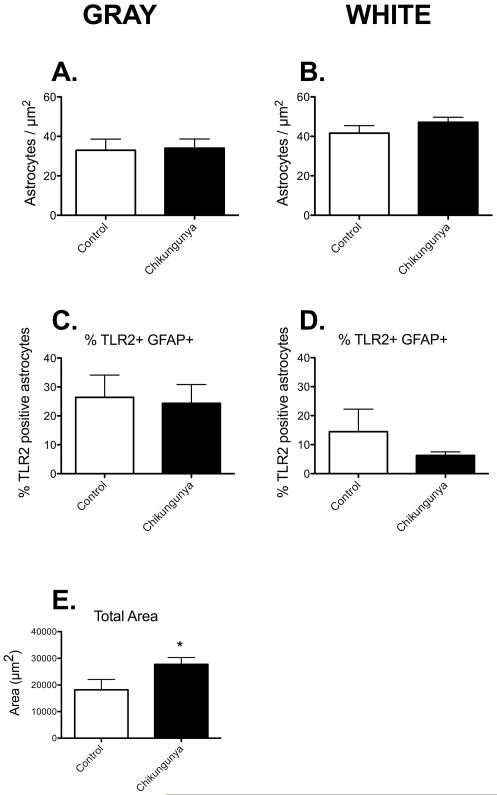
Our previous studies with macaque infection have shown altered numbers of astrocytes and innate immune activation within grey and white matter [18-20]. Therefore, we set out to determine if similar changes in either numbers of astrocytes per unit area, or proportion of astrocytes double positive for TLR2 was altered following infection with Chikungunya virus. We were surprised to note no changes in the number of GFAP+ cells per unit area in either grey (Figure 2A) or white matter (Figure 2B). We were even more surprised to note that there was no significant change in the proportion of astrocytes that were double positive for TLR2 expression (Figures 2C & D, p=0.18 for white matter astrocytes). It should be noted that the basal proportion of TLR2 positive astrocytes in cynomolgus macaques was almost double that observed in other macaque species [17,19]. However, the density of the TLR2 positive puncta was significantly increased in grey matter (Figure 2E). Much of this was contained within astrocyte processes (see Fig 1B), but by no means all.
Figure 2. Chikungunya infection affects TLR2 expression on astrocytes.
There were no significant differences in GFAP-expression in gray and white matter astrocytes of control animals and those infected with Chikugunya (A&B). Quantifying the subset of GFAP-positive astrocytes that were also positive for TLR2, we found no changes in double-labeled astrocytes after infection (C&D). There was an increase the total area of TLR2 expression in the gray matter of infected animals (E). Data is shown as mean ± SEM. Significance is set at *p < 0.05.
Chikungunya infection is associated with white matter astrocyte hypertrophy
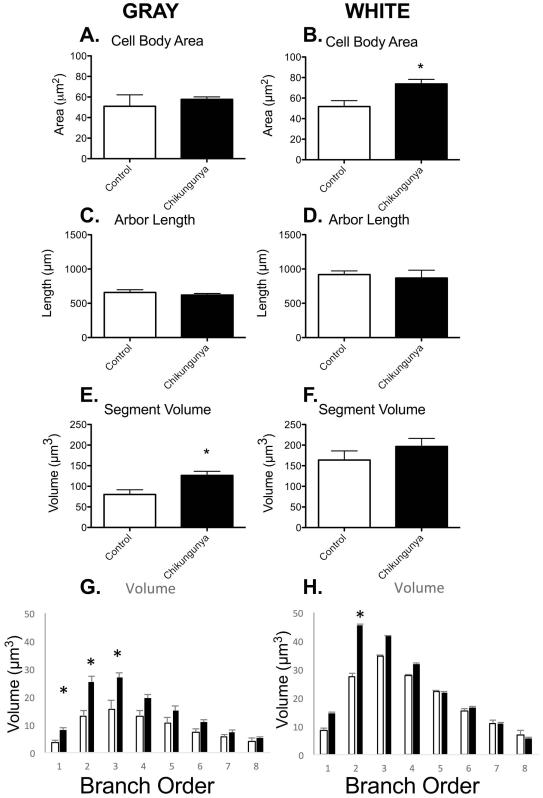
To determine the nature of this innate immune activation on the astrocytes, we performed analyses of key morphometric parameters, as is routine in this group [18-20,25]. There was no change in the size of the cell body in grey matter astrocytes following subcutaneous infection with Chikungunya virus (Figure 3A). However, there was significant hypertrophy of white matter astrocytes (Figure 3B). This was in contrast to examinations of the processes emanating from the cell body. While the total length of arbor was not altered in either grey (Figure 3C) or white matter (Figure 3D), the grey matter processes were apparently swollen, with significantly increased volume of processes (Figure 3E). There was no alteration in the volume of white matter astrocyte processes (Figure 3F). Interestingly, the increased volume in grey matter processes was primarily found in first through third order processes, indicating that the swelling was immediately outside the cell bodies, rather than in distal processes (Figure 3G). The only significant change in white matter astrocytes was in second order branches (Figure 3H).
Figure 3. Cell body hypertrophy of white matter astrocytes in macaques infected with Chikugunya virus.
There was cell body hypertrophy in white matter astrocytes of animals infected with Chikigunya virus (B). There were no differences in total arbor length (C, D) and surface area (not shown) observed between Chikugunya-infected animals and controls. However, total arbor volume was increased in gray matter astrocytes of infected animals indicating hypertrophy or swelling of the processes (E). No significant changes were observed in the total arbor volume in white matter astrocytes (F). This hypertrophy was mainly confined to the first three branches (G). Data is shown as mean ± SEM. Significance is set at *p < 0.05.
Chikungunya infection results in less complex astrocytes
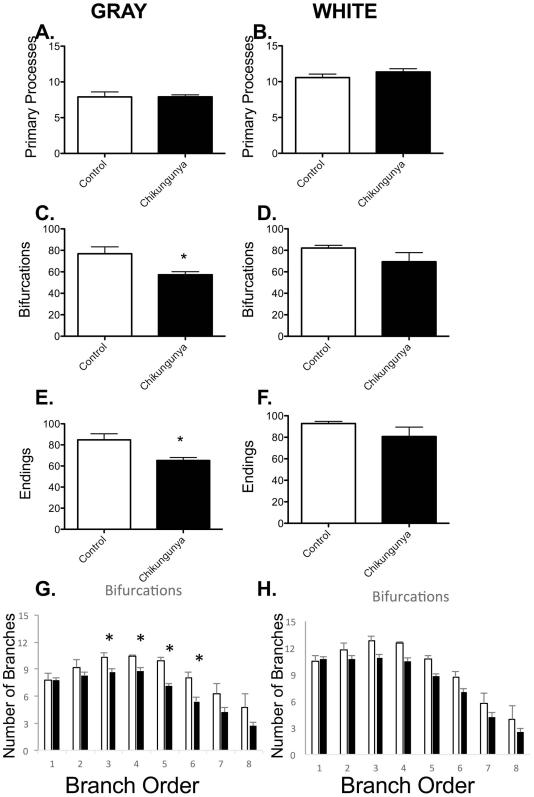
The number of neuronal synapses an astrocyte can integrate with is directly proportional to the number of astrocyte tips. For this reason, we quantified the branching of astrocytes in both grey and white matter. While there were no alterations in the numbers of primary processes leaving astrocyte cell bodies in either gray (Figure 4A) or white matter (Figure 4B), the pattern of branching of these processes was altered. Grey matter astrocytes were found to have fewer bifurcations (Figure 4C) and tips (Figure 4E) following Chikungunya infection. There were no such changes in white matter astrocytes (Figures 4D and F). To determine the nature of these increased branches in grey matter astrocytes, we examined the segment distribution. There were decreased numbers of bifurcations at third through sixth branch orders (Figure 4G), indicating that the pruning of astrocyte processes occurred in existing astrocyte processes, rather than through loss or merging of processes. As anticipated, there was no significant alteration in the branching pattern in white matter astrocytes (Figure 4H).
Figure 4. Decreased complexity of gray matter astrocytes in Chikugunya-infected macaques.
There was no change in the number of processes leaving the cell bodies in either gray (A) or white matter (B) astrocytes. Gray matter astrocytes in CHIK-infected cynomolgus macaques had fewer bifurcations (C) and end points in their processes (E). These differences were no observed in white matter astrocytes (D&F). The decreased branching appeared to cluster between third and sixth order branches in gray matter (G). Data is shown as mean ± SEM. Significance is set at *p < 0.05.
DISCUSSION
Chikungunya is an emerging global health threat, with increasing incidence of neurological complications [12]. Cultured gliomas are capable of being infected with Chikungunya virus [2], and recent studies have demonstrated that cultured astrocytes and oligodendrocytes are highly susceptible to Chikungunya infection [7,8]. Astrocytes are commonly the first cells activated in brain following viral infection [37]. To determine how astrocytes within brain would be impacted, we performed a retrospective analysis of tissues available at the TNPRC archive.
In this study we examined the innate immune activation of astrocytes following subcutaneous Chikungunya infection. This was followed up with measures of morphometric activation of grey matter astrocytes. Grey matter astrocytes had increased TLR2 expression, although the proportion of astrocytes double positive for GFAP and TLR2 was not altered.
Glial cells express several pattern recognition receptors (PRRs), including TLRs, involved in detecting viral particles as well as damage-associated molecular patterns (DAMPs; [13]. These cells can then be induced to express high levels of cytokines and chemokines in response to Chikungunya infection [8]. Virus in the CNS upregulates several genes associated with the activation of the innate immune pathway [24]. TLRs 2,3,4,7,8 and 9 are known to be involved in antiviral immunity [34]. Specifically, TLR2 and TLR4 are activated by viral surface glycoproteins [10]. Though we had observed significant innate immune activation in previous studies, we did not observe any changes in TLR2 staining in GFAP-positive cells in macaques infected with La Reunion strain Chikungunya virus compared to controls in either white or gray matter. Chikungunya is an ssRNA virus and replicates using a dsRNA intermediate. Therefore, potential PRRs for Chikungunya virus may include TLR3, which detects dsRNA, and TLRs 7 and 8, which are triggered by ssRNA [28]. Gene profiling studies indicate that CNS infection with alphavirus is accompanied by upregulation of proinflammatory cytokines and chemokines as well as iNOS and TNF-alpha, which can have direct or indirect neurotoxic effects [30]. Endogenous DAMP molecules released from damaged neurons can bind to TLR2 on nearby glia, and in turn, activate glial cells during CNS trauma and infection [23]. It is interesting that there was no increase in the proportion of TLR2 immunopositive astrocytes, as each of our previous studies showing morphometric changes in astrocytes were combined with increased TLR2 expression [32,17,20,19], although this is the first study with cynomolgus macaques.
Morphometric Experiments
We also performed measures of morphometric activation of grey and white matter astrocytes. White matter astrocytes had swollen cell bodies, but not processes, whereas grey matter astrocytes tended to have swollen cell processes but not cell bodies. Additionally, the grey matter astrocytes had fewer branches following Chikungunya infection, which did not alter the overall length of the astrocyte arbor.
Bioinformatic analysis of Chikungunya-infected rodents before and after infection showed alterations in processes involved in integrin signaling and cytoskeleton dynamics, which are involved in cell growth, immune response, and blood-brain barrier permeability [11]. These analyses also detected altered expression of proteins involved in integrin recycling and the maintenance of cellular adhesion, which could lead to blood-brain barrier disruption, facilitating the migration of Chikungunya virus through the BBB [3].
The downstream effects of astrocyte activation has been reviewed recently [21]. In summary, the composition and volume of the space between astrocytes is a function of the numbers and sizes of astrocytes [29]. Excitotoxicity will lead to neuronal dysfunction[9] and damage to synapses [6,26]. Retraction of astrocyte processes from the microvascular endothelium would impact the energy supply to the brain [1].
This study highlights the importance of astrocytes in Chikungunya neurological infection. Astrocyte activation and immune responses have increasingly become targets of therapeutic interventions in CNS injury and disease. Further research can evaluate potential disease markers and the development of new therapeutic targets to prevent innate immune activation in the CNS.
Author Summary.
With the first case of Chikungunya acquired in the US in 2014, it is only a matter of time before an epidemic breaks out. Chikungunya is transmitted by bites of the Aedes mosquitoes that now inhabit much of the American continents. With new strains of Chikungunya becoming increasingly neuropathogenic, it is critical that we know the mechanisms of neuropathogenesis. Here, we used advanced computer-assisted camera lucida technology to discover morphometric parameters of glial activation. We report that astrocytes are activated in both gray and white matter with swelling in white matter, and increased volume of the astrocyte processes in gray matter. Altered astrocyte-neuron connections are therefore likely following Chikungunya infection, with downstream events likely. Our study provides novel insights in the neuropathogenesis of this emerging pathogen, and the potential new therapeutic target to prevent associated symptoms.
Acknowledgements
This work was supported by a grant from the National Institute of Allergy and Infectious Disease (NIAID) through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (WRCE), National Institutes of Health (NIH) grant U54 AI057156. This work was supported in part through the NIH/OD grant OD-011104-51 (Tulane National Primate Research Center Base grant).
Footnotes
Author Contributions
All authors contributed to the writing of the manuscript. PJD performed pathologic analyses, KML, FMI, KBC performed immunohistochemistry, fluorescence and Neurolucida studies, under the guidance and supervision of AGM. KR-L, SCW and CJR designed and performed the infection studies.
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 2.Abraham R, Mudaliar P, Padmanabhan A, Sreekumar E. Induction of cytopathogenicity in human glioblastoma cells by chikungunya virus. PLoS One. 2013;8(9):e75854. doi: 10.1371/journal.pone.0075854. doi:10.1371/journal.pone.0075854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaire PD, Seyed Sadr M, Chaineau M, Seyed Sadr E, Konefal S, Fotouhi M, Maret D, Ritter B, Del Maestro RF, McPherson PS. Interplay between Rab35 and Arf6 controls cargo recycling to coordinate cell adhesion and migration. J Cell Sci. 2013;126:722–731. doi: 10.1242/jcs.112375. Pt 3. doi:10.1242/jcs.112375. [DOI] [PubMed] [Google Scholar]
- 4.Arpino C, Curatolo P, Rezza G. Chikungunya and the nervous system: what we do and do not know. Reviews in medical virology. 2009;19(3):121–129. doi: 10.1002/rmv.606. doi:10.1002/rmv.606. [DOI] [PubMed] [Google Scholar]
- 5.Chhabra M, Mittal V, Bhattacharya D, Rana U, Lal S. Chikungunya fever: a re-emerging viral infection. Indian journal of medical microbiology. 2008;26(1):5–12. doi: 10.4103/0255-0857.38850. [DOI] [PubMed] [Google Scholar]
- 6.Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res. 2012;10(5):392–406. doi: 10.2174/157016212802138832. doi:CHIVR-EPUB-20120511-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Das T, Hoarau JJ, Bandjee MC, Maquart M, Gasque P. Multifaceted innate immune responses engaged by astrocytes, microglia and resident dendritic cells against Chikungunya neuroinfection. J Gen Virol. 2015;96:294–310. doi: 10.1099/vir.0.071175-0. Pt 2. doi:10.1099/vir.0.071175-0. [DOI] [PubMed] [Google Scholar]
- 8.Das T, Jaffar-Bandjee MC, Hoarau JJ, Krejbich Trotot P, Denizot M, Lee-Pat-Yuen G, Sahoo R, Guiraud P, Ramful D, Robin S, Alessandri JL, Gauzere BA, Gasque P. Chikungunya fever: CNS infection and pathologies of a re-emerging arbovirus. Prog Neurobiol. 2010;91(2):121–129. doi: 10.1016/j.pneurobio.2009.12.006. doi:10.1016/j.pneurobio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Ernst T, Jiang CS, Nakama H, Buchthal S, Chang L. Lower brain glutamate is associated with cognitive deficits in HIV patients: a new mechanism for HIV-associated neurocognitive disorder. Journal of magnetic resonance imaging : JMRI. 2010;32(5):1045–1053. doi: 10.1002/jmri.22366. doi:10.1002/jmri.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finberg RW, Kurt-Jones EA. Viruses and Toll-like receptors. Microbes Infect. 2004;6(15):1356–1360. doi: 10.1016/j.micinf.2004.08.013. doi:10.1016/j.micinf.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 11.Fraisier C, Koraka P, Belghazi M, Bakli M, Granjeaud S, Pophillat M, Lim SM, Osterhaus A, Martina B, Camoin L, Almeras L. Kinetic analysis of mouse brain proteome alterations following Chikungunya virus infection before and after appearance of clinical symptoms. PLoS One. 2014;9(3):e91397. doi: 10.1371/journal.pone.0091397. doi:10.1371/journal.pone.0091397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin DE. Emergence and re-emergence of viral diseases of the central nervous system. Prog Neurobiol. 2010;91(2):95–101. doi: 10.1016/j.pneurobio.2009.12.003. doi:10.1016/j.pneurobio.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayakawa K, Miyamoto N, Seo JH, Pham LD, Kim KW, Lo EH, Arai K. High-mobility group box 1 from reactive astrocytes enhances the accumulation of endothelial progenitor cells in damaged white matter. J Neurochem. 2012 doi: 10.1111/jnc.12120. doi:10.1111/jnc.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzere BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debre P, Autran B, Gasque P. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184(10):5914–5927. doi: 10.4049/jimmunol.0900255. doi:10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 15.Lahariya C, Pradhan SK. Emergence of chikungunya virus in Indian subcontinent after 32 years: A review. Journal of vector borne diseases. 2006;43(4):151–160. [PubMed] [Google Scholar]
- 16.Lee KM, Chiu KB, Didier PJ, Baker KC, MacLean AG. Naltrexone treatment reverses astrocyte atrophy and immune dysfunction in self-harming macaques. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.07.017. doi:10.1016/j.bbi.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KM, Chiu KB, Renner NA, Sansing HA, Didier PJ, MacLean AG. Form follows function: astrocyte morphology and immune dysfunction in SIV neuroAIDS. J Neurovirol. 2014;20(5):474–484. doi: 10.1007/s13365-014-0267-1. doi:10.1007/s13365-014-0267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KM, Chiu KB, Renner NA, Sansing HA, Didier PJ, MacLean AG. Form follows function: astrocyte morphology and immune dysfunction in SIV neuroAIDS. J Neurovirol. 2014 doi: 10.1007/s13365-014-0267-1. doi:10.1007/s13365-014-0267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KM, Chiu KB, Sansing HA, Didier PJ, Ficht TA, Arenas-Gamboa AM, Roy CJ, Maclean AG. Aerosol-induced brucellosis increases TLR-2 expression and increased complexity in the microanatomy of astroglia in rhesus macaques. Frontiers in cellular and infection microbiology. 2013;3:86. doi: 10.3389/fcimb.2013.00086. doi:10.3389/fcimb.2013.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KM, Chiu KB, Sansing HA, Inglis FM, Baker KC, Maclean AG. Astrocyte atrophy and immune dysfunction in self-harming macaques. PLoS One. 2013;8(7):e69980. doi: 10.1371/journal.pone.0069980. doi:10.1371/journal.pone.0069980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee KM, MacLean AG. New advances on glial activation in health and disease. World J Virol. 2015;4(2):42–55. doi: 10.5501/wjv.v4.i2.42. doi:10.5501/wjv.v4.i2.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberheim NA, Goldman SA, Nedergaard M. Heterogeneity of astrocytic form and function. Methods Mol Biol. 2012;814:23–45. doi: 10.1007/978-1-61779-452-0_3. doi:10.1007/978-1-61779-452-0_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park C, Cho IH, Kim D, Jo EK, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. Toll-like receptor 2 contributes to glial cell activation and heme oxygenase-1 expression in traumatic brain injury. Neurosci Lett. 2008;431(2):123–128. doi: 10.1016/j.neulet.2007.11.057. doi:10.1016/j.neulet.2007.11.057. [DOI] [PubMed] [Google Scholar]
- 24.Priya R, Patro IK, Parida MM. TLR3 mediated innate immune response in mice brain following infection with Chikungunya virus. Virus Res. 2014;189C:194–205. doi: 10.1016/j.virusres.2014.05.010. doi:10.1016/j.virusres.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Renner NA, Sansing HA, Inglis FM, Mehra S, Kaushal D, Lackner AA, Maclean AG. Transient acidification and subsequent proinflammatory cytokine stimulation of astrocytes induce distinct activation phenotypes. J Cell Physiol. 2013;228(6):1284–1294. doi: 10.1002/jcp.24283. doi:10.1002/jcp.24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi DJ. Astrocytes join the plasticity party. Nature neuroscience. 2012;15(5):649–651. doi: 10.1038/nn.3095. doi:10.1038/nn.3095 nn.3095 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy CJ, Adams AP, Wang E, Plante K, Gorchakov R, Seymour RL, Vinet-Oliphant H, Weaver SC. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis. 2014;209(12):1891–1899. doi: 10.1093/infdis/jiu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz O, Albert ML. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol. 2010;8(7):491–500. doi: 10.1038/nrmicro2368. doi:10.1038/nrmicro2368. [DOI] [PubMed] [Google Scholar]
- 29.Shao Y, Enkvist MO, McCarthy KD. Glutamate blocks astroglial stellation: effect of glutamate uptake and volume changes. Glia. 1994;11(1):1–10. doi: 10.1002/glia.440110103. doi:10.1002/glia.440110103. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Bhattacharya B, Puri RK, Maheshwari RK. Venezuelan equine encephalitis virus infection causes modulation of inflammatory and immune response genes in mouse brain. BMC Genomics. 2008;9:289. doi: 10.1186/1471-2164-9-289. doi:10.1186/1471-2164-9-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon F, Parola P, Grandadam M, Fourcade S, Oliver M, Brouqui P, Hance P, Kraemer P, Ali Mohamed A, de Lamballerie X, Charrel R, Tolou H. Chikungunya infection: an emerging rheumatism among travelers returned from Indian Ocean islands. Report of 47 cases. Medicine. 2007;86(3):123–137. doi: 10.1097/MD/0b013e31806010a5. doi:10.1097/MD/0b013e31806010a5. [DOI] [PubMed] [Google Scholar]
- 32.Snook ER, Fisher-Perkins JM, Sansing HA, Lee KM, Alvarez X, MacLean AG, Peterson KE, Lackner AA, Bunnell BA. Innate immune activation in the pathogenesis of a murine model of globoid cell leukodystrophy. Am J Pathol. 2014;184(2):382–396. doi: 10.1016/j.ajpath.2013.10.011. doi:10.1016/j.ajpath.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Symes AJ, Eilertsen M, Millar M, Nariculam J, Freeman A, Notara M, Feneley MR, Patel HR, Masters JR, Ahmed A. Quantitative analysis of BTF3, HINT1, NDRG1 and ODC1 protein over-expression in human prostate cancer tissue. PLoS One. 2013;8(12):e84295. doi: 10.1371/journal.pone.0084295. doi:10.1371/journal.pone.0084295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. doi:10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- 35.Tsetsarkin K, Higgs S, McGee CE, De Lamballerie X, Charrel RN, Vanlandingham DL. Infectious clones of Chikungunya virus (La Reunion isolate) for vector competence studies. Vector borne and zoonotic diseases. 2006;6(4):325–337. doi: 10.1089/vbz.2006.6.325. doi:10.1089/vbz.2006.6.325. [DOI] [PubMed] [Google Scholar]
- 36.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med. 2015;372(13):1231–1239. doi: 10.1056/NEJMra1406035. doi:10.1056/NEJMra1406035. [DOI] [PubMed] [Google Scholar]
- 37.Zlotnik I. The reaction of astrocytes to acute virus infections of the central nervous system. Br J Exp Pathol. 1968;49(6):555–564. [PMC free article] [PubMed] [Google Scholar]